Optimized Modeling and Design of a PCM-Enhanced H2 Storage
Abstract
:1. Introduction
2. Problem Statement
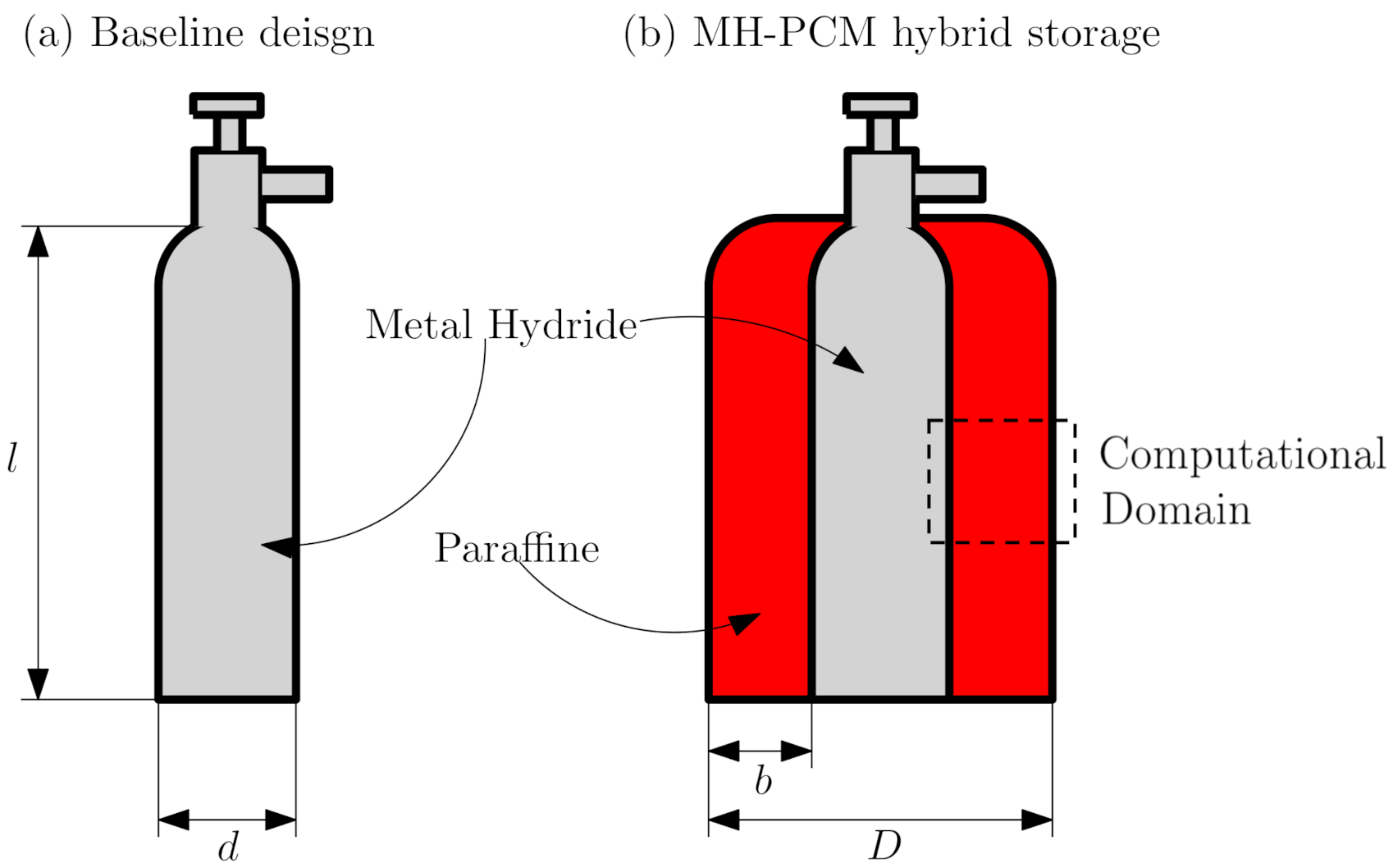
3. Hybrid Metal Hydride PCM-TES Design
| (a) Baseline Design | |||
|---|---|---|---|
| k | W/(mK) | [34] | |
| m/s | [34] | ||
| 1/K | [34] | ||
| kg/m | [34] | ||
| Ra | —– | Equation (2) | |
| Nu | —– | Equation (2) | |
| Pr | —– | [34] | |
| 293 | K | ||
| W/(mK) | |||
| 22 | W | ||
| (b) MH–PCM integrated system | |||
| k | W/(mK) | [35] | |
| m/s | [35] | ||
| 1/K | [35] | ||
| 800 | kg/m | [35] | |
| Ra | —– | Equation (2) | |
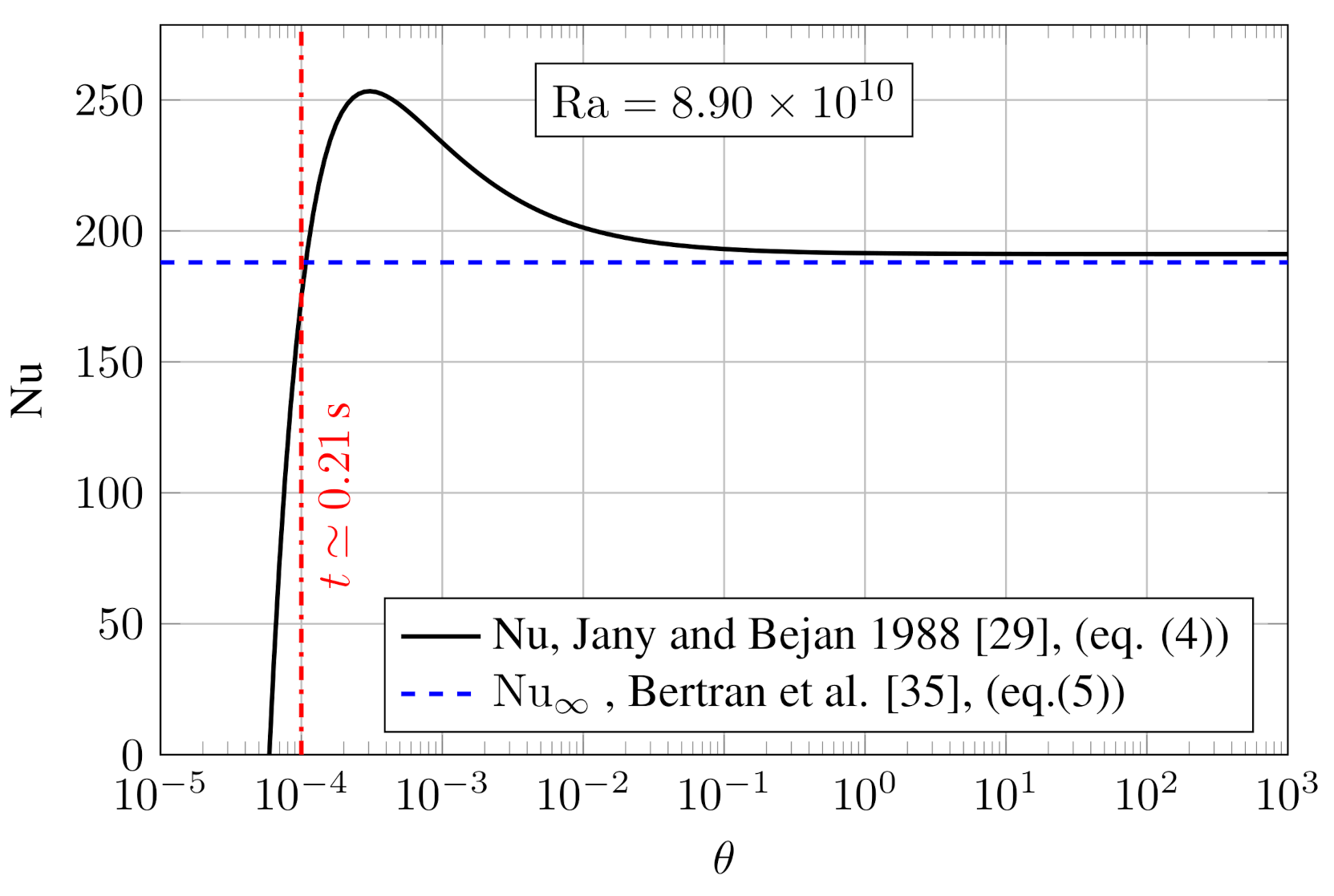
| Nu | 188 | —– | Equation (5) |
| Pr | 60 | —– | [35] |
| 302 | K | [35] | |
| 53 | W/(mK) | ||
| 208 | W | ||
4. Multidimensional Modeling of PCM Melting & Solidification
- is a relaxation parameter, chosen to be in the order of 0.5, to ensure that the relaxation of is faster than the other dynamics;
- is the non-dimensional Stefan number, given by , with the specific heat and the latent heat.
- is the characteristic temperature difference: we have fixed ;
5. Numerical Results & Discussion
5.1. 1D Stefan Problem
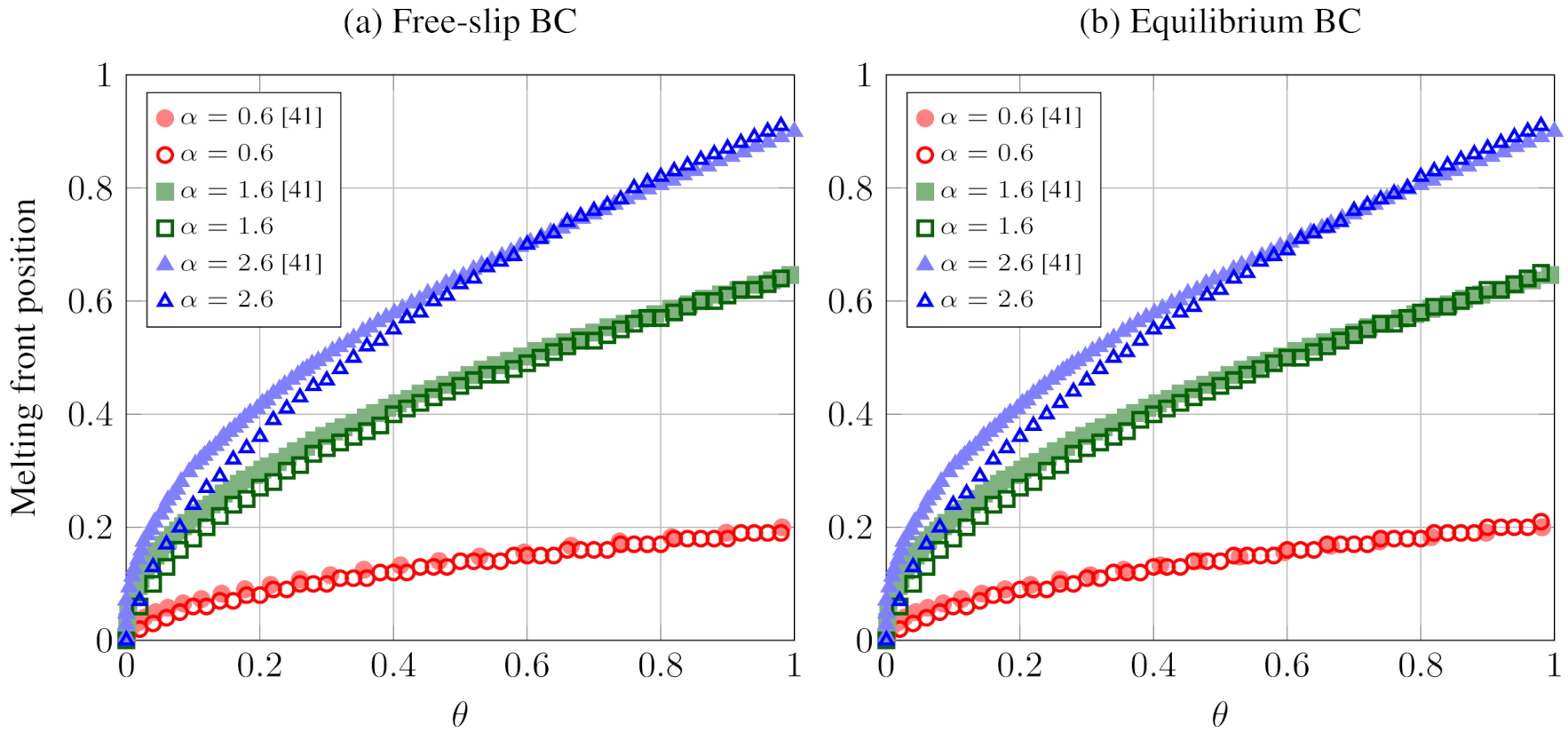
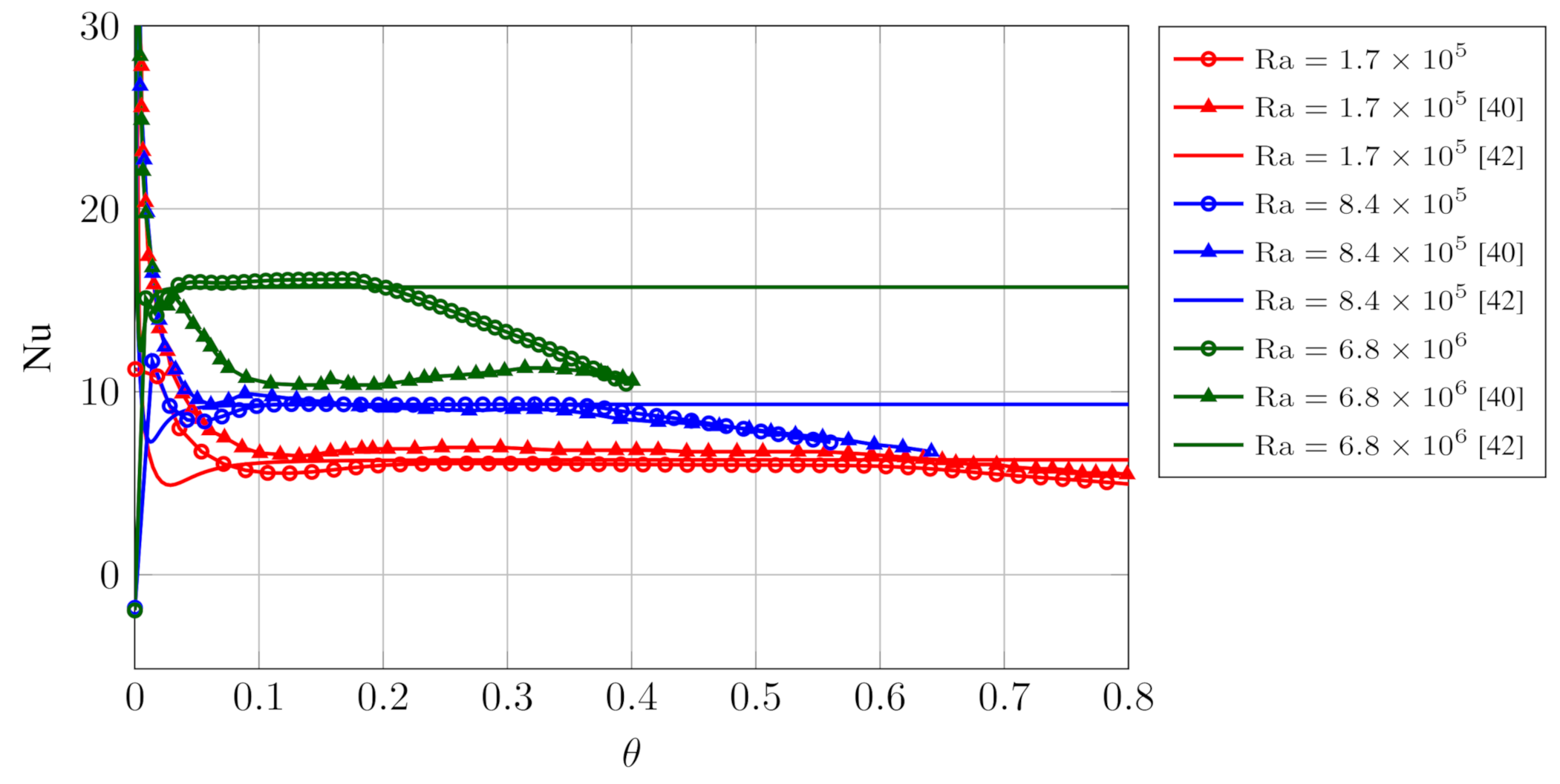
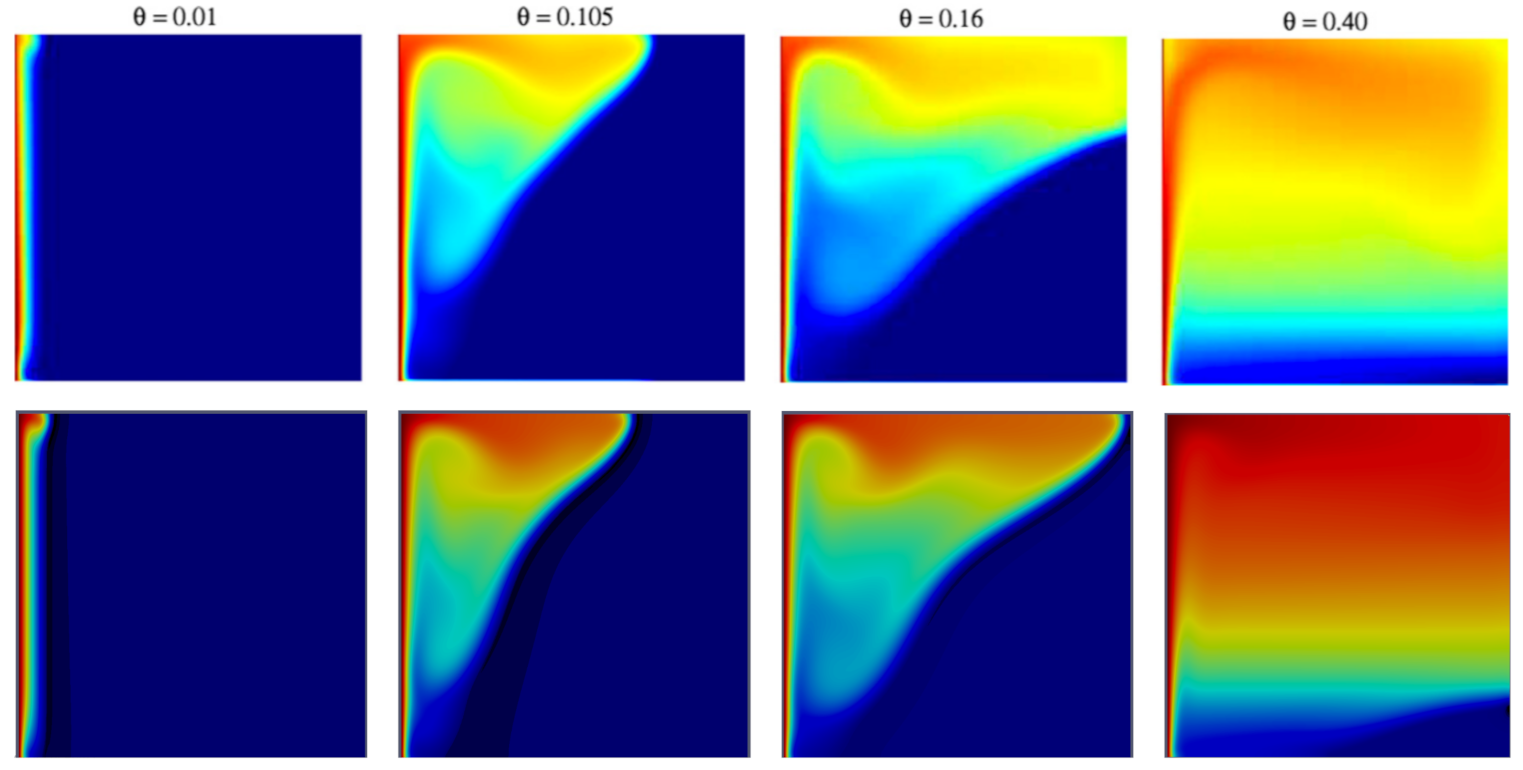
5.2. 2D Melting: The Effects of Gravity and Buoyancy
- : the end of the conduction-dominated phase, with the formation of a rounded sack on top of the computational domain, due to the inception of convective motions;
- : advance of the interface towards the cold wall, with the formation of a rounded profile in the upper part of the computational domain, due to the melting front pointing downwards;
- : the melting front reaches the cold wall: this corresponds to the end of the plateau region of in Figure 4;
- : the progressive shrinkage of the solid phase.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Physical Parameters | ||
| Specific heat of the liquid phase (melting process) | J/(kgK) | |
| Empirical constants for evaluation | ||
| Lattice discretized directions | ||
| , , | Force terms in the LB Equations (6) and (7) | |
| D | Diameter | m |
| Enthalpy variation for MH absorption/desorption | J/mol | |
| Entropy variation for MH absorption/desorption | J/(molK) | |
| Lattice populations for fluid flow | ||
| f+, f− | Frequency factors for melting/solidification | |
| Gravitational acceleration | m/s | |
| g | magnitude of the gravitational acceleration | m/s |
| Lattice populations for temperature evolution | ||
| H | Height of the computational domain | m |
| , | Switch functions for (local) melting/solidification | |
| k | Heat conduction coefficient | W/(mK) |
| MH heat conduction coefficient | W/(mK) | |
| Reference length | m | |
| Latent heat of fusion | J/kg | |
| LHV | Hydrogen Lower Heating Value | J/kg or J/mol |
| Molecular weight | g/mol | |
| Mass of the H stored in the MH | kg | |
| Metal hydride mass | kg | |
| PCM mass | kg | |
| p | Pressure | bar |
| Reference pressure | bar | |
| Q | Thermal energy | J |
| Thermal power exchanged at the canister surface | W | |
| Reaction term for liquid/solid phase change | ||
| Universal gas constant | J/(molK) | |
| T | Temperature | K |
| t | Time | s |
| Critical temperature (melting/solidification) | K | |
| Environment reference temperature | K | |
| Temperature of the wall | K | |
| Temperature of the liquid phase | K | |
| Melting/solidification activation energy | K | |
| Control parameter for phase transition interface width | K | |
| Temperature of the solid phase | K | |
| PCM volume | m | |
| Gravimetric density of MH storage | % | |
| Storage power | W | |
| Greek Letters | ||
| Thermal diffusivity | m/s | |
| Coefficient of thermal volumetric expansion | 1/K | |
| Thermal convection coefficient | W/m | |
| Kinematic viscosity | m/s | |
| phase field variable for solid/liquid evolution | ||
| Dimensionless time | ||
| Time when the top wall is fully bathed by liquid | ||
| Fluid relaxation time | ||
| Thermal relaxation time | ||
| Frequency factor for the drag force | ||
| Acronyms | ||
| 1D | One Dimensional | |
| 2D | Two Dimensional | |
| Bi | Biot number | |
| CHP | Combined Heat and Power | |
| Fo | Fourier number | |
| LBM | Lattice Boltzmann Method | |
| MH | Metal Hydride | |
| Nu | Nusselt number | |
| PCM | Phase Change Material | |
| Pr | Prandtl number | |
| Ra | Rayleigh number | |
| St | Stefan number | |
| TES | Thermal energy Storage |
References
- Boyle, G. Renewable Energy: Power for a Sustainable Future; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Facci, A.L.; Krastev, V.K.; Falcucci, G.; Ubertini, S. Smart integration of photovoltaic production, heat pump and thermal energy storage in residential applications. Sol. Energy 2019, 192, 133–143. [Google Scholar] [CrossRef]
- Ubertini, S.; Facci, A.L.; Andreassi, L. Hybrid hydrogen and mechanical distributed energy storage. Energies 2017, 10, 2035. [Google Scholar]
- Chiappini, D.; Facci, A.L.; Tribioli, L.; Ubertini, S. SOFC management in distributed energy systems. J. Fuel Cell Sci. Technol. 2011, 8, 031015. [Google Scholar]
- Facci, A.L.; Sánchez, D.; Jannelli, E.; Ubertini, S. Trigenerative micro compressed air energy storage: Concept and thermodynamic assessment. Appl. Energy 2015, 158, 243–254. [Google Scholar]
- Facci, A.L.; Cigolotti, V.; Jannelli, E.; Ubertini, S. Technical and economic assessment of a SOFC-based energy system for combined cooling, heating and power. Appl. Energy 2017, 192, 563–574. [Google Scholar]
- Facci, A.L.; Loreti, G.; Ubertini, S.; Barbir, F.; Chalkidis, T.; Eßling, R.P.; Peters, T.; Skoufa, E.; Bove, R. Numerical assessment of an automotive derivative CHP fuel cell system. Energy Procedia 2017, 105, 1564–1569. [Google Scholar]
- Facci, A.L.; Ubertini, S. Analysis of a fuel cell combined heat and power plant under realistic smart management scenarios. Appl. Energy 2018, 216, 60–72. [Google Scholar]
- Loreti, G.; Facci, A.L.; Peters, T.; Ubertini, S. Numerical modeling of an automotive derivative polymer electrolyte membrane fuel cell cogeneration system with selective membranes. Int. J. Hydrog. Energy 2019, 44, 4508–4523. [Google Scholar]
- Loreti, G.; Facci, A.L.; Baffo, I.; Ubertini, S. Combined heat, cooling, and power systems based on half effect absorption chillers and polymer electrolyte membrane fuel cells. Appl. Energy 2019, 235, 747–760. [Google Scholar]
- Abe, J.O.; Popoola, A.; Ajenifuja, E.; Popoola, O. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrog. Energy 2019, 44, 15072–15086. [Google Scholar]
- Minutillo, M.; Forcina, A.; Jannelli, N.; Lavadera, A.L. Assessment of a sustainable energy chain designed for promoting the hydrogen mobility by means of fuel-cell powered bicycles. Energy 2018, 153, 200–210. [Google Scholar]
- Andreassi, L.; Falcucci, G.; Facci, A.L.; Ubertini, S. Environmental and Health Impact of Electric and Hydrogen Light Vehicles: The Case of an Italian Small City; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2019. [Google Scholar] [CrossRef]
- Hirscher, M.; Yartys, V.A.; Baricco, M.; von Colbe, J.B.; Blanchard, D.; Bowman, R.C., Jr.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P.; et al. Materials for hydrogen-based energy storage–past, recent progress and future outlook. J. Alloy Compd. 2020, 827, 153548. [Google Scholar]
- Lototskyy, M.; Sekhar, B.S.; Muthukumar, P.; Linkov, V.; Pollet, B. Niche applications of metal hydrides and related thermal management issues. J. Alloy Compd. 2015, 645, S117–S122. [Google Scholar]
- Afzal, M.; Mane, R.; Sharma, P. Heat transfer techniques in metal hydride hydrogen storage: A review. Int. J. Hydrog. Energy 2017, 42, 30661–30682. [Google Scholar]
- Shafiee, S.; McCay, M.H. Different reactor and heat exchanger configurations for metal hydride hydrogen storage systems—A review. Int. J. Hydrog. Energy 2016, 41, 9462–9470. [Google Scholar]
- Succi, S. The Lattice Boltzmann Equation for Fluid Dynamics and Beyond; Clarendon: Oxford, UK, 2001. [Google Scholar]
- Montessori, A.; La Rocca, M.; Falcucci, G.; Succi, S. Regularized lattice BGK versus highly accurate spectral methods for cavity flow simulations. Int. J. Mod. Phys. C 2014, 25, 1441003. [Google Scholar]
- Falcucci, G.; Amati, G.; Krastev, V.K.; Montessori, A.; Yablonsky, G.S.; Succi, S. Heterogeneous catalysis in pulsed-flow reactors with nanoporous gold hollow spheres. Chem. Eng. Sci. 2017, 166, 274–282. [Google Scholar]
- Krastev, V.; Falcucci, G. Simulating engineering flows through complex porous media via the lattice Boltzmann method. Energies 2018, 11, 715. [Google Scholar]
- Montessori, A.; Falcucci, G. Lattice Boltzmann Modeling of Complex Flows for Engineering Applications; Morgan & Claypool Publishers: Bristol, UK, 2018; pp. 2053–2571. [Google Scholar] [CrossRef]
- Succi, S.; Amati, G.; Bernaschi, M.; Falcucci, G.; Lauricella, M.; Montessori, A. Towards exascale lattice Boltzmann computing. Comput. Fluids 2019, 181, 107–115. [Google Scholar]
- Venturi, S.; Di Francesco, S.; Geier, M.; Manciola, P. A new collision operator for lattice Boltzmann shallow water model: A convergence and stability study. Adv. Water Resour. 2020, 135, 103474. [Google Scholar]
- Durbin, D.; Malardier-Jugroot, C. Review of hydrogen storage techniques for on board vehicle applications. Int. J. Hydrog. Energy 2013, 38, 14595–14617. [Google Scholar] [CrossRef]
- Rizzi, P.; Pinatel, E.; Luetto, C.; Florian, P.; Graizzaro, A.; Gagliano, S.; Baricco, M. Integration of a PEM fuel cell with a metal hydride tank for stationary applications. J. Alloy Compd. 2015, 645, S338–S342. [Google Scholar] [CrossRef]
- Planet, H. MyH2-2000 Technical Specifications. Available online: https://www.h2planet.eu/en/detail/MyH22000 (accessed on 26 November 2020).
- Zhao, W.; Yang, Y.; Bao, Z.; Yan, D.; Zhu, Z. Methods for measuring the effective thermal conductivity of metal hydride beds: A review. Int. J. Hydrog. Energy 2020, 45, 6680–6700. [Google Scholar] [CrossRef]
- Escobar, A.R.G. TiMn2 Based Metal Hydrides for Hydrogen Compression Applications: Numerical and Experimental Approach. Ph.D. Thesis, Univesidad Autonoma de Madrid, Madrid, Spain, 2018. [Google Scholar]
- Laurencelle, F.; Goyette, J. Simulation of heat transfer in a metal hydride reactor with aluminium foam. Int. J. Hydrog. Energy 2007, 32, 2957–2964. [Google Scholar] [CrossRef]
- Sánchez, A.R.; Klein, H.P.; Groll, M. Expanded graphite as heat transfer matrix in metal hydride beds. Int. J. Hydrog. Energy 2003, 28, 515–527. [Google Scholar]
- Ron, M.; Bershadsky, E.; Josephy, Y. Thermal conductivity of PMH compacts, measurements and evaluation. Int. J. Hydrog. Energy 1992, 17, 623–630. [Google Scholar]
- Davids, M.; Lototskyy, M.; Malinowski, M.; van Schalkwyk, D.; Parsons, A.; Pasupathi, S.; Swanepoel, D.; van Niekerk, T. Metal hydride hydrogen storage tank for light fuel cell vehicle. Int. J. Hydrog. Energy 2019, 44, 29263–29272. [Google Scholar]
- Kreith, F.; Manglik, R.M.; Bohn, M.S. Principles of Heat Transfer; Cengage Learning: Boston, MA, USA, 2012. [Google Scholar]
- Madruga, S.; Curbelo, J. Dynamic of plumes and scaling during the melting of a Phase Change Material heated from below. Int. J. Heat Mass Transf. 2018, 126, 206–220. [Google Scholar]
- Jany, P.; Bejan, A. Scaling theory of melting with natural convection in an enclosure. Int. J. Heat Mass Transf. 1988, 31, 1221–1235. [Google Scholar]
- Krüger, T.; Kusumaatmaja, H.; Kuzmin, A.; Shardt, O.; Silva, G.; Viggen, E.M. The Lattice Boltzmann Method; Springer: Berlin/Heidelberg, Germany, 2017; Chapter 8. [Google Scholar]
- Succi, S. The Lattice Boltzmann Equation: For Complex States of Flowing Matter; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Miller, W.; Succi, S.; Mansutti, D. Lattice Boltzmann model for anisotropic liquid-solid phase transition. Phys. Rev. Lett. 2001, 86, 3578. [Google Scholar]
- Huber, C.; Parmigiani, A.; Chopard, B.; Manga, M.; Bachmann, O. Lattice Boltzmann model for melting with natural convection. Int. J. Heat Fluid Flow 2008, 29, 1469–1480. [Google Scholar]
- Di Ilio, G.; Ubertini, S.; Succi, S.; Falcucci, G. Nanofluid Heat Transfer in Wavy-Wall Channels with Different Geometries: A Finite-Volume Lattice Boltzmann Study. J. Sci. Comput. 2020, 83, 56. [Google Scholar]
- Bertrand, O.; Binet, B.; Combeau, H.; Couturier, S.; Delannoy, Y.; Gobin, D.; Lacroix, M.; Le Quéré, P.; Médale, M.; Mencinger, J.; et al. Melting driven by natural convection A comparison exercise: First results. Int. J. Therm. Sci. 1999, 38, 5–26. [Google Scholar]
| Volume of H stored | 2 | Sm |
|---|---|---|
| Mass of H stored | kg | |
| Energy stored | ≃5.5 | kWh |
| Canister weight | 14 | kg |
| Canister volume | m | |
| Canister diameter | m | |
| Canister length | m | |
| H storage pressure | 10–12 | bar |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Facci, A.L.; Lauricella, M.; Succi, S.; Villani, V.; Falcucci, G. Optimized Modeling and Design of a PCM-Enhanced H2 Storage. Energies 2021, 14, 1554. https://doi.org/10.3390/en14061554
Facci AL, Lauricella M, Succi S, Villani V, Falcucci G. Optimized Modeling and Design of a PCM-Enhanced H2 Storage. Energies. 2021; 14(6):1554. https://doi.org/10.3390/en14061554
Chicago/Turabian StyleFacci, Andrea Luigi, Marco Lauricella, Sauro Succi, Vittorio Villani, and Giacomo Falcucci. 2021. "Optimized Modeling and Design of a PCM-Enhanced H2 Storage" Energies 14, no. 6: 1554. https://doi.org/10.3390/en14061554
APA StyleFacci, A. L., Lauricella, M., Succi, S., Villani, V., & Falcucci, G. (2021). Optimized Modeling and Design of a PCM-Enhanced H2 Storage. Energies, 14(6), 1554. https://doi.org/10.3390/en14061554







