Compatibility of 3D-Printed Oxide Ceramics with Molten Chloride Salts for High-Temperature Thermal Energy Storage in Next-Generation CSP Plants
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and 3D-Printed Ceramics
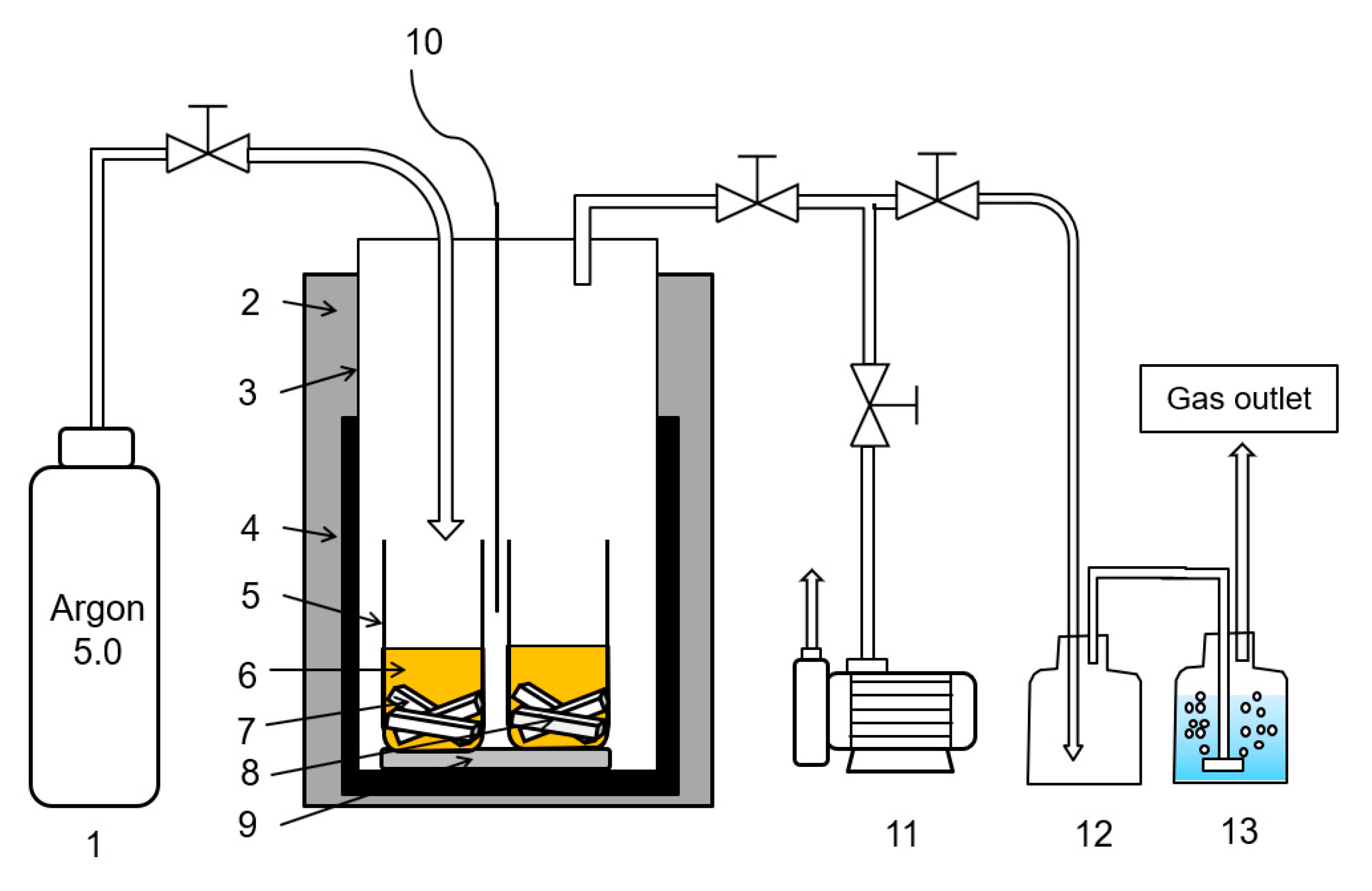
2.2. High-Temperature Tests
2.3. Corrosion and Mechanical Analysis
2.3.1. Mechanical Tests
2.3.2. Material Analysis
3. Results and Discussion
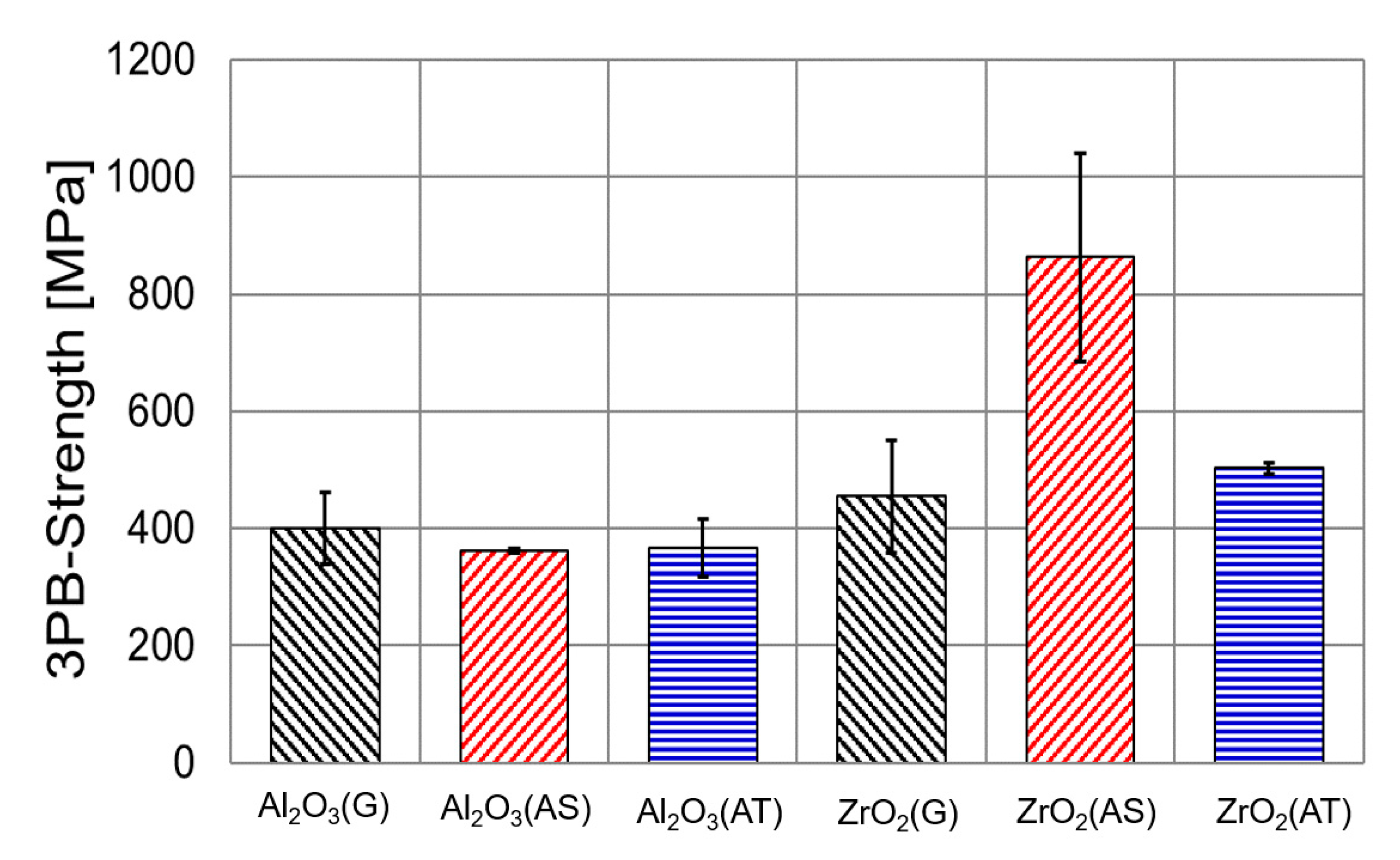
3.1. Mechanical Tests
3.2. Material Analysis
3.2.1. XRD Analysis
3.2.2. SEM-EDX Analysis
4. Conclusions
- The 3D-printed oxide ceramics (ZrO2, Al2O3) showed good compatibility in molten chlorides (i.e., corrosion resistance in molten chlorides).
- 3D-printed Al2O3 maintained its mechanical properties after exposure in the strongly corrosive molten chloride salt. At 700 °C for 600 h, no indication of reaction or phase change (XRD) between the molten chloride salt and the 3D-printed Al2O3 was observed.
- Mechanical tests showed that the 3D-printed ZrO2 had enhanced mechanical stability after exposure in molten chlorides. Material tests (XRD and SEM-EDX) showed that the 3D-printed ZrO2 on the surface changed its crystal structure and shape after exposure in the molten chloride salt due to T→M phase transformation, which may be the reason for the enhanced mechanical properties.
- The exposure tests of the 3D-printed ceramics in Ar without molten salt at 700 °C for 600 h showed no mechanical strength changes. Thus, the change of ZrO2 crystal structure and shape is attributed to the molten chloride salt investigated in this work. The thermodynamic calculation shows that the reactions of the Y2O3 and ZrO2 with gaseous MgCl2 could be the main reason for the phase transformation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehos, M.; Turchi, C.; Vidal, J.; Wagner, M.; Ma, Z.; Ho, C.; Kolb, W.; Andraka, C.; Kruizenga, A. Concentrating Solar Power Gen3 Demonstration Roadmap (Technical Report: NREL/TP-5500-67464); National Renewable Energy Laboratory: Golden, CO, USA, 2017.
- Guo, L.; Liu, Q.; Yin, H.; Pana, T.J.; Tang, Z. Excellent corrosion resistance of 316 stainless steel in purified NaCl-MgCl2 eutectic salt at high temperature. Corr. Sci. 2020, 166, 108473. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, P.; Wang, J. Effects of alloying elements on the corrosion behavior of Ni-based alloys in molten NaCl-KCl-MgCl2 salt at different temperatures. Corr. Sci. 2018, 143, 187–199. [Google Scholar] [CrossRef]
- Grégoire, B.; Oskay, C.; Meißner, T.M.; Galetz, M.C. Corrosion mechanisms of ferritic-martensitic P91 steel and Inconel 600 nickel-based alloy in molten chlorides. Part II: NaCl-KCl-MgCl2 ternary system. Sol. Energy Mater. Sol. Cells 2020, 216, 110675. [Google Scholar] [CrossRef]
- Ding, W.; Shi, H.; Xiu, Y.; Bonk, A.; Weisenburger, A.; Jianu, A.; Bauer, T. Hot corrosion behavior of commercial alloys in thermal energy storage material of molten MgCl2/KCl/NaCl under inert atmosphere. Sol. Energy Mater. Sol. Cells 2018, 184, 22–30. [Google Scholar] [CrossRef]
- Ding, W.; Bonk, A.; Bauer, T. Corrosion behavior of metallic alloys in molten chloride salts for thermal energy storage in concentrated solar power plants—A review. Front. Chem. Sci. Eng. 2018, 12, 564–576. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Klammer, N.; Vidal, J. Purification strategy and effect of impurities on corrosivity of dehydrated carnallite for thermal solar applications. RSC Adv. 2019, 9, 41664–41671. [Google Scholar] [CrossRef] [Green Version]
- Schulte-Fischedick, J.; Dreißigacker, V.; Tamme, R. An innovative ceramic high temperature plate-fin heat exchanger for EFCC processes. Appl. Therm. Eng. 2007, 27, 1285–1294. [Google Scholar] [CrossRef]
- Van Loo, K.; Lapauw, T.; Ozalp, N.; Ström, E.; Lambrinou, K.; Vleugels, J. Compatibility of SiC- and MAX phase based ceramics with a KNO3-NaNO3 molten solar salt. Sol. Energy Mater. Sol. Cells 2019, 195, 228–240. [Google Scholar] [CrossRef]
- Olson, L.C.; Fuentes, R.E.; Martinez-Rodrigueza, M.J.; Ambrosek, J.W.; Sridharan, K.; Anderson, M.H.; Garcia-Diaz, B.L.; Gray, J.; Allen, T. Impact of corrosion test container material in molten fluorides. J. Sol. Energy Eng. 2015, 137, 061007. [Google Scholar] [CrossRef]
- Jagadeeswara Rao, C.; Venkatesh, P.; Prabhakara Reddy, B.; Ningshen, S.; Mallika, C.; Kamachi Mudali, U. Corrosion behavior of Yttria-stabilized Zirconia-coated 9Cr-1Mo steel in molten UCl3-LiCl-KCl salt. J. Therm. Spray Tech. 2017, 26, 569–580. [Google Scholar] [CrossRef]
- Ravi Shankar, A.; Kamachi Mudali, U.; Sole, R.; Khatak, H.S.; Raj, B. Plasma-sprayed yttria-stabilized zirconia coatings on type 316L stainless steel for pyrochemical reprocessing plant. J. Nucl. Mater. 2008, 372, 226–232. [Google Scholar] [CrossRef]
- Vidal, J.; Klammer, N. Molten Chloride technology pathway to meet the U.S. DOE SunShot initiative with Gen3 CSP. AIP Conf. Proc. 2019, 2126, 080006. [Google Scholar]
- He, X.; Robb, K.R.; Sulejmanovic, D.; Keiser, J.R.; Qu, J. Effects of particle size and concentration of magnesium oxide on the lubricating performance of a chloride molten salt for concentrating solar power. ACS Sustain. Chem. Eng. 2021, in press. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Li, J.; Liu, C.; Lao, C.; Fu, Y.; Liu, C.; Li, Y.; Wang, P.; He, Y. 3D printing of ceramics: A review. J. Eur. Ceram. Soc. 2019, 39, 661–687. [Google Scholar] [CrossRef]
- Ding, W.; Shi, Y.; Kessel, F.; Koch, D.; Bauer, T. Characterization of corrosion resistance of C/C–SiC composite in molten chloride mixture MgCl2/NaCl/KCl at 700 °C. NPJ Mater. Degrad. 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Scheithauer, U.; Schwarzer, E.; Moritz, T.; Michaelis, A. Additive Manufacturing of Ceramic Heat Exchanger: Opportunities and Limits of the Lithography-Based Ceramic Manufacturing (LCM). J. Mater. Eng. Perform. 2018, 27, 14–20. [Google Scholar] [CrossRef]
- Liang, Z.Y.; Tang, B.; Gui, Y.; Zhao, Q.X. Corrosion resistance of 3D-printed and cold-rolled titanium alloys at 600 °C in air and air-SO2 environments, Mater. Today Commun. 2020, 24, 101055. [Google Scholar]
- LITHOZ, LCM Technology Material Overview. Available online: https://www.lithoz.com/application/files/2315/5197/6789/2019_1_Materialfolder_EN_Print-1.pdf (accessed on 7 October 2020).
- DIN EN 843-1: 2008-08 Beuth.de: Advanced Technical Ceramics—Mechanical Properties of Monolithic Ceramics at Room Temperature—Part 1: Determination of Flexural Strength; Deutsches Institut fur Normung E.V. (DIN): Berlin, Germany, 2008.
- Ding, W.; Shi, H.; Xiu, Y.; Bonk, A.; Weisenburger, A.; Jianu, A.; Bauer, T. Molten chloride salts for high-temperature thermal energy storage: Mitigation strategies against corrosion of structural materials. Sol. Energy Mater. Sol. Cells 2019, 193, 298–313. [Google Scholar] [CrossRef]
- Gomez-Vidal, J.C.; Fernandez, A.G.; Tirawat, R.; Turchi, C.; Huddleston, W. Corrosion resistance of alumina-forming alloys against molten chlorides for energy production. II: Pre-oxidation treatment and isothermal corrosion tests. Sol. Energy Mater. Sol. Cells 2017, 166, 222–233. [Google Scholar] [CrossRef] [Green Version]
- Mulaudzi, M.G. Ab Initio Study of Structural Stability and Electronic Properties of ZrO2-xSx for 0 ≤ x ≤ 2. Ph.D. Thesis, University of Limpopo, Mankweng, South Africa, 2015. [Google Scholar]
- Swab, J.J. Role of Oxide Additives in Stabilizing Zirconia for Coating Applications; Report ARL-TR-2591; Army Research Laboratory: Adelphi, MD, USA, 2001. [Google Scholar]
- Scott, H.G. Phase relations in the Zirconia-Yttria system. J. Mater. Sci. 1975, 10, 1527–1535. [Google Scholar] [CrossRef]
- Wang, X.; Rincon, J.D.; Li, P.; Zhao, Y.; Vidal, J. Thermophysical properties experimentally tested for NaCl-KCl-MgCl2 eutectic molten salt as a next-generation high-temperature heat transfer fluids in concentrated solar power systems. ASME J. Sol. Energy Eng. 2021, 143, 041005. [Google Scholar] [CrossRef]
- Kipouros, G.J.; Sadoway, D.R. A thermochemical analysis of the production of anhydrous MgCl2. J. Light Met. 2001, 1, 111–117. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, W.; Shi, Y.; Braun, M.; Kessel, F.; Frieß, M.; Bonk, A.; Bauer, T. Compatibility of 3D-Printed Oxide Ceramics with Molten Chloride Salts for High-Temperature Thermal Energy Storage in Next-Generation CSP Plants. Energies 2021, 14, 2599. https://doi.org/10.3390/en14092599
Ding W, Shi Y, Braun M, Kessel F, Frieß M, Bonk A, Bauer T. Compatibility of 3D-Printed Oxide Ceramics with Molten Chloride Salts for High-Temperature Thermal Energy Storage in Next-Generation CSP Plants. Energies. 2021; 14(9):2599. https://doi.org/10.3390/en14092599
Chicago/Turabian StyleDing, Wenjin, Yuan Shi, Markus Braun, Fiona Kessel, Martin Frieß, Alexander Bonk, and Thomas Bauer. 2021. "Compatibility of 3D-Printed Oxide Ceramics with Molten Chloride Salts for High-Temperature Thermal Energy Storage in Next-Generation CSP Plants" Energies 14, no. 9: 2599. https://doi.org/10.3390/en14092599
APA StyleDing, W., Shi, Y., Braun, M., Kessel, F., Frieß, M., Bonk, A., & Bauer, T. (2021). Compatibility of 3D-Printed Oxide Ceramics with Molten Chloride Salts for High-Temperature Thermal Energy Storage in Next-Generation CSP Plants. Energies, 14(9), 2599. https://doi.org/10.3390/en14092599






