Evaluation of the Performance Degradation of a Metal Hydride Tank in a Real Fuel Cell Electric Vehicle
Abstract
:1. Introduction
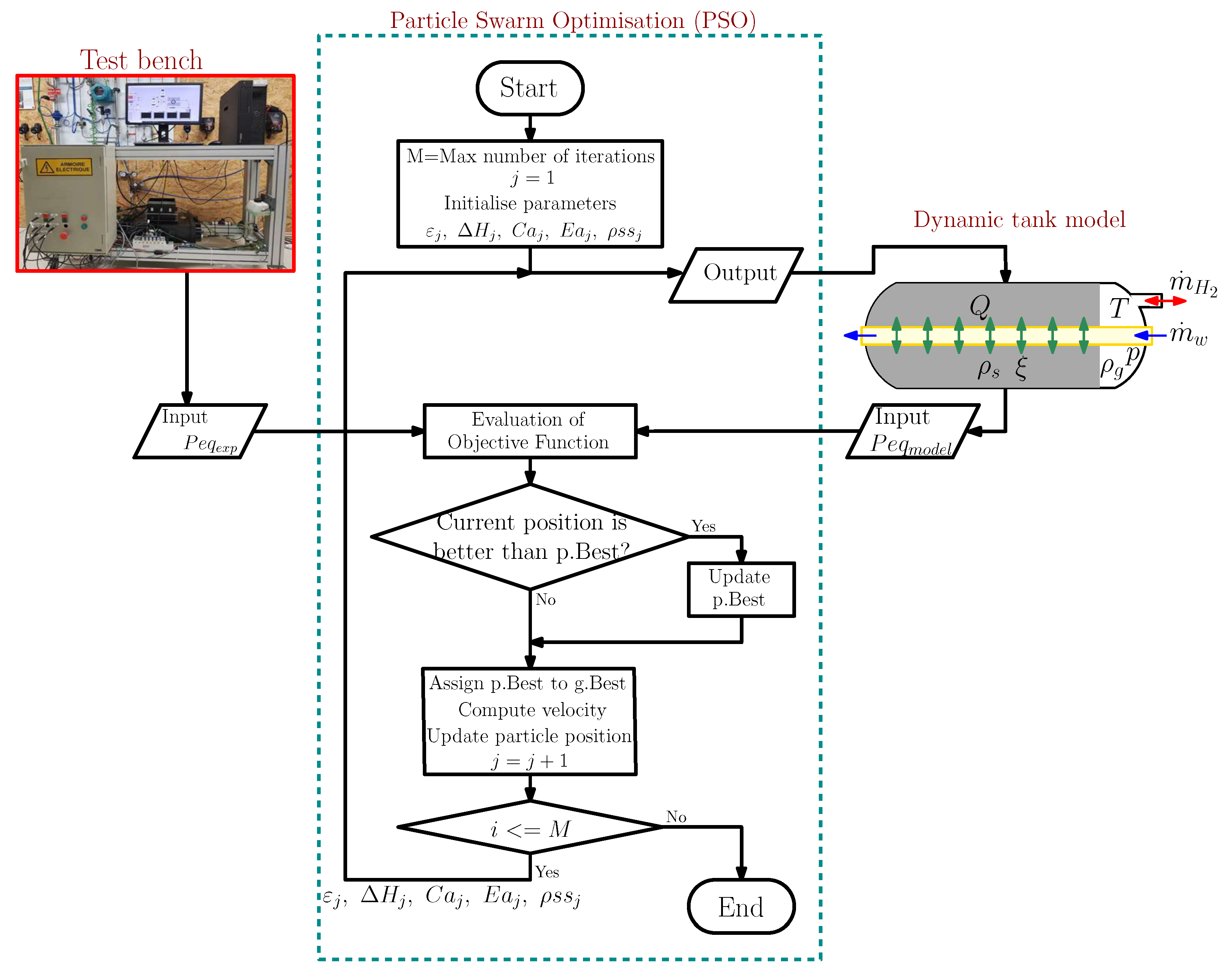
2. Modelling Process
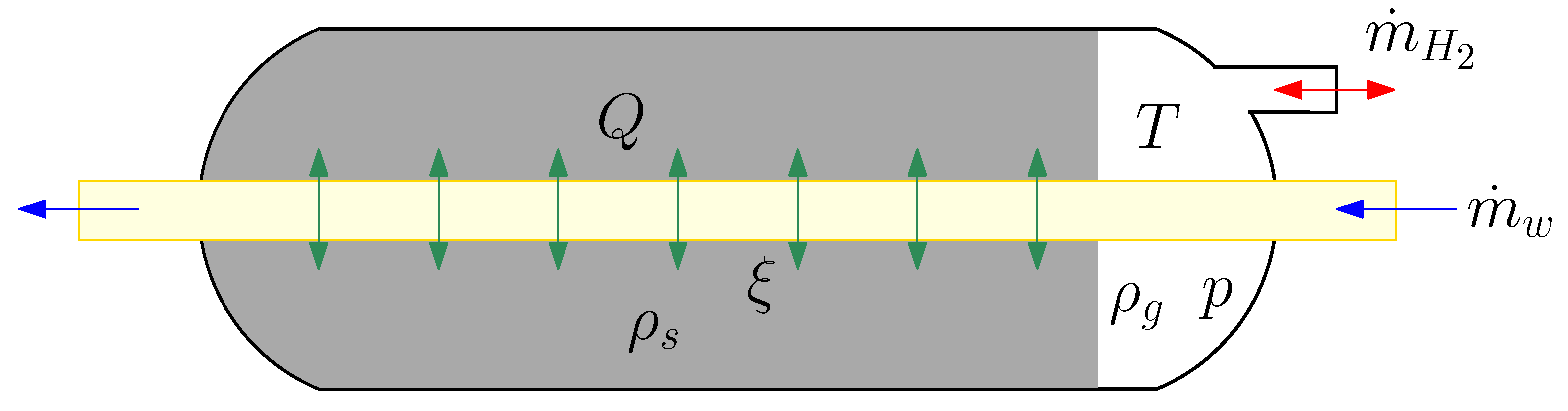
2.1. Dynamic Modelling of the Hydride Tank
- The gas phase behaves as a thermodynamically ideal gas.
- The solid phase is isotropic and has a uniform porosity.
- There is a thermal equilibrium between the gas and the solid particles.
- The thermophysical properties are constant.
- The equilibrium gas pressure is calculated by the Van’t Hoff equation.
2.2. Experimental Dynamic Tank Characterisation
2.3. Identification of the Physical Parameters of The Model
3. Evaluation of Tank Performances in Healthy and Degraded States
3.1. Hydride Tank Used in a Real FCEV
3.2. Results of the Experimental Dynamic Characterisation of the Tank
3.3. Result of the Identification of the Model’s Physical Parameters
4. Correlation between Tank Degradation and Model Physical Parameters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Physics Constants | |
| Porosity of MH | |
| Density | |
| Hydrogen atoms per metal atom | |
| Hydrogen kinetics | |
| R | Universal gas constant |
| Specific heat capacity | |
| T | Temperature |
| Heat transfer coefficient | |
| Hydrogen molar mass | |
| Enthalpy of reaction | |
| Entropy of reaction | |
| P | Pressure |
| C | Constant rate |
| E | Activation energy |
| Q | Heat flow |
| t | Time |
| D | Diameter |
| L | Length |
| V | Volume |
| m | Mass |
| Mass flow rate | |
| Volume flow rate | |
| Subscripts | |
| a | Absorption |
| Equilibrium | |
| g | Gas |
| s | Solid |
| Inlet | |
| Outlet | |
| Metal hydride | |
| M | Metal |
| Reference | |
| 0 | Empty |
| w | Water |
Appendix A
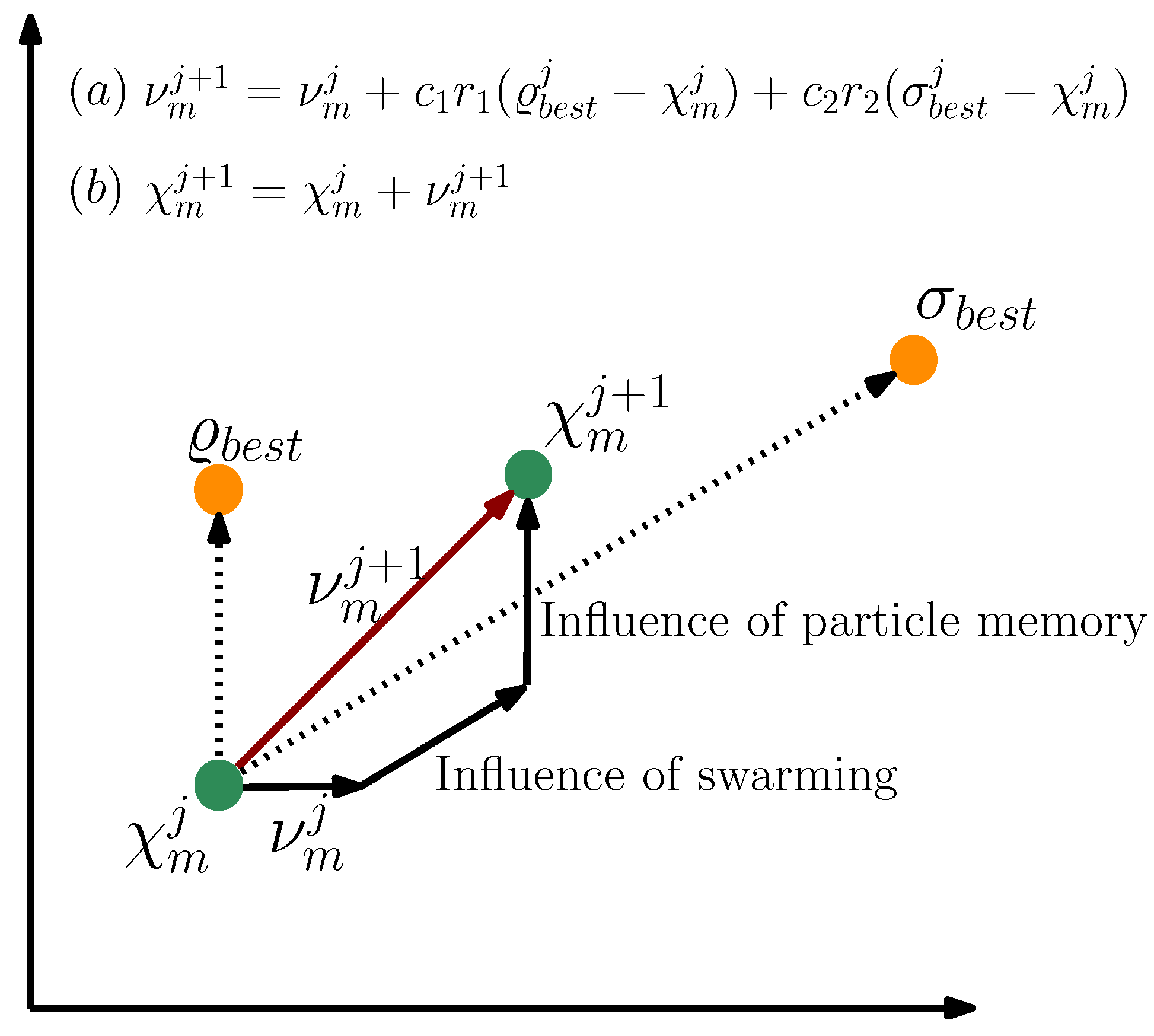
References
- Xin, G.; Yuan, H.; Yang, K.; Jiang, L.; Liu, X.; Wang, S. Promising hydrogen storage properties of cost-competitive La(Y)–Mg–Ca–Ni AB3-type alloys for stationary applications. RSC Adv. 2016, 6, 21742–21748. [Google Scholar] [CrossRef]
- Yang, C.C.; Wang, C.C.; Li, M.M.; Jiang, Q. A start of the renaissance for nickel metal hydride batteries: A hydrogen storage alloy series with an ultra-long cycle life. J. Mater. Chem. A 2017, 5, 1145–1152. [Google Scholar] [CrossRef]
- Manzetti, S.; Mariasiu, F. Electric vehicle battery technologies: From present state to future systems. Renew. Sustain. Energy Rev. 2015, 51, 1004–1012. [Google Scholar] [CrossRef]
- Joseph, B.; Iadecola, A.; Schiavo, B.; Cognigni, A.; Olivi, L.; Saini, N.L. Large atomic disorder in nanostructured LaNi5 alloys: A La L3-edge extended X-ray absorption fine structure study. J. Phys. Chem. Solids 2010, 71, 1069–1072. [Google Scholar] [CrossRef] [Green Version]
- Balogun, M.S.; Wang, Z.M.; Chen, H.X.; Deng, J.Q.; Yao, Q.R.; Zhou, H.Y. Effect of Al content on structure and electrochemical properties of LaNi4.4-xCo0.3Mn0.3Alx hydrogen storage alloys. Int. J. Hydrogen Energy 2013, 38, 10926–10931. [Google Scholar] [CrossRef]
- Wjihi, S.; Sellaoui, L.; Bouzid, M.; Dhaou, H.; Knani, S.; Jemni, A.; Ben Lamine, A. Theoretical study of hydrogen sorption on LaNi5 using statistical physics treatment: Microscopic and macroscopic investigation. Int. J. Hydrogen Energy 2017, 42, 2699–2712. [Google Scholar] [CrossRef]
- Chabane, D.; Harel, F.; Djerdir, A.; Ibrahim, M.; Candusso, D.; Elkedim, O.; Fenineche, N. Influence of the key parameters on the dynamic behaviour of the hydrogen absorption by LaNi5. Int. J. Hydrogen Energy 2017, 42, 1412–1419. [Google Scholar] [CrossRef] [Green Version]
- Dhaou, M.H.; Belkhiria, S.; Sdiri, N.; Mallah, A.; Al-Thoyaib, S.; Jemni, A.; Ben Nasrallah, S. Thermodynamic and electric study of the LaNi3,6Al0,4Co0,7Mn0,3 alloy. Int. J. Hydrogen Energy 2017, 42, 2209–2214. [Google Scholar] [CrossRef]
- Borzone, E.M.; Blanco, M.V.; Baruj, A.; Meyer, G.O. Stability of LaNi5-xSnx cycled in hydrogen. Int. J. Hydrogen Energy 2014, 39, 8791–8796. [Google Scholar] [CrossRef]
- Spodaryk, M.; Shcherbakova, L.; Sameljuk, A.; Wichser, A.; Zakaznova-Herzog, V.; Holzer, M.; Braem, B.; Khyzhun, O.; Mauron, P.; Remhof, A.; et al. Description of the capacity degradation mechanism in LaNi5-based alloy electrodes. J. Alloys Compd. 2015, 621, 225–231. [Google Scholar] [CrossRef]
- Pinatel, E.R.; Palumbo, M.; Massimino, F.; Rizzi, P.; Baricco, M. Hydrogen sorption in the LaNi5-xAlx-H system (0 ≤ x ≤ 1). Intermetallics 2015, 62, 7–16. [Google Scholar] [CrossRef]
- Luo, S.; Clewley, J.D.; Flanagan, T.B.; Bowman, R.C.; Cantrell, J.S. Split Plateaux in the LaNi5–H System and the Effect of Sn Substitution on Splitting. J. Alloys Compd. 1997, 253–254, 226–231. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Wu, J.; Wang, Q.D. Disproportionation of LaNi5 and TiFe in 4 MPa H2 at 300 °C. J. Alloys Compd. 1992, 185, 1–6. [Google Scholar] [CrossRef]
- Han, J.; Lee, J.Y. An investigation of the intrinsic degradation mechanism of LaNi5 by thermal desorption technique. Int. J. Hydrogen Energy 1988, 13, 577–581. [Google Scholar] [CrossRef]
- Benham, M.J.; Ross, D.K.; Lartigue, C.; Percheron-Guégan, A. Inelastic Neutron Scattering Studies of Multiply Cycled Lanthanum-Nickel Hydride*. Z. Für Phys. Chem. 1986, 147, 219–229. [Google Scholar] [CrossRef]
- Wan, C.; Ju, X.; Qi, Y.; Zhang, Y.; Wang, S.; Liu, X.; Jiang, L. Synchrotron XRD and XANES studies of cerium-doped NaAlH4: Elucidation of doping induced structure changes and electronic state. J. Alloys Compd. 2009, 481, 60–64. [Google Scholar] [CrossRef]
- Peng, X.; Liu, B.; Fan, Y.; Zhu, X.; Peng, Q.; Zhang, Z. Microstructures and electrochemical hydrogen storage characteristics of La0.7Ce0.3Ni4.2Mn0.9-xCu0.37(Fe0.43B0.57)x (x = 0–0.20) alloys. J. Power Sources 2013, 240, 178–183. [Google Scholar] [CrossRef]
- Casini, J.C.S.; Silva, F.M.; Guo, Z.P.; Liu, H.K.; Faria, R.N.; Takiishi, H. Effects of substituting Cu for Sn on the microstructure and hydrogen absorption properties of Co-free AB5 alloys. Int. J. Hydrogen Energy 2016, 41, 17022–17028. [Google Scholar] [CrossRef]
- Zhu, D.; Ait-Amirat, Y.; N’Diaye, A.; Djerdir, A. New dynamic modeling of a real embedded metal hydride hydrogen storage system. Int. J. Hydrogen Energy 2019, 44, 29203–29211. [Google Scholar] [CrossRef]
- Higel, C.; Harel, F.; Candusso, D.; Faivre, S.; Ravey, A.; Guilbert, D.; N’diaye, A.; Gaillard, A.; Bouquain, D.; Djerdir, A.; et al. Part 1: Mobypost vehicle’s powertrain modeling, simulation and sizing. In Proceedings of the 5th International Conference on Fundamentals and Development of Fuel Cells, Karlsruhe, Germany, 15–17 April 2013; p. 2357414. [Google Scholar]
- Faivre, S.; Ravey, A.; Guilbert, D.; Ndiaye, A.; Gaillard, A.; Bouquain, D.; Djerdir, A.; Higel1, C.; Harel, F.; Candusso, D. Part 2—Mobypost vehicle’s powertrain desing and experimental validation. In Proceedings of the 5th International Conference on Fundamentals and Development of Fuel Cells, Karlsruhe, Germany, 15–17 April 2013. [Google Scholar]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Ren, J.; Musyoka, N.M.; Langmi, H.W.; Mathe, M.; Liao, S. Current research trends and perspectives on materials-based hydrogen storage solutions: A critical review. Int. J. Hydrogen Energy 2017, 42, 289–311. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, Z.; Cheng, H.; Li, K.; Yan, K.; Han, X.; Wang, Y.; Liu, Y. Long-term hydrogen storage performance and structural evolution of LaNi4Al alloy. J. Alloys Compd. 2018, 731, 172–180. [Google Scholar] [CrossRef]
- Chabane, D.; Harel, F.; Djerdir, A.; Candusso, D.; Elkedim, O.; Fenineche, N. Energetic modeling, simulation and experimental of hydrogen desorption in a hydride tank. Int. J. Hydrogen Energy 2019, 44, 1034–1046. [Google Scholar] [CrossRef]
- Afzal, M.; Mane, R.; Sharma, P. Heat transfer techniques in metal hydride hydrogen storage: A review. Int. J. Hydrogen Energy 2017, 42, 30661–30682. [Google Scholar] [CrossRef]
- Tong, L.; Xiao, J.; Yang, T.; Bénard, P.; Chahine, R. Complete and reduced models for metal hydride reactor with coiled-tube heat exchanger. Int. J. Hydrogen Energy 2019, 44, 15907–15916. [Google Scholar] [CrossRef]
- Busqué, R.; Torres, R.; Grau, J.; Roda, V.; Husar, A. Effect of metal hydride properties in hydrogen absorption through 2D-axisymmetric modeling and experimental testing in storage canisters. Int. J. Hydrogen Energy 2017, 42, 19114–19125. [Google Scholar] [CrossRef] [Green Version]
- Busqué, R.; Torres, R.; Grau, J.; Roda, V.; Husar, A. Mathematical modeling, numerical simulation and experimental comparison of the desorption process in a metal hydride hydrogen storage system. Int. J. Hydrogen Energy 2018, 43, 16929–16940. [Google Scholar] [CrossRef] [Green Version]
- Mellouli, S.; Ben Khedher, N.; Askri, F.; Jemni, A.; Ben Nasrallah, S. Numerical analysis of metal hydride tank with phase change material. Appl. Therm. Eng. 2015, 90, 674–682. [Google Scholar] [CrossRef]
- Askri, F.; Ben Salah, M.; Jemni, A.; Ben Nasrallah, S. Optimization of hydrogen storage in metal-hydride tanks. Int. J. Hydrogen Energy 2009, 34, 897–905. [Google Scholar] [CrossRef]
- Abdin, Z.; Webb, C.J.; Gray, E.M.A. One-dimensional metal-hydride tank model and simulation in Matlab–Simulink. Int. J. Hydrogen Energy 2018, 43, 5048–5067. [Google Scholar] [CrossRef]
- Chaise, A.; De Rango, P.; Marty, P.; Fruchart, D. Experimental and numerical study of a magnesium hydride tank. Int. J. Hydrogen Energy 2010, 35, 6311–6322. [Google Scholar] [CrossRef]
- Suárez, S.; Chabane, D.; N’Diaye, A.; Ait-Amirat, Y.; Djerdir, A. Static and dynamic characterization of metal hydride tanks for energy management applications. Renew. Energy 2022, 191, 59–70. [Google Scholar] [CrossRef]
- Kennedy, J.; Eberhart, R. Particle swarm optimization. In Proceedings of the ICNN’95—International Conference on Neural Networks, Perth, WA, Australia, 27 November–1 December 1995; Volume 4, pp. 1942–1948. [Google Scholar] [CrossRef]
- Kennedy, J. Particle swarm: Social adaptation of knowledge. In Proceedings of the IEEE Conference on Evolutionary Computation, (ICEC 1997), Indianapolis, IN, USA, 3–16 April 1997; pp. 303–308. [Google Scholar] [CrossRef]
- Storn, R. Differrential evolution—A simple and efficient adaptive scheme for global optimization over continuous spaces. Tech. Rep. Int. Comput. Sci. Inst. 1995, 11, 341–359. [Google Scholar]
- Chabane, D.; Harel, F.; Djerdir, A.; Candusso, D.; ElKedim, O.; Fenineche, N. A new method for the characterization of hydrides hydrogen tanks dedicated to automotive applications. Int. J. Hydrogen Energy 2016, 41, 11682–11691. [Google Scholar] [CrossRef]
- Suarez, S.H.; Chabane, D.; N’Diaye, A.; Ait-Amirat, Y.; Djerdir, A. Dynamic and Static characterization of the absorption process in metal hydride tanks for Mobile Applications. In Proceedings of the 2021 IEEE Vehicle Power and Propulsion Conference, VPPC 2021-Proceeding, Gijón, Spain, 25 October–14 November 2021. [Google Scholar] [CrossRef]
- Shi, Y.; Eberhart, R.C. Empirical study of particle swarm optimization. In Proceedings of the 1999 Congress on Evolutionary Computation-CEC99, Washington, DC, USA, 6–9 July 1999; Volume 3, pp. 1945–1950. [Google Scholar] [CrossRef]








| Definition | Equation | Ref. |
|---|---|---|
| Mass balance for the gas | [27] | |
| Mass balance for the metal alloy | [28] | |
| Kinetics of the process | [29] | |
| [29] | ||
| Equilibrium pressure | [30] | |
| Experimental equilibrium pressure | [31] | |
| Mass of hydrogen | [25] | |
| Gravimetric storage capacity | [32] | |
| Energy balance | [33] | |
| Heat exchanged | [25] |
| Parameter | Healthy State | Degraded State | Variation (%) | Unit |
|---|---|---|---|---|
| Pabs | 3.06 | 3.77 | 23.2 | bar |
| Pdes | 1.47 | 2.26 | 53.7 | bar |
| Hysteresis | 0.75 | 0.51 | −32 | |
| Temperature | 21 | 21 | 0 | C |
| Concentration | 1.52 | 0.81 | −47.4 | % |
| Parameters | Range | State | (%) | Units | |
|---|---|---|---|---|---|
| Healthy | Degraded | ||||
| [0.2, 0.7] | 0.5021 | 0.3553 | 29.3 | - | |
| [40, 70] | 58.020 | 43.858 | 24.4 | 1/s | |
| [20, 22] | 20.989 | 20.681 | 1.4 | kJ/mol | |
| [7, 10] | 9.970 | 7.996 | 19.8 | 1/s | |
| [15, 20] | 16.510 | 16.193 | 1.9 | kJ/mol | |
| [8400, 8600] | 8473 | 8446 | 0.31 | kg/m3 | |
| [6000, 36,000] | −31,660 | −6753 | 78.5 | J/mol | |
| [6000, 36,000] | 31,800 | 13,856 | 56.4 | J/mol | |
| Simulation error | <2.5 | 1.8095 | 1.503 | - | % |
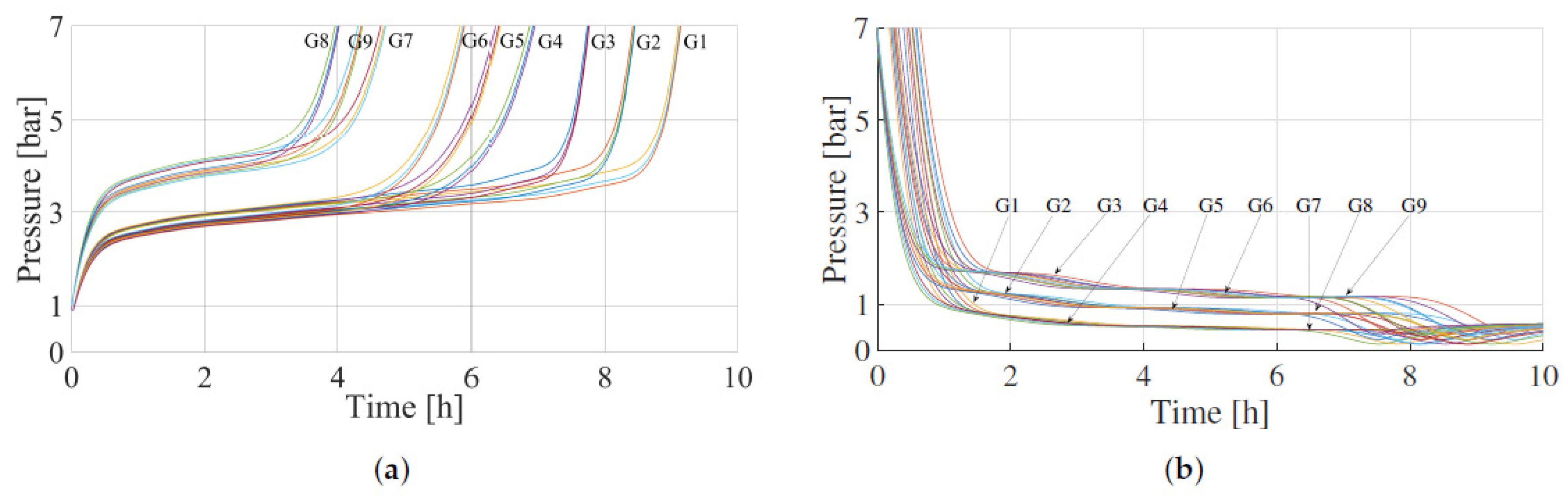
| Absorption | Desorption | Simulation Groups | ||||
|---|---|---|---|---|---|---|
| (kg/m3) | (J/mol) | (kg/m3) | (J/mol) | |||
| 8470 | 0.45 | 30,000 | 8470 | 0.45 | 30,000 | G1 |
| 20,000 | 22,000 | |||||
| 10,000 | 14,000 | |||||
| 0.40 | 30,000 | 0.40 | 30,000 | G2 | ||
| 20,000 | 22,000 | |||||
| 10,000 | 14,000 | |||||
| 0.35 | 30,000 | 0.35 | 30,000 | G3 | ||
| 20,000 | 22,000 | |||||
| 10,000 | 14,000 | |||||
| 8460 | 0.45 | 30,000 | 8460 | 0.45 | 30,000 | G4 |
| 20,000 | 22,000 | |||||
| 10,000 | 14,000 | |||||
| 0.40 | 30,000 | 0.40 | 30,000 | G5 | ||
| 20,000 | 22,000 | |||||
| 10,000 | 14,000 | |||||
| 0.35 | 30,000 | 0.35 | 30,000 | G6 | ||
| 20,000 | 22,000 | |||||
| 10,000 | 14,000 | |||||
| 8450 | 0.45 | 30,000 | 8450 | 0.45 | 30,000 | G7 |
| 20,000 | 22,000 | |||||
| 10,000 | 14,000 | |||||
| 0.40 | 30,000 | 0.40 | 30,000 | G8 | ||
| 20,000 | 22,000 | |||||
| 10,000 | 14,000 | |||||
| 0.35 | 30,000 | 0.35 | 30,000 | G9 | ||
| 20,000 | 22,000 | |||||
| 10,000 | 14,000 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suárez, S.H.; Chabane, D.; N’Diaye, A.; Ait-Amirat, Y.; Elkedim, O.; Djerdir, A. Evaluation of the Performance Degradation of a Metal Hydride Tank in a Real Fuel Cell Electric Vehicle. Energies 2022, 15, 3484. https://doi.org/10.3390/en15103484
Suárez SH, Chabane D, N’Diaye A, Ait-Amirat Y, Elkedim O, Djerdir A. Evaluation of the Performance Degradation of a Metal Hydride Tank in a Real Fuel Cell Electric Vehicle. Energies. 2022; 15(10):3484. https://doi.org/10.3390/en15103484
Chicago/Turabian StyleSuárez, Santiago Hernán, Djafar Chabane, Abdoul N’Diaye, Youcef Ait-Amirat, Omar Elkedim, and Abdesslem Djerdir. 2022. "Evaluation of the Performance Degradation of a Metal Hydride Tank in a Real Fuel Cell Electric Vehicle" Energies 15, no. 10: 3484. https://doi.org/10.3390/en15103484






