Optimization of Silver Nanoparticle Separation Method from Drilling Waste Matrices
Abstract
1. Introduction
- (a)
- Losses may arise in the solid waste sample preparation stage. Sample homogenization and fragmentation, e.g., during grinding, may change the matrix structure of the starting material.
- (b)
- Due to the organic–inorganic nature of nanomaterials, their characterization requires the use of various analytical methods.
- (c)
- A broader characterization of nanoparticles requires the use of several types of methods. For example, a Scanning Electron Microscope (SEM) can provide structure information but cannot quantify NPs
- (d)
- The concentrations of the analyzed nanoparticles are often very low, close to or below the detection limit of the instruments used.
- (e)
- The complex and unknown matrix of the samples analyzed can make it very difficult to correctly analyze NPs.
2. Materials and Methods
2.1. Chemicals
2.2. Instrument
2.3. Drilling Waste Samples Preparation
- 01 01 02 (wastes from the extraction of minerals other than metal ores),
- 01 04 12 (this group includes wastes from washing and cleaning of minerals other than those mentioned in 01 04 07 and 01 04 11),
- 01 05 05* (petroleum-containing drilling fluids and wastes),
- 01 05 06* (waste and drilling fluids that contain dangerous substances),
- 01 05 07 (barite-containing drilling fluids and wastes other than those mentioned in 01 05 05 and 01 05 06),
- 01 05 08 (chloride-containing drilling fluids and wastes other than those mentioned in 01 05 05 and 01 05 06), and
- 01 05 99 (other wastes not listed).
3. Results
3.1. Water Extracts of Drilling Waste—Centrifugation, DW(1–5)-W
3.2. Drilling Waste—Cloud Point Extraction (CPE), Centrifugation, and DW(1–4)-E
4. Discussion
4.1. Water Extracts of Drilling Waste
4.2. Drilling Waste
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fakoya, M.F.; Shah, S.N. Emergence of nanotechnology in the oil and gas industry: Emphasis on the application of silica nanoparticles. Petroleum 2017, 3, 391–405. [Google Scholar] [CrossRef]
- Cheraghian, G. Application of Nano-Particles of Clay to Improve Drilling Fluid. IJNN 2017, 13, 177–186. [Google Scholar]
- Cheraghian, G.; Hemmati, M.; Bazgir, S. Application of TiO2 and fumed silica nanoparticles and improve the performance of drilling fluids. AIP Conf. Proc. 2014, 1590, 266. [Google Scholar] [CrossRef]
- Munawar, K.; Jan, B.M.; Tong, C.W.; Berawi, M.A. Advanced nanomaterials in oil and gas industry: Design, application and challenges. Appl. Energy 2017, 191, 287–310. [Google Scholar] [CrossRef]
- Wilk, K.; Kasza, P.; Czupski, M. Zastosowanie Nanocieczy jako Dodatków Wspomagających Proces Wypierania ropy Naftowej. Nafta-Gaz 2014, 1, 14–20. Available online: http://www.archiwum.inig.pl/INST/nafta-gaz/nafta-gaz/Nafta-Gaz-2014-01-03.pdf (accessed on 8 February 2021).
- Zhe, Z.; Yuxiu, A. Nanotechnology for the Oil and Gas Industry—An Overview of Recent progress. Nanotechnol. Rev. 2018, 7, 341–353. Available online: https://www.degruyter.com/document/doi/10.1515/ntrev-2018-0061/html (accessed on 8 February 2021). [CrossRef]
- Krasodomski, M.; Krasodomski, W.; Ziemiański, L. Nanotechnologia a przemysł naftowy. Nafta-Gaz 2009, 65, 83–92. [Google Scholar]
- Zima, G. Analiza wpływu nanomateriałów na właściwości osadu filtracyjnego. Nafta-Gaz 2017, 73, 312–320. Available online: http://archiwum.inig.pl/INST/nafta-gaz/nafta-gaz/Nafta-Gaz-2017-05-03.pdf (accessed on 8 February 2021). [CrossRef]
- Krasodomski, W.; Rembiesa-Śmiszek, A.; Skibińska, A. Nanocząstki w środkach smarowych. Nafta-Gaz 2013, 69, 220–225. Available online: http://www.archiwum.inig.pl/INST/nafta-gaz/nafta-gaz/Nafta-Gaz-2013-03-02.pdf (accessed on 8 February 2021).
- Das, A.; Kamle, M.; Bharti, A.; Kumar, P. Nanotechnology and it’s applications in environmental remediation: An overview. Vegetos 2019, 32, 227–237. [Google Scholar] [CrossRef]
- Li, D.; Hong, B.; Fang, W.; Guo, Y.; Lin, R. Preparation of well-dispersed silver nanoparticles for oil-based nanofluids. Ind. Eng. Chem. Res. 2010, 49, 1697–1702. [Google Scholar] [CrossRef]
- Noeiaghaei, T.; Mukherjee, A.; Dhami, N.; Chae, S.-R. Biogenic deterioration of concrete and its mitigation technologies. Constr. Build. Mater. 2017, 149, 575–586. [Google Scholar] [CrossRef]
- Husin, H.; Ahmad, N.; Jamil, N.; Chyuan, O.H.; Roslan, A. Evaluation on the Presence of Nano Silver Particle in Improving a Conventional Water-based Drilling Fluid, IOP Conference Series: Materials Science and Engineering, 358. In Proceedings of the 3rd International Conference on Global Sustainability and Chemical Engineering (ICGSCE), Putrajaya, Malaysia, 15–16 February 2017. [Google Scholar]
- Husin, H.; Elraies, K.A.; Choi, H.J.; Aman, Z. Influence of Graphene Nanoplatelet and Silver Nanoparticle on the Rheological Properties of Water-Based Mud. Appl. Sci. 2018, 8, 1386. [Google Scholar] [CrossRef]
- Bundschuh, M.; Filser, J.; Lüderwald, S.; Mckee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the environment: Where do we come from, where do we go to? Environ. Sci. Eur. 2018, 30, 6. Available online: https://enveurope.springeropen.com/articles/10.1186/s12302-018-0132-6 (accessed on 8 February 2021). [CrossRef]
- Alsaba, M.T.; Al Dushaishi, M.F.; Abbas, A.K. A comprehensive review of nanoparticles applications in the oil and gas industry. J. Petrol. Explor. Prod. Technol. 2020, 10, 1389–1399. [Google Scholar] [CrossRef]
- Resnik, D.B. How should engineered nanomaterials be regulated for public and environmental health? AMA J. Ethics 2019, 21, E363–E369. [Google Scholar] [CrossRef] [PubMed]
- ASTM E2909-13 American Standard. Standard Guide for Investigation/Study/Assay Tab-Delimited Format for Nanotechnologies (ISA-TAB-nano): Standard File Format for the Submission and Exchange of Data on Nanomaterials and Characterizations; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- ISO/TS 80004-1:2015 European Standard. Nanotechnologies—Vocabulary—Part 1: Core terms ASTM E249009 Standard Guide for Measurement of Particle Size Distribution of Nanomaterials in Suspension by Photon Correlation Spectroscopy (PCS); European Committee for Standardization: Brussels, Belgium, 2015. [Google Scholar]
- ISO/TR 13014:2012 European Standard. Nanotechnologies—Guidance on Physicochemical Characterization of Engineered Nanoscale Materials for Toxicological Assessment; European Committee for Standardization: Brussels, Belgium, 2012. [Google Scholar]
- ISO 10808:2010 European Standard. Nanotechnologies—Characterization of Nanoparticles in Inhalation Exposure Chambers for Inhalation Toxicity Testing; European Committee for Standardization: Brussels, Belgium, 2010. [Google Scholar]
- ASTM E2524-08:2013 American Standard. Standard Test Method for Analysis of Hemolytic Properties of Nanoparticles; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- ISO/TS 12901-1:2012 European Standard. Nanotechnologies—Occupational Risk Management Applied to Engineered Nanomaterials—Part 1: Principles and Approaches; European Committee for Standardization: Brussels, Belgium, 2012. [Google Scholar]
- ASTM E2996-15 American Standard. Standard Guide for Workforce Education in Nanotechnology Health and Safety; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- ISO/TR 12885:2018 European Standard. Nanotechnologies—Health and Safety Practices in Occupational Settings; European Committee for Standardization: Brussels, Belgium, 2018. [Google Scholar]
- ISO/TS 11308:2020 European Standard. Nanotechnologies—Characterization of Carbon Nanotube Samples Using Thermogravimetric Analysis; European Committee for Standardization: Brussels, Belgium, 2020. [Google Scholar]
- ISO/TR 13121:2011 European Standard. Nanotechnologies—Nanomaterial Risk Evaluation; European Committee for Standardization: Brussels, Belgium, 2011. [Google Scholar]
- Campos, A.; López, I. Current Status and Perspectives in Nanowaste Management. In Handbook of Environmental Materials Management; Hussain, C., Ed.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Part, F.; Zecha, G.; Causon, T.; Sinner, E.-K.; Huber-Humer, M. Current Limitations and Challenges In Nanowaste Detection, Characterisation And Monitoring. Waste Manag. 2015, 43, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Younis, S.A.; El-Fawal, E.M.; Serp, P. Nano-wastes and the Environment: Potential Challenges and Opportunities of Nano-waste Management Paradigm for Greener Nanotechnologies. In Handbook of Environmental Materials Management; Hussain, C.M., Ed.; Springer International Publishing AG: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Gajec, M.; Kukulska-Zając, E.; Król, A. Determination of silver nanoparticles in liquid environmental samples. Appl. Ecol. Environ. Res. 2020, 18, 5775–5788. [Google Scholar] [CrossRef]
- Liu, J.F.; Yu, S.J.; Yin, Y.G.; Chao, J.B. Methods for separation, identification, characterization and quantification of silver nanoparticles. TrAC Trends Anal. Chem. 2012, 33, 95–106. [Google Scholar] [CrossRef]
- Torrent, L.; Laborda, F.; Marguí, E.; Hidalgo, M.; Iglesias, M. Combination of cloud point extraction with single particle inductively coupled plasma mass spectrometry to characterize silver nanoparticles in soil leachates. Anal. Bioanal. Chem. 2019, 411, 5317–5329. Available online: https://link.springer.com/article/10.1007%2Fs00216-019-01914-y (accessed on 8 February 2021). [CrossRef]
- El Hadri, H.; Hackley, V.A. Investigation of cloud point extraction for the analysis of metallic nanoparticles in a soil matrix. Environ. Sci. Nano 2017, 4, 105–116. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5427641/ (accessed on 8 February 2021). [CrossRef]
- Majedi, S.M.; Kelly, B. Evaluation of a cloud point extraction approach for the preconcentration and quantification of trace CuO nanoparticles in environmental waters. Anal. Chim. Acta 2014, 814, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Hedayatipour, M.; Jaafarzadeh, N.; Ahmadmoazzam, M. Removal optimization of heavy metals from effluent of sludge dewatering process in oil and gas well drilling by nanofiltration. J. Environ. Manag. 2017, 203, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Navratilova, J.; Gondikas, A. Sample Preparation for the Analysis of Nanomaterials in Water. Fundam. Water Chem. Part. Ecol. Nanomater. Colloids 2019. [Google Scholar] [CrossRef]
- Regulation of the Minister of Climate of 2 January 2020 on the Catalogue of Wastes (Dz.U. 2020 poz. 10). Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20200000010 (accessed on 8 February 2021).
- PN-EN 12457-2:2006 Polish Standard. Charakteryzowanie Odpadów—Wymywanie—Badanie Zgodności w Odniesieniu do Wymywania Ziarnistych Materiałów Odpadowych i Osadów—Część 2: Jednostopniowe Badanie Porcjowe Przy Stosunku cieczy do fazy stałej 10 l/kg w Przypadku Materiałów o Wielkości Cząstek Poniżej 4 mm (bez redukcji lub z redukcją wielkości); Polski Komitet Normalizacji: Warsaw, Poland, 2006. [Google Scholar]
- PN-EN 12457-4:2006 Polish Standard. Charakteryzowanie Odpadów—Wymywanie—Badanie Zgodności w Odniesieniu do Wymywania Ziarnistych Materiałów Odpadowych i Osadów—Część 3: Dwustopniowe Badanie Porcjowe Przy Stosunku cieczy do Fazy stałej 2 l/kg i 8 l/kg dla Materiałów o Wysokiej Zawartości fazy stałej i Wielkości Cząstek Poniżej 4 mm (bez redukcji lub z redukcją wielkości); Polski Komitet Normalizacji: Warsaw, Poland, 2006. [Google Scholar]




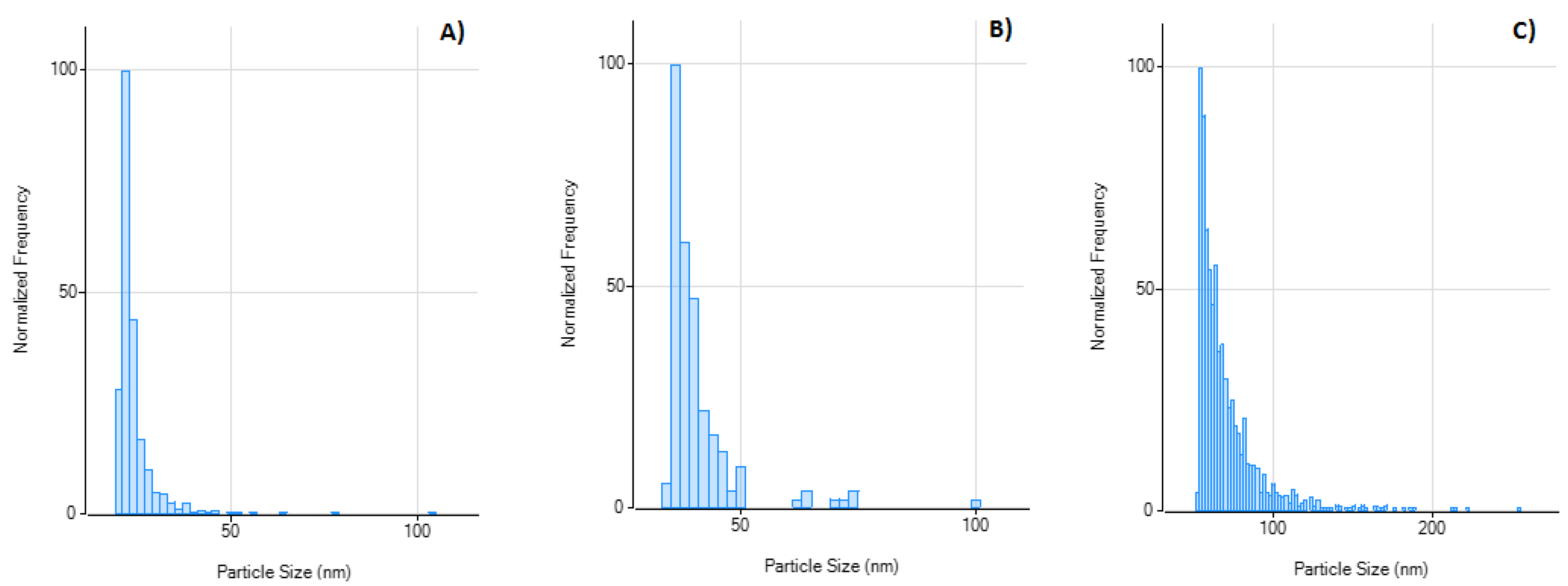
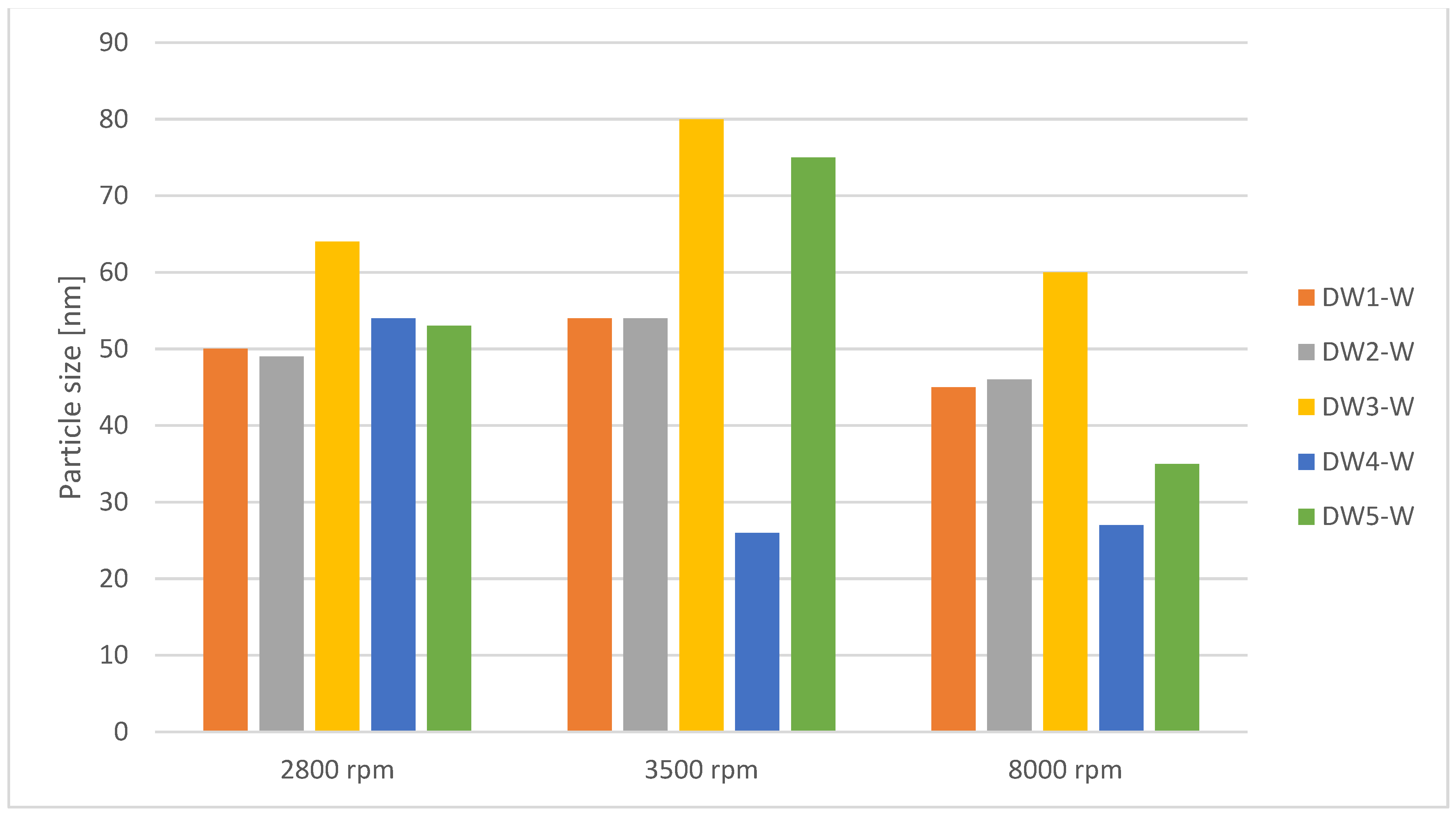
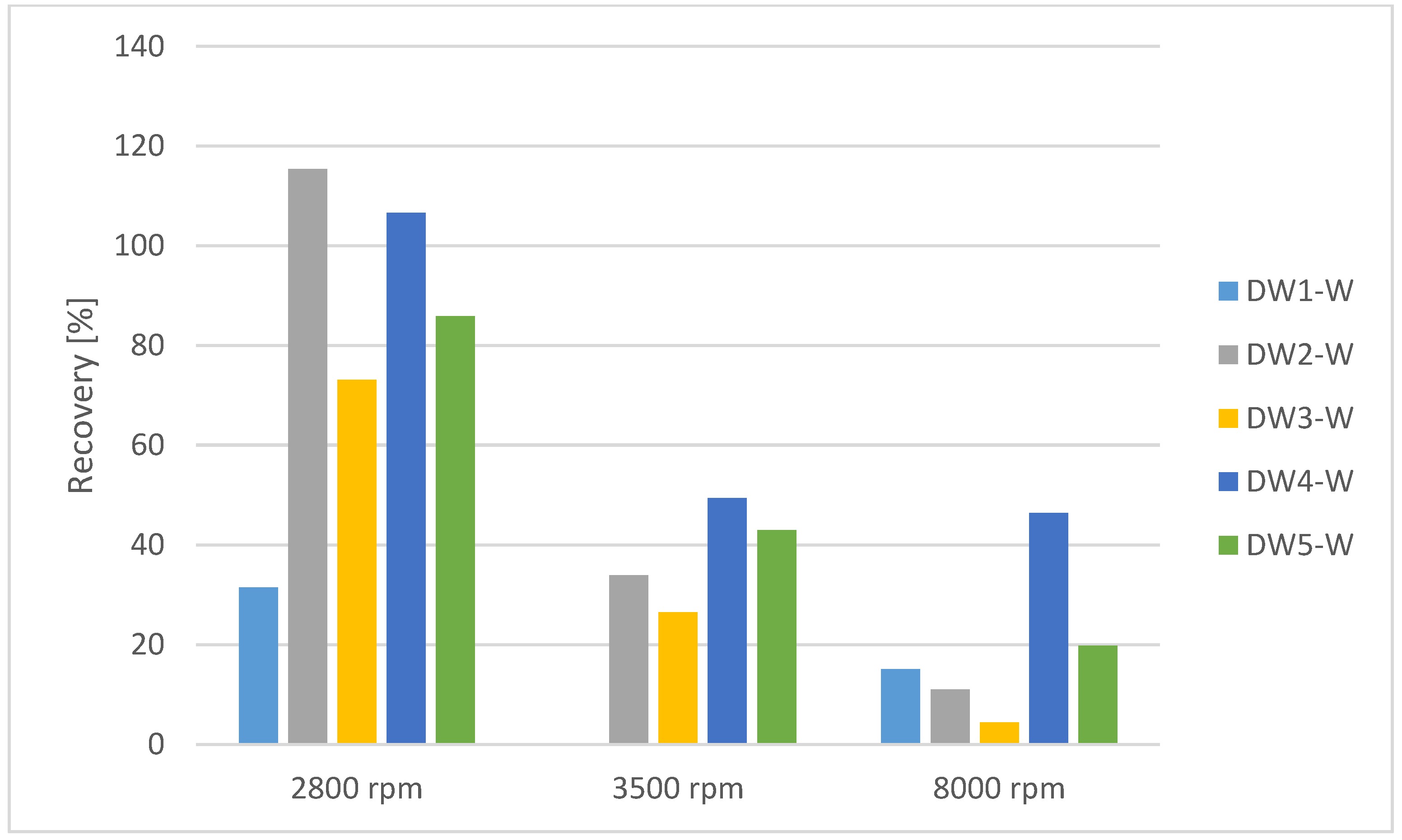


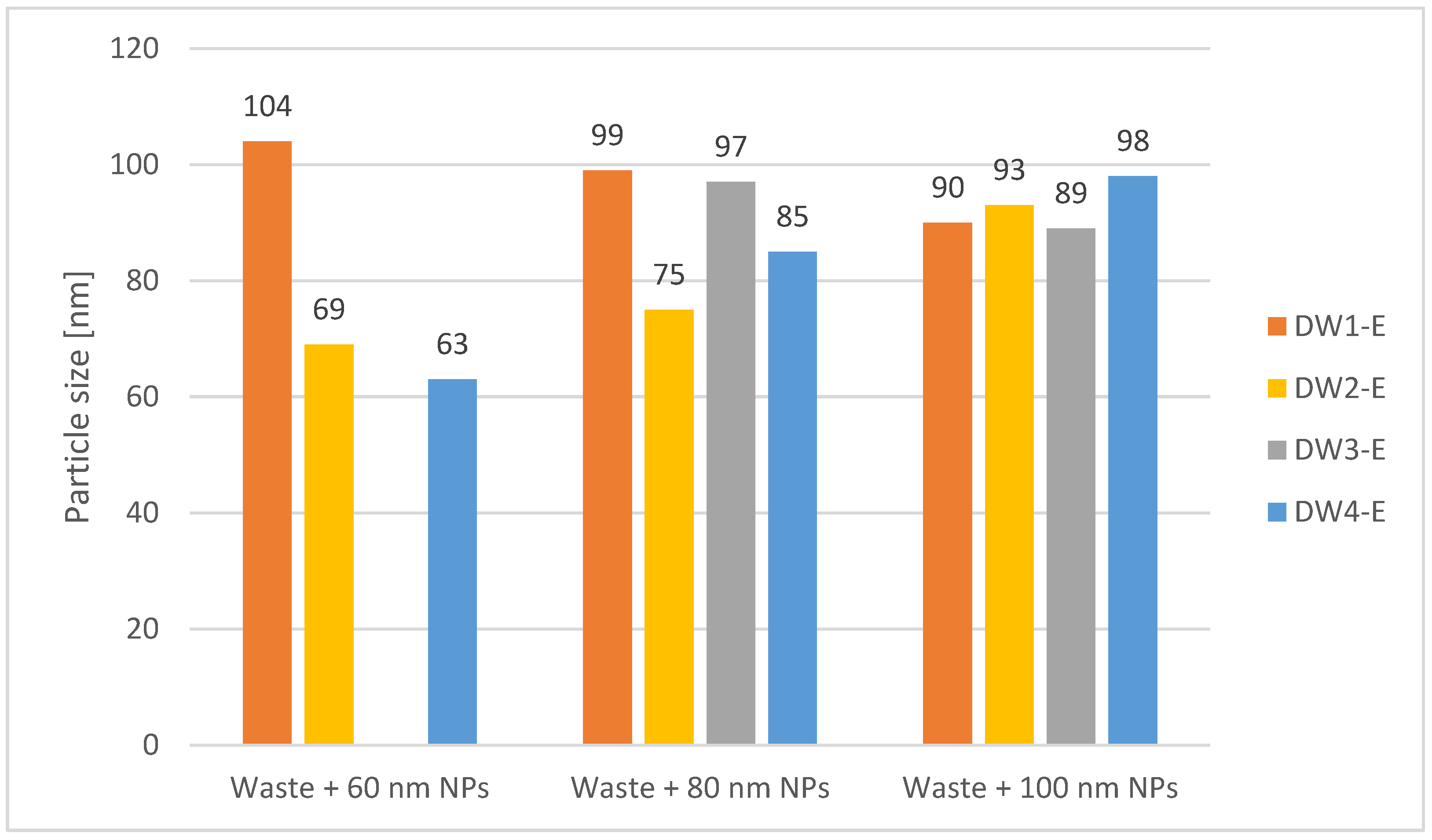
| Instrument Setting | Value | Unit |
|---|---|---|
| uRF power | 1550 | W |
| Mist chamber temperature | 2.0 | °C |
| Nebulizer pump velocity | 0.1 | rpm |
| Sampling depth | 8.0 | mm |
| Integration time | 0.1 | ms |
| Data collection time | 60 | s |
| Density of particles | 10.5 | g/mL |
| Carrier gas flow velocity | 1.05 | L/min |
| Data collection mode | TRA | - |
| Monitored weight | 107Ag | - |
| Mass fraction of Ag | 1 | - |
| Sample Identification | Sample Description | Waste Code |
|---|---|---|
| DW1 | Drilling waste after application of synthetic fluid; solid (powder) and brown in color and has a characteristic organic odor. | 01 05 06* |
| DW2 | Drilling waste (borings) after using a polymer-potassium fluid; semi-solid sample (brownish-gray mass containing fine borings), with a characteristic cement tang. | 01 05 08 |
| DW3 | Drilling waste (borings) after the use of barite fluid; waste after initial cleaning on vibrating screens; a sample of a semi-solid, greasy consistency (gray-brown mass containing fine borings), with a characteristic tang. | 01 05 07 |
| DW4 | Drilling waste (borings) after application of synthetic fluid (third interval from a depth of about 3000 m BGL); waste after cleaning by decantation centrifuges; sample of semi-solid, homogeneous consistency, dark brown color, and characteristic organic odor. | 01 05 05* |
| DW5 | Drilling waste (borings) after application of synthetic fluid (fourth interval from a depth of about 3500 m BGL); waste after cleaning by decantation centrifuges; sample of semi-solid, greasy, homogeneous consistency, dark brown color, and characteristic organic odor. | 01 05 05* |
| Matrix, Sample Preparation Method | Mean Particle Diameter (nm) | Particle Diameter Median (nm) | Most Common Particle Size (nm) | Ionic Concentration of Ag (µg/L) |
|---|---|---|---|---|
| Water extract of waste having the code 01 05 06* (DW1-W) | 23 | 22 | 22 | 0.034 |
| Water extract of waste having the code 01 05 08 (DW2-W) | 40 | 37 | 36 | 0.332 |
| Water extract of waste having the code 01 05 07 (DW3-W) | 69 | 63 | 54 | 1.47 |
| Water extract of waste having the code 01 05 05* (DW4-W) | 19 | 18 | 18 | 0.023 |
| Water extract of waste having the code 01 05 0* (DW5-W) | 25 | 22 | 22 | 0.030 |
| Matrix | Size of AgNPs Introduced into the Matrix (nm) | Separation Method | Mean Particle Diameter (nm) | Particle Diameter Median (nm) | Most Common Particle Size (nm) |
|---|---|---|---|---|---|
| Water extract DW1-W | 60 | centrifugation: 2800 rpm, 15 min | 52 | 50 | 44 |
| Water extract DW2-W | 60 | centrifugation: 2800 rpm, 15 min | 53 | 49 | 42 |
| Water extract DW3-W | 60 | centrifugation: 2800 rpm, 15 min | 67 | 64 | 56 |
| Water extract DW4-W | 60 | centrifugation: 2800 rpm, 15 min | 57 | 54 | 44 |
| Water extract DW5-W | 60 | centrifugation: 2800 rpm, 15 min | 55 | 53 | 50 |
| Water extract DW1-W | 60 | centrifugation: 3500 rpm, 15 min | 57 | 54 | 40 |
| Water extract DW2-W | 60 | centrifugation: 3500 rpm, 15 min | 57 | 54 | 48 |
| Water extract DW3-W | 60 | centrifugation: 3500 rpm, 15 min | 84 | 80 | 70 |
| Water extract DW4-W | 60 | centrifugation: 3500 rpm, 15 min | 29 | 26 | 24 |
| Water extract DW5-W | 60 | centrifugation: 3500 rpm, 15 min | 76 | 75 | 38 |
| Water extract DW1-W | 60 | centrifugation: 8000 rpm, 15 min | 49 | 45 | 38 |
| Water extract DW2-W | 60 | centrifugation: 8000 rpm, 15 min | 49 | 46 | 44 |
| Water extract DW3-W | 60 | centrifugation: 8000 rpm, 15 min | 70 | 60 | 56 |
| Water extract DW4-W | 60 | centrifugation: 8000 rpm, 15 min | 28 | 27 | 24 |
| Water extract DW5-W | 60 | centrifugation: 8000 rpm, 15 min | 43 | 35 | 34 |
| Matrix | Size of AgNPs Introduced into the Matrix (nm) | Separation Method | Mean Particle Diameter (nm) | Particle Diameter Median (nm) | Most Common Particle Size (nm) |
|---|---|---|---|---|---|
| Water extract DW1-W | 60 | centrifugation: 2800 rpm, 5 min | 66 | 62 | 52 |
| Water extract DW2-W | 60 | centrifugation: 2800 rpm, 5 min | 67 | 63 | 52 |
| Water extract DW3-W | 60 | centrifugation: 2800 rpm, 5 min | 88 | 81 | 72 |
| Water extract DW4-W | 60 | centrifugation: 2800 rpm, 5 min | 50 | 48 | 28 |
| Water extract DW5-W | 60 | centrifugation: 2800 rpm, 5 min | 57 | 54 | 38 |
| Water extract DW1-W | 60 | centrifugation: 2800 rpm, 30 min | 59 | 58 | 46 |
| Water extract DW2-W | 60 | centrifugation: 2800 rpm, 30 min | 59 | 55 | 52 |
| Water extract DW3-W | 60 | centrifugation: 2800 rpm, 30 min | 67 | 65 | 62 |
| Water extract DW4-W | 60 | centrifugation: 2800 rpm, 30 min | 28 | 27 | 26 |
| Water extract DW5-W | 60 | centrifugation: 2800 rpm, 30 min | 41 | 34 | 34 |
| Matrix | Size of AgNPs Introduced into the Matrix (nm) | Separation Method | Mean Particle Diameter (nm) | Particle Diameter Median (nm) | Most Common Particle Size (nm) |
|---|---|---|---|---|---|
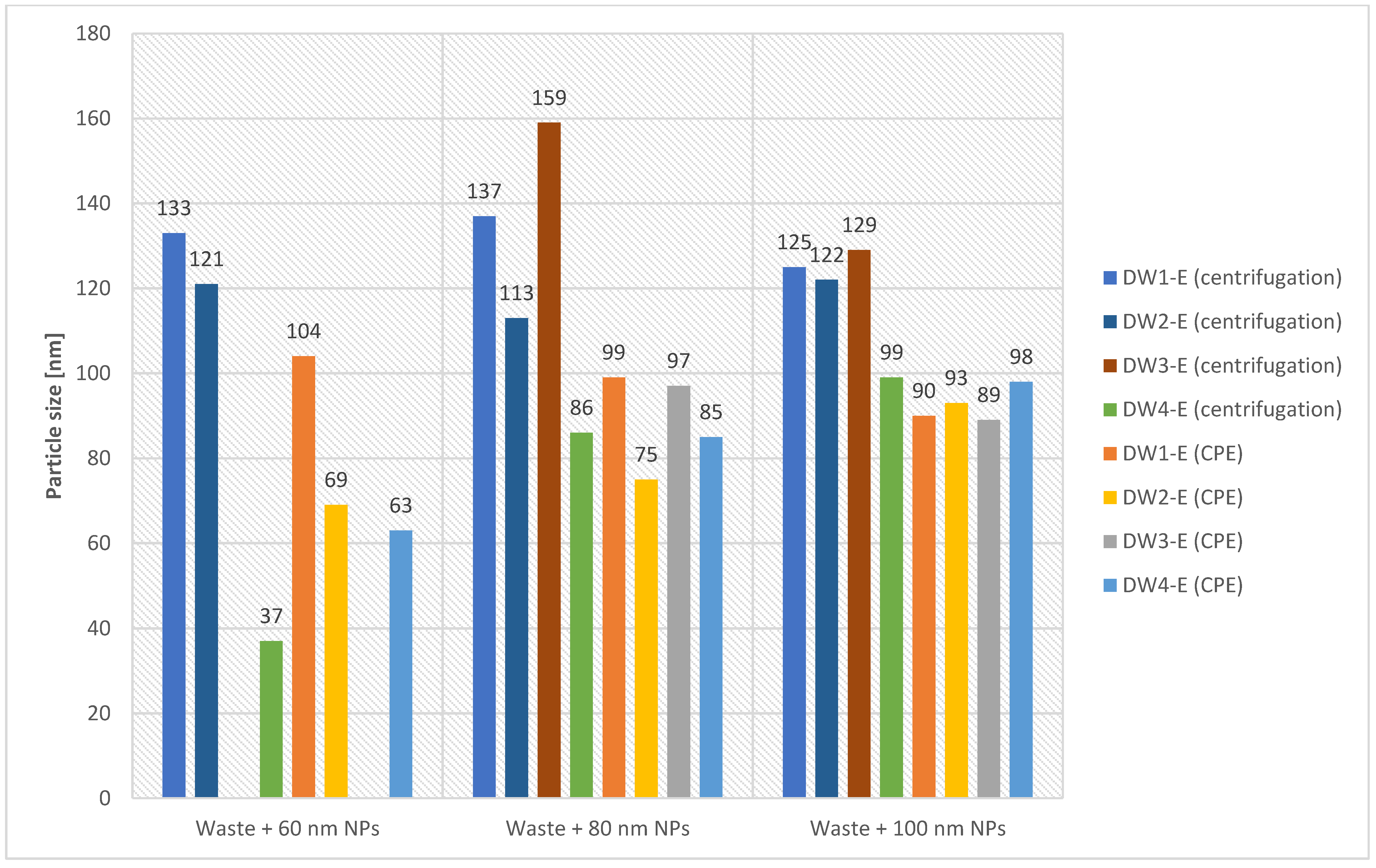
| DW1-E | 60 | CPE | 110 | 104 | 96 |
| DW1-E | 80 | CPE | 105 | 99 | 90 |
| DW1-E | 100 | CPE | 94 | 90 | 82 |
| DW2-E | 60 | CPE | 73 | 69 | 64 |
| DW2-E | 80 | CPE | 78 | 75 | 70 |
| DW2-E | 100 | CPE | 97 | 93 | 86 |
| DW3-E | 80 | CPE | 100 | 97 | 86 |
| DW3-E | 100 | CPE | 94 | 89 | 82 |
| DW4-E | 60 | CPE | 68 | 63 | 58 |
| DW4-E | 80 | CPE | 96 | 85 | 70 |
| DW4-E | 100 | CPE | 101 | 98 | 106 |
| Matrix | Size of AgNPs Introduced into the Matrix (nm) | Separation Method | Mean Particle Diameter (nm) | Particle Diameter Median (nm) | Most Common Particle Size (nm) |
|---|---|---|---|---|---|
| DW1-E | 60 | centrifugation 2800 rpm, 12 min | 137 | 133 | 124 |
| DW1-E | 80 | centrifugation 2800 rpm, 12 min | 143 | 137 | 118 |
| DW1-E | 100 | centrifugation 2800 rpm, 12 min | 130 | 125 | 108 |
| DW2-E | 60 | centrifugation 2800 rpm, 12 min | 127 | 121 | 108 |
| DW2-E | 80 | centrifugation 2800 rpm, 12 min | 120 | 113 | 98 |
| DW2-E | 100 | centrifugation 2800 rpm, 12 min | 132 | 122 | 104 |
| DW3-E | 80 | centrifugation 2800 rpm, 12 min | 178 | 159 | 128 |
| DW3-E | 100 | centrifugation 2800 rpm, 12 min | 137 | 129 | 110 |
| DW4-E | 60 | centrifugation 2800 rpm, 12 min | 44 | 37 | 36 |
| DW4-E | 80 | centrifugation 2800 rpm, 12 min | 89 | 86 | 42 |
| DW4-E | 100 | centrifugation 2800 rpm, 12 min | 99 | 99 | 44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajec, M.; Kukulska-Zając, E.; Król, A. Optimization of Silver Nanoparticle Separation Method from Drilling Waste Matrices. Energies 2021, 14, 1950. https://doi.org/10.3390/en14071950
Gajec M, Kukulska-Zając E, Król A. Optimization of Silver Nanoparticle Separation Method from Drilling Waste Matrices. Energies. 2021; 14(7):1950. https://doi.org/10.3390/en14071950
Chicago/Turabian StyleGajec, Monika, Ewa Kukulska-Zając, and Anna Król. 2021. "Optimization of Silver Nanoparticle Separation Method from Drilling Waste Matrices" Energies 14, no. 7: 1950. https://doi.org/10.3390/en14071950
APA StyleGajec, M., Kukulska-Zając, E., & Król, A. (2021). Optimization of Silver Nanoparticle Separation Method from Drilling Waste Matrices. Energies, 14(7), 1950. https://doi.org/10.3390/en14071950






