Physicochemical Properties of Torrefied and Pyrolyzed Food Waste Biochars as Fuel: A Pilot-Scale Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
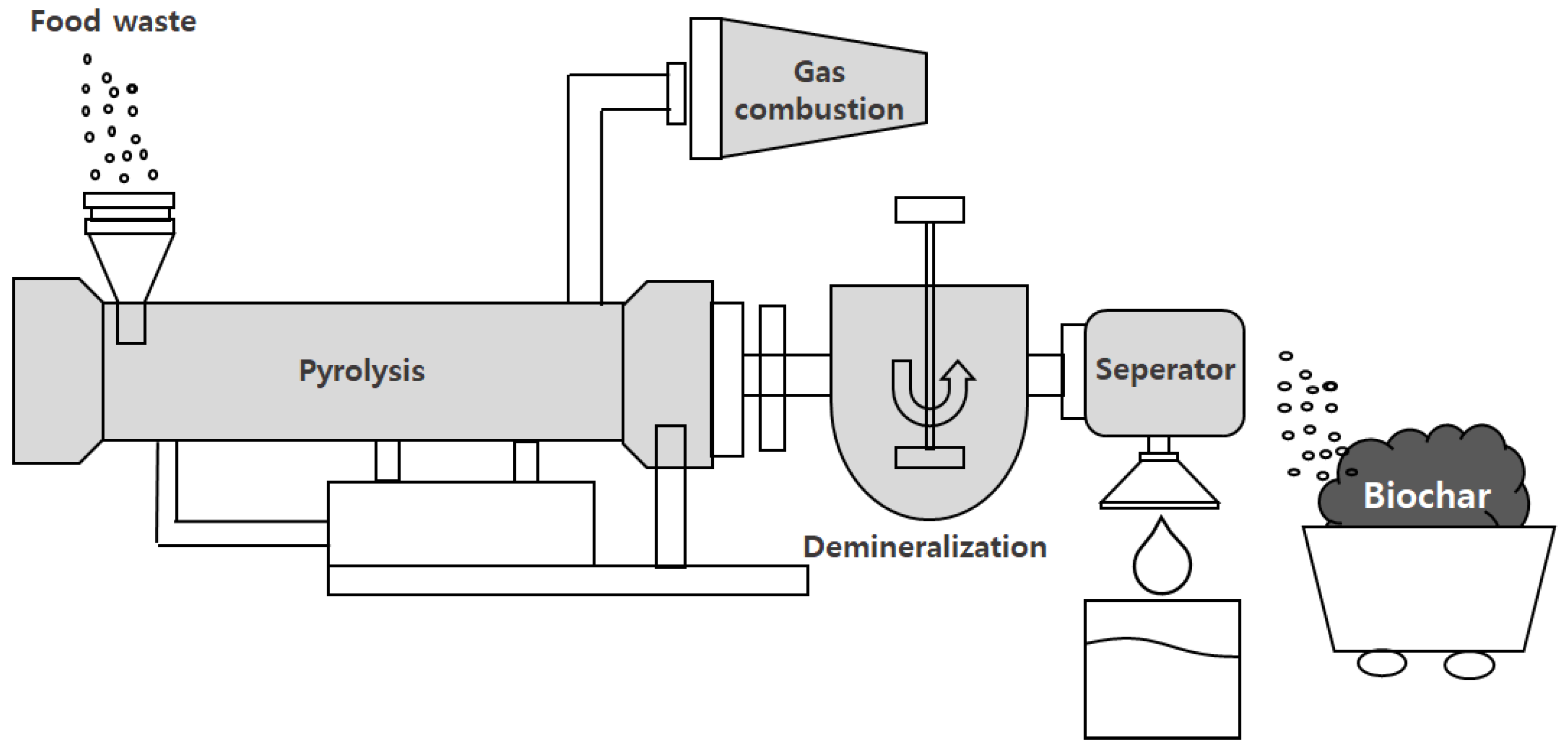
2.2. Pyrolysis Furnace
2.3. Sample Analysis
2.4. Pyrolysis and Demineralization Process
2.5. Statistical Analyses
3. Results and Discussion
3.1. NCV and Chloride Ion Analysis
3.2. AAEM Component Analysis and Removal Efficiency
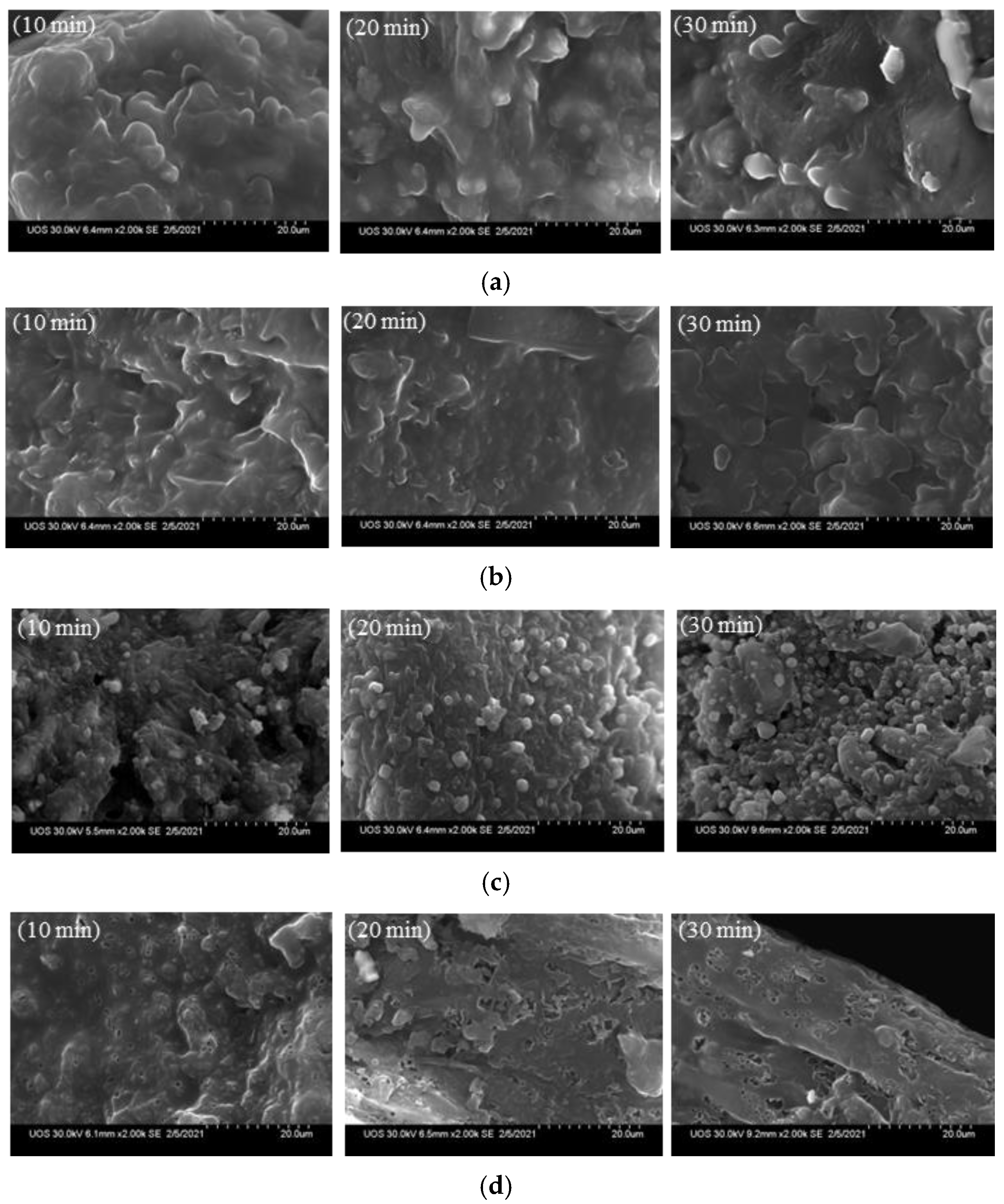
3.3. SEM Analysis
3.4. Analysis of Heavy Metals
3.5. Analysis of Demineralized Water
3.6. Proximate and Ultimate Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Z.; Koh, S.K.; Ng, W.C.; Lim, R.C.J.; Wang, C.-H. Potential application of gasification to recycle food waste and rehabilitate acidic soil from secondary forests on degraded land in Southeast Asia. J. Environ. Manag. 2016, 172, 40–48. [Google Scholar] [CrossRef]
- Opatokun, S.A.; Yousef, L.F.; Strezov, V. Agronomic assessment of pyrolysed food waste digestate for sandy soil management. J. Environ. Manag. 2017, 187, 24–30. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, Y.; Zhang, Q.; Rong, H.; Liu, Y.; Ziao, B.; Guo, D.; Laghari, M.; Ruan, R. Gas-carrying enhances the combustion temperature of the biomass particles. Energy 2022, 239, 121956. [Google Scholar] [CrossRef]
- Mir, M.A.; Hussain, A.; Verma, C. Design considerations and operational performance of anaerobic digester: A review. Cogent Eng. 2016, 3, 1181696. [Google Scholar] [CrossRef]
- Poudel, J.; Ohm, T.-I.; Oh, S.C. A study on torrefaction of food waste. Fuel 2015, 140, 275–281. [Google Scholar] [CrossRef]
- Elkhalifa, S.; Al-Ansari, T.; Mackey, H.R.; McKay, G. Food waste to biochars through pyrolysis: A review. Resour. Conserv. Recycl. 2019, 144, 310–320. [Google Scholar] [CrossRef]
- Lee, Y.-E.; Jo, J.-H.; Kim, S.-M.; Yoo, Y.-S. Recycling possibility of the salty food waste by pyrolysis and water scrubbing. Energies 2017, 10, 210. [Google Scholar] [CrossRef] [Green Version]
- Jori, F.; Bastos, A.D.S. Role of wild suids in the epidemiology of African swine fever. Ecohealth 2009, 6, 296–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, W.; Gao, G.F. Emerging H5N8 avian influenza viruses. Science 2021, 21, 784–786. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kang, Y.-G.; Luyima, D.; Park, S.-J.; Oh, T.-K.; Lee, C.H. Characteristics of food waste: Water and salinity contents. Korean J. Agric. Sci. 2020, 47, 375–380. [Google Scholar]
- Williams, P.T. Dioxins and furans from the incineration of municipal solid waste: An overview. J. Energy Inst. 2005, 78, 38–48. [Google Scholar] [CrossRef]
- Katami, T.; Yasuhara, A.; Shibamoto, T. Formation of dioxins from incineration of foods found in domestic garbage. Environ. Sci. Technol. 2004, 38, 1062–1065. [Google Scholar] [CrossRef]
- Knapczyk, A.; Francik, S.; Jewiarz, M.; Zawi´slak, A.; Francik, R. Thermal treatment of biomass: A bibliometric analysis—The torrefaction case. Energies 2021, 14, 162. [Google Scholar] [CrossRef]
- van der Stelta, M.J.C.; Gerhauser, H.; Kiel, J.H.A.; Ptasinski, K.J. Biomass upgrading by torrefaction for the production of biofuels: A review. Biomass Bioenergy 2011, 35, 3748–3762. [Google Scholar] [CrossRef]
- Chen, D.; Zheng, Z.; Fu, K.; Zeng, Z.; Wang, J.; Lu, M. Torrefaction of biomass stalk and its effect on the yield and quality of pyrolysis products. Fuel 2015, 159, 27–32. [Google Scholar] [CrossRef]
- Chen, W.-H.; Wang, C.-W.; Ong, H.C.; Show, P.L.; Hsieh, T.-H. Torrefaction, pyrolysis and two-stage thermodegradation of hemicellulose, cellulose and lignin. Fuel 2019, 258, 116168. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, B.-J. Characteristics of products from the pyrolysis of oil palm fiber and its pellets in nitrogen and carbon dioxide atmospheres. Energy 2016, 94, 569–578. [Google Scholar] [CrossRef]
- Cui, B.; Chen, Z.; Guo, D.; Liu, Y. Investigations on the pyrolysis of microalgal-bacterial granular sludge: Products, kinetics, and potential mechanisms. Bioresour. Technol. 2021, 23, 126328. [Google Scholar] [CrossRef]
- Opatokun, S.A.; Strezov, V.; Kan, T. Product based evaluation of pyrolysis of foodwaste and its digestate. Energy 2015, 92, 349–354. [Google Scholar] [CrossRef]
- Opatokun, S.A.; Kan, T.; Al Shoaibi, A.; Srinivasakannan, C.; Strezov, V. Characterization of food waste and its digestate as feedstock for thermochemical processing. Energy Fuels 2016, 30, 1589–1597. [Google Scholar] [CrossRef]
- Jo, J.-H.; Kim, S.-S.; Sim, J.-W.; Lee, Y.-E.; Yoo, Y.-S. Pyrolysis characteristics and kinetics of food wastes. Energies 2017, 10, 1191. [Google Scholar] [CrossRef] [Green Version]
- BS EN 15359; Solid Recovered Fuels—Specifications and Classes; BSI Standards Publication: London, UK, 2011.
- Jeong, Y.; Lee, Y.-E.; Kim, I.-T. Characterization of sewage sludge and food waste-based biochar for co-firing in a coal-fired power plant: A case study in Korea. Sustainability 2020, 12, 9411. [Google Scholar] [CrossRef]
- Uemura, Y.; Omar, W.N.; Tsutsui, T.; Yusup, S.B. Torrefaction of oil palm wastes. Fuel 2011, 90, 2585–2591. [Google Scholar] [CrossRef]
- Samad, N.A.F.A.; Jamin, N.A.; Saleh, S. Torrefaction of municipal solid waste in Malaysia. Energy Procedia 2017, 138, 313–318. [Google Scholar] [CrossRef] [Green Version]
- Wannapeera, J.; Fungtammasan, B.; Worasuwannarak, N. Effects of temperature and holding time during torrefaction on the pyrolysis behaviors of woody biomass. J. Anal. Appl. Pyrolysis 2011, 92, 99–105. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, H.; Wang, X.; Du, W.; Mikulčić, H.; Duić, N. Study on extracting available salt from straw/woody biomass ashes and predicting its slagging/fouling tendency. J. Clean. Prod. 2017, 155, 164–171. [Google Scholar] [CrossRef]
- Yu, J.; Guo, Q.; Gong, Y.; Wang, J.; Yu, G. A review of the effects of alkali and alkaline earth metal species on biomass gasification. Fuel Process. Technol. 2021, 214, 106723. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, Y.; Ma, Q.; Zhou, H.; Luo, X.; Liu, X.; Wang, S. Evolution of the chemical composition, functional group, pore structure and crystallographic structure of bio-char from palm kernel shell pyrolysis under different temperatures. J. Anal. Appl. Pyrolysis 2017, 127, 350–359. [Google Scholar] [CrossRef]
- Cao, X.; Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef] [PubMed]
- Magdziarz, A.; Dalai, A.K.; Koziński, J.A. Chemical composition, character and reactivity of renewable fuel ashes. Fuel 2016, 176, 135–145. [Google Scholar] [CrossRef]
- Lee, Y.-E.; Jo, J.-H.; Kim, I.-T.; Yoo, Y.-S. Influence of NaCl concentration on food-waste biochar structure and templating effects. Energies 2018, 11, 2341. [Google Scholar] [CrossRef] [Green Version]
- Iacovidou, E.; Hahladakis, J.; Deans, I.; Velis, C.; Purnell, P. Technical properties of biomass and solid recovered fuel (SRF) co-fired with coal: Impact on multi-dimensional resource recovery value. Waste Manag. 2018, 73, 535–545. [Google Scholar] [CrossRef]
- Ministry of Environment. Enforcement Rule of the Act on the Promotion of Saving and Recycling of Resources; Ministry of Environment: Sejong City, Korea, 2020.
- Jeong, Y.; Lee, Y.-E.; Shin, D.-C.; Ahn, K.-H.; Jung, J.; Kim, I.-T. Demineralization of food waste biochar for effective alleviation of alkali and alkali earth metal species. Processes 2021, 9, 47. [Google Scholar] [CrossRef]
- Lampropoulos, A.; Kaklidis, N.; Athanasiou, C.; Montes-Morán, M.A.; Arenillas, A.; Menéndez, J.A.; Binas, V.D.; Konsolakis, M.; Marnellos, G.E. Effect of olive kernel thermal treatment (torrefaction vs. slow pyrolysis) on the physicochemical characteristics and the CO2 or H2O gasification performance of as-prepared biochars. Int. J. Hydrogen Energy 2021, 46, 29126–29141. [Google Scholar] [CrossRef]
- Chen, R.; Sheng, Q.; Dai, X.; Dong, B. Upgrading of sewage sludge by low temperature pyrolysis: Biochar fuel properties and combustion behavior. Fuel 2021, 300, 121007. [Google Scholar] [CrossRef]
- Deng, J.; Wang, G.-J.; Kuang, J.-H.; Zhang, Y.-L.; Luo, Y.-H. Pretreatment of agricultural residues for co-gasification via torrefaction. J. Anal. Appl. Pyrolysis 2009, 86, 331–337. [Google Scholar] [CrossRef]
- Angın, D. Effect of pyrolysis temperature and heating rate on biochar obtained from pyrolysis of safflower seed press cake. Bioresour. Technol 2013, 128, 593–597. [Google Scholar] [CrossRef] [PubMed]





| Properties | Values | |
|---|---|---|
| Elements (wt.%, dry) | C | 43.10 |
| H | 6.91 | |
| N | 3.14 | |
| O | 38.45 | |
| S | 0.62 | |
| Cl | 1.62 | |
| Others | 6.16 | |
| Moisture (%, wet) | 9.91 | |
| Volatile matter (%, wet) | 70.19 | |
| Ash (%, wet) | 8.78 | |
| Fixed C | 11.13 | |
| HHV (MJ·kg−1) | 18.8 | |
| Metal | Raw | 270 °C | 450 °C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 min | 20 min | 30 min | 10 min | 20 min | 30 min | ||||||||
| Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | ||
| Cd (mg·kg−1) | 0.26 ± 0.04 | 0.39 ± 0.03 | 0.43 ± 0.02 | 0.35 ± 0.04 | 0.51 ± 0.03 | 0.36 ± 0.04 | 0.43 ± 0.02 | 0.48 ± 0.03 | 0.58 ± 0.02 | 0.48 ± 0.03 | 0.55 ± 0.01 | 0.54 ± 0.02 | 0.60 ± 0.02 |
| Pb (mg·kg−1) | N.D. | 2.61 ± 0.16 | 2.32 ± 0.17 | N.D. | N.D. | 1.74 ± 0.24 | N.D. | N.D. | 1.51 ± 0.27 | 1.53 ± 0.16 | 2.50 ± 0.37 | 3.01 ± 0.18 | 2.30 ± 0.29 |
| Cr6+ (mg·kg−1) | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Hg (mg·kg−1) | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Sample | Proximate Analysis (wt.%) | Ultimate Analysis (wt.%) | Ratios | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisture | Volatile Matter | Ash | Fixed Carbon | C | H | N | O | S | FC/VM | FC + VM | C/N | H/C | O/C | |
| Raw | 9.91 ± 0.06 | 70.19 ± 0.15 | 8.78 ± 0.22 | 11.13 ± 0.03 | 43.10 ± 0.22 | 6.91 ± 0.43 | 3.14 ± 0.11 | 38.45 ± 0.09 | 0.62 ± 0.06 | 0.16 | 81.32 | 13.73 | 0.16 | 0.89 |
| 270 °C 20 min Before | 3.51 ± 0.06 | 69.89 ± 0.12 | 12.52 ± 0.38 | 14.08 ± 0.24 | 50.31 ± 0.00 | 6.45 ± 0.06 | 4.84 ± 0.89 | 28.44 ± 0.29 | 0.69 ± 0.05 | 0.20 | 83.97 | 10.39 | 0.13 | 0.57 |
| 270 °C 20 min After | 1.53 ± 0.02 | 74.01 ± 0.27 | 9.39 ± 0.39 | 15.07 ± 0.28 | 53.06 ± 0.28 | 6.65 ± 0.26 | 4.34 ± 0.19 | 26.94 ± 0.02 | 0.69 ± 0.01 | 0.20 | 89.08 | 12.23 | 0.13 | 0.51 |
| 450 °C 20 min Before | 3.30 ± 0.08 | 45.48 ± 0.51 | 25.81 ± 0.14 | 25.41 ± 0.25 | 55.98 ± 0.12 | 4.71 ± 0.07 | 4.22 ± 0.18 | 14.42 ± 0.04 | 0.28 ± 0.39 | 0.56 | 70.89 | 13.27 | 0.08 | 0.26 |
| 450 °C 20 min After | 1.53 ± 0.02 | 48.10 ± 0.21 | 23.12 ± 0.75 | 27.25 ± 1.20 | 59.30 ± 0.00 | 4.82 ± 0.02 | 4.11 ± 0.15 | 13.99 ± 0.37 | 0.27 ± 0.38 | 0.57 | 75.35 | 14.43 | 0.08 | 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, K.-H.; Shin, D.-C.; Jung, J.; Jeong, Y.; Lee, Y.-E.; Kim, I.-T. Physicochemical Properties of Torrefied and Pyrolyzed Food Waste Biochars as Fuel: A Pilot-Scale Study. Energies 2022, 15, 333. https://doi.org/10.3390/en15010333
Ahn K-H, Shin D-C, Jung J, Jeong Y, Lee Y-E, Kim I-T. Physicochemical Properties of Torrefied and Pyrolyzed Food Waste Biochars as Fuel: A Pilot-Scale Study. Energies. 2022; 15(1):333. https://doi.org/10.3390/en15010333
Chicago/Turabian StyleAhn, Kwang-Ho, Dong-Chul Shin, Jinhong Jung, Yoonah Jeong, Ye-Eun Lee, and I-Tae Kim. 2022. "Physicochemical Properties of Torrefied and Pyrolyzed Food Waste Biochars as Fuel: A Pilot-Scale Study" Energies 15, no. 1: 333. https://doi.org/10.3390/en15010333






