Studies on the CO2 Capture by Coal Fly Ash Zeolites: Process Design and Simulation
Abstract
:1. Introduction
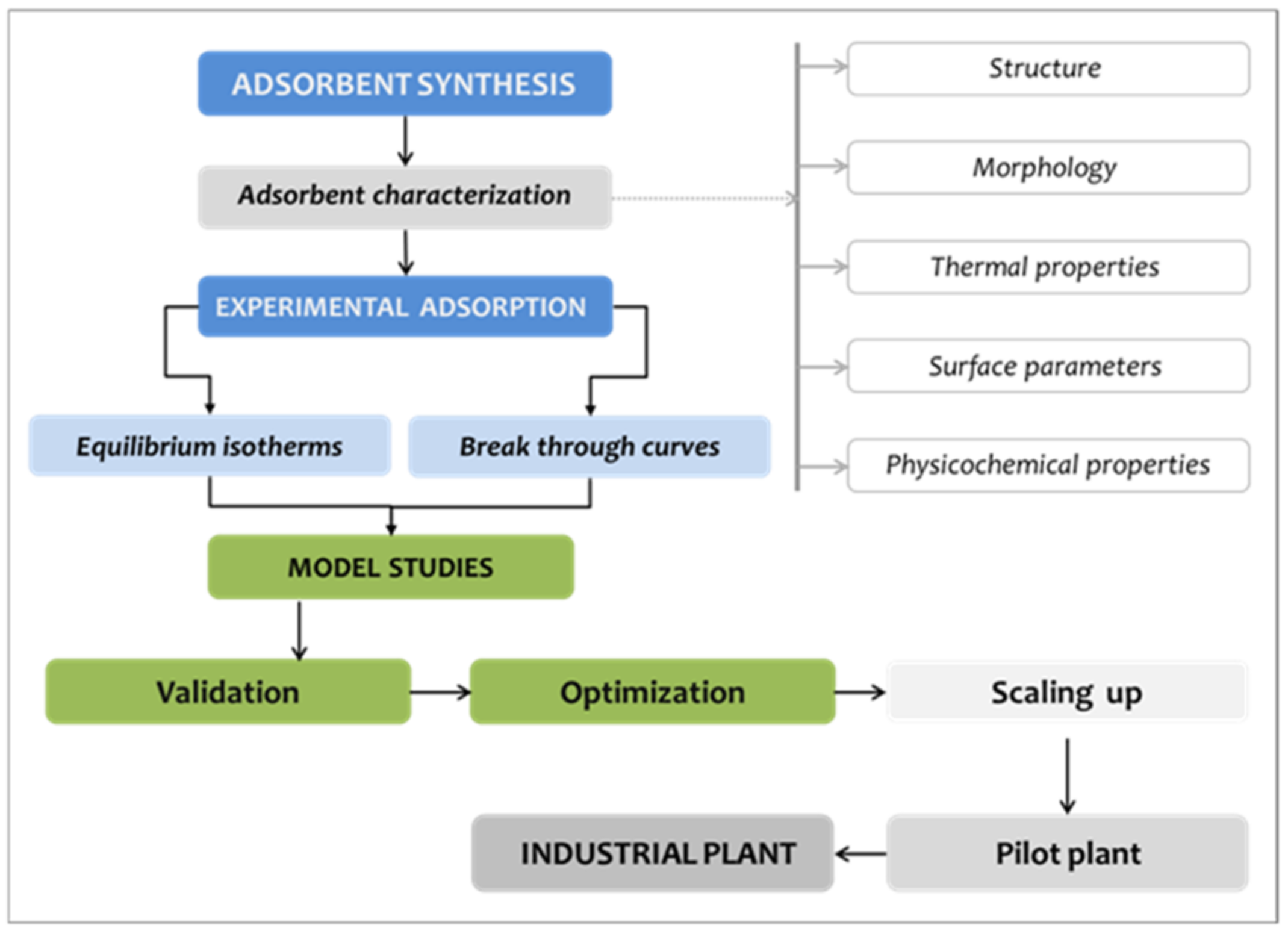
2. Materials and Methods
2.1. Material Characterization
2.2. Experimental Studies on CO2 Adsorption
3. Results
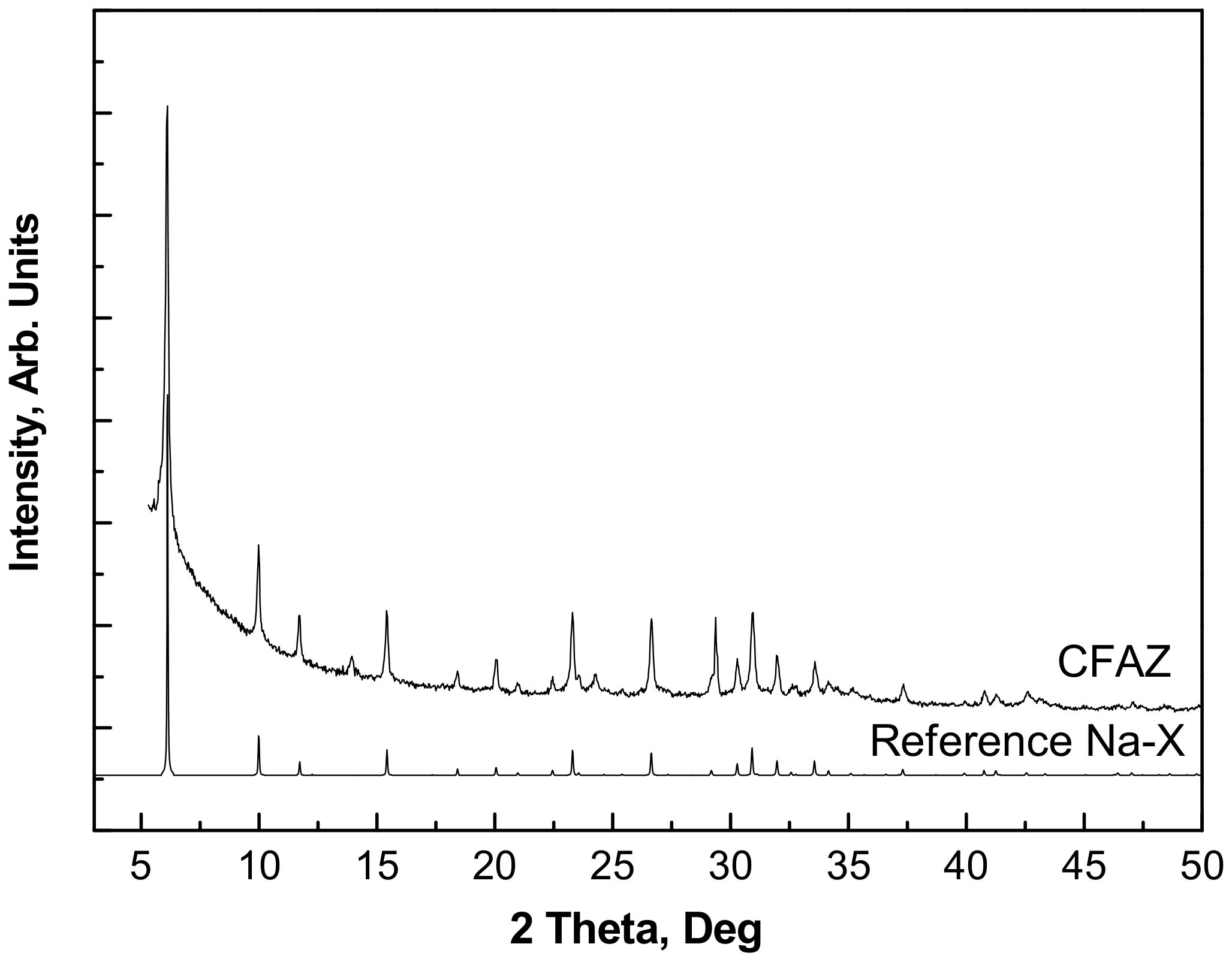
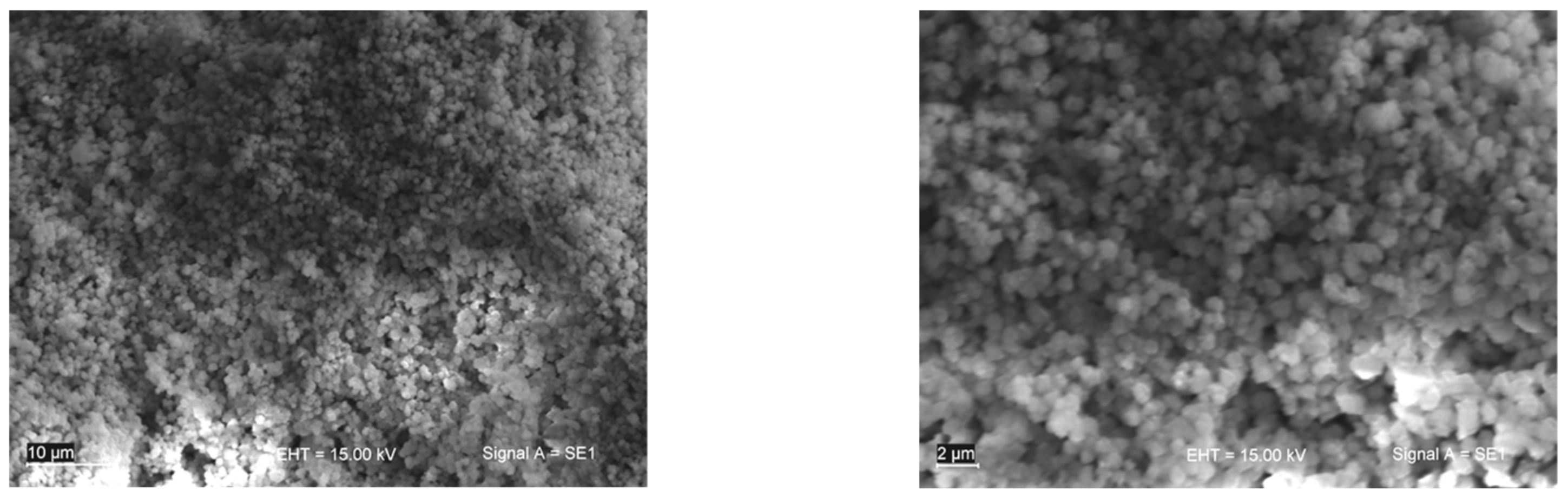
3.1. Material Characterization
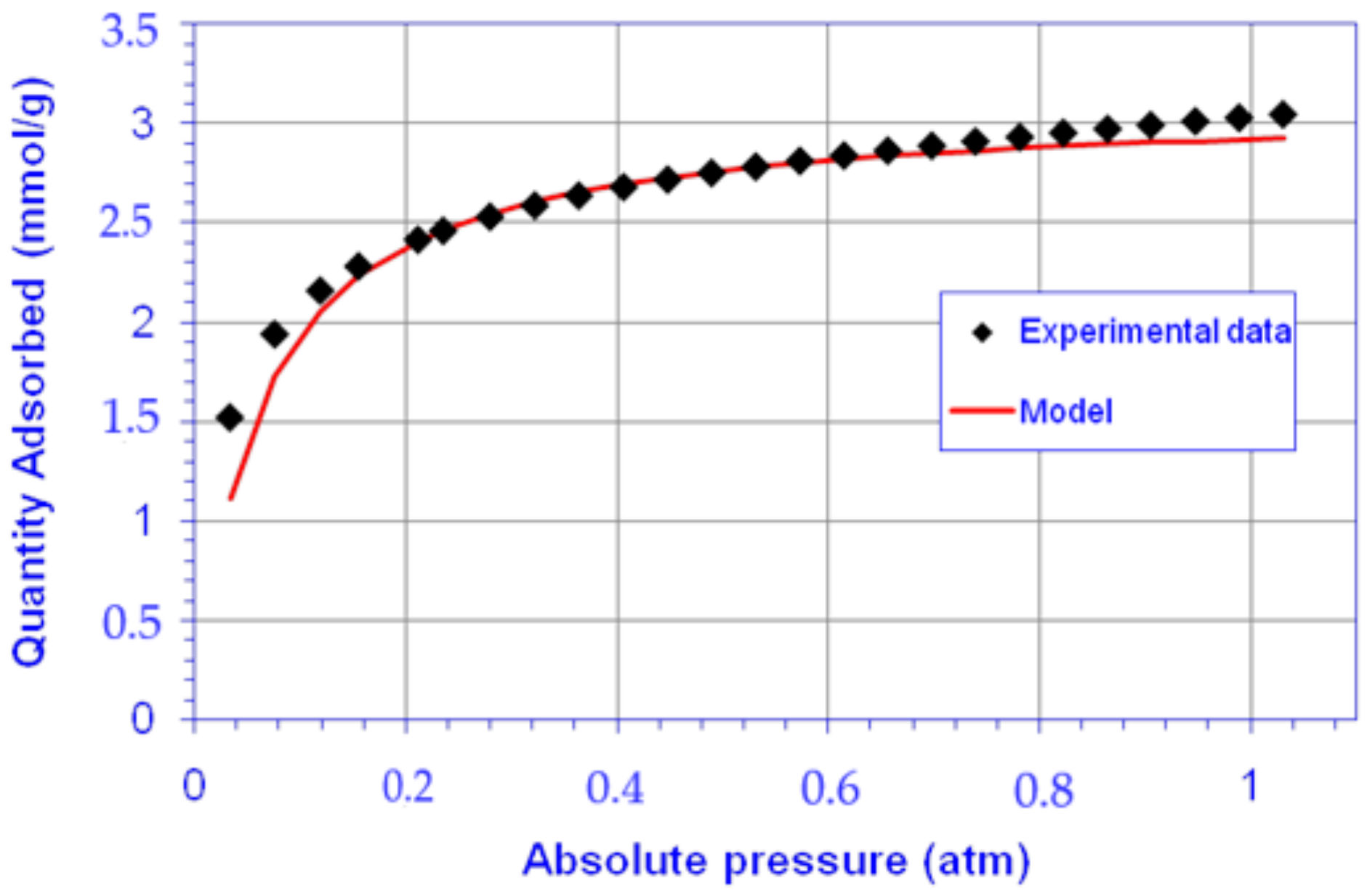
3.2. CO2 Adsorption Studies onto CFAZ
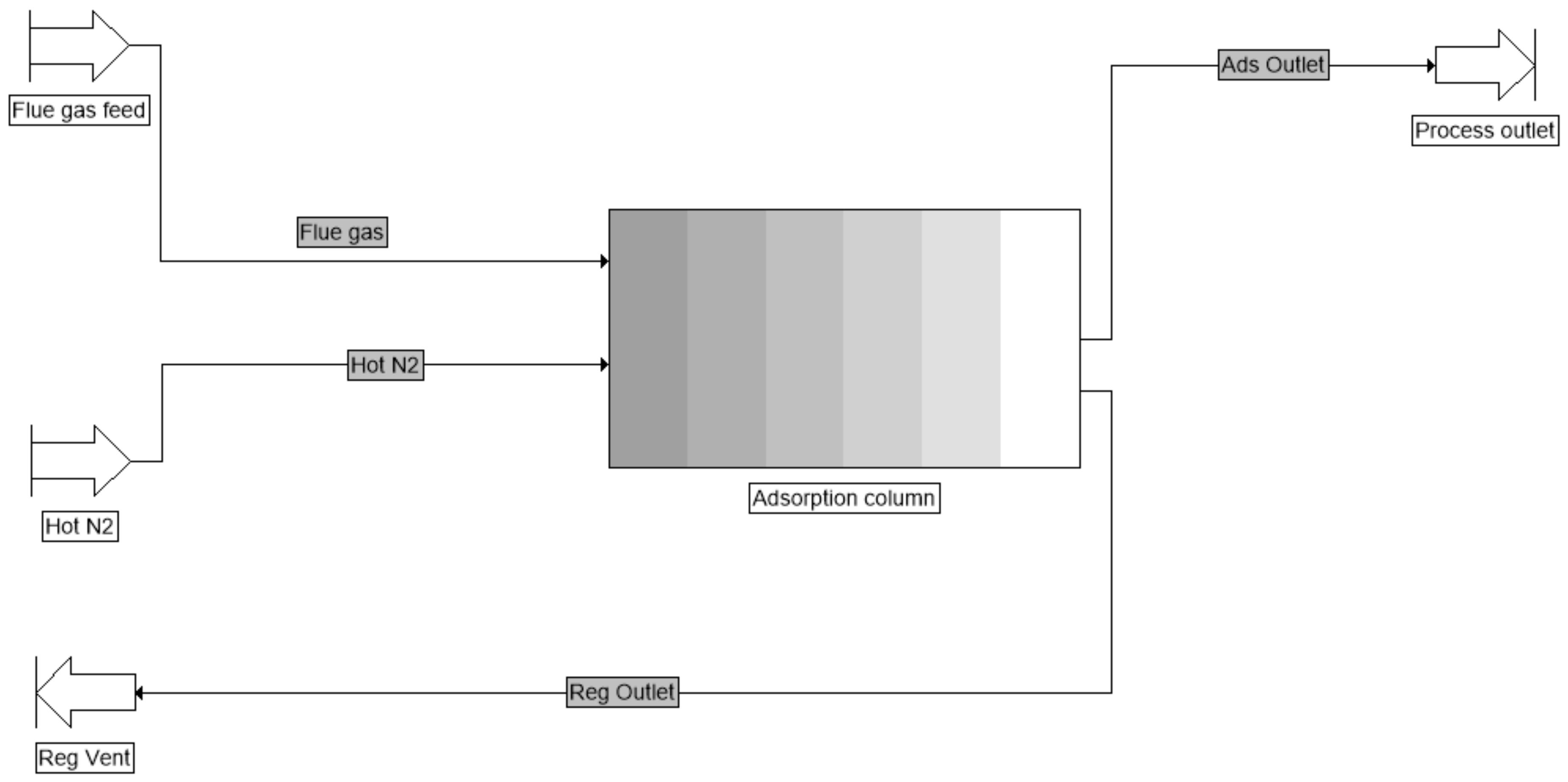
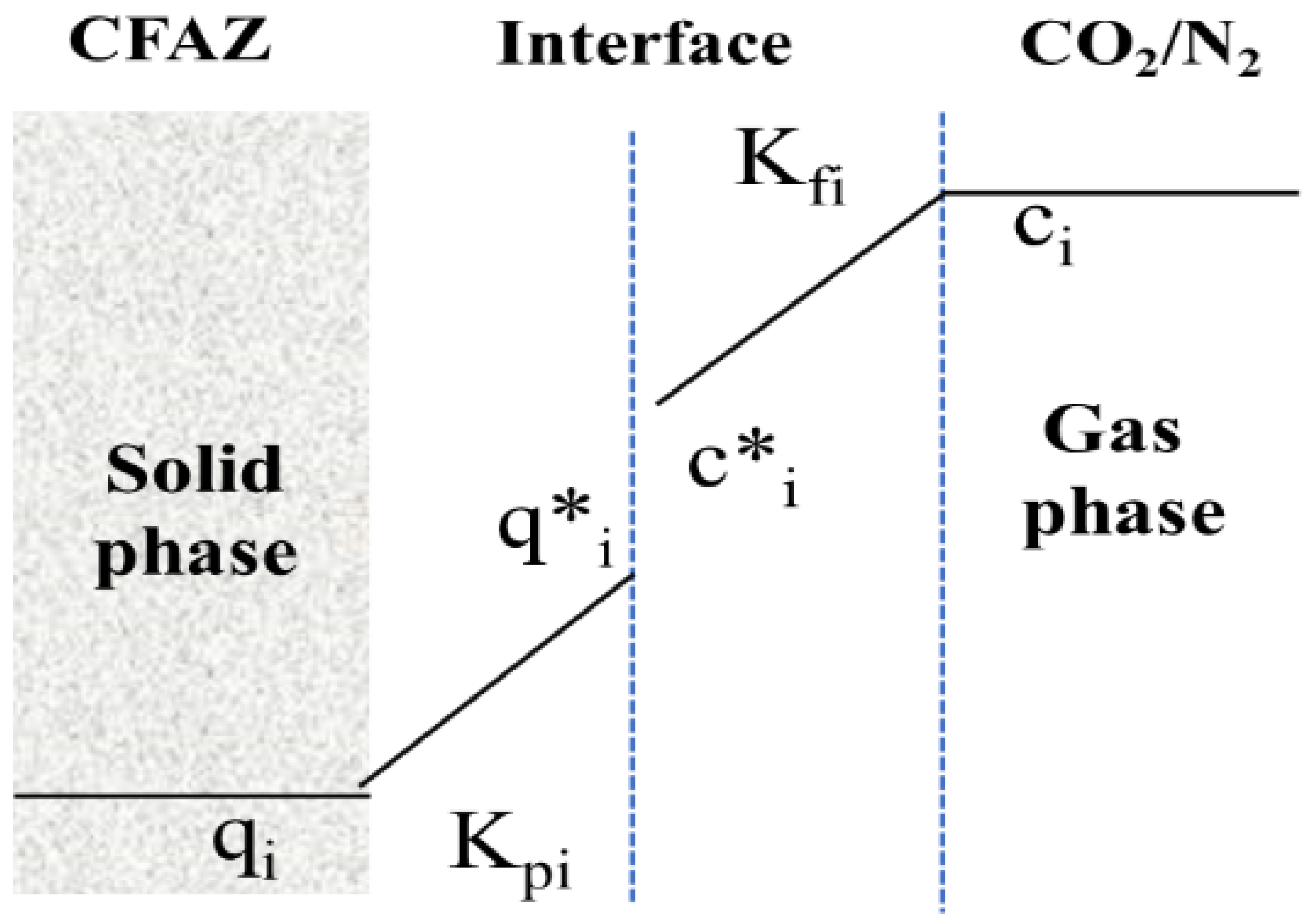
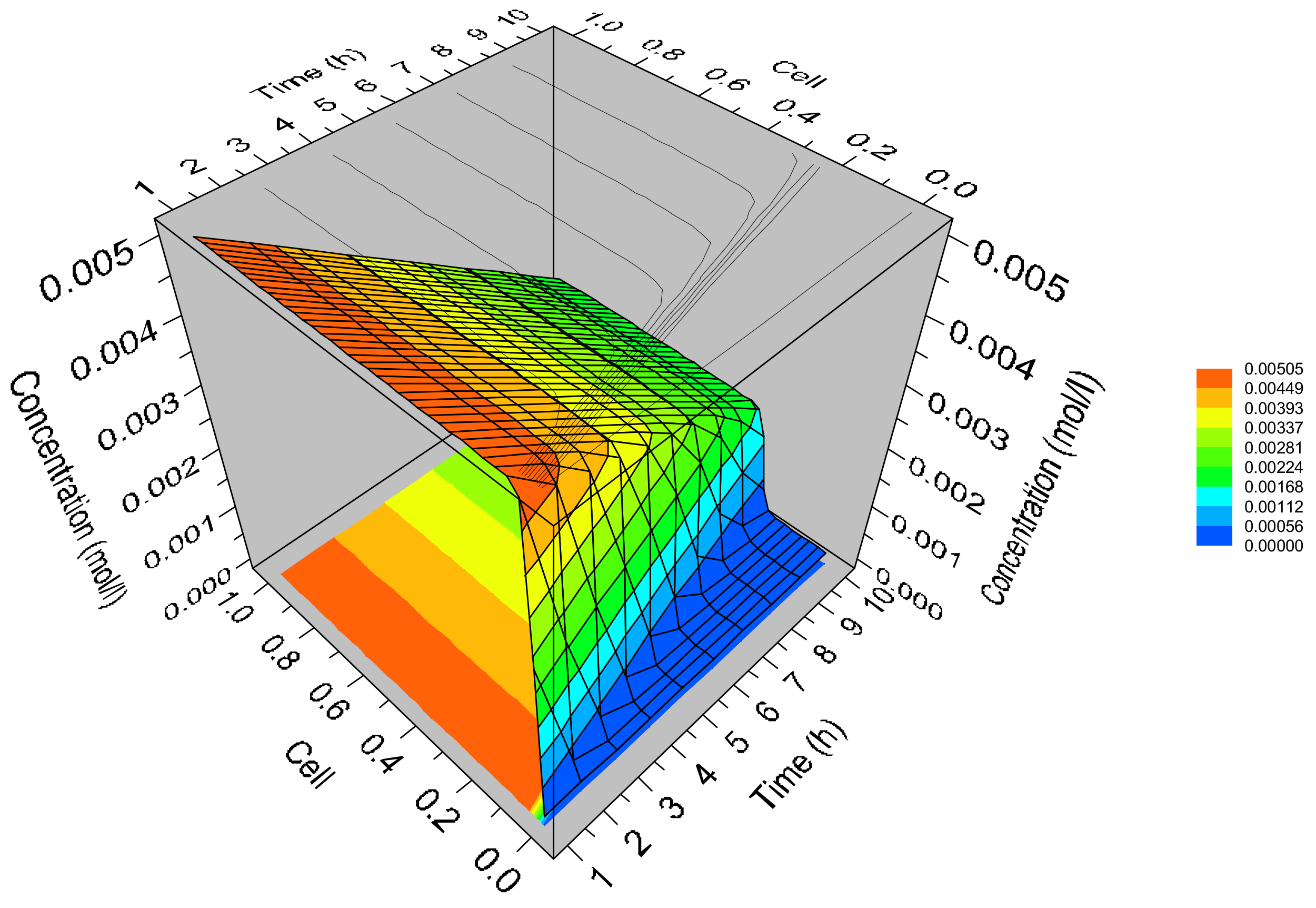
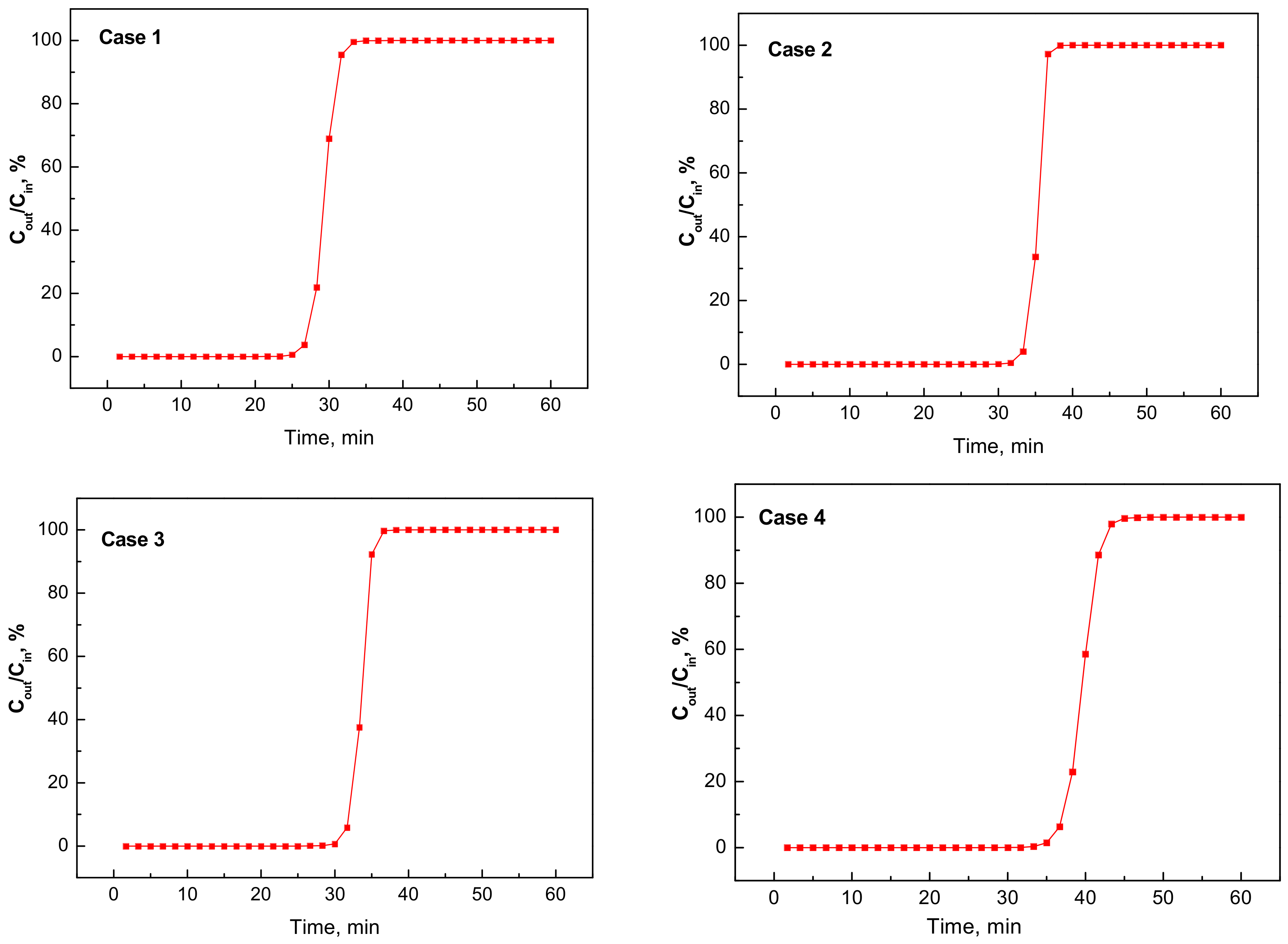
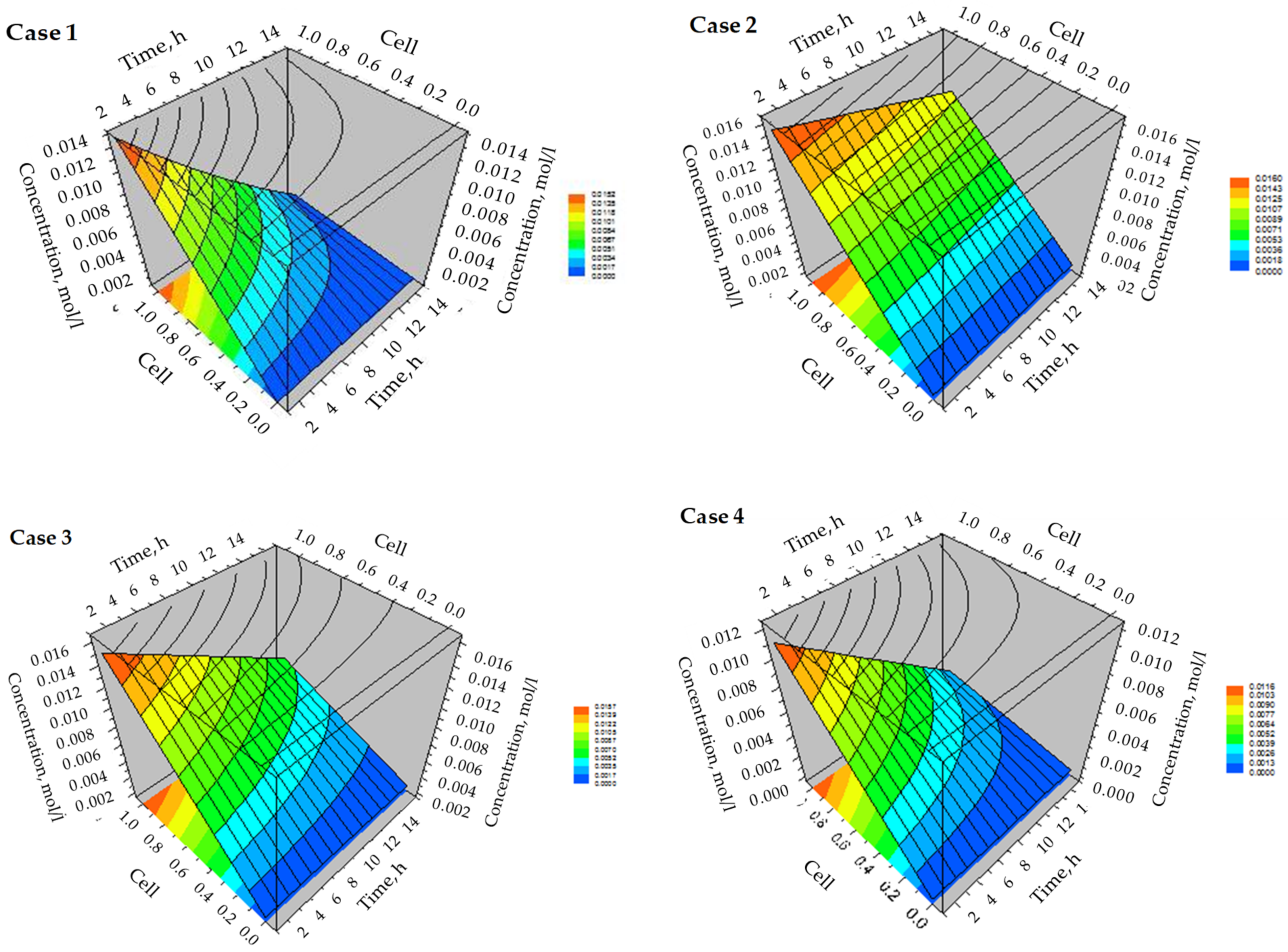
3.3. Simulation of Dynamic Thermal-Swing Adsorption in the CFAZ-CO2 System
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Energy Agency: IEA. Available online: https://www.iea.org (accessed on 13 November 2021).
- United Nations Climate Change. Available online: https://www.unfccc.int (accessed on 13 November 2021).
- Herzog, H.J. Peer reviewed: What future for carbon capture and sequestration? New technologies could reduce carbon dioxide emissions to the atmosphere while still allowing the use of fossil fuels. Environ. Sci. Technol. 2001, 35, 148A–153A. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Z.-W.; Rongwong, W.; Liu, H.; Fu, K.; Gao, H.; Cao, F.; Zhang, R.; Sema, T.; Henni, A.; Sumon, K.; et al. Recent progress and new developments in post-combustion carbon-capture technology with amine based solvents. Int. J. Greenh. Gas Con. 2015, 40, 26–54. [Google Scholar] [CrossRef] [Green Version]
- Plaza, J.M.; Van Wagenera, D.; Rochellea, G.T. Modeling CO2 capture with aqueous monoethanolamine. Energ. Proc. 2009, 1, 1171–1178. [Google Scholar] [CrossRef] [Green Version]
- Kárászová, M.; Zach, B.; Petrusová, Z.; Červenka, V.; Bobák, M.; Šyc, M.; Izák, P. Post-combustion carbon capture by membrane separation. Review. Sep. Purif. Technol. 2020, 238, 116448. [Google Scholar] [CrossRef]
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-combustion CO2 capture using solid sorbents: A review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Berger, A.H.; Bhown, A.S. Optimizing solid sorbents for CO2 capture. Energ. Proc. 2013, 37, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Hollman, G.G.; Steenbruggen, G.; Jurkovičová, M.J. A two-step process for the synthesis of zeolites from coal fly ash. Fuel 1999, 78, 1225–1230. [Google Scholar] [CrossRef]
- Boycheva, S.; Behunová, D.; Václavíková, M. Smart- and zero-energy utilization of coal ash from thermal power plants in the context of circular economy and related to soil recovery. J. Environ. Eng. 2020, 146, 04020081. [Google Scholar]
- Yao, Z.T.; Ji, X.S.; Sarker, P.K.; Tang, J.H.; Ge, L.Q.; Xia, M.S.; Xi, Y.Q. A comprehensive review on the applications of coal fly ash. Earth-Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Mendoza, E.Y.M.; Santos, A.S.; López, E.V.; Drozd, V.; Durygin, A.; Chen, J.; Saxena, S.K. Iron oxides as efficient sorbents for CO2 capture. J. Mat. Res. Technol. 2019, 8, 2944–2956, doiorg/101016/jjmrt201905002. [Google Scholar] [CrossRef]
- Boycheva, S.; Zgureva, D.; Lazarova, H.; Popova, M. Comparative studies of carbon capture onto coal fly ash zeolites Na-X and Na–Ca-X. Chemosphere 2021, 271, 129505. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, L.; Zhao, L.; Deng, S.; Li, S.; Zhang, Y. A comprehensive performance evaluation of temperature swing adsorption for post-combustion carbon dioxide capture. Renew. Sustain. Energ. Rev. 2019, 114, 109285. [Google Scholar] [CrossRef]
- Chue, K.T.; Kim, J.N.; Yoo, Y.J.; Cho, S.H.; Yang, R.T. Comparison of activated carbon and zeolite 13X for CO2 recovery from flue gas by Pressure Swing Adsorption. Ind. Eng. Chem. Res. 1995, 34, 591–598. [Google Scholar] [CrossRef]
- Azarabadi, H.; Lackner, K.S. A sorbent-focused techno-economic analysis of direct air capture. Appl. Energ. 2019, 250, 959–975. [Google Scholar] [CrossRef]
- Baiker, A. Utilization of carbon dioxide in heterogeneous catalytic synthesis. Appl. Organometal. Chem. 2000, 14, 751–762, doiorg/101002/1099. [Google Scholar] [CrossRef]
- Marchese, M.; Buffo, G.; Santarelli, M.; Lanzini, A. CO2 from direct air capture as carbon feedstock for Fischer-Tropsch chemicals and fuels: Energy and economic analysis. J. CO2 Utiliz. 2021, 46, 101487. [Google Scholar] [CrossRef]
- Popova, M.; Boycheva, S.; Lazarova, H.; Zgureva, D.; Lázár, K.; Szegedi, Á. VOC oxidation and CO2 adsorption on dual adsorption/catalytic system based on fly ash zeolites. Catal. Tod. 2020, 357, 518–525. [Google Scholar] [CrossRef]
- Boycheva, S.; Marinov, I.; Miteva, S.; Zgureva, D. Conversion of coal fly ash into nanozeolite Na-X by applying ultrasound assisted hydrothermal and fusion-hydrothermal alkaline activation. Sustain. Chem. Pharm. 2020, 15, 100217. [Google Scholar] [CrossRef]
- Zgureva, D.; Boycheva, S. Experimental and model investigations of CO2 adsorption onto fly ash zeolite surface in dynamic conditions. Sustain. Chem. Pharm. 2020, 15, 100222. [Google Scholar] [CrossRef]
- International Zeolite Association (IZA). Available online: https://www.iza-online.org (accessed on 30 November 2021).
- Uppili, S.; Thomas, K.J.; Crompton, E.M.; Ramamurthy, V. Probing zeolites with organic molecules: supercages of X and Y zeolites are superpolar. Langmuir 2000, 16, 265–274. [Google Scholar] [CrossRef]
- Osatiashtiani, A.; Puertolas, B.; Oliveira, C.C.S.; Manayil, J.C.; Barbero, B.; Isaacs, M.; Michailof, C.; Heracleous, E.; Pérez-Ramírez, J.; Lee, A.F.; et al. On the influence of Si:Al ratio and hierarchical porosity of FAU zeolites in solid acid catalysed esterification pretreatment of bio-oil. Biomass Conv. Bioref. 2017, 7, 331–342. [Google Scholar] [CrossRef] [Green Version]
- Nikolakis, V.; Xomeritakis, G.; Abibi, A.; Dickson, M.; Tsapatsis, M.; Vlachos, D.G. Growth of a faujasite-type zeolite membrane and its application in the separation of saturated/unsaturated hydrocarbon mixtures. J. Membr. Sci. 2001, 184, 209–219. [Google Scholar] [CrossRef]
- Julbe, A.; Drobek, M. Zeolite X: Type. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Su, F.; Lu, C. CO2 capture from gas stream by zeolite 13X using a dual-column temperature/vacuum swing adsorption. Energy Environ. Sci. 2012, 5, 9021–9027. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore sizedistribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental methods in chemical engineering: Specific surface area and pore size distribution measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Bolis, V. Fundamentals in adsorption at the solid-gas interface. Concepts and thermodynamic. In Calorimetry and Thermal Methods in Catalysis; Auroux, A., Ed.; Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 2013; Volume 154, pp. 3–50. [Google Scholar]
- Ruthven, D.M. Adsorption (Chemical Engineering). In Encyclopedia of Physical Science and Technology, 3rd ed.; Meyers, R.A., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 251–271. [Google Scholar] [CrossRef]
- Chou, C.-T.; Wu, B.-C.; Wu, T.-L.; Yang, H.-S.; Shen, C.-H. Concentrating high purity CO2 from syngas after oxy-fuel combustion by pressure swing adsorption process. Comp. Aided Chem. Eng. 2018, 43, 1425–1431 doiorg/101016/B978. [Google Scholar]
- Perry, R.H.; Green, D.W.; Maloney, J.O. Perry’s Chemical Engineers Handbook, 7th ed.; McGraw-Hill: New York, NY, USA, 2007. [Google Scholar]
- Herraiz, L.; Palfi, E.; Sánchez, E.F.; Mathieu, L. Rotary Adsorption: Selective recycling of CO2 in combined cycle gas turbine power plants. Front. Energ. Res. 2020, 8, 308. [Google Scholar] [CrossRef]
- Kalvachev, Y.; Zgureva, D.; Boycheva, S.; Barbov, B.; Petrova, N. Synthesis of carbon dioxide adsorbents by zeolitization of fly ash. J. Therm. Anal. Cal. 2016, 124, 101–106. [Google Scholar] [CrossRef]
- Boycheva, S.; Zgureva, D. Studies on the CO2 adsorption onto coal fly ash zeolites at elevated Pressures. In Proceedings of the 17th International Conference on Environmental Science and Technology (CEST 2021), Athens, Greece, 1–4 September 2021. [Google Scholar]
- Kareem, F.A.A.; Shariff, A.M.; Ullah, S.; Dreisbach, F.; Keong, L.K.; Mellon, N.; Garg, S. Experimental measurements and modeling of supercritical CO2 adsorption on 13X and 5A zeolites. J. Nat. Gas Sci. Eng. 2018, 50, 115–127, doiorg/101016/jjngse201711016. [Google Scholar] [CrossRef]




| Sample | SBET, m2/g | Smicro, m2/g | Sextern, m2/g | Vmicro, cm3/g | Vmeso, cm3/g | Vtotal, cm3/g | dmicro, Å | dmeso, Å |
|---|---|---|---|---|---|---|---|---|
| CFAZ | 486 | 334 | 166 | 0.13 | 0.17 | 0.31 | 14 | 42 |
| Sample | qm, mol/kg | K, atm−1 | K, kPa−1 | R2 | Cads, mmol/g (100 kPa) | Cads,dyn, mmol/g |
|---|---|---|---|---|---|---|
| CFAZ | 3.0972 | 16.53 | 0.1244 | 0.9998 | 3.026 | 2.8 |
| Group | Parameter | Symbol | Dimension | Value |
|---|---|---|---|---|
| Adsorption column | Column type | Lengthwise flow column | ||
| Diameter of the adsorption column | D | cm | 0.8 | |
| Length of the adsorption column | L | cm | 11.15 | |
| Bed void ratio | ε | m3/m3 | 0.73 | |
| Initial temperature | Tin | °C | 24 | |
| Initial pressure | Pin | atm | 2 | |
| Temperature of the wall | Twall | °C | 24 | |
| Adsorbent | Density of the material | ρ | kg/m3 | 0.8 |
| Specific heat of the solid | cp | J/kg·K | 950 | |
| Particle diameter | dp | mm | 0.002 | |
| Particle surface/volume ratio | R | m2/m3 | 300,000 | |
| Adsorption isotherm correlation | Langmuir | |||
| Model parameter 1 | qi | mol/kg | 3.0537 | |
| Model parameter 2 | K | atm−1 | 21.53156 | |
| Adsorbate | Volume flow rate | Qads | L/min | 0.03 |
| Gas mixture volume ratio | N2/CO2 | vol%/vol% | 90/10 | |
| Adsorbate pressure | Pads | atm | 2 | |
| Adsorbate temperature | Tads | °C | 24 | |
| Number of discretization cells | z | - | 10 | |
| Simulation time | t | s | 3600 | |
| Case Number | D, m | L, m | Vcol, m3 | ε | VCFAZ, m3 | mCFAZ, kg | Pads, atm | qCO2, kmol |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.0 | 13 | 10.42 | 0.570 | 4.48 | 3585 | 4 | 8.04 |
| 2 | 1.5 | 5 | 8.84 | 0.493 | 4.48 | 3584 | 4 | 9.64 |
| 3 | 2.0 | 2 | 6.28 | 0.287 | 4.48 | 3584 | 4 | 9.19 |
| 4 | 2.0 | 2 | 6.28 | 0.287 | 4.48 | 3584 | 3 | 8.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boycheva, S.; Marinov, I.; Zgureva-Filipova, D. Studies on the CO2 Capture by Coal Fly Ash Zeolites: Process Design and Simulation. Energies 2021, 14, 8279. https://doi.org/10.3390/en14248279
Boycheva S, Marinov I, Zgureva-Filipova D. Studies on the CO2 Capture by Coal Fly Ash Zeolites: Process Design and Simulation. Energies. 2021; 14(24):8279. https://doi.org/10.3390/en14248279
Chicago/Turabian StyleBoycheva, Silviya, Ivan Marinov, and Denitza Zgureva-Filipova. 2021. "Studies on the CO2 Capture by Coal Fly Ash Zeolites: Process Design and Simulation" Energies 14, no. 24: 8279. https://doi.org/10.3390/en14248279
APA StyleBoycheva, S., Marinov, I., & Zgureva-Filipova, D. (2021). Studies on the CO2 Capture by Coal Fly Ash Zeolites: Process Design and Simulation. Energies, 14(24), 8279. https://doi.org/10.3390/en14248279







