Thermogravimetric Study of the Kinetics of the Reaction C + CO2 under Pore-Diffusion Control
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermal Analysis
2.3. Kinetic Models for Processing TG Curves
3. Results
3.1. Thermogravimetric Curves
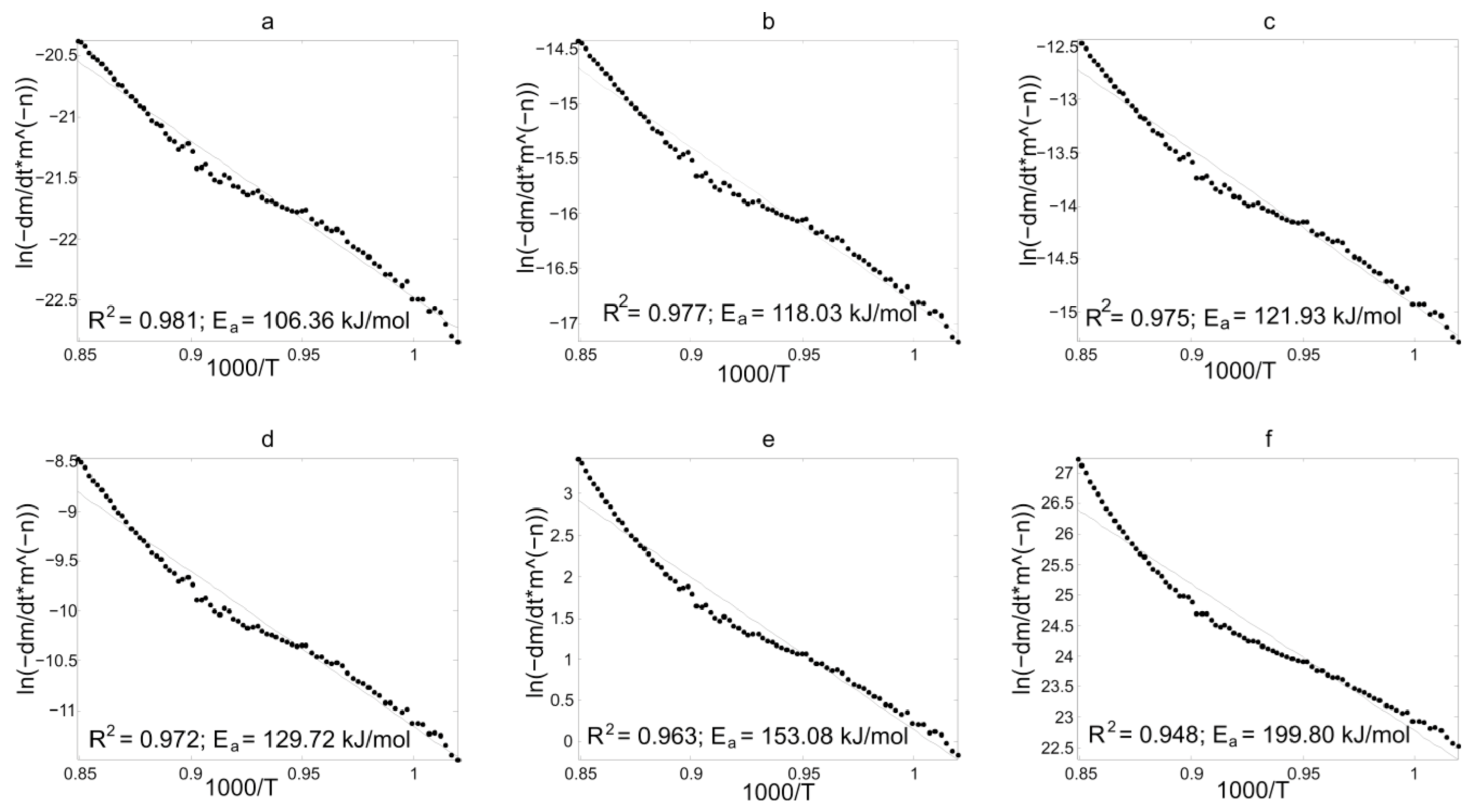
3.2. Kinetic Analysis
3.2.1. External Diffusion Model
3.2.2. Pore-Diffusion Model
3.2.3. Kinetic Model
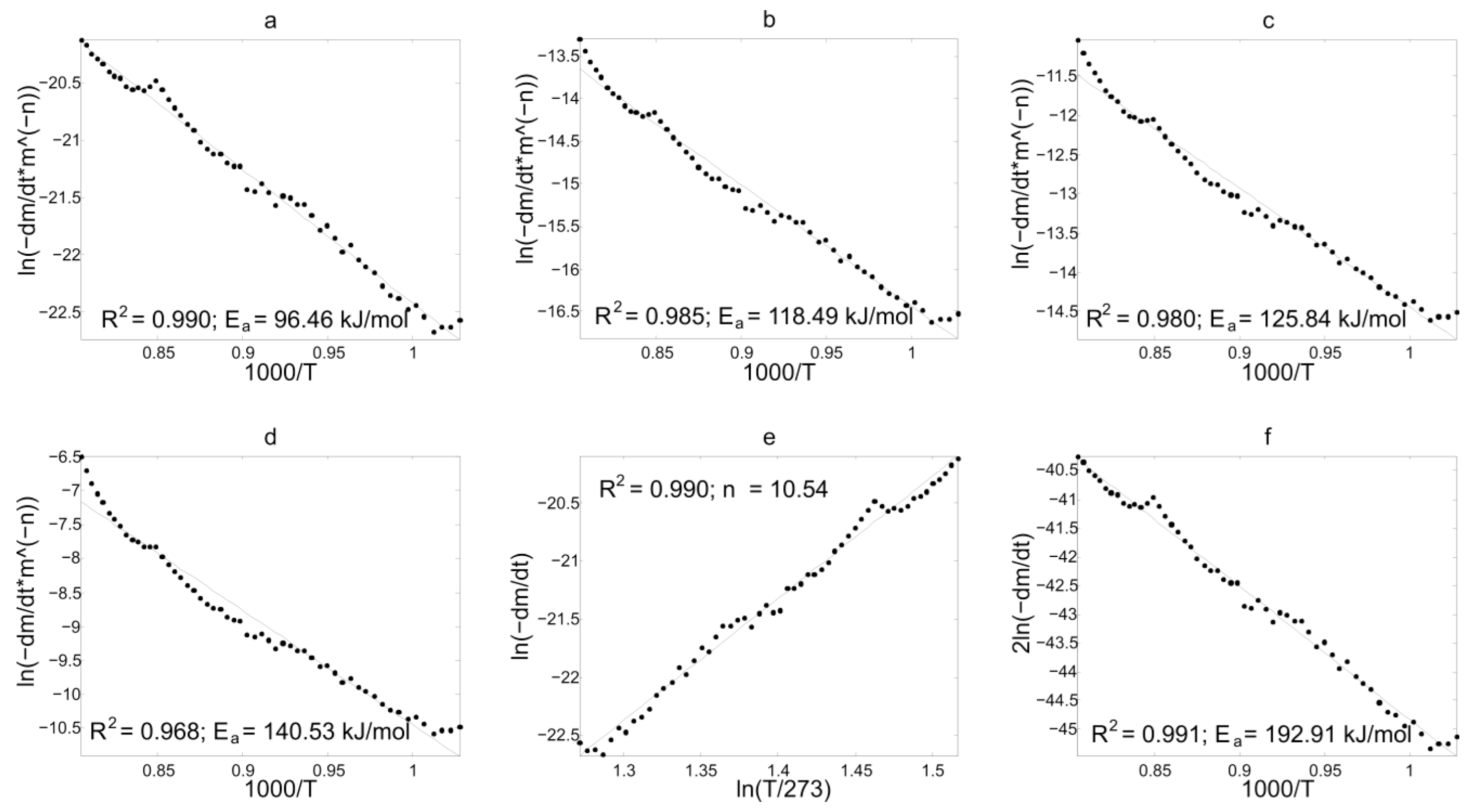
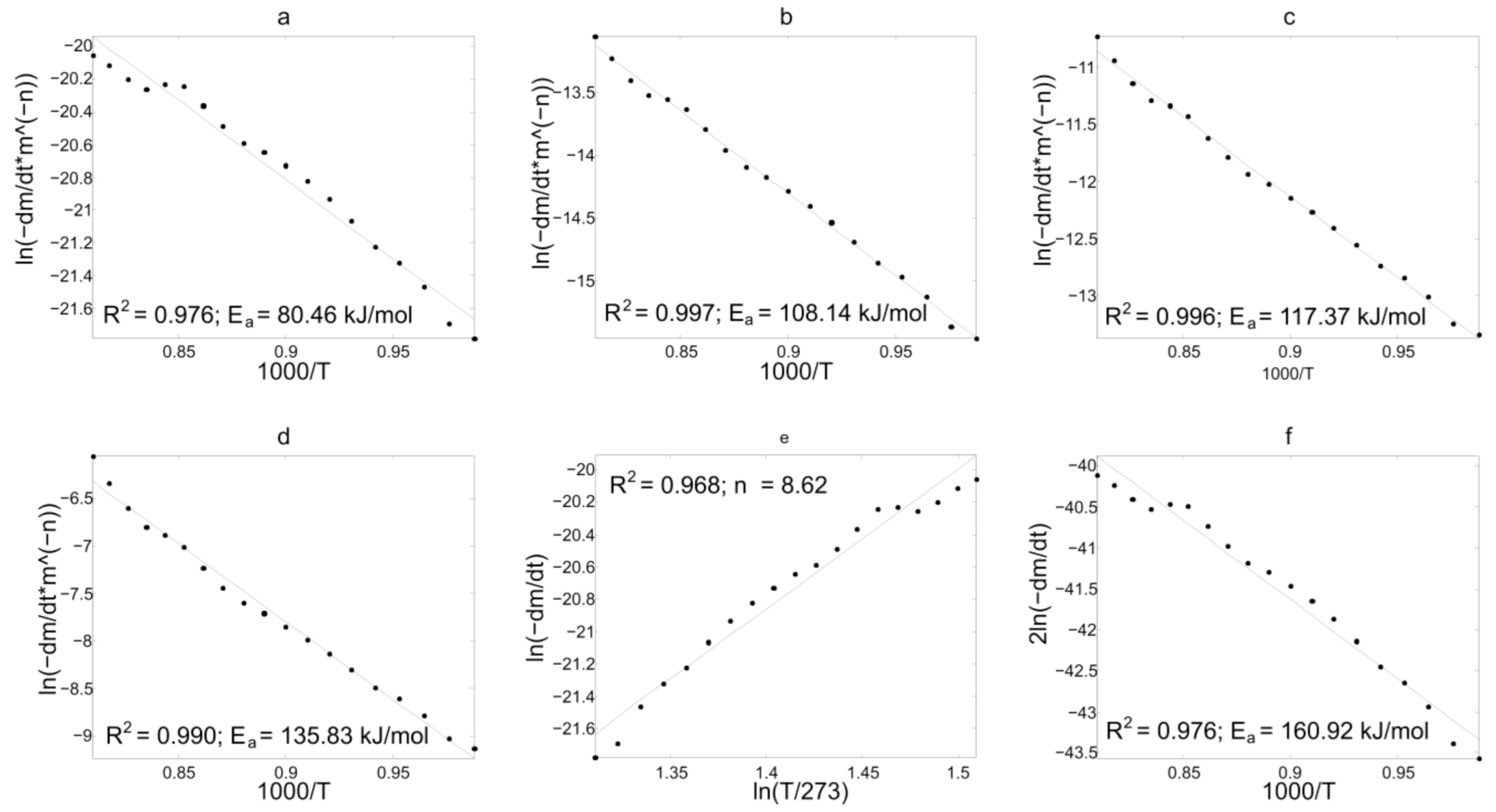
3.3. Discussion of Kinetic Analysis Results
3.3.1. Results of Experimental TG-Curves Processing
3.3.2. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Notation
| mC | carbon mass |
| t | time |
| k0 | pre-exponential factor |
| Ea | activation energy |
| R | universal gas constant |
| T | temperature |
| S | surface area |
| C | oxidizer concentration |
| n | exponent in the formula for the dependence of reaction surface on sample mass (reaction order with respect to fuel) |
| NuD | mass transfer Nusselt number |
| D | diffusion coefficient |
| L | characteristic linear crucible size |
| p | exponent in the formula for the dependence of the diffusion coefficient on temperature |
References
- World Energy Outlook 2019; International Energy Agency: Paris, France, 2019.
- Cormos, A.-M.; Dinca, C.; Cormos, C.-C. Multi-fuel multi-product operation of IGCC power plants with carbon capture and storage (CCS). Appl. Therm. Eng. 2015, 74, 20–27. [Google Scholar] [CrossRef]
- Kler, A.M.; Tyurina, E.A.; Mednikov, A.S.; Kler, A.M.; Mednikov, A.S. A plant for methanol and electricity production: Technical-economic analysis. Energy 2018, 165, 890–899. [Google Scholar] [CrossRef]
- Hurt, R.H.; Haynes, B.S. On the origin of power-law kinetics in carbon oxidation. Proc. Combust. Inst. 2005, 30, 2161–2168. [Google Scholar] [CrossRef]
- Di Blasi, C. Combustion and gasification rates of lignocellulosic chars. Prog. Energy Combust. Sci. 2009, 35, 121–140. [Google Scholar] [CrossRef]
- Mianowski, A.; Radko, T.; Siudyga, T. The reactivity of cokes in Boudouard–Bell reactions in the context of an Ergun model. J. Therm. Anal. Calorim. 2015, 122, 1013–1021. [Google Scholar] [CrossRef]
- Wang, S.; Wu, L.; Hu, X.; Zhang, L.; O’Donnell, K.M.; Buckley, C.E.; Li, C.-Z. An X-ray photoelectron spectroscopic perspective for the evolution of O-containing structures in char during gasification. Fuel Process. Technol. 2018, 172, 209–215. [Google Scholar] [CrossRef]
- Huo, W.; Zhou, Z.; Chen, X.; Dai, Z.; Yu, G. Study on CO2 gasification reactivity and physical characteristics of biomass, petroleum coke and coal chars. Bioresour. Technol. 2014, 159, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, G.; Schürz, M.; Steffen, K.; Meyer, B. Kinetic study on CO2 gasification of brown coal and biomass chars: Reaction order. Fuel 2016, 173, 311–319. [Google Scholar] [CrossRef]
- Kuznetsov, A.P.; Orenbakh, M.S. Formation and change of coke structure in the process of fossil coal burning. Teploenergetika 1975, 3, 23–27. (In Russian) [Google Scholar]
- Jayaraman, K.; Gokalp, I.; Bonifaci, E.; Merlo, N. Kinetics of steam and CO2 gasification of high ash coal–char produced under various heating rates. Fuel 2015, 154, 370–379. [Google Scholar] [CrossRef]
- Vorobiev, N.; Geier, M.; Schiemann, M.; Scherer, V. Experimentation for char combustion kinetics measurements: Bias from char preparation. Fuel Process. Technol. 2016, 151, 155–165. [Google Scholar] [CrossRef]
- Russell, N.V.; Gibbins, J.R.; Man, C.K.; Williamson, J. Coal char thermal deactivation under pulverized fuel combustion conditions. Energy Fuels 2000, 14, 883–888. [Google Scholar] [CrossRef]
- Zolin, A.; Jensen, A.D.; Jensen, P.A.; Dam-Johansen, K. Experimental study of char thermal deactivation. Fuel 2002, 81, 1065–1075. [Google Scholar] [CrossRef]
- Karlström, O.; Brink, A.; Hupa, M. Desorption kinetics of CO in char oxidation and gasification in O2, CO2 and H2O. Combust. Flame 2015, 162, 788–796. [Google Scholar] [CrossRef]
- Ralnikov, P.A.; Abaimov, N.A.; Ryzhkov, A.F. Investigation of coal entrained-flow gasification in O2-CO2 mixtures for oxy-fuel IGCC. J. Phys. Conf. Ser. 2018, 1128, 012007. [Google Scholar] [CrossRef]
- Gonzalez, V.; Rusig, S.; Schurz, M.; Krzack, S.; Kleeberg, J.; Guhl, S.; Meyer, B. Experimental investigations on lignite char gasification kinetics using a pressurized drop tube reactor. Fuel 2018, 224, 348–356. [Google Scholar] [CrossRef]
- Rußig, S.; Gonzalez, V.; Schurz, M.; Krzack, S.; Kleeberg, J.; Guhl, S.; Meyer, B. Particle residence time measurement in a pressurized drop-tube reactor with radioactive tracer. Fuel 2019, 252, 37–46. [Google Scholar] [CrossRef]
- Ryzhkov, A.F.; Silin, V.E.; Bogatova, T.F.; Nadir, S.M. Ignition and combustion features of biofuels. J. Eng. Phys. Thermophys. 2011, 84, 888–897. [Google Scholar] [CrossRef]
- Vershinina, K.Y.; Shlegel, N.; Strizhak, P. Impact of environmentally attractive additives on the ignition delay times of slurry fuels: Experimental study. Fuel 2019, 238, 275–288. [Google Scholar] [CrossRef]
- Pielsticker, S.; Gövert, B.; Kreitzberg, T.; Habermehl, M.; Hatzfeld, O.; Kneer, R. Development of a rapidly responding fluidized bed reactor by theoretical and experimental evaluation of combustion reactions. Fuel 2018, 223, 462–469. [Google Scholar] [CrossRef]
- Schröder, E. Experiments on the pyrolysis of large beechwood particles in fixed beds. J. Anal. Appl. Pyrolysis 2004, 71, 669–694. [Google Scholar] [CrossRef]
- Gerasimov, G.; Khaskhachikh, V.; Potapov, O. Experimental study of kukersite oil shale pyrolysis by solid heat carrier. Fuel Process. Technol. 2017, 158, 123–129. [Google Scholar] [CrossRef]
- Boyko, E.A. Complex Thermal Analysis of Solid Organic Fuels; Krasnoyarsk State Technical University: Krasnoyarsk, Russia, 2005. [Google Scholar]
- Merzhanov, A.G. On quasi-stationary theory of thermal explosion. Doklady USSR 1961, 40, 637–640. (In Russian) [Google Scholar]
- Sánchez-Rodriguez, D.; Farjas, J.; Roura, P. The critical conditions for thermal explosion in a system heated at a constant rate. Combust. Flame 2017, 186, 211–219. [Google Scholar] [CrossRef]
- Khudyakova, G.I. Experimental Study of Thermochemical Conversion of Charcoal by Thermogravimetric Analysis. Ph.D. Thesis, Ural Federal University, Yekaterinburg, Russia, 2015. (In Russian). [Google Scholar]
- Branca, C.; Di Blasi, C. Self-heating effects in the thermogravimetric analysis of wood char oxidation. Fuel 2020, 276, 118012. [Google Scholar] [CrossRef]
- L’vov, B.V. Thermal Decomposition of Solid and Liquid Substances; Polytechnic Univ. Publ.: Saint-Petersburg, Russia, 2006. (In Russian) [Google Scholar]
- Meng, X.; De Jong, W.; Badri, F.S.; Benito, P.; Basile, F.; Verkooijen, A.H. Combustion study of partially gasified willow and DDGS chars using TG analysis and COMSOL modeling. Biomass Bioenergy 2012, 39, 356–369. [Google Scholar] [CrossRef]
- Schulze, S.; Nikrityuk, P.; Abosteif, Z.; Guhl, S.; Richter, A.; Meyer, B. Heat and mass transfer within thermogravimetric analyser: From simulation to improved estimation of kinetic data for char gasification. Fuel 2017, 187, 338–348. [Google Scholar] [CrossRef]
- Kumar, M.; Ghoniem, A.F. Application of a validated gasification model to determine the impact of coal particle grinding size on carbon conversion. Fuel 2013, 108, 565–577. [Google Scholar] [CrossRef]
- Chevarin, F.; Azari, K.; Lemieux, L.; Ziegler, D.; Fafard, M.; Alamdari, H. Active pore sizes during the CO2 gasification of carbon anode at 960 °C. Fuel 2016, 178, 93–102. [Google Scholar] [CrossRef]
- Hurt, R.H.; Sarofim, A.F.; Longwell, J.P. Gasification-induced densification of carbons: From soot to form coke. Combust. Flame 1993, 95, 430–432. [Google Scholar] [CrossRef]
- Mitchell, R.E.; Ma, L.; Kim, B. On the burning behavior of pulverized coal chars. Combust. Flame 2007, 151, 426–436. [Google Scholar] [CrossRef]
- Gómez-Barea, A.; Ollero, P.; Arjona, R.; De Castro, P.A.O. Reaction-diffusion model of TGA gasification experiments for estimating diffusional effects. Fuel 2005, 84, 1695–1704. [Google Scholar] [CrossRef]
- Nowak, B.; Karlström, O.; Backman, P.; Brink, A.; Zevenhoven, M.; Voglsam, S.; Winter, F.; Hupa, M. Mass transfer limitation in thermogravimetry of biomass gasification. J. Therm. Anal. Calorim. 2013, 111, 183–192. [Google Scholar] [CrossRef]
- Jess, A.; Andresen, A.-K. Influence of mass transfer on thermogravimetric analysis of combustion and gasification reactivity of coke. Fuel 2010, 89, 1541–1548. [Google Scholar] [CrossRef]
- Mani, T.; Mahinpey, N.; Murugan, P. Reaction kinetics and mass transfer studies of biomass char gasification with CO2. Chem. Eng. Sci. 2011, 66, 36–41. [Google Scholar] [CrossRef]
- Gomez, A.; Mahinpey, N. Kinetic study of coal steam and CO2 gasification: A new method to reduce interparticle diffusion. Fuel 2015, 148, 160–167. [Google Scholar] [CrossRef]
- Morin, M.; Pécate, S.; Masi, E.; Hémati, M. Kinetic study and modelling of char combustion in TGA in isothermal conditions. Fuel 2017, 203, 522–536. [Google Scholar] [CrossRef]
- Shi, L.; Liu, Q.; Guo, X.; He, W.; Liu, Z. Pyrolysis of coal in TGA: Extent of volatile condensation in crucible. Fuel Process. Technol. 2014, 121, 91–95. [Google Scholar] [CrossRef]
- Zeldovich, Y.B. Selected Works. Chemical Physics and Hydrodynamics; Princeton Univ. Press: Oxfordshire, UK, 1992; Volume I. [Google Scholar]
- Annamalai, K.; Ryan, W. Interactive processes in gasification and combustion—II. Isolated carbon, coal and porous char particles. Prog. Energy Combust. Sci. 1993, 19, 383–446. [Google Scholar] [CrossRef]
- Huo, W.; Zhou, Z.; Wang, F.; Wang, Y.; Yu, G. Experimental study of pore diffusion effect on char gasification with CO2 and steam. Fuel 2014, 131, 59–65. [Google Scholar] [CrossRef]
- Piddubniak, O.; Ledakowicz, S.; Nowicki, L. New approach to a problem of heat transfer with chemical reaction in a cylinder of finite dimensions. Int. J. Heat Mass Transf. 2011, 54, 338–344. [Google Scholar] [CrossRef]
- Buczyński, R.; Czerski, G.; Zubek, K.; Weber, R.; Grzywacz, P. Evaluation of carbon dioxide gasification kinetics on the basis of non-isothermal measurements and CFD modelling of the thermogravimetric analyser. Fuel 2018, 228, 50–61. [Google Scholar] [CrossRef]
- Korotkikh, A.G.; Slyusarskiy, K.V.; Ditts, A.A. Kinetics of coal char gasification in a carbon dioxide medium. Russ. J. Phys. Chem. B 2016, 10, 576–581. [Google Scholar] [CrossRef]
- Urych, B.; Smoliński, A. Kinetics of sewage sludge pyrolysis and air gasification of its chars. Energy Fuels 2016, 30, 4869–4878. [Google Scholar] [CrossRef]
- Slyusarskiy, K.V.; Larionov, K.B.; Osipov, V.I.; Yankovsky, S.A.; Gubin, V.E.; Gromov, A.A. Non-isothermal kinetic study of bituminous coal and lignite conversion in air and in argon/air mixtures. Fuel 2017, 191, 383–392. [Google Scholar] [CrossRef]
- Zhao, B.; Xu, X.; Li, H.; Chen, X.; Zeng, F. Kinetics evaluation and thermal decomposition characteristics of co-pyrolysis of municipal sewage sludge and hazelnut shell. Bioresour. Technol. 2018, 247, 21–29. [Google Scholar] [CrossRef]
- Levin, A.A.; Kozlov, A.N. Verification of the stage scheme of low-grade solid fuel gasification. J. Phys. Conf. Ser. 2018, 1128, 012071. [Google Scholar] [CrossRef]
- Senneca, O.; Ontyd, C.; Cerciello, F.; Schiemann, M.; Scherer, V. Extension of the thermal annealing concepts developed for coal combustion to conversion of lignocellulosic biomass. Energy Fuels 2020, 34, 3661–3670. [Google Scholar] [CrossRef]
- Mularski, J.; Pawlak-Kruczek, H.; Modlinski, N. A review of recent studies of the CFD modelling of coal gasification in entrained flow gasifiers, covering devolatilization, gas-phase reactions, surface reactions, models and kinetics. Fuel 2020, 271, 117620. [Google Scholar] [CrossRef]
- Hirschfelder, J.; Curtiss, C.; Bird, R. The Molecular Theory of Gases and Liquids; Wiley: New York, NY, USA, 1954. [Google Scholar]
- Thiele, E.W. Relation between catalytic activity and size of particle. Ind. Eng. Chem. 1939, 31, 916–920. [Google Scholar] [CrossRef]
- Irfan, M.F.; Usman, M.R.; Kusakabe, K. Coal gasification in CO2 atmosphere and its kinetics since 1948: A brief review. Energy 2011, 36, 12–40. [Google Scholar] [CrossRef]
- Hecht, E.S.; Shaddix, C.R.; Molina, A.; Haynes, B.S. Effect of CO2 gasification reaction on oxy-combustion of pulverized coal char. Proc. Combust. Inst. 2011, 33, 1699–1706. [Google Scholar] [CrossRef]
- Veca, E.; Adrover, A. Isothermal kinetics of char-coal gasification with pure CO2. Fuel 2014, 123, 151–157. [Google Scholar] [CrossRef]
- López-González, D.; Fernandez-Lopez, M.; Valverde, J.; Sanchez-Silva, L. Gasification of lignocellulosic biomass char obtained from pyrolysis: Kinetic and evolved gas analyses. Energy 2014, 71, 456–467. [Google Scholar] [CrossRef]
- Cortazar, M.; Lopez, G.; Alvarez, J.; Arregi, A.; Amutio, M.; Bilbao, J.; Olazar, M. Experimental study and modeling of biomass char gasification kinetics in a novel thermogravimetric flow reactor. Chem. Eng. J. 2020, 396, 125200. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Favergeon, L.; Koga, N.; Moukhina, E.; Pérez-Maqueda, L.A.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for analysis of multi-step kinetics. Thermochim. Acta 2020, 689, 178597. [Google Scholar] [CrossRef]
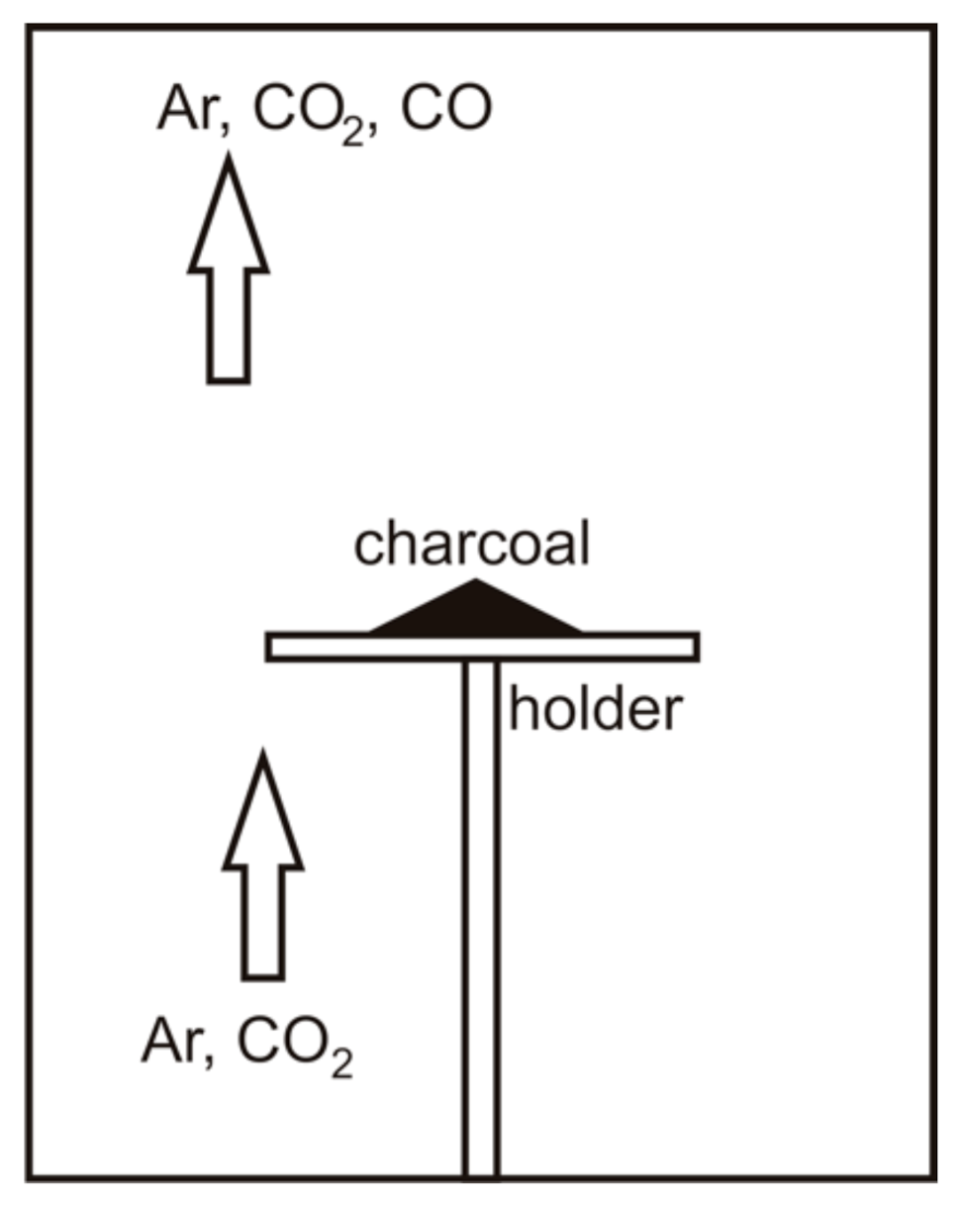
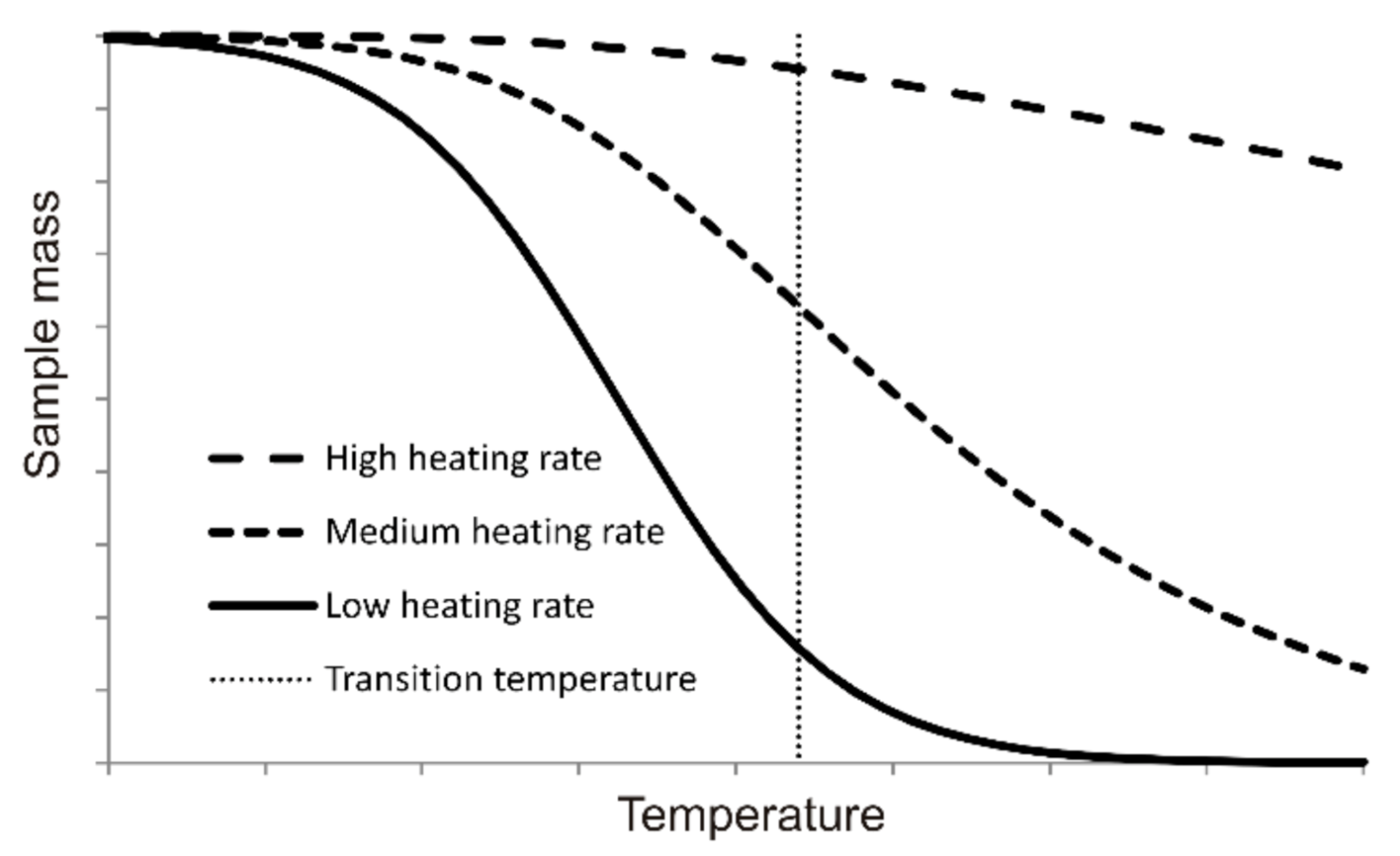
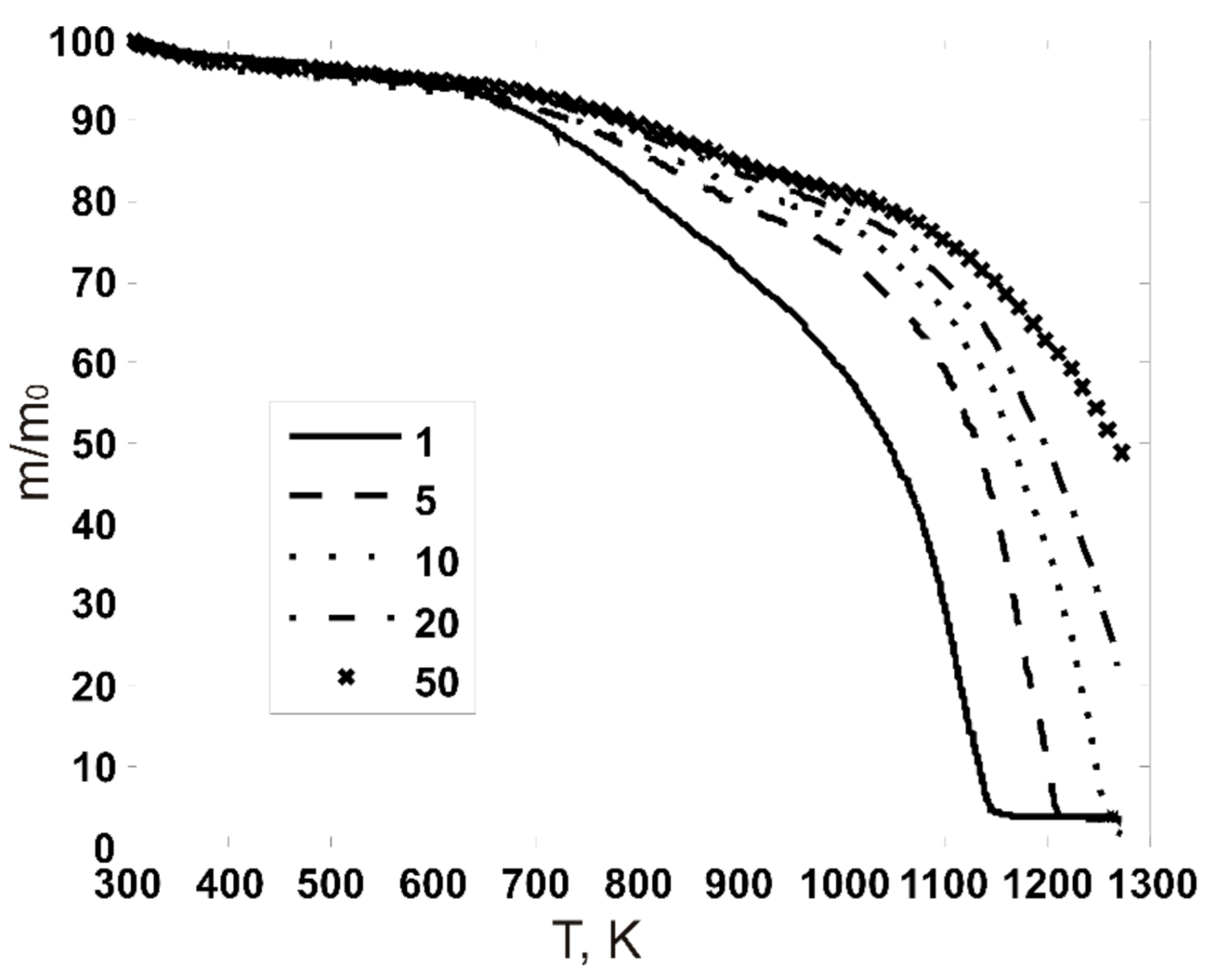



| Technical Characteristics (% wt., Dry Basis) | Pine | Charcoal |
|---|---|---|
| Fixed carbon | 10.23 | 58.72 |
| Ash content | 0.17 | 1.99 |
| Volatile yield | 89.6 | 39.29 |
| Elemental composition (% wt., dry ash-free basis) | ||
| C | 46.60 | 79.84 |
| H | 6.32 | 5.79 |
| O | 47.08 | 14.37 |
| Brutto-formula | CH1.62O0.76 | CH0.46O0.14 |
| Model (Expression) | Heating Rate, K min−1 | ||||
|---|---|---|---|---|---|
| 1 | 5 | 10 | 20 | 50 | |
| n = 0 (2) | 118.46 | 104.64 | 106.36 | 96.46 | 80.46 |
| n = 1/2 (2) | 157.84 | 116.99 | 118.04 | 118.49 | 108.14 |
| n = 2/3 (2) | 170.97 | 121.11 | 121.93 | 125.84 | 117.37 |
| n = 1 (2) | 197.23 | 129.34 | 129.72 | 140.53 | 135.83 |
| n = 2 (2) | 187.59 | 154.03 | 153.08 | 184.60 | 191.20 |
| Z (6) | 236.92 | 209.28 | 212.71 | 192.92 | 160.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donskoy, I.; Kozlov, A. Thermogravimetric Study of the Kinetics of the Reaction C + CO2 under Pore-Diffusion Control. Energies 2021, 14, 1886. https://doi.org/10.3390/en14071886
Donskoy I, Kozlov A. Thermogravimetric Study of the Kinetics of the Reaction C + CO2 under Pore-Diffusion Control. Energies. 2021; 14(7):1886. https://doi.org/10.3390/en14071886
Chicago/Turabian StyleDonskoy, Igor, and Aleksandr Kozlov. 2021. "Thermogravimetric Study of the Kinetics of the Reaction C + CO2 under Pore-Diffusion Control" Energies 14, no. 7: 1886. https://doi.org/10.3390/en14071886
APA StyleDonskoy, I., & Kozlov, A. (2021). Thermogravimetric Study of the Kinetics of the Reaction C + CO2 under Pore-Diffusion Control. Energies, 14(7), 1886. https://doi.org/10.3390/en14071886







