Increasing the Biogas Potential of Rapeseed Straw Using Pulsed Electric Field Pre-Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate
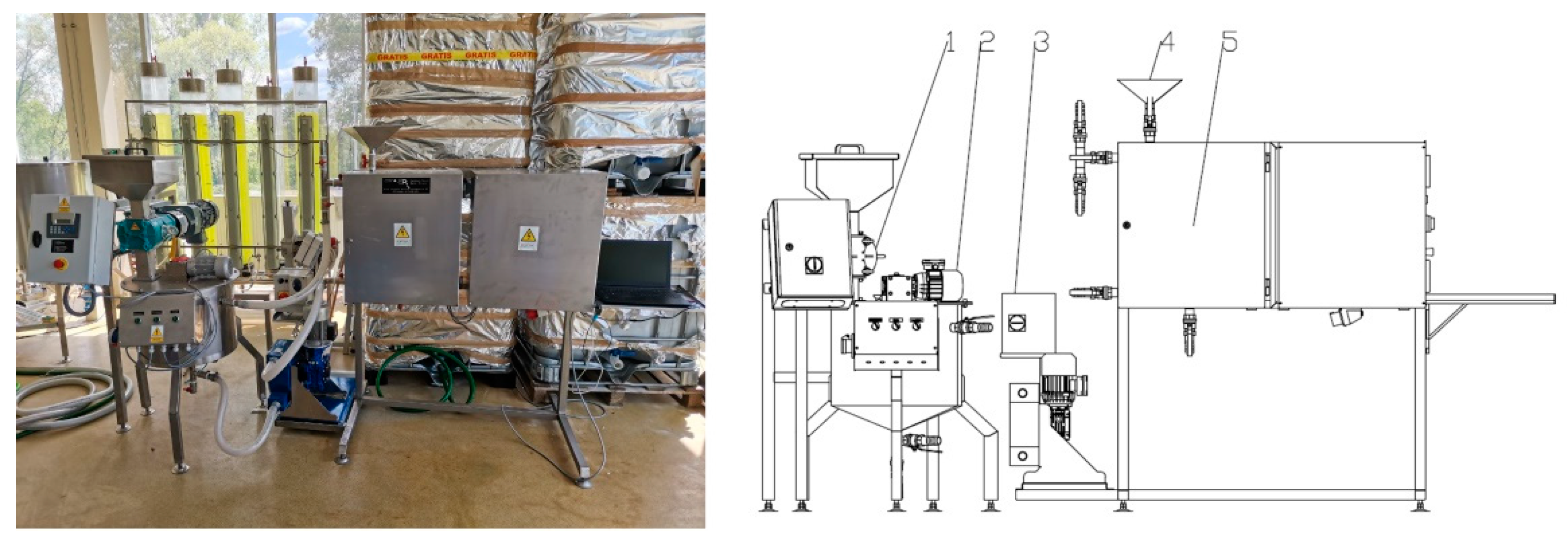
2.2. Equipment
2.3. Pretreatment
2.4. Determination of the Cellulose, Hemicellulose, and Lignin
2.5. Analytical Methods
2.6. Kinetic Evaluations
3. Results and Discussion
3.1. Pretreatment Efficiency
3.2. Biogas and Methane Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, P.; Sakuragi, K.; Makino, H. Extraction Techniques in Sustainable Biofuel Production: A Concise Review. Fuel Process. Technol. 2019, 193, 295–303. [Google Scholar] [CrossRef]
- Alalwan, H.A.; Alminshid, A.H.; Aljaafari, H.A.S. Promising Evolution of Biofuel Generations. Subject Review. Renew. Energy Focus 2019, 28, 127–139. [Google Scholar] [CrossRef]
- Scaramuzzino, C.; Garegnani, G.; Zambelli, P. Integrated Approach for the Identification of Spatial Patterns Related to Renewable Energy Potential in European Territories. Renew. Sustain. Energy Rev. 2019, 101, 1–13. [Google Scholar] [CrossRef]
- Olatunji, K.O.; Ahmed, N.A.; Ogunkunle, O. Optimization of Biogas Yield from Lignocellulosic Materials with Different Pretreatment Methods: A Review. Biotechnol. Biofuels 2021, 14, 1–34. [Google Scholar] [CrossRef]
- Chintagunta, A.D.; Zuccaro, G.; Kumar, M.; Kumar, S.P.J.; Garlapati, V.K.; Postemsky, P.D.; Kumar, N.S.S.; Chandel, A.K.; Simal-Gandara, J. Biodiesel Production from Lignocellulosic Biomass Using Oleaginous Microbes: Prospects for Integrated Biofuel Production. Front. Microbiol. 2021, 12, 2080. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Paritosh, K.; Vivekanand, V. Lignocellulose to Bio-Hydrogen: An Overview on Recent Developments. Int. J. Hydrogen Energy 2020, 45, 18195–18210. [Google Scholar] [CrossRef]
- Tuong, T.; Tran, A.; Kim, T.; Le, P.; Mai, T.P.; Nguyen, D.Q. Bioethanol Production from Lignocellulosic Biomass. Xiandai Huagong/Mod. Chem. Ind. 2019, 31, 40–44. [Google Scholar]
- Liu, X.; Xu, W.; Mao, L.; Zhang, C.; Yan, P.; Xu, Z.; Zhang, Z.C. Lignocellulosic Ethanol Production by Starch-Base Industrial Yeast under PEG Detoxification. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Gholizadeh, M. Biomass Pyrolysis: A Review of the Process Development and Challenges from Initial Researches up to the Commercialisation Stage. J. Energy Chem. 2019, 39, 109–143. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A Review on Hydrothermal Liquefaction of Biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Bekchanov, M.; Mondal, M.A.H.; de Alwis, A.; Mirzabaev, A. Why Adoption Is Slow despite Promising Potential of Biogas Technology for Improving Energy Security and Mitigating Climate Change in Sri Lanka? Renew. Sustain. Energy Rev. 2019, 105, 378–390. [Google Scholar] [CrossRef]
- Baskar, G.; Renganathan, S.; Zakaria, Z.A. Biofuels and Bioenergy: Opportunities and Challenges, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Kumar, P.; Kumar, V.; Singh, J.; Kumar, P. Electrokinetic Assisted Anaerobic Digestion of Spent Mushroom Substrate Supplemented with Sugar Mill Wastewater for Enhanced Biogas Production. Renew. Energy 2021, 179, 418–426. [Google Scholar] [CrossRef]
- Mirmohamadsadeghi, S.; Karimi, K.; Azarbaijani, R.; Parsa Yeganeh, L.; Angelidaki, I.; Nizami, A.S.; Bhat, R.; Dashora, K.; Vijay, V.K.; Aghbashlo, M.; et al. Pretreatment of Lignocelluloses for Enhanced Biogas Production: A Review on Influencing Mechanisms and the Importance of Microbial Diversity. Renew. Sustain. Energy Rev. 2021, 135, 110173. [Google Scholar] [CrossRef]
- Igliński, B.; Buczkowski, R. Potencjał Techniczny i Możliwości Wykorzystania Biogazu Utylizacyjnego Na Przykładzie Województwa Warmińsko-Mazurskiego. Rynek Energii 2017, 4, 56–62. [Google Scholar]
- Wu, D.; Wei, Z.; Zhao, Y.; Zhao, X.; Mohamed, T.A.; Zhu, L.; Wu, J.; Meng, Q.; Yao, C.; Zhao, R. Improved Lignocellulose Degradation Efficiency Based on Fenton Pretreatment during Rice Straw Composting. Bioresour. Technol. 2019, 294, 122132. [Google Scholar] [CrossRef]
- Kamaraj, M.; Ramachandran, K.K.; Aravind, J. Biohydrogen Production from Waste Materials: Benefits and Challenges. Int. J. Environ. Sci. Technol. 2020, 17, 559–576. [Google Scholar] [CrossRef]
- Enshaeieh, M.; Abdoli, A.; Madani, M.; Bayat, M. Recycling of Lignocellulosic Waste Materials to Produce High-Value Products: Single Cell Oil and Xylitol. Int. J. Environ. Sci. Technol. 2015, 12, 837–846. [Google Scholar] [CrossRef][Green Version]
- Brahim, M.; Checa Fernandez, B.L.; Regnier, O.; Boussetta, N.; Grimi, N.; Sarazin, C.; Husson, E.; Vorobiev, E.; Brosse, N. Impact of Ultrasounds and High Voltage Electrical Discharges on Physico-Chemical Properties of Rapeseed Straw’s Lignin and Pulps. Bioresour. Technol. 2017, 237, 11–19. [Google Scholar] [CrossRef] [PubMed]
- López-Linares, J.C.; Ballesteros, I.; Tourán, J.; Cara, C.; Castro, E.; Ballesteros, M.; Romero, I. Optimization of Uncatalyzed Steam Explosion Pretreatment of Rapeseed Straw for Biofuel Production. Bioresour. Technol. 2015, 190, 97–105. [Google Scholar] [CrossRef]
- Talebnia, F.; Mighani, M.; Rahimnejad, M.; Angelidaki, I. Ethanol Production from Steam Exploded Rapeseed Straw and the Process Simulation Using Artificial Neural Networks. Biotechnol. Bioprocess Eng. 2015, 20, 139–147. [Google Scholar] [CrossRef]
- Díaz, M.J.; Cara, C.; Ruiz, E.; Romero, I.; Moya, M.; Castro, E. Hydrothermal Pre-Treatment of Rapeseed Straw. Bioresour. Technol. 2010, 101, 2428–2435. [Google Scholar] [CrossRef]
- Guo, J.; Huang, K.; Cao, R.; Zhang, J.; Xu, Y. Aliphatic Extractive Effects on Acetic Acid Catalysis of Typical Agricultural Residues to Xylo-Oligosaccharide and Enzymatic Hydrolyzability of Cellulose. Biotechnol. Biofuels 2021, 14, 97. [Google Scholar] [CrossRef]
- Ahmed, B.; Aboudi, K.; Tyagi, V.K.; Álvarez-Gallego, C.J.; Fernández-Güelfo, L.A.; Romero-García, L.I.; Kazmi, A.A. Improvement of Anaerobic Digestion of Lignocellulosic Biomass by Hydrothermal Pretreatment. Appl. Sci. 2019, 9, 3853. [Google Scholar] [CrossRef]
- Karuppiah, T.; Azariah, V.E. Biomass Pretreatment for Enhancement of Biogas Production. Anaerobic Digestion 2019, 150, 111509. [Google Scholar]
- Li, M.; Pu, Y.; Ragauskas, A.J. Current Understanding of the Correlation of Lignin Structure with Biomass Recalcitrance. Front. Chem. 2016, 4, 45. [Google Scholar] [CrossRef]
- Szwarc, D.; Szwarc, K. Use of a Pulsed Electric Field to Improve the Biogas Potential of Maize Silage. Energies 2020, 14, 119. [Google Scholar] [CrossRef]
- Kovačić, Đ.; Kralik, D.; Rupčić, S.; Jovičić, D.; Spajić, R.; Tišma, M. Electroporation of Harvest Residues for Enhanced Biogas Production in Anaerobic Co-Digestion with Dairy Cow Manure. Bioresour. Technol. 2019, 274, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of Lignocellulosic Materials as Substrates for Fermentation Processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Nandal, P.; Singh, J.; Verma, M.L. Nanobiotechnological Advancements in Lignocellulosic Biomass Pretreatment. Mater. Sci. Energy Technol. 2020, 3, 308–318. [Google Scholar] [CrossRef]
- Dahunsi, S.O. Mechanical Pretreatment of Lignocelluloses for Enhanced Biogas Production: Methane Yield Prediction from Biomass Structural Components. Bioresour. Technol. 2019, 280, 18–26. [Google Scholar] [CrossRef]
- Kong, X.; Du, J.; Ye, X.; Xi, Y.; Jin, H.; Zhang, M.; Guo, D. Enhanced Methane Production from Wheat Straw with the Assistance of Lignocellulolytic Microbial Consortium TC-5. Bioresour. Technol. 2018, 263, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Wyman, V.; Henríquez, J.; Palma, C.; Carvajal, A. Lignocellulosic Waste Valorisation Strategy through Enzyme and Biogas Production. Bioresour. Technol. 2018, 247, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, M.; Rusanowska, P.; Krzywik, A.; Dudek, M.; Nowicka, A.; Ebowski, M.D. Application of Hydrodynamic Cavitation for Improving Methane Fermentation of Sida Hermaphrodita Silage. Energies 2019, 12, 526. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, Y.; Lu, X.; Zhang, X.; He, H.; Pan, F.; Zhou, L.; Liu, X.; Ji, X.; Zhang, S. Cascade Utilization of Lignocellulosic Biomass to High-Value Products. Green Chem. 2019, 21, 3499–3535. [Google Scholar] [CrossRef]
- Safavi, S.M.; Unnthorsson, R. Enhanced Methane Production from Pig Slurry with Pulsed Electric Field Pre-Treatment. Environ. Technol. 2018, 39, 479–489. [Google Scholar] [CrossRef]
- Golberg, A.; Sack, M.; Teissie, J.; Pataro, G.; Pliquett, U.; Saulis, G.; Stefan, T.; Miklavcic, D.; Vorobiev, E.; Frey, W. Energy-Efficient Biomass Processing with Pulsed Electric Fields for Bioeconomy and Sustainable Development. Biotechnol. Biofuels 2016, 9, 1–22. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Cydzik-Kwiatkowska, A.; Rusanowska, P.; Głowacka, K. Operation Mode and External Carbon Dose as Determining Factors in Elemental Composition and Morphology of Aerobic Granules. Arch. Environ. Prot. 2016, 42, 74–79. [Google Scholar] [CrossRef][Green Version]
- Kuşçu, Ö.S.; Çömlekçi, S.; Çört, N. Disintegration of Sewage Sludge Using Pulsed Electrical Field Technique: PEF Optimization, Simulation, and Anaerobic Digestion. Environ. Technol. 2021, 1–16. [Google Scholar] [CrossRef]
- Deng, Y.D.; Gao, Y.; Men, Y.K.; Du, B.X.; Wang, Y.N.; Liu, C.H. Effect of DC Corona on Performance of Pulsed Electric Field Pretreatment on Waste Activated Sludge. In Proceedings of the 2016 IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Toronto, ON, Canada, 16–19 October 2016; pp. 747–750. [Google Scholar]
- El Achkar, J.H.; Lendormi, T.; Salameh, D.; Louka, N.; Maroun, R.G.; Lanoisellé, J.L.; Hobaika, Z. Influence of Pretreatment Conditions on Lignocellulosic Fractions and Methane Production from Grape Pomace. Bioresour. Technol. 2018, 247, 881–889. [Google Scholar] [CrossRef]
- Kim, Y.N.; Kwon, H.J.; Lee, D.U. Effects of Pulsed Electric Field (PEF) Treatment on Physicochemical Properties of Panax Ginseng. Innov. Food Sci. Emerg. Technol. 2019, 58, 102232. [Google Scholar] [CrossRef]
- Liu, Z.; Esveld, E.; Vincken, J.P.; Bruins, M.E. Pulsed Electric Field as an Alternative Pre-Treatment for Drying to Enhance Polyphenol Extraction from Fresh Tea Leaves. Food Bioprocess Technol. 2019, 12, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chen, T.; Qin, X.; Wu, Q.; Zhao, Y.; Bai, S.; Peng, W.; Feng, B. Effect of High-Voltage Pulsed Electric Field (HPEF) Pretreatment on Biogas Production Rates of Hybrid Pennisetum by Anaerobic Fermentation. Nat. Gas Ind. B 2018, 5, 48–53. [Google Scholar] [CrossRef]
- Zou, L.; Ma, C.; Liu, J.; Li, M.; Ye, M.; Qian, G. Pretreatment of Food Waste with High Voltage Pulse Discharge towards Methane Production Enhancement. Bioresour. Technol. 2016, 222, 82–88. [Google Scholar] [CrossRef] [PubMed]






| Parameters | Value |
|---|---|
| Hydration [%] | 7.92 |
| Dry weight [%] | 92.08 |
| Organic dry matter [% TS] | 92.15 |
| Total carbon (TC) [mg C/g TS] | 446.32 |
| Total organic carbon (TOC) [mg C/g TS] | 369.43 |
| Total nitrogen (TN) [mg N/g TS] | 18.59 |
| C/N | 24.01 |
| Disintegration Time [min] | Q [NmL/g VS] | Qe [Nml/g VS] | r [NmL/g VS /d] | k [1/d] | R2 |
|---|---|---|---|---|---|
| 0 | 372.63 | 369.03 | 81.36 | 0.22 | 0.99 |
| 1 | 379.50 | 369.61 | 95.32 | 0.26 | 0.98 |
| 2 | 393.50 | 387.95 | 78.03 | 0.20 | 0.98 |
| 3 | 401.43 | 407.57 | 66.17 | 0.16 | 0.99 |
| 4 | 426.60 | 423.88 | 77.01 | 0.18 | 0.98 |
| 5 | 427.53 | 412.87 | 115.47 | 0.28 | 0.98 |
| 6 | 420.8 | 407.17 | 117.06 | 0.29 | 0.98 |
| 7 | 420.6 | 407.51 | 103.81 | 0.26 | 0.98 |
| 8 | 413.5 | 400.54 | 114.37 | 0.29 | 0.98 |
| Pre-Treatment Time [min] | Ein (Wh/g TS) | Eout (Wh/g TS) | ET (Wh/g TS) |
|---|---|---|---|
| 0 | - | - | - |
| 1 | 0.05 | 0.04 | −0.11 |
| 2 | 0.11 | 0.12 | 0.01 |
| 3 | 0.13 | 0.14 | 0.01 |
| 4 | 0.22 | 0.28 | 0.06 |
| 5 | 0.26 | 0.29 | 0.03 |
| 6 | 0.33 | 0.30 | −0.03 |
| 7 | 0.39 | 0.29 | −0.10 |
| 8 | 0.44 | 0.25 | −0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szwarc, D.; Głowacka, K. Increasing the Biogas Potential of Rapeseed Straw Using Pulsed Electric Field Pre-Treatment. Energies 2021, 14, 8307. https://doi.org/10.3390/en14248307
Szwarc D, Głowacka K. Increasing the Biogas Potential of Rapeseed Straw Using Pulsed Electric Field Pre-Treatment. Energies. 2021; 14(24):8307. https://doi.org/10.3390/en14248307
Chicago/Turabian StyleSzwarc, Dawid, and Katarzyna Głowacka. 2021. "Increasing the Biogas Potential of Rapeseed Straw Using Pulsed Electric Field Pre-Treatment" Energies 14, no. 24: 8307. https://doi.org/10.3390/en14248307
APA StyleSzwarc, D., & Głowacka, K. (2021). Increasing the Biogas Potential of Rapeseed Straw Using Pulsed Electric Field Pre-Treatment. Energies, 14(24), 8307. https://doi.org/10.3390/en14248307






