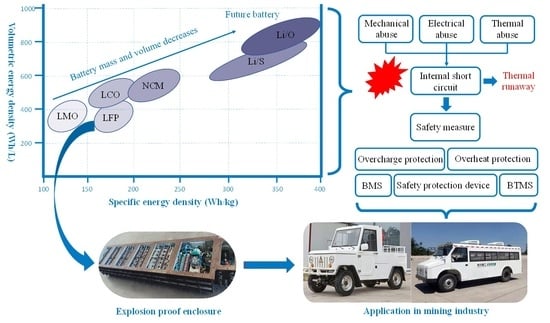
Large-Scale Li-Ion Battery Research and Application in Mining Industry
Abstract
:1. Introduction
2. LIB Cathode Material
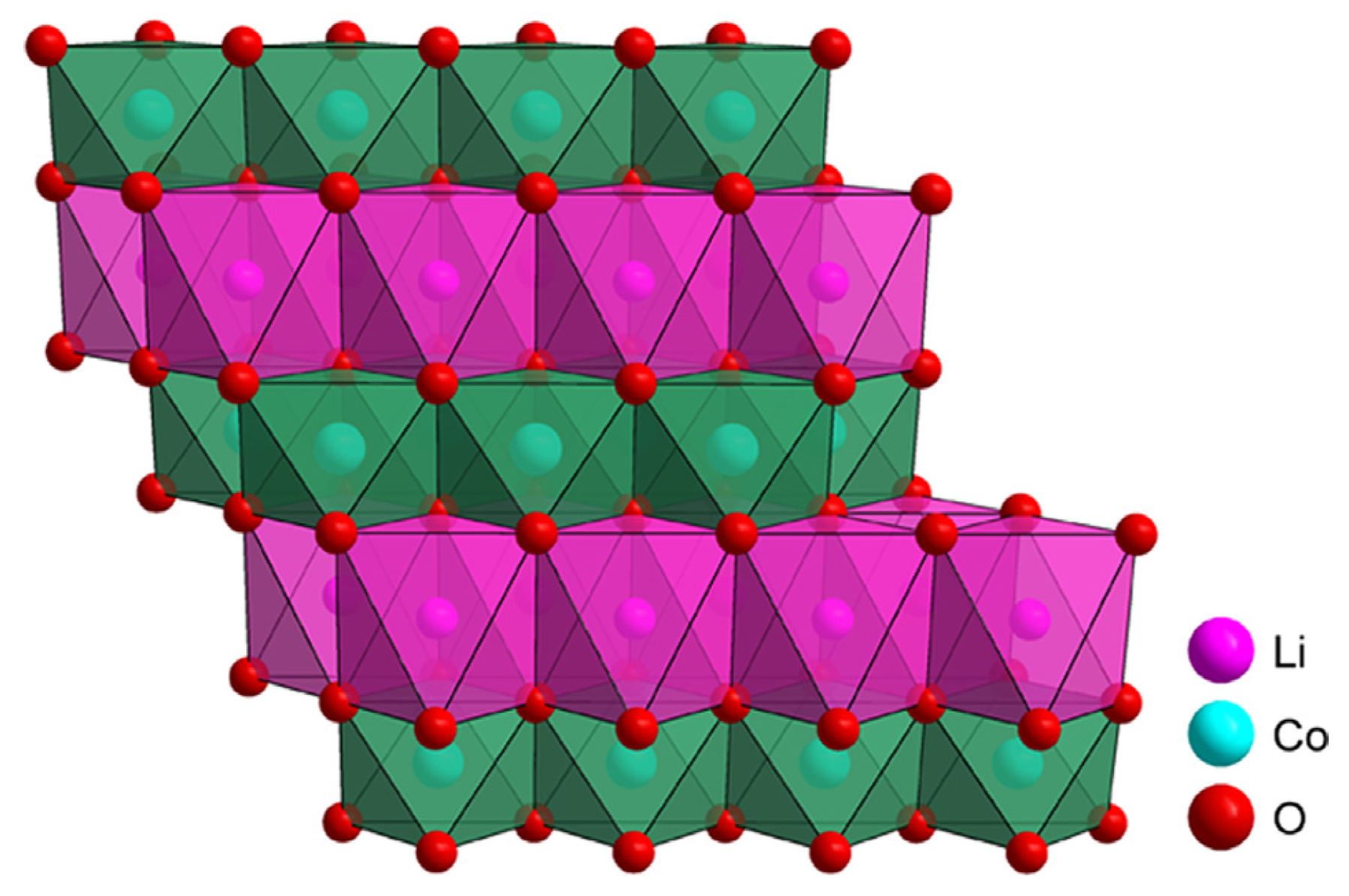
2.1. Lithium Cobalt Oxide (LCO)
2.2. Lithium Manganate Oxide (LMO)
2.3. Lithium Iron Phosphate (LFP)
2.4. Lithium Nickel Manganese Cobalt Oxides (NCM)
3. LIB Thermal Runaway
3.1. Thermal Runaway Mechanism
3.1.1. Decomposition of SEI Layer
3.1.2. Reaction between Anode and Electrolyte (SEI Film Regeneration)
3.1.3. Separator Melt Down
3.1.4. Decomposition of Cathode
3.1.5. Decomposition of Electrolyte Solution
3.1.6. The Reaction between Anode and Binder
3.1.7. The Burning of Electrolyte and Gas Release
3.2. Thermal Runaway Inducements
3.2.1. Mechanical Abuse
3.2.2. Electrical Abuse
3.2.3. Thermal Abuse
4. LIB Safety Measures
4.1. Overcharge Protection
4.2. Overheat Protection
4.3. Battery Management System (BMS)
4.4. Battery Thermal Management System (BTMS)
4.5. Safety Protection Device
5. Research and Application Status of Large-Scale LIB in Mining Industry
5.1. Research of Large-Scale LIB in Mining Industry
5.2. Application of Large-Scale LIB in Mining Industry
6. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3C | Computer, communication, and consumer electronics |
| ARC | Accelerating rate calorimeter |
| BMS | Battery management system |
| BTMS | Battery thermal management system |
| CCTEG | China Coal Technology Engineering Group |
| CID | Current interrupt device |
| DBBB | 2,5-Di-tert-butyl-1,4-bis benzene |
| DEC | Diethyl carbonate |
| DMC | Dimethyl carbonate |
| EC | Ethylene carbonate |
| EKF | Extended Kalman filter |
| EMC | Methyl ethyl carbonate |
| EPL | Equipment protection level |
| EV | Electronic vehicle |
| GMG | Global mining guidelines |
| IEC | International electrotechnical commission |
| ISC | Internal short circuit |
| KF | Kalman filter |
| LCO | Lithium cobalt oxide |
| LFP | Lithium iron phosphate |
| LIB | Lithium-ion battery |
| LMO | Lithium manganate oxide |
| MPSACC | Mining products safety and certification center |
| MPT | Methylphenothiazine |
| MSHA | Mine safety and health administration |
| NCA | Nickel cobalt aluminum |
| NCM | Lithium nickel manganese cobalt oxide |
| NIOSH | National institute for occupational safety and health |
| OCV | Open circuit voltage |
| PCM | Phase change material |
| PE | Polyethylene |
| PET | Polyethylene terephthalate |
| PI | Polyimide |
| PP | Polypropylene |
| PTC | Positive temperature coefficient element |
| PVDF | Polyvinylidene fluoride |
| RLS | Recursive least squares filter |
| SEI | Solid electrolyte interphase |
| SOC | State of charge |
| SOH | State of health |
| TEMPO | 2,2,6,6-tetramethylpiperidinyl-oxide |
| TGA | Thermal gravimetric analyzer |
| TM | Transmission metal |
| TR | Thermal runaway |
| UKF | Unscented Kalman filter |
References
- Reddy, M.V.; Mauger, A.; Julien, C.M.; Paolella, A.; Zaghib, K. Brief history of early lithium-battery development. Materials 2020, 13, 1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Wang, Z.; Wang, C.; Huang, L. Overcharge investigation of large format lithium-ion pouch cells with Li(Ni0.6Co0.2Mn0.2)O2 cathode for electric vehicles: Thermal runaway features and safety management method. J. Electrochem. Soc. 2018, 165, 3613–3629. [Google Scholar] [CrossRef]
- Annual Report on The Development of China’s Auto Industry; Equipment Industry Development Center of the Ministry of Industry and Information Technology of China: Beijing, China, 2021.
- GB 31241-2014; Lithium Ion Cells and Batteries Used in Portable Electronic Equipments-Satety Requirements. Standards Press of China: Beijing, China, 2014.
- GB 38031-2020; Electric Vechicles Traction Battery Safety Requirements. Standards Press of China: Beijing, China, 2020.
- Wang, B.; Jin, J.; Yuan, X. Development status and key technology of mine electric driving trackless transportation vehicles. Coal Sci. Technol. 2015, 43, 74–76. [Google Scholar]
- He, F.; Cui, X.; Shen, W.S.; Kapoor, A. Modelling of electric vehicles for underground mining personnel transport. In Proceedings of the IEEE 8th Conference on Industrial Electronics and Applications (ICIEA), Melbourne, VIC, Australia, 19–21 June 2013; pp. 870–875. [Google Scholar]
- Zhang, Y. Technical analysis of safety performance for mining flam—Proof trackless vehicles powered by lithium battery. Coal Sci. Technol. 2017, 45, 4. [Google Scholar]
- Zhang, G.; Zhang, L.; Rao, Z.; Li, Y. Manufacture and performance tests of lithium iron phosphate batteries used as electric vehicle power. J. Automot. Saf. Energy 2011, 2, 68–71. [Google Scholar]
- Aziz, N.A.A.; Abdullah, T.K.; Mohamad, A.A. Synthesis of LiCoO2 via sol-gel method for aqueous rechargeable lithium batteries. Ionics 2017, 8, 1–10. [Google Scholar]
- Li, Z.; Li, A.; Zhang, H.; Ning, F.; Li, W. Multi-scale stabilization of high-voltage LiCoO2 enabled by nanoscale solid electrolyte coating. Energy Storage Mater. 2020, 29, 71–77. [Google Scholar] [CrossRef]
- Dong, Y.; Su, P.; He, G.; Zhao, H.; Bai, Y. Constructing compatible interface between Li7La3Zr2O12 solid electrolyte and LiCoO2 cathode for stable cycling performances at 4.5 V. Nanoscale 2021, 13, 7822–7830. [Google Scholar] [CrossRef]
- Zhang, T.; Li, D.; Tao, Z.; Chen, J. Understanding electrode materials of rechargeable lithium batteries via DFT calculations. Prog. Nat. Sci. Mater. Int. 2013, 22, 256–272. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Qian, D.; Wang, Z.; Meng, Y.S. Recent progress in cathode materials research for advanced lithium ion batteries. Mater. Sci. Eng. R. Rep. 2012, 73, 51–65. [Google Scholar] [CrossRef]
- Zhou, Q.H.; Xu, H.H.; Lv, L.; Liu, W.H.; Liang, Y.; Li, H.L.; Chen, T. Study on the decline mechanism of cathode material LiCoO2 for Li-ion battery. Vacuum 2020, 177, 109313. [Google Scholar] [CrossRef]
- Tan, J.H.; Wang, Z.Z.; Li, G.Z.; Hu, H.C.; Li, J.; Han, R.; Zhang, D.Y. Electrochemically driven phase transition in LiCoO2 cathode. Materials 2021, 14, 242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.S.; Li, X.N.; Liu, W.F.; Yue, H.Y.; Shi, Z.P.; Yin, Y.H.; Yang, S.T. Olivine LiFePO4 as an additive into LiCoO2 electrodes for LIBs to improve high-voltage performances. J. Alloy. Compd. 2021, 869, 159188. [Google Scholar] [CrossRef]
- Du, M.; Li, Q.; Pang, H. Oxalate-derived porous prismatic nickel/nickel oxide nanocomposites toward lithium-ion battery. J. Colloid Interface Sci. 2020, 580, 614–622. [Google Scholar] [CrossRef]
- Kawashima, K.; Ohnishi, T.; Takada, K. High-Rate Capability of LiCoO2 Cathodes. Acs. Appl. Energy Mater. 2020, 3, 11803–11810. [Google Scholar] [CrossRef]
- Mahara, Y.; Makimura, Y.; Oka, H.; Nakano, H.; Tajima, S.; Nonaka, T.; Sasaki, T. Appearance of the 4 V signal without transformation to spinel-related oxides from loose-crystalline rock-salt LiMnO2. J. Power Sources 2021, 497, 229788. [Google Scholar] [CrossRef]
- Zhenfei, C.; Yangzhou, M.; Xuanning, H.; Xiaohui, Y.; Zexin, Y.; Shihong, Z.; Guangsheng, S.; Youlong, X.; Cuie, W.; Weidong, Y. High electrochemical stability al-doped spinel limn_2o_4 cathode material for li-ion batteries. J. Energy Storage 2020, 27, 101036.1–101036.8. [Google Scholar]
- Dong, W.J.; Huang, X.Y.; Jin, Y.; Xie, M.; Zhao, W.; Huang, F.Q. Building an artificial solid electrolyte interphase on spinel lithium manganate for high performance aqueous lithium-ion batteries. Dalton Trans. 2020, 49, 8136–8142. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef]
- Yang, S.; Song, Y.; Zavalij, P.Y.; Whittingham, M.S. Reactivity, stability and electrochemical behavior of lithium iron phosphates. Electrochem. Commun. 2002, 4, 239–244. [Google Scholar] [CrossRef]
- Yamada, A.; Chung, S.C.; Hinokuma, K. Optimized LiFePO4 for lithium battery cathodes. J. Electrochem. Soc. 2001, 148, 224–229. [Google Scholar] [CrossRef]
- Beletskii, E.V.; Alekseeva, E.V.; Spiridonova, D.Y.V.; Yankin, A.N.; Levin, O.V. Overcharge cycling effect on the surface layers and crystalline structure of LiFePO4 cathodes of Li-Ion batteries. Energies 2019, 12, 4652. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.G.; Liu, T.; Wang, C.Y. Thermally modulated lithium iron phosphate batteries for mass-market electric vehicles. Nat. Energy 2021, 6, 176–185. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, K. Electronic structure and comparative properties of LiNixMnyCozO2 cathode materials. J. Phys. Chem. C 2017, 121, 6002–6010. [Google Scholar] [CrossRef]
- Min, K.; Kim, K.; Jung, C.; Seo, S.-W.; Song, Y.Y.; Lee, H.S.; Shin, J.; Shin, J. A comparative study of structural changes in lithium nickel cobalt manganese oxide as a function of Ni content during delithiation process. J. Power Sources 2016, 315, 111–119. [Google Scholar] [CrossRef]
- Yan, W.; Yang, S.; Huang, Y.; Yang, Y.; Yuan, G. A review on dopingcoating of nickel-rich cathode materials for lithium-ion batteries. J. Alloy. Compd. 2019, 819, 153048. [Google Scholar] [CrossRef]
- Fan, L.; Guo, X.T.; Hang, X.X.; Pang, H. Synthesis of truncated octahedral zinc-doped manganese hexacyanoferrates and low-temperature calcination activation for lithium-ion battery. J. Colloid Interface Sci. 2022, 607, 1898–1907. [Google Scholar] [CrossRef]
- Chiba, K.; Yoshizawa, A.; Isogai, Y. Thermal safety diagram for lithium-ion battery using single-crystal and polycrystalline particles LiNi0.8Co0.1Mn0.1O2. J. Energy Storage 2020, 32, 101775. [Google Scholar] [CrossRef]
- Park, S.; Jo, C.; Kim, H.J.; Kim, S.; Myung, S.T.; Kang, H.K.; Kim, H.; Song, J.; Yu, J.S.; Kwon, K. Understanding the role of trace amount of Fe incorporated in Ni-rich Li Ni1-x-yCoxMny O-2 cathode material. J. Alloy. Compd. 2020, 835, 155342. [Google Scholar] [CrossRef]
- Watanabe, S.; Kinoshita, M.; Nakura, K. Capacity fade of LiNi(1−x−y) CoxAlyO2 cathode for lithium-ion batteries during accelerated calendar and cycle life test. I. Comparison analysis between LiNi(1−x−y) CoxAlyO2 and LiCoO2 cathodes in cylindrical lithium-ion cells during long term storage test. J. Power Sources 2014, 247, 412–422. [Google Scholar] [CrossRef]
- Chu, C.T.; Chang, L.M.; Yin, D.M.; Zhang, D.Y.; Cheng, Y.; Wang, L.M. Large-sized nickel-cobalt-manganese composite oxide agglomerate anode material for long-life-span lithium-ion batteries. ACS Appl. Energy Mater. 2021, 4, 13811–13818. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Liu, C.; Li, H.; Kong, X.; Zhao, J. Modeling analysis of the effect of battery design on internal short circuit hazard in LiNi0.8Co0.1Mn0.1O2/SiOx-graphite lithium ion batteries. Int. J. Heat Mass Transf. 2020, 153, 119590. [Google Scholar] [CrossRef]
- Kim, G.-H.; Pesaran, A.; Spotnitz, R. A three-dimensional thermal abuse model for lithium-ion cells. J. Power Sources 2007, 170, 476–489. [Google Scholar] [CrossRef]
- Nguyen, T.T.D.; Abada, S.; Lecocq, A.; Bernard, J.; Petit, M.; Marlair, G.; Grugeon, S.; Laruelle, S. Understanding the thermal runaway of Ni-Rich lithium-ion batteries. World Electr. Veh. J. 2019, 10, 79. [Google Scholar] [CrossRef] [Green Version]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Zhao, L.; Watanabe, I.; Doi, T.; Okada, S.; Yamaki, J.I. TG-MS analysis of solid electrolyte interphase (SEI) on graphite negative-electrode in lithium-ion batteries. J. Power Sources 2006, 161, 1275–1280. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Chen, C. Thermal stability of delithiated LiMn2O4 with electrolyte for lithium-ion batteries. J. Electrochem. Soc. 2007, 154, 263. [Google Scholar] [CrossRef]
- Ren, D.; Hsu, H.; Li, R.; Feng, X.; Guo, D.; Han, X.; Lu, L. A comparative investigation of aging effects on thermal runaway behavior of lithium-ion batteries. eTransportation 2019, 2, 100034. [Google Scholar] [CrossRef]
- Yoon, T.; Milien, M.S.; Parimalam, B.S.; Lucht, B.L. Thermal decomposition of the solid electrolyte interphase (SEI) on silicon electrodes for lithium ion batteries. Chem. Mater. 2017, 29, 3237–3245. [Google Scholar] [CrossRef]
- Saqib, N.; Ganim, C.M.; Shelton, A.E.; Porter, J.M. On the decomposition of carbonate-based lithium-ion battery electrolytes studied using operando infrared spectroscopy. J. Electrochem. Soc. 2018, 165, 4051–4057. [Google Scholar] [CrossRef]
- Maleki, H.; Deng, G.; Anani, A.; Howard, J. Thermal stability studies of li-ion cells and components. J. Electrochem. Soc. 2019, 146, 3224–3229. [Google Scholar] [CrossRef]
- Ryou, M.H.; Lee, J.N.; Lee, D.J.; Kim, W.K.; Jeong, Y.K.; Choi, J.W.; Park, J.K.; Lee, Y.M. Effects of lithium salts on thermal stabilities of lithium alkyl carbonates in SEI layer. Electrochim. Acta 2012, 83, 259–263. [Google Scholar] [CrossRef]
- Zhang, Z.; Fouchard, D.; Rea, J.R. Differential scanning calorimetry material studies: Implications for the safety of lithium-ion cells. J. Power Sources 1998, 70, 16–20. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, K. Rational design on separators and liquid electrolytes for safer lithium-ion batteries. J. Energy Chem. 2020, 43, 58–70. [Google Scholar] [CrossRef] [Green Version]
- Roth, E.P.; Doughty, D.H.; Pile, D.L. Effects of separator breakdown on abuse response of 18650 Li-ion cells. J. Power Sources 2007, 174, 579–583. [Google Scholar] [CrossRef]
- Deimede, V.; Elmasides, C. Separators for lithium-ion batteries: A review on the production processes and recent developments. Energy Technol. 2015, 3, 453–468. [Google Scholar] [CrossRef]
- Choi, J.A.; Kim, S.H.; Kim, D.W. Enhancement of thermal stability and cycling performance in lithium-ion cells through the use of ceramic-coated separators. J. Power Sources 2010, 195, 6192–6196. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, K.; Jow, T. An inorganic composite membrane as the separator of Li-ion batteries. J. Power Sources 2005, 140, 361–364. [Google Scholar] [CrossRef]
- Liu, K.; Zhuo, D.; Lee, H.W.; Liu, W.; Lin, D.; Lu, Y.; Cui, Y. Extending the life of lithium-based rechargeable batteries by reaction of lithium dendrites with a novel silica nanoparticle sandwiched separator. Adv. Mater. 2017, 29, 1603987. [Google Scholar] [CrossRef]
- Peng, P.; Jiang, F. Thermal safety of lithium-ion batteries with various cathode materials: A numerical study. Int. J. Heat Mass Transf. 2016, 103, 1008–1016. [Google Scholar] [CrossRef]
- Spotnitz, R.; Franklin, J. Abuse behavior of high-power, lithium-ion cells. J. Power Sources 2003, 113, 81–100. [Google Scholar] [CrossRef]
- MacNeil, D.D.; Dahn, J.R. The reaction of charged cathodes with nonaqueous solvents and electrolytes. J. Electrochem. Soc. 2001, 148, 1205. [Google Scholar] [CrossRef]
- Wang, Q.; Mao, B.; Stoliarov, S.I.; Sun, J. A review of lithium ion battery failure mechanisms and fire prevention strategies. Prog. Energy Combust. Sci. 2019, 73, 95–131. [Google Scholar] [CrossRef]
- Lamb, J.; Orendorff, C.J.; Roth, E.P.; Langendorf, J. Studies on the thermal breakdown of common Li-Ion battery electrolyte components. J. Electrochem. Soc. 2015, 162, 2131–2135. [Google Scholar] [CrossRef]
- Roth, E.P.; Orendorff, C.J. How electrolytes influence battery safety. Electrochem. Soc. Interface 2012, 21, 45. [Google Scholar] [CrossRef]
- Pasquier, A.D.; Disma, F.; Bowmer, T.; Gozdz, A.S.; Amatucci, G.; Tarascon, J.M. Differential scanning calorimetry study of the reactivity of carbon anodes in plastic Li-Ion batteries. J. Electrochem. Soc. 2019, 145, 472–477. [Google Scholar] [CrossRef]
- Markevich, E.; Salitra, G.; Aurbach, D. Influence of the PVdF binder on the stability of LiCoO2 electrodes. Electrochem. Commun. 2005, 7, 1298–1304. [Google Scholar] [CrossRef]
- Biensan, P.; Simon, B.; Peres, J.P.; De Guibert, A.; Broussely, M.; Bodet, J.M.; Perton, F. On safety of lithium-ion cells. J. Power Sources 1999, 81, 906–912. [Google Scholar] [CrossRef]
- See, K.W.; Wang, Y.; Zhang, Y.; Zhang, N.; Zang, C. Study on influencing factors of mine explosion-proof lithium-ion battery power supply safety design. Coal Sci. Technol. 2020, 48, 153–165. [Google Scholar]
- Fernandes, Y.; Bry, A.; De Persis, S. Identification and quantification of gases emitted during abuse tests by overcharge of a commercial Li-ion battery. J. Power Sources 2018, 389, 106–119. [Google Scholar] [CrossRef]
- Wen, J.; Yu, Y.; Chen, C. A Review on lithium-ion batteries safety issues: Existing problems and possible solutions. Mater. Express 2012, 2, 197–212. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Lai, W.-J.; Ali, M.Y.; Pan, J. Mechanical behavior of representative volume elements of lithium-ion battery modules under various loading conditions. J. Power Sources 2014, 248, 789–808. [Google Scholar] [CrossRef]
- Sahraei, E.; Kahn, M.; Meier, J.; Wierzbicki, T. Modelling of cracks developed in lithium-ion cells under mechanical loading. RSC Adv. 2015, 5, 80369–80380. [Google Scholar] [CrossRef]
- Sahraei, E.; Bosco, E.; Dixon, B.; Lai, B. Microscale failure mechanisms leading to internal short circuit in Li-ion batteries under complex loading scenarios. J. Power Sources 2016, 319, 56–65. [Google Scholar] [CrossRef]
- Kalnaus, S.; Wang, Y.; Turner, J.A. Mechanical behavior and failure mechanisms of Li-ion battery separators. J. Power Sources 2017, 348, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Cannarella, J.; Liu, X.; Leng, C.Z.; Sinko, P.D.; Gor, G.Y.; Arnold, C.B. Mechanical properties of a battery separator under compression and tension. J. Electrochem. Soc. 2014, 161, 3117–3122. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Han, G.Y.; Yoon, K.J.; Park, J.H. Preparation of a trilayer separator and its application to lithium-ion batteries. J. Power Sources 2010, 195, 8302–8305. [Google Scholar] [CrossRef]
- Yamanaka, T.; Takagishi, Y.; Tozuka, Y.; Yamaue, T. Modeling lithium ion battery nail penetration tests and quantitative evaluation of the degree of combustion risk. J. Power Sources 2019, 416, 132–140. [Google Scholar] [CrossRef]
- Finegan, D.P.; Tjaden, B.; Heenan, T.M.M.; Jervis, R.; Michiel, M.D.; Rack, A.; Hinds, G.; Brett, D.J.L.; Shearing, P.R. Tracking internal temperature and structural dynamics during nail penetration of lithium-ion cells. J. Electrochem. Soc. 2017, 164, 3285–3291. [Google Scholar] [CrossRef]
- Huang, S.; Du, X.; Richter, M.; Ford, J.; Cavalheiro, G.M.; Du, Z.; White, R.T.; Zhang, G. Understanding Li-Ion cell internal short circuit and thermal runaway through small, slow and in situ sensing nail penetration. J. Electrochem. Soc. 2020, 167, 90526. [Google Scholar] [CrossRef]
- Wang, H.; Kumar, A.; Simunovic, S.; Allu, S.; Kalnaus, S.; Turner, J.A.; Helmers, J.C.; Rules, E.T.; Winchester, C.S.; Gorney, P. Progressive mechanical indentation of large-format Li-ion cells. J. Power Sources 2017, 341, 156–164. [Google Scholar] [CrossRef] [Green Version]
- Avdeev, I.; Gilaki, M. Structural analysis and experimental characterization of cylindrical lithium-ion battery cells subject to lateral impact. J. Power Sources 2014, 271, 382–391. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Lin, C.; Su, Y.; Yang, S. State of charge-dependent failure prediction model for cylindrical lithium-ion batteries under mechanical abuse. Appl. Energy 2019, 251, 113365. [Google Scholar]
- Wu, M.S.; Chiang, P.C.J.; Lin, J.C.; Jan, Y.S. Correlation between electrochemical characteristics and thermal stability of advanced lithium-ion batteries in abuse tests—Short-circuit tests. Electrochim. Acta 2004, 49, 1803–1812. [Google Scholar] [CrossRef]
- Li, J.; Sun, D.; Jin, X.; Shi, W.; Sun, C. Lithium-ion battery overcharging thermal characteristics analysis and an impedance-based electro-thermal coupled model simulation. Appl. Energy 2019, 254, 113574. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, J.; Zhu, X.; Wang, H.; Huang, L.; Wang, Y.; Xu, S. Overcharge-to-thermal-runaway behavior and safety assessment of commercial lithium-ion cells with different cathode materials: A comparison study. J. Energy Chem. 2021, 55, 484–498. [Google Scholar] [CrossRef]
- Bugryniec, P.J.; Davidson, J.N.; Cumming, D.J.; Brown, S.F. Pursuing safer batteries: Thermal abuse of LiFePO4 cells. J. Power Sources 2019, 414, 557–568. [Google Scholar] [CrossRef] [Green Version]
- Liao, Z.; Zhang, S.; Li, K.; Zhao, M.; Qiu, Z.; Han, D.; Zhang, G.; Habetler, T.G. Hazard analysis of thermally abused lithium-ion batteries at different state of charges. J. Energy Storage 2020, 27, 101065. [Google Scholar] [CrossRef]
- Ren, D.; Feng, X.; Liu, L.; Hsu, H.; Lu, L.; Wang, L.; He, X.; Ouyang, M. Investigating the relationship between internal short circuit and thermal runaway of lithium-ion batteries under thermal abuse condition. Energy Storage Mater. 2021, 34, 563–573. [Google Scholar] [CrossRef]
- Xu, G.; Huang, L.; Lu, C.; Zhou, X.; Cui, G. Revealing the multilevel thermal safety of lithium batteries. Energy Storage Mater. 2020, 31, 72–86. [Google Scholar] [CrossRef]
- IEC 62133-2:2017; Safety requirements for portable sealed secondary cells, and for batteries made from them, for use in portable applications–Part 2: Lithium systems. In Proceedings of the International Conference on Connected Vehicles and Expo (ICCVE), Las Vegas, NV, USA, 2–6 December 2017. IEC: Geneva, Switzerland; pp. 437–442.
- IEC 62619:2017; Secondary Cells and Batteries Containing Alkaline or Other Non-Acid Electrolytes–Safety Requirements for Secondary Lithium Cells and Batteries, For Use In Industrial Applications. IEC: Geneva, Switzerland, 2017.
- IEC 62281-2019; Safety of Primary and Secondary Lithium Cells and Batteries During Transport. IEC: Geneva, Switzerland, 2019.
- Chombo, P.V.; Laoonual, Y. A review of safety strategies of a Li-ion battery. J. Power Sources 2020, 478, 228649. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Redfern, P.C.; Curtiss, L.A.; Amine, K. Molecular engineering towards safer lithium-ion batteries: A highly stable and compatible redox shuttle for overcharge protection. Energy Environ. Sci. 2012, 5, 8204. [Google Scholar] [CrossRef]
- Buhrmester, C.; Moshurchak, L.; Wang, R.L.; Dahn, J.R. Phenothiazine Molecules. J. Electrochem. Soc. 2006, 153, 288. [Google Scholar] [CrossRef]
- Buhrmester, C.; Moshurchak, L.; Wang, R.; Dahn, J. The use of 2,2,6,6-tetramethylpiperinyl-oxides and derivatives for redox shuttle additives in Li-Ion Cells. J. Electrochem. Soc. 2006, 153, 1800. [Google Scholar] [CrossRef]
- Zheng, Y.; Ouyang, M.; Lu, L.; Li, J.; Han, X.; Xu, L. On-line equalization for lithium-ion battery packs based on charging cell voltages: Part 1. Equalization based on remaining charging capacity estimation. J. Power Sources 2014, 247, 676–686. [Google Scholar] [CrossRef]
- Love, C.T.; Johannes, M.D.; Swider-Lyons, K.E. Thermal stability of delithiated Al-substituted Li(Ni1/3Co1/3Mn1/3)O2 cathodes. ECS Trans. 2010, 25, 231–240. [Google Scholar] [CrossRef]
- Choudhury, S.; Azizi, M.; Raguzin, I.; Göbel, M.; Michel, S.; Simon, F.; Willomitzer, A.; Mechtcherine, V.; Stamm, M.; Ionov, L. Effect of fibrous separators on the performance of lithium–sulfur batteries. Phys. Chem. Chem. Phys. 2017, 19, 11239–11248. [Google Scholar] [CrossRef]
- Miao, Y.-E.; Zhu, G.-N.; Hou, H.; Xia, Y.-Y.; Liu, T. Electrospun polyimide nanofiber-based nonwoven separators for lithium-ion batteries. J. Power Sources 2013, 226, 82–86. [Google Scholar] [CrossRef]
- Zeng, Z.; Wu, B.; Xiao, L.; Jiang, X.; Chen, Y.; Ai, X.; Yang, H.; Cao, Y. Safer lithium ion batteries based on nonflammable electrolyte. J. Power Sources 2015, 279, 6–12. [Google Scholar] [CrossRef]
- Smith, J.; Singh, R.; Hinterberger, M.; Mochizuki, M. Battery thermal management system for electric vehicle using heat pipes. Int. J. Therm. Sci. 2018, 134, 517–529. [Google Scholar] [CrossRef]
- Bandhauer, T.M.; Garimella, S.; Fuller, T.F. A critical review of thermal issues in lithium-ion batteries. J. Electrochem. Soc. 2011, 158, 1–25. [Google Scholar] [CrossRef]
- Katoch, S.S.; Eswaramoorthy, M. A detailed review on electric vehicles battery thermal management system. IOP Conf. Ser. Mater. Sci. Eng. 2020, 912, 42005. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, M.; Tao, P.; Song, C.; Wu, J.; Wang, J.; Deng, T.; Shang, W. Temperature effect and thermal impact in lithium-ion batteries: A review. Prog. Nat. Sci. Mater. Int. 2018, 28, 653–666. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, K.H.; Ko, D.C.; Lee, S.B.; Kim, B.M. Design of integrated safety vent in prismatic lithium-ion battery. J. Mech. Sci. Technol. 2017, 31, 2505–2511. [Google Scholar] [CrossRef]
- Finegan, D.P.; Darcy, E.; Keyser, M.; Tjaden, B.; Heenan, T.; Jervis, R.; Bailey, J.J.; Vo, N.T.; Magdysyuk, O.V.; Drakopoulos, M. Thermal runaway: Identifying the cause of rupture of Li-Ion batteries during thermal runaway. Adv. Sci. 2018, 5, 1700369. [Google Scholar] [CrossRef] [Green Version]
- Kuchta, J.M. Investigation of Fire and Explosion Accidents in the Chemical, Mining, and Fuel-Related Industries–A Manual; U.S. Department of the Interior, Bureau of Mines: Washington, DC, USA, 1986.
- MSHA. Requirements for explosion testing per 30 CFR, 18.62. In ASTP 2137; U.S. Department of Labor, Mine Safety and Health Administration: Arlington, VA, USA. Available online: https://arlweb.msha.gov/TECHSUPP/ACC/StandardTestProcs/ASTP2137.pdf (accessed on 1 August 2019).
- Wo, L.; Zhang, Y.; He, J. Study on safety of large volume lithium ion battery applied in flameproof enclosure. Coal Sci. Technol. 2018, 46, 145–148. [Google Scholar]
- Dubaniewicz, T.H.; Zlochower, I.; Barone, T.; Thomas, R.; Yuan, L. Thermal runaway pressures of iron phosphate lithium-ion cells as a function of free space within sealed enclosures. Min. Metall. Explor. 2020, 38, 539–547. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Xie, B.; Zhao, X.; Zhu, K. Analysis on explosion-proof techniques and standards for lithium-ion battery power supply used in underground coal mine. Coal Sci. Technol. 2020, 48, 203–208. [Google Scholar]
- Mao, B.; Liu, C.; Yang, K.; Li, S.; Liu, P.; Zhang, M.; Meng, X.; Gao, F.; Duan, Q.; Wang, Q.; et al. Thermal runaway and fire behaviors of a 300 Ah lithium ion battery with LiFePO4 as cathode. Renew. Sustain. Energy Rev. 2021, 139, 110717. [Google Scholar] [CrossRef]
- Meng, L.; See, K.W.; Wang, G.; Wang, Y.; Zhang, Y.; Zang, C.; Xie, B. Explosion-proof lithium-ion battery pack–In-depth investigation and experimental study on the design criteria. Energy 2022, 249, 23715. [Google Scholar] [CrossRef]
- GB 3836; Explosive Atmospheres. Standards Press of China: Beijing, China, 2017.
- Safety Requirements for Mining Lithium ion Battery (Trial); China Mining Products Safety Approval and Certification Center: Beijing, China, 2020.
- Safety Requirements for Mine Flameproof (Intrinsically Safe) Lithium ion Battery Power Supply (Trial); China Mining Products Safety Approval and Certification Center: Beijing, China, 2020.
- Mitchell, P. Will Electrification Spark the Next Wave of Mining Innovation; EY: London, UK, 2019. [Google Scholar]
- Electric Vehicle Council. State of Electric Vehicles. Available online: https://electricvehiclecouncil.com.au (accessed on 18 April 2022).
- GMG Recomended Practices for Battery Electric Vehicles in Underground Mining—2nd ed. 2020. Available online: https://gmggroup.org/wp-content/uploads/2018/11/20180621_UG_Mining_BEV_GMG-WG-v02-r01.pdf (accessed on 18 April 2022).
- Shang, X.-F.; Cheol, C.M. A study on corporate culture of BYD. Int. J. Adv. Cult. Technol. 2020, 8, 135–140. [Google Scholar]
- Li, J.L.; Du, Z.J.; Ruther, R.E.; An, S.J.; David, L.A.; Hays, K.; Wood, M.; Phillip, N.D.; Sheng, Y.P.; Mao, C.Y.; et al. Toward low-cost, high-energy density, and high-power density lithium-ion batteries. JOM 2017, 69, 1484–1496. [Google Scholar] [CrossRef] [Green Version]
- He, J.R.; Bhargav, A.; Manthiram, A. High-energy-density, long-life lithium-sulfur batteries with practically necessary parameters enabled by low-cost Fe-Ni nanoalloy catalysts. ACS Nano 2021, 15, 8583–8591. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.X.; Rong, X.H.; Hu, Y.S.; Chen, L.Q.; Li, H. Research and development of advanced battery materials in China. Energy Storage Mater. 2019, 23, 144–153. [Google Scholar] [CrossRef]
- Shen, X.; Liu, H.; Cheng, X.B.; Yan, C.; Huang, J.Q. Beyond lithium ion batteries: Higher energy density battery systems based on lithium metal anodes. Energy Storage Mater. 2018, 12, 161–175. [Google Scholar] [CrossRef]
- Matsuda, S.; Yamaguchi, S.; Yasukawa, E.; Asahina, H.; Kakuta, H.; Otani, H.; Kimura, S.; Kameda, T.; Takayanagi, Y.; Tajika, A.; et al. Effect of electrolyte filling technology on the performance of porous carbon electrode-based lithium-oxygen batteries. ACS Appl. Energy Mater. 2021, 4, 2563–2569. [Google Scholar] [CrossRef]
- Li, S.L.; Jiang, Z.D.; Hou, X.Y.; Xu, J.; Xu, M.T.; Yu, X.B.; Ma, Z.F.; Yang, J.; Yuan, X.X. High performance Li-O2 batteries enabled with manganese sulfide as cathode catalyst. J. Electrochem. Soc. 2020, 167, 20520. [Google Scholar] [CrossRef]
- Yang, Y.F.; Wang, W.K.; Li, L.X.; Li, B.C.; Zhang, J.P. Stable cycling of Li-S batteries by simultaneously suppressing Li-dendrite growth and polysulfide shuttling enabled by a bioinspired separator. J. Mater. Chem. A 2020, 8, 3692–3700. [Google Scholar] [CrossRef]
- Guo, X.T.; Wang, S.B.; Yang, B.; Xu, Y.X.; Liu, Y.; Pang, H. Porous pyrrhotite Fe7S8 nanowire/SiOx/nitrogen-doped carbon matrix for high-performance Li-ion-battery anodes. J. Colloid Interface Sci. 2020, 561, 801–807. [Google Scholar] [CrossRef] [PubMed]






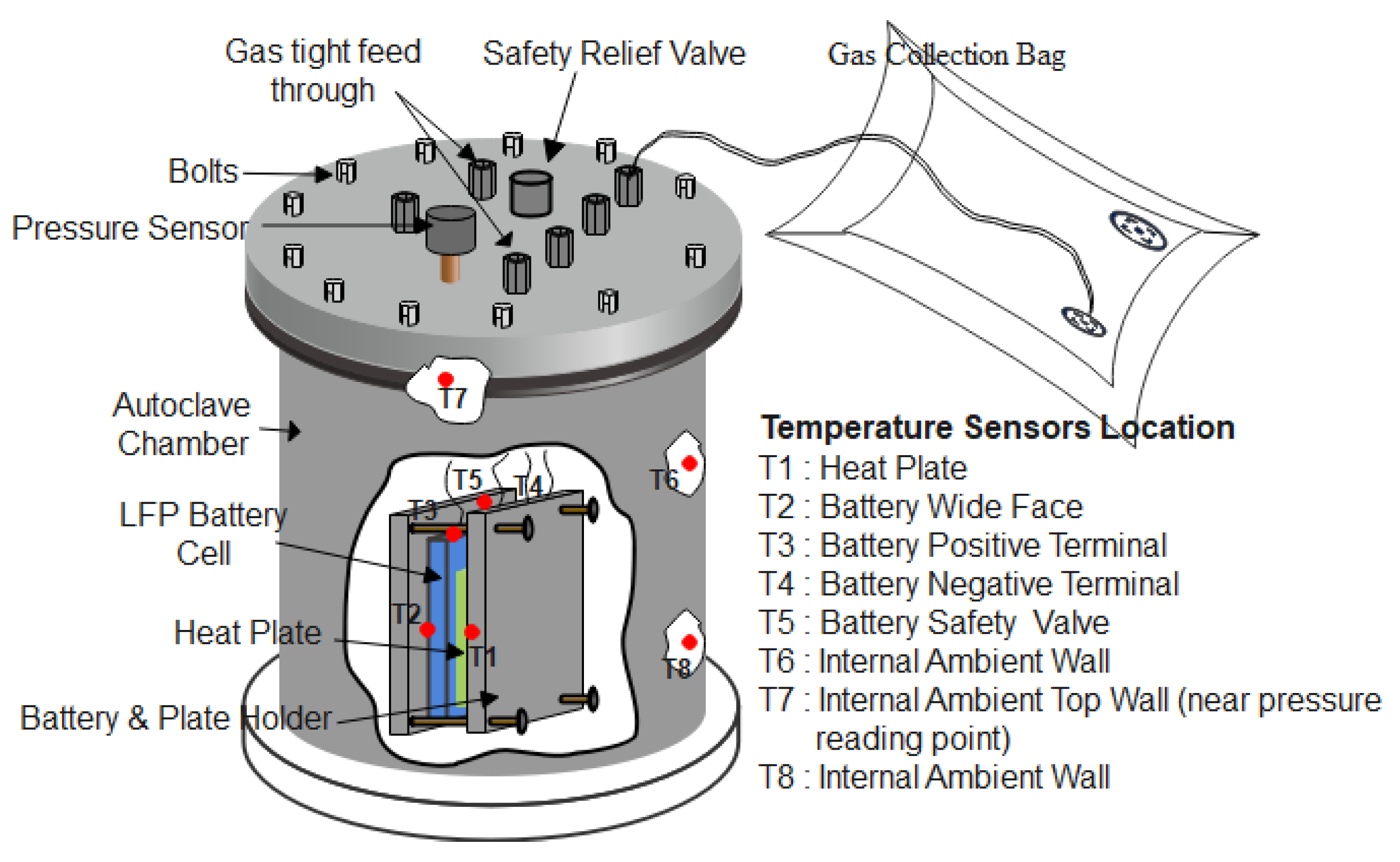










| Cathode Materials | Decomposition Equation | DecompositionTemperature |
|---|---|---|
| LFP | 190–285 °C | |
| LMO | 150–400 °C | |
| NCA | 160–200 °C | |
| NCM | 175–199 °C | |
| LCO | 220–500 °C |
| Solution | Boiling Point |
|---|---|
| EC | 517 K |
| DEC | 400 K |
| DMC | 364 K |
| EMC | 380 K |
| Gas Composition | M1-72 Ah V, % | M1-100 Ah V, % | M1-180 Ah V, % | M2-90 Ah V, % | M2-280 Ah V, % | M3-120 Ah V, % | M3-202 Ah V, % |
|---|---|---|---|---|---|---|---|
| CO2 | 7.52 | 7.99 | 11.95 | 5.69 | 14.18 | 6.69 | 11.9 |
| C2H4 | 4.19 | 4.18 | 5.88 | 0.85 | 2.30 | 1.99 | 1.8 |
| C2H2 | 0.07 | 0.18 | 0.19 | 0.05 | 0.07 | 0.05 | 0.1 |
| C2H6 | 0.56 | 0.9 | 1.31 | 0.09 | 0.65 | 0.41 | 0.55 |
| C3H6 | 0.92 | 1.76 | 2.8 | 0.05 | 0.56 | 0.23 | 0.47 |
| C3H8 | N.A | 0.21 | 0.37 | N.A | 0.20 | 0.1 | 0.16 |
| H2 | 12.79 | 17.91 | 23.39 | 5.74 | 33.99 | 15.46 | 29.89 |
| O2 | 1.1 | 0.45 | 1.48 | 11.04 | 3.36 | 0.58 | 0.72 |
| N2 | 67.91 | 61.64 | 45.23 | 75.52 | 36.79 | 72.14 | 48.06 |
| CH4 | 2.06 | 2.12 | 3.35 | 0.26 | 2.56 | 1.02 | 2.64 |
| CO | 2.89 | 2.66 | 4.04 | 0.73 | 5.33 | 1.34 | 3.7 |
| SoC Estimation Techniques | |||
|---|---|---|---|
| Direct Measurement | Book-Keeping Estimation | Adaptive System | Hybrid Methods |
|
|
|
|
| SoH Estimation Techniques | ||
|---|---|---|
| Experimental Methods | Model-Based Methods | Machine/Deep Learning Methods |
|
|
|
| Time | Research Group | Contribution |
|---|---|---|
| 1986 | Kuchta, J.M. | Observed the lowest spontaneous combustion temperature of CH4 |
| 2005 | MSHA | The ignition temperature of coal dust cloud is 440 °C to 640 °C |
| 2017 | MPSACC | Studied LIB safety performance requirements, chassis design, body structure, automatic protection and monitoring system, etc. |
| 2018 | MPSACC | Conducted short circuit and overcharge experiments on 60 Ah LFP battery |
| 2020 | CCTEG | Analysis on explosion—proof techniques and standards for lithium—ion battery power supply used in underground coal mine |
| 2020 | NIOSH | Measured TR pressures of LFP cells as a function of free space within sealed enclosures and observed an inverse power relationship. |
| 2021 | Binbin Mao | Conducted 300 Ah large-scale LFP batteries under external heating. TR can be divided to 4 stages. |
| 2022 | CCTEG | Conducted nail penetration test for 202 Ah large-scale battery under encapsulation protection. |
| EPL | Protection Type | Sign | Group | Standards |
|---|---|---|---|---|
| ‘Ma’ | Intrinsically safe | ‘ia’ | I | GB 3836.4 & IEC 60079.11 |
| Encapsulation | ‘ma’ | I | GB 3836.9 & IEC 60079.18 | |
| Special | ‘s’ | I | GB 3836.24 & IEC 60079.33 | |
| 2 independent EPL Mb | I | GB 3836.23 & IEC 60079.14 | ||
| ‘Mb’ | Intrinsically safe | ‘ib’ | I | GB 3836.4 & IEC 60079.11 |
| Explosion-proof | ‘d’ | I | GB 3836.2 & IEC 60079.1 | |
| Increased safe | ‘e’ | I | GB 3836.3 & IEC 60079.7 | |
| Positive-pressure | ‘p’,‘px’ | I | GB 3836.5 & IEC 60079.2 | |
| Encapsulation | ‘m’,‘mb’ | I | GB 3836.9 & IEC 60079.18 | |
| Sand filling | ‘q’ | I | GB 3836.7 & IEC 60079.5 | |
| Special | ‘s’ | I | GB 3836.24 & IEC 60079.33 |
| Recommended Industry Standard | Topic | Citation |
|---|---|---|
| E/ECE/324/Rev.2/Add.99/Rev.2 | Safety requirements of vehicle electric power train | United Nations, 2013 |
| ST/SG/AC.10/11/Rev.5 | Criteria, test methods and procedures for classifying dangerous goods | United Nations, 2019 |
| J2288_200806 | Standardized test method to determine the expected life cycles of BEV battery modules | SAE International, 2008 |
| UL 1642 | Requirements to reduce the risk of and injury from fire or explosion when lithium batteries are used or removed from a product and discarded | UL, 2012b |
| UL 2580 | Evaluates the ability of the electrical energy storage assembly to safely withstand simulated abuse conditions and prevents exposure of persons to hazards as a result of the abuse | UL, 2013 |
| CAN/CSA-E62660-1:15 | Performance and life testing of rechargeable lithium-ion cells for propulsion of BEVs and hybrid electric vehicles | CSA Group, 2015a |
| CAN/CSA-E62660-2:15 | Test procedures to observe the reliability and abuse behavior of rechargeable lithium-ion cells for propulsion of BEVs and hybrid electric vehicles | CSA Group, 2105b |
| IEC 62133–2:2017 | Requirements and tests for safe operation of portable sealed rechargeable lithium cells and LIBs containing non-acid electrolyte | International Electrotechnical Commission, 2017 |
| IEC 61508:2010 | Aspects to be considered when electrical/electronic/programmable electronic systems are used to carry out safety functions | International Electrotechnical Commission, 2010 |
| IEC 62061:2005 (plus amendments) | Requirements and recommendations for the design, integration, and validation of safety-related electronical, electronic and programmable electronic control systems for machines | International Electrotechnical Commission, 2015 |
| US CFR Parts 100–177 | United States Code of Federal Regulations on Transportation | United States Office of the Federal Register, 2012 |
| Canada TDG | Transportation of dangerous goods regulations | Transport Canada, 2016 |
| IMDG 2014,2016 | International Maritime Dangerous Goods Code. IMDG 2014 in force as of January 2016; IMDG 2016 in force as of January 2018 | International Maritime Organization, 2017 |
| IATA Dangerous Goods Regulations | International Air Transport Association Dangerous Goods Regulations | International Air Transport Association, 2018 |
| ISO 14990–1:2016 | General safety requirements for electrical equipment and components incorporated into earth-moving machines as defined in 1506165:2012 | International Organization for Standardization, 2016a |
| ISO 6165:2012 | Terms and definitions and an identification structure for classifying earth-moving machinery | International Organization for Standardization, 2012a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, L.; Wang, G.; See, K.W.; Wang, Y.; Zhang, Y.; Zang, C.; Zhou, R.; Xie, B. Large-Scale Li-Ion Battery Research and Application in Mining Industry. Energies 2022, 15, 3884. https://doi.org/10.3390/en15113884
Meng L, Wang G, See KW, Wang Y, Zhang Y, Zang C, Zhou R, Xie B. Large-Scale Li-Ion Battery Research and Application in Mining Industry. Energies. 2022; 15(11):3884. https://doi.org/10.3390/en15113884
Chicago/Turabian StyleMeng, Lingyu, Guofa Wang, Khay Wai See, Yunpeng Wang, Yong Zhang, Caiyun Zang, Rulin Zhou, and Bin Xie. 2022. "Large-Scale Li-Ion Battery Research and Application in Mining Industry" Energies 15, no. 11: 3884. https://doi.org/10.3390/en15113884
APA StyleMeng, L., Wang, G., See, K. W., Wang, Y., Zhang, Y., Zang, C., Zhou, R., & Xie, B. (2022). Large-Scale Li-Ion Battery Research and Application in Mining Industry. Energies, 15(11), 3884. https://doi.org/10.3390/en15113884