Abstract
This article presents experimental studies that have shown the effectiveness of using a composition of fly ash and metakaolin as an active mineral admixture to cement. It is shown that composite ash–metakaolin additives (AMAd) have on the one hand increased pozzolanic activity and surface energy, and, on the other hand, moderated water demand and provided a significant increase in strength, which is especially important for low-cement concretes. The obtained experimental–statistical models make it possible to determine the effect of the AMAd composition, its content in combination with the addition of a superplasticizer on the rheological properties, the kinetics of structure formation and the main physical and mechanical properties of cement.
1. Introduction
Portland cement is one of the most commonly used mineral binders, but its production is associated with high CO2 emissions. The carbon footprint of the cement industry affects the natural environment, which results in climate change. Nowadays, in many areas, measures are being taken to reduce the environmental impact and energy consumption of the construction sector. In the cement industry, this means the need for effective mineral additives, including industrial waste materials use. Thanks to such contributions, it is possible to considerably reduce the cost and environmental impact of concrete and thus reduce the carbon footprint of the construction sector.
One of the ways to save Portland cement is the introduction of active mineral additives into cement or concrete mix. The effect of mineral additives on the strength of concrete is associated with a modified indicator (C/W)′, [1], calculated by the formula:
where C is the Portland cement consumption, kg/m3, Kc.e.—admixture cementing efficiency coefficient, numerically equal to the possible savings of Portland cement per 1 kg of admixture, ma—admixture consumption, W—water consumption in the concrete mixture.
To date, a significant number of studies have been carried out aimed at improving the efficiency of mineral additives introduced into cement during its production or directly into the concrete mix. To the greatest extent, the methods of mineral additives activation have been developed in relation to cement. The main ones are regrinding, vibration activation, turbulent, acoustic, ultrasonic, thermal, electric-thermal and electric-pulse processing [2,3]. There are a number of recommendations for the activation of mineral additives by modifying their surface with various chemicals, including surfactants, halogen-containing and organic-silicon substances [4]. When choosing a method for activating mineral additives, it is necessary to take into account, along with the effect of increasing their activity, the manufacturability and energy intensity of the processing of the powdered component, the possible productivity of the aggregates and the controllability and stability of the achieved parameters of the activated material. The most developed mechanical and mechanical–chemical activation methods, based on the regrinding of powdered components, have a number of disadvantages. The main ones are high energy intensity and the need to install a rather complex technological line in the conditions of the reinforced concrete plant, including a grinding unit, dust collection and other equipment [5].
Coal sour fly ash is among the most common and relatively well-studied mineral admixtures for cement concrete [4]. The pozzolanic activity of fly ash is relatively low (10–50 mg CaO/g), and it depends mainly on the content and composition of the vitreous phase, as well as dispersion [4,6]. For example, the activity of fly ash from the Pridneprovskaya thermal power station (Ukraine) was about 30 mg/g, and after grinding to complete passage through a No. 008 sieve, it was 63 mg/g [7]. Such a fine grinding of ash as a way to increase activity, however, is very energy intensive and difficult to implement.
The big advantage of fly ash as an active additive in cement concretes is its low water requirement. The introduction of ash into concrete mixture, unlike other active mineral additives, does not worsen, but, as a rule, improves the workability [8].
Furthermore, paste containing fly ash has a lower hydration heat compared with pure Portland cement-based paste [9]. The addition of fly ash could also contribute to reducing the permeability of concrete [10]. Concrete with a high content of fly ash has good durability in certain harsh conditions, such as the marine environment [11].
Metakaolin, a product of kaolin clays calcination and a pozzolanic mineral admixture, can serve as a highly reactive cementitious component of mineral binders, concrete and mortars. The most applicable areas of metakaolin in cement-based composites are self-leveling and self-compacting concrete and high-strength concrete, due to the high aesthetical appearance of metakaolin–architectural concrete finishing plasters.
The results of the Dadsetan and Bai et al. [12] research show that the addition of metakaolin can improve the transition zone of concrete, positively affecting concrete strength. Metakaolin also increases concrete durability in aggressive environments. According to Ferreira et al. [13], metakaolin enhances the chloride penetration resistance of concrete. Moreover, the addition of metakaolin can reduce the porosity and increase the matrix density [10]. Bucher et al. [14] found beneficial effects of combining metakaolin with limestone filler. Fine pores formed in the material structure improved carbonation resistance.
The MK content in concrete is recommended in the amount of 5–10% per cement weight. A higher MK content (such as microsilica) leads to a sharp increase in the concrete mixtures’ water demand, the need for an increased amount of superplasticizers and a decrease in the crack resistance of the concrete. This disadvantage of metakaolin can be offset by the addition of fly ash, of which the glassy surface of the particles leads to a certain plasticizing effect.
In addition to experimental studies, the literature can find various numerical models describing cements containing mineral additives, including fly ash and metakaolin blends. Hydration models were proposed to estimate the degree of hydration and the development of the concrete microstructure with mineral additives [15,16,17]. With the help of a numerical model, Kinomura and Ishida [18] described the hydration process of cement–fly ash blends taking into account the development of micro-pore structures. Other simulations have also been developed, which can be helpful in analyzing the development of concrete strength when mineral additives are used. The literature also provides models that predict the carbonation of concretes modified with mineral additives [19,20].
On the one hand, it can be assumed that composite ash–metakaolin additives (AMAd) should have increased pozzolanic activity and surface energy, whereas on the other hand, should also have moderate water demand and provide a significant increase in strength, which is especially important for low-cement concretes. This is the essence of the working hypothesis underlying this study.
2. Materials and Methods
Portland cement CEM I 42.5, fly ash and metakaolin, with the chemical composition and specific surface area given in Table 1, were used in the first research.

Table 1.
Characteristics of raw materials.
The mineralogical composition of the Portland cement clinker used was: C3S—57.10, C2S—21.27, C3A—6.87%, C4A—12.19%. The most significant oxides contained in the ash SiO2 and Al2O3, according to microscopic analysis, are in a vitreous form. Its composition also contains quartz and mullite (3Al2O3∙2SiO2). Losses on ignition in the ash are represented by unburned carbonaceous particles contained in fine fractions.
A naphthalene–formaldehyde superplasticizer was introduced into cement containing ash–metakaolin mineral additives.
Based on the concept [8] that the effectiveness of mineral additives in cement system is influenced by their surface energy and pozzolanic activity, the corresponding characteristics of these properties were studied.
The powders’ wetting heat as one of the criteria of surface energy was determined by the calorimetric method in water (Q1) and benzene (Q2) [8] with the determination of the hydrophilicity coefficient β12 = Q1/Q2.
The pozzolanic activity of the composite ash–metakaolin admixture was determined by the titration method by the CaO absorption from a saturated solution [21,22,23].
Features of cement–water pastes with additives of AMAd hydration were studied using differential thermal analysis [24]. To determine the degree of hydration by calcined cement stone samples, the amount of hydrated water was found [25]. Structure formation was characterized by measuring the kinetics of changes in plastic strength [26].
The physical and mechanical properties of concrete mixtures and hardened concrete with a composite active mineral admixture were determined in accordance with EN191–1 [23].
In our studies, to determine the calculated dependences, mathematical planning of experiments was used to obtain the necessary experimental–statistical models [27].
3. Results and Discussion
Wetting heat.Figure 1 shows the experimental results of determining the heat Q and wetting coefficient β of ash–metakaolin and their mixtures, depending on the volume ratio X of metakaolin (Vmk) in the total volume of metakaolin and fly ash (Vmk + Va):
These data indicate that the addition of MK to fly ash increases the total surface energy of the mixture not according to the linear law of mixtures, but in accordance with a power law of the type:
where A and β are constants.
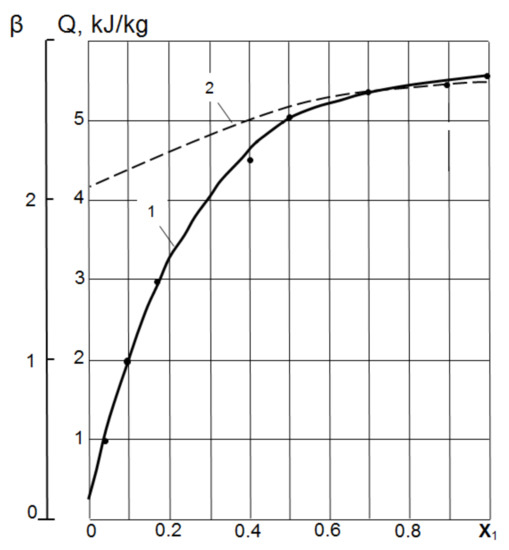
Figure 1.
Influence of the MK volume concentration in a mixture of MK and fly ash on the heat of wetting Q and coefficient β: 1—heat of wetting Q in mixtures MK-Ash; 2—coefficient β in the mixtures MK-Ash.
Figure 1.
Influence of the MK volume concentration in a mixture of MK and fly ash on the heat of wetting Q and coefficient β: 1—heat of wetting Q in mixtures MK-Ash; 2—coefficient β in the mixtures MK-Ash.
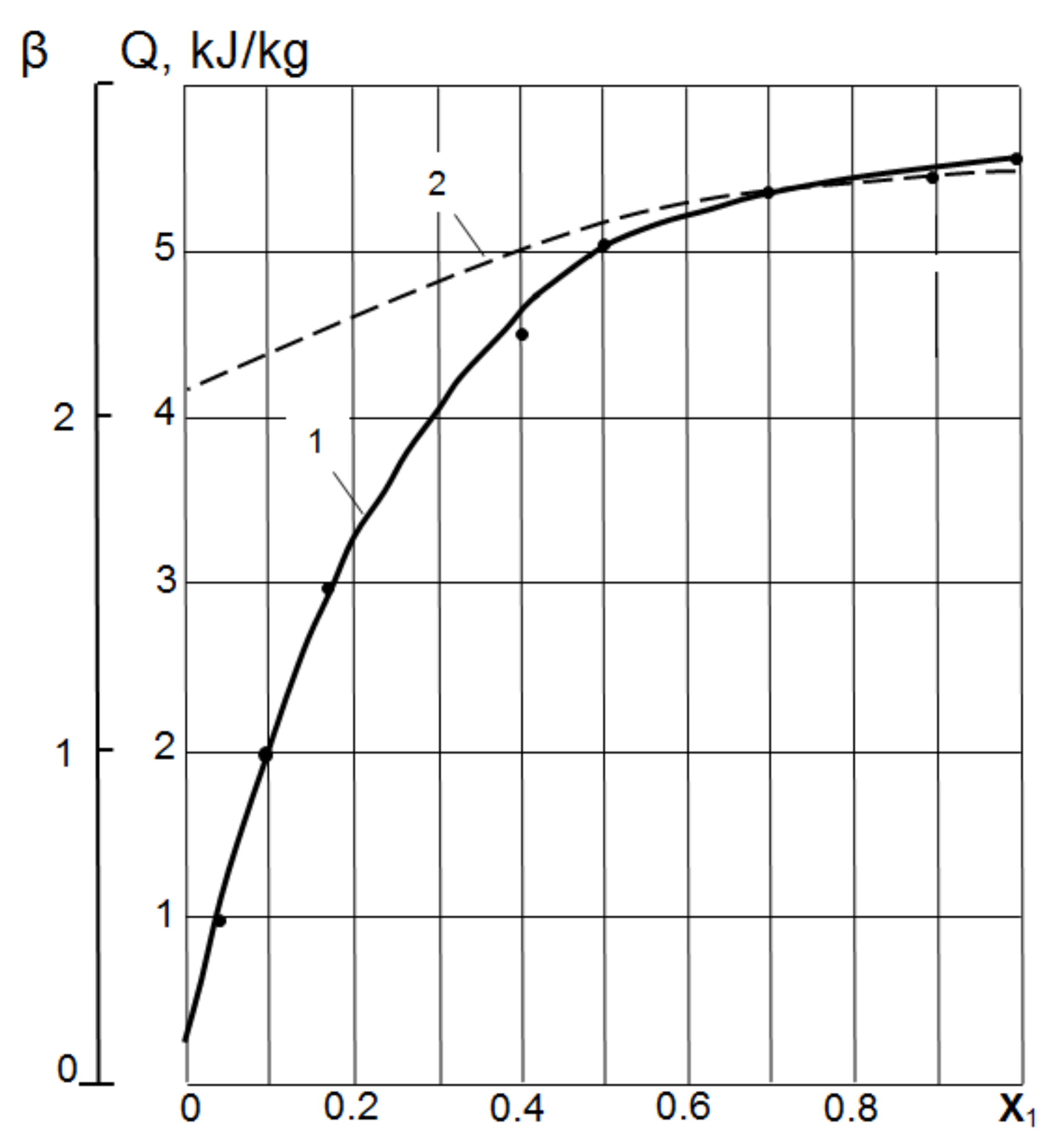
At X = 0.2, the values of Q are 50–58, and at X = 0.4 it is already about 75% of the pure MK wetting heat. The mechanism of the disproportionate increase in the energy characteristics of MK–fly ash mixtures with an increase in the share of MK can be associated with the formation of cluster structures, the cores of which are relatively large ash particles, and the peripheral shell is formed by MK particles [28].
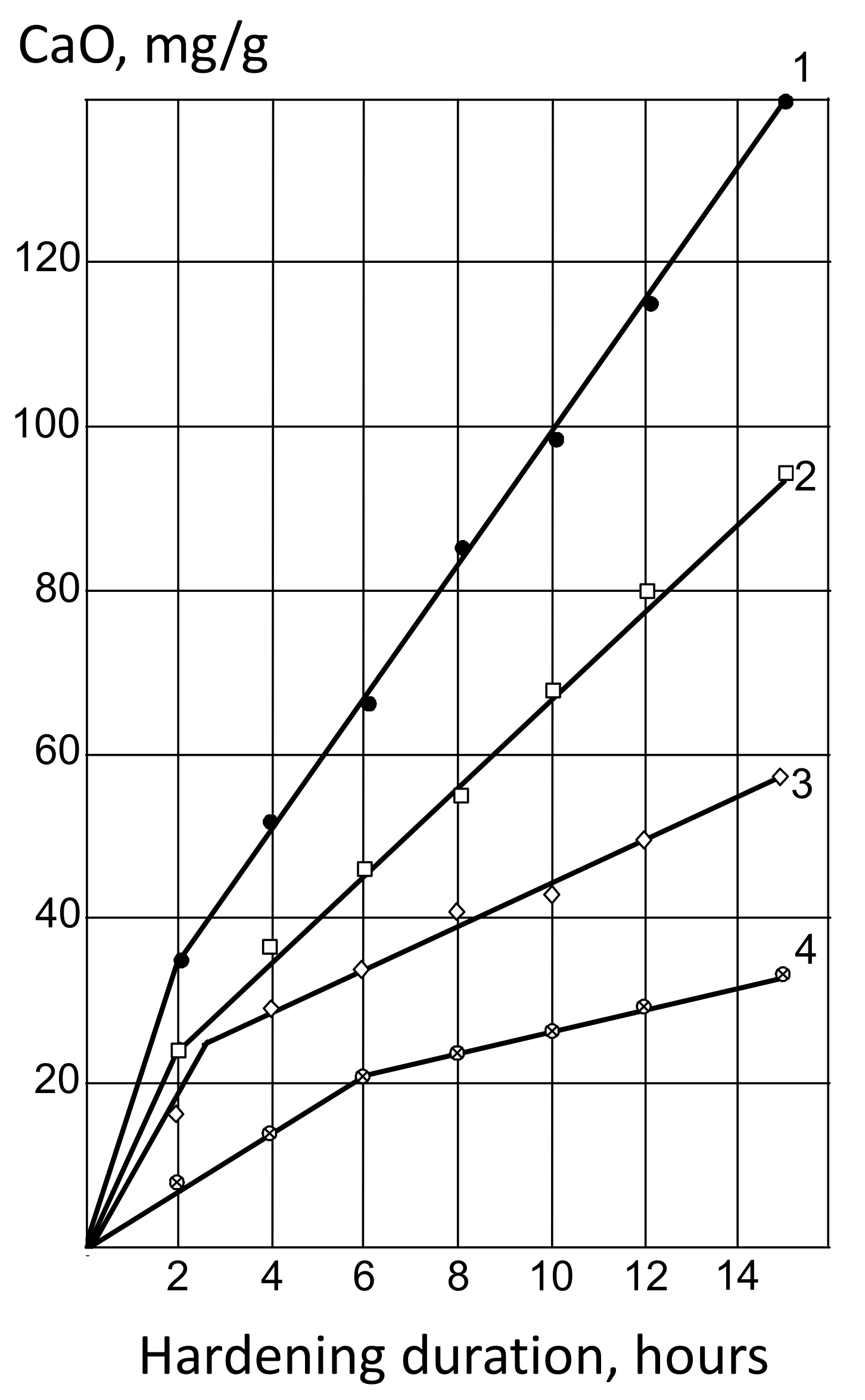
Pozzolanic activity and hydration degree. Pozzolanic activity and the kinetics of CaO uptake from a saturated solution were determined for the AMAd of various compositions. When studying the kinetics CaO by active mineral materials absorption, a saturated CaO solution and the powders studied were mixed beforehand and thermostated at a temperature of 80 °C and; after a certain holding time, the concentration of Ca(OH)2 was measured by titration.
The results of the experiments are shown in Table 2 and in Figure 2. The pozzolanic activity of MK, as follows from the data presented, is 3.5–4.5 times higher than that of fly ash. Regrinding ash to Ssp = 420–450 m2/kg increases its activity only by 1.5 times, whereas mixing with 20–30% MK allows for increasing lime absorption by almost 2 times.

Table 2.
Pozzolanic activity of MK, fly ash and their mixtures.
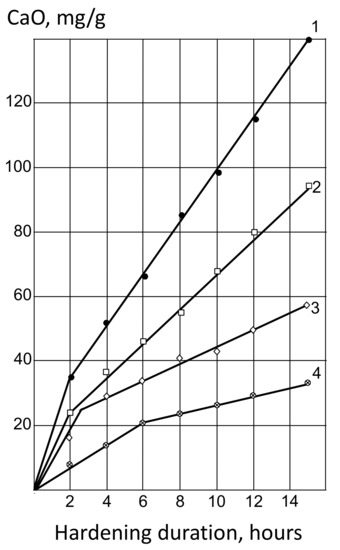
Figure 2.
Kinetics of CaO by active admixture absorption: 1—MK; 2—MK—50%, A—50%; 3—MK–20%, A—80%; 4—A.
On the graphs of the kinetics of CaO by MK absorption, fly ash and their mixtures (Figure 2) into two periods are traced; the first, corresponding to a sharp initial change in Ca(OH)2 concentration, and the second, characterized by a slower process rate. The duration of the first period in MK and ash–metakaolin mixtures is approximately 3–4 times shorter than in fly ash.
The separation of the process of absorption of CaO by colloidal silica into two periods was previously noted in the work of P. E. Halstead and S. D. Lowrence [29]. They interpreted the first period as Ca(OH)2 adsorption on SiO2 particles. The decrease in the concentration of Ca(OH)2 occurs due to the precipitation of calcium hydrosilicate from the solution, which is formed as a result of a chemical reaction.
According to some researchers [30], the pozzolanic reaction of ash also begins with the adsorption of calcium hydroxide on its surface. Under normal conditions, this film on ash particles forms in concrete within 24 h, regardless of the aggregate type. The thin boundary layers that arise in this case serve as conductors of calcium ions, under the influence of which a gradual erosion of the ash particles surface develops. Grooves are formed in it, where the products of the pozzolanic reaction settle.
In accordance with this mechanism, there are active centers on the surface of the ash. Ca (OH)2 particles initially diffuse to these centers. Subsequently, the precipitation of Ca(OH)2 on the active sites begins and a spherical film of calcium hydroxide appears. The chemical interaction between Ca (OH)2 particles and the active glassy phase completes the pozzolanic reaction of the ash.
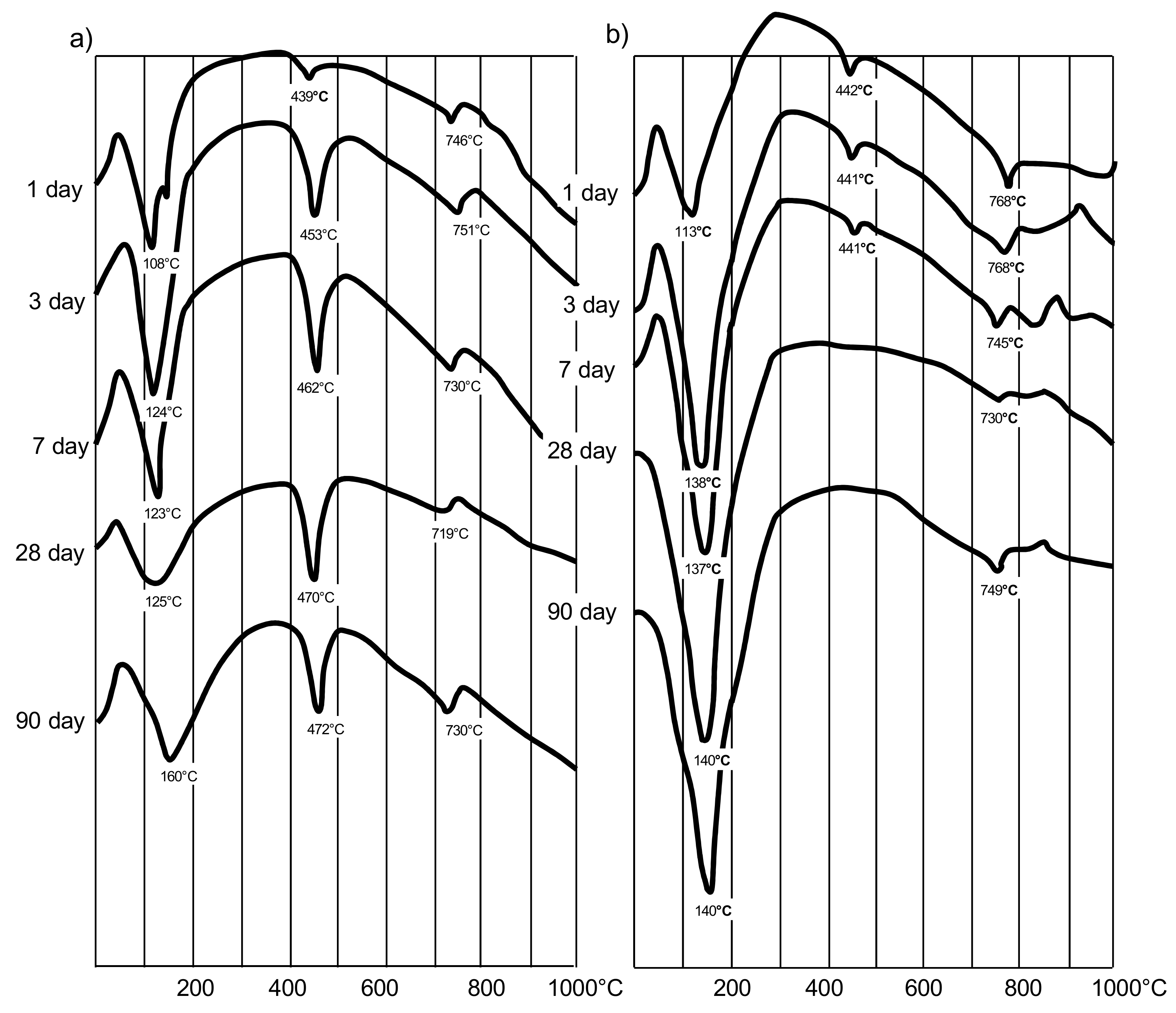
Differential thermal analysis was carried out to study the features of hydration of cement with AMAd. As follows from the thermograms (Figure 3), the introduction of MK significantly accelerates the binding of Ca (OH)2, which is released during the hydrolysis of alite and the formation of low-basic hydrosilicates. Almost complete absorption of Ca(OH)2 and, accordingly, the formation of hydrate neoplasms occurs when the content of AMAd, including 20–30% MK, is up to 40% by weight.
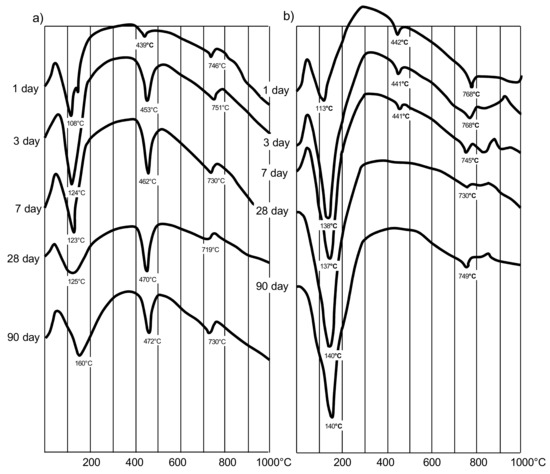
Figure 3.
DTA curves: (a)—cement stone containing 40% fly ash (a) by weight of cement; (b)—cement stone containing 40% AMAd (30% MK is part of AMAd).
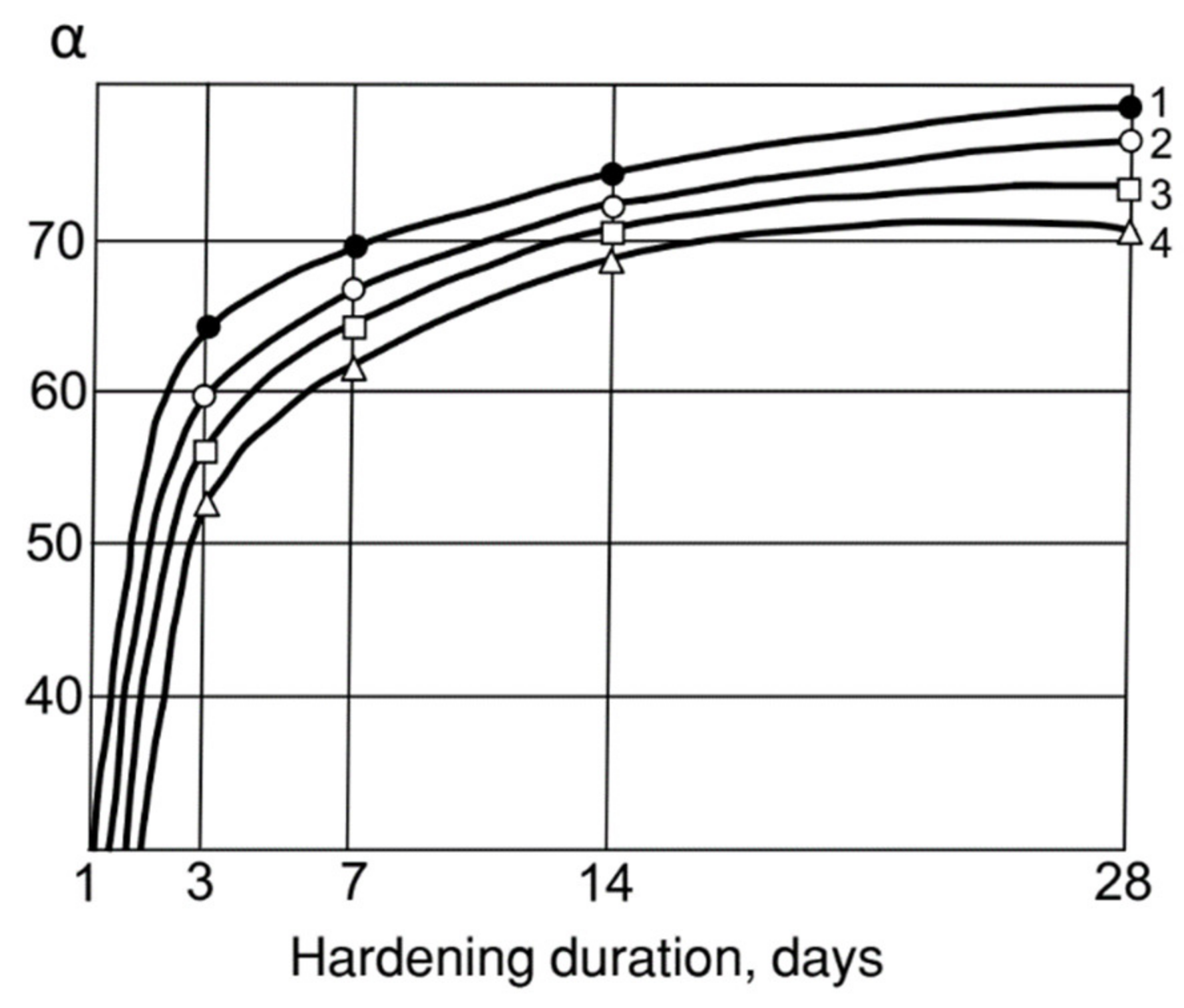
By accelerating the Ca(OH)2 binding, MK contributes to a more complete hydrolysis of clinker minerals and an increase in the degree of cement hydration. As can be seen from Figure 4, the introduction of 30% MK brings the cement hydration degree up to 0.8 in 28 days, whereas already at 3 days of age it is 0.65, i.e., 25% higher than cement without MK. The hydration–accelerating effect of AMAd containing only 30% MK is quite close to the pure MK effect.
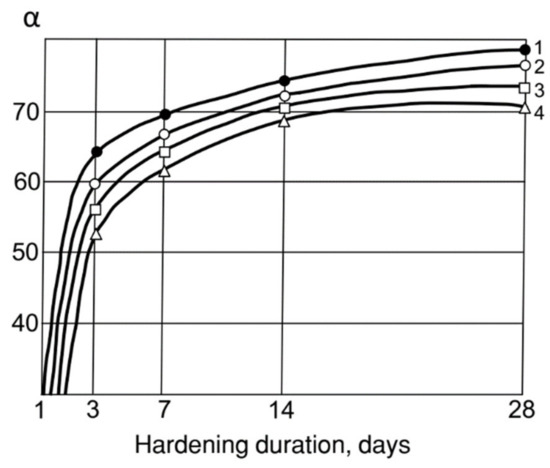
Figure 4.
Influence of MK, fly ash and their mixtures on the degree of hydration (α). cement: 1—30% MK; 2—30% AMAd (30% MS: 70% Ash); 3—30% fly Ash; 4—CEM I.
Structure formation and properties of cement-water pastes. To establish the influence of the main compositional factors on the viscosity of cement-water pastes filled with AMAd, their normal consistency, the kinetics of structure formation, as well as the strength of the cement stone and algorithmic experiments were performed in accordance with the standard plan B4 [27]. The conditions for planning experiments are given in Table 3.

Table 3.
Conditions for planning experiments in the study of effective viscosity.
As a result of experimental data processing, the effective viscosity equation in Pa∙s was obtained, which is adequate at 95% confidence probability:
The resulting regression equation can be considered a mathematical model of the viscosity of the investigated system MK–ash–Portland cement–water–superplasticizer under the accepted compositional restrictions, which are of interest for the technology of low-cement concretes. In the investigated range of compositions, the viscosity, as the analysis of the model shows, varies over a wide range (at the extreme points: x1 = +1; x2 = +1; x3 = +1; x4 = −1 and x1 = −1; x2 = −1; x3 = −1; x4 = +1, it differs by more than 35 times). According to the influence on the magnitude, the factors can be ranked in the series x1 > x4 > x3 > x2. When MK is added to the ash, the viscosity of the paste increases non-uniformly as the volume concentration of MK in the AMAd increases (x1). Therefore, if with an increase in x1 to 0.5, the viscosity increases by 66 Pa∙s, then from 0.5 to 1 by 177 Pa∙s. At x1 < 0.5, it is possible to prevent an increase in the viscosity of the pastes at a superplasticizer concentration in the water, up to 0.01. Complete replacement of fly ash–MK is not compensated even at double the superplasticizer concentration.
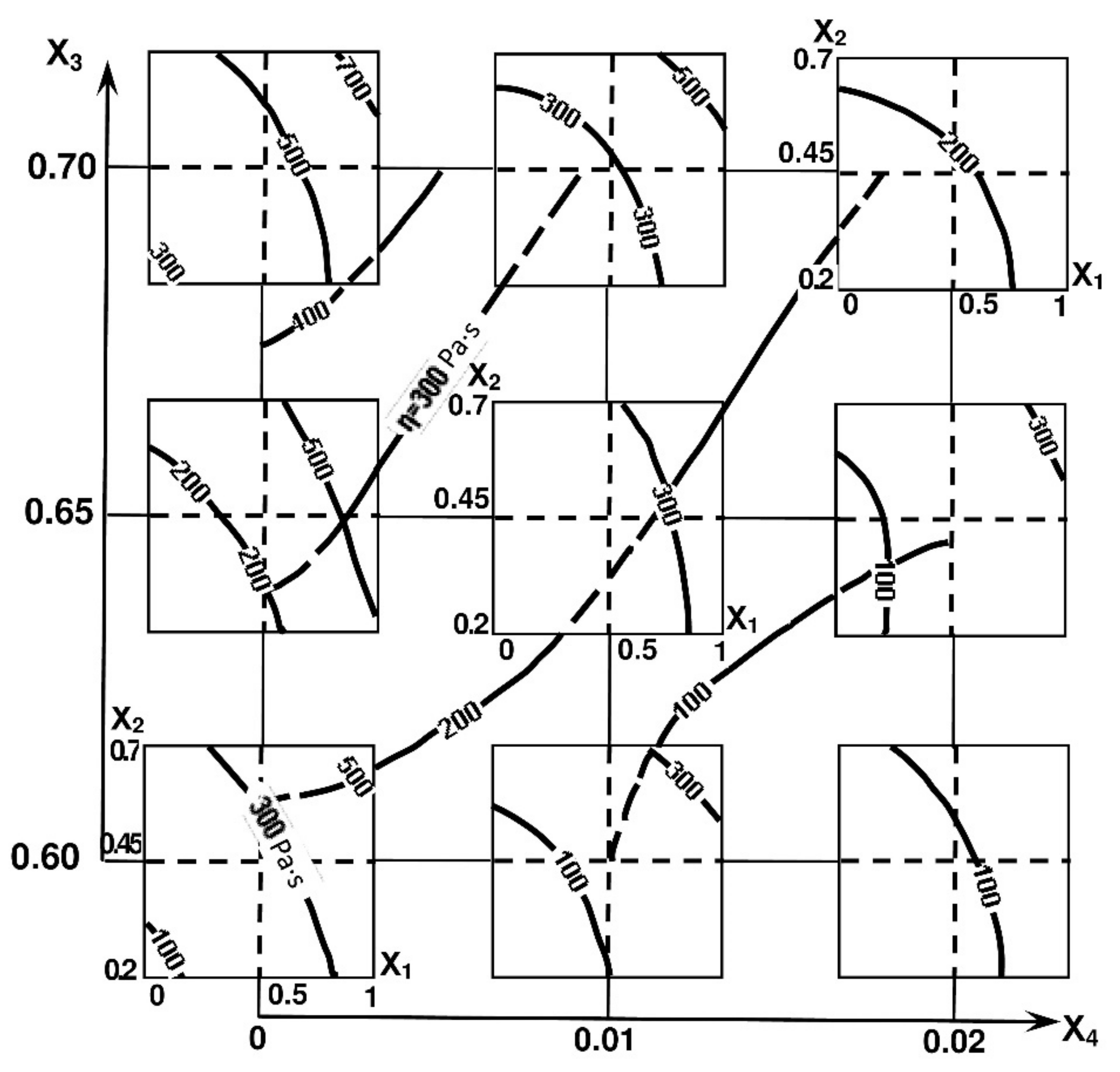
The ability to control the viscosity of cement-water pastes filled with AMAd can be judged from the diagrams of viscosity isolines (Figure 5). It follows from them that the main technological method for reducing the viscosity of cement-water systems containing MK and AMAd is the use of an appropriate superplasticizer amount.
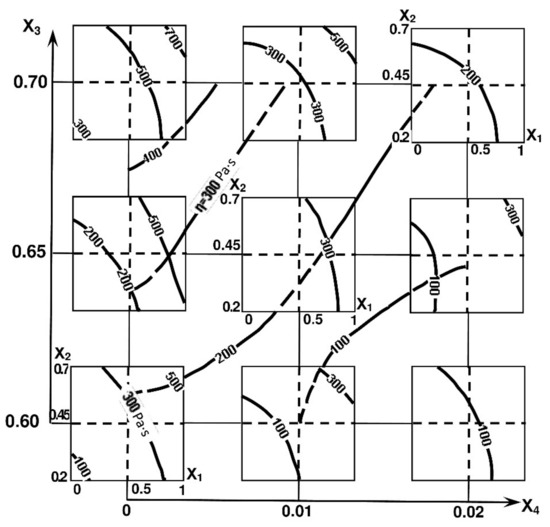
Figure 5.
Isolines of cement-water pastes with AMAd viscosity.
Another important conclusion follows from the analysis of the viscosity model: the introduction of MK into compositions with ash leads to a decrease in the viscosity of cement-water pastes compared to the pastes containing pure MK viscosity.
The influence of the superplasticizer admixture increases with the increase in the volume concentration of MK.
A change in the rheological properties of the filled cement-water pastes also manifests itself at their normal consistency (Table 4).

Table 4.
Normal consistency of filled cement-water pastes.
It follows from Table 4 that the introduction of AMAd in combination with a superplasticizer makes it possible not to significantly increase the normal consistency, and consequently, the water demand of cement pastes. At the same time, when cement pastes are filled with only MK, their normal consistency remains much higher, even with an increased concentration of the superplasticizer.
The kinetics of structure formation of cement pastes of normal consistency, filled with AMAd, were studied by measuring the plastic strength with a conical plastometer and ultrasonic waves velocity.
A smooth increase in plastic strength throughout the first section of plastograms (Figure 5) corresponds to the period of the coagulation structure formation [8,31]. The particles of the mineral admixture form coagulation contacts with the hydrated cement particles. For globular-type structures formed in this case, the strength of contacts (Rc) depends on a number of factors [19]:
where —is the chemical interaction constant; Fr is the resulting force of interaction between particles; —degree of filling; Ssp—is the specific surface of the particles involved in the interaction.
With the introduction of both MK and AMAd, one can expect an increase in the strength of coagulation contacts, which is confirmed by plastograms (Figure 6). It is characteristic that at approximately equal volume concentrations of metakaolin and ash–metakaolin additives, the absolute values of the cement pastes plastic strength and the kinetics of its change are quite close.
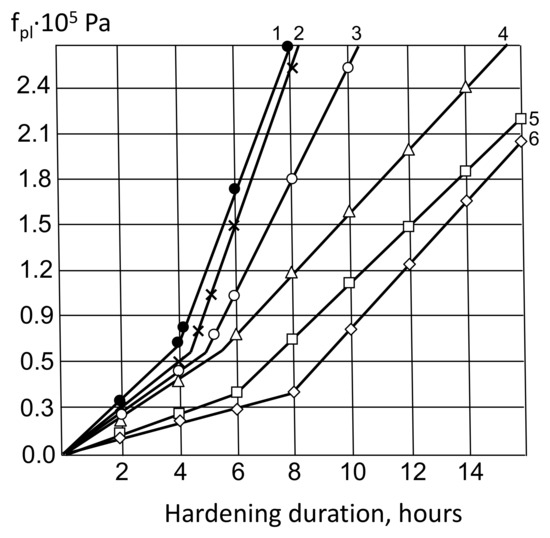
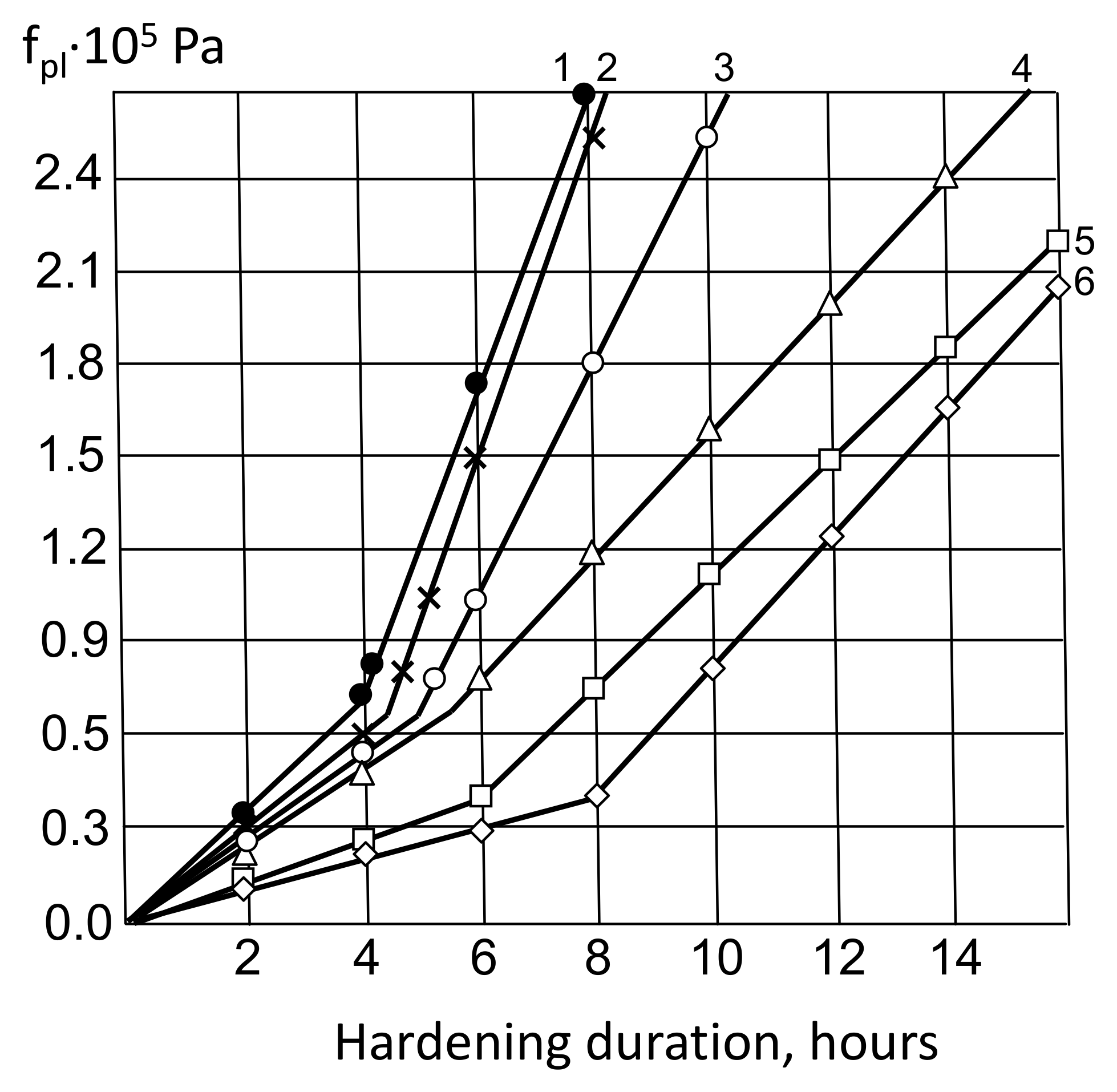
Figure 6.
Kinetics of cement-water pastes plastic strength change (fpl): 1—X1 = 1; X2 = 0.2; X4 = 0; 2—X1 = 0.5; X2 = 0.2; X4 = 0; 3—X1 = 0.2; X2 = 0.5; X4 = 0; 4—X1 = 1; X2 = 0.2; X4 = 0; 5—X1 = 0; X2 = 0; X4 = 0; 6—X1 = 0; X2 = 0.5; X4 = 0.
The first section of the plastograms corresponds approximately to the setting time of cement pastes, and the inflection point corresponds to the final setting (Table 5). The introduction of ash into cement pastes slows down their setting, which can be explained by its stabilizing effect on the hydration of the aluminate phase [32].

Table 5.
Setting time of filled cement-water pastes.
MK can both slow down and accelerate the setting, and in particular, the beginning of it. In accordance with the physical mechanism, the setting of the cement paste is caused by an increase in the volume of hydration products, with a corresponding decrease in the distance between the particles until cohesive forces begin to appear, proportional to the number of particles in contact with each other [33]. A decrease in the distance between hydrated particles and strengthening of the coagulation structure with the introduction of MK is typical when the water content of cement pastes is limited due to the superplasticizer.
The Section 2 of the plastograms corresponds to the period of hardening of the coagulation structure and the beginning of the formation of the crystallization structure. In this area, the introduction of MK and AMAd in combination with a superplasticizer causes an avalanche-like increase in plastic strength, which is consistent with the theoretical assumptions about their effect on the process of crystallization of neoplasms. Already approximately 2–3 h after the setting final, the plastic strength of cement pastes filled with MK and AMAd, with the addition of a superplasticizer, increases almost four times and reaches 2.7 Pa. For other studied pastes, such a value of plastic strength is achieved after a much longer period of time.
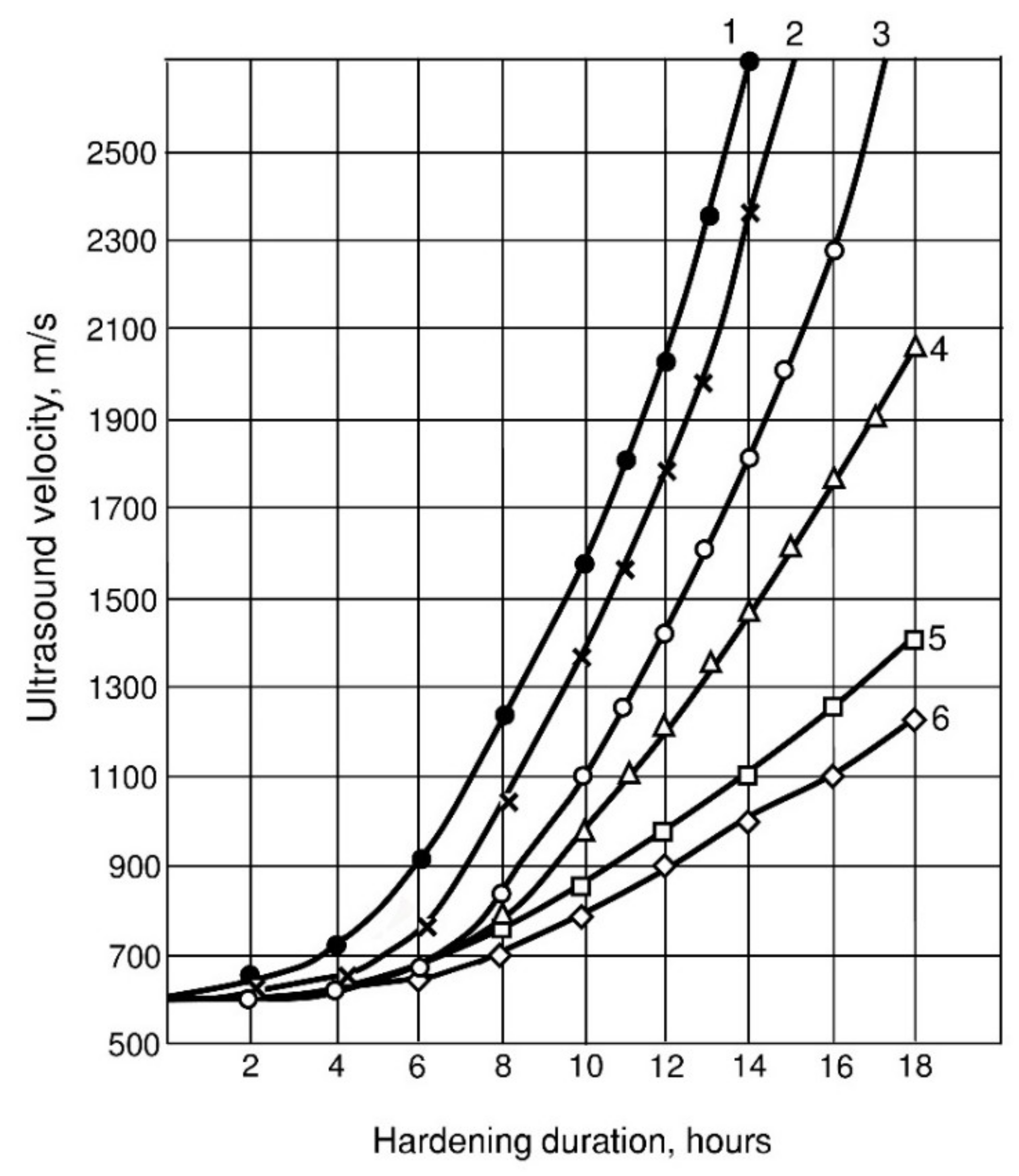
The kinetics of the rate of passage of ultrasonic waves through the hardening cement-water pastes is also consistent with the kinetics of plastic strength growth. The initial period of formation of the coagulation structure on the ultrasound velocity curves is characterized by a horizontal section (Figure 7), the length of which coincides well with the beginning of setting.
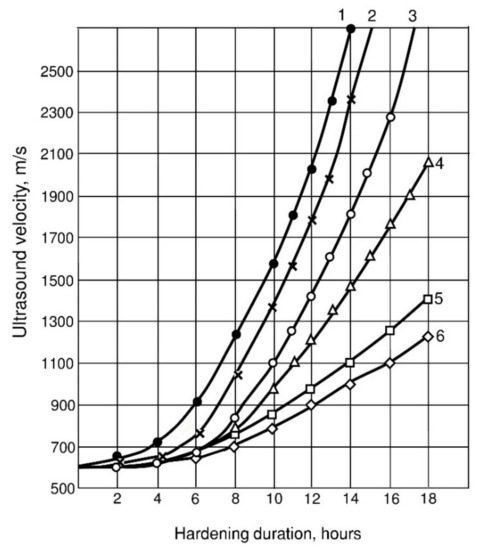
Figure 7.
Kinetics of ultrasound velocity through cement-water pastes: 1—X1 = 1; X2 = 0.2; X4 = 0.02; 2—X1 = 0; X2 = 0.2; X4 = 0.02; 3—X1 = 0.2; X2 = 0.5; X4 = 0.02; 4—X1 = 1; X2 = 0.2; X4 = 0; 5—X1 = 0; X2 = 0; X4 = 0; 6—X1 = 0; X2 = 0.5; X4 = 0.
The active influence of the ash–metakaolin admixture (AMAd) on the hydration and structure formation of cement-water pastes suggests its significant role in the synthesis of the cement stone strength.
Statistical processing of the results obtained during the implementation of the experimental plan B3 in accordance with the planning conditions (Table 3) made it possible to obtain adequate regression equations for the 28-day strength of cement-ash binders at 95% probability level:
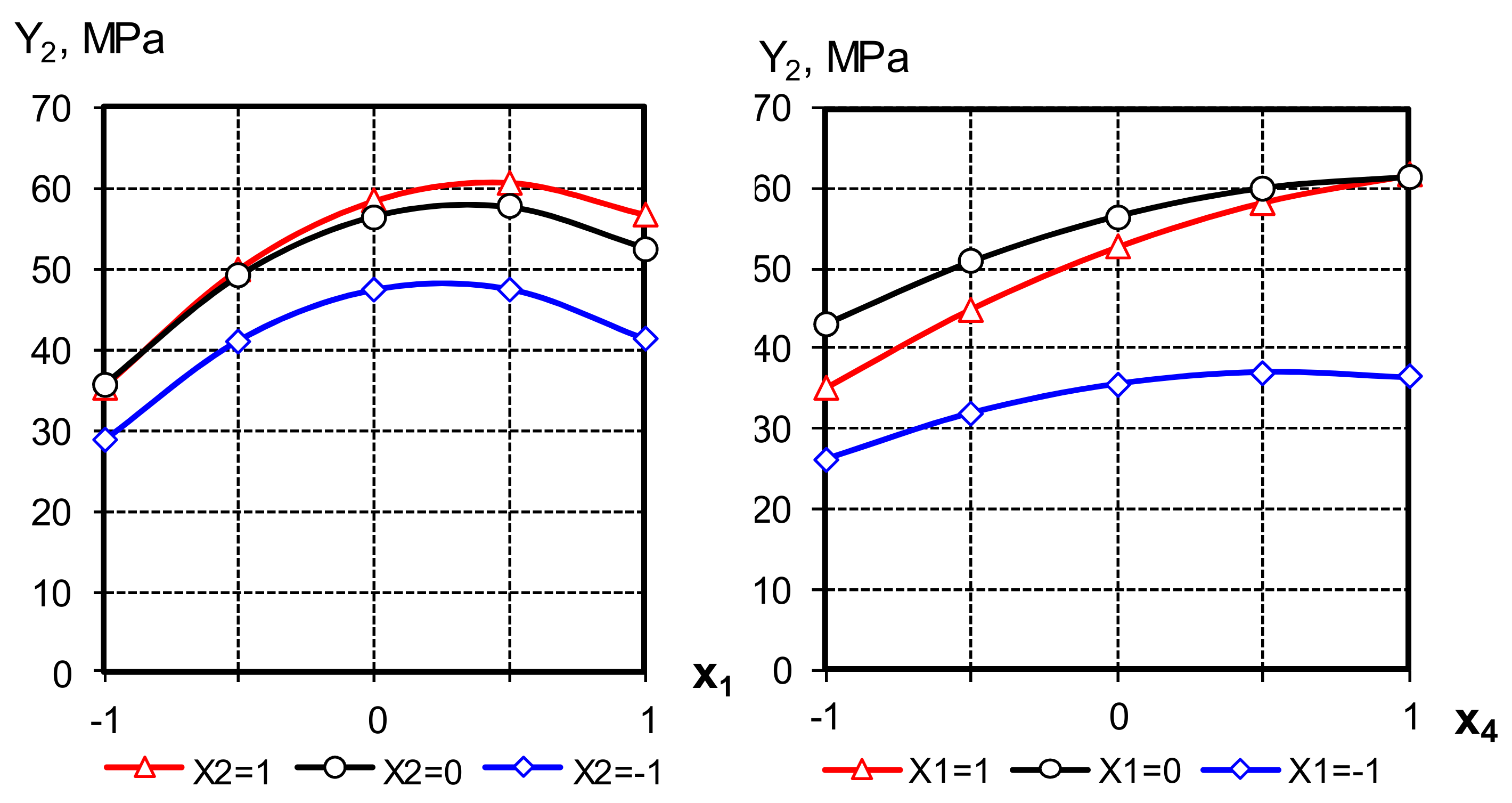
The strength was determined by testing standard samples of prisms with a size of 40 × 40 × 160 mm. The composition of the mortar: one mass part of binder and three mass parts of standard sand. The graphical dependences obtained as a result of the mathematical model analysis are shown in Figure 8.
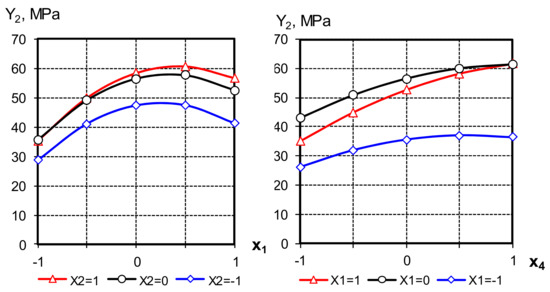
Figure 8.
Graphical dependences of the variable factors on the cement strength influence.
The strength of cements, as follows from the model analysis, can be significantly increased by filling them with AMAd, using an aqueous solution of a superplasticizer for mixing.
The analysis of the equations obtained allows us to note the non-linear influence of all the studied factors on the output parameter. Ranking in order of increasing impact on strength allows you to arrange the studied factors in the following sequence: X1 > X4 > X2.
The compressive strength of cement in 28 days with the addition of 70% AMAd reaches 60 MPa, i.e., by 20% better than cement without additives. The most significant effects of the interaction of factors X1 and X4, X2 and X4 show that with an increase in the MK content in the AMAd composition, as well as the AMAd filling degree of binder filling degree, it is advisable to simultaneously increase the superplasticizer concentration to increase its strength [34].
4. Conclusions
- The addition of MK to fly ash increases the total surface energy according to a power law. With a volume content of MK 0.2, the value of the wetting heat (surface energy parameter) increases to 55–57%, with MK 0.4-up to 75%.
- The introduction of 20–30% addition of metakaolin to fly ash makes it possible to increase its pozzolanic activity by almost two times, providing a higher effect than the regrinding of ash to a specific surface of 420–450 m2/kg.
- The increased activity of ash–metakaolin mixtures makes it possible to significantly increase the degree of cement hydration, and the amount of low-basic hydrosilicates in its hydration products. The introduction of 30% MK brings the degree of hydration of the cement in 28 days up to 0.8, whereas already at 3 days of age it is 0.65, i.e., 25% higher than cement without MK.
- An experimental–statistical model of the viscosity of cement-water pastes is obtained, taking into account its change with the introduction of an ash–metakaolin admixture of various compositions and a superplasticizer. Changing the volume ratio of the components of the AMAd and the concentration of the superplasticizer in the mix makes it possible to regulate the viscosity of cement pastes in a wide range as an indicator that determines the workability of mortar and concrete mixtures.
- Activation of the hydration capacity and structure formation of Portland cement with the introduction of an ash–metakaolin additive has a positive effect on its strength. As follows from the obtained mathematical model, the compressive strength of cement in 28 days with the addition of 70% AMAd reaches 60 MPa, i.e., by 20% better than cement without additives. The experimental–statistical model makes it possible to predict the change in strength depending on the amount and composition of the AMAd additive, taking into account the influence of the superplasticizing admixture.
Author Contributions
Conceptualization, L.D. and V.Z.; methodology, L.D.; validation, M.S.; formal analysis, L.D. and I.H.; investigation, L.D. and V.Z.; data curation, V.Z.; writing—original draft preparation, L.D. and V.Z.; writing—review and editing, L.D.,V.Z., I.H. and M.S.; visualization, V.Z.; supervision, L.D.; project administration, I.H.; funding acquisition, I.H. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been financed by the Polish National Agency for Academic Exchange under the International Academic Partnership Program within the framework of the grant: E-mobility and sustainable materials and technologies EMMAT (PPI/APM/2018/1/00027).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Smith, I.A. The design of fly ash concretes. Proc. Inst. Civ. Eng. 1967, 36, 769–790. [Google Scholar] [CrossRef]
- Kokubu, I. Zola i zol’nyye tsementy. In Metakaolin and Fly Ash as Mineral Admixtures for Concrete; Stroyizdat: Moscow, Russia, 1973; pp. 405–416. [Google Scholar]
- Neville, F.M. Properties of Concrete, 4th ed.; Wiley & Sons: New York, NY, USA, 1996; p. 844. [Google Scholar]
- Bapat, J.D. Mineral Admixtures in Cement and Concrete; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar] [CrossRef]
- Wilińska, I.; Pacewska, B.; Ostrowski, A. Investigation of different ways of activation of fly ash–cement mixtures. J. Therm. Anal. 2019, 138, 4203–4213. [Google Scholar] [CrossRef] [Green Version]
- Moropoulou, A.; Bakolas, A.; Aggelakopoulou, E. Evaluation of pozzolanic activity of natural and artificial pozzolans by thermal analisis. Thermochim. Acta 2004, 420, 135–140. [Google Scholar] [CrossRef]
- Sergeev, A.M. Ispol’zovaniye v Stroitel’stve Otkhodov Energeticheskoy Promyshltnnosti [Use in Construction of Waste of the Power Industry]; Budivelnik: Kyiv, Ukrainian, 1984; p. 120. [Google Scholar]
- Dvorkin, L.I.; Solomatov, V.I.; Vyrovoj, V.N.; Chudnovskij, S.M. Czementnye Betony s Mineralnymi Napolnitelyami [Cement-Baset Concrete with Mineral Fillers]; Budivelnik: Kyiv, Russia, 1991; p. 136. [Google Scholar]
- Snelson, D.G.; Wild, S.; O’Farrell, M. Heat of hydration of Portland Cement–Metakaolin–Fly ash (PC–MK–PFA) blends. Cem. Concr. Res. 2008, 38, 832–840. [Google Scholar] [CrossRef]
- Saboo, N.; Shivhare, S.; Kori, K.K.; Chandrappa, A.K. Effect of fly ash and metakaolin on pervious concrete properties. Constr. Build. Mater. 2019, 223, 322–328. [Google Scholar] [CrossRef]
- Moffatt, E.G.; Thomas, M.D.; Fahim, A. Performance of high-volume fly ash concrete in marine environment. Cem. Concr. Res. 2017, 102, 127–135. [Google Scholar] [CrossRef]
- Dadsetan, S.; Bai, J. Mechanical and microstructural properties of self-compacting concrete blended with metakaolin, ground granulated blast-furnace slag and fly ash. Constr. Build. Mater. 2017, 146, 658–667. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Castro-Gomes, J.P.; Costa, P.; Malheiro, R. Effect of metakaolin on the chloride ingress properties of concrete. KSCE J. Civ. Eng. 2016, 20, 1375–1384. [Google Scholar] [CrossRef]
- Bucher, R.; Diederich, P.; Escadeillas, G.; Cyr, M. Service life of metakaolin-based concrete exposed to carbonation: Comparison with blended cement containing fly ash, blast furnace slag and limestone filler. Cem. Concr. Res. 2017, 99, 18–29. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Lee, H.-S. Modeling the hydration of concrete incorporating fly ash or slag. Cem. Concr. Res. 2010, 40, 984–996. [Google Scholar] [CrossRef]
- Wang, X.-Y. Analysis of Hydration-Mechanical-Durability Properties of Metakaolin Blended Concrete. Appl. Sci. 2017, 7, 1087. [Google Scholar] [CrossRef]
- Kunther, W.; Dai, Z.; Skibsted, J. Thermodynamic modeling of hydrated white Portland cement–metakaolin–limestone blends utilizing hydration kinetics from 29Si MAS NMR spectroscopy. Cem. Concr. Res. 2016, 86, 29–41. [Google Scholar] [CrossRef]
- Kinomura, K.; Ishida, T. Enhanced hydration model of fly ash in blended cement and application of extensive modeling for continuous hydration to pozzolanic micro-pore structures. Cem. Concr. Compos. 2020, 114, 103733. [Google Scholar] [CrossRef]
- Wang, X.-Y. Analysis of hydration and strength optimization of cement-fly ash-limestone ternary blended concrete. Constr. Build. Mater. 2018, 166, 130–140. [Google Scholar] [CrossRef]
- Shi, Z.; Lothenbach, B.; Geiker, M.R.; Kaufmann, J.; Leemann, A.; Ferreiro, S.; Skibsted, J. Experimental studies and thermo-dynamic modeling of the carbonation of Portland cement, metakaolin and limestone mortars. Cem. Concr. Res. 2016, 88, 60–72. [Google Scholar] [CrossRef]
- Donatello, S.; Tyrer, M.; Cheeseman, C. Comparison of test methods to assess pozzolanic activity. Cem. Concr. Compos. 2010, 32, 121–127. [Google Scholar] [CrossRef] [Green Version]
- De Silva, P.S.; Glasser, F.P. Pozzolanic activation of metakaolin. Adv. Cem. Res. 1992, 4, 167–178. [Google Scholar] [CrossRef]
- EN 196–5:2011; Methods of Testing Cement. Pozzolanicity Test for Pozzolanic Cement. NSAI: Nashua, NH, USA, 2011.
- Sorrentino, F.; Castanet, R. Application of thermal analysis to the cement industry. J. Therm. Anal. 1992, 38, 2137–2146. [Google Scholar] [CrossRef]
- Zhao, D.; Khoshnazar, R. Hydration and microstructural development of calcined clay cement paste in the presence of calcium-silicate-hydrate (C–S–H) seed. Cem. Concr. Compos. 2021, 122, 104162. [Google Scholar] [CrossRef]
- Rebinder, P.A.; Cemenenko, N.A. O metode pogruzheniya konusa dlya kharakteristiki strukturno-mekhanicheskikh svoystv plastichno-vyazkikh tel [The method of dipping the cone to characterize structural and mechanical properties of the plastic-viscous bodies]. Proc. USSR Acad. Sci. 1949, LXIV, 835–838. (In Russian) [Google Scholar]
- Dvorkin, L.; Dvorkin, O.; Ribakov, Y. Multi-Parametric Concrete Composition Design; Nova Science Pub Inc.: New York, NY, USA, 2013; 233p. [Google Scholar]
- Dvorkin, L.I.; Dvorkin, O.L.; Korneichuk, Y.A. Effektyvnye Tcementno-Zolnye Betony [Effective Cement-Fly Ash Concrete]; RSTU: Rovno, Ukrainian, 1998; p. 196. [Google Scholar]
- Halstead, P.E.; Lawrence, S.D. Kinetika Reaktsiy v Sisteme CaO∙SiO2∙N2O. [Kinetics of Reactions in the CaO∙SiO2∙H2O System]. In Proceedings of the 4th Congress on the Cement Chemistry, Washington, DC, USA, 2–7 October 1960; Stroyizdat: Moscow, Russia, 1964; pp. 261–264. [Google Scholar]
- Rakhimova, N.; Rakhimov, R. A review on alkali-activated slag cements incorporated with supplementary materials. J. Sustain. Cem. Mater. 2014, 3, 61–74. [Google Scholar] [CrossRef]
- Kokubu, M. Tsement s dobavleniyem letuchey zoly (osnovnoy doklad) [Cement with the addition of fly ash (main re-port)]. In Proceedings of the Sixth International Congress on Cement Chemistry, Gothenburg, Sweden, 2–6 June 1997; Stroyizdat: Moscow, Russia, 1976; pp. 405–416. [Google Scholar]
- Nawy, E.G. Fundamentals of High-Strenght High-Performance Concrete; Longman Group Limited: Harlow, UK, 1996; p. 360. [Google Scholar]
- Wild, S.; Khatib, J.M.; Roose, L.J. Chemical shrinkage and autogenous shrinkage of Portland cement–metacaolin pastes. Adv. Cement Res. 1998, 10, 109–119. [Google Scholar] [CrossRef]
- Dvorkin, L.; Lushnikova, N.; Runova, R.; Troyan, V. Metakaolin in Building Mortars and Concrete [Metakaolin v Budivelnych Rozchynach i Betonach]; KNUBA: Kyiv, Ukrainian, 2007; p. 216. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).