A Combination Method of Liquid Hot Water and Phosphotungstic Acid Pretreatment for Improving the Enzymatic Saccharification Efficiency of Rice Straw
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Pretreatment Method
2.3. Catalyst Recycling Method
2.4. Enzymatic Saccharification Method
2.5. Analytical Methods
2.5.1. Quantitative Analysis of Chemical Composition
2.5.2. Relative Crystallinity of Straw Cellulose
2.5.3. FT-IR Analysis
3. Results and Discussion
3.1. Effect of Reaction Conditions of Two-Step Pretreatment on Chemical Composition
3.1.1. Initial LHW Pretreatment
3.1.2. Second Pretreatment with PTA
| Run | Sample | Pretreatment Condition | Solid Recovery 1 (%) | Chemical Composition (%) | Composition Changes (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp. (°C) | Time (min) | Solid/Liquid Ratio (g/mL) | Cellulose | Hemicellulose | Lignin | Hemicellulose Removal | Lignin Removal | Cellulose Retention | |||
| — | Control | — | — | — | — | 44.47 ± 0.03 | 21.64 ± 0.31 | 17.18 ± 0.20 | — | — | — |
| 1 | 1 | 160 | 90 | 1:20 | 83.12 ± 0.17 | 52.95 ± 0.36 | 9.74 ± 0.26 | 19.97 ± 0.21 | 62.57 ± 0.97 d 2 | 3.37 ± 1.04 d | 98.96 ± 0.83 a |
| 2 | 2 | 170 | 90 | 1:20 | 81.78 ± 0.44 | 53.70 ± 0.21 | 7.67 ± 0.20 | 20.29 ± 0.14 | 71.01 ± 0.79 b | 3.43 ± 1.10 d | 98.75 ± 0.77 a |
| 3 | 3 | 180 | 90 | 1:20 | 78.46 ± 0.16 | 54.15 ± 0.30 | 7.25 ± 0.14 | 20.99 ± 0.18 | 73.71 ± 0.47 a | 4.15 ± 0.94 d | 95.54 ± 0.35 c |
| 4 | 4 | 190 | 90 | 1:20 | 76.12 ± 0.27 | 54.83 ± 0.30 | 7.57 ± 0.33 | 20.91 ± 0.52 | 73.36 ± 1.10 a | 7.36 ± 2.01 abc | 93.85 ± 0.59 d |
| 5 | 5 | 200 | 90 | 1:20 | 72.60 ± 0.34 | 52.51 ± 0.13 | 7.85 ± 0.30 | 21.65 ± 0.33 | 73.65 ± 1.02 a | 8.51 ± 1.16 ab | 85.72 ± 0.17 g |
| 6 | 6 | 180 | 30 | 1:20 | 87.68 ± 0.25 | 50.09 ± 0.18 | 13.84 ± 0.26 | 18.77 ± 0.47 | 43.90 ± 1.07 e | 4.22 ± 2.47 d | 98.76 ± 0.27 a |
| 7 | 7 | 180 | 60 | 1:20 | 82.65 ± 0.31 | 53.19 ± 0.46 | 8.37 ± 0.20 | 19.93 ± 0.41 | 68.04 ± 0.59 c | 4.11 ± 1.93 d | 98.86 ± 0.47 a |
| 8 | 8 | 180 | 120 | 1:20 | 73.21 ± 0.47 | 53.18 ± 0.23 | 7.49 ± 0.45 | 22.08 ± 0.18 | 74.65 ± 1.51 a | 5.92 ± 0.92 bcd | 87.56 ± 0.75 f |
| 9 | 9 | 180 | 180 | 1:20 | 72.26 ± 0.20 | 51.85 ± 0.08 | 7.85 ± 0.30 | 22.57 ± 0.37 | 73.79 ± 0.82 a | 5.09 ± 1.22 cd | 84.25 ± 0.19 h |
| 10 | 10 | 180 | 90 | 1:10 | 81.17 ± 0.16 | 53.00 ± 0.33 | 9.77 ± 0.26 | 20.13 ± 0.26 | 63.36 ± 0.82 d | 4.90 ± 0.99 cd | 96.75 ± 0.35 b |
| 11 | 11 | 180 | 90 | 1:30 | 75.21 ± 0.26 | 55.09 ± 0.23 | 7.48 ± 0.24 | 20.90 ± 0.40 | 74.01 ± 0.69 a | 8.41 ± 1.74 ab | 93.17 ± 0.53 d |
| 12 | 12 | 180 | 90 | 1:40 | 74.34 ± 0.20 | 54.58 ± 0.14 | 7.67 ± 0.42 | 21.08 ± 0.30 | 73.88 ± 1.67 a | 8.81 ± 1.14 a | 91.24 ± 0.40 e |
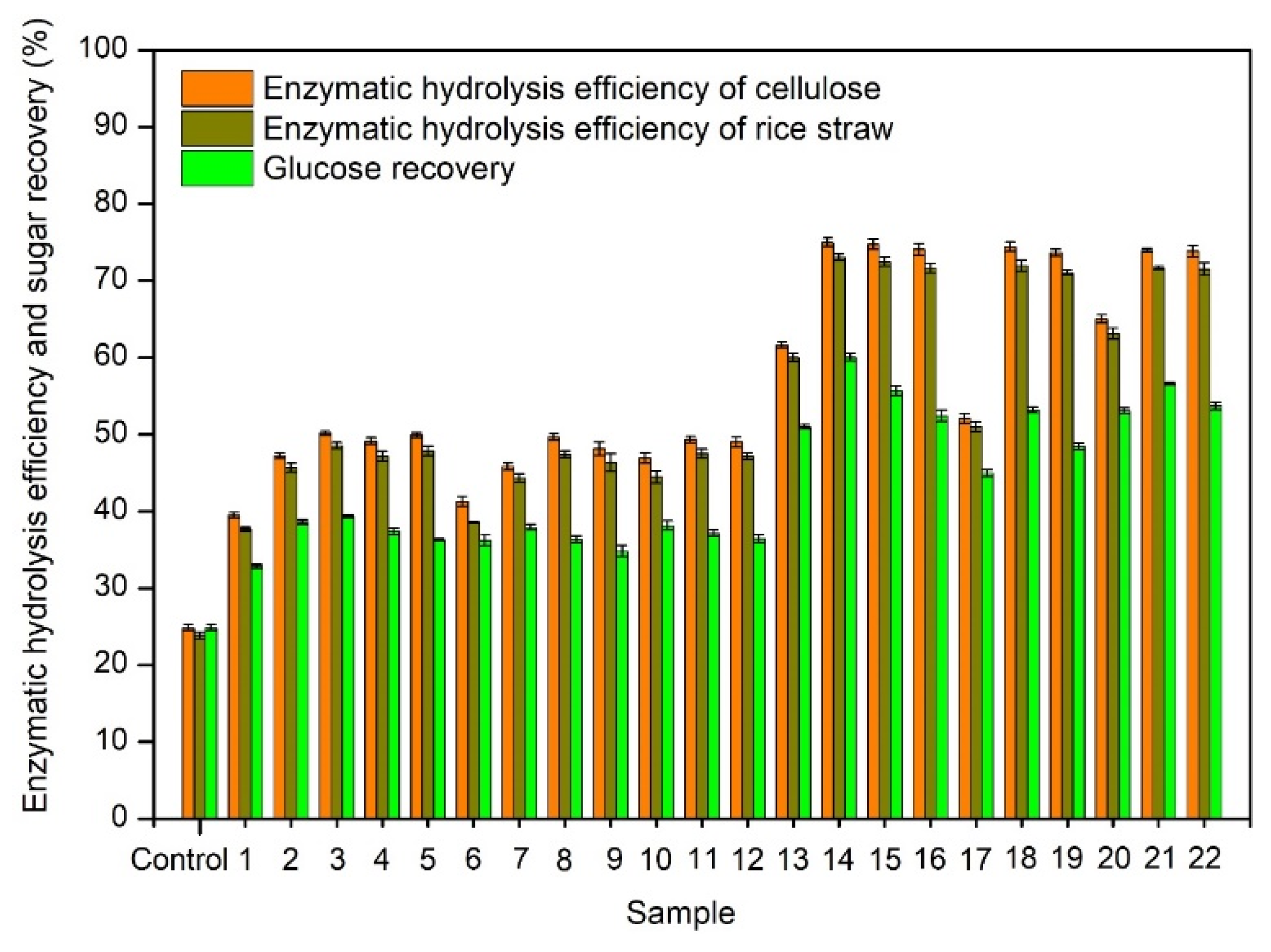
3.2. Enzymatic Hydrolysis Efficiency
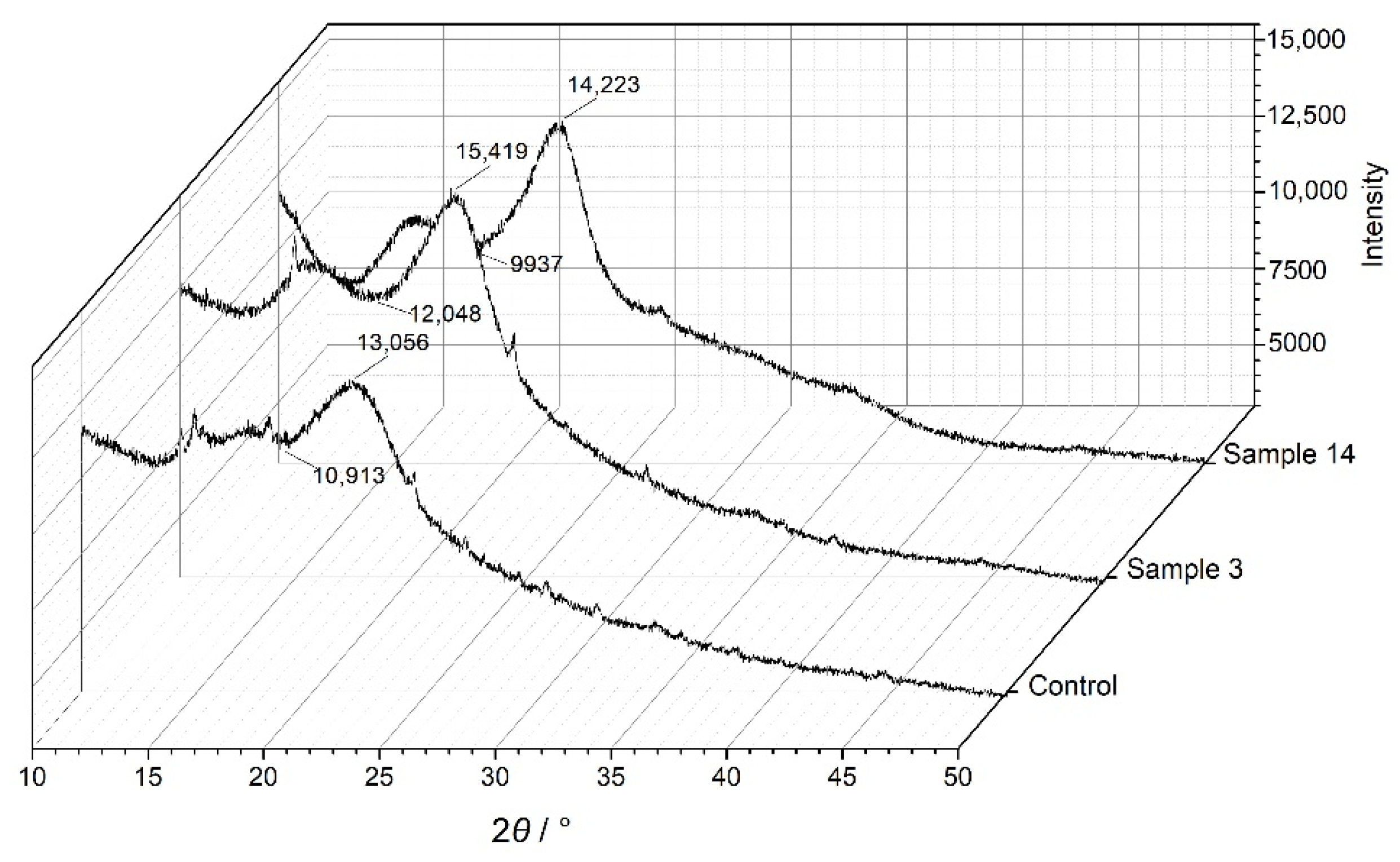
3.3. XRD Analysis
3.4. FT-IR Analysis
3.5. Reuse Performance of PTA Catalyst
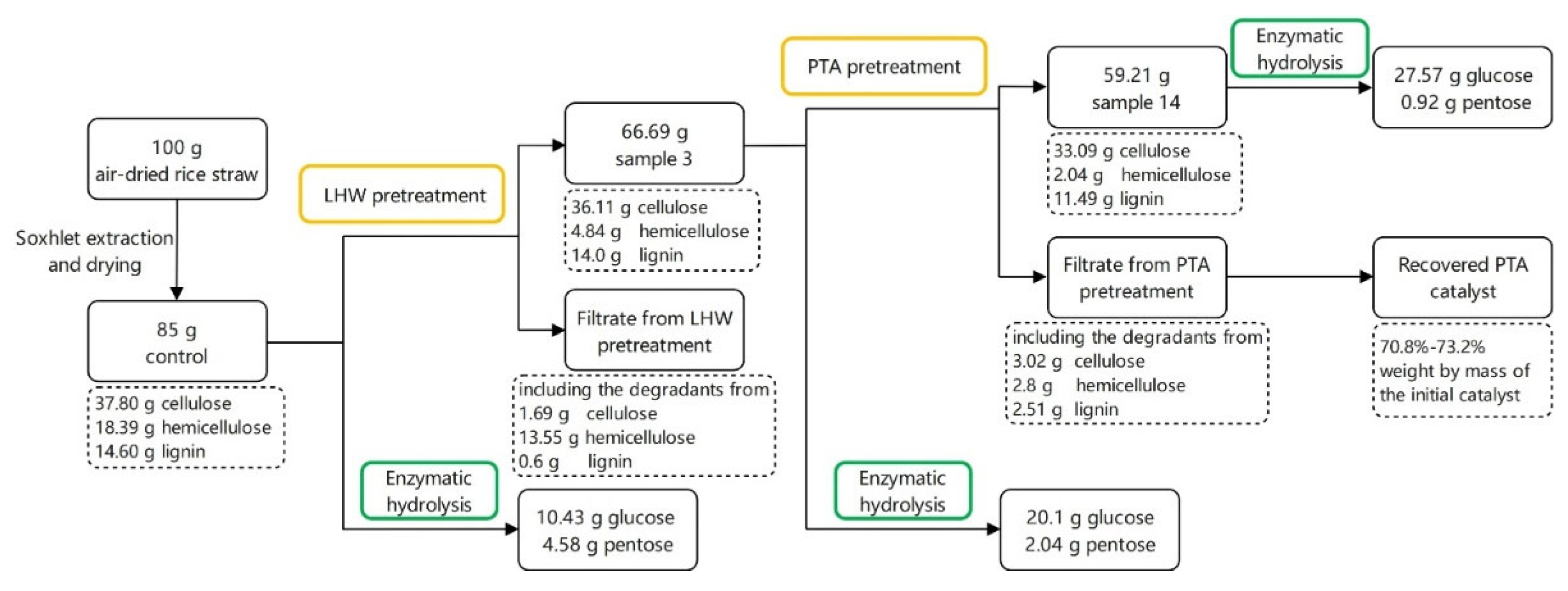
3.6. Mass Balance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, T.Q.; Shen, Y.Q.; Liu, Y. State of the art of straw treatment technology: Challenges and solutions forward. Bioresour. Technol. 2020, 313, 123656. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.W.; Tan, X.C.; Gu, B.H.; Lin, J.C. Evaluation of potential amounts of crop straw available for bioenergy production and bio-technology spatial distribution in China under ecological and cost constraints. J. Clean. Prod. 2021, 292, 125958. [Google Scholar] [CrossRef]
- Pandiyan, K.; Singh, A.; Singh, S.; Saxena, A.K.; Nain, L. Technological interventions for utilization of crop residues and weedy biomass for second generation bio-ethanol production. Renew. Energy 2019, 132, 723–741. [Google Scholar] [CrossRef]
- Liu, C.G.; Xiao, Y.; Xia, X.X.; Zhao, X.Q.; Peng, L.C.; Srinophakun, P.; Bai, F.W. Cellulosic ethanol production: Progress, challenges and strategies for solutions. Biotechnol. Adv. 2019, 37, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Soltanian, S.; Aghbashlo, M.; Almasi, F.; Hosseinzadeh-Bandbafha, H.; Nizami, A.S.; Ok, Y.S.; Lam, S.S.; Tabatabaei, M. A critical review of the effects of pretreatment methods on the exergetic aspects of lignocellulosic biofuels. Energy Convers. Manag. 2020, 212, 112792. [Google Scholar] [CrossRef]
- Dziekońska-Kubczak, U.; Berłowska, J.; Dziugan, P.; Patelski, P.; Balcerek, M.; Pielech-Przybylska, K.; Robak, K. Two-stage pretreatment to improve saccharification of oat straw and jerusalem artichoke biomass. Energies 2019, 12, 1715. [Google Scholar] [CrossRef] [Green Version]
- Woiciechowski, A.L.; Neto, C.J.D.; de-Souza-Vandenberghe, L.P.; de-Carvalho-Neto, D.P.; Sydney, A.C.N.; Letti, L.A.J.; Karp, S.G.; Torres, L.A.Z.; Soccol, C.R. Lignocellulosic biomass: Acid and alkaline pretreatments and their effects on biomass recalcitrance–conventional processing and recent advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar] [CrossRef]
- Zahoor; Wang, W.; Tan, X.S.; Imtiaz, M.; Wang, Q.F.; Miao, C.L.; Yuan, Z.H.; Zhuang, X.S. Rice straw pretreatment with KOH/urea for enhancing sugar yield and ethanol production at low temperature. Ind. Crops Prod. 2021, 170, 113776. [Google Scholar] [CrossRef]
- Wu, Q.Q.; Ma, Y.L.; Chang, X.; Sun, Y.G. Optimization and kinetic analysis on the sulfuric acid–catalyzed depolymerization of wheat straw. Carbohydr. Polym. 2015, 129, 79–86. [Google Scholar] [CrossRef]
- Sherif, A.; Hussen, A.; Firemichael, D. Hydolysis of multi substrate biomass using para-toluenesulphonic acid for bioethanol production: A promising option over the sulfuric acid treatment. Biomass Bioenergy 2021, 144, 105922. [Google Scholar] [CrossRef]
- Sabanci, B.; Buyukkileci, A.O. Comparison of liquid hot water, very dilute acid and alkali treatments for enhancing enzymatic digestibility of hazelnut tree pruning residues. Bioresour. Technol. 2018, 261, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Ilanidis, D.; Stagge, S.; Jönsson, L.J.; Martín, C. Effects of operational conditions on auto-catalyzed and sulfuric-acid-catalyzed hydrothermal pretreatment of sugarcane bagasse at different severity factor. Ind. Crop. Product. 2021, 159, 113077. [Google Scholar] [CrossRef]
- Sun, Y.G.; Ma, Y.L.; Wang, L.Q.; Wang, F.Z.; Wu, Q.Q.; Pan, G.Y. Physicochemical properties of corn stalk after treatment using steam explosion coupled with acid or alkali. Carbohydr. Polym. 2015, 117, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Sarker, A.R.; Pattnaik, F.; Nanda, S.; Dalai, A.K.; Meda, V.; Naik, S. Hydrothermal pretreatment technologies for lignocellulosic biomass: A review of steam explosion and subcritical water hydrolysis. Chemosphere 2021, 284, 131372. [Google Scholar] [CrossRef] [PubMed]
- Mansir, N.; Taufiq-Yap, Y.H.; Rashid, U.; Lokman, I.M. Investigation of heterogeneous solid acid catalyst performance on low grade feedstocks for biodiesel production: A review. Energy Convers. Manag. 2017, 141, 171–182. [Google Scholar] [CrossRef]
- De-Meirelesa, A.L.P.; da-Silva-Rocha, K.A.; Kozhevnikova, E.F.; Kozhevnikov, I.V.; Gusevskaya, E.V. Heteropoly acid catalysts for the valorization of biorenewables: Isomerization of caryophyllene oxide in green solvents. Mol. Catal. 2018, 458, 213–222. [Google Scholar] [CrossRef]
- Zhang, L.B.; Zheng, W.X.; Wang, Z.M.; Ma, Y.B.; Jiang, L.; Wang, T.F. Efficient degradation of lignin in raw wood via pretreatment with heteropoly acids in γ-valerolactone/water. Bioresour. Technol. 2018, 261, 70–75. [Google Scholar] [CrossRef]
- Shatalov, A.A. Highly efficient hydrolysis of plant hemicelluloses by mixed-addenda Keggin-type (Mo-V-P)-heteropolyacids in diluted aqueous solution. Carbohydr. Polym. 2019, 206, 80–85. [Google Scholar] [CrossRef]
- Wu, X.H.; Zhang, T.T.; Liu, N.; Zhao, Y.J.; Tian, G.Y.; Wang, Z.J. Sequential extraction of hemicelluloses and lignin for wood fractionation using acid hydrotrope at mild conditions. Ind. Crop. Product. 2020, 145, 112086. [Google Scholar] [CrossRef]
- Wang, X.Q.; Duan, C.; Feng, X.M.; Qin, X.Y.; Wang, W.L.; Wang, J.; Xu, Y.J.; Ni, Y.H. Combining phosphotungstic acid pretreatment with mild alkaline extraction for selective separation of hemicelluloses from hardwood kraft pulp. Sep. Purif. Technol. 2021, 266, 118562. [Google Scholar] [CrossRef]
- Gallina, G.; Cabeza, Á.; Grénman, H.; Biasi, P.; García-Serna, J.; Salmi, T. Hemicellulose extraction by hot pressurized water pretreatment at 160 °C for 10 different woods: Yield and molecular weight. J. Supercrit. Fluids 2018, 133, 716–725. [Google Scholar] [CrossRef] [Green Version]
- Cebreiros, F.; Ferrari, M.D.; Lareo, C. Combined autohydrolysis and alkali pretreatments for cellulose enzymatic hydrolysis of Eucalyptus grandis wood. Biomass Convers. Biorefinery 2017, 8, 33–42. [Google Scholar] [CrossRef]
- Tian, D.; Shen, F.; Yang, G.; Deng, S.H.; Long, L.L.; He, J.S.; Zhang, J.; Huang, C.R.; Luo, L. Liquid hot water extraction followed by mechanical extrusion as a chemical-free pretreatment approach for cellulosic ethanol production from rigid hardwood. Fuel 2019, 252, 589–597. [Google Scholar] [CrossRef]
- Serna-Loaiza, S.; Zikeli, F.; Adamcyk, J.; Friedl, A. Towards a wheat straw biorefinery: Combination of organosolv and liquid hot water for the improved hydrolysis of lignin and hemicellulose. Bioresour. Technol. Rep. 2021, 14, 100667. [Google Scholar] [CrossRef]
- Xia, F.; Gong, J.W.; Lu, J.; Cheng, Y.; Zhai, S.R.; An, Q.D.; Wang, H.S. Combined liquid hot water with sodium carbonate-oxygen pretreatment to improve enzymatic saccharification of reed. Bioresour. Technol. 2020, 297, 122498. [Google Scholar] [CrossRef]
- Araújo, D.; Vilarinho, M.; Machado, A. Effect of combined dilute-alkaline and green pretreatments on corncob fractionation: Pretreated biomass characterization and regenerated cellulose film production. Ind. Crop. Product. 2019, 141, 111785. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. Technical Report NREL/TP-510-42618. Available online: https://www.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 3 August 2012).
- Inoue, H.; Yano, S.; Endo, T.; Sakaki, T.; Sawayama, S. Combining hot-compressed water and ball milling pretreatments to improve the efficiency of the enzymatic hydrolysis of eucalyptus. Biotechnol. Biofuels 2008, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.F.S.; Akbar, M.; Xu, Z.; Wang, H. A review on the role of pretreatment technologies in the hydrolysis of lignocellulosic biomass of corn stover. Biomass Bioenergy 2021, 155, 106276. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, J.H.; Kim, J.C.; Jang, S.K.; Kwak, H.W.; Koo, B.; Choi, I.G. Production of succinic acid from liquid hot water hydrolysate derived from Quercus mongolica. Biomass Bioenergy 2021, 150, 106103. [Google Scholar] [CrossRef]
- Arzami, A.N.; Ho, T.M.; Mikkonen, K.S. Valorization of cereal by-product hemicelluloses: Fractionation and purity considerations. Food Res. Int. 2022, 151, 110818. [Google Scholar] [CrossRef]
- Chen, B.H.; Wang, Z.Q.; Jin, Z.C.; Gou, Z.C.; Tang, S.S.; Yu, X.X.; Chen, H.; Chen, G.; Su, Y.J. Optimized phosphotungstic acid pretreatment for enhancing cellulase adsorption and biomass saccharification in corn stover. Biomass Convers. Biorefin. 2021. [Google Scholar] [CrossRef]
- Xie, J.X.; Xu, J.; Cheng, A.; Zhu, S.Y.; Wang, B. Phosphotungstic acid assisted with neutral deep eutectic solvent boost corn straw pretreatment for enzymatic saccharification and lignin extraction. Ind. Crop. Product. 2021, 172, 114058. [Google Scholar] [CrossRef]
- Dionísio, S.R.; Santoro, D.C.J.; Bonan, C.I.D.G.; Soares, L.B.; Biazi, L.E.; Rabelo, S.C.; Ienczak, J.L. Second-generation ethanol process for integral use of hemicellulosic and cellulosic hydrolysates from diluted sulfuric acid pretreatment of sugarcane bagasse. Fuel 2021, 304, 121290. [Google Scholar] [CrossRef]
- Sidana, A.; Kaur, S.; Yadav, S.K. Assessment of the ability of Meyerozyma guilliermondii P14 to produce second-generation bioethanol from giant reed (Arundo donax) biomass. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Zhuang, X.S.; Wang, W.; Yu, Q.; Qi, W.; Wang, Q.; Tan, X.S.; Zhou, G.X.; Yuan, Z.H. Liquid hot water pretreatment of lignocellulosic biomass for bioethanol production accompanying with high valuable products. Bioresour. Technol. 2016, 199, 68–75. [Google Scholar] [CrossRef]
- Nitsos, C.K.; Choli-Papadopoulou, T.; Matis, K.A.; Triantafyllidis, K.S. Optimization of hydrothermal pretreatment of hardwood and softwood lignocellulosic residues for selective hemicellulose recovery and improved cellulose enzymatic hydrolysis. ACS Sustain. Chem. Eng. 2016, 4, 4529–4544. [Google Scholar] [CrossRef]
- Li, M.F.; Chen, C.Z.; Sun, R.C. Effect of pretreatment severity on the enzymatic hydrolysis of bamboo in hydrothermal deconstruction. Cellulose 2014, 21, 4105–4117. [Google Scholar] [CrossRef]
- Dimitrellos, G.; Lyberatos, G.; Antonopoulou, G. Does acid addition improve liquid hot water pretreatment of lignocellulosic biomass towards biohydrogen and biogas production? Sustainability 2020, 12, 8935. [Google Scholar] [CrossRef]
- Ghaffar, S.H.; Fan, M.Z. Differential behaviour of nodes and internodes of wheat straw with various pre-treatments. Biomass Bioenergy 2015, 83, 373–382. [Google Scholar] [CrossRef]
- Zhang, S.M.; Fang, G.Z.; Chen, H.T.; Lang, Q. The effect of degradation of soda lignin using Pd/SO42−/ZrO2 as a catalyst: Improved reactivity and antioxidant activity. Polymers 2019, 11, 1218. [Google Scholar] [CrossRef] [Green Version]
- Tsegaye, B.; Balomajumder, C.; Roy, P. Organosolv pretreatments of rice straw followed by microbial hydrolysis for efficient biofuel production. Renew. Energy 2020, 148, 923–934. [Google Scholar] [CrossRef]
- An, Q.; Lin, H.N.; Wang, Y.T.; Deng, M.C.; Zhu, M.J. Improved saccharification of pretreated lignocellulose by Clostridium thermocellum with the addition of surfactant, low loading of cellulose. Process Biochem. 2021, 111, 267–273. [Google Scholar] [CrossRef]
- Sorn, V.; Chang, K.L.; Phitsuwan, P.; Ratanakhanokchai, K.; Dong, C.D. Effect of m39icrowave-assisted ionic liquid/acidic ionic liquid pretreatment on the morphology, structure, and enhanced delignification of rice straw. Bioresour. Technol. 2019, 293, 121929. [Google Scholar] [CrossRef] [PubMed]


| Run | Sample | Pretreatment Condition | Solid Recovery 1 (%) | Chemical Composition (%) | Composition Changes (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp. (°C) | Time (min) | Concentration (mM) | Cellulose | Hemicellulose | Lignin | Hemicellulose Removal | Lignin Removal | Cellulose Retention | |||
| 3 | 3 | — | — | — | — | 54.15 ± 0.30 | 7.25 ± 0.14 | 20.99 ± 0.18 | 73.71 ± 0.47 f 2 | 4.15 ± 0.94 e | 95.54 ± 0.35 a |
| 13 | 13 | 120 | 60 | 20 | 91.76 ± 0.15 | 55.78 ± 0.22 | 3.43 ± 0.07 | 20.29 ± 0.24 | 88.58 ± 0.24 de | 14.96 ± 0.82 b | 90.30 ± 0.42 c |
| 14 | 14 | 130 | 60 | 20 | 88.79 ± 0.16 | 55.88 ± 0.25 | 3.44 ± 0.05 | 19.40 ± 0.36 | 89.24 ± 0.16 cd | 21.33 ± 1.16 a | 87.54 ± 0.36 d |
| 15 | 15 | 140 | 60 | 20 | 82.64 ± 0.18 | 52.75 ± 0.13 | 3.39 ± 0.05 | 20.78 ± 0.30 | 89.85 ± 0.12 bc | 21.59 ± 0.87 a | 76.92 ± 0.30 f |
| 16 | 16 | 150 | 60 | 20 | 78.48 ± 0.33 | 50.12 ± 0.11 | 3.52 ± 0.17 | 21.85 ± 0.17 | 89.98 ± 0.44 b | 21.69 ± 0.37 a | 69.40 ± 0.40 i |
| 17 | 17 | 130 | 30 | 20 | 95.70 ± 0.19 | 55.80 ± 0.08 | 3.47 ± 0.10 | 20.47 ± 0.32 | 87.97 ± 0.33 e | 10.55 ± 1.14 d | 94.22 ± 0.12 b |
| 18 | 18 | 130 | 90 | 20 | 79.25 ± 0.28 | 50.76 ± 0.19 | 3.38 ± 0.17 | 21.72 ± 0.17 | 90.28 ± 0.42 ab | 21.41 ± 0.76 a | 70.97 ± 0.21 h |
| 19 | 19 | 130 | 120 | 20 | 72.93 ± 0.33 | 46.43 ± 0.05 | 3.48 ± 0.16 | 23.53 ± 0.38 | 90.80 ± 0.38 a | 21.61 ± 1.51 a | 59.75 ± 0.27 j |
| 20 | 20 | 130 | 60 | 10 | 90.54 ± 0.18 | 54.67 ± 0.17 | 3.46 ± 0.20 | 21.04 ± 0.31 | 88.63 ± 0.61 de | 13.02 ± 1.23 c | 87.33 ± 0.21 d |
| 21 | 21 | 130 | 60 | 30 | 84.87 ± 0.12 | 53.77 ± 0.19 | 3.50 ± 0.18 | 20.31 ± 0.21 | 89.22 ± 0.48 cd | 21.30 ± 0.89 a | 80.50 ± 0.19 e |
| 22 | 22 | 130 | 60 | 40 | 80.65 ± 0.16 | 51.60 ± 0.27 | 3.42 ± 0.07 | 21.24 ± 0.16 | 90.00 ± 0.21 b | 21.76 ± 0.49 a | 73.42 ± 0.25 g |
| Run | Solid Recovery (%) | Catalyst Recovery (%) | Glucose Yield (mg/g) | Enzymatic Hydrolysis Efficiency of Cellulose (%) |
|---|---|---|---|---|
| First test | 88.79 ± 0.16 a 1 | 73.24 ± 2.30 a | 465.55 ± 5.15 a | 74.98 ± 0.53 a |
| Sequence 1 | 89.13 ± 0.55 a | 70.81 ± 4.33 a | 469.09 ± 8.24 a | 75.55 ± 1.33 a |
| Sequence 2 | 88.44 ± 0.45 a | 71.02 ± 2.32 a | 461.59 ± 3.61 a | 74.34 ± 0.58 a |
| Sequence 3 | 88.92 ± 0.42 a | 72.98 ± 3.07 a | 462.06 ± 6.12 a | 74.42 ± 0.99 a |
| Sequence 4 | 88.84 ± 1.20 a | 71.62 ± 3.50 a | 466.58 ± 5.55 a | 74.38 ± 1.04 a |
| Sequence 5 | 88.41 ± 0.54 a | 73.06 ± 2.08 a | 464.80 ± 6.48 a | 74.86 ± 1.04 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Mei, T.; Zhu, C.; Shang, H.; Gao, S.; Qin, L.; Chen, H. A Combination Method of Liquid Hot Water and Phosphotungstic Acid Pretreatment for Improving the Enzymatic Saccharification Efficiency of Rice Straw. Energies 2022, 15, 3636. https://doi.org/10.3390/en15103636
Zhang S, Mei T, Zhu C, Shang H, Gao S, Qin L, Chen H. A Combination Method of Liquid Hot Water and Phosphotungstic Acid Pretreatment for Improving the Enzymatic Saccharification Efficiency of Rice Straw. Energies. 2022; 15(10):3636. https://doi.org/10.3390/en15103636
Chicago/Turabian StyleZhang, Shengming, Tiehan Mei, Chonghao Zhu, Huimin Shang, Shushan Gao, Liyuan Qin, and Haitao Chen. 2022. "A Combination Method of Liquid Hot Water and Phosphotungstic Acid Pretreatment for Improving the Enzymatic Saccharification Efficiency of Rice Straw" Energies 15, no. 10: 3636. https://doi.org/10.3390/en15103636
APA StyleZhang, S., Mei, T., Zhu, C., Shang, H., Gao, S., Qin, L., & Chen, H. (2022). A Combination Method of Liquid Hot Water and Phosphotungstic Acid Pretreatment for Improving the Enzymatic Saccharification Efficiency of Rice Straw. Energies, 15(10), 3636. https://doi.org/10.3390/en15103636






