Subcritical Hydrothermal Co-Liquefaction of Process Rejects at a Wastepaper-Based Paper Mill with Waste Soybean Oil
Abstract
1. Introduction
2. Experiment Section
2.1. Materials
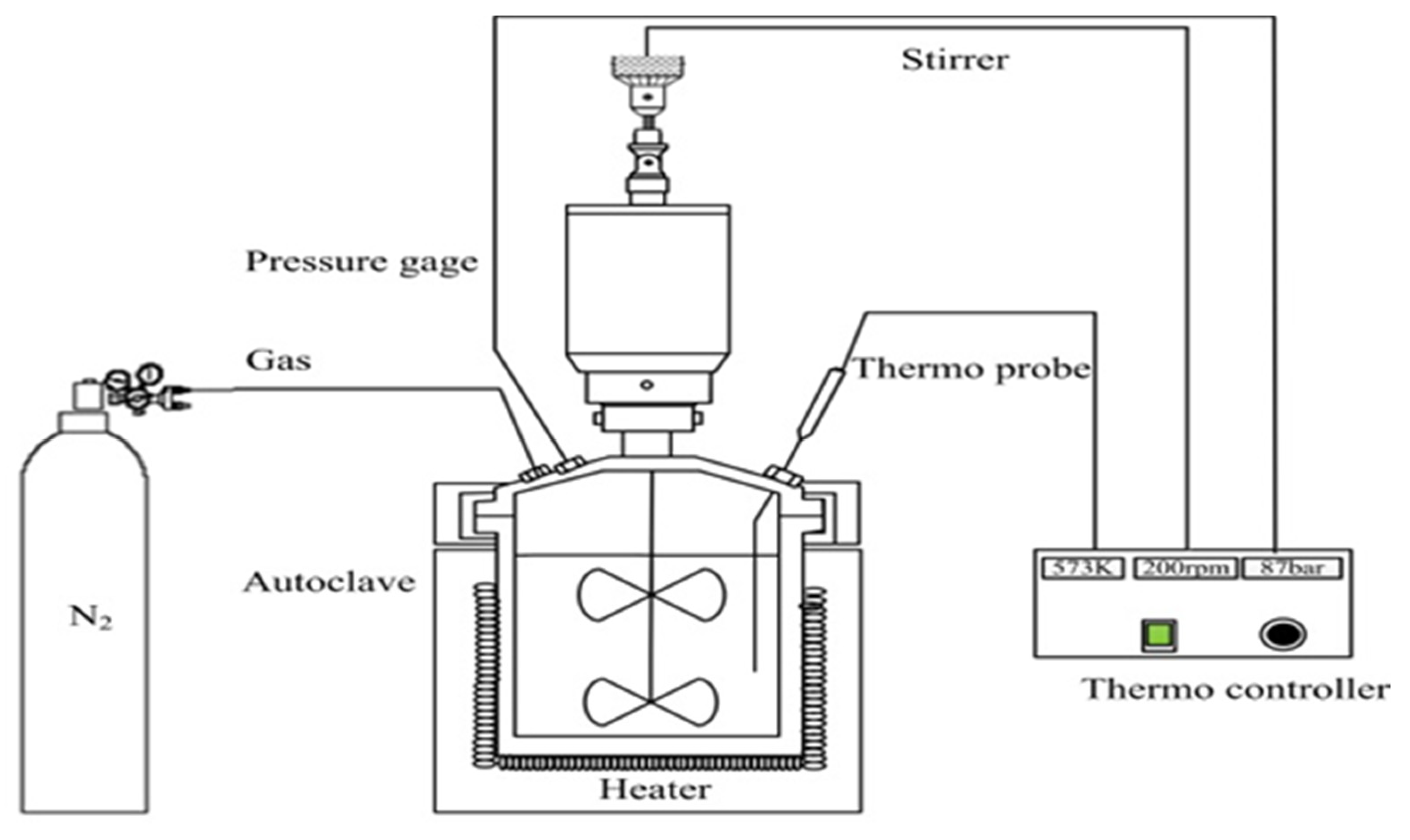
2.2. Experimental Equipment
2.3. Subcritical Hydrothermal Liquefaction (SHTL) Experiment
2.4. Subcritical Hydrothermal Liquefaction (SHTL) Experiment with WSO
2.5. Product Analysis
3. Results and Discussion
3.1. Characteristic Analysis of the Solid-Phase Products from SHTL with and without WSO
3.2. Elemental Analysis of Solid-Phase Products
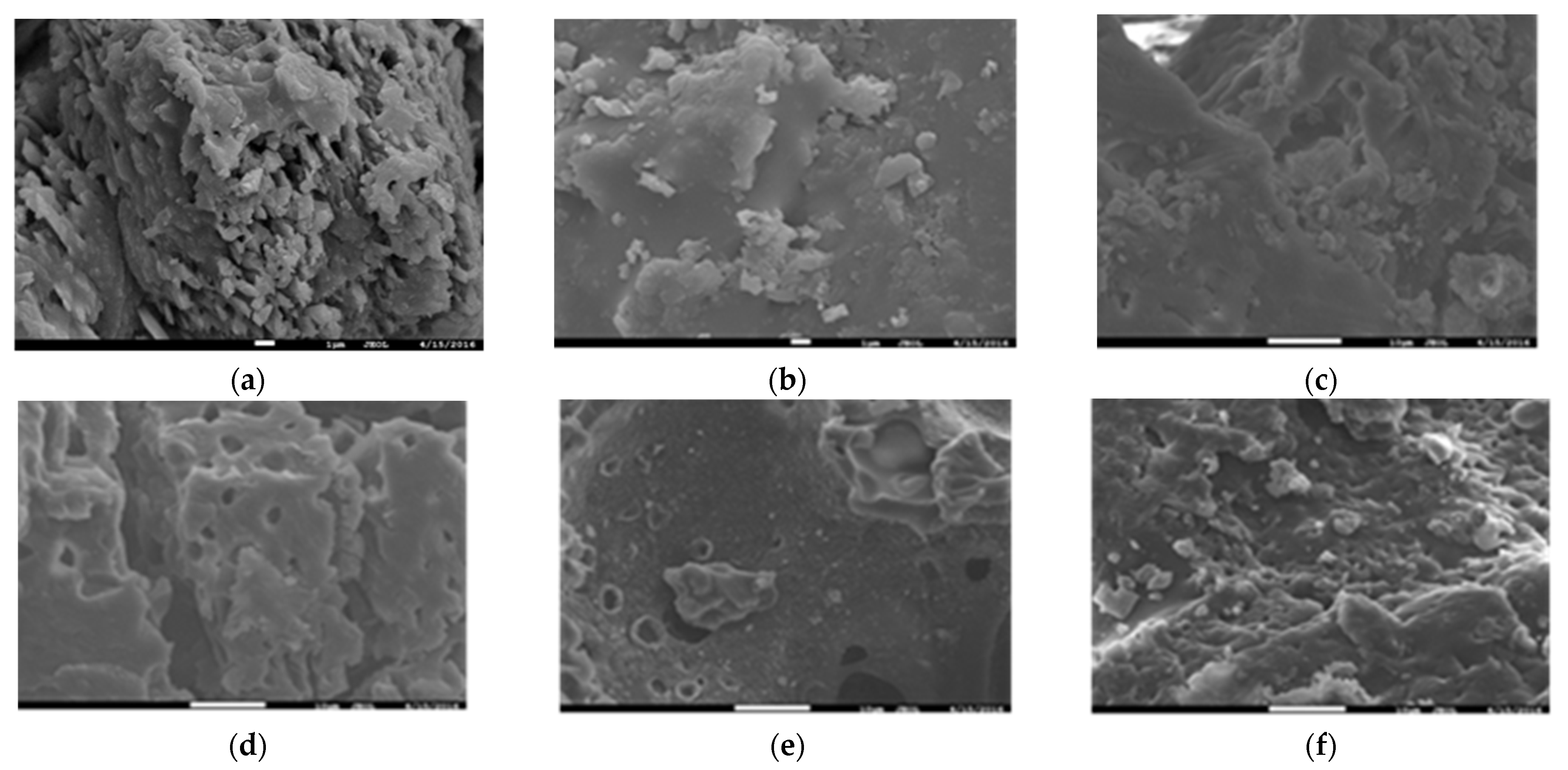
3.3. SEM Image of Solid Phase Products
3.4. Characteristics of Liquid-Phase Product
3.5. Elemental Analysis of Oil-Phase Products
3.6. Composition Analysis of Oil-Phase Products
3.7. Simulated Distillation Analysis of Oil-Phase Products
3.8. pH Value of Oil-Phase and Liquid-Phase Products
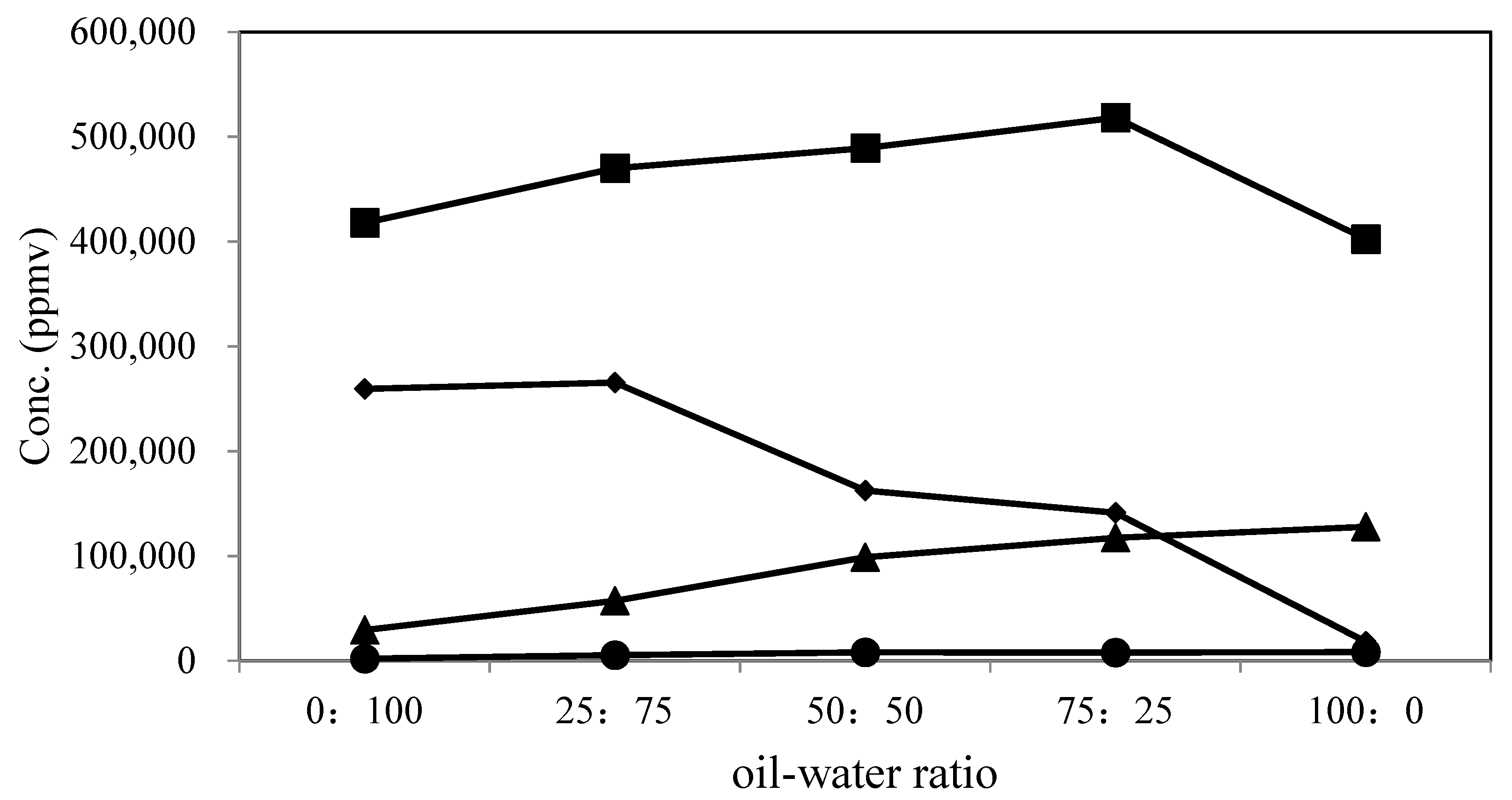
3.9. Gas-Phase Product Analysis
3.10. Yield and Conversion Rate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Setiawan, Y.; Surachman, A. Reject waste pellets of paper mills as fuel and their contribution to greenhouse gas (GHG). Int. J. Technol. 2015, 5, 847–855. [Google Scholar] [CrossRef]
- Zhu, Z.; Toor, S.S.; Rosendahl, L.; Yu, D.H.; Chen, G.Y. Influence of alkali catalyst on product yield and properties via hydrothermal liquefaction of barley straw. Energy 2015, 80, 284–292. [Google Scholar] [CrossRef]
- Brand, S.; Hardi, F.; Kim, J.; Suh, D.J. Effect of heating rate on biomass liquefaction: Differences between subcritical water and supercritical ethanol. Energy 2014, 68, 420–427. [Google Scholar] [CrossRef]
- Christensen, P.R.; Morup, A.J.; Mamakhel, A.; Glasius, M.; Becker, J.; Iversen, B.B. Effects of heterogeneous catalyst in hydrothermal liquefaction of dried distillers grains with solubles. Fuel 2014, 123, 158–166. [Google Scholar] [CrossRef]
- Chang, C.C.; Chen, C.P.; Yang, C.S.; Chen, Y.H.; Huang, M.; Chang, C.Y.; Shie, J.L.; Yuan, M.H.; Chen, Y.H.; Ho, C.; et al. Conversion of waste bamboo chopsticks to bio-oil via catalytic hydrothermal liquefaction using K2CO3. Sustain. Environ. Res. 2016, 26, 262–267. [Google Scholar] [CrossRef]
- King, J.W.; Holliday, R.L.; List, G.R. Hydrolysis of soybean oil in a subcritical water flow reactor. Green Chem. 1999, 1, 261–265. [Google Scholar] [CrossRef]
- Fangwei, C.; Luo, H.; Colosi, L.M. Slow pyrolysis as a platform for negative emissions technology: An integration of machine learning models, life cycle assessment, and economic analysis. Energy Convers. Manag. 2020, 223, 113258. [Google Scholar]
- Kruse, A.; Dinjus, E. Hot compressed water as reaction medium and reactant properties and synthesis reactions. J. Supercrit. Fluids 2007, 39, 362–380. [Google Scholar] [CrossRef]
- Uematsu, M.; Franck, E.U. Static dielectric constant of water and steam. J. Phys. Chem. Ref. Data 1980, 9, 1291–1306. [Google Scholar] [CrossRef]
- Mathanker, A.; Pudasainee, D.; Kumar, A.; Gupt, R. Hydrothermal liquefaction of lignocellulosic biomass feedstock to produce biofuels: Parametric study and products characterization. Fuel 2020, 271, 117534. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Williams, P.T. Hydrothermal gasification and oxidation as effective flameless conversion technologies for organic wastes. J. Energy Inst. 2008, 81, 102–109. [Google Scholar] [CrossRef]
- Tekin, K.; Karagöz, S. Non-catalytic and catalytic hydrothermal liquefaction of biomass. Res. Chem. Intermed. 2013, 39, 485–498. [Google Scholar] [CrossRef]
- Kayukova, G.P.; Mikhailova, A.N.; Kosachev, I.P.; Nasyrova, Z.R.; Gareev, B.I.; Vakhin, A.V. Catalytic hydrothermal conversion of heavy oil in the porous media. Energy Fuels 2021, 35, 1297–1307. [Google Scholar] [CrossRef]
- MacDermid-Watts, K.; Pradhan, R.; Dutta, A. Catalytic hydrothermal carbonization treatment of biomass for enhanced activated carbon: A review. Waste Biomass Valor 2020, 12, 2171–2186. [Google Scholar] [CrossRef]
- Mackintosh, A.F.; Shin, T.; Yang, H.; Choe, K. Hydrothermal polymerization catalytic process effect of various organic wastes on reaction time, yield, and temperature. Processes 2020, 8, 303. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, S.; Chauhan, P.K.; Verma, M.; Bahuguna, V.; Joshi, H.C.; Ahmad, W.; Negi, P.; Sharma, N.; Ramola, B.; et al. Low-temperature catalyst based hydrothermal liquefaction of harmful macroalgal blooms, and aqueous phase nutrient recycling by microalgae. Sci. Rep. 2019, 9, 11384. [Google Scholar] [CrossRef] [PubMed]
- Fangwei, C.; Porter, M.D.; Colosi, L.M. Is hydrothermal treatment coupled with carbon capture and storage an energy-producing negative emissions technology? Energy Convers. Manag. 2020, 203, 112252. [Google Scholar]
- Nam, H.; Choi, J.; Capareda, S.C. Comparative study of vacuum and fractional distillation using pyrolytic microalgae (Nannochloropsis oculata) bio-oil. Algal. Res. 2016, 17, 87–96. [Google Scholar] [CrossRef]
- Reddy, H.K.; Muppaneni, T.; Ponnusamy, S.; Sudasinghe, N.; Pegallapati, A.; Selvaratnam, T.; Seger, M.; Dungan, B.; Nirmalakhandan, N.; Schaub, T.; et al. Temperature effect on hydrothermal liquefaction of Nannochloropsis gaditana and Chlorella sp. Appl. Energy 2016, 165, 943–951. [Google Scholar] [CrossRef]
- Li, J.; Zhou, W.; Leng, L.; Yuan, X.Z. Beneficial synergistic effect on bio-oil production from co-liquefaction of sewage sludge and lignocellulosic biomass. Bioresour. Technol. 2017, 251, 49–56. [Google Scholar]
- Zhang, L.; Champagne, P.; Xu, C.C. Bio-crude production from secondary pulp/paper-mill sludge and waste newspaper via co-liquefaction in hot-compressed water. Energy 2021, 36, 2142–2150. [Google Scholar] [CrossRef]
- You, Y.D.; Shie, J.L.; Chang, C.Y.; Huang, S.H.; Pai, C.Y.; Chang, C.H.; Yu, Y.H. Economic cost analysis of biodiesel production: Case in soybean oil. Energy Fuels 2008, 22, 182–189. [Google Scholar] [CrossRef]
- Mehta, P.S.; Anand, K. Estimation of a lower heating value of vegetable oil and biodiesel fuel. Energy Fuels 2009, 23, 3893–3898. [Google Scholar] [CrossRef]
- Chang, C.C.; Chen, Y.H.; Lin, Y.S.; Hung, Z.S.; Yuan, M.H.; Chang, C.Y.; Li, Y.S.; Shie, J.L.; Chen, Y.H.; Wang, Y.C.; et al. A pilot plant study on the autoclaving of food wastes for resource recovery and reutilization. Sustainability 2018, 10, 3566. [Google Scholar] [CrossRef]
- Gollakotaa, A.R.K.; Kishoreb, N.; Gua, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Kong, X.; Liu, C.; Han, Y.; Lei, M.; Fan, Y.; Li, M.; Xiao, R. Cu/CuMgAlOx-Catalyzed guaiacol hydrodeoxygenation in supercritical methanol: A modeling mechanistic insight for lignin-derivatives upgrading. Energy Fuels 2020, 35, 1511–1522. [Google Scholar] [CrossRef]
- Brown, T.M.; Duan, P.; Savage, P.E. Hydrothermal liquefaction and gasification of Nannochloropsis sp. Energy Fuels 2010, 24, 3639–3646. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chang, C.C.; Chang, C.Y.; Yuan, M.H.; Ji, D.R.; Shie, J.L.; Lee, C.H.; Chen, Y.H.; Chang, W.R.; Yang, T.Y.; et al. Production of a solid biofuel from waste bamboo chopsticks by torrefaction for cofiring with coal. J. Anal. Appl. Pyrolysis 2017, 126, 315–322. [Google Scholar] [CrossRef]
- Chang, C.C.; Manh, V.D.; Hsu, W.L.; Liu, B.L.; Chang, C.Y.; Chen, Y.H.; Yuan, M.H.; Lin, C.F.; Yu, C.P.; Chen, Y.H.; et al. A case study on the electricity generation using a micro gas turbine fueled by biogas from a sewage treatment plant. Energies 2019, 12, 2424. [Google Scholar] [CrossRef]
- Chang, C.C.; Teng, S.; Yuan, M.H.; Ji, D.R.; Chang, C.Y.; Chen, Y.H.; Shie, J.L.; Ho, C.; Tian, S.Y.; Andrade-Tacca, C.A.D.; et al. Esterification of jatropha oil with isopropanol via ultrasonic irradiation. Energies 2018, 11, 1456. [Google Scholar] [CrossRef]
- Wu, X.F.; Zhou, Q.; Li, M.F.; Li, S.X.; Bian, J.; Peng, F. Conversion of poplar into bio-oil via subcritical hydrothermal liquefaction: Structure and antioxidant capacity. Bioresour. Technol. 2018, 270, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.D.; Lopez-Ruiz, J.A.; Flake, M.; Cooper, A.R.; Elkasabi, Y.; Morgano, M.T.; Dagle, V.L.; Albrecht, K.O.; Dagle, R.A. Cleanup and conversion of biomass liquefaction aqueous phase to C3–C5 olefins over ZnxZryOz catalyst. Catalysts 2019, 9, 923. [Google Scholar] [CrossRef]

| Item | PRWPM | WSO |
|---|---|---|
| Proximate analysis (%) | ||
| Moisture | 51.06 (0.74) a | - |
| Combustible | 45.04 (1.40) | - |
| Ash | 4.19 (1.0) | - |
| Fixed Carbon | 0.64 (0.02) | - |
| Heating value analysis (MJ kg−1) | ||
| High heating value of wet basis | 30.52 (0.35) | 40.03 (0.17) |
| Low heating value of wet basis | 14.94 (0.17) | 37.01 [21] |
| Ultimate analysis (dry basis, weight %) | ||
| C | 55.97 (0.20) | 78.69 (0.08) 77.56 [22] |
| H | 8.45 (0.01) | 11.65 (0.04) 13.22 [22] |
| N | 0.26 (0.03) | 0.13 (0.01) 0.025 [22] |
| S | 0.13 (0.01) | 0.02 (0.001) <0.0001 [22] |
| O b | 35.19 | 9.2 [15] |
| Sample | Temp. (K) | Time (Hour) | Oil-Water Ratio (Oil: Water) | MJ/kg | C | H | N | S | O a | Volatile | Fixed Carbon | Ash |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dry PRWPM | - | - | - | 30.52 (0.35) b | 55.97 (0.20) | 8.45 (0.01) | 0.26 (0.03) | 0.13 (0.01) | 35.19 | 90.32 (1.31) | 1.27 (0.04) | 8.4 (2.00) |
| Solid-phase products | 573 | 2 | 0:100 | 36.98 (2.43) | 80.68 (0.40) | 11.71 (0.55) | 0.38 (0.01) | 0.08 (0.01) | 7.15 | 92.42 (0.68) | 0.44 (0.05) | 7.14 (0.63) |
| 573 | 2 | 25:75 | 37.14 (0.17) | 82.67 (0.80) | 12.98 (0.95) | 0.13 (0.02) | 0.06 (0.02) | 4.16 | 98.13 (0.28) | 0.20 (0.49) | 1.67 (0.73) | |
| 573 | 2 | 50:50 | 37.23 (0.27) | 81.17 (0.13) | 13.50 (0.003) | 0.09 (0.01) | 0.03 (0.006) | 5.21 | 98.65 (0.10) | 0.39 (0.01) | 0.96 (0.10) | |
| 573 | 2 | 75:25 | 38.04 (0.29) | 79.64 (0.64) | 13.14 (0.20) | 0.11 (0.04) | 0.01 (0.002) | 7.10 | 98.77 (0.33) | 0.56 (0.03) | 0.67 (0.35) | |
| 573 | 2 | 100:0 | 33.85 (0.20) | 65.00 (0.35) | 6.79 (0.03) | 0.13 (0.01) | 0.05 (0.004) | 28.03 | 98.94 (1.40) | 0.34 (0.06) | 0.72 (0.06) |
| Sample | Oil-Water Ratio (Oil: Water) | Oil -Phase HHV MJ kg−1 | Oil-Phase pH | C | O b | H | N | S | Liquid-Phase pH |
|---|---|---|---|---|---|---|---|---|---|
| Raw WSO | - | 40.03 (0.17) a | - | 78.69 (0.08) | 9.51 | 11.65 (0.04) | 0.13 (0.01) | 0.02 (0.004) | - |
| oil-phase product | 0:100 | ND | ND | ND | ND | ND | ND | ND | 4.14 |
| 25:75 | 42.02 (0.25) | 4.85 | 63.04 (0.10) | 25.57 | 11.1 (0.05) | 0.21 (0.01) | 0.08 (0.01) | 4.09 | |
| 50:50 | 40.99 (0.28) | 4.8 | 72.37 (0.80) | 15.88 | 11.51 (0.02) | 0.19 (0.01) | 0.05 (0.003) | 4.24 | |
| 75:25 | 40.74 (0.46) | 5.0 | 73.8 (0.11) | 14.40 | 11.56 (0.01) | 0.19 (0.002) | 0.05 (0.003) | 4.48 | |
| 100:0 | 38.56 (0.76) | 4.8 | 78.21 (0.14) | 10.04 | 11.58 (0.004) | 0.15 (0.006) | 0.02 (0.008) | ND |
| Conc. (ppmv) | ||||||
|---|---|---|---|---|---|---|
| Sample | Oil-Water Ratio (Oil: Water) | CO2 | H2 | CO | CH4 | Total Volume Yield (L) |
| Gas-phase product | 0:100 | 417,918 (58.97) a | 259,497 (36.62) | 29,266 (4.13) | 1981 (0.28) | 2.1 |
| 25:75 | 469,868 (58.88) | 265,468 (33.27) | 57,179 (7.17) | 5517 (0.69) | 3 | |
| 50:50 | 489,036 (64.48) | 162,578 (21.44) | 98,751 (13.02) | 8020 (1.06) | 2.7 | |
| 75:25 | 518,159 (66.04) | 141,219 (18.00) | 117,362 (14.96) | 7928 (1.01) | 2.8 | |
| 100:0 | 402,315 (72.27) | 18,215 (3.27) | 127,890 (22.97) | 8260 (1.48) | 1.3 | |
| Oil-Water Ratio | Solid | Oil | Liquid | Gas | Yield | Conversion Rate |
|---|---|---|---|---|---|---|
| 0:100 | 5.86 | - | 88.33 | 0.42 | 94.60 | 35.56 |
| 25:75 | 7.23 | 21.41 | 67.53 | 0.65 | 96.82 | 20.43 |
| 50:50 | 4.67 | 40.62 | 44.09 | 0.67 | 90.05 | 48.63 |
| 75:25 | 4.97 | 62.08 | 20.99 | 0.80 | 88.83 | 45.33 |
| 100:0 | 0.57 | 97.32 | - | 0.28 | 98.16 | 93.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shie, J.-L.; Yang, W.-S.; Liau, Y.-R.; Liau, T.-H.; Yang, H.-R. Subcritical Hydrothermal Co-Liquefaction of Process Rejects at a Wastepaper-Based Paper Mill with Waste Soybean Oil. Energies 2021, 14, 2442. https://doi.org/10.3390/en14092442
Shie J-L, Yang W-S, Liau Y-R, Liau T-H, Yang H-R. Subcritical Hydrothermal Co-Liquefaction of Process Rejects at a Wastepaper-Based Paper Mill with Waste Soybean Oil. Energies. 2021; 14(9):2442. https://doi.org/10.3390/en14092442
Chicago/Turabian StyleShie, Je-Lueng, Wei-Sheng Yang, Yi-Ru Liau, Tian-Hui Liau, and Hong-Ren Yang. 2021. "Subcritical Hydrothermal Co-Liquefaction of Process Rejects at a Wastepaper-Based Paper Mill with Waste Soybean Oil" Energies 14, no. 9: 2442. https://doi.org/10.3390/en14092442
APA StyleShie, J.-L., Yang, W.-S., Liau, Y.-R., Liau, T.-H., & Yang, H.-R. (2021). Subcritical Hydrothermal Co-Liquefaction of Process Rejects at a Wastepaper-Based Paper Mill with Waste Soybean Oil. Energies, 14(9), 2442. https://doi.org/10.3390/en14092442





