Heat-Up Performance of Catalyst Carriers—A Parameter Study and Thermodynamic Analysis
Abstract
:1. Introduction
- Three-Way Catalytic Converter: TWCs represent an efficient, safe and reliable method for exhaust gas cleaning in SI engines and are operated within a narrow air-to-fuel ratio window, near stoichiometric conditions. The basic functionalities of a TWC can be described as follows: oxidation reactions of HC and CO and reduction reactions of NOx using HC, CO or hydrogen as reductants. Oxidation reactions are primarily catalyzed through platinum group metals, platinum (Pt), and palladium (Pd), while rhodium (Rh) supports the reduction reactions. Furthermore, cerium can act as an oxygen buffer. Light-off temperatures of TWCs are reported to be typically in a range between 548 K, e.g., [2,3] and 573 K, [4]. In stationary engines, the TWC technology is also known as non-selective catalytic reduction (NSCR).
- Diesel Oxidation Catalyst: The main function of DOCs is the oxidation of HC, CO and partly of particulate matter (PM), in lean-burn engines such as diesel engines. In addition, DOCs are employed to control the nitrogen dioxide (NO2) share in the exhaust gas to support the continuous regeneration of particulate filters and SCR-reactions. Commonly used catalytically active components are Pt and Pd or a combination of both on Al2O3. Due to the installation order, close to the engine, light-off issues are only of minor relevance, in the literature reported light-off temperatures for CO oxidation range from 443 K to 493 K [5].
- Nitrogen Oxide Storage Catalyst: This technology is applied in lean-burn gasoline engines as well as in diesel engines. The functionality of NSC can be divided into two phases: the storage of NO2 under lean conditions, e.g., on barium oxide, and a subsequent and periodically repeating reduction during rich conditions, e.g., on Rh. Since it is only possible to store NO2, NO must be oxidized prior storing. The typical activity window of NSCs is between 423 K and 723 K. In a temperature range below 573 K the efficiency of NSCs is essentially determined by the oxidation of NO to NO2 [5].
- Selective Catalytic Reduction: The SCR of NOx with ammonia (NH3) is a widespread solution to meet upcoming emission regulations for lean-burn combustion engines. Due to the toxicity of ammonia, exclusively SCR systems with precursor substances, e.g., urea-water solution, are available or being developed. Ammonia is generated within mixing sections upstream of the converter by two reactions, namely, thermolysis of urea, and hydrolysis of the before formed intermediate isocyanic acid cf. [6]. The determining factors for the efficiency of SCR systems are sufficient ammonia generation, adequate homogenization upstream of the catalytic converter and the converter temperature. Due to the installation position of SCR converters, e.g., downstream a DOC, a particulate filter and a mixing section, the heat economy deserves distinct attention. Catalytically active sites can be copper or iron, which are incorporated in zeolites, or vanadium-based formulations. Operation temperatures for SCR-converters must be commonly between 453 K and 723 K [5,7]. Furthermore, the necessary ammonia generation upstream the converter typically requires a temperature of approximately 473 K cf. [6].
2. Problem Statement and Scope of this Study
3. Literature Review
- large open frontal area (OFA),
- high geometric surface area (GSA),
- low thermal mass/heat capacity,
- thermal stability,
- low coefficient of thermal expansion,
- thermal shock resistance,
- mechanical strength,
- chemical resistance,
- coatability and washcoat compatibility.
3.1. Ceramic Catalyst Substrates
3.2. Metal Catalyst Substrates
4. Methodology
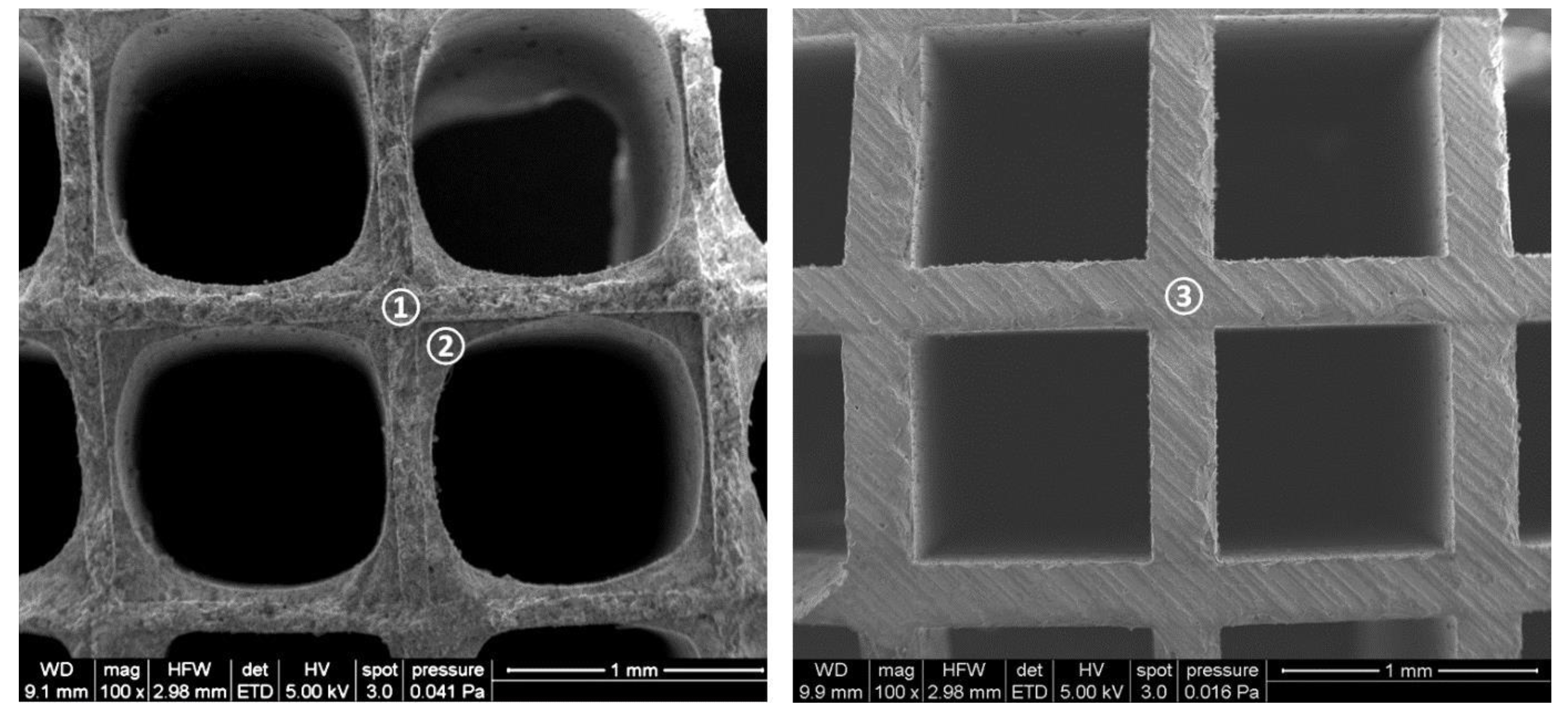
4.1. Investigated Monoliths
4.2. Simulation Model
- The exhaust gas is an ideal gas with properties of dry air, ; nevertheless, the temperature dependency of heat conductivity , heat capacity , dynamic viscosity , and density is included, see Equations (3)–(6).
- All catalytic converters are placed in adiabatic housing, which implies that the heat losses are neglected and no temperature gradients occur within solid cells.
- Reaction enthalpies are not considered to maintain independence from engine-out exhaust gas compositions.
- Heat conductivity, heat capacity and density of solid cells are constant and present typical values for cordierite: , and .
- Ambient temperature and initial temperature of solid cells at are 293 K and pressure is constant at 101,325 Pa.
5. Discussion of Results
5.1. Geometric Parameter Study
5.2. Thermodynamic Analysis
- laminar flows with
- Prandtl numbers () between 0.1 and ∞, and
5.3. Steady State Heat-Up
5.4. Optimization of Dimensions for Improved Heat-Up Performance
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Latin Letters | ||
| area | m2 | |
| free edge length | m | |
| mean isobaric heat capacity | J·kg−1·K−1 | |
| isobaric heat capacity | J·kg−1·K−1 | |
| diameter | m | |
| length | m | |
| mass | kg | |
| gravimetric flow rate | kg·s−1 | |
| Nusselt number | - | |
| pressure | Pa | |
| Peclet number | - | |
| Prandtl number | - | |
| heat | J | |
| heat flux | W | |
| heat flux density | W·m−2·K−1 | |
| specific gas constant | J·kg−1·K−1 | |
| Reynolds number | - | |
| temperature | K | |
| time | s | |
| velocity | m·s−1 | |
| uniformity index | - | |
| volume | m3 | |
| volumetric flow rate | m3·s−1 | |
| conversion | % | |
| Greek Letters | ||
| heat transfer coefficient | W·m−2·K−1 | |
| difference | - | |
| heat conductivity | W·m−1·K−1 | |
| dynamic viscosity | kg·m−1·s−1 | |
| density | kg·m−3 | |
| Subscripts | ||
| average | ||
| catalytic converter | ||
| cell | ||
| conductive | ||
| convective | ||
| exhaust gas | ||
| hydraulic | ||
| inflowing | ||
| internal | ||
| logarithmic | ||
| heat loss | ||
| mean | ||
| monolith | ||
| open | ||
| outflowing | ||
| solid | ||
| Definitions/Abbreviations | ||
| 1D | one-dimensional | |
| Al2O3 | aluminum oxide | |
| CI | compression ignited | |
| CO | carbon monoxide | |
| CO2 | carbon dioxide | |
| cpsi | cells per square inch | |
| Cu | copper | |
| DOC | diesel oxidation catalyst | |
| EGR | exhaust gas recirculation | |
| Fe | iron | |
| GHSV | gas hourly space velocity | |
| GSA | geometric surface area | |
| HC | hydrocarbons | |
| ICE | internal combustion engines | |
| NH3 | ammonia | |
| NO | nitrogen monoxide | |
| NO2 | nitrogen dioxide | |
| NOx | nitrogen oxides | |
| NSC | nitrogen storage catalyst | |
| NSCR | non-selective catalytic reduction | |
| OFA | open frontal area | |
| Pd | palladium | |
| PM | particulate matter | |
| Pt | platinum | |
| Rh | rhodium | |
| SCR | selective catalytic reduction | |
| SI | spark-ignited | |
| TWC | three-way catalytic converter |
Appendix A







References
- Moeltner, L.; Hohensinner, M.; Schallhart, V. Experimental and Numerical Analysis of Low Temperature NOx—Conversion in Urban Busses. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2016. [Google Scholar] [CrossRef]
- Bresch-Pietri, D.; Leroy, T.; Petit, N. Control-oriented Time-varying Input-delayed Temperature Model for SI Engine Exhaust Catalyst. In Proceedings of the 2013 American Control Conference, Washington, DC, USA, 17–19 June 2013; pp. 2189–2195. [Google Scholar]
- Sabatini, S.; Kil, I.; Dekar, J.; Hamilton, T.; Wuttke, J.; Smith, M.A.; Hoffman, M.A.; Onori, S. A New Semi-Empirical Temperature Model for the Three Way Catalytic Converter. IFAC-PapersOnLine 2015, 48, 434–440. [Google Scholar] [CrossRef]
- Chang, H.-L.; Chen, H.-Y.; Koo, K.; Rieck, J.S.; Blakeman, P. Gasoline Cold Start Concept (gCSC™) Technology for Low Temperature Emission Control. SAE Int. J. Fuels Lubr. 2014, 7, 480–488. [Google Scholar] [CrossRef]
- Basshuysen, R. “Handbuch Verbrennungsmotor” ATZ/MTZ Fachbuch, 8th ed.; Springer: Berlin, Germany, 2017; ISBN 978-3-658-10901-1. [Google Scholar]
- Schallhart, V.; Moeltner, L. Effect of Spray-Exhaust Gas Interactions on Ammonia Generation in SCR Mixing Sections. SAE Int. J. Fuels Lubr. 2018, 11, 181–200. [Google Scholar] [CrossRef]
- Moeltner, L.; Hohensinner, M.; Schallhart, V. Aging Effects of Catalytic Converters in Diesel Exhaust Gas Systems and Their Influence on Real Driving NOx Emissions for Urban Buses. SAE Int. J. Commer. Veh. 2018, 11, 171–190. [Google Scholar] [CrossRef]
- Marsh, P.; Acke, F.; Konieczny, R.; Brück, R.; Hirth, P. Application Guideline to Define Catalyst Layout for Maximum Catalytic Efficiency. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2001. [Google Scholar] [CrossRef]
- Gulati, S.T. Design Considerations for Advanced Ceramic Catalyst Supports. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2000. [Google Scholar] [CrossRef]
- Gottschalk, W.; Kirstein, G.; Magnor, O.; Schultalbers, M.; Wetten, R. Investigations on a Catalyst Heating Strategy by Variable Valve Train for SI Engines. SAE Int. J. Engines 2012, 5, 1177–1200. [Google Scholar] [CrossRef]
- Szolak, R.; Danckert, B.; Susdorf, A.; Beutel, P.; Pautsch, K.; Ewert, C.; Rümmele, F.; Kakadiya, A.; Schaadt, A. CatVap®—A New Heating Measure for Exhaust Aftertreatment System; Springer Viewag: Wiesbaden, Germany, 2020; pp. 37–52. [Google Scholar]
- Holy, G.; Bruck, R.; Hirth, P. Improved Catalyst Systems for SULEV Legislation: First Practical Experience. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2000. [Google Scholar]
- Gelner, A.D.; Pastoetter, C.; Beck, H.A.; Härtl, M.; Wachtmeister, G. Fuel Dosing on a Diesel Oxidation Catalyst for After-Treatment System Heating on a Heavy-Duty Engine Powered by Polyoxymethylene Dimethyl Ethers. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2020. [Google Scholar]
- Della Torre, A.; Montenegro, G.; Onorati, A.; Cerri, T. CFD Investigation of the Impact of Electrical Heating on the Light-off of a Diesel Oxidation Catalyst. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2018. [Google Scholar] [CrossRef]
- Breuer, J.; Hirth, P.; Bruck, R.; Kruse, C. Electrically Heated Catalyst for Future USA and European Legislation. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 1996. [Google Scholar] [CrossRef]
- Wiehl, J.; Vogt, C.D. Keramische Ultradünnwandsubstrate für moderne Katalysatoren. MTZ Mot. Z. 2003, 64, 112–119. [Google Scholar] [CrossRef]
- Madhusoodana, C.D.; Das, R.N.; Umarji, A.M.; Panda, P.K.; Kannan, T.S. Thermal Expansion and Thermal Shock Resistance of Cordierite Honeycombs Used in Catalytic Converters. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 1999. [Google Scholar] [CrossRef]
- McLaren, M.G.; Ott, W.R. Ceramic Monolithic Substrates-State of the Art. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 1974. [Google Scholar] [CrossRef]
- Teague, M. Ceramic Substrate Technology for Automotive Catalysts. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 1976. [Google Scholar] [CrossRef]
- Gulati, S.T. Ceramic Converter Technology for Automotive Emissions Control. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 1991. [Google Scholar] [CrossRef]
- Lundsager, C.B.; Turner, G.J.; Pentecost, J.L. Thermoplastics Process for Catalyst Substrates. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 1976. [Google Scholar] [CrossRef]
- Day, J.P. The Design of a New Ceramic Catalyst Support. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 1990. [Google Scholar] [CrossRef]
- Machida, M.; Yamada, T.; Makino, M. Study of Ceramic Catalyst Optimization for Emission Purification Efficiency. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 1994. [Google Scholar] [CrossRef]
- Bach, C.; Eggenschwiler, P.D. Ceramic Foam Catalyst Substrates for Diesel Oxidation Catalysts: Pollutant Conversion and Operational Issues. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2011. [Google Scholar] [CrossRef] [Green Version]
- Dimopoulos, P.; Bach, C.; Vogt, U.; Herrmann, K. Ceramic Foams as Catalyst Substrates: Pre-catalyst Application Homogenising the Exhaust Flow upstream of Aftertreatment Devices. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2007. [Google Scholar]
- Jatkar, A.D. A New Catalyst Support Structure for Automotive Catalytic Converters. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 1997. [Google Scholar] [CrossRef]
- Stankiewicz, E.P.; Sherman, A.J.; Zinn, A.A.; Scott, D.J. Properties and Performance of UltraCatTM Open-Cell Silicon Carbide Foam Catalyst Substrates. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 1998. [Google Scholar] [CrossRef]
- Lakshmikantha, M.; Keck, M. Optimization of Exhaust Systems. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2002. [Google Scholar]
- Pannone, G.M.; Mueller, J.D. A Comparison of Conversion Efficiency and Flow Restriction Performance of Ceramic and Metallic Catalyst Substrates. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2001. [Google Scholar] [CrossRef]
- Tamura, N.; Matsumoto, S.; Kawabata, M.; Kojima, M.; Machida, M. The Development of an Automotive Catalyst using a Thin Wall (4 mil/400cpsi) Substrate. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 1996. [Google Scholar]
- Aoki, Y.; Miyairi, Y.; Ichikawa, Y.; Abe, F. Product Design and Development of Ultra Thin Wall Ceramic Catalytic Substrate. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2002. [Google Scholar] [CrossRef]
- Schmidt, J.; Franz, J.; Merdes, N.; Brady, M.J.; Mueller, W.; Lindner, D.; Bog, T.; Clark, D.; Buckel, T.; Stoepler, W.; et al. Utilization of Advanced Three-Way Catalyst Formulations on Ceramic Ultra Thin Wall Substrates for Future Legislation. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2002. [Google Scholar] [CrossRef]
- Knon, H.; Brenscheidt, T.; Flörchinger, P. Keramische Ultradünnwandträger für zukünftige Emissionsanforderungen. MTZ Mot. Z. 2001, 62, 662–666. [Google Scholar] [CrossRef]
- Twigg, M. Advanced Exhaust Emissions Control—A Selective Review of the Detroit 2000 SAE World Congress. Platin. Metals Rev. 2000, 44, 67. [Google Scholar]
- Tanaka, M.; Nishimura, M.; Murata, M.; Itou, K. The Innovative Reinforcement Technology of Thin Wall Ceramic Substrate Having High Coatability. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2000. [Google Scholar] [CrossRef]
- Umehara, K.; Makino, M.; Brayer, M.; Becker, E.R.; Watson, R. Prediction of Catalytic Performance for Ultra Thin Wall and High Cell Density Substrates. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2000. [Google Scholar]
- Lafyatis, D.S.; Will, N.S.; Martin, A.P.; Rieck, J.S.; Cox, J.P.; Evans, J.M. Use of High Cell Density Substrates and High Technology Catalysts to Significantly Reduce Vehicle Emissions. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2000. [Google Scholar]
- Hirose, S.; Yamamoto, H.; Suenobu, H.; Sakamoto, H.; Katsube, F.; Busch, P.; Martín, A.; Kai, R.; Vogt, C.D. Development of High Porosity Cordierite Honeycomb Substrate for SCR Application to Realize High NOx Conversion Efficiency and System Compactness. SAE Int. J. Mater. Manuf. 2014, 7, 682–687. [Google Scholar] [CrossRef]
- Andreassi, L.; Cordiner, S.; Mulone, V. Cell Shape Influence on Mass Transfer and Backpressure Losses in an Automotive Catalytic Converter. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2004. [Google Scholar] [CrossRef]
- Day, J.P. Substrate Effects on Light-Off—Part II Cell Shape Contributions. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 1997. [Google Scholar] [CrossRef]
- Shamim, T.; Shen, H. Effect of Geometric Parameters on the Performance of Automotive Catalytic Converters. Int. J. Sci. Technol. 2003, 14, 15–22. [Google Scholar]
- Anderson, M.J. Ultra Thin Wall Mat Design and Optimization with Hybrid Mats. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2004. [Google Scholar] [CrossRef]
- Maus, W.; Brück, R. The Conical Catalytic Converter and Its Potential for Future Close-Coupled Converter Concepts. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 1998. [Google Scholar] [CrossRef]
- Bonnefoy, F.; De Meyer, J.; Steenackers, P. High Flexibility in Converter Design by Using Modular Block Metallic Substrates. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2000. [Google Scholar] [CrossRef]
- Kikuchi, S.; Hatcho, S.; Okayama, T.; Inose, S.; Ikeshima, K. High Cell Density and Thin Wall Substrate for Higher Conversion Ratio Catalyst. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 1999. [Google Scholar] [CrossRef]
- Moore, W.J.; Hummel, D.O.; Trafara, G.; Holland-Moritz, K. Physikalische Chemie; Walter de Gruyter GmbH: Berlin, Germany, 1986. [Google Scholar]
- Kleiber, M.; Joh, R. “VDI-Wärmeatlas”, Verein Deutscher Ingenieure VDI-Gesellschaft Verfahrenstechnik und Chemieingenieurwesen; Springer: Berlin, Germany, 2006; ISBN 13-978-3-540-25504-8. [Google Scholar]















| cpsi cells·m−2 | 300 4.65·105 | 400 6.2·105 | 600 9.3·105 | 750 1.16·106 | 900 1.4·106 | 1200 1.86·106 |
|---|---|---|---|---|---|---|
| 2 mil 50.8 μm | - | - | ● | ● | ● | ● |
| 3 mil 76.2 μm | - | ● | ● | - | - | - |
| 4 mil 101.6 μm | - | ● | ● | - | - | - |
| 6 mil 152.4 μm | ● | ● | - | - | - | - |
| 8 mil 203.2 μm | ● | - | - | - | - | - |
| Geometries | m/kg | α/W·m−2·K−1 | Aint/m2 | tTavg 473 K/s | UI/- |
|---|---|---|---|---|---|
| 300 cpsi, 8 mil 4.65·105 cells·m−2, 203.2 μm | 1.324 | 118.61 | 5.81 | 38.2 | 0.942 |
| 300 cpsi, 6 mil 4.65·105 cells·m−2, 152.4 μm | 1.024 | 114.02 | 6.05 | 29.1 | 0.940 |
| 400 cpsi, 6 mil 6.20·105 cells·m−2, 152.4 μm | 1.172 | 133.94 | 6.86 | 33.1 | 0.940 |
| 400 cpsi, 4 mil 6.20·105 cells·m−2, 101.6 μm | 0.798 | 128.12 | 7.17 | 22.5 | 0.940 |
| 400 cpsi, 3 mil 6.20·105 cells·m−2, 76.2 μm | 0.605 | 125.39 | 7.32 | 17.1 | 0.940 |
| 600 cpsi, 4 mil 9.30·105 cells·m−2, 101.6 μm | 0.968 | 159.93 | 8.61 | 27.2 | 0.938 |
| 600 cpsi, 3 mil 9.30·105 cells·m−2, 76.2 μm | 0.735 | 155.70 | 8.84 | 20.6 | 0.937 |
| 600 cpsi, 2 mil 9.30·105 cells·m−2, 50.8 μm | 0.497 | 151.69 | 9.08 | 13.9 | 0.937 |
| 750 cpsi, 2 mil 1.16·106 cells·m−2, 50.8 μm | 0.554 | 170.60 | 10.09 | 15.5 | 0.936 |
| 900 cpsi, 2 mil 1.40·106 cells·m−2, 50.8 μm | 0.605 | 187.90 | 10.99 | 16.9 | 0.935 |
| 1200 cpsi, 2 mil 1.86·106 cells·m−2, 50.8 μm | 0.695 | 219.12 | 12.56 | 19.3 | 0.934 |
| Geometries | lmono/inch, lmono/m | m/KG | Aint/m2 (const.) | tTavg 473 k/ S | UI/- |
|---|---|---|---|---|---|
| 400 cpsi, 4 mil 6.20·105 cells·m−2, 101.6 μm | 6.00 0.152 | 0.798 | 7.17 | 22.5 | 0.940 |
| 600 cpsi, 4 mil 9.30·105 cells·m−2, 101.6 μm | 5.00 0.127 | 0.806 | 7.17 | 22.7 | 0.939 |
| 600 cpsi, 3 mil 9.30·105 cells·m−2, 76.2 μm | 4.86 0.124 | 0.596 | 7.17 | 16.8 | 0.939 |
| 600 cpsi, 2 mil 9.30·105 cells·m−2, 50.8 μm | 4.74 0.120 | 0.392 | 7.17 | 11.0 | 0.939 |
| 750 cpsi, 2 mil 1.16·106 cells·m−2, 50.8 μm | 4.27 0.108 | 0.393 | 7.17 | 11.1 | 0.938 |
| 900 cpsi, 2 mil 1.40·106 cells·m−2, 50.8 μm | 3.92 0.099 | 0.395 | 7.17 | 11.1 | 0.937 |
| 1200 cpsi, 2 mil 1.86·106 cells·m−2, 50.8 μm | 3.42 0.087 | 0.397 | 7.17 | 11.1 | 0.937 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steiner, T.; Neurauter, D.; Moewius, P.; Pfeifer, C.; Schallhart, V.; Moeltner, L. Heat-Up Performance of Catalyst Carriers—A Parameter Study and Thermodynamic Analysis. Energies 2021, 14, 964. https://doi.org/10.3390/en14040964
Steiner T, Neurauter D, Moewius P, Pfeifer C, Schallhart V, Moeltner L. Heat-Up Performance of Catalyst Carriers—A Parameter Study and Thermodynamic Analysis. Energies. 2021; 14(4):964. https://doi.org/10.3390/en14040964
Chicago/Turabian StyleSteiner, Thomas, Daniel Neurauter, Peer Moewius, Christoph Pfeifer, Verena Schallhart, and Lukas Moeltner. 2021. "Heat-Up Performance of Catalyst Carriers—A Parameter Study and Thermodynamic Analysis" Energies 14, no. 4: 964. https://doi.org/10.3390/en14040964
APA StyleSteiner, T., Neurauter, D., Moewius, P., Pfeifer, C., Schallhart, V., & Moeltner, L. (2021). Heat-Up Performance of Catalyst Carriers—A Parameter Study and Thermodynamic Analysis. Energies, 14(4), 964. https://doi.org/10.3390/en14040964








