Role of Monovalent and Divalent Ions in Low-Salinity Water Flood in Carbonate Reservoirs: An Integrated Analysis through Zeta Potentiometric and Simulation Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Samples for Zeta Potentiometric Study
2.1.1. Rock Samples
2.1.2. Fluid Samples
2.2. Experimental Design for Zeta Potential Analysis
2.3. Simulation Model and Properties
2.3.1. Aqueous and Mineral Reactions
2.3.2. Grid Model and Petrophysical Properties
3. Results and Discussions
3.1. Zeta Potentiometric Analysis
3.1.1. Diluted Brines
3.1.2. Smart/Modified Brines
3.2. Simulation Results
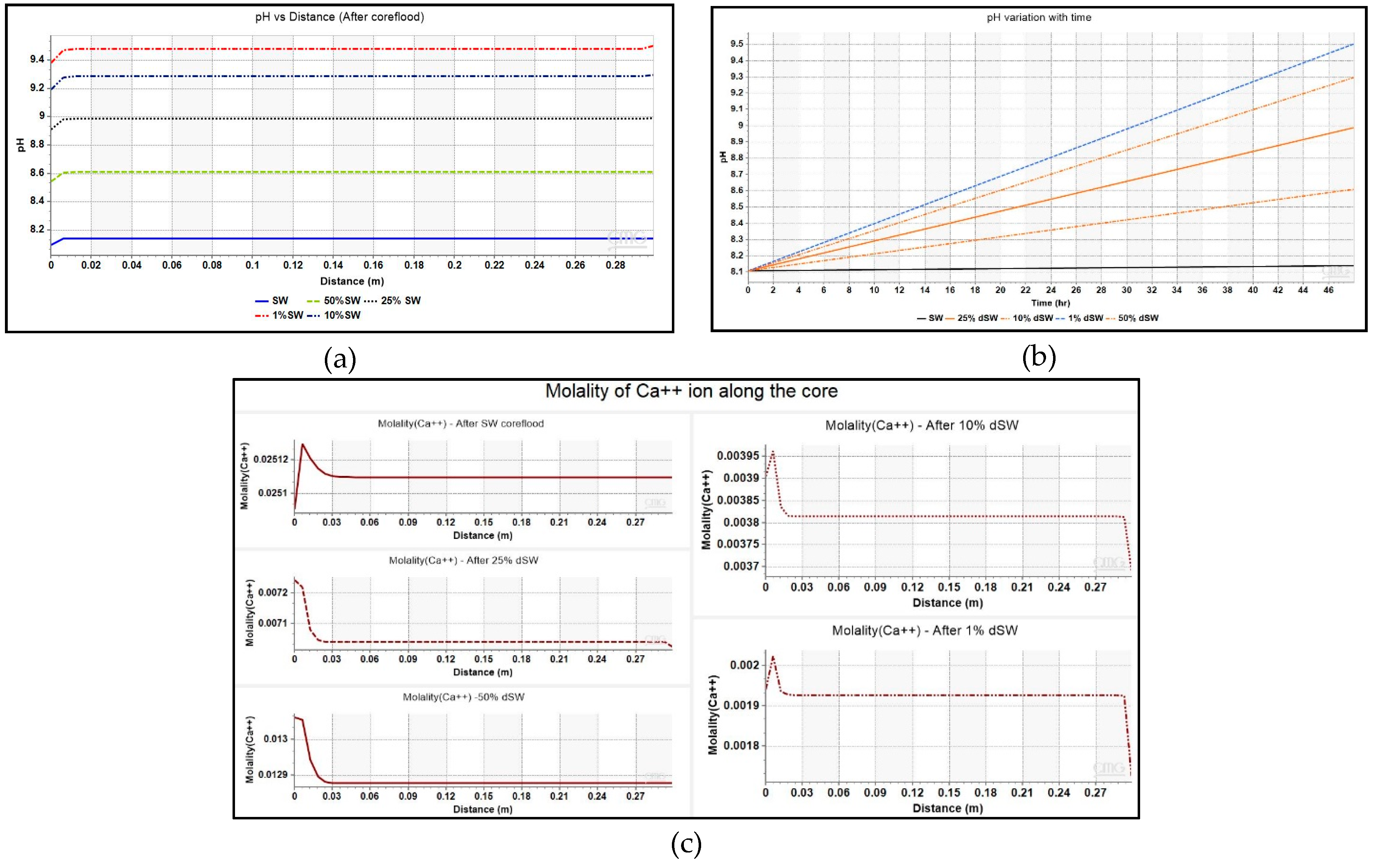
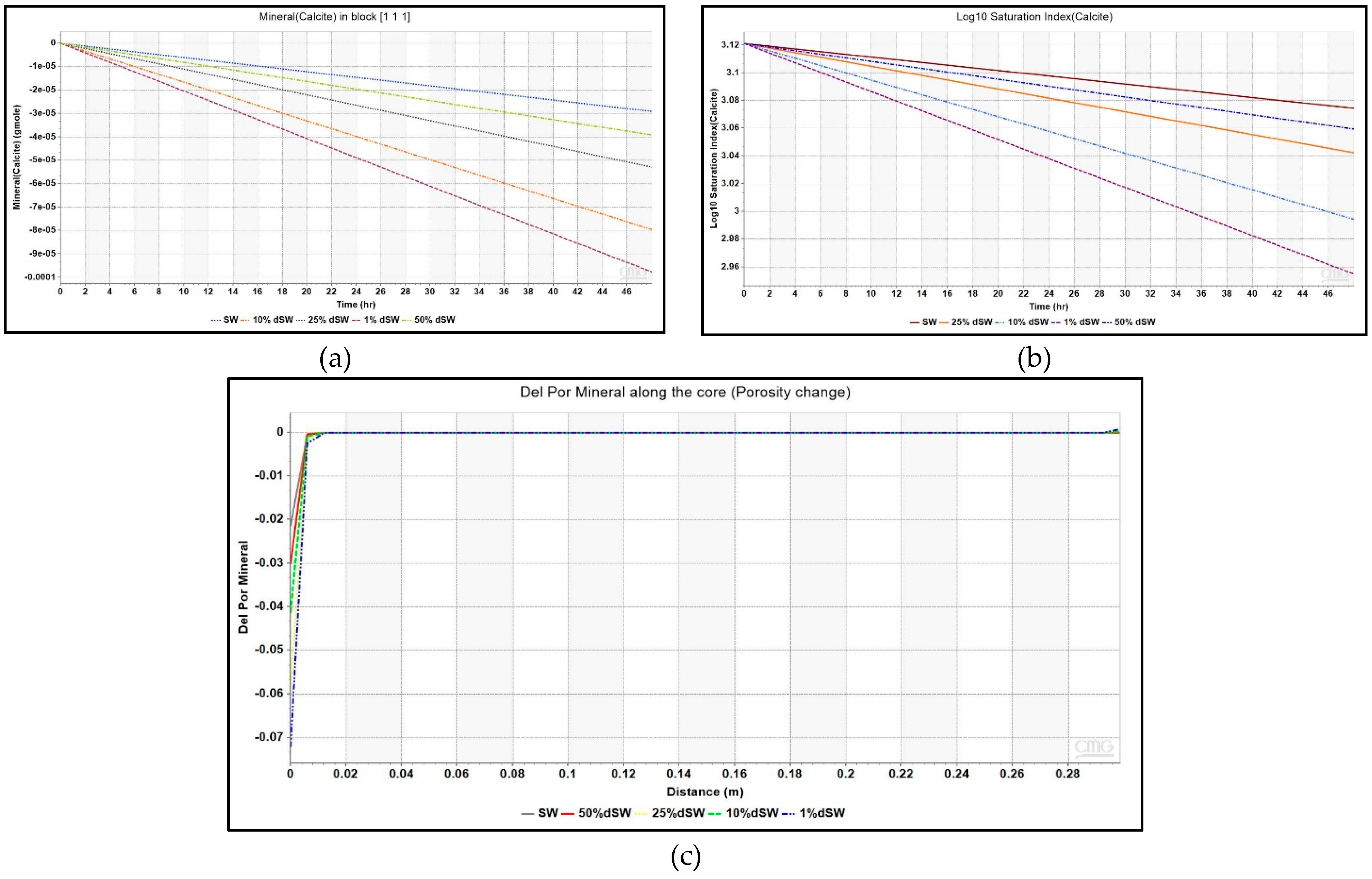
3.2.1. Diluted Brines and Underlying Mechanism
3.2.2. Smart/Modified Brines and Underlying Mechanism
4. Conclusions
- (1)
- Additional oil recovery is obtained from low-salinity waterflooding as observed from the increasing trend of zeta potential and oil recovery. Maximum incremental oil recovery is given by 1% dSW which correlated with the analysis of pH, moles of calcite, saturation index and Ca2+ ion profiles presented in this paper showed that rock dissolution as the underlying mechanism.
- (2)
- The impact of PDIs has been studied in this paper and following conclusions can be drawn from it: (a) For enhanced oil recovery, brine spiked with Ca2+ ion is preferred such that the magnitude of zeta potential is close to zero which ensures minimum attractive forces between adhered oil molecules and the rock surface. (b) Less active role is observed for Mg ion as compared to Ca ion due to the relatively lower solubility of MgSO4 in brine at ambient conditions. (c) Brines can also be spiked with divalent anions, SO42+ and PO43− owing to the oil detachment from repulsive forces due to their adsorption.
- (3)
- Additional oil recovery of 20.5% resulted from seawater depleted of Na+ and Cl− ions from sea water whereas seawater depleted of only Ca2+ ions gave no additional oil. The underlying mechanism is explained by the resultant increase in electrostatic repulsive forces from expansion of electrical double layer but needs to be further confirmed by surface complexation modeling of surface and electrostatic interactions.
- (4)
- As seen from simulation study, only the injection gridblock had evidence of calcite dissolution taking place. Therefore, the applicability of rock dissolution mechanism in field conditions is highly doubtful as it may not propagate further into the reservoir.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Awolayo, A.N.; Sarma, H.K.; Nghiem, L.X. Brine-Dependent Recovery Processes in Carbonate and Sandstone Petroleum Reservoirs: Review of Laboratory-Field Studies, Interfacial Mechanisms and Modeling Attempts. Energies 2018, 11, 3020. [Google Scholar] [CrossRef] [Green Version]
- Awolayo, A.N. Impact of Multi-ion Interactions on Oil Mobilization by Smart Waterflooding in Carbonate Reservoirs. J. Pet. Environ. Biotechnol. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Al-Shalabi, E.W.; Sepehrnoori, K. A Comprehensive Review of Low Salinity/engineered Water Injections and Their Applications in Sandstone and Carbonate Rocks. J. Pet. Sci. Eng. 2016, 139, 137–161. [Google Scholar] [CrossRef]
- Dehaghani, A.H.S.; Badizad, M.H. Impact of ionic composition on modulating wetting preference of calcite surface: Implication for chemically tuned water flooding. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 568, 470–480. [Google Scholar] [CrossRef]
- Fathi, S.J.; Austad, T.; Strand, S. Water-Based Enhanced Oil Recovery (EOR) by “Smart Water”: Optimal Ionic Composition for EOR in Carbonates. Energy Fuels 2011, 25, 5173–5179. [Google Scholar] [CrossRef] [Green Version]
- Mahani, H.; Keya, A.L.; Berg, S.; Bartels, W.-B.; Nasralla, R.A.; Rossen, W.R. Insights into the Mechanism of Wettability Alteration by Low-Salinity Flooding (LSF) in Carbonates. Energy Fuels 2015, 29, 1352–1367. [Google Scholar] [CrossRef]
- Hiorth, A.; Cathles, L.M.; Madland, M.V. The Impact of Pore Water Chemistry on Carbonate Surface Charge and Oil Wettability. Transp. Porous Media 2010, 85, 1–21. [Google Scholar] [CrossRef]
- Austad, T.; Strand, S.; Puntervold, T. Is Wettability Alteration of Carbonates by Seawater Caused by Rock Dissolution. In Paper SCA2009-43 presented at the International Symposium of the Society of Core Analysts, Noordwijk, The Netherlands, 27–30 September 2009; pp. 27–30. [Google Scholar]
- Mahani, H.; Keya, A.L.; Berg, S.; Nasralla, R. Electrokinetics of Carbonate/Brine Interface in Low-Salinity Waterflooding: Effect of Brine Salinity, Composition, Rock Type, and pH on ζ-Potential and a Surface-Complexation Model. SPE J. 2017, 22, 053–068. [Google Scholar] [CrossRef]
- Glover, P. Geophysical Properties of the Near Surface Earth: Electrical Properties. In Treatise on Geophysics; Elsevier: Amsterdam, The Netherlands, 2015; Volume 11, pp. 89–137. [Google Scholar]
- Jackson, M.D.; Al-Mahrouqi, D.; Vinogradov, J. Zeta potential in oil-water-carbonate systems and its impact on oil recovery during controlled salinity water-flooding. Sci. Rep. 2016, 6, 37363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Hashim, H.; Kasha, A.A.; Abdallah, W.A.; Sauerer, B. Impact of Modified Seawater on Zeta Potential and Morphology of Calcite and Dolomite Aged with Stearic Acid. Energy Fuels 2018, 32, 1644–1656. [Google Scholar] [CrossRef]
- Gopani, P.H.; Singh, N.; Sarma, H.K.; Mattey, P.S.; Negi, D.S. A Zeta Potentiometric Study On Effects of Ionic Composition And Rock-Saturation On Surface-Charge Interactions In Low-Salinity Water Flooding For Carbonate Formation. In Proceedings of the SPE-200804-MS at the 2020 SPE Western Regional Meeting, Bakersfield, CA, USA. (Meeting Postponed to a Later Date due to Pandemic).
- Luxbacher, T. The Streaming Potential Technique. In The Zeta Guide—Principles of the Streaming Potential Technique, 1st ed.; Chapter 4.1; Anton Paar GmbH: Austria, Europe, 2014; pp. 27–30. [Google Scholar]
- Dang, C.T.Q.; Nghiem, L.X.; Chen, Z.; Nguyen, Q.P. Modeling Low Salinity Waterflooding: Ion Exchange, Geochemistry and Wettability Alteration. In Proceedings of the SPE Annual Technical Conference and Exhibition, Society of Petroleum Engineers (SPE), New Orleans, LA, USA, 30 September–2 October 2013. [Google Scholar]
- Kharaka, Y.; Gunter, W.; Aggarwal, P.; Perkins, E.; DeBraal, J. SOLMINEQ.88; A Computer Program for Geochemical Modeling of Water-Rock Interactions; US Geological Survey: Reston, VA, USA, 1988.
- Rollinson, H. CM Bethke Geochemical Reaction Modeling. New York, Oxford (Oxford University Press). 1996, xvii+ 397 pp. Price£ 37.95. ISBN 0-19-509475-1. Mineral. Mag. 1997, 61, 611–612. [Google Scholar] [CrossRef]
- Kakati, A.; Jha, N.K.; Kumar, G.; Sangwai, J.S. Application of Low Salinity Water Flooding for Light Paraffinic Crude Oil Reservoir. In Proceedings of the SPE Symposium: Production Enhancement and Cost Optimisation, Kuala Lumpur, Malaysia, 7–8 November 2017; Society of Petroleum Engineers (SPE), 2017. [Google Scholar] [CrossRef]
- Singh, N.; Gopani, P.H.; Sarma, H.K.; Mattey, P.S.; Negi, D.S. Characterization of Rock and Fluid Properties for Low-Salinity Water Flooding of Highly Paraffinic Oil in a Deep Low-Permeability High-Pressure High-Temperature Offshore Carbonate Reservoir. In International Conference on Offshore Mechanics and Arctic Engineering; American Society of Mechanical Engineers, 2020; Volume 84430, p. V011T11A008. [Google Scholar]
- Strand, S.; Austad, T.; Puntervold, T.; Høgnesen, E.J.; Olsen, M.; Barstad, S.M.F. “Smart water” for oil recovery from fractured limestone: A preliminary study. Energy Fuels 2008, 22, 3126–3133. [Google Scholar] [CrossRef] [Green Version]
- Alroudhan, A.; Vinogradov, J.; Jackson, M. Zeta potential of intact natural limestone: Impact of potential-determining ions Ca, Mg and SO4. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 493, 83–98. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Tweheyo, M.T.; Austad, T. Wettability alteration and improved oil recovery by spontaneous imbibition of seawater into chalk: Impact of the potential determining ions Ca2+, Mg2+, and SO42−. Colloids Surfaces A Physicochem. Eng. Asp. 2007, 301, 199–208. [Google Scholar] [CrossRef]
- Montenegro, A.; Brovkin, V.; Eby, M.; Archer, D.; Weaver, A.J. Long term fate of anthropogenic carbon. Geophys. Res. Lett. 2007, 34. [Google Scholar] [CrossRef]
- Fathi, S.J.; Austad, T.; Strand, S. “Smart Water” as a Wettability Modifier in Chalk: The Effect of Salinity and Ionic Composition. Energy Fuels 2010, 24, 2514–2519. [Google Scholar] [CrossRef] [Green Version]
- Shariatpanahi, S.F.; Strand, S.; Austad, T. Evaluation of Water-Based Enhanced Oil Recovery (EOR) by Wettability Alteration in a Low-Permeable Fractured Limestone Oil Reservoir. Energy Fuels 2010, 24, 5997–6008. [Google Scholar] [CrossRef]
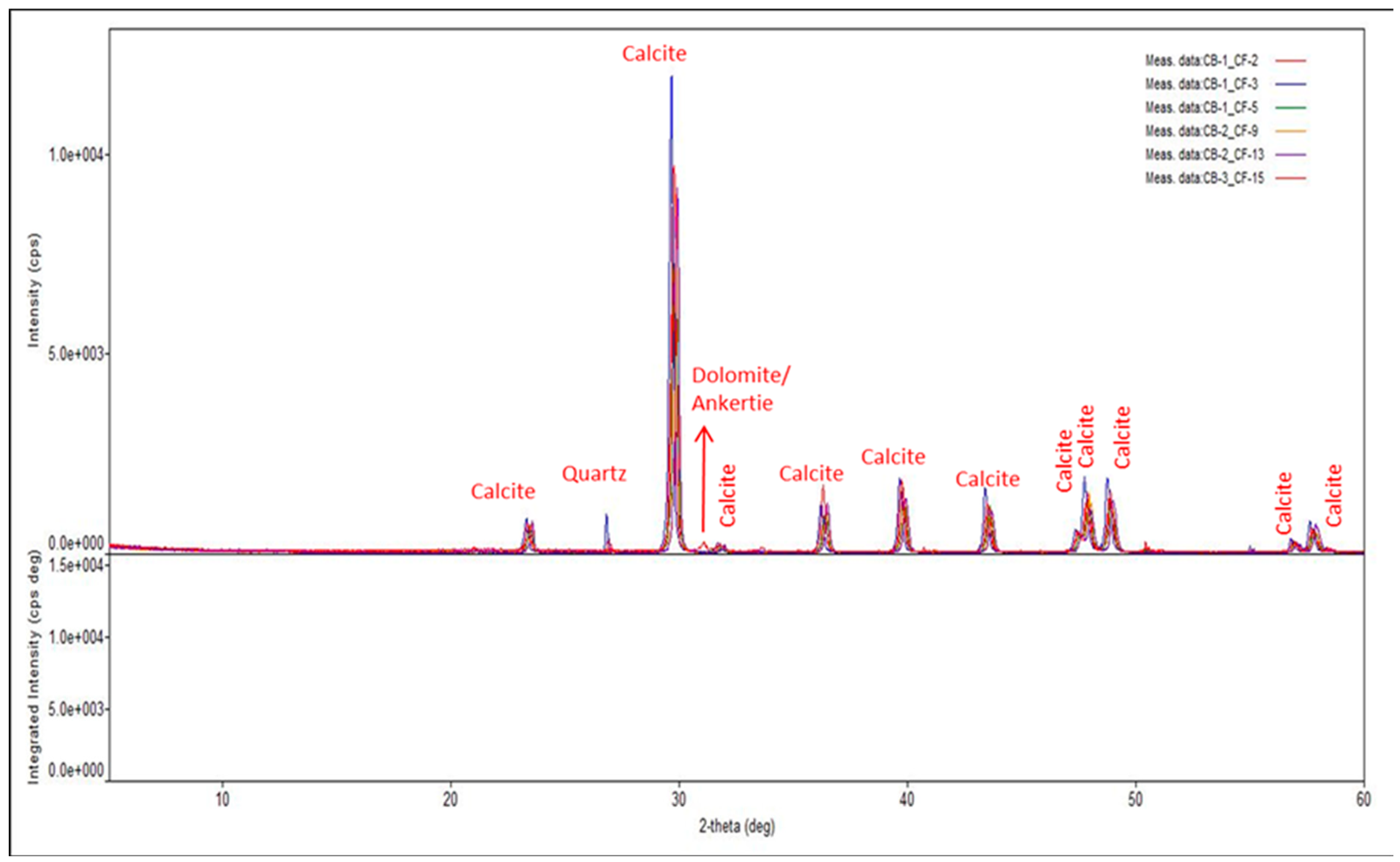

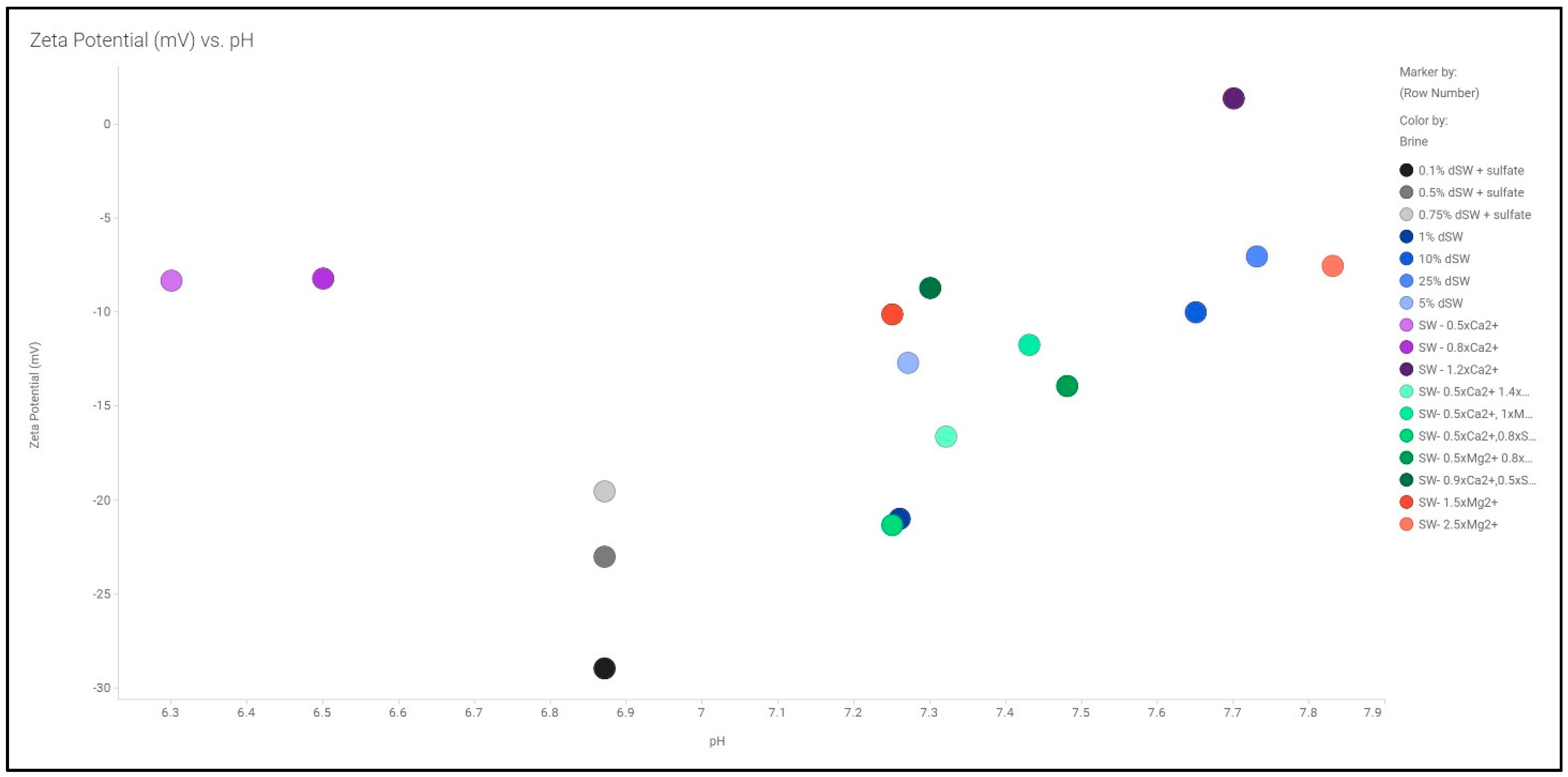

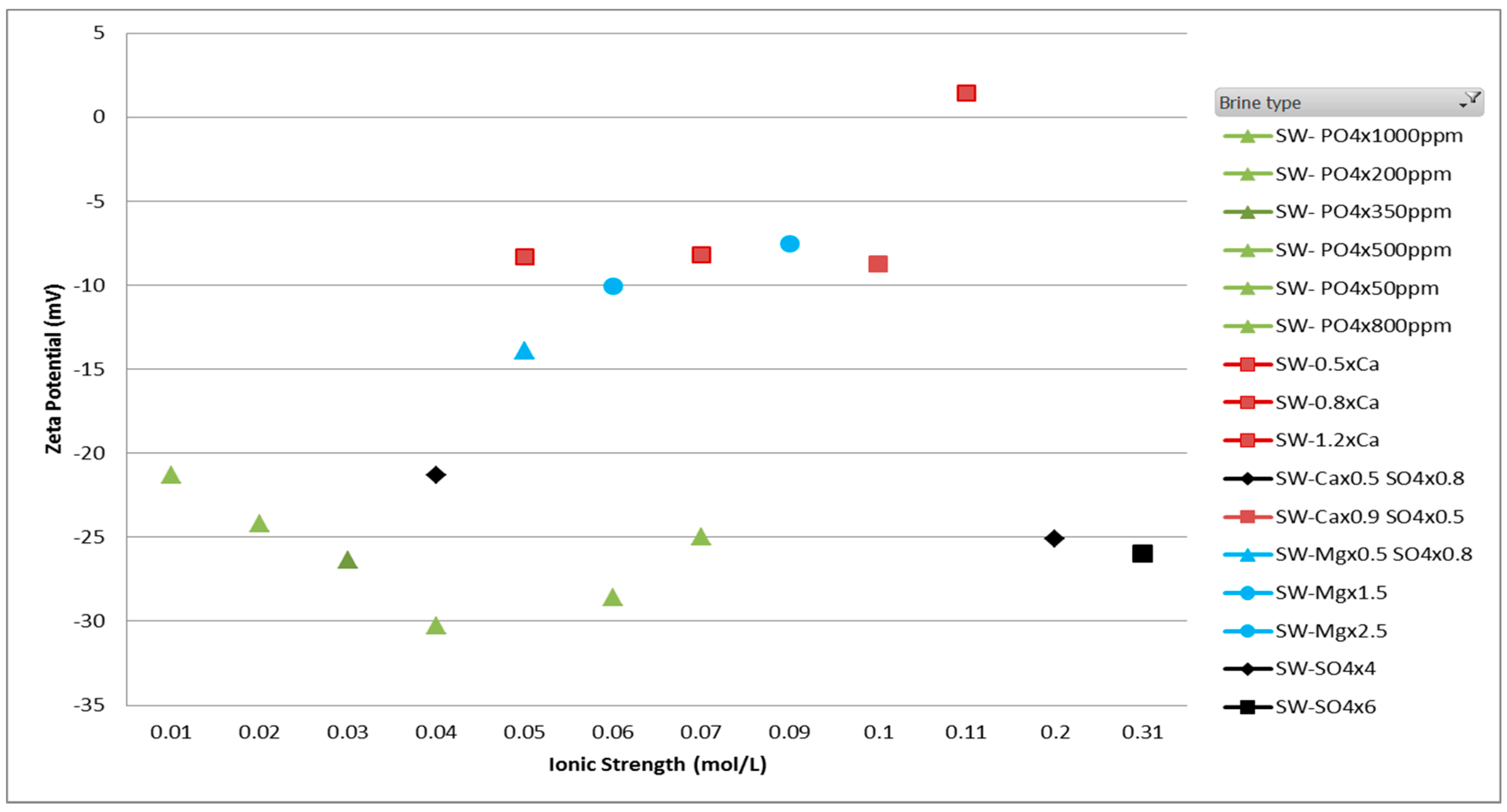
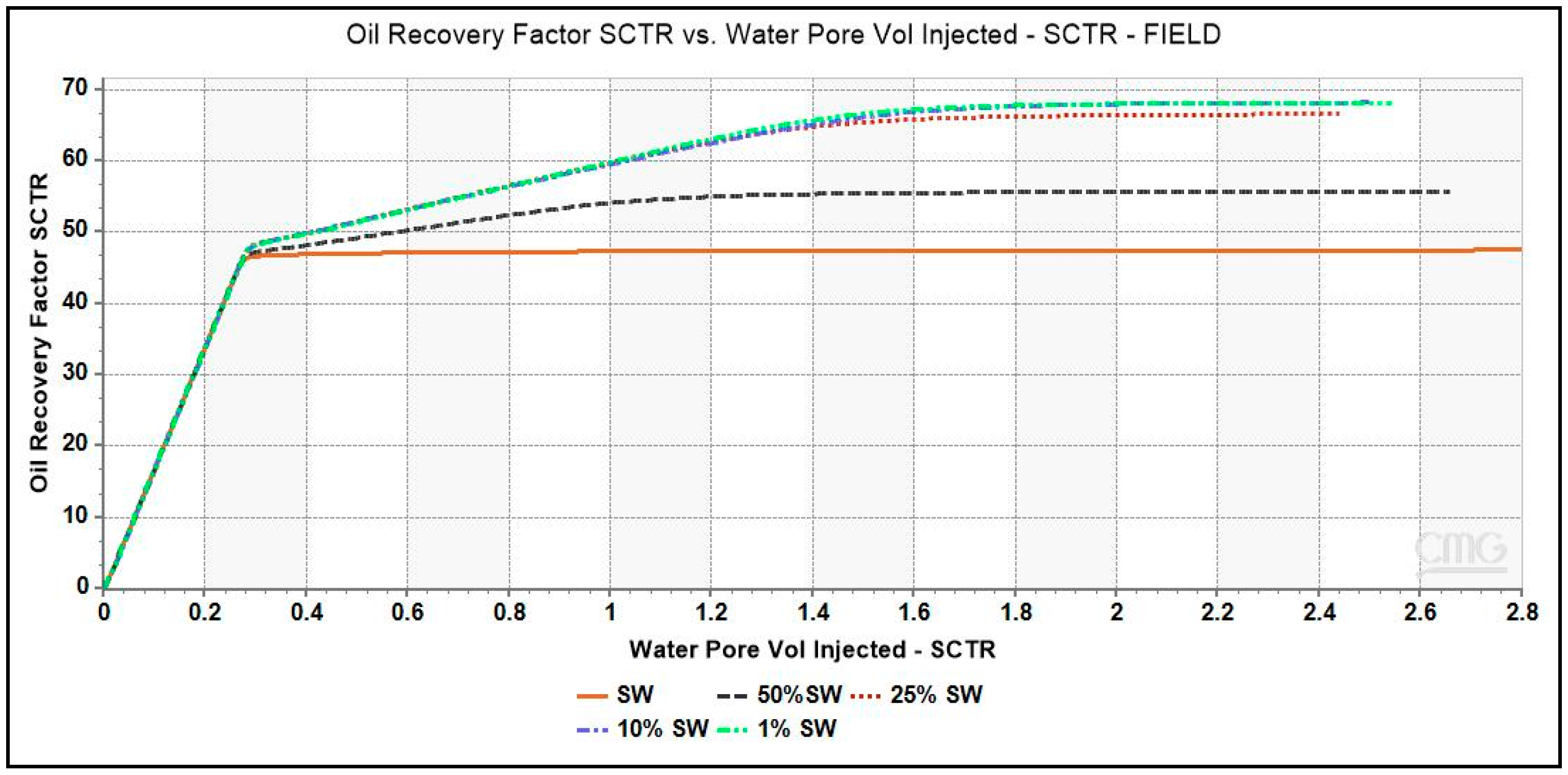

| Ions | SW | 50%SW | 25%SW | 10%SW | 1%SW |
|---|---|---|---|---|---|
| Na+ (ppm) | 11,638.00 | 5819 | 2909.5 | 1163.8 | 116.38 |
| Ca2+ (ppm) | 1101 | 550.5 | 275.25 | 110.1 | 11.01 |
| Mg2+ (ppm) | 265 | 132.5 | 66.25 | 26.5 | 2.65 |
| Cl− (ppm) | 20,913.00 | 10,456.50 | 5228.25 | 2091.3 | 209.13 |
| SO42− (ppm) | 1198 | 599 | 299.5 | 119.8 | 11.98 |
| HCO3− (ppm) | 857 | 428.5 | 214.25 | 85.7 | 8.57 |
| Sr (ppm) | 50 | 25 | 12.5 | 5 | 0.5 |
| Total Dissolved Solids (ppm) | 36,022.00 | 18,011.00 | 9005.5 | 3602.2 | 360.22 |
| Ionic Strength (mol/L) | 0.66 | 0.33 | 0.17 | 0.07 | 0.01 |
| pH | 8 | 7.8 | 7.73 | 7.65 | 7.26 |
| Modified-Brines | Na− (ppm) | Ca2+ (ppm) | Mg2+ (ppm) | Cl− (ppm) | SO42− (ppm) | HCO3− (ppm) | Sr+ (ppm) | Total Dissolved Solids (ppm) | Ionic Strength (mol/L) |
|---|---|---|---|---|---|---|---|---|---|
| SW—Sea Water | 11,638 | 1101 | 265 | 20,913 | 1198 | 857 | 50 | 36,022 | 0.66 |
| SW—1.5 × Mg2+ | 116.4 | 11 | 400.2 | 1368.4 | 12 | 8.6 | 0.5 | 1917 | 0.06 |
| SW—2.5 × Mg2+ | 116.4 | 11 | 665.2 | 2141.3 | 12 | 8.6 | 0.5 | 2954.9 | 0.09 |
| SW—0.9 × Ca2+, 0.5 × SO42− | 403.4 | 1001.9 | 2.7 | 1963.8 | 611 | 8.6 | 0.5 | 3991.8 | 0.1 |
| SW—0.5 × Ca2+, 0.8 × SO42− | 575.6 | 143.5 | 2.7 | 443.8 | 970.4 | 8.6 | 0.5 | 2145 | 0.05 |
| SW—0.5 × Ca2+, 1 × Mg2+, 0.8 × SO42− | 575.6 | 143.5 | 267.7 | 1216.6 | 970.4 | 8.6 | 0.5 | 3182.9 | 0.08 |
| SW—0.5 × Ca2+, 1.4 × SO42− | 920 | 143.5 | 2.7 | 443.8 | 1689.2 | 8.6 | 0.5 | 3208.2 | 0.07 |
| SW—0.5 × Mg2+, 0.8 × SO42− | 575.6 | 11 | 135.2 | 595.6 | 970.4 | 8.6 | 0.5 | 2296.8 | 0.05 |
| SW—4 × SO42− | 2412.5 | 11 | 2.7 | 209.1 | 4804 | 8.6 | 0.5 | 7448.4 | 0.16 |
| SW—6 × SO42− | 3560.6 | 11 | 2.7 | 209.1 | 7200 | 8.6 | 0.5 | 10,992.5 | 0.23 |
| SW—1.2 × Ca2+ | 116.4 | 1332.2 | 2.7 | 2548.7 | 12 | 8.6 | 0.5 | 4021 | 0.11 |
| SW—0.8 × Ca2+ | 116.4 | 891.8 | 2.7 | 1768.8 | 12 | 8.6 | 0.5 | 2801 | 0.07 |
| SW—0.5 × Ca2+ | 116.4 | 561.5 | 2.7 | 1183.9 | 12 | 8.6 | 0.5 | 1886 | 0.05 |
| Aqueous Reactions | |
|---|---|
| 1 | ‘H+’ + ‘OH−’ = ‘H2O’ |
| 2 | ‘H+’ + ‘CO3−’ = ‘HCO3−’ |
| 3 | ‘CaSO4’ = ‘Ca++’ + ‘SO4−’ |
| 4 | ‘MgSO4’ = ‘Ca++’ + ‘SO4−’ |
| Mineral reactions | |
| 6 | ‘Calcite’ + ‘H+’ = ‘Ca++’ + ‘HCO3−’ |
| 7 | ‘Dolomite’ + ‘H+’ = ‘Ca++’ + ‘Mg++’ + 2 ‘HCO3−’ |
| Component | Proportion (%) |
|---|---|
| C5 | 3.6 |
| C6 | 5.3 |
| C7+ | 91.1 |
| Properties of the Crude Oil | |
|---|---|
| Specific gravity | 0.85 |
| API gravity | 36 |
| Pour point | 33 °C |
| Wax Appearance Temperature | 55 °C |
| Wax Content (% wt) | 20 |
| Viscosity @ 40 °C (cP) | 4–8 |
| Property | Value |
|---|---|
| Porosity (%) | 15.8 |
| Porosity Type | Single |
| kx (mD) | 0.14 |
| ky (mD) | 0.14 |
| kz (mD) | 0.05 |
| Swirr (%) | 42 |
| Length (inch) | 12 |
| Width (inch) | 1 |
| Height (inch) | 1 |
| Number of grids in × dir. | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gopani, P.H.; Singh, N.; Sarma, H.K.; Mattey, P.; Srivastava, V.R. Role of Monovalent and Divalent Ions in Low-Salinity Water Flood in Carbonate Reservoirs: An Integrated Analysis through Zeta Potentiometric and Simulation Studies. Energies 2021, 14, 729. https://doi.org/10.3390/en14030729
Gopani PH, Singh N, Sarma HK, Mattey P, Srivastava VR. Role of Monovalent and Divalent Ions in Low-Salinity Water Flood in Carbonate Reservoirs: An Integrated Analysis through Zeta Potentiometric and Simulation Studies. Energies. 2021; 14(3):729. https://doi.org/10.3390/en14030729
Chicago/Turabian StyleGopani, Paras H., Navpreet Singh, Hemanta K. Sarma, Padmaja Mattey, and Vivek R. Srivastava. 2021. "Role of Monovalent and Divalent Ions in Low-Salinity Water Flood in Carbonate Reservoirs: An Integrated Analysis through Zeta Potentiometric and Simulation Studies" Energies 14, no. 3: 729. https://doi.org/10.3390/en14030729
APA StyleGopani, P. H., Singh, N., Sarma, H. K., Mattey, P., & Srivastava, V. R. (2021). Role of Monovalent and Divalent Ions in Low-Salinity Water Flood in Carbonate Reservoirs: An Integrated Analysis through Zeta Potentiometric and Simulation Studies. Energies, 14(3), 729. https://doi.org/10.3390/en14030729







