Experimental Study on Mechanical Properties of Hydrate-Bearing Sand: The Influence of Sand-Water Mixing Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Low-Field NMR
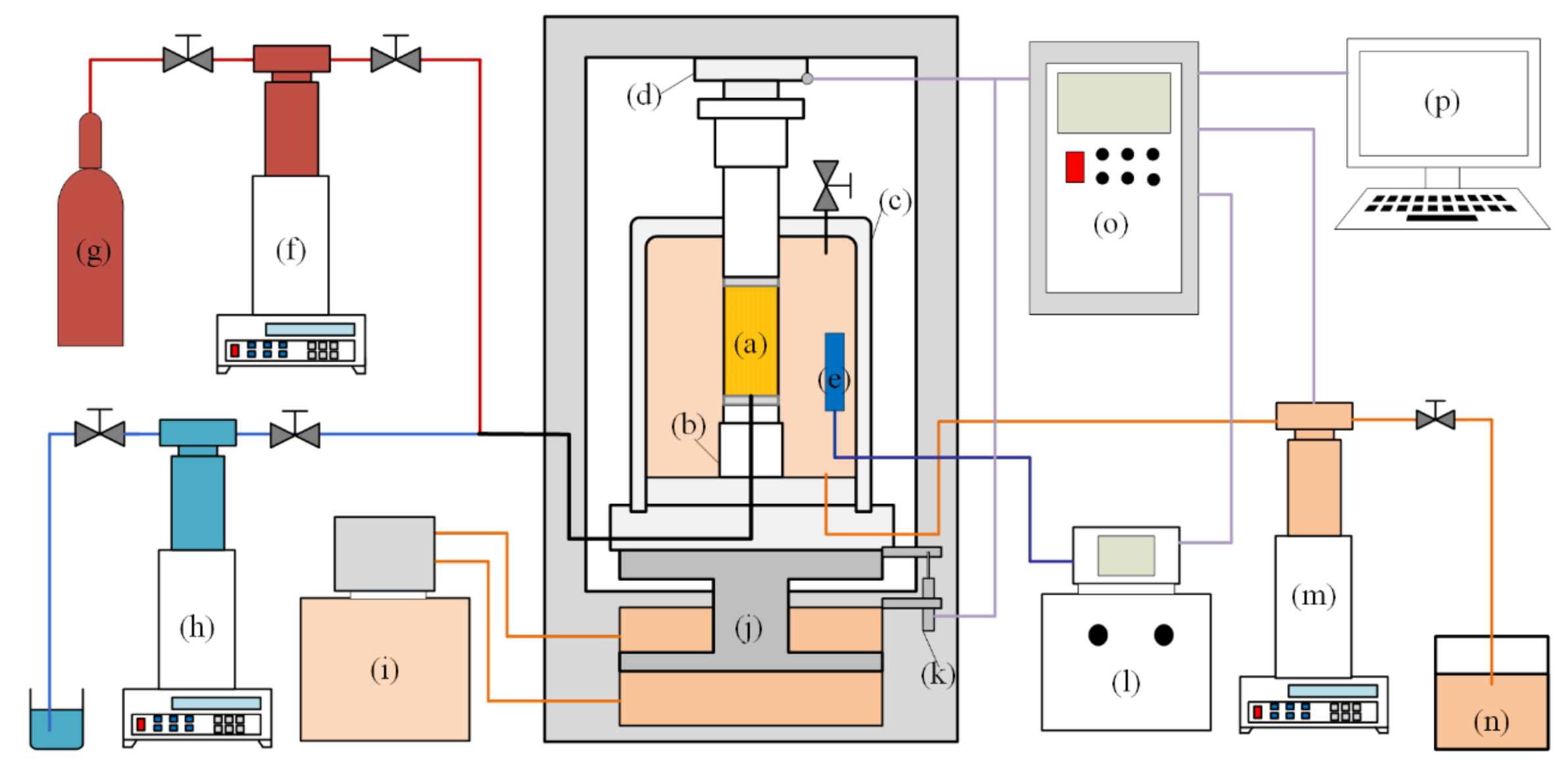
2.2.2. Triaxial Test Apparatus
2.2.3. Drained Triaxial Test
3. Experimental Results
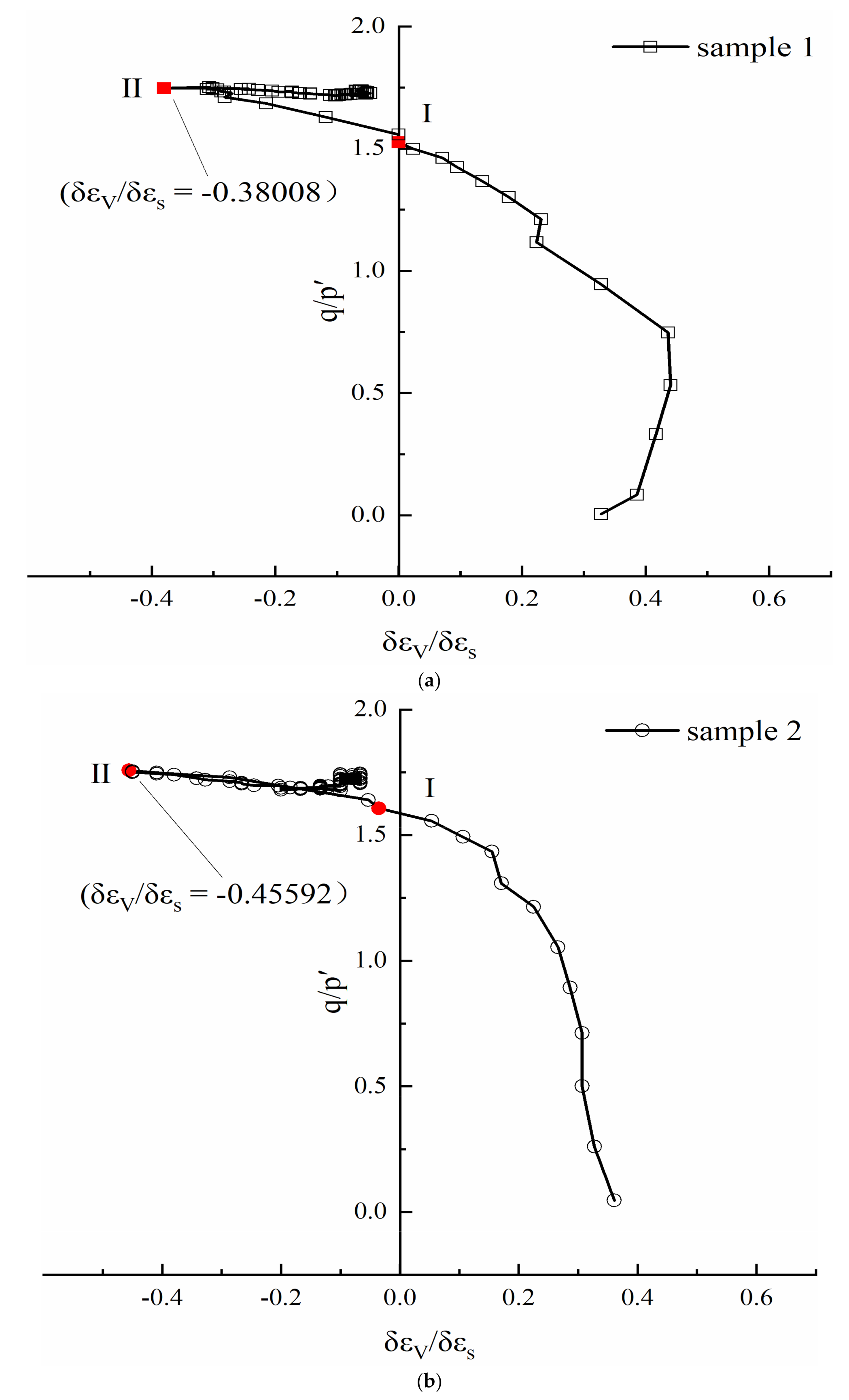
3.1. Stress-Strain Relationship and Dilatancy
3.2. Measurement of Saturation Profile
4. Discussion
5. Conclusions
- Most of the water of sample 1 concentrates in the middle section, and the water content of sample 3 distributes mainly at the bottom. Nevertheless, water in sample 2 distributes uniformly. The reason for water’s uneven distribution is that liquid water would show a downward movement driven by gravity. The hydrate distribution habit would be determined by water (ice) distribution before hydrate formation.
- Compared with the sample with the evenly distributed hydrate, the sample with unevenly distributed hydrate shows a lower initial modulus of elasticity and lower strength. Furthermore, for these two kinds of host-sand-and-water-mixture prepared samples, the sample with evenly distributed hydrate shows strain-softening behavior, while the sample with unevenly distributed hydrate shows strain-hardening behavior. The peak strength of the sample with evenly distributed hydrate is higher than the 15% strain strength of the sample with unevenly distributed hydrate. During the shearing process with increasing strain, the stress of the sample with unevenly distributed hydrate is higher than that of the sample with evenly distributed hydrate.
- All three samples show the characteristic of contraction at first, and then dilation. Sample 2 has the largest dilation, and sample 3 has the smallest one.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HBS | hydrate-bearing sediments |
| NMR | nuclear magnetic resonance |
| THF | tetrahydrofuran |
| hydrate saturation | |
| volume of hydrate | |
| pore volume of the host sand | |
| mass of the hydrate | |
| density of the hydrate | |
| volume of the methane gas consumed by the samples | |
| molar mass of methane hydrate | |
| P | hydrate formation pressure |
| T | hydrate formation temperature |
| Z | compressibility factor |
| R | gas constant |
| GIT | Green Imaging Technologies |
| LVDT | linear variable displacement transducer |
| DHK | double half-k-space |
| SPRITE | single-point ramped imaging with T1 enhancement |
References
- Boswell, R. Is Gas Hydrate Energy Within Reach? Science 2009, 325, 957–958. [Google Scholar] [CrossRef] [PubMed]
- Sloan, E.D. Fundamental principles and applications of natural gas hydrates. Nature 2003, 426, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Dawe, R.A.; Thomas, S. A Large Potential Methane Source—Natural Gas Hydrates. Energy Sources Part A Recover. Util. Environ. Eff. 2007, 29, 217–229. [Google Scholar] [CrossRef]
- Milkov, A.V. Global estimates of hydrate-bound gas in marine sediments: How much is really out there? Earth-Sci. Rev. 2004, 66, 183–197. [Google Scholar] [CrossRef]
- Englezos, P. Clathrate hydrates. Ind. Eng. Chem. Res. 1993, 32, 1251–1274. [Google Scholar] [CrossRef]
- Li, X.-S.; Xu, C.-G.; Zhang, Y.; Ruan, X.-K.; Li, G.; Wang, Y. Investigation into gas production from natural gas hydrate: A review. Appl. Energy 2016, 172, 286–322. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Liu, W.; Lang, C.; Liu, R.; Song, Y.; Li, Y. Viscosity investigation on metastable hydrate suspension in oil-dominated systems. Chem. Eng. Sci. 2021, 238, 116608. [Google Scholar] [CrossRef]
- Liu, Z.; Song, Y.; Liu, W.; Liu, R.; Lang, C.; Li, Y. Rheology of methane hydrate slurries formed from water-in-oil emulsion with different surfactants concentrations. Fuel 2020, 275, 117961. [Google Scholar] [CrossRef]
- Yang, M.; Zhao, J.; Zheng, J.; Song, Y. Hydrate reformation characteristics in natural gas hydrate dissociation process: A review. Appl. Energy 2019, 256, 113878. [Google Scholar] [CrossRef]
- Yousif, M.H.; Li, P.M.; Selim, M.S.; Sloan, E.D. Depressurization of natural gas hydrates in berea sandstone cores. J. Incl. Phenom. Mol. Recognit. Chem. 1990, 8, 71–88. [Google Scholar] [CrossRef]
- Kim, H.C.; Bishnoi, P.R.; Heidemann, R.A.; Rizvi, S.S.H. Kinetics of methane hydrate decomposition. Chem. Eng. Sci. 1987, 42, 1645–1653. [Google Scholar] [CrossRef]
- Ohgaki, K.; Takano, K.; Sangawa, H.; Matsubara, T.; Nakano, S. Methane Exploitation by Carbon Dioxide from Gas Hydrates—Phase Equilibria for CO2-CH4 Mixed Hydrate System. J. Chem. Eng. Jpn. 1996, 29, 478–483. [Google Scholar] [CrossRef] [Green Version]
- Glasby, G.P. Potential impact on climate of the exploitation of methane hydrate deposits offshore. Mar. Pet. Geol. 2003, 20, 163–175. [Google Scholar] [CrossRef]
- Rabbani, E.; Davarpanah, A.; Memariani, M. An experimental study of acidizing operation performances on the wellbore productivity index enhancement. J. Pet. Explor. Prod. Technol. 2018, 8, 1243–1253. [Google Scholar] [CrossRef] [Green Version]
- Yun, T.S.; Santamarina, C.J.; Ruppel, C. Mechanical properties of sand, silt, and clay containing tetrahydrofuran hydrate. J. Geophys. Res. Solid Earth 2007, 112, 1–13. [Google Scholar] [CrossRef]
- Wu, P.; Li, Y.; Sun, X.; Liu, W.; Song, Y. Mechanical Characteristics of Hydrate-Bearing Sediment: A Review. Energy Fuels 2020, 35, 1041–1057. [Google Scholar] [CrossRef]
- Hyodo, M.; Yoneda, J.; Yoshimoto, N.; Nakata, Y. Mechanical and dissociation properties of methane hydrate-bearing sand in deep seabed. Soils Found. 2013, 53, 299–314. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, Y.; Wu, P.; Shen, S.; Liu, T.; Leng, S.; Chang, Y.; Zhao, J. Physical and mechanical properties of the overburden layer on gas hydrate-bearing sediments of the South China sea. J. Pet. Sci. Eng. 2020, 189, 107020. [Google Scholar] [CrossRef]
- Wu, P.; Li, Y.; Sun, X.; Liu, W.; Song, Y. Pore-Scale 3D Morphological Modeling and Physical Characterization of Hydrate-Bearing Sediment Based on Computed Tomography. J. Geophys. Res. Solid Earth 2020, 125. [Google Scholar] [CrossRef]
- Winters, W.J.; Pecher, I.A.; Waite, W.F.; Mason, D.H. Physical properties and rock physics models of sediment containing natural and laboratory-formed methane gas hydrate. Am. Mineral. 2004, 89, 1221–1227. [Google Scholar] [CrossRef]
- Waite, W.F.; Santamarina, J.C.; Cortes, D.D.; Dugan, B.; Espinoza, D.N.; Germaine, J.; Jang, J.; Jung, J.W.; Kneafsey, T.J.; Shin, H.; et al. Physical properties of hydrate-bearing sediments. Rev. Geophys. 2009, 47, RG4003. [Google Scholar] [CrossRef]
- Stern, L.A.; Kirby, S.H.; Durham, W.B. Polycrystalline methane hydrate: Synthesis from superheated ice, and low-temperature mechanical properties. Energy Fuels 1998, 12, 201–211. [Google Scholar] [CrossRef]
- Zhou, M.; Soga, K.; Yamamoto, K. Upscaled Anisotropic Methane Hydrate Critical State Model for Turbidite Hydrate-Bearing Sediments at East Nankai Trough. J. Geophys. Res. Solid Earth 2018, 123, 6277–6298. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Kou, X.; Wang, Y.; Li, L. Analysis of Hydrate Heterogeneous Distribution Effects on Mechanical Characteristics of Hydrate-Bearing Sediments. Energy Fuels 2021, 35. [Google Scholar] [CrossRef]
- Ma, L.; Qi, J.; Yu, F.; Yao, X. Experimental study on variability in mechanical properties of a frozen sand as determined in triaxial compression tests. Acta Geotech. 2016, 11, 61–70. [Google Scholar] [CrossRef]
- Gao, S.; House, W.; Chapman, W.G. NMR/MRI Study of Clathrate Hydrate Mechanisms. J. Phys. Chem. B 2005, 109, 19090–19093. [Google Scholar] [CrossRef]
- Salahi, A.; Dehghan, A.N.; Sheikhzakariaee, S.J.; Davarpanah, A. Sand production control mechanisms during oil well production and construction. Pet. Res. 2021. [Google Scholar] [CrossRef]
- Shen, S.; Sun, X.; Wang, L.; Song, Y.; Li, Y. Effect of temperature on the mechanical properties of hydrate-bearing sand under different confining pressures. Energy Fuels 2021, 35, 4106–4117. [Google Scholar] [CrossRef]
- Kleinberg, R.L.; Flaum, C.; Griffin, D.D.; Brewer, P.G.; Malby, G.E.; Peltzer, E.T.; Yesinowski, J.P. Deep sea NMR: Methane hydrate growth habit in porous media and its relationship to hydraulic permeability, deposit accumulation, and submarine slope stability. J. Geophys. Res. Solid Earth 2003, 108. [Google Scholar] [CrossRef]
- Shakerian, M.; Afrough, A.; Vashaee, S.; Marica, F.; Balcom, B.J. Monitoring gas hydrate formation with magnetic resonance imaging in a metallic core holder. E3S Web Conf. 2019, 89, 2008. [Google Scholar] [CrossRef]
- Kajiyama, S.; Hyodo, M.; Nishimura, A. Mechanical characteristics and localized deformation of Methane Hydrate-bearing sand using high pressure plane strain shear tests. Jpn. Geotech. Soc. Spec. Publ. 2016, 2, 2549–2552. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liu, C.; Liu, L.; Sun, J.; Liu, H.; Meng, Q. Experimental study on evolution behaviors of triaxial-shearing parameters for hydrate-bearing intermediate fine sediment. Adv. Geo-Energy Res. 2018, 2, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, K.; Masui, A.; Sakamoto, Y.; Aoki, K.; Tenma, N.; Yamaguchi, T. Triaxial compressive properties of artificial methane-hydrate-bearing sediment. J. Geophys. Res. Solid Earth 2011, 116, 1–11. [Google Scholar] [CrossRef]
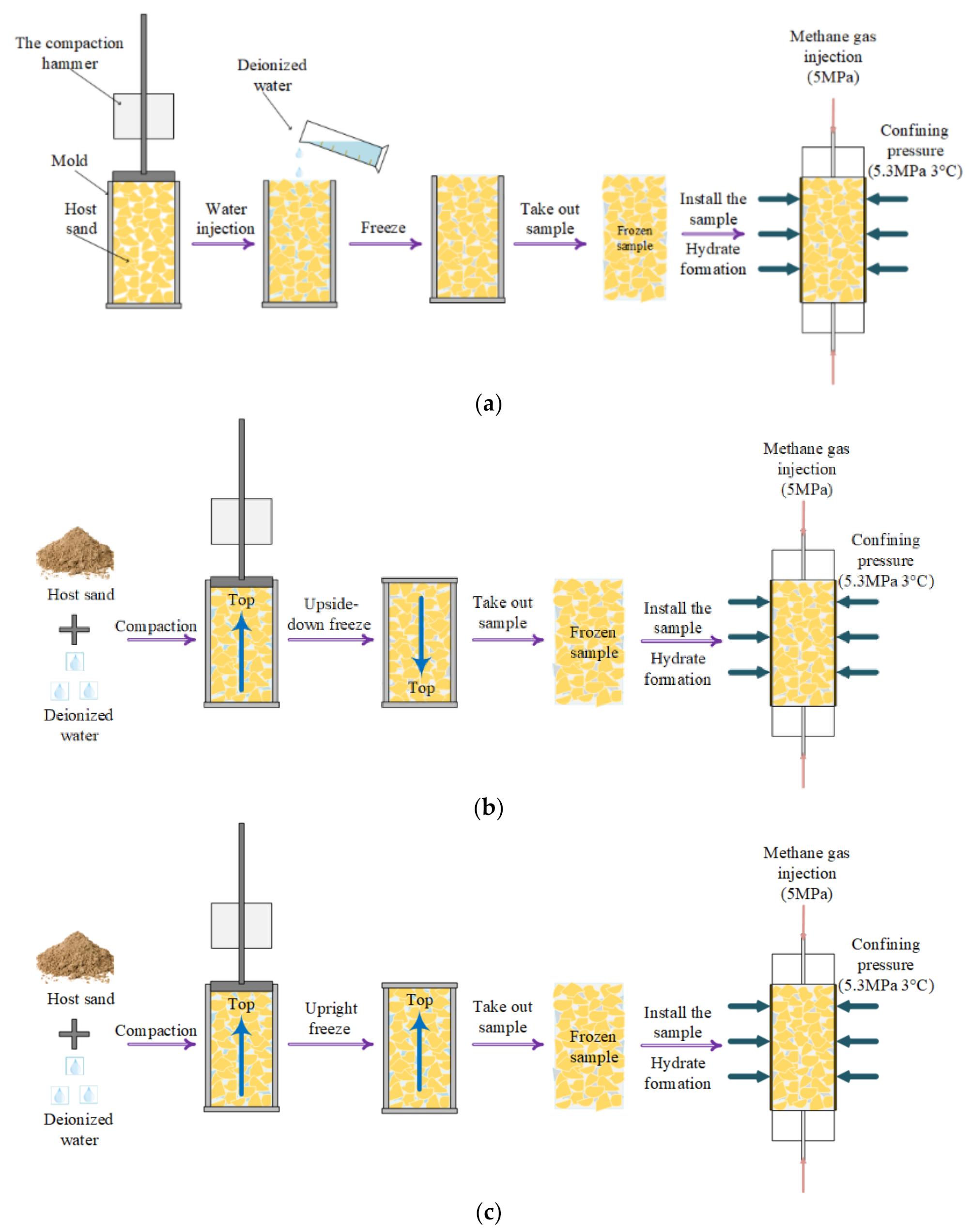





| Sand–Water Mixing Method | Method 1 | Method 2 | Method 3 |
|---|---|---|---|
| Initial elastic modulus (100 MPa) | 5.724 | 7.168 | 5.466 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Pan, D.; Shen, S.; You, Z.; Zhao, Y.; Sun, X. Experimental Study on Mechanical Properties of Hydrate-Bearing Sand: The Influence of Sand-Water Mixing Methods. Energies 2021, 14, 2554. https://doi.org/10.3390/en14092554
Liu W, Pan D, Shen S, You Z, Zhao Y, Sun X. Experimental Study on Mechanical Properties of Hydrate-Bearing Sand: The Influence of Sand-Water Mixing Methods. Energies. 2021; 14(9):2554. https://doi.org/10.3390/en14092554
Chicago/Turabian StyleLiu, Weiguo, Dedong Pan, Shi Shen, Zeshao You, Yuechao Zhao, and Xiang Sun. 2021. "Experimental Study on Mechanical Properties of Hydrate-Bearing Sand: The Influence of Sand-Water Mixing Methods" Energies 14, no. 9: 2554. https://doi.org/10.3390/en14092554






