Use of Hydrogen as Fuel: A Trend of the 21st Century
Abstract
:1. Introduction
2. Hydrogen as a Sustainable Energy Source
2.1. Physical Properties of Hydrogen
2.2. Chemical Properties of Hydrogen
3. International Standardization of Hydrogen
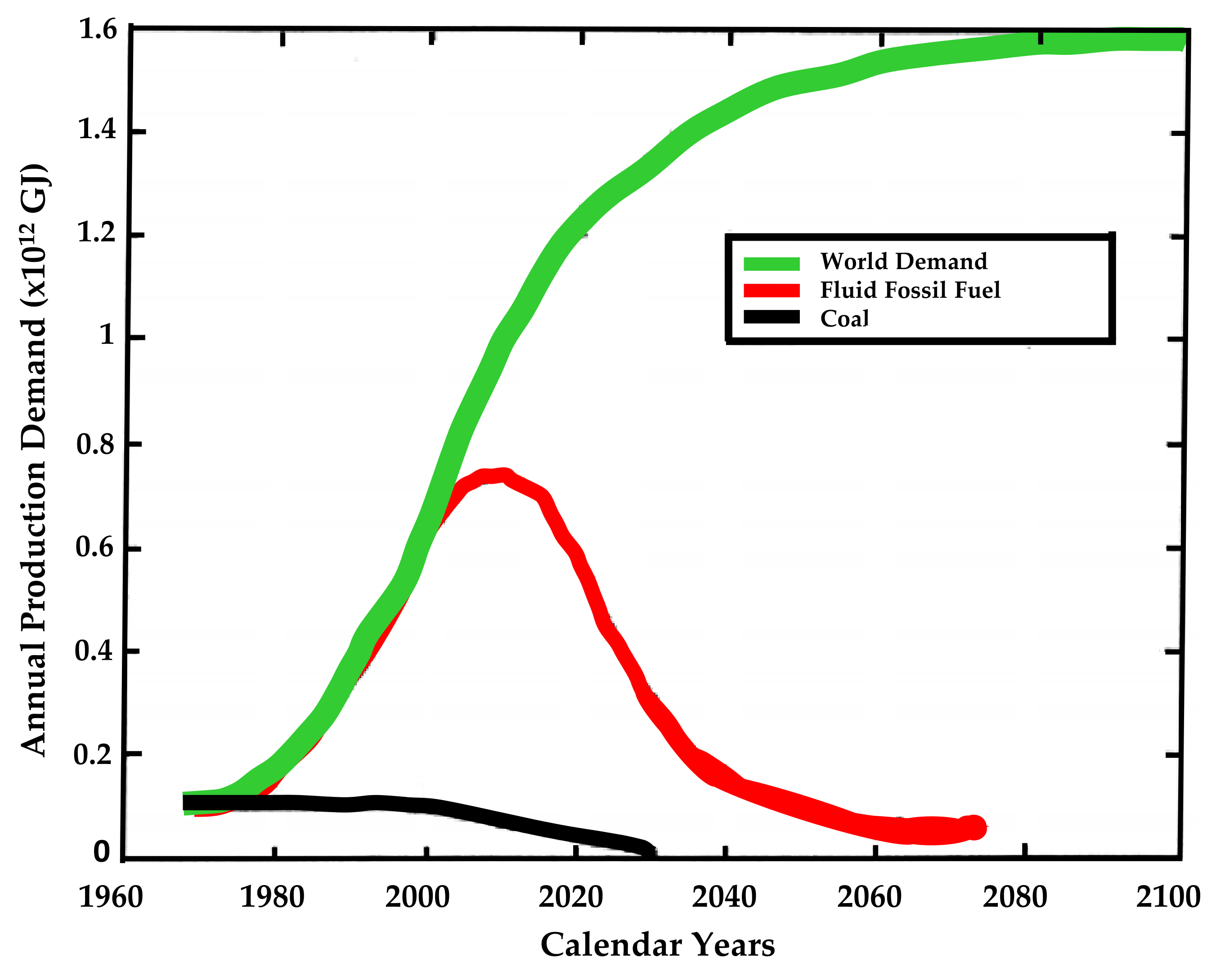
4. Hydrogen Use Prospects
- Use in existing industrial processes that use H2 as a raw material, being characterized by a high demand for H2 in their processes;
- Use in the transport sector, where this fuel is already used, although the current demand for refueling at H2 fueling stations is low, indicating a greater number of fueling stations and a greater number of H2 vehicles for this increase in use;
- Volume proportional use: volume for heating energy production through gas distribution systems by mixing in a methane or 100% H2 gas distribution network;
- The regulatory change on the future use of H2, however, must be regulated by each of these sectors independently and/or through a set of policy measures for the growth of demand for use and consequent production of H2 across the globe [53].
5. Hydrogen Production
6. Hydrogen Storage and Transport
7. Green Hydrogen Production
8. International Hydrogen Market
9. Conclusions and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weidner, T.; Yang, A.; Hamm, M.W. Energy optimisation of plant factories and greenhouses for different climatic conditions. Energy Convers. Manag. 2021, 243, 114336. [Google Scholar] [CrossRef]
- Veziroğlu, T.N.; Şahin, S. 21st Century’s energy: Hydrogen energy system. Energy Convers. Manag. 2008, 49, 1820–1831. [Google Scholar] [CrossRef]
- Burke, A.; Fishel, S. A coal elimination treaty 2030: Fast tracking climate change mitigation, global health and security. Earth System Gov. 2020, 3, 100046. [Google Scholar] [CrossRef]
- Nicolay, S.; Karpuk, S.; Liu, Y.; Elham, A. Conceptual design and optimization of a general aviation aircraft with fuel cells and hydrogen. Int. J. Hydrog. Energy 2021, 46, 32676–32694. [Google Scholar] [CrossRef]
- Estevão, T.E.R. Hydrogen as fuel. Master’s Degree, Faculty of Mechanical Engineering University of Porto, Porto, Portugal, 2008. [Google Scholar]
- Zhao, L.; Wang, D.; Qi, W. Comparative study on air dilution and hydrogen-enriched air dilution employed in a SI engine fueled with iso-butanol-gasoline. Int. J. Hydrog. Energy 2020, 45, 10895–10905. [Google Scholar] [CrossRef]
- Davies, A.; Simmons, M.D. Demand for “advantaged” hydrocarbons during the 21st century energy transition. Energy Rep. 2021, 7, 4483–4497. [Google Scholar] [CrossRef]
- Boretti, A. Production of hydrogen for export from wind and solar energy, natural gas, and coal in Australia. Int. J. Hydrog. Energy 2020, 45, 3899–3904. [Google Scholar] [CrossRef]
- Bruce, S.; Temminghoff, M.; Hayward, J.; Schmidt, E.; Munnings, C.; Palfreyman, D.; Hartley, P. National Hydrogen Roadmap. Commonwealth Scientific and Industrial Research Organisation. Available online: http://doi.org/10.25919/5b8055bc08acb (accessed on 9 December 2021).
- Nadaleti, W.C.; Santos, G.B.; Lourenço, V.A. The potential and economic viability of hydrogen production from the use of hydroelectric and wind farms surplus energy in Brazil: A national and pioneering analysis. Int J. Hydrogen Energy 2020, 45, 1373–1384. [Google Scholar] [CrossRef]
- France unveils national hydrogen plan as tool for energy transition. Fuel Cells Bull. 2018, 2018, 10. [CrossRef]
- Green hydrogen plans for German region in GET H2 initiative. Fuel Cells Bull. 2019, 2019, 11–12. [CrossRef]
- Michalski, J.; Bünger, U.; Crotogino, F.; Donadei, S.; Schneider, G.-S.; Pregger, T.; Cao, K.-K.; Heide, D. Hydrogen generation by electrolysis and storage in salt caverns: Potentials, economics and systems aspects with regard to the German energy transition. Int. J. Hydrog. Energy 2017, 42, 13427–13443. [Google Scholar] [CrossRef]
- Behling, N.; Williams, M.C.; Managi, S. Fuel cells and the hydrogen revolution: Analysis of a strategic plan in Japan. Econ. Anal. Policy 2015, 48, 204–221. [Google Scholar] [CrossRef]
- Li, Y.; Shi, X.; Phoumin, H. A strategic roadmap for large-scale green hydrogen demonstration and commercialisation in China: A review and survey analysis. Int. J. Hydrog. Energy 2021, (in press). [Google Scholar] [CrossRef]
- Barrett, S. Gasunie plans first 1 MW P2G hydrogen plant in Netherlands. Fuel Cells Bull. 2017, 8, 14–20. [Google Scholar] [CrossRef]
- Delpierre, M.; Quist, J.; Mertens, J.; Prieur-Vernat, A.; Cucurachi, S. Assessing the environmental impacts of wind-based hydrogen production in the Netherlands using ex-ante LCA and scenarios analysis. J. Clean. Prod. 2021, 299, 126866. [Google Scholar] [CrossRef]
- Cowell, R.; Webb, J. Making useful knowledge for heat decarbonisation: Lessons from local energy planning in the United Kingdom. Energy Res. Soc. Sci. 2021, 75, 102010. [Google Scholar] [CrossRef]
- Yousif, M.; Hamad, T.A.; Hamad, A.A.A.; Agll, S.G.B.; Bauer, C.; Clum, A.; Shivaprasad, N.; Thomas, M.; Sheffield, J.W. A design for hydrogen production and dispensing for northeastern United States, along with its infrastructural development timeline. Int. J. Hydrog. Energy 2014, 39, 9943–9961. [Google Scholar] [CrossRef]
- Nadaleti, W.C.; Lourenço, V.A.; Americo, G. Green hydrogen-based pathways and alternatives: Towards the renewable energy transition in South America’s regions—Part A. Int. J. Hydrog. Energy 2021, 46, 22247–22255. [Google Scholar] [CrossRef]
- IRENA. Hydrogen: A Renewable Energy Perspective. 2019. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2019/Sep/IRENA_Hydrogen_2019.pdf (accessed on 9 December 2021).
- IRENA. REmap: Roadmap for a Renewable Energy Future. 2016. Available online: https://www.irena.org/publications/2016/Mar/REmap-Roadmap-for-A-Renewable-Energy-Future-2016-Edition (accessed on 9 December 2021).
- Vieira, B.; Nadaleti, W.C.; Sarto, E. The effect of the addition of castor oil to residual soybean oil to obtain biodiesel in Brazil: Energy matrix diversification. Renew. Energy 2021, 165, 657–667. [Google Scholar] [CrossRef]
- Oliveira, T.D.; Gurgel, A.C.; Tonry, S. Potential trading partners of a Brazilian emissions trading scheme: The effects of linking with a developed region (Europe) and two developing regions (Latin America and China). Technol. Forecast. Soc. Change 2021, 171, 2021–120947. [Google Scholar] [CrossRef]
- Dagdougui, H.; Ouammi, A.; Sacile, R. A regional decision support system for onsite renewable hydrogen production from solar and wind energy sources. Int. J. Hydrog. Energy 2011, 36, 14324–14334. [Google Scholar] [CrossRef]
- Ortiz-Imedio, R.; Ortiz, A.; Ortiz, I. Comprehensive analysis of the combustion of low carbon fuels (hydrogen, methane and coke oven gas) in a park ignition engine through CFD modeling. Energy Convers. Manag. 2022, 251, 114918. [Google Scholar] [CrossRef]
- Balat, H.; Kirtay, E. Hydrogen from biomass—Present scenario and future prospects. Int. J. Hydrog. Energy 2010, 35, 7416–7426. [Google Scholar] [CrossRef]
- Sarıkoç, E. Effect of H2 addition to methanol-gasoline blend on an SI engine at various lambda values and engine loads: A case of performance, combustion, and emission characteristics. Fuel 2021, 297, 120732. [Google Scholar] [CrossRef]
- Sierens, R.; Rosseel, E. Variable composition hydrogen/natural gas mixtures for increased engine efficiency and decreased emissions. In Proceedings of the Spring Engine Technology Conference, Fort Lauderadale, FL, USA, 26 April 1998. 98-ICE-105. [Google Scholar]
- Cracknell, R.F.; Alcock, J.L.; Rowson, J.J.; Shirvill, L.C.; Üngüt, A. Safety considerations in retailing hydrogen. SAE Tech. Pap. 2002, 1, 1928. [Google Scholar] [CrossRef]
- Al-Rousan, A.A. Reduction of fuel consumption in gasoline engines by introducing HHO gas into intake manifold. Int. J. Hydrog. Energy 2010, 35, 12930–12935. [Google Scholar] [CrossRef]
- Schoenung, S. Hydrogen vehicle fueling alternatives: An analysis developed for the International Energy Agency. SAE Tech. Pap. 2001, 01, 2528. [Google Scholar] [CrossRef]
- Taghavifar, H.; Nemati, A.; Salvador, F.J.; Morena, J.d.L. 1D energy, exergy, and performance assessment of turbocharged diesel/hydrogen RCCI engine at different levels of diesel, hydrogen, compressor pressure ratio, and combustion duration. Int. J. Hydrog. Energy 2021, 46, 22180–22194. [Google Scholar] [CrossRef]
- Yilmaz, A.C.; Uludamar, E.; Aydin, K. Effect of hydroxy (HHO) gas addition on performance and exhaust emissions in compression ignition engines. Int. J. Hydrog. Energy 2010, 35, 11366–11372. [Google Scholar] [CrossRef]
- Sadeghzadeh, K.; Salehi, M.B. Mathematical analysis of fuel cell strategic technologies development solutions in the automotive industry by the TOPSIS multi-criteria decision making method. Int. J. Hydrog. Energy 2011, 36, 13272–13280. [Google Scholar] [CrossRef]
- Yang, X.; Wang, T.; Zhang, Y.; Zhang, H.; Wu, Y.; Zhang, J. Hydrogen effect on flame extinction of hydrogen-enriched methane/air premixed flames: An assessment from the combustion safety point of view. Energy 2022, 239, 122248. [Google Scholar] [CrossRef]
- Wang, L.Q.; Ma, H.H.; Shen, Z.W.; Chen, D.G. Experimental study of DDT in hydrogen-methane-air mixtures in a tube filled with square orifice plates. Process. Saf. Environ. 2018, 116, 228–234. [Google Scholar] [CrossRef]
- Gürsu, S.; Sheriff, S.A.; Vezirocglu, T.N.; Sheffield, J.W. Review of slush hydrogen production and utilization Technologies. Int. J. Hydrog. Energy 1994, 19, 491–496. [Google Scholar] [CrossRef]
- Ciccarelli, G.; Ginsberg, T.; Boccio, J.; Economos, C.; Sato, K.; Kinoshita, M. Detonation cell size measurements and predictions in hydrogen-air-steam mixtures at elevated temperatures. Combust. Flame 1994, 99, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Motores, C.I.C. Apostila de Motores a Combustão Interna; Universidade Federal de Pelotas: Pelotas, Brasil, 2013. [Google Scholar]
- Cai, P.; Zhang, C.; Jing, Z.; Peng, Y.; Jing, J.; Sun, H. Effects of Fischer-Tropsch diesel blending in petrochemical diesel on combustion and emissions of a common-rail diesel engine. Fuel 2021, 305, 121587. [Google Scholar] [CrossRef]
- Haynes, W.M. Handbook of Chemistry and Physics, 95th ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 4–17. [Google Scholar]
- Souza, M.M.V.M. Tecnologia do Hidrogênio; Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro: Editora Synergia: Rio de Janeiro, Brazil, 2008; pp. 154–196. [Google Scholar]
- Wu, H.W.; Wu, Z.Y. Combustion characteristics and optimal factors determination with Taguchi method for diesel engines port-injecting hydrogen. Energy 2012, 47, 411–420. [Google Scholar] [CrossRef]
- Chauhan, N.S.; Singh, V.K. Fundamentals and use of hydrogen as a fuel. ISST J. Mech. Eng. 2015, 6, 63–68. [Google Scholar]
- ISO—International Organization for Standardization. Available online: https://www.iso.org/home.html (accessed on 21 January 2020).
- Yang, Y.; Wang, G.; Zhang, S.; Zhang, L.; Lin, L. Review of hydrogen standards for China. E3S Web of Conf. 2019, 118, 03032. [Google Scholar] [CrossRef]
- Felseghi, R.A.; Carcadea, E.; Raboaca, M.S.; Trufin, C.N.; Filote, C. Hydrogen fuel cell technology for the sustainable future of stationary applications. Energies 2019, 12, 4593. [Google Scholar] [CrossRef] [Green Version]
- Apostolou, D.; Xydis, G. A literature review on hydrogen refueling stations and infrastructure, Current status and future prospectus. Renew. Sustain. Energy Rev. 2019, 113, 109292. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Smart energy solutions with hydrogen options. Int. J. Hydrog. Energy 2018, 43, 8579–8599. [Google Scholar] [CrossRef]
- Sharma, S.; Agarwal, S.; Jain, A. Significance of hydrogen as economic and environmentally friendly fuel. Energies 2021, 14, 7389. [Google Scholar] [CrossRef]
- Hydrogen Council. Path to Hydrogen Competitiveness a Cost Perspective; Hydrogen Council: Brussels, Belgium, 2020; pp. 1–88. [Google Scholar]
- Newborough, M.; Cooley, G. Developments in the global hydrogen market: Electrolyser deployment rationale and renewable hydrogen strategies and policies. Fuel Cells Bull. 2020, 2020, 16–22. [Google Scholar] [CrossRef]
- Grand View Research. Available online: http://www.grandviewresearch.com/industry-analysis/green-hydrogen-market (accessed on 31 July 2021).
- Markets and markets. Available online: http://www.marketsandmarkets.com/PressReleases/hydrogen.asp (accessed on 3 June 2021).
- Hydrogen generation market size and share: North America, Europe, and APAC industry forecasts 2026. Focus on Catalysts 2021, 9, 2. [CrossRef]
- Upham, P.; Bögel, P.; Dütschke, E.; Burghard, U.; Oltra, C.; Sala, R.; Lores, M.; Brinkmann, J. The revolution is conditional? The conditionality of hydrogen fuel cell expectations in five European countries. Energy Res. Soc. Sci. 2020, 70, 101722. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Velazquez, A.A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 2, 463–491. [Google Scholar] [CrossRef] [Green Version]
- IRENA. International Renewable Energy Agency. Hydrogen: A Renewable Energy Perspective. 2019. Available online: https://www.irena.org/publications/2019/Sep/Hydrogen-A-renewable-energy-perspective (accessed on 9 December 2021).
- Hydrogen Council. Hydrogen Scaling up, a Sustainable Pathway for the Global Energy Transition; Hydrogen Council: Brussels, Belgium, 2017; pp. 1–80. [Google Scholar]
- Djalante, R. Key assessments from the IPCC special report on global warming of 1.5 °C and the implications for the Sendai framework for disaster risk reduction. Prog. Disaster Sci. 2019, 1, 100001. [Google Scholar] [CrossRef]
- UNEP. Emissions Gap Report 2018. UNEP—UN Environment Program. Available online: https://www.unep.org/resources/emissions-gap-report-2018 (accessed on 4 May 2021).
- Hydrogen Council. Hydrogen Scaling Up. Available online: https://hydrogencouncil.com/wp-content/uploads/2017/11/Hydrogen-scaling-up-Hydrogen-Council.pdf (accessed on 24 May 2021).
- OEC. Hydrogen in Malaysia. OEC—The Observatory of Economic Complexity. Available online: https://oec.world/en/profile/bilateral-product/hydrogen/reporter/mys?redirect=true (accessed on 4 August 2021).
- Viktorsson, L.; Heinonen, J.T.; Skulason, J.B.; Unnthorsson, R. A Step towards the Hydrogen economy—A life cycle cost analysis of a hydrogen refueling station. Energies 2017, 10, 763. [Google Scholar] [CrossRef]
- Aschilean, I.; Rasoi, G.; Raboaca, M.S.; Filote, C.; Culcer, M. Design and concept of an energy system based on renewable sources for greenhouse sustainable agriculture. Energies 2018, 11, 1201. [Google Scholar] [CrossRef] [Green Version]
- ONU. UN News: Global Perspective, Human Stories. Available online: https://news.un.org/en/ (accessed on 31 December 2021).
- Singla, M.K.; Nijhawan, P.; Oberoi, A.S. Hydrogen fuel and fuel cell technology for cleaner future: A review. Environ. Sci. Pollut. Res. 2021, 28, 15607–15626. [Google Scholar] [CrossRef]
- Dündar-Tekkaya, E.; Yürüm, Y. Mesoporous MCM-41 material for hydrogen storage: A short review. Int. J. Hydrogen Energy 2016, 41, 9789–9795. [Google Scholar] [CrossRef]
- Zhu, J.; Dai, L.; Yu, Y.; Cao, J.; Wang, L. Direct electrochemical route from oxides to TiMn2 hydrogen storage alloy. Chin. J. Chem. Eng. 2015, 23, 1865–1870. [Google Scholar] [CrossRef]
- International Energy Agency. The Future of Hydrogen. Report Prepared by the IEA, Japan. 2019. Available online: http://www.iea.org/reports/the-future-of-hydrogen (accessed on 9 December 2021).
- HyUnder. Assessment of the Potential, the Actors and Relevant Business Cases for Large Scale and Long Term Storage of Renewable Electricity by Hydrogen Underground Storage in Europe (Executive Summary). 2–14. Available online: http://hyunder.eu/wp-content/uploads/2016/01/D8.1_HyUnder-Executive-Summary.pdf (accessed on 9 December 2021).
- Zgonnik, V. The occurrence and geoscience of natural hydrogen: A comprehensive review. Earth-Sci. Rev. 2020, 203, 103140. [Google Scholar] [CrossRef]
- Bünger, U.; Landinger, H.; Pschorr-Schoberer, E.; Schmidt, P.; Weindorf, W.; Jöhrens, J.; Lambrecht, U.; Naumann, K.; Lischke, A. Power-to-Gas (PtG) in Transport: Status Quo and Perspectives for Development. Report to the Federal Ministry of Transport and Digital Infrastructure (BMVI), Germany. 2014. Available online: https://www.bmvi.de/SharedDocs/EN/Documents/MKS/mks-studie-ptg-transport-status-quo-and-perspectives-for-development.pdf?__blob=publicationFile (accessed on 9 December 2021).
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrog. Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Sreedhar, I.; Kamani, K.M.; Kamani, B.M.; Reddy, B.M.; Venugopal, A. A Bird’s Eye view on process and engineering aspects of hydrogen storage. Renew. Sustain. Energy Rev. 2018, 91, 838–860. [Google Scholar] [CrossRef]
- Suh, M.P.; Park, H.J.; Prasad, T.K.; Lim, D.W. Hydrogen storage in metal–organic frameworks. Chem. Rev. 2012, 112, 782–835. [Google Scholar] [CrossRef]
- Jain, A.; Agarwal, S.; Ichikawa, T. Catalytic tuning of sorption kinetics of lightweight hydrides: A review of the materials and mechanism. Catalysts 2018, 8, 651. [Google Scholar] [CrossRef] [Green Version]
- Frank, E.D.; Elgowainy, A.; Khalid, Y.S.; Peng, J.-K.; Reddi, K. Refueling-station costs for metal hydride storage tanks on board hydrogen fuel cell vehicles. Int. J. Hydrog. Energy 2019, 44, 29849–29861. [Google Scholar] [CrossRef]
- Rao, P.M.P.; Jhala, P.P. Project: Green hydrogen-energy source of the future an analysis of the technology scenario. Preprint 2021. [Google Scholar] [CrossRef]
- Dincer, I. Green methods for hydrogen production. Int. J. Hydrog. Energy 2012, 37, 1954–1971. [Google Scholar] [CrossRef]
- International Energy Agency. The Future of Hydrogen; International Energy Agency: Paris, France, 2019. [Google Scholar]
- Jovan, D.J.; Dolanc, G. Can green hydrogen production be economically viable under current market conditions. Energies 2020, 13, 6599. [Google Scholar] [CrossRef]
- Karp, I.M. Hydrogen: State of the art and directions of future use. Int. J. Biosens. Bioelectron. 2021, 7, 25–28. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Int. J. Hydrog. Energy 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrog. Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Albrecht, U.; Altmann, M.; Barth, F.; Bünger, U.; Fraile, D.; Lanoix, J.-C.; Pschorr-Schoberer, E.; Vanhoudt, W.; Weindorf, W.; Zerta, M.; et al. Study on Hydrogen from Renewable Resources in the EU; FCH: Brussels, Belgium, 2015. [Google Scholar]
- European Biogas Association. Annual Report; European Biogas Association: Brussels, Belgium, 2015. [Google Scholar]
- Di Marcoberardino, G.; Vitali, D.; Spinelli, F.; Binotti, M.; Manzolini, G. Green hydrogen production from raw biogas: A techno-economic investigation of conventional processes using pressure swing adsorption unit. Processes 2018, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Braga, L.B.; Silveira, J.L.; da Silva, M.E.; Tuna, C.E.; Machin, E.B.; Pedroso, D.T. Hydrogen production by biogas steam reforming: A technical, economic and ecological analysis. Renew. Sustain. Energy Rev. 2013, 28, 166–173. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Binotti, M.; Di Marcoberardino, G.; Biassoni, M.; Manzolini, G. Solar hydrogen production with cerium oxides thermochemical cycle. AIP Conf. Proc. 2017, 1850, 100002. [Google Scholar] [CrossRef] [Green Version]
- Göransson, K.; Söderlind, U.; He, J.; Zhang, W. Review of syngas production via biomass DFBGs. Renew. Sustain. Energy Rev. 2011, 15, 482–492. [Google Scholar] [CrossRef]
- Ugarte, P.; Durán, P.; Lasobras, J.; Soler, J.; Menéndez, M.; Herguido, J. Dry reforming of biogas in fluidized bed: Process intensification. Int. J. Hydrog. Energy 2017, 42, 13589–13597. [Google Scholar] [CrossRef] [Green Version]
- Ferraren-De Cagalitan, D.D.T.; Abundo, M.L.S. A review of biohydrogen production technology for application towards hydrogen fuel cells. Renew. Sustain. Energy Rev. 2021, 151, 111413. [Google Scholar] [CrossRef]
- Ohkubo, T.; Hideshima, Y.; Shudo, Y. Estimation of hydrogen output from a full-scale plant for production of hydrogen from biogas. Int. J. Hydrog. Energy 2010, 35, 13021–13027. [Google Scholar] [CrossRef]
- Araki, S.; Hino, N.; Mori, T.; Hikazudani, S. Durability of a Ni based monolithic catalyst in the autothermal reforming of biogas. Int. J. Hydrog. Energy 2009, 34, 4727–4734. [Google Scholar] [CrossRef]
- Debowski, M.; Korzeniewska, E.; Filipkowska, Z.; Zielinski, M.; Kwiatkowski, R. Possibility of hydrogen production during cheese whey fermentation process by different strains of psychrophilic bacteria. Int. J. Hydrog. Energy 2014, 39, 1972–1978. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Y.; Chen, M.; Zeng, R.J. Hydrogen super saturation in thermophilic mixed culture fermentation. Int. J. Hydrog. Energy 2012, 37, 17809–17816. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, W.; Tang, C.; Suo, Y.; Gao, L.; Yuan, X. Bioreactor performance and methanogenic population dynamics in a low-temperature (5e18C) anaerobic fixed-bed reactor. Bioresour. Technol. 2012, 104, 136–143. [Google Scholar] [CrossRef]
- Scherer, S.; Neuhaus, K. Life at low temperatures. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 210–262. [Google Scholar]
- Feller, G.; Gerday, C. Psychrophilic enzymes: Hot topics in cold adaptation. Nat. Rev. Microbiol. 2003, 1, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Xing, D.; Ren, N.; Logan, B.E. Syntrophic interactions drive the hydrogen production from glucose at low temperature in microbial electrolysis cells. Bioresour. Technol. 2012, 124, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Touloupakis, E.; Faraloni, C.; Silva Benavides, A.M.; Torzillo, G. Recent achievements in microalgal photobiological hydrogen production. Energies 2021, 14, 7170. [Google Scholar] [CrossRef]
- Melis, A. Photosynthetic H2 metabolism in Chlamydomonas reinhardtii (unicellular green algae). Planta 2007, 226, 1075–1086. [Google Scholar] [CrossRef]
- Amaro, H.M.; Esquível, M.G.; Pinto, T.S.; Malcata, F.X. Hydrogen production by microalgae. In Natural and Artificial Photosynthesis: Solar Power as an Energy Source, 1st ed.; Razeghifard, R., Ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 231–241. [Google Scholar]
- Jimenez-Llanos, J.; Ramirez-Carmona, M.; Rendon-Castrillon, L.; Ocampo-Lopez, C. Sustainable biohydrogen production by Chlorella sp. microalgae: A review. Int. J. Hydrog. Energy 2020, 45, 8310–8328. [Google Scholar] [CrossRef]
- Nagarajan, D.; Dong, C.D.; Chen, C.Y.; Lee, D.J.; Chang, J.S. Biohydrogen production from microalgae - Major bottlenecks and future research perspectives. Biotechnol. J. 2021, 16, 2000124. [Google Scholar] [CrossRef] [PubMed]
- Melis, A. Green alga hydrogen production: Progress, challenges and prospects. Int. J. Hydrog. Energy 2002, 27, 1217–1228. [Google Scholar] [CrossRef]
- Tsygankov, A.A.; Kosourov, S.N.; Tolstygina, I.V.; Ghirardi, M.L.; Seibert, M. Hydrogen production by sulfur-deprived Chlamydomonas reinhardtii under photoautotrophic conditions. Int. J. Hydrog. Energy 2006, 31, 1574–1584. [Google Scholar] [CrossRef]
- Rashid, N.; Lee, K.; Mahmood, Q. Bio-hydrogen production by Chlorella vulgaris under diverse photoperiods. Bioresour. Technol. 2011, 102, 2101–2104. [Google Scholar] [CrossRef] [PubMed]
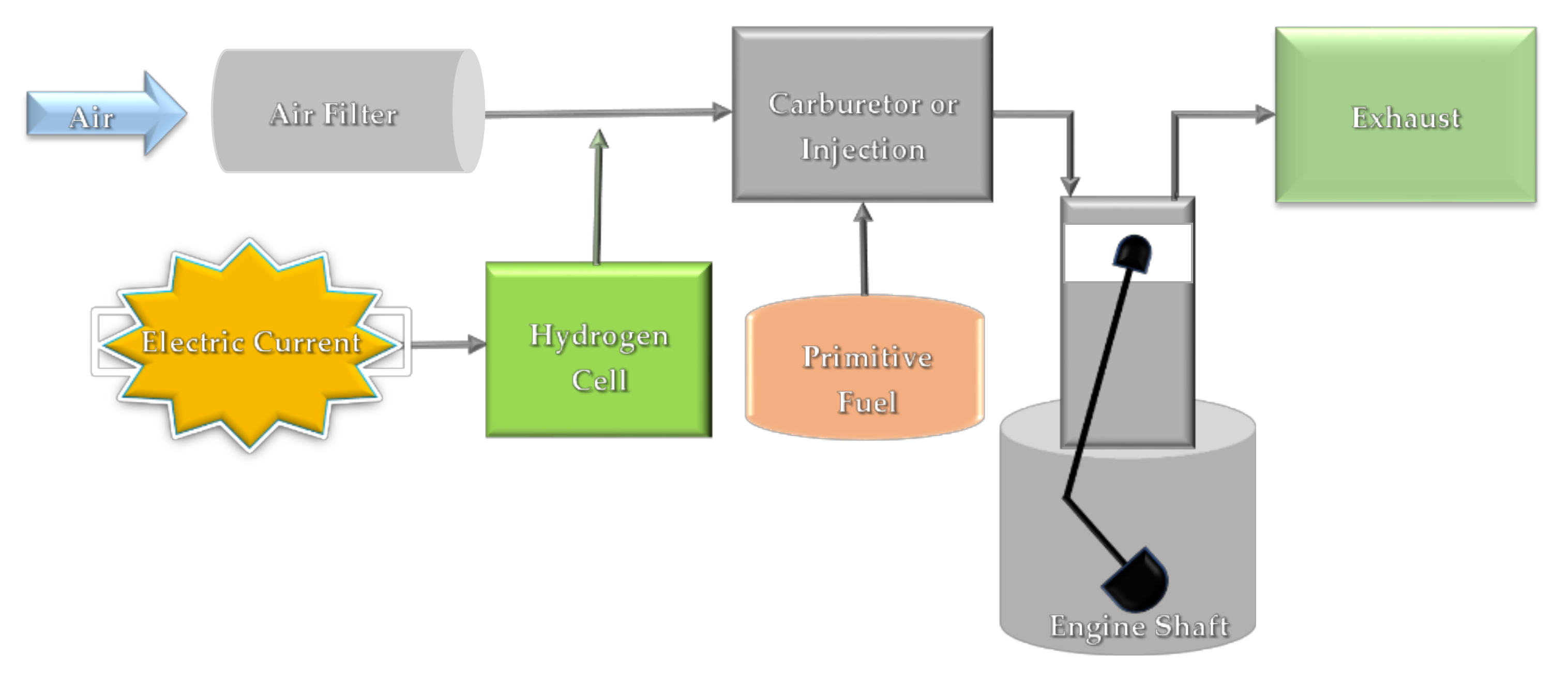



| Fuel | Calorific Value (MJ/kg) |
|---|---|
| Hydrogen | 119.93 |
| Methane | 50.02 |
| Propane | 45.60 |
| Gasoline | 44.50 |
| Diesel | 42.50 |
| Ethanol | 27.00 |
| Methanol | 18.50 |
| Data | Hydrogen | Methane | Propane | Gasoline | Unit |
|---|---|---|---|---|---|
| Lower Detonability Limit (LDL) in Air | 11–18 | 6.3 | 3.1 | 1.1 | % (v/v) |
| Upper Detonability Limit (UDL in Air | 59 | 13.5 | 7 | 3.3 | % (v/v) |
| Lower Flammable Limit (LFL) in Air | 4 | 5.3 | 2.1 | 1.4 | % (v/v) |
| Upper Flammable Limit (UFL) in Air | 75 | 15 | 9.5 | 7.6 | % (v/v) |
| Maximum Laminar Burning Velocity | 3.46 | 0.43 | 0.47 | - | m/s |
| Maximum Concentration | 42.5 | 10.2 | 4.3 | - | % (v/v) |
| Stoichiometric Laminar Burning Velocity | 2.37 | 0.42 | 0.46 | 0.42 | m/s |
| Stoichiometric Concentration | 29.5 | 9.5 | 4.1 | 1.8 | % (v/v) |
| Density (NTP) | 0.084 | 0.65 | 2.01 | - | kg/m3 |
| Ignition Limit in Air (NTP) | 4.0–77.0 | 4.4–16.5 | 1.7–10.9 | - | % (v/v) |
| Ignition Temperature | 560 | 540 | 487 | 228–471 | C |
| Minimum Ignition Energy in Air | 0.02 | 0.29 | 0.26 | 0.24 | mJ |
| Maximum Combustion Rate in Air | 3.46 | 0.43 | 0.47 | - | m/s |
| Detonation Limits in Air | 18–59 | 6.3–14 | 1.1–1.3 | - | % (v/v) |
| Stoichiometric Rate in Air | 29.5 | 9.5 | 4.0 | - | % (v/v) |
| Organization | Area | Norm | Application | Source |
|---|---|---|---|---|
| ISO | Fuel Hydrogen quality | PAS 15594:2004-TS 15869:2009 | Gaseous hydrogen (H2) and hydrogen mixtures—fuel tanks for land vehicles | [46] |
| ISO 14687-1:1999 | All uses of H2 as a fuel for road vehicles excluding proton-exchange membrane fuel cells (PEMFC) | [46,47] | ||
| ISO 14687-2:2012 | PEMFC use for road vehicles | [46,47] | ||
| ISO 14683-3:2014 | PEMFC use for stationary devices | [46] | ||
| Safety in the use of hydrogen | ISO/TR 15916:2015 | General issues on safety of H2 powered systems | [46,47] | |
| ISO 16110-1:2017 | Safety of H2 generation systems integrated with fuel processing technologies | [46,47] | ||
| ISO/TS 19883:2017 | Safety of systems based on pressure swing adsorption to separate and purify H2 | [46] | ||
| ISO 23273:2013 | Safety of H2-fueled road vehicles | [46] | ||
| Hydrogen production and purification | ISO 22734-1:2008 | Industrial/commercial uses of H2 generation systems based on the electrolysis of water | [46] | |
| ISO 22734-2:2011 | Residential uses of H2 generation systems based on the electrolysis of water | [46] | ||
| Hydrogen storage, transport and fueling | ISO 13985:2006 | Liquid H2—Land vehicle fuel tanks | [46,47] | |
| ISO 16111:2018 | Devices to store H2 for transport absorbed in reversible metal hydride | [46,47] | ||
| ISO 19881:2018 | Containers for gaseous H2 as a fuel for land vehicles | [46] | ||
| ISO 19882:2018 | Pressure relief devices to be used in fuel tanks of H2-powered vehicles | [46] | ||
| ISO 13984:1999 | Systems for liquid H2 fueling and delivery on all types of land vehicles | [46,47] | ||
| ISO 17268:2012 | Refueling connectors for gaseous H2 land vehicles | [46,47] | ||
| ISO/TS 198801:2016 | Fueling stations delivering gaseous H2 to light-duty land vehicles | [46] | ||
| ISO 19880-3:2018 | High-pressure gas valves for gaseous H2 stations | [46] | ||
| Testing | ISO 2626:1973 | Copper—H2 embrittlement (HE) test | [46] | |
| ISO 7539-11:2013 | Tests for assessing metal and alloy resistance to HE and H2-assisted cracking | [46] | ||
| ISO 11114-4:2017 | Tests for qualifying steels to be used to manufacture cylinders and valves resistant to HE | [46,47] | ||
| ISO 15330:1999 | Preloading test to detect HE by the parallel bearing surface method | [46] | ||
| ISO 16573:2015 | Method for assessing resistance of high-strength steel to HE | [46] | ||
| ISO 17081:2014 | Method to measure H2 permeation, uptake and transport in metals and alloys electrochemically | [46] | ||
| ISO/TR 11954:2008 | Procedure to measure the maximum speed of fuel cell vehicles using compressed H2 | [46] | ||
| ISO 15859-2:2004 | Limits for the composition of H2 for space systems as well as sampling and test requirements to verify | [46] | ||
| ISO 23828:2013 | Procedure to measure the energy consumption of fuel cell vehicles using compressed H2 | [46,47] | ||
| ISO 16110-2:2010 | Methods to assess the performance of H2 generation systems integrated with fuel processing technologies | [46,47] | ||
| ISO 26142:2010 | H2 detection apparatus—Stationary applications | [46] | ||
| IEC | Terminology | IEC 60050-485:2020 | General terminology relating to all applications of fuel cell technologies | [46] |
| Safety in the use of hydrogen | IEC 62282-3-100:2019 | Safety of stationary fuel cell power systems (FCPS) | [46] | |
| IEC 62282-4-101:2014 | Safety of FCPS intended for use in industrial electric trucks | [46] | ||
| IEC 62282-5-100:2018 | Safety of portable FCPS | [46] | ||
| IEC 62282-6-100:2010 | Safety of micro FCPS | [46] | ||
| IEC PAS 62282-6-150:2011 | Safety of micro FCPS using H2 released by the reaction of water-reactive compounds in indirect PEMFC | [46] | ||
| Hydrogen application | IEC 62282-2:2012 | Safety in construction, operation and testing of fuel cell modules | [46] | |
| IEC 62282-3-300:2012 | Safety in the installation of stationary FCPS | [46] | ||
| IEC 62282-3-400:2016 | Small-sized stationary FCPS with combined production of heat and power | [46] | ||
| IEC 62282-6-300:2012 | Fuel cartridge interchangeability in micro FCPS | [46] | ||
| IEC 62282-6-400:2019 | Interchangeability of power and data between micro FCPS and electronic devices | [46] | ||
| Testing | IEC/TS 62282-3-200:2016 | Methods to assess the performance of stationary FCPS | [46] | |
| IEC 62282-3-201:2017 | Methods to assess the performance of small stationary FCPS | [46] | ||
| IEC 62282-4-102:2017 | Methods to assess the performance of FCPS for industrial electric trucks | [46] | ||
| IEC 62282-6-200:2016 | Methods to assess the performance of micro FCPS | [46] | ||
| IEC/TS 62282-7-1:2017 | Single cell performance tests for polymer electrolyte fuel cells | [46] | ||
| IEC/TS 62282-7-2:2021 | Single cell and stack performance tests for solid oxide fuel cells | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farias, C.B.B.; Barreiros, R.C.S.; da Silva, M.F.; Casazza, A.A.; Converti, A.; Sarubbo, L.A. Use of Hydrogen as Fuel: A Trend of the 21st Century. Energies 2022, 15, 311. https://doi.org/10.3390/en15010311
Farias CBB, Barreiros RCS, da Silva MF, Casazza AA, Converti A, Sarubbo LA. Use of Hydrogen as Fuel: A Trend of the 21st Century. Energies. 2022; 15(1):311. https://doi.org/10.3390/en15010311
Chicago/Turabian StyleFarias, Charles Bronzo Barbosa, Robson Carmelo Santos Barreiros, Milena Fernandes da Silva, Alessandro Alberto Casazza, Attilio Converti, and Leonie Asfora Sarubbo. 2022. "Use of Hydrogen as Fuel: A Trend of the 21st Century" Energies 15, no. 1: 311. https://doi.org/10.3390/en15010311
APA StyleFarias, C. B. B., Barreiros, R. C. S., da Silva, M. F., Casazza, A. A., Converti, A., & Sarubbo, L. A. (2022). Use of Hydrogen as Fuel: A Trend of the 21st Century. Energies, 15(1), 311. https://doi.org/10.3390/en15010311









