High-Pressure Torsion of Non-Equilibrium Hydrogen Storage Materials: A Review
Abstract
:1. Introduction
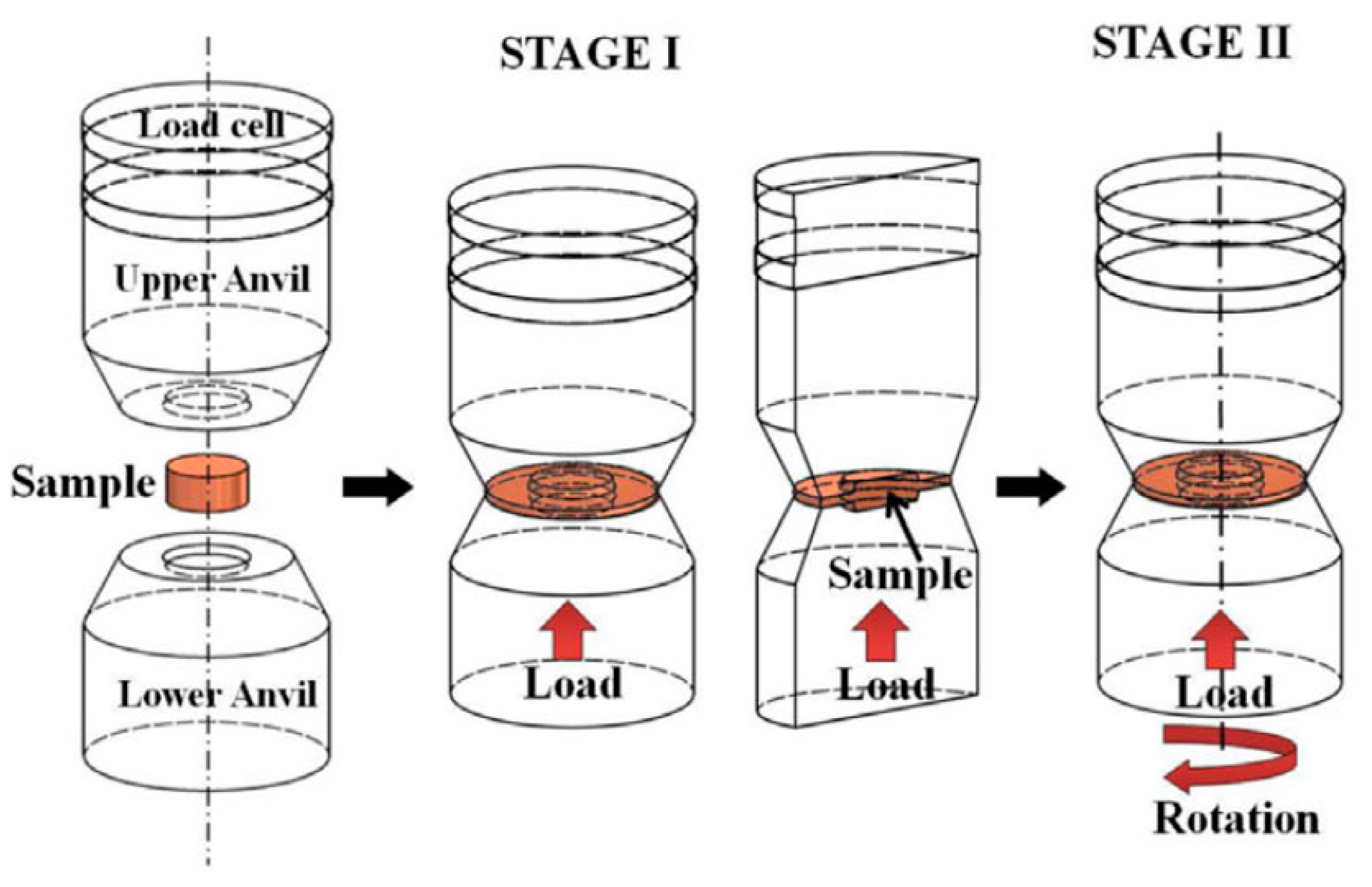
2. The High-Pressure Torsion Procedure
3. Nanostructured Mg-Based Hydrogen Storage Systems
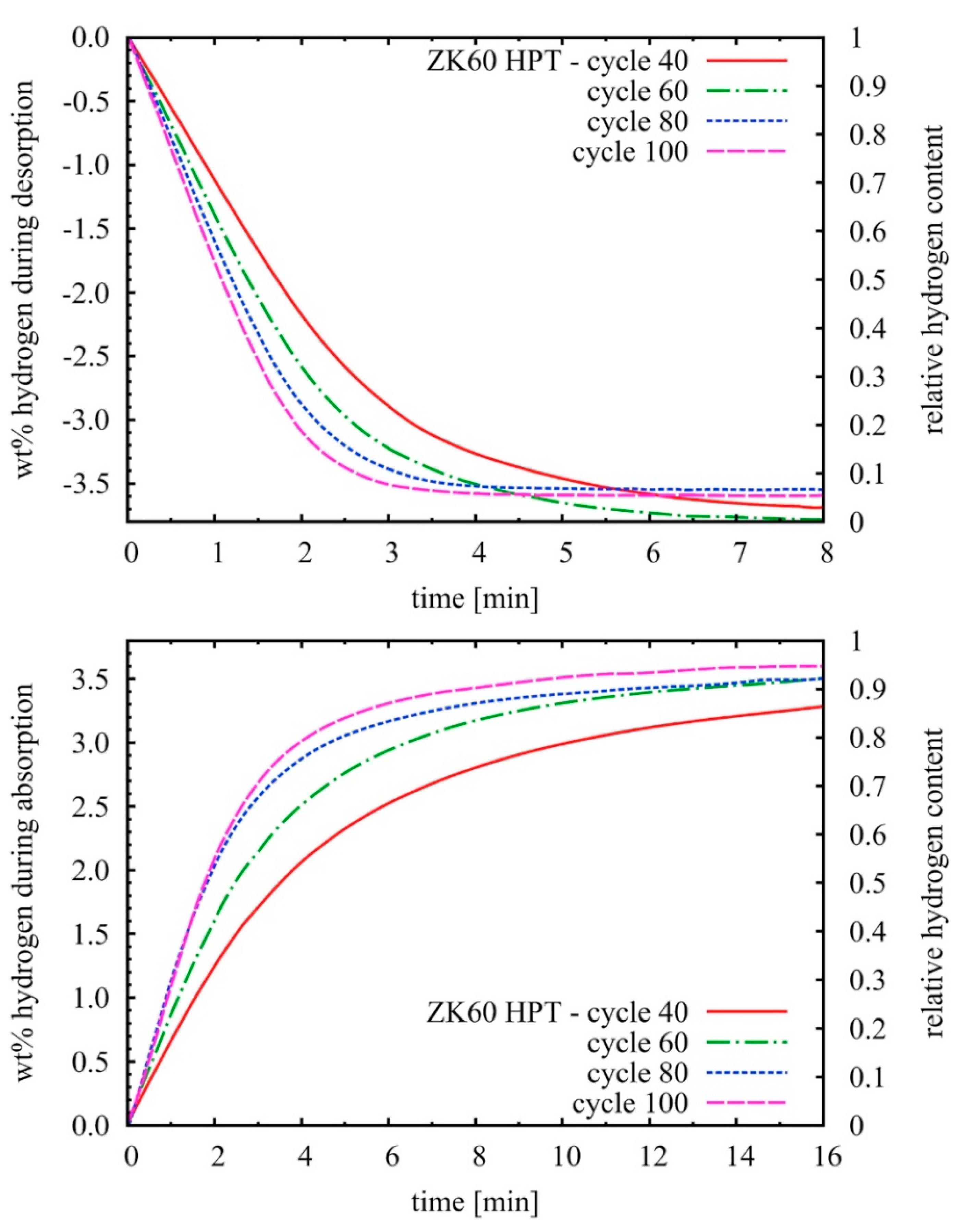
3.1. Elemental Mg and MgH2
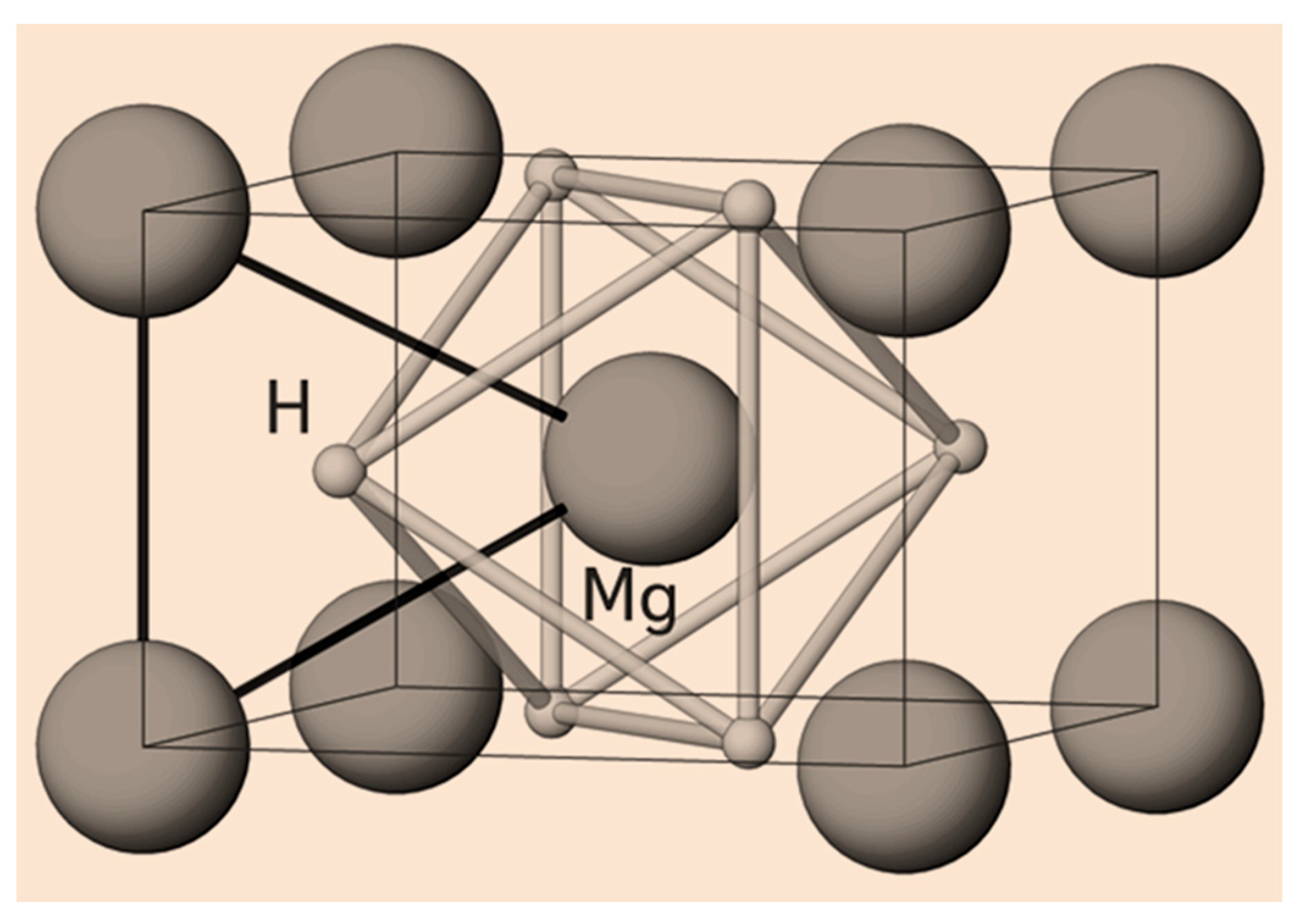
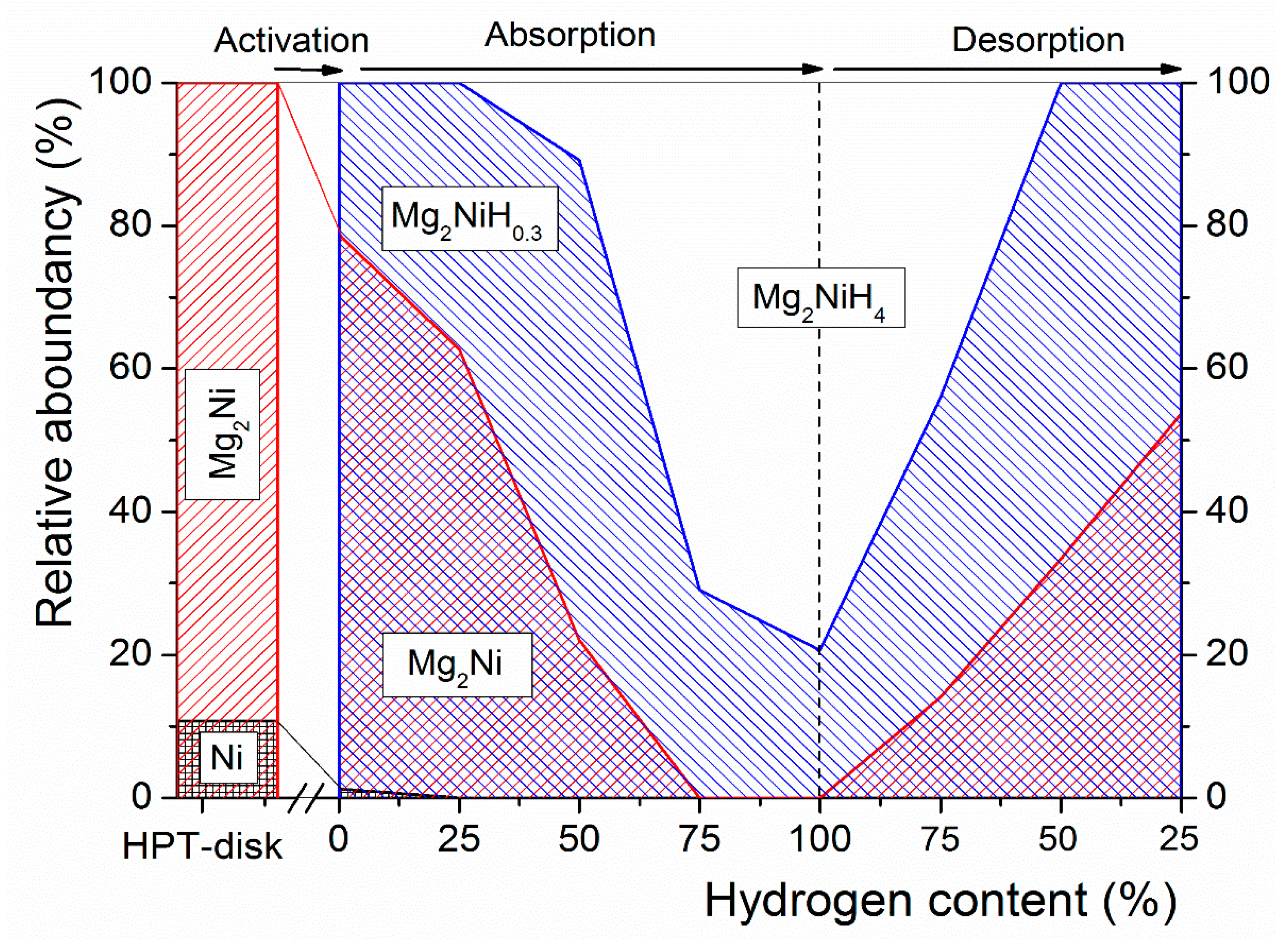
3.2. Mg-Ni System
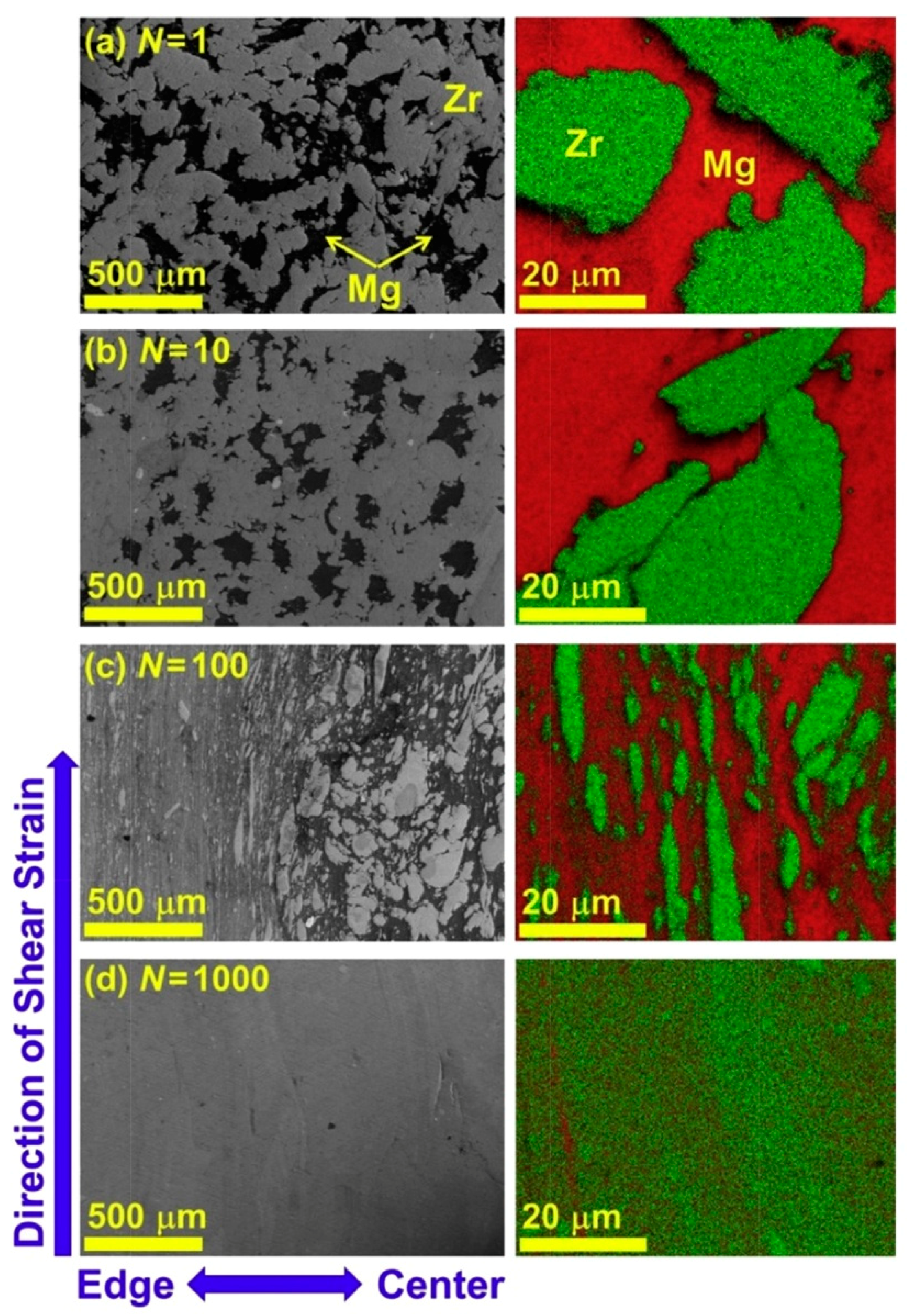
3.3. Formation of New Hydrogen Storage Phases by HPT in Other Mg-Based Systems
3.4. Nanocrystalline Mg Catalyzed by Nanotube Additives
4. Hydrogen Storage in the Non-Equilibrium Mg-(Ni-Cu)-(Y-Ce) System
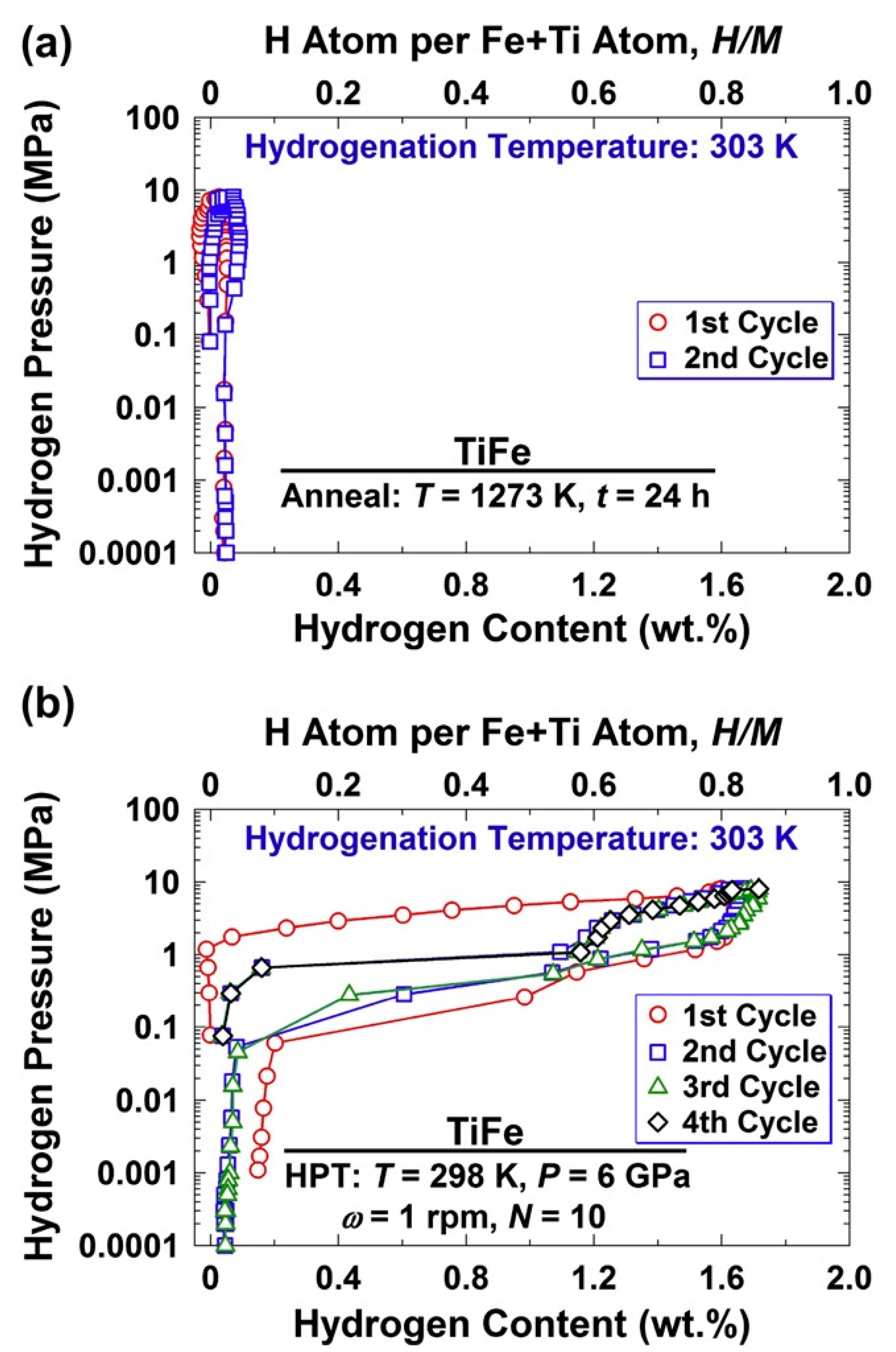
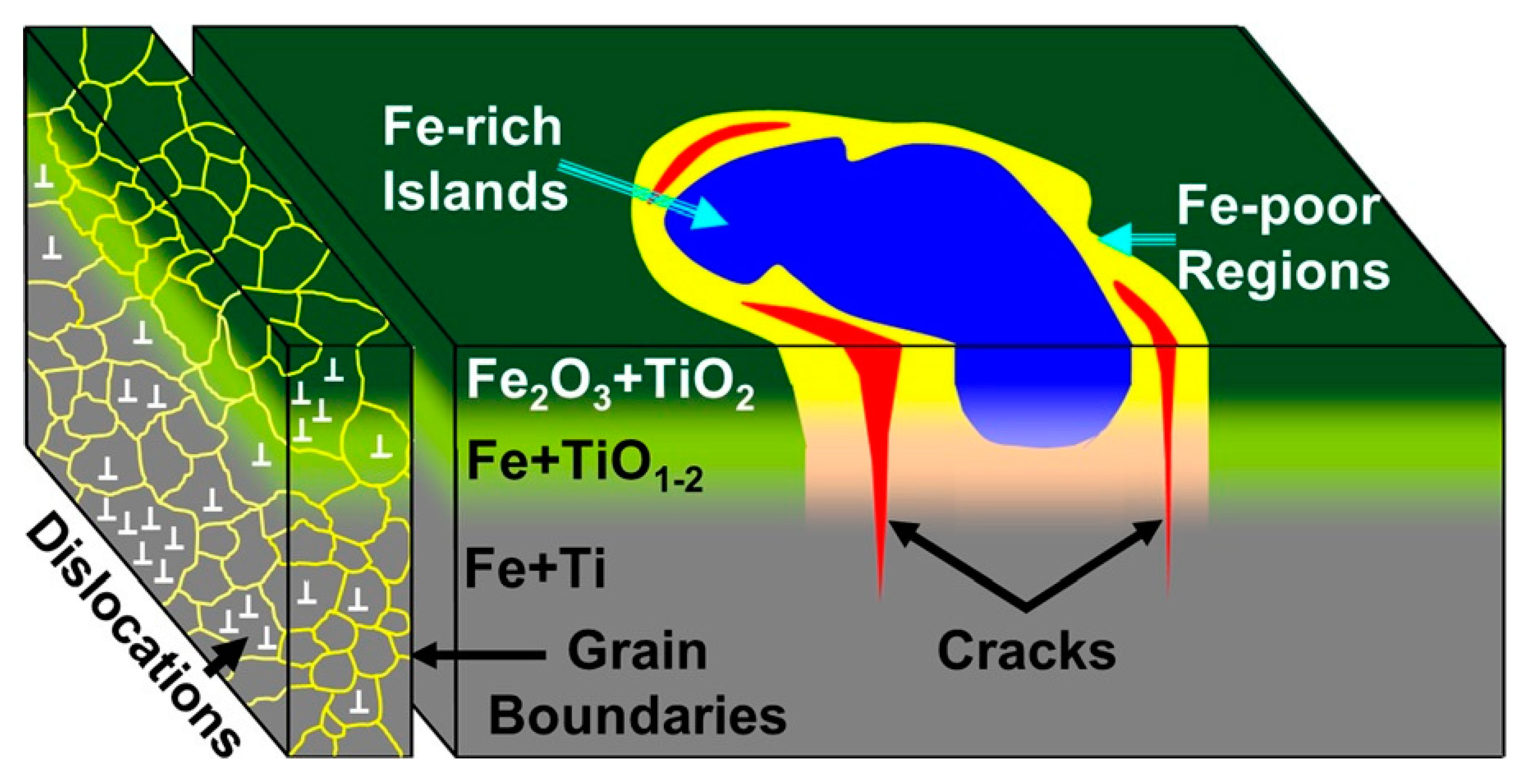
5. Non-Magnesium-Based Materials
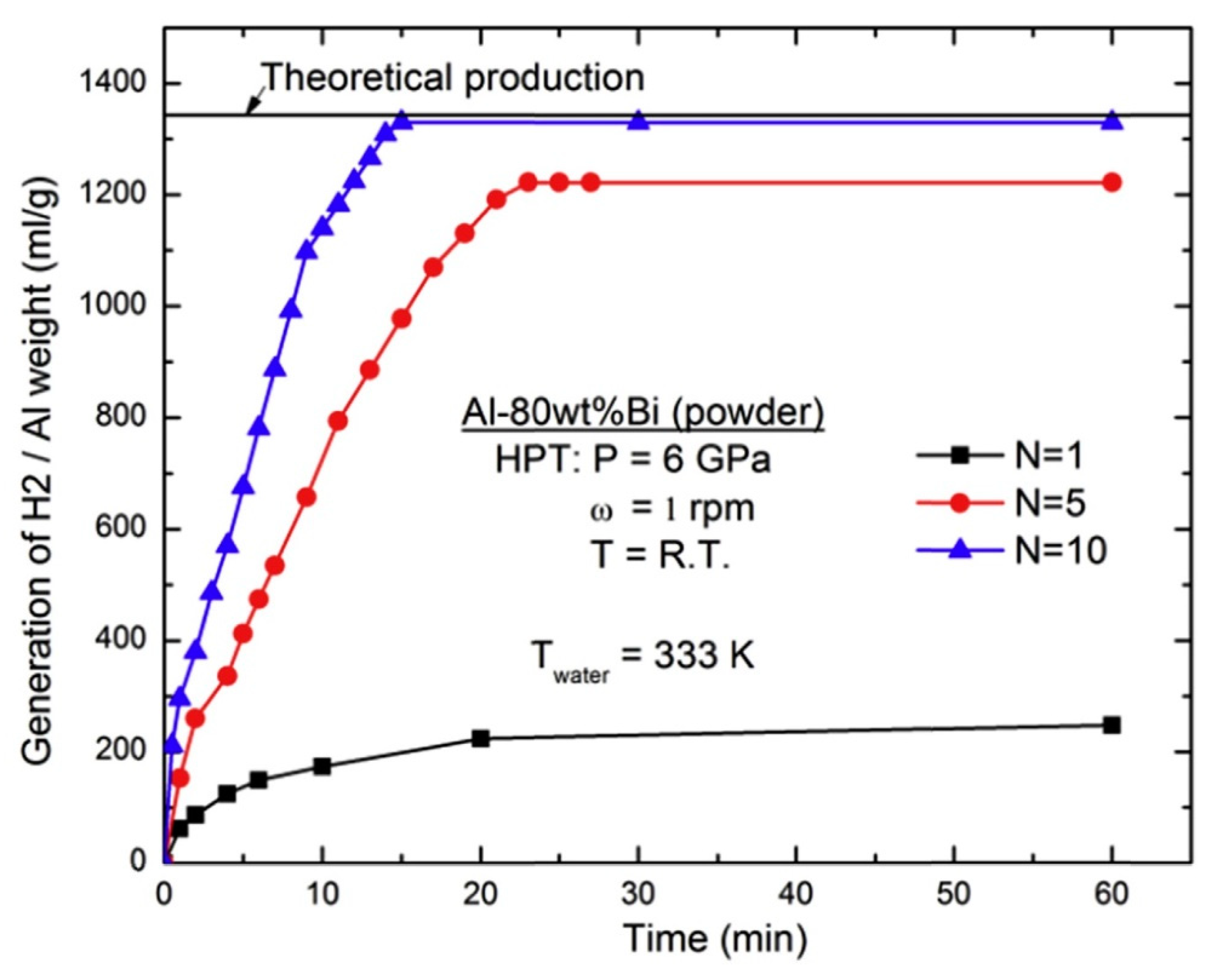
6. Improvement of Hydrogen Production by HPT
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen Energy, Economy and Storage: Review and Recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Jain, I.P. Hydrogen the Fuel for 21st Century. Int. J. Hydrogen Energy 2009, 34, 7368–7378. [Google Scholar] [CrossRef]
- International Energy Agency. Key World Energy Statistics 2020. Available online: https://www.iea.org/reports/key-world-energy-statistics-2020 (accessed on 5 December 2020).
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen Production, Storage, Transportation and Key Challenges with Applications: A Review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Ren, J.; Musyoka, N.M.; Langmi, H.W.; Mathe, M.; Liao, S. Current Research Trends and Perspectives on Materials-Based Hydrogen Storage Solutions: A Critical Review. Int. J. Hydrogen Energy 2017, 42, 289–311. [Google Scholar] [CrossRef]
- Klebanoff, L. Hydrogen Storage Technology: Materials and Application; CRC Press: Boca Raton, FL, USA, 2013; ISBN 978-1-138-19929-3. [Google Scholar]
- Sharma, S.; Ghoshal, S.K. Hydrogen the Future Transportation Fuel: From Production to Applications. Renew. Sustain. Energy Rev. 2015, 43, 1151–1158. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-Storage Materials for Mobile Applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Rusman, N.A.A.; Dahari, M. A Review on the Current Progress of Metal Hydrides Material for Solid-State Hydrogen Storage Applications. Int. J. Hydrogen Energy 2016, 41, 12108–12126. [Google Scholar] [CrossRef]
- Yang, J.; Sudik, A.; Wolverton, C.; Siegel, D.J. High Capacity Hydrogen Storage Materials: Attributes for Automotive Applications and Techniques for Materials Discovery. Chem Soc. Rev. 2010, 39, 656–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niaz, S.; Manzoor, T.; Pandith, A.H. Hydrogen Storage: Materials, Methods and Perspectives. Renew. Sustain. Energy Rev. 2015, 50, 457–469. [Google Scholar] [CrossRef]
- US Department of Energy (DOE). Target Explanation Document: Onboard Hydrogen Storage for Light-Duty Fuel Cell Vehicles. Available online: https://www.energy.gov/sites/prod/files/2017/05/f34/fcto_targets_onboard_hydro_storage_explanation.pdf (accessed on 5 December 2020).
- Wang, H.; Lin, H.J.; Cai, W.T.; Ouyang, L.Z.; Zhu, M. Tuning Kinetics and Thermodynamics of Hydrogen Storage in Light Metal Element Based Systems—A Review of Recent Progress. J. Alloys Compd. 2016, 658, 280–300. [Google Scholar] [CrossRef]
- Pasquini, L. Design of Nanomaterials for Hydrogen Storage. Energies 2020, 13, 3503. [Google Scholar] [CrossRef]
- Varin, R.A.; Czujko, T.; Wronski, Z.S. Nanomaterials for Solid State Hydrogen Storage; Springer Science: New York, NY, USA, 2009. [Google Scholar]
- Crivello, J.-C.; Dam, B.; Denys, R.V.; Dornheim, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; de Jongh, P.; Latroche, M.; Milanese, C.; et al. Review of Magnesium Hydride-Based Materials: Development and Optimisation. Appl. Phys. A 2016, 122, 97. [Google Scholar] [CrossRef] [Green Version]
- Khafidz, N.Z.A.K.; Yaakob, Z.; Lim, K.L.; Timmiati, S.N. The Kinetics of Lightweight Solid-State Hydrogen Storage Materials: A Review. Int. J. Hydrogen Energy 2016, 41, 13131–13151. [Google Scholar] [CrossRef]
- Aguey-Zinsou, K.-F.; Ares-Fernández, J.-R. Hydrogen in Magnesium: New Perspectives toward Functional Stores. Energy Environ. Sci. 2010, 3, 526–543. [Google Scholar] [CrossRef]
- Huot, J.; Cuevas, F.; Deledda, S.; Edalati, K.; Filinchuk, Y.; Grosdidier, T.; Hauback, B.C.; Heere, M.; Jensen, T.R.; Latroche, M.; et al. Mechanochemistry of Metal Hydrides: Recent Advances. Materials 2019, 12, 2778. [Google Scholar] [CrossRef] [Green Version]
- Valiev, R.Z.; Islamgaliev, R.K.; Alexandrov, I.V. Bulk Nanostructured Materials from Severe Plastic Deformation. Prog. Mater. Sci. 2000, 45, 103–189. [Google Scholar] [CrossRef]
- Edalati, K.; Akiba, E.; Horita, Z. High-Pressure Torsion for New Hydrogen Storage Materials. Sci. Technol. Adv. Mater. 2018, 19, 185–193. [Google Scholar] [CrossRef]
- Leiva, D.R.; Jorge, A.M., Jr.; Ishikawa, T.T.; Botta, W.J. Hydrogen Storage in Mg and Mg-Based Alloys and Composites Processed by Severe Plastic Deformation. Mater. Trans. 2019, 60, 1561–1570. [Google Scholar] [CrossRef] [Green Version]
- Edalati, K. Metallurgical Alchemy by Ultra-Severe Plastic Deformation via High-Pressure Torsion Process. Mater. Trans. 2019, 60, 1221–1229. [Google Scholar] [CrossRef] [Green Version]
- Edalati, K.; Horita, Z. A Review on High-Pressure Torsion (HPT) from 1935 to 1988. Mater. Sci. Eng. A 2016, 652, 325–352. [Google Scholar] [CrossRef]
- Wei, P.T.; Lu, C.; Tieu, K.; Deng, G.Y. A Study of Plastic Deformation Behavior during High Pressure Torsion Process by Crystal Plasticity Finite Element Simulation. IOP Conf. Ser. Mater. Sci. Eng. 2014, 63, 012045. [Google Scholar] [CrossRef] [Green Version]
- Zhilyaev, A.; Langdon, T. Using High-Pressure Torsion for Metal Processing: Fundamentals and Applications. Prog. Mater. Sci. 2008, 53, 893–979. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Zhilyaev, A.P.; Langdon, T.G. Bulk Nanostructured Materials: Fundamentals and Applications; Wiley-Blackwell: Hoboken, NJ, USA, 2013; ISBN 978-1-118-09540-9. [Google Scholar]
- Straumal, B.B.; Mazilkin, A.A.; Baretzky, B.; Schütz, G.; Rabkin, E.; Valiev, R.Z. Accelerated Diffusion and Phase Transformations in Co-Cu Alloys Driven by the Severe Plastic Deformation. Mater. Trans. 2012, 53, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Edalati, K.; Horita, Z. High-Pressure Torsion of Pure Metals: Influence of Atomic Bond Parameters and Stacking Fault Energy on Grain Size and Correlation with Hardness. Acta Mater. 2011, 59, 6831–6836. [Google Scholar] [CrossRef]
- Boucharat, N.; Rösner, H.; Wilde, G. Nanocrystallization Mechanisms of Al-Rich Glass-Forming Alloys. J. Non Cryst. Solids 2008, 354, 592–596. [Google Scholar] [CrossRef]
- Révész, Á.; Hóbor, S.; Szabó, P.J.; Zhilyaev, A.P.; Kovács, Z. Deformation Induced Crystallization in an Amorphous Cu60Zr20Ti20 Alloy by High Pressure Torsion. Mater. Sci. Eng. A 2007, 460–461, 459–463. [Google Scholar] [CrossRef]
- Révész, Á.; Schafler, E.; Kovács, Z. Structural Anisotropy in a Zr57Ti5Cu20Al10Ni8 Bulk Metallic Glass Deformed by High Pressure Torsion at Room Temperature. Appl. Phys. Lett. 2008, 92, 011910. [Google Scholar] [CrossRef]
- Révész, Á.; Horváth, A.; Ribárik, G.; Schafler, E.; Browne, D.J.; Kovács, Z. Crystallization of Cu60Zr20Ti20 Bulk Metallic Glass by High Pressure Torsion. Rev. Adv. Mater. Sci. 2019, 58, 304–312. [Google Scholar] [CrossRef] [Green Version]
- Kovács, Z.; Schafler, E.; Révész, Á. Volume Changes in Vitreloy Bulk Metallic Glass during Room Temperature High-Pressure Torsion. J. Mater. Res. 2008, 23, 3409–3414. [Google Scholar] [CrossRef]
- Révész, Á.; Kovács, Z. Severe Plastic Deformation of Amorphous Alloys. Mater. Trans. 2019, 60, 1283–1293. [Google Scholar] [CrossRef] [Green Version]
- Gunderov, D.; Astanin, V. Influence of HPT Deformation on the Structure and Properties of Amorphous Alloys. Metals 2020, 10, 415. [Google Scholar] [CrossRef] [Green Version]
- Edalati, K.; Uehiro, R.; Fujiwara, K.; Ikeda, Y.; Li, H.-W.; Sauvage, X.; Valiev, R.Z.; Akiba, E.; Tanaka, I.; Horita, Z. Ultra-Severe Plastic Deformation: Evolution of Microstructure, Phase Transformation and Hardness in Immiscible Magnesium-Based Systems. Mater. Sci. Eng. A 2017, 701, 158–166. [Google Scholar] [CrossRef]
- Bachmaier, A.; Pippan, R. High-Pressure Torsion Deformation Induced Phase Transformations and Formations: New Material Combinations and Advanced Properties. Mater. Trans. 2019, 60, 1256–1269. [Google Scholar] [CrossRef] [Green Version]
- Edalati, K.; Hashiguchi, Y.; Pereira, P.H.R.; Horita, Z.; Langdon, T.G. Effect of Temperature Rise on Microstructural Evolution during High-Pressure Torsion. Mater. Sci. Eng. A 2018, 714, 167–171. [Google Scholar] [CrossRef] [Green Version]
- Edalati, K.; Miresmaeili, R.; Horita, Z.; Kanayama, H.; Pippan, R. Significance of Temperature Increase in Processing by High-Pressure Torsion. Mater. Sci. Eng. A 2011, 528, 7301–7305. [Google Scholar] [CrossRef]
- Henits, P.; Révész, Á.; Kovács, Z. Free Volume Simulation for Severe Plastic Deformation of Metallic Glasses. Mech. Mater. 2012, 50, 81–87. [Google Scholar] [CrossRef]
- Hóbor, S.; Révész, Á.; Szabó, P.J.; Zhilyaev, A.P.; Kis, V.K.; Lábár, J.L.; Kovács, Z. High Pressure Torsion of Amorphous Cu60Zr30Ti10 Alloy. J. Appl. Phys. 2008, 104, 033525. [Google Scholar] [CrossRef]
- Hóbor, S.; Kovács, Z.; Révész, Á. Macroscopic Thermoplastic Model Applied to the High Pressure Torsion of Metallic Glasses. J. Appl. Phys. 2009, 106, 023531. [Google Scholar] [CrossRef]
- Edalati, K.; Horita, Z. Continuous High-Pressure Torsion. J. Mater. Sci. 2010, 45, 4578–4582. [Google Scholar] [CrossRef]
- Edalati, K.; Horita, Z. Scaling-Up of High Pressure Torsion Using Ring Shape. Mater. Trans. 2009, 50, 92–95. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Yoon, E.Y.; Lee, D.J.; Lee, J.H.; Kim, H.S. Mechanical Properties of Copper after Compression Stage of High-Pressure Torsion. Mater. Sci. Eng. A 2011, 528, 4840–4844. [Google Scholar] [CrossRef]
- Grau-Crespo, R.; Smith, K.C.; Fisher, T.S.; de Leeuw, N.H.; Waghmare, U.V. Thermodynamics of Hydrogen Vacancies in MgH2 from First-Principles Calculations and Grand-Canonical Statistical Mechanics. Phys. Rev. B 2009, 80, 174117. [Google Scholar] [CrossRef] [Green Version]
- Züttel, A. Materials for Hydrogen Storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]
- Zaluski, L.; Zaluska, A.; Tessier, P.; Stroem-Olsen, J.O.; Schulz, R. Nanocrystalline Hydrogen Absorbing Alloys. Mater. Sci. Forum 1996, 225–227, 853–858. [Google Scholar] [CrossRef]
- Oelerich, W.; Klassen, T.; Bormann, R. Metal Oxides as Catalysts for Improved Hydrogen Sorption in Nanocrystalline Mg-Based Materials. J. Alloys Compd. 2001, 315, 237–242. [Google Scholar] [CrossRef]
- Fátay, D.; Révész, Á.; Spassov, T. Particle Size and Catalytic Effect on the Dehydriding of MgH2. J. Alloys Compd. 2005, 399, 237–241. [Google Scholar] [CrossRef]
- Fátay, D.; Spassov, T.; Delchev, P.; Ribárik, G.; Révész, A. Microstructural Development in Nanocrystalline MgH2 during H-Absorption/Desorption Cycling. Int. J. Hydrogen Energy 2007, 32, 2914–2919. [Google Scholar] [CrossRef]
- Révész, Á.; Fátay, D. Microstructural Evolution of Ball-Milled MgH2 during a Complete Dehydrogenation–Hydrogenation Cycle. J. Power Sources 2010, 195, 6997–7002. [Google Scholar] [CrossRef]
- Paidar, V. Magnesium Hydrides and Their Phase Transitions. Int. J. Hydrogen Energy 2016, 41, 9769–9773. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, Q.; Chen, H.; Zang, X.; Zhou, X.; Wang, R.; Jiang, X.; Yang, B.; Jiang, R. Crystalline Structure, Energy Calculation and Dehydriding Thermodynamics of Magnesium Hydride from Reactive Milling. Int. J. Hydrogen Energy 2015, 40, 11484–11490. [Google Scholar] [CrossRef]
- Leiva, D.R.; Jorge, A.M.; Ishikawa, T.T.; Huot, J.; Fruchart, D.; Miraglia, S.; Kiminami, C.S.; Botta, W.J. Nanoscale Grain Refinement and H-Sorption Properties of MgH2 Processed by High-Pressure Torsion and Other Mechanical Routes. Adv. Eng. Mater. 2010, 12, 786–792. [Google Scholar] [CrossRef]
- Edalati, K.; Kitabayashi, K.; Ikeda, Y.; Matsuda, J.; Li, H.-W.; Tanaka, I.; Akiba, E.; Horita, Z. Bulk Nanocrystalline Gamma Magnesium Hydride with Low Dehydrogenation Temperature Stabilized by Plastic Straining via High-Pressure Torsion. Scr. Mater. 2018, 157, 54–57. [Google Scholar] [CrossRef]
- Panda, S.; Fundenberger, J.-J.; Zhao, Y.; Zou, J.; Toth, L.S.; Grosdidier, T. Effect of Initial Powder Type on the Hydrogen Storage Properties of High-Pressure Torsion Consolidated Mg. Int. J. Hydrogen Energy 2017, 42, 22438–22448. [Google Scholar] [CrossRef]
- Edalati, K.; Yamamoto, A.; Horita, Z.; Ishihara, T. High-Pressure Torsion of Pure Magnesium: Evolution of Mechanical Properties, Microstructures and Hydrogen Storage Capacity with Equivalent Strain. Scr. Mater. 2011, 64, 880–883. [Google Scholar] [CrossRef]
- Révész, Á.; Gajdics, M. Correlation between Microstructure and Hydrogen Storage Properties of Nanocrystalline Magnesium Subjected to High-Pressure Torsion. Mater. Sci. Forum 2017, 885, 67–73. [Google Scholar] [CrossRef]
- Grill, A.; Horky, J.; Panigrahi, A.; Krexner, G.; Zehetbauer, M. Long-Term Hydrogen Storage in Mg and ZK60 after Severe Plastic Deformation. Int. J. Hydrogen Energy 2015, 40, 17144–17152. [Google Scholar] [CrossRef]
- Zeng, K.; Klassen, T.; Oelerich, W.; Bormann, R. Thermodynamic Analysis of the Hydriding Process of Mg–Ni Alloys. J. Alloys Compd. 1999, 283, 213–224. [Google Scholar] [CrossRef]
- Liang, G.; Boily, S.; Huot, J.; Van Neste, A.; Schulz, R. Mechanical Alloying and Hydrogen Absorption Properties of the Mg–Ni System. J. Alloys Compd. 1998, 267, 302–306. [Google Scholar] [CrossRef]
- Zaluska, A.; Zaluski, L.; Ström-Olsen, J.O. Structure, Catalysis and Atomic Reactions on the Nano-Scale: A Systematic Approach to Metal Hydrides for Hydrogen Storage. Appl. Phys. A 2001, 72, 157–165. [Google Scholar] [CrossRef]
- Massalski, T.B.; Murray, J.L.; Bennett, L.H.; Baker, H. Binary Alloy Phase Diagrams; American Society for Metals: Materials Park, OH, USA, 1986; ISBN 978-0-87170-261-6. [Google Scholar]
- Reilly, J.J., Jr.; Wishwall, R.H., Jr. Reaction of Hydrogen with Alloys of Magnesium and Nickel and the Formation of Mg2NiH4. Inorg. Chem. 1968, 7, 2254–2256. [Google Scholar] [CrossRef]
- Simchi, H.; Kaflou, A.; Simchi, A. Synergetic Effect of Ni and Nb2O5 on Dehydrogenation Properties of Nanostructured MgH2 Synthesized by High-Energy Mechanical Alloying. Int. J. Hydrogen Energy 2009, 34, 7724–7730. [Google Scholar] [CrossRef]
- Xie, L.; Li, J.; Zhang, T.; Kou, H. Understanding the Dehydrogenation Process of MgH2 from the Recombination of Hydrogen Atoms. Int. J. Hydrogen Energy 2016, 41, 5716–5724. [Google Scholar] [CrossRef]
- Urretavizcaya, G.; García, G.; Serafini, D.; Meyer, G. Mg-Ni Alloys For Hydrogen Storage Obtained By Ball Milling. Lat. Am. Appl. Res. 2002, 32, 289–294. [Google Scholar]
- Varin, R.A.; Czujko, T.; Mizera, J. Microstructural Evolution during Controlled Ball Milling of (Mg2Ni+MgNi2) Intermetallic Alloy. J. Alloys Compd. 2003, 350, 332–339. [Google Scholar] [CrossRef]
- Révész, Á.; Gajdics, M.; Spassov, T. Microstructural Evolution of Ball-Milled Mg–Ni Powder during Hydrogen Sorption. Int. J. Hydrogen Energy 2013, 38, 8342–8349. [Google Scholar] [CrossRef]
- Orimo, S.; Ikeda, K.; Fujii, H.; Fujikawa, Y.; Kitano, Y.; Yamamoto, K. Structural and Hydriding Properties of the Mg-Ni-H System with Nano- and/or Amorphous Structures. Acta Mater. 1997, 45, 2271–2278. [Google Scholar] [CrossRef]
- Kusadome, Y.; Ikeda, K.; Nakamori, Y.; Orimo, S.; Horita, Z. Hydrogen Storage Capability of MgNi2 Processed by High Pressure Torsion. Scr. Mater. 2007, 57, 751–753. [Google Scholar] [CrossRef]
- Révész, Á.; Kánya, Z.; Verebélyi, T.; Szabó, P.J.; Zhilyaev, A.P.; Spassov, T. The Effect of High-Pressure Torsion on the Microstructure and Hydrogen Absorption Kinetics of Ball-Milled Mg70Ni30. J. Alloys Compd. 2010, 504, 83–88. [Google Scholar] [CrossRef]
- Hongo, T.; Edalati, K.; Arita, M.; Matsuda, J.; Akiba, E.; Horita, Z. Significance of Grain Boundaries and Stacking Faults on Hydrogen Storage Properties of Mg2Ni Intermetallics Processed by High-Pressure Torsion. Acta Mater. 2015, 92, 46–54. [Google Scholar] [CrossRef]
- Grosdidier, T.; Fundenberger, J.J.; Zou, J.X.; Pan, Y.C.; Zeng, X.Q. Nanostructured Mg Based Hydrogen Storage Bulk Materials Prepared by High Pressure Torsion Consolidation of Arc Plasma Evaporated Ultrafine Powders. Int. J. Hydrogen Energy 2015, 40, 16985–16991. [Google Scholar] [CrossRef]
- Gajdics, M.; Calizzi, M.; Pasquini, L.; Schafler, E.; Révész, Á. Characterization of a Nanocrystalline Mg–Ni Alloy Processed by High-Pressure Torsion during Hydrogenation and Dehydrogenation. Int. J. Hydrogen Energy 2016, 41, 9803–9809. [Google Scholar] [CrossRef]
- Révész, Á.; Gajdics, M.; Schafler, E.; Calizzi, M.; Pasquini, L. Dehydrogenation-Hydrogenation Characteristics of Nanocrystalline Mg2Ni Powders Compacted by High-Pressure Torsion. J. Alloys Compd. 2017, 702, 84–91. [Google Scholar] [CrossRef]
- Dębski, A.; Terlicka, S.; Gąsior, W.; Gierlotka, W.; Pęska, M.; Polański, M. Thermodynamic Properties of Mg-Pd Liquid Alloys. J. Mol. Liq. 2020, 317, 114024. [Google Scholar] [CrossRef]
- Edalati, K.; Emami, H.; Staykov, A.; Smith, D.J.; Akiba, E.; Horita, Z. Formation of Metastable Phases in Magnesium–Titanium System by High-Pressure Torsion and Their Hydrogen Storage Performance. Acta Mater. 2015, 99, 150–156. [Google Scholar] [CrossRef]
- Edalati, K.; Emami, H.; Ikeda, Y.; Iwaoka, H.; Tanaka, I.; Akiba, E.; Horita, Z. New Nanostructured Phases with Reversible Hydrogen Storage Capability in Immiscible Magnesium–Zirconium System Produced by High-Pressure Torsion. Acta Mater. 2016, 108, 293–303. [Google Scholar] [CrossRef]
- Kitabayashi, K.; Edalati, K.; Li, H.; Akiba, E.; Horita, Z. Phase Transformations in MgH2–TiH2 Hydrogen Storage System by High-Pressure Torsion Process. Adv. Eng. Mater. 2020, 22, 1900027. [Google Scholar] [CrossRef]
- Edalati, K.; Uehiro, R.; Ikeda, Y.; Li, H.-W.; Emami, H.; Filinchuk, Y.; Arita, M.; Sauvage, X.; Tanaka, I.; Akiba, E.; et al. Design and Synthesis of a Magnesium Alloy for Room Temperature Hydrogen Storage. Acta Mater. 2018, 149, 88–96. [Google Scholar] [CrossRef]
- López Gómez, E.I.; Edalati, K.; Coimbrão, D.D.; Antiqueira, F.J.; Zepon, G.; Cubero-Sesin, J.M.; Botta, W.J. FCC Phase Formation in Immiscible Mg–Hf (Magnesium–Hafnium) System by High-Pressure Torsion. AIP Adv. 2020, 10, 055222. [Google Scholar] [CrossRef]
- De Marco, M.O.; Li, Y.; Li, H.-W.; Edalati, K.; Floriano, R. Mechanical Synthesis and Hydrogen Storage Characterization of MgVCr and MgVTiCrFe High-Entropy Alloy. Adv. Eng. Mater. 2020, 22, 1901079. [Google Scholar] [CrossRef]
- Aminorroaya, S.; Liu, H.K.; Cho, Y.; Dahle, A. Microstructure and Activation Characteristics of Mg–Ni Alloy Modified by Multi-Walled Carbon Nanotubes. Int. J. Hydrogen Energy 2010, 35, 4144–4153. [Google Scholar] [CrossRef]
- Hou, X.; Hu, R.; Zhang, T.; Kou, H.; Li, J. Hydrogenation Thermodynamics of Melt-Spun Magnesium Rich Mg–Ni Nanocrystalline Alloys with the Addition of Multiwalled Carbon Nanotubes and TiF3. J. Power Sources 2016, 306, 437–447. [Google Scholar] [CrossRef]
- Chen, B.-H.; Kuo, C.-H.; Ku, J.-R.; Yan, P.-S.; Huang, C.-J.; Jeng, M.-S.; Tsau, F.-H. Highly Improved with Hydrogen Storage Capacity and Fast Kinetics in Mg-Based Nanocomposites by CNTs. J. Alloys Compd. 2013, 568, 78–83. [Google Scholar] [CrossRef]
- Su, W.; Zhu, Y.; Zhang, J.; Liu, Y.; Yang, Y.; Mao, Q.; Li, L. Effect of Multi-Wall Carbon Nanotubes Supported Nano-Nickel and TiF3 Addition on Hydrogen Storage Properties of Magnesium Hydride. J. Alloys Compd. 2016, 669, 8–18. [Google Scholar] [CrossRef]
- Shahi, R.R.; Bhatnagar, A.; Pandey, S.K.; Dixit, V.; Srivastava, O.N. Effects of Ti-Based Catalysts and Synergistic Effect of SWCNTs-TiF3 on Hydrogen Uptake and Release from MgH2. Int. J. Hydrogen Energy 2014, 39, 14255–14261. [Google Scholar] [CrossRef]
- Amirkhiz, B.S.; Danaie, M.; Barnes, M.; Simard, B.; Mitlin, D. Hydrogen Sorption Cycling Kinetic Stability and Microstructure of Single-Walled Carbon Nanotube (SWCNT) Magnesium Hydride (MgH2) Nanocomposites. J. Phys. Chem. C 2010, 114, 3265–3275. [Google Scholar] [CrossRef] [Green Version]
- Verón, M.G.; Troiani, H.; Gennari, F.C. Synergetic Effect of Co and Carbon Nanotubes on MgH2 Sorption Properties. Carbon 2011, 49, 2413–2423. [Google Scholar] [CrossRef]
- Chuang, Y.-S.; Hwang, S.-J. Synthesis and Hydrogen Absorption/Desorption Properties of Mg–Nb2O5 -SWCNT/MWCNT Nanocomposite Prepared by Reactive Milling. J. Alloys Compd. 2016, 656, 835–842. [Google Scholar] [CrossRef]
- Gajdics, M.; Spassov, T.; Kis, V.K.; Schafler, E.; Révész, Á. Microstructural and Morphological Investigations on Mg-Nb2O5-CNT Nanocomposites Processed by High-Pressure Torsion for Hydrogen Storage Applications. Int. J. Hydrogen Energy 2020, 45, 7917–7928. [Google Scholar] [CrossRef]
- Révész, Á.; Spassov, T.; Kis, V.K.; Schafler, E.; Gajdics, M. The Influence of Preparation Conditions on the Hydrogen Sorption of Mg-Nb2O5-CNT Produced by Ball Milling and Subsequent High-Pressure Torsion. J. Nanosci. Nanotechnol. 2020, 20, 4587–4590. [Google Scholar] [CrossRef]
- Gajdics, M.; Spassov, T.; Kis, V.K.; Béke, F.; Novák, Z.; Schafler, E.; Révész, Á. Microstructural Investigation of Nanocrystalline Hydrogen-Storing Mg-Titanate Nanotube Composites Processed by High-Pressure Torsion. Energies 2020, 13, 563. [Google Scholar] [CrossRef] [Green Version]
- Hara, M.; Morozumi, S.; Watanabe, K. Effect of a Magnesium Depletion on the Mg–Ni–Y Alloy Hydrogen Absorption Properties. J. Alloys Compd. 2006, 414, 207–214. [Google Scholar] [CrossRef]
- Yang, T.; Wang, P.; Li, Q.; Xia, C.; Yin, F.; Liang, C.; Zhang, Y. Hydrogen Absorption and Desorption Behavior of Ni Catalyzed Mg–Y–C–Ni Nanocomposites. Energy 2018, 165, 709–719. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, D.; Li, B.; Ren, H.; Guo, S.; Wang, X. Electrochemical Hydrogen Storage Characteristics of Nanocrystalline Mg20Ni10-xCox (X = 0–4) Alloys Prepared by Melt-Spinning. J. Alloys Compd. 2010, 491, 589–594. [Google Scholar] [CrossRef]
- Si, T.Z.; Liu, Y.F.; Zhang, Q.A. Hydrogen Storage Properties of the Supersaturated Mg12YNi Solid Solution. J. Alloys Compd. 2010, 507, 489–493. [Google Scholar] [CrossRef]
- Kalinichenka, S.; Röntzsch, L.; Baehtz, C.; Kieback, B. Hydrogen Desorption Kinetics of Melt-Spun and Hydrogenated Mg90Ni10 and Mg80Ni10Y10 Using in Situ Synchrotron, X-Ray Diffraction and Thermogravimetry. J. Alloys Compd. 2010, 496, 608–613. [Google Scholar] [CrossRef]
- Spassov, T.; Köster, U. Thermal Stability and Hydriding Properties of Nanocrystalline Melt-Spun Mg63Ni30Y7 Alloy. J. Alloys Compd. 1998, 279, 279–286. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Z.; Zhou, H.; Ni, C.; Deng, J.; Yao, Q. Hydrogen Storage Properties and Thermal Stability of Amorphous Mg70(RE25Ni75)30 Alloys. J. Alloys Compd. 2013, 563, 1–5. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Jiang, C.J.; Liu, D.D. Comparative Investigations on the Hydrogenation Characteristics and Hydrogen Storage Kinetics of Melt-Spun Mg10NiR (R = La, Nd and Sm) Alloys. Int. J. Hydrogen Energy 2012, 37, 10709–10714. [Google Scholar] [CrossRef]
- Lin, H.-J.; He, M.; Pan, S.-P.; Gu, L.; Li, H.-W.; Wang, H.; Ouyang, L.-Z.; Liu, J.-W.; Ge, T.-P.; Wang, D.-P.; et al. Towards Easily Tunable Hydrogen Storage via a Hydrogen-Induced Glass-to-Glass Transition in Mg-Based Metallic Glasses. Acta Mater. 2016, 120, 68–74. [Google Scholar] [CrossRef]
- Spassov, T.; Köster, U. Hydrogenation of Amorphous and Nanocrystalline Mg-Based Alloys. J. Alloys Compd. 1999, 287, 243–250. [Google Scholar] [CrossRef]
- Kalinichenka, S.; Röntzsch, L.; Kieback, B. Structural and Hydrogen Storage Properties of Melt-Spun Mg–Ni–Y Alloys. Int. J. Hydrogen Energy 2009, 34, 7749–7755. [Google Scholar] [CrossRef]
- Wu, Y.; Solberg, J.K.; Yartys, V.A. The Effect of Solidification Rate on Microstructural Evolution of a Melt-Spun Mg–20Ni–8Mm Hydrogen Storage Alloy. J. Alloys Compd. 2007, 446–447, 178–182. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Ren, H.; Ding, X.; Liu, X.; Chen, L. An Investigation on Hydrogen Storage Kinetics of Nanocrystalline and Amorphous Mg2Ni1-xCox (X = 0–0.4) Alloy Prepared by Melt Spinning. J. Alloys Compd. 2011, 509, 2808–2814. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Ren, H.; Guo, S.; Zhao, D.; Wang, X. Hydrogenation and Dehydrogenation Behaviours of Nanocrystalline Mg20Ni10-xCux (X = 0 − 4) Alloys Prepared by Melt Spinning. Int. J. Hydrogen Energy 2010, 35, 2040–2047. [Google Scholar] [CrossRef]
- Yang, T.; Wang, P.; Xia, C.; Li, Q.; Liang, C.; Zhang, Y. Characterization of Microstructure, Hydrogen Storage Kinetics and Thermodynamics of a Melt-Spun Mg86Y10Ni4 Alloy. Int. J. Hydrogen Energy 2019, 44, 6728–6737. [Google Scholar] [CrossRef]
- Wu, Y.; Lototskyy, M.V.; Solberg, J.K.; Yartys, V.A. Effect of Microstructure on the Phase Composition and Hydrogen Absorption-Desorption Behaviour of Melt-Spun Mg-20Ni-8Mm Alloys. Int. J. Hydrogen Energy 2012, 37, 1495–1508. [Google Scholar] [CrossRef]
- Denys, R.V.; Poletaev, A.A.; Maehlen, J.P.; Solberg, J.K.; Tarasov, B.P.; Yartys, V.A. Nanostructured Rapidly Solidified LaMg11Ni Alloy. II. In Situ Synchrotron X-Ray Diffraction Studies of Hydrogen Absorption–Desorption Behaviours. Int. J. Hydrogen Energy 2012, 37, 5710–5722. [Google Scholar] [CrossRef]
- Révész, Á.; Kis-Tóth, Á.; Varga, L.K.; Lábár, J.L.; Spassov, T. High Glass Forming Ability Correlated with Microstructure and Hydrogen Storage Properties of a Mg–Cu–Ag–Y Glass. Int. J. Hydrogen Energy 2014, 39, 9230–9240. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Lin, H.-J.; Edalati, K.; Li, W.; Li, L.; Zhu, Y. Superior Hydrogenation Properties in a Mg65Ce10Ni20Cu5 Nanoglass Processed by Melt-Spinning Followed by High-Pressure Torsion. Scr. Mater. 2018, 152, 137–140. [Google Scholar] [CrossRef]
- Révész, Á.; Kis-Tóth, Á.; Varga, L.K.; Schafler, E.; Bakonyi, I.; Spassov, T. Hydrogen Storage of Melt-Spun Amorphous Mg65Ni20Cu5Y10 Alloy Deformed by High-Pressure Torsion. Int. J. Hydrogen Energy 2012, 37, 5769–5776. [Google Scholar] [CrossRef]
- Révész, Á.; Kis-Tóth, Á.; Szommer, P.; Spassov, T. Hydrogen Storage, Microstructure and Mechanical Properties of Strained Mg65Ni20Cu5Y10 Metallic Glass. Mater. Sci. Forum 2013, 729, 74–79. [Google Scholar] [CrossRef]
- Spaepen, F. A Microscopic Mechanism for Steady State Inhomogeneous Flow in Metallic Glasses. Acta Metall. 1977, 25, 407–415. [Google Scholar] [CrossRef]
- Reilly, J.J.; Wiswall, R.H. Formation and Properties of Iron Titanium Hydride. Inorg. Chem. 1974, 13, 218–222. [Google Scholar] [CrossRef]
- Edalati, K.; Matsuda, J.; Iwaoka, H.; Toh, S.; Akiba, E.; Horita, Z. High-Pressure Torsion of TiFe Intermetallics for Activation of Hydrogen Storage at Room Temperature with Heterogeneous Nanostructure. Int. J. Hydrogen Energy 2013, 38, 4622–4627. [Google Scholar] [CrossRef]
- Edalati, K.; Matsuda, J.; Arita, M.; Daio, T.; Akiba, E.; Horita, Z. Mechanism of Activation of TiFe Intermetallics for Hydrogen Storage by Severe Plastic Deformation Using High-Pressure Torsion. Appl. Phys. Lett. 2013, 103, 143902. [Google Scholar] [CrossRef]
- Edalati, K.; Matsuda, J.; Yanagida, A.; Akiba, E.; Horita, Z. Activation of TiFe for Hydrogen Storage by Plastic Deformation Using Groove Rolling and High-Pressure Torsion: Similarities and Differences. Int. J. Hydrogen Energy 2014, 39, 15589–15594. [Google Scholar] [CrossRef]
- Lv, P.; Liu, Z.; Dixit, V. Improved Hydrogen Storage Properties of TiFe Alloy by Doping (Zr+2V) Additive and Using Mechanical Deformation. Int. J. Hydrogen Energy 2019, 44, 27843–27852. [Google Scholar] [CrossRef]
- Emami, H.; Edalati, K.; Matsuda, J.; Akiba, E.; Horita, Z. Hydrogen Storage Performance of TiFe after Processing by Ball Milling. Acta Mater. 2015, 88, 190–195. [Google Scholar] [CrossRef]
- Edalati, K.; Matsuo, M.; Emami, H.; Itano, S.; Alhamidi, A.; Staykov, A.; Smith, D.J.; Orimo, S.; Akiba, E.; Horita, Z. Impact of Severe Plastic Deformation on Microstructure and Hydrogen Storage of Titanium-Iron-Manganese Intermetallics. Scr. Mater. 2016, 124, 108–111. [Google Scholar] [CrossRef]
- Gómez, E.I.L.; Edalati, K.; Antiqueira, F.J.; Coimbrão, D.D.; Zepon, G.; Leiva, D.R.; Ishikawa, T.T.; Cubero-Sesin, J.M.; Botta, W.J. Synthesis of Nanostructured TiFe Hydrogen Storage Material by Mechanical Alloying via High-Pressure Torsion. Adv. Eng. Mater. 2020, 22, 2000011. [Google Scholar] [CrossRef]
- Edalati, K.; Shao, H.; Emami, H.; Iwaoka, H.; Akiba, E.; Horita, Z. Activation of Titanium-Vanadium Alloy for Hydrogen Storage by Introduction of Nanograins and Edge Dislocations Using High-Pressure Torsion. Int. J. Hydrogen Energy 2016, 41, 8917–8924. [Google Scholar] [CrossRef]
- Edalati, K.; Novelli, M.; Itano, S.; Li, H.-W.; Akiba, E.; Horita, Z.; Grosdidier, T. Effect of Gradient-Structure versus Uniform Nanostructure on Hydrogen Storage of Ti-V-Cr Alloys: Investigation Using Ultrasonic SMAT and HPT Processes. J. Alloys Compd. 2018, 737, 337–346. [Google Scholar] [CrossRef]
- Novelli, M.; Edalati, K.; Itano, S.; Li, H.-W.; Akiba, E.; Horita, Z.; Grosdidier, T. Microstructural Details of Hydrogen Diffusion and Storage in Ti–V–Cr Alloys Activated through Surface and Bulk Severe Plastic Deformation. Int. J. Hydrogen Energy 2020, 45, 5326–5336. [Google Scholar] [CrossRef]
- Iwaoka, H.; Horita, Z. Hydrogen Behavior in Ultrafine-Grained Palladium Processed by High-Pressure Torsion. Int. J. Hydrogen Energy 2013, 38, 14879–14886. [Google Scholar] [CrossRef]
- Iwaoka, H.; Arita, M.; Horita, Z. Hydrogen Diffusion in Ultrafine-Grained Palladium: Roles of Dislocations and Grain Boundaries. Acta Mater. 2016, 107, 168–177. [Google Scholar] [CrossRef]
- Krystian, M.; Setman, D.; Mingler, B.; Krexner, G.; Zehetbauer, M.J. Formation of Superabundant Vacancies in Nano-Pd–H Generated by High-Pressure Torsion. Scr. Mater. 2010, 62, 49–52. [Google Scholar] [CrossRef]
- Hongo, T.; Edalati, K.; Iwaoka, H.; Arita, M.; Matsuda, J.; Akiba, E.; Horita, Z. High-Pressure Torsion of Palladium: Hydrogen-Induced Softening and Plasticity in Ultrafine Grains and Hydrogen-Induced Hardening and Embrittlement in Coarse Grains. Mater. Sci. Eng. A 2014, 618, 1–8. [Google Scholar] [CrossRef]
- Zhang, F.; Yonemoto, R.; Arita, M.; Horita, Z. Hydrogen Generation from Pure Water Using Al–Sn Powders Consolidated through High-Pressure Torsion. J. Mater. Res. 2016, 31, 775–782. [Google Scholar] [CrossRef]
- Zhang, F.; Edalati, K.; Arita, M.; Horita, Z. Fast Hydrolysis and Hydrogen Generation on Al-Bi Alloys and Al-Bi-C Composites Synthesized by High-Pressure Torsion. Int. J. Hydrogen Energy 2017, 42, 29121–29130. [Google Scholar] [CrossRef]
- Zhang, F.; Edalati, K.; Arita, M.; Horita, Z. Hydrolytic Hydrogen Production on Al–Sn–Zn Alloys Processed by High-Pressure Torsion. Materials 2018, 11, 1209. [Google Scholar] [CrossRef] [Green Version]
- Razavi-Khosroshahi, H.; Edalati, K.; Hirayama, M.; Emami, H.; Arita, M.; Yamauchi, M.; Hagiwara, H.; Ida, S.; Ishihara, T.; Akiba, E.; et al. Visible-Light-Driven Photocatalytic Hydrogen Generation on Nanosized TiO2-II Stabilized by High-Pressure Torsion. ACS Catal. 2016, 6, 5103–5107. [Google Scholar] [CrossRef]
- Edalati, K.; Wang, Q.; Eguchi, H.; Razavi-Khosroshahi, H.; Emami, H.; Yamauchi, M.; Fuji, M.; Horita, Z. Impact of TiO2-II Phase Stabilized in Anatase Matrix by High-Pressure Torsion on Electrocatalytic Hydrogen Production. Mater. Res. Lett. 2019, 7, 334–339. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Révész, Á.; Gajdics, M. High-Pressure Torsion of Non-Equilibrium Hydrogen Storage Materials: A Review. Energies 2021, 14, 819. https://doi.org/10.3390/en14040819
Révész Á, Gajdics M. High-Pressure Torsion of Non-Equilibrium Hydrogen Storage Materials: A Review. Energies. 2021; 14(4):819. https://doi.org/10.3390/en14040819
Chicago/Turabian StyleRévész, Ádám, and Marcell Gajdics. 2021. "High-Pressure Torsion of Non-Equilibrium Hydrogen Storage Materials: A Review" Energies 14, no. 4: 819. https://doi.org/10.3390/en14040819




