Recent Advancements in Chalcogenides for Electrochemical Energy Storage Applications
Abstract
:1. Introduction
2. Types of Electrochemical Energy Storage Devices
2.1. Batteries
2.2. Supercapacitors
3. Chalcogenides for Electrochemical Energy Storage Devices
3.1. Synthesis of Chalcogenides
3.2. Applications of Chalcogenides in Batteries
3.2.1. Applications of Chalcogenides in Metal-Ion Batteries
Li-Ion
Na-Ion
K-Ion
Mg-Ion
Al-Ion

Zn-Ion (ZIB)
Hybrid Metal Ion
3.2.2. Applications of Chalcogenides in Metal–Sulfur Batteries
Li–S Batteries
Na-S Batteries
Mg–S Batteries
3.2.3. Applications of Chalcogenides in Metal–Air Batteries
Li–Air
Zn–Air
Al–Air
3.3. Applications of Chalcogenides in Supercapacitors
3.3.1. Applications of Chalcogenides in Pseudocapacitors
Metal Tellurides
Metal Selenides
Metal Sulfides
3.3.2. Applications of Chalcogenides in Hybrid Capacitors
Metal Sulfides
Metal Selenides
Metal Tellurides
3.4. Chalcogenides for Flexible Devices
3.4.1. Flexible Supercapacitors
3.4.2. Flexible Batteries
4. Conclusions and Future Remark
- (i).
- Although several studies on modified TMCs have been published, their architecture-dependent feature modification has not been thoroughly examined. TMCs, for example, can be made in a variety of morphologies, including hierarchical, core-shell, and surface ornamentations.
- (ii).
- Advanced characterization methods such as in situ spectroscopy and imaging are appealing to the research industry because they provide real-time information about the reaction methods involved. These techniques can be used to establish a relationship between the material’s composition, structure, characteristics, and electrochemical performance. Furthermore, these techniques would aid in determining the underlying electrochemical reactions as well as monitoring the structural changes in the material during applications.
- (iii).
- Doping various heteroatoms such as oxygen, nitrogen, phosphorous, etc., into electroactive materials has been shown to improve their capacitive performance. Although this is relatively prevalent in carbon-based electroactive materials, it is quite rare in TMCs. Heteroatoms have been discovered to improve the electroactive materials’ electronic conductivity, which could aid rate performance in electrochemical energy storage applications. Furthermore, because of their affinity for various ionic species, these heteroatoms can act as anchoring points for ions within the electrolytes, thus increasing charge storage capabilities dramatically. For high-performance storage applications, heteroatom-doped TMC-based hybrid electroactive materials will be of great interest.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Badwal, S.P.S.; Giddey, S.S.; Munnings, C.; Bhatt, A.I.; Hollenkamp, A.F. Emerging electrochemical energy conversion and storage technologies. Front. Chem. 2014, 2, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Gao, M.-R.; Xu, Y.-F.; Jiang, J.; Yu, S.-H. Nanostructured metal chalcogenides: Synthesis, modification, and applications in energy conversion and storage devices. Chem. Soc. Rev. 2013, 42, 2986–3017. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Yu, L.; Lou, X.W. Metal Sulfide Hollow Nanostructures for Electrochemical Energy Storage. Adv. Energy Mater. 2016, 6, 1501333. [Google Scholar] [CrossRef]
- Yuan, H.; Kong, L.; Li, T.; Zhang, Q. A review of transition metal chalcogenide/graphene nanocomposites for energy storage and conversion. Chin. Chem. Lett. 2017, 28, 2180–2194. [Google Scholar] [CrossRef]
- Choi, W.; Choudhary, N.; Han, G.H.; Park, J.; Akinwande, D.; Lee, Y.H. Recent development of two-dimensional transition metal dichalcogenides and their applications. Mater. Today 2017, 20, 116–130. [Google Scholar] [CrossRef]
- Kulandaivalu, S.; Sulaiman, Y. Recent Advances in Layer-by-Layer Assembled Conducting Polymer Based Composites for Supercapacitors. Energies 2019, 12, 2107. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.; Ji, M.; Xia, J.; Zhu, H. Recent Advanced Materials for Electrochemical and Photoelectrochemical Synthesis of Ammonia from Dinitrogen: One Step Closer to a Sustainable Energy Future. Adv. Energy Mater. 2020, 10, 1902020. [Google Scholar] [CrossRef]
- Luo, F.; Li, J.; Yuan, H.; Xiao, D. Rapid synthesis of three-dimensional flower-like cobalt sulfide hierarchitectures by microwave assisted heating method for high-performance supercapacitors. Electrochim. Acta 2014, 123, 183–189. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Liu, W.; Yue, H.; Shi, Z.; Yin, Y.; Yang, S. Olivine LiFePO4 as an additive into LiCoO2 electrodes for LIBs to improve high-voltage performances. J. Alloys Compd. 2021, 869, 159188. [Google Scholar] [CrossRef]
- Abraham, K.M. Intercalation positive electrodes for rechargeable sodium cells. Solid State Ionics 1982, 7, 199–212. [Google Scholar] [CrossRef]
- Rajabathar, J.R.; Al-lohedan, H.A.; Arunachalam, P.; Issa, Z.A.; Gnanamani, M.K.; Appaturi, J.N.; Ibrahim, S.N.; Mohammed Dahan, W. Unexpected discovery of low-cost maricite NaFePO4 as a high-performance electrode for Na-ion batteries. J. Alloys Compd. 2021, 850, 540–545. [Google Scholar]
- Rajabathar, J.R.; Al-lohedan, H.A.; Arunachalam, P.; Issa, Z.A.; Gnanamani, M.K.; Appaturi, J.N.; Ibrahim, S.N.; Mohammed Dahan, W. Challenges for Na-ion negative electrodes. J. Alloys Compd. 2021, 850, A1011. [Google Scholar]
- Rajabathar, J.R.; Al-lohedan, H.A.; Arunachalam, P.; Issa, Z.A.; Gnanamani, M.K.; Appaturi, J.N.; Ibrahim, S.N.; Mohammed Dahan, W. Sodium-Ion Batteries. J. Alloys Compd. 2021, 850, 947–958. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagde, K.R.; Dhoble, S.J. Li-S ion batteries: A substitute for Li-ion storage batteries. In Energy Materials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 335–371. [Google Scholar]
- Evers, S.; Yim, T.; Nazar, L.F. Understanding the nature of absorption/adsorption in nanoporous polysulfide sorbents for the Li–S battery. J. Phys. Chem. C 2012, 116, 19653–19658. [Google Scholar] [CrossRef]
- Moy, D.; Narayanan, S.R. Mixed Conduction Membranes Suppress the Polysulfide Shuttle in Lithium-Sulfur Batteries. J. Electrochem. Soc. 2017, 164, A560–A566. [Google Scholar] [CrossRef]
- Moy, D.; Manivannan, A.; Narayanan, S.R. Direct Measurement of Polysulfide Shuttle Current: A Window into Understanding the Performance of Lithium-Sulfur Cells. J. Electrochem. Soc. 2014, 162, A1–A7. [Google Scholar] [CrossRef]
- Li, H.; Tsai, C.; Koh, A.L.; Cai, L.; Contryman, A.W.; Fragapane, A.H.; Zhao, J.; Han, H.S.; Manoharan, H.C.; Abild-Pedersen, F.; et al. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies. Nat. Mater. 2016, 15, 48–53. [Google Scholar] [CrossRef]
- Ji, G.; Yu, Y.; Yao, Q.; Qu, B.; Chen, D.; Chen, W.; Xie, J.; Lee, J.Y. Promotion of reversible Li+ storage in transition metal dichalcogenides by Ag nanoclusters. NPG Asia Mater. 2016, 8, e247. [Google Scholar] [CrossRef]
- Lin, H.; Yang, L.; Jiang, X.; Li, G.; Zhang, T.; Yao, Q.; Zheng, G.W.; Lee, J.Y. Electrocatalysis of polysulfide conversion by sulfur-deficient MoS2 nanoflakes for lithium–sulfur batteries. Energy Environ. Sci. 2017, 10, 1476–1486. [Google Scholar] [CrossRef] [Green Version]
- Clark, S.; Latz, A.; Horstmann, B. A Review of Model-Based Design Tools for Metal-Air Batteries. Batteries 2018, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.; Lu, Q.; Liao, K.; Shao, Z. Towards practically accessible aprotic Li-air batteries: Progress and challenges related to oxygen-permeable membranes and cathodes. Energy Storage Mater. 2022, 45, 869–902. [Google Scholar] [CrossRef]
- Lu, S.-H.; Lu, H.-C. Pouch-type hybrid Li-air battery enabled by flexible composite lithium-ion conducting membrane. J. Power Source 2021, 489, 229431. [Google Scholar] [CrossRef]
- Parveen, N.; Khan, Z.; Ansari, S.A.; Park, S.; Senthilkumar, S.T.; Kim, Y.; Ko, H.; Cho, M.H. Feasibility of using hollow double walled Mn2O3 nanocubes for hybrid Na-air battery. Chem. Eng. J. 2019, 360, 415–422. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Q. Materials Design for Rechargeable Metal-Air Batteries. Matter 2019, 1, 565–595. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, Y.; Cui, C.; Lin, C.; Li, Y.; Bu, D.; Yan, G.; Liu, D.; Wu, Q.; Song, X.-M. Photo-assisted Al-air batteries based on gel-state electrolyte. J. Power Source 2022, 533, 231377. [Google Scholar] [CrossRef]
- Wu, P.; Zhao, Q.; Yu, H.; Tang, Z.; Li, Y.; Huang, D.; Sun, D.; Wang, H.; Tang, Y. Modification on water electrochemical environment for durable Al-Air Battery: Achieved by a Low-Cost sucrose additive. Chem. Eng. J. 2022, 438, 135538. [Google Scholar] [CrossRef]
- Zhang, L.; Shao, Q.; Zhang, J. An overview of non-noble metal electrocatalysts and their associated air cathodes for Mg-air batteries. Mater. Rep. Energy 2021, 1, 100002. [Google Scholar] [CrossRef]
- Vaghefinazari, B.; Snihirova, D.; Wang, C.; Wang, L.; Deng, M.; Höche, D.; Lamaka, S.V.; Zheludkevich, M.L. Exploring the effect of sodium salt of Ethylenediaminetetraacetic acid as an electrolyte additive on electrochemical behavior of a commercially pure Mg in primary Mg-air batteries. J. Power Source 2022, 527, 231176. [Google Scholar] [CrossRef]
- Leong, K.W.; Wang, Y.; Ni, M.; Pan, W.; Luo, S.; Leung, D.Y.C. Rechargeable Zn-air batteries: Recent trends and future perspectives. Renew. Sustain. Energy Rev. 2022, 154, 111771. [Google Scholar] [CrossRef]
- Qian, M.; Guo, M.; Qu, Y.; Xu, M.; Liu, D.; Hou, C.; Isimjan, T.T.; Yang, X. Energy barrier engineering of oxygen reduction reaction synergistically promoted by binary Zn-Cu pair sites for advanced Zn–air batteries. J. Alloys Compd. 2022, 907, 164527. [Google Scholar] [CrossRef]
- Hang, B.T.; Watanabe, T.; Egashira, M.; Watanabe, I.; Okada, S.; Yamaki, J. The effect of additives on the electrochemical properties of Fe/C composite for Fe/air battery anode. J. Power Source 2006, 155, 461–469. [Google Scholar] [CrossRef]
- Hang, B.T.; Hayashi, H.; Yoon, S.-H.; Okada, S.; Yamaki, J. Fe2O3-filled carbon nanotubes as a negative electrode for an Fe–air battery. J. Power Source 2008, 178, 393–401. [Google Scholar] [CrossRef]
- Inoishi, A.; Ida, S.; Uratani, S.; Okano, T.; Ishihara, T. High capacity of an Fe–air rechargeable battery using LaGaO3-based oxide ion conductor as an electrolyte. Phys. Chem. Chem. Phys. 2012, 14, 12818–12822. [Google Scholar] [CrossRef]
- Lyu, Z.; Zhou, Y.; Dai, W.; Cui, X.; Lai, M.; Wang, L.; Huo, F.; Huang, W.; Hu, Z.; Chen, W. Recent advances in understanding of the mechanism and control of Li 2 O 2 formation in aprotic Li–O2 batteries. Chem. Soc. Rev. 2017, 46, 6046–6072. [Google Scholar] [CrossRef]
- Geng, D.; Ding, N.-N.; Hor, T.S.A.; Chien, S.W.; Liu, Z.; Zong, Y. Cobalt sulfide nanoparticles impregnated nitrogen and sulfur co-doped graphene as bifunctional catalyst for rechargeable Zn–air batteries. RSC Adv. 2015, 5, 7280–7284. [Google Scholar] [CrossRef]
- Olabi, A.G.; Sayed, E.T.; Wilberforce, T.; Jamal, A.; Alami, A.H.; Elsaid, K.; Rahman, S.M.; Shah, S.K.; Abdelkareem, M.A. Metal-Air Batteries—A Review. Energies 2021, 14, 7373. [Google Scholar] [CrossRef]
- Bao, S.-J.; Li, C.M.; Guo, C.-X.; Qiao, Y. Biomolecule-assisted synthesis of cobalt sulfide nanowires for application in supercapacitors. J. Power Source 2008, 180, 676–681. [Google Scholar] [CrossRef]
- Subramanian, A.; Punnoose, D.; Raman, V.; Gopi, C.V.V.M.; Rao, S.S.; Khan, M.A.; Kim, H.-J. Layer by layer approach to enhance capacitance using metal sulfides for supercapacitor applications. Mater. Lett. 2018, 231, 64–67. [Google Scholar] [CrossRef]
- Sajedi-Moghaddam, A.; Saievar-Iranizad, E.; Pumera, M. Two-dimensional transition metal dichalcogenide/conducting polymer composites: Synthesis and applications. Nanoscale 2017, 9, 8052–8065. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, T.; Na, J.; Yi, J.W.; Kim, J.; Kim, M.; Bando, Y.; Yamauchi, Y.; Lin, J. Layered transition metal dichalcogenide/carbon nanocomposites for electrochemical energy storage and conversion applications. Nanoscale 2020, 12, 8608–8625. [Google Scholar] [CrossRef] [PubMed]
- Cherusseri, J.; Choudhary, N.; Sambath Kumar, K.; Jung, Y.; Thomas, J. Recent trends in transition metal dichalcogenide based supercapacitor electrodes. Nanoscale Horiz. 2019, 4, 840–858. [Google Scholar] [CrossRef]
- Ahmad, M.; Hussain, I.; Nawaz, T.; Li, Y.; Chen, X.; Ali, S.; Imran, M.; Ma, X.; Zhang, K. Comparative study of ternary metal chalcogenides (MX.; M= Zn–Co–Ni; X= S, Se, Te): Formation process, charge storage mechanism and hybrid supercapacitor. J. Power Source 2022, 534, 231414. [Google Scholar] [CrossRef]
- Teli, A.M.; Beknalkar, S.A.; Mane, S.M.; Bhat, T.S.; Kambale, B.B.; Patil, S.B.; Sadale, S.B.; Shin, J.C. Electrodeposited crumpled MoS2 nanoflakes for asymmetric supercapacitor. Ceram. Int. 2022, in press. [Google Scholar] [CrossRef]
- Sharma, G.K.; Ranjan, B.; Kaur, D. Electrochemical kinetics of 2D-MoS2 sputtered over stainless-steel mesh: Insights into the Na+ ions storage for flexible supercapacitors. Ceram. Int. 2022, in press. [Google Scholar] [CrossRef]
- Gao, Y.-P.; Huang, K.-J.; Wu, X.; Hou, Z.-Q.; Liu, Y.-Y. MoS2 nanosheets assembling three-dimensional nanospheres for enhanced-performance supercapacitor. J. Alloys Compd. 2018, 741, 174–181. [Google Scholar] [CrossRef]
- Thanh, T.D.; Chuong, N.D.; Van Hien, H.; Kshetri, T.; Kim, N.H.; Lee, J.H. Recent advances in two-dimensional transition metal dichalcogenides-graphene heterostructured materials for electrochemical applications. Prog. Mater. Sci. 2018, 96, 51–85. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, L.; Lian, J. Arrays of hierarchical nickel sulfides/MoS2 nanosheets supported on carbon nanotubes backbone as advanced anode materials for asymmetric supercapacitor. J. Power Source 2017, 343, 373–382. [Google Scholar] [CrossRef]
- Sun, T.; Li, Z.; Liu, X.; Ma, L.; Wang, J.; Yang, S. Facile construction of 3D graphene/MoS2 composites as advanced electrode materials for supercapacitors. J. Power Source 2016, 331, 180–188. [Google Scholar] [CrossRef]
- Ray, S.K.; Pant, B.; Park, M.; Hur, J.; Lee, S.W. Cavity-like hierarchical architecture of WS2/α-NiMoO4 electrodes for supercapacitor application. Ceram. Int. 2020, 46, 19022–19027. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Shu, T.; Yuan, J.; Lu, G.; Lin, B.; Gao, Z.; Wei, F.; Ma, C.; Qi, J.; et al. Two-dimensional hierarchical MoS2 lamella inserted in CoS2 flake as an advanced supercapacitor electrode. J. Energy Storage 2022, 51, 104299. [Google Scholar] [CrossRef]
- Bhol, P.; Swain, S.; Altaee, A.; Saxena, M.; Samal, A.K. Cobalt–iron decorated tellurium nanotubes for high energy density supercapacitor. Mater. Today Chem. 2022, 24, 100871. [Google Scholar] [CrossRef]
- Rathore, H.K.; Hariram, M.; Ganesha, M.K.; Singh, A.K.; Das, D.; Kumar, M.; Awasthi, K.; Sarkar, D. Charge storage mechanism in vanadium telluride/carbon nanobelts as electroactive material in an aqueous asymmetric supercapacitor. J. Colloid Interface Sci. 2022, 621, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Theerthagiri, J.; Karuppasamy, K.; Durai, G.; Rana, A.U.H.S.; Arunachalam, P.; Sangeetha, K.; Kuppusami, P.; Kim, H.-S. Recent Advances in Metal Chalcogenides (MX; X = S, Se) Nanostructures for Electrochemical Supercapacitor Applications: A Brief Review. Nanomaterials 2018, 8, 256. [Google Scholar] [CrossRef] [Green Version]
- Miao, C.; Xia, G.; Zhu, K.; Ye, K.; Wang, Q.; Yan, J.; Cao, D.; Gong, F.; Wang, G. Enhanced supercapacitor performance of bimetallic metal selenides via controllable synergistic engineering of composition. Electrochim. Acta 2021, 370, 137802. [Google Scholar] [CrossRef]
- Lei, H.; Zhou, J.; Zhao, R.; Peng, H.; Xu, Y.; Wang, F.; Hamouda, H.A.; Zhang, W.; Ma, G. Design and assembly of a novel asymmetric supercapacitor based on all-metal selenides electrodes. Electrochim. Acta 2020, 363, 137206. [Google Scholar] [CrossRef]
- Amiri, M.; Saeed Hosseiny Davarani, S.; Ebrahim Moosavifard, S.; Fu, Y.-Q. Cobalt-molybdenum selenide double-shelled hollow nanocages derived from metal-organic frameworks as high performance electrodes for hybrid supercapacitor. J. Colloid Interface Sci. 2022, 616, 141–151. [Google Scholar] [CrossRef]
- Liu, Q.; Hong, X.; You, X.; Zhang, X.; Zhao, X.; Chen, X.; Ye, M.; Liu, X. Designing heterostructured metal sulfide core-shell nanoneedle films as battery-type electrodes for hybrid supercapacitors. Energy Storage Mater. 2020, 24, 541–549. [Google Scholar] [CrossRef]
- Wang, Q.; Qu, Z.; Chen, S.; Zhang, D. Metal organic framework derived P-doping CoS@C with sulfide defect to boost high-performance asymmetric supercapacitors. J. Colloid Interface Sci. 2022, in press. [Google Scholar] [CrossRef]
- Lu, L.; Xu, Q.; Chen, Y.; Zhou, Y.; Jiang, T.; Zhao, Q. Preparation of metal sulfide electrode materials derived based on metal organic framework and application of supercapacitors. J. Energy Storage 2022, 49, 104073. [Google Scholar] [CrossRef]
- Yu, S.; Xiong, X.; Ma, J.; Qian, H. One-step preparation of cobalt nickel oxide hydroxide @ cobalt sulfide heterostructure film on Ni foam through hydrothermal electrodeposition for supercapacitors. Surf. Coat. Technol. 2021, 426, 127791. [Google Scholar] [CrossRef]
- Sahoo, S.; Krishnamoorthy, K.; Pazhamalai, P.; Mariappan, V.K.; Kim, S.-J. Copper molybdenum sulfide: A novel pseudocapacitive electrode material for electrochemical energy storage device. Int. J. Hydrogen Energy 2018, 43, 12222–12232. [Google Scholar] [CrossRef]
- Rajabathar, J.R.; Al-lohedan, H.A.; Arunachalam, P.; Issa, Z.A.; Gnanamani, M.K.; Appaturi, J.N.; Ibrahim, S.N.; Mohammed Dahan, W. Synthesis and characterization of metal chalcogenide modified graphene oxide sandwiched manganese oxide nanofibers on nickel foam electrodes for high performance supercapacitor applications. J. Alloys Compd. 2021, 850, 156346. [Google Scholar] [CrossRef]
- Nagaraju, M.; Chandra Sekhar, S.; Ramulu, B.; Arbaz, S.J.; Yu, J.S. In situ deposited cobalt-magnesium selenates as an advanced electrode for electrochemical energy storage. J. Magnes. Alloys 2022, in press. [Google Scholar] [CrossRef]
- Sakthivel, M.; Sukanya, R.; Chen, S.-M.; Pandi, K.; Ho, K.-C. Synthesis and characterization of bimetallic nickel-cobalt chalcogenides (NiCoSe2, NiCo2S4, and NiCo2O4) for non-enzymatic hydrogen peroxide sensor and energy storage: Electrochemical properties dependence on the metal-to-chalcogen composition. Renew. Energy 2019, 138, 139–151. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Guo, X.; Liu, Y.; Zheng, Y.; Zhang, M.; Li, R.; Peng, Z.; Zhang, Y.; Zhang, T. One-step microwave-hydrothermal preparation of NiS/rGO hybrid for high-performance symmetric solid-state supercapacitor. Appl. Surf. Sci. 2020, 514, 146080. [Google Scholar] [CrossRef]
- Nandhini, S.; Muralidharan, G. Facile microwave-hydrothermal synthesis of NiS nanostructures for supercapacitor applications. Appl. Surf. Sci. 2018, 449, 485–491. [Google Scholar] [CrossRef]
- Ansari, A.; Badhe, R.A.; Babar, D.G.; Garje, S.S. One pot solvothermal synthesis of bimetallic copper iron sulfide (CuFeS2) and its use as electrode material in supercapacitor applications. Appl. Surf. Sci. Adv. 2022, 9, 100231. [Google Scholar] [CrossRef]
- Qu, J.; Bai, Y.; Li, X.; Song, K.; Zhang, S.; Wang, X.; Wang, X.; Dai, S. Rational design of NiSe2@rGO nanocomposites for advanced hybrid supercapacitors. J. Mater. Res. Technol. 2021, 15, 6155–6161. [Google Scholar] [CrossRef]
- Gowrisankar, A.; Sherryn, A.L.; Selvaraju, T. In situ integrated 2D reduced graphene oxide nanosheets with MoSSe for hydrogen evolution reaction and supercapacitor application. Appl. Surf. Sci. Adv. 2021, 3, 100054. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, D.; Liu, H.; Umar, A.; Wu, X. High performance hybrid supercapacitor based on hierarchical MoS2/Ni3S2 metal chalcogenide. Chin. Chem. Lett. 2019, 30, 1105–1110. [Google Scholar] [CrossRef]
- Manikandan, R.; Justin Raj, C.; Nagaraju, G.; Velayutham, R.; Moulton, S.E.; Puigdollers, J.; Chul Kim, B. Selenium enriched hybrid metal chalcogenides with enhanced redox kinetics for high-energy density supercapacitors. Chem. Eng. J. 2021, 414, 128924. [Google Scholar] [CrossRef]
- Kim, Y.; Samuel, E.; Joshi, B.; Park, C.; Lee, H.S.; Yoon, S.S. Flexible metallized carbon nanofibers decorated with two-dimensional NiGa2S4 nanosheets as supercapacitor electrodes. Chem. Eng. J. 2021, 420, 130497. [Google Scholar] [CrossRef]
- Zhang, S.; Dai, P.; Liu, H.; Yan, L.; Song, H.; Liu, D.; Zhao, X. Metal-organic framework derived porous flakes of cobalt chalcogenides (CoX, X = O, S, Se and Te) rooted in carbon fibers as flexible electrode materials for pseudocapacitive energy storage. Electrochim. Acta 2021, 369, 137681. [Google Scholar] [CrossRef]
- Shaikh, S.; Rabinal, M.K. Rapid ambient growth of copper sulfide microstructures: Binder free electrodes for supercapacitor. J. Energy Storage 2020, 28, 101288. [Google Scholar] [CrossRef]
- Gohar, R.S.; Ahmad, I.; Shah, A.; Majeed, S.; Najam-Ul-Haq, M.; Ashiq, M.N. Fabrication of transition-metal oxide and chalcogenide nanostructures with enhanced electrochemical performances. J. Energy Storage 2020, 31, 101621. [Google Scholar] [CrossRef]
- Maity, C.K.; Goswami, N.; Verma, K.; Sahoo, S.; Nayak, G.C. A facile synthesis of boron nitride supported zinc cobalt sulfide nano hybrid as high-performance pseudocapacitive electrode material for asymmetric supercapacitors. J. Energy Storage 2020, 32, 101993. [Google Scholar] [CrossRef]
- Ghosh, S.; Samanta, P.; Murmu, N.C.; Kuila, T. Investigation of electrochemical charge storage in nickel-cobalt-selenide/reduced graphene oxide composite electrode and its hybrid supercapacitor device. J. Alloys Compd. 2020, 835, 155432. [Google Scholar] [CrossRef]
- Zhang, F.; Tang, Y.; Liu, H.; Ji, H.; Jiang, C.; Zhang, J.; Zhang, X.; Lee, C.-S. Uniform Incorporation of Flocculent Molybdenum Disulfide Nanostructure into Three-Dimensional Porous Graphene as an Anode for High-Performance Lithium Ion Batteries and Hybrid Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 4691–4699. [Google Scholar] [CrossRef]
- Wang, R.; Wang, S.; Jin, D.; Zhang, Y.; Cai, Y.; Ma, J.; Zhang, L. Engineering layer structure of MoS2-graphene composites with robust and fast lithium storage for high-performance Li-ion capacitors. Energy Storage Mater. 2017, 9, 195–205. [Google Scholar] [CrossRef]
- Wang, C.; Zhan, C.; Ren, X.; Lv, R.; Shen, W.; Kang, F.; Huang, Z.-H. MoS2/carbon composites prepared by ball-milling and pyrolysis for the high-rate and stable anode of lithium ion capacitors. RSC Adv. 2019, 9, 42316–42323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, J.; Yang, L.; Zhang, H.; Liu, J.; Hu, R.; Zhu, M. Engineering layer structure of MoS2/polyaniline/graphene nanocomposites to achieve fast and reversible lithium storage for high energy density aqueous lithium-ion capacitors. J. Power Source 2020, 450, 227680. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Jia, Q.-C.; Kong, L.-B. Multi-dimensional hybrid heterostructure MoS2@C nanocomposite as a highly reversible anode for high-energy lithium-ion capacitors. Appl. Surf. Sci. 2020, 531, 147222. [Google Scholar] [CrossRef]
- Wang, R.; Wang, S.; Peng, X.; Zhang, Y.; Jin, D.; Chu, P.K.; Zhang, L. Elucidating the Intercalation Pseudocapacitance Mechanism of MoS2–Carbon Monolayer Interoverlapped Superstructure: Toward High-Performance Sodium-Ion-Based Hybrid Supercapacitor. ACS Appl. Mater. Interfaces 2017, 9, 32745–32755. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Wang, L.; Wang, R.; He, B.; Gong, Y.; Hu, X. Ultrafast Na+-storage in TiO2-coated MoS2@N-doped carbon for high-energy sodium-ion hybrid capacitors. Energy Storage Mater. 2019, 23, 95–104. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, H.; Zhang, L.; Li, Q.; Xu, M.; Bao, S. A rough endoplasmic reticulum-like VSe2/rGO anode for superior sodium-ion capacitors. Inorg. Chem. Front. 2019, 6, 2935–2943. [Google Scholar] [CrossRef]
- Tang, J.; Huang, X.; Lin, T.; Qiu, T.; Huang, H.; Zhu, X.; Gu, Q.; Luo, B.; Wang, L. MXene derived TiS2 nanosheets for high-rate and long-life sodium-ion capacitors. Energy Storage Mater. 2020, 26, 550–559. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Y.-G.; Kong, L.-B. Synthesis of polyvalent ion reaction of MoS2/CoS2-RGO anode materials for high-performance sodium-ion batteries and sodium-ion capacitors. J. Colloid Interface Sci. 2020, 575, 42–53. [Google Scholar] [CrossRef]
- Xu, D.; Wang, H.; Qiu, R.; Wang, Q.; Mao, Z.; Jiang, Y.; Wang, R.; He, B.; Gong, Y.; Li, D.; et al. Coupling of bowl-like VS2 nanosheet arrays and carbon nanofiber enables ultrafast Na+-Storage and robust flexibility for sodium-ion hybrid capacitors. Energy Storage Mater. 2020, 28, 91–100. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Y.-G.; Kong, L.-B. High-capacity and fast Na-ion diffusion rate three-dimensional MoS2/SnS2-RGO anode for advanced sodium-ion batteries and sodium-ion capacitors. Solid State Ionics 2020, 355, 115416. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, M.; Zhang, X.; Wan, B.; Li, X.; Gou, H.; Wang, Y.; Yin, F.; Wang, G. 2D Sandwiched Nano Heterostructures Endow MoSe2/TiO2−x/Graphene with High Rate and Durability for Sodium Ion Capacitor and Its Solid Electrolyte Interphase Dependent Sodiation/Desodiation Mechanism. Small 2020, 16, 2004457. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Jiang, P.; He, H.; Chen, C.; Liu, Y.; Zhang, M. Encapsulation of MoSe2 in carbon fibers as anodes for potassium ion batteries and nonaqueous battery–supercapacitor hybrid devices. Nanoscale 2019, 11, 13511–13520. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Sun, Z.; Li, C.; Tian, Z.; Lu, C.; Shao, Y.; Li, J.; Sun, J.; Liu, Z. Designing 3D Biomorphic Nitrogen-Doped MoSe2/Graphene Composites toward High-Performance Potassium-Ion Capacitors. Adv. Funct. Mater. 2020, 30, 1903878. [Google Scholar] [CrossRef]
- Huang, K.J.; Wang, L.; Liu, Y.J.; Liu, Y.M.; Wang, H.B.; Gan, T.; Wang, L.L. Layered MoS2-graphene composites for supercapacitor applications with enhanced capacitive performance. Int. J. Hydrogen Energy 2013, 38, 14027–14034. [Google Scholar] [CrossRef]
- Huang, K.J.; Wang, L.; Zhang, J.Z.; Xing, K. Synthesis of molybdenum disulfide/carbon aerogel composites for supercapacitors electrode material application. J. Electroanal. Chem. 2015, 752, 33–40. [Google Scholar] [CrossRef]
- Wang, L.; Ma, Y.; Yang, M.; Qi, Y. Hierarchical hollow MoS2 nanospheres with enhanced electrochemical properties used as an Electrode in Supercapacitor. Electrochim. Acta 2015, 186, 391–396. [Google Scholar] [CrossRef]
- Gigot, A.; Fontana, M.; Serrapede, M.; Castellino, M.; Bianco, S.; Armandi, M.; Bonelli, B.; Pirri, C.F.; Tresso, E.; Rivolo, P. Mixed 1T-2H Phase MoS2/Reduced Graphene Oxide as Active Electrode for Enhanced Supercapacitive Performance. ACS Appl. Mater. Interfaces 2016, 8, 32842–32852. [Google Scholar] [CrossRef]
- Liang, X.; Nie, K.; Ding, X.; Dang, L.; Sun, J.; Shi, F.; Xu, H.; Jiang, R.; He, X.; Liu, Z.; et al. Highly Compressible Carbon Sponge Supercapacitor Electrode with Enhanced Performance by Growing Nickel-Cobalt Sulfide Nanosheets. ACS Appl. Mater. Interfaces 2018, 10, 10087–10095. [Google Scholar] [CrossRef]
- Tang, J.; Shen, J.; Li, N.; Ye, M. A free template strategy for the synthesis of CoS2-reduced graphene oxide nanocomposite with enhanced electrode performance for supercapacitors. Ceram. Int. 2014, 40, 15411–15419. [Google Scholar] [CrossRef]
- Huang, K.J.; Zhang, J.Z.; Liu, Y.; Liu, Y.M. Synthesis of reduced graphene oxide wrapped-copper sulfide hollow spheres as electrode material for supercapacitor. Int. J. Hydrogen Energy 2015, 40, 10158–10167. [Google Scholar] [CrossRef]
- Zhao, T.; Yang, W.; Zhao, X.; Peng, X.; Hu, J.; Tang, C.; Li, T. Facile preparation of reduced graphene oxide/copper sulfide composite as electrode materials for supercapacitors with high energy density. Compos. Part B Eng. 2018, 150, 60–67. [Google Scholar] [CrossRef]
- Xiao, W.; Zhou, W.; Feng, T.; Zhang, Y.; Liu, H.; Yu, H.; Tian, L.; Pu, Y. One-pot solvothermal synthesis of flower-like copper sulfide/reduced graphene oxide composite superstructures as high-performance supercapacitor electrode materials. J. Mater. Sci. Mater. Electron. 2017, 28, 5931–5940. [Google Scholar] [CrossRef]
- Boopathiraja, R.; Parthibavarman, M.; Prabhu, S.; Ramesh, R. A facile one step hydrothermal induced hexagonal shaped CuS/rGO nanocomposites for asymmetric supercapacitors. Mater. Today Proc. 2019, 26, 3507–3513. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Guo, X.; Jin, K.; Chen, Z.; Liu, Y.; Yin, L.; Li, L.; Yin, K.; Sun, L.; et al. Ni-Co Selenide Nanosheet/3D Graphene/Nickel Foam Binder-Free Electrode for High-Performance Supercapacitor. ACS Appl. Mater. Interfaces 2019, 11, 7946–7953. [Google Scholar] [CrossRef]
- Amin, B.G.; Masud, J.; Nath, M. Facile one-pot synthesis of NiCo2Se4-rGO on Ni foam for high performance hybrid supercapacitors. RSC Adv. 2019, 9, 37939–37946. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Samanta, P.; Samanta, P.; Murmu, N.C.; Kuila, T. Investigation of Electrochemical Charge Storage Efficiency of NiCo2Se4/RGO Composites Derived at Varied Duration and Its Asymmetric Supercapacitor Device. Energy Fuels 2020, 34, 13056–13066. [Google Scholar] [CrossRef]
- Zhang, K.; Wei, Y.; Huang, J.; Xiao, Y.; Yang, W.; Hu, T.; Yuan, K.; Chen, Y. A generalized one-step in situ formation of metal sulfide/reduced graphene oxide nanosheets toward high-performance supercapacitors. Sci. China Mater. 2020, 63, 1898–1909. [Google Scholar] [CrossRef]
- Sarkar, S.; Howli, P.; Das, B.; Das, N.S.; Samanta, M.; Das, G.C.; Chattopadhyay, K.K. Novel Quaternary Chalcogenide/Reduced Graphene Oxide-Based Asymmetric Supercapacitor with High Energy Density. ACS Appl. Mater. Interfaces 2017, 9, 22652–22664. [Google Scholar] [CrossRef]
- Huang, K.J.; Wang, L.; Zhang, J.Z.; Wang, L.L.; Mo, Y.P. One-step preparation of layered molybdenum disulfide/multi-walled carbon nanotube composites for enhanced performance supercapacitor. Energy 2014, 67, 234–240. [Google Scholar] [CrossRef]
- Sun, P.; Wang, R.; Wang, Q.; Wang, H.; Wang, X. Uniform MoS2 nanolayer with sulfur vacancy on carbon nanotube networks as binder-free electrodes for asymmetrical supercapacitor. Appl. Surf. Sci. 2019, 475, 793–802. [Google Scholar] [CrossRef]
- Huang, K.J.; Zhang, J.Z.; Xing, K. One-step synthesis of layered CuS/multi-walled carbon nanotube nanocomposites for supercapacitor electrode material with ultrahigh specific capacitance. Electrochim. Acta 2014, 149, 28–33. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, X.; Wang, W.; Cheng, J.; Yan, H.; Tang, C.; Kim, J.K.; Luo, Y. Hierarchical, porous CuS microspheres integrated with carbon nanotubes for high-performance supercapacitors. Sci. Rep. 2015, 5, 16584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, X.; Liu, X.; Lu, Y.; Cheng, J.; Luo, R.; Yu, Q.; Wei, X.; Yan, H.; Ji, X.; Kim, J.K.; et al. Copper sulfide nanoneedles on CNT backbone composite electrodes for high-performance supercapacitors and Li-S batteries. J. Solid State Electrochem. 2017, 21, 349–359. [Google Scholar] [CrossRef]
- Muralee Gopi, C.V.V.; Ravi, S.; Rao, S.S.; Eswar Reddy, A.; Kim, H.J. Carbon nanotube/metal-sulfide composite flexible electrodes for high-performance quantum dot-sensitized solar cells and supercapacitors. Sci. Rep. 2017, 7, 46519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azad, M.; Hussain, Z.; Baig, M.M. MWCNTs/NiS2 decorated Ni foam based electrode for high-performance supercapacitors. Electrochim. Acta 2020, 345, 136196. [Google Scholar] [CrossRef]
- Ma, X.; Kang, Z. A facile electrodeposition technique for synthesis of nickel sulfides/carbon nanotubes nanocomposites as high performance electrodes for supercapacitor. Mater. Lett. 2019, 236, 468–471. [Google Scholar] [CrossRef]
- Pandit, B.; Karade, S.S.; Sankapal, B.R. Hexagonal VS2 Anchored MWCNTs: First Approach to Design Flexible Solid-State Symmetric Supercapacitor Device. ACS Appl. Mater. Interfaces 2017, 9, 44880–44891. [Google Scholar] [CrossRef]
- Zang, X.; Shen, C.; Kao, E.; Warren, R.; Zhang, R.; Teh, K.S.; Zhong, J.; Wei, M.; Li, B.; Chu, Y.; et al. Titanium Disulfide Coated Carbon Nanotube Hybrid Electrodes Enable High Energy Density Symmetric Pseudocapacitors. Adv. Mater. 2018, 30, 1704754. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Li, J.; Long, C.; Wei, T.; Ning, G.; Yan, J.; Fan, Z. Nickel sulfide/graphene/carbon nanotube composites as electrode material for the supercapacitor application in the sea flashing signal system. J. Mar. Sci. Appl. 2014, 13, 462–466. [Google Scholar] [CrossRef]
- Yang, W.; He, L.; Tian, X.; Yan, M.; Yuan, H.; Liao, X.; Meng, J.; Hao, Z.; Mai, L. Carbon-MEMS-Based Alternating Stacked MoS2@rGO-CNT Micro-Supercapacitor with High Capacitance and Energy Density. Small 2017, 13, 1700639. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, J.; Myung, Y.; Lee, S.M.; Kim, H.J.; Ko, Y.J.; Yang, M.; Son, S.U. Yolk-Shell Polystyrene@Microporous Organic Network: A Smart Template with Thermally Disassemblable Yolk to Engineer Hollow MoS2/C Composites for High-Performance Supercapacitors. ACS Omega 2017, 2, 7658–7665. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.S.; Long, J.Y.; Zhou, Q.F.; Gong, Y.; Lin, J.H. One-step synthesis of MnS/MoS2/C through the calcination and sulfurization of a bi-metal-organic framework for a high-performance supercapacitor and its photocurrent investigation. Dalt. Trans. 2018, 47, 5390–5405. [Google Scholar] [CrossRef]
- Miao, C.; Xiao, X.; Gong, Y.; Zhu, K.; Cheng, K.; Ye, K.; Yan, J.; Cao, D.; Wang, G.; Xu, P. Facile Synthesis of Metal-Organic Framework-Derived CoSe2 Nanoparticles Embedded in the N-Doped Carbon Nanosheet Array and Application for Supercapacitors. ACS Appl. Mater. Interfaces 2020, 12, 9365–9375. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lu, W.; Li, Z.; Ning, J.; Zhong, Y.; Hu, Y. Hierarchical MoS2/NiCo2S4@C urchin-like hollow microspheres for asymmetric supercapacitors. Chem. Eng. J. 2020, 380, 122544. [Google Scholar] [CrossRef]
- Kshetri, T.; Singh, T.I.; Lee, Y.S.; Khumujam, D.D.; Kim, N.H.; Lee, J.H. Metal organic framework-derived cobalt telluride-carbon porous structured composites for high-performance supercapacitor. Compos. Part B Eng. 2021, 211, 108624. [Google Scholar] [CrossRef]
- Shi, Z.; Yue, L.; Wang, X.; Lei, X.; Sun, T.; Li, Q.; Guo, H.; Yang, W. 3D mesoporous hemp-activated carbon/Ni3S2 in preparation of a binder-free Ni foam for a high performance all-solid-state asymmetric supercapacitor. J. Alloys Compd. 2019, 791, 665–673. [Google Scholar] [CrossRef]
- Zhai, S.; Fan, Z.; Jin, K.; Zhou, M.; Zhao, H.; Zhao, Y.; Ge, F. Synthesis of zinc sulfide/copper sulfide/porous carbonized cotton nanocomposites for flexible supercapacitor and recyclable photocatalysis with high performance. J. Colloid Interface Sci. 2020, 575, 306–316. [Google Scholar] [CrossRef]
- Yang, M.; Jeong, J.; Suk, Y.; Gill, B. High-performance supercapacitor based on three-dimensional MoS2/graphene aerogel composites. Compos. Sci. Technol. 2015, 121, 123–128. [Google Scholar] [CrossRef]
- Li, P.; Jeong, J.Y.; Jin, B.; Zhang, K.; Park, J.H. Vertically Oriented MoS2 with Spatially Controlled Geometry on Nitrogenous Graphene Sheets for High-Performance Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1703300. [Google Scholar] [CrossRef]
- Cui, C.; Wei, Z.; Xu, J.; Zhang, Y.; Liu, S.; Liu, H.; Mao, M.; Wang, S.; Ma, J.; Dou, S. Three-dimensional carbon frameworks enabling MoS2 as anode for dual ion batteries with superior sodium storage properties. Energy Storage Mater. 2018, 15, 22–30. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Hu, X.D.; Wang, H.; Ye, M.Y.; Sang, Z.Y.; Ji, H.M.; Li, X.L.; Dai, Y. Superelastic 3D few-layer MoS2/carbon framework heterogeneous electrodes for highly reversible sodium-ion batteries. Nano Energy 2018, 48, 526–535. [Google Scholar] [CrossRef]
- Liu, Y.; He, X.; Hanlon, D.; Harvey, A.; Coleman, J.N.; Li, Y. Liquid Phase Exfoliated MoS2 Nanosheets Percolated with Carbon Nanotubes for High Volumetric/Areal Capacity Sodium-Ion Batteries. ACS Nano 2016, 10, 8821–8828. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yang, H.; Zhou, J.; Luo, Z.; Fang, G.; Liu, S.; Pan, A.; Liang, S. Nitrogen doped hollow MoS2/C nanospheres as anode for long-life sodium-ion batteries. Chem. Eng. J. 2017, 327, 522–529. [Google Scholar] [CrossRef]
- Sun, D.; Ye, D.; Liu, P.; Tang, Y.; Guo, J.; Wang, L.; Wang, H. MoS2/Graphene Nanosheets from Commercial Bulky MoS2 and Graphite as Anode Materials for High Rate Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1702383. [Google Scholar] [CrossRef]
- Li, G.; Luo, D.; Wang, X.; Seo, M.H.; Hemmati, S.; Yu, A.; Chen, Z. Enhanced Reversible Sodium-Ion Intercalation by Synergistic Coupling of Few-Layered MoS2 and S-Doped Graphene. Adv. Funct. Mater. 2017, 27, 1702562. [Google Scholar] [CrossRef]
- Kong, D.; Cheng, C.; Wang, Y.; Huang, Z.; Liu, B.; Von Lim, Y.; Ge, Q.; Yang, H.Y. Fe3O4 quantum dot decorated MoS2 nanosheet arrays on graphite paper as free-standing sodium-ion battery anodes. J. Mater. Chem. A 2017, 5, 9122–9131. [Google Scholar] [CrossRef]
- Geng, X.; Jiao, Y.; Han, Y.; Mukhopadhyay, A.; Yang, L.; Zhu, H. Freestanding Metallic 1T MoS2 with Dual Ion Diffusion Paths as High Rate Anode for Sodium-Ion Batteries. Adv. Funct. Mater. 2017, 27, 1702998. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Liu, J.; Ouyang, L.; Liu, J.; Hu, R.; Yang, L.; Zhu, M. MoS2/cotton-derived carbon fibers with enhanced cyclic performance for sodium-ion batteries. Appl. Surf. Sci. 2017, 413, 169–174. [Google Scholar] [CrossRef]
- Wang, L.; Yang, G.; Wang, J.; Peng, S.; Yan, W.; Ramakrishna, S. Controllable Design of MoS2 Nanosheets Grown on Nitrogen-Doped Branched TiO2/C Nanofibers: Toward Enhanced Sodium Storage Performance Induced by Pseudocapacitance Behavior. Small 2020, 16, 1904589. [Google Scholar] [CrossRef]
- Pang, Y.; Zhang, S.; Liu, L.; Liang, J.; Sun, Z.; Wang, Y.; Xiao, C.; Ding, D.; Ding, S. Few-layer MoS2 anchored at nitrogen-doped carbon ribbons for sodium-ion battery anodes with high rate performance. J. Mater. Chem. A 2017, 5, 17963–17972. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, S.; Wang, H.; Zhu, Z.; Liu, Q.; Xu, E.; Li, D.; Tong, G.; Jiang, Y. A novel carbon-decorated hollow flower-like MoS2 nanostructure wrapped with RGO for enhanced sodium-ion storage. Chem. Eng. J. 2018, 343, 180–188. [Google Scholar] [CrossRef]
- Ye, H.; Wang, L.; Deng, S.; Zeng, X.; Nie, K.; Duchesne, P.N.; Wang, B.; Liu, S.; Zhou, J.; Zhao, F.; et al. Amorphous MoS3 Infiltrated with Carbon Nanotubes as an Advanced Anode Material of Sodium-Ion Batteries with Large Gravimetric, Areal, and Volumetric Capacities. Adv. Energy Mater. 2017, 7, 1601602. [Google Scholar] [CrossRef]
- Niu, F.; Yang, J.; Wang, N.; Zhang, D.; Fan, W.; Yang, J.; Qian, Y. MoSe2-Covered N,P-Doped Carbon Nanosheets as a Long-Life and High-Rate Anode Material for Sodium-Ion Batteries. Adv. Funct. Mater. 2017, 27, 1700522. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, X.; Fu, Y.; Du, K. Ultrathin molybdenum diselenide nanosheets anchored on multi-walled carbon nanotubes as anode composites for high performance sodium-ion batteries. J. Power Source 2015, 296, 2–9. [Google Scholar] [CrossRef]
- Wang, S.; Gong, F.; Yang, S.; Liao, J.; Wu, M.; Xu, Z.; Chen, C.; Yang, X.; Zhao, F.; Wang, B.; et al. Graphene Oxide-Template Controlled Cuboid-Shaped High-Capacity VS4 Nanoparticles as Anode for Sodium-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1801806. [Google Scholar] [CrossRef]
- Sun, R.; Wei, Q.; Li, Q.; Luo, W.; An, Q.; Sheng, J.; Wang, D.; Chen, W.; Mai, L. Vanadium Sulfide on Reduced Graphene Oxide Layer as a Promising Anode for Sodium Ion Battery. ACS Appl. Mater. Interfaces 2015, 7, 20902–20908. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, N.; Xue, P.; Liu, Y.; Tang, B.; Bai, Z.; Dou, S. Co9S8@carbon nanospheres as high-performance anodes for sodium ion battery. Chem. Eng. J. 2018, 343, 512–519. [Google Scholar] [CrossRef]
- Pan, Y.; Cheng, X.; Huang, Y.; Gong, L.; Zhang, H. CoS2 Nanoparticles Wrapping on Flexible Freestanding Multichannel Carbon Nanofibers with High Performance for Na-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 35820–35828. [Google Scholar] [CrossRef]
- Freitas, F.S.; Gonçalves, A.S.; De Morais, A.; Benedetti, J.E.; Nogueira, A.F. Graphene-like MoS2 as a low-cost counter electrode material for dye-sensitized solar cells. NanoGe J. Energy Sustain. 2012, 11002–11003. [Google Scholar]
- Yao, S.; Cui, J.; Lu, Z.; Xu, Z.L.; Qin, L.; Huang, J.; Sadighi, Z.; Ciucci, F.; Kim, J.K. Unveiling the Unique Phase Transformation Behavior and Sodiation Kinetics of 1D van der Waals Sb2S3 Anodes for Sodium Ion Batteries. Adv. Energy Mater. 2017, 7, 1602149. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, G.; Lin, Y.; Wang, Y.; Ou, X.; Zheng, F.; Yang, C.; Wang, J.H.; Liu, M. Enhancing Sodium Ion Battery Performance by Strongly Binding Nanostructured Sb2S3 on Sulfur-Doped Graphene Sheets. ACS Nano 2016, 10, 10953–10959. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Li, C.; Ge, X.; Li, Z.; Miao, X.; Yin, L. ZnS-Sb2S3@C Core-Double Shell Polyhedron Structure Derived from Metal-Organic Framework as Anodes for High Performance Sodium Ion Batteries. ACS Nano 2017, 11, 6474–6482. [Google Scholar] [CrossRef]
- Ge, P.; Cao, X.; Hou, H.; Li, S.; Ji, X. Rodlike Sb2Se3 Wrapped with Carbon: The Exploring of Electrochemical Properties in Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 34979–34989. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Calas, A.; Tang, C.; Li, F.; Zhou, L.; Mai, L. Ultralong Sb2Se3 Nanowire-Based Free-Standing Membrane Anode for Lithium/Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 35219–35226. [Google Scholar] [CrossRef]
- Xiong, X.; Yang, C.; Wang, G.; Lin, Y.; Ou, X.; Wang, J.H.; Zhao, B.; Liu, M.; Lin, Z.; Huang, K. SnS nanoparticles electrostatically anchored on three-dimensional N-doped graphene as an active and durable anode for sodium-ion batteries. Energy Environ. Sci. 2017, 10, 1757–1763. [Google Scholar] [CrossRef]
- Lin, Y.; Qiu, Z.; Li, D.; Ullah, S.; Hai, Y.; Xin, H.; Liao, W.; Yang, B.; Fan, H.; Xu, J.; et al. NiS2@CoS2 nanocrystals encapsulated in N-doped carbon nanocubes for high performance lithium/sodium ion batteries. Energy Storage Mater. 2018, 11, 67–74. [Google Scholar] [CrossRef]
- Jiang, Y.; Wei, M.; Feng, J.; Ma, Y.; Xiong, S. Enhancing the cycling stability of Na-ion batteries by bonding SnS2 ultrafine nanocrystals on amino-functionalized graphene hybrid nanosheets. Energy Environ. Sci. 2016, 9, 1430–1438. [Google Scholar] [CrossRef]
- Gao, S.; Chen, G.; Dall’Agnese, Y.; Wei, Y.; Gao, Z.; Gao, Y. Flexible MnS–Carbon Fiber Hybrids for Lithium-Ion and Sodium-Ion Energy Storage. Chem.-A Eur. J. 2018, 24, 13535–13539. [Google Scholar] [CrossRef]
- Wu, T.H.; Lin, Y.C.; Hou, B.W.; Liang, W.Y. Nanostructured β−NiS catalyst for enhanced and stable electro−oxidation of urea. Catalysts 2020, 10, 1280. [Google Scholar] [CrossRef]
- Liu, M.; Jiao, Y.; Zhan, S.; Wang, H. Ni3S2 nanowires supported on Ni foam as efficient bifunctional electrocatalyst for urea-assisted electrolytic hydrogen production. Catal. Today 2020, 355, 596–601. [Google Scholar] [CrossRef]
- Hao, P.; Zhu, W.; Li, L.; Tian, J.; Xie, J.; Lei, F.; Cui, G.; Zhang, Y.; Tang, B. Nickel incorporated Co9S8 nanosheet arrays on carbon cloth boosting overall urea electrolysis. Electrochim. Acta 2020, 338, 135883. [Google Scholar] [CrossRef]
- Khalafallah, D.; Zou, Q.; Zhi, M.; Hong, Z. Tailoring hierarchical yolk-shelled nickel cobalt sulfide hollow cages with carbon tuning for asymmetric supercapacitors and efficient urea electrocatalysis. Electrochim. Acta 2020, 350, 136399. [Google Scholar] [CrossRef]
- Yang, M.; Ding, C.; Liu, Y.; Bai, Q. Enhanced electro-oxidation of urea using Ni-NiS debris via confinement in carbon derived from glucose. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126425. [Google Scholar] [CrossRef]
- He, M.; Hu, S.; Feng, C.; Wu, H.; Liu, H.; Mei, H. Interlaced rosette-like MoS2/Ni3S2/NiFe-LDH grown on nickel foam: A bifunctional electrocatalyst for hydrogen production by urea-assisted electrolysis. Int. J. Hydrogen Energy 2020, 45, 23–35. [Google Scholar] [CrossRef]
- Xiong, P.; Ao, X.; Chen, J.; Li, J.G.; Lv, L.; Li, Z.; Zondode, M.; Xue, X.; Lan, Y.; Wang, C. Nickel diselenide nanoflakes give superior urea electrocatalytic conversion. Electrochim. Acta 2019, 297, 833–841. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Fan, L.Z. MOF-derived CoSe2 microspheres with hollow interiors as high-performance electrocatalysts for the enhanced oxygen evolution reaction. J. Mater. Chem. A 2017, 5, 15310–15314. [Google Scholar] [CrossRef]
- Liu, T.; Sun, X.; Asiri, A.M.; He, Y. One-step electrodeposition of Ni-Co-S nanosheets film as a bifunctional electrocatalyst for efficient water splitting. Int. J. Hydrogen Energy 2016, 41, 7264–7269. [Google Scholar] [CrossRef]
- Zhao, X.; Li, X.; Yan, Y.; Xing, Y.; Lu, S.; Zhao, L.; Zhou, S.; Peng, Z.; Zeng, J. Electrical and structural engineering of cobalt selenide nanosheets by Mn modulation for efficient oxygen evolution. Appl. Catal. B Environ. 2018, 236, 569–575. [Google Scholar] [CrossRef]
- Yu, J.; Tian, Y.; Zhou, F.; Zhang, M.; Chen, R.; Liu, Q.; Liu, J.; Xu, C.Y.; Wang, J. Metallic and superhydrophilic nickel cobalt diselenide nanosheets electrodeposited on carbon cloth as a bifunctional electrocatalyst. J. Mater. Chem. A 2018, 6, 17353–17360. [Google Scholar] [CrossRef]
- Chi, J.Q.; Yan, K.L.; Xiao, Z.; Dong, B.; Shang, X.; Gao, W.K.; Li, X.; Chai, Y.M.; Liu, C.G. Trimetallic Ni–Fe–Co selenides nanoparticles supported on carbon fiber cloth as efficient electrocatalyst for oxygen evolution reaction. Int. J. Hydrogen Energy 2017, 42, 20599–20607. [Google Scholar] [CrossRef]
- Yao, M.; Hu, H.; Sun, B.; Wang, N.; Hu, W.; Komarneni, S. Self-Supportive Mesoporous Ni/Co/Fe Phosphosulfide Nanorods Derived from Novel Hydrothermal Electrodeposition as a Highly Efficient Electrocatalyst for Overall Water Splitting. Small 2019, 15, 1905201. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.F.; Yuan, C.Z.; Zhou, X.; Liu, Y.N.; Zhao, Z.W.; Zhao, S.J.; Xu, A.W. Selenium phosphorus co-doped cobalt oxide nanosheets anchored on Co foil: A self-supported and stable bifunctional electrode for efficient electrochemical water splitting. Electrochim. Acta 2018, 292, 247–255. [Google Scholar] [CrossRef]
- Li, J.G.; Xie, K.; Sun, H.; Li, Z.; Ao, X.; Chen, Z.; Ostrikov, K.K.; Wang, C.; Zhang, W. Template-Directed Bifunctional Dodecahedral CoP/CN@MoS2 Electrocatalyst for High Efficient Water Splitting. ACS Appl. Mater. Interfaces 2019, 11, 36649–36657. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Shen, S.; Zhong, Y.; Zhang, K.; Wu, J.; Wang, X.; Xia, X.; Tu, J. Assembling Co9S8 nanoflakes on Co3O4 nanowires as advanced core/shell electrocatalysts for oxygen evolution reaction. J. Energy Chem. 2017, 26, 1203–1209. [Google Scholar] [CrossRef] [Green Version]
- Muthurasu, A.; Maruthapandian, V.; Kim, H.Y. Metal-organic framework derived Co3O4/MoS2 heterostructure for efficient bifunctional electrocatalysts for oxygen evolution reaction and hydrogen evolution reaction. Appl. Catal. B Environ. 2019, 248, 202–210. [Google Scholar] [CrossRef]
- Wang, S.; Xu, L.; Lu, W. Synergistic effect: Hierarchical Ni3S2 @Co(OH)2 heterostructure as efficient bifunctional electrocatalyst for overall water splitting. Appl. Surf. Sci. 2018, 457, 156–163. [Google Scholar] [CrossRef]
- Yan, J.; Chen, L.; Liang, X. Co9S8 nanowires@NiCo LDH nanosheets arrays on nickel foams towards efficient overall water splitting. Sci. Bull. 2019, 64, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Zhan, C.; Liu, Z.; Zhou, Y.; Guo, M.; Zhang, X.; Tu, J.; Ding, L.; Cao, Y. Triple hierarchy and double synergies of NiFe/Co9S8/carbon cloth: A new and efficient electrocatalyst for the oxygen evolution reaction. Nanoscale 2019, 11, 3193–3199. [Google Scholar] [CrossRef]
- Que, R.; Ji, G.; Liu, D.; Li, M.; Wang, X.; Jiang, S.P. Nickel Foam-Supported CoCO 3 @CoSe Nanowires with a Heterostructure Interface for Overall Water Splitting with Low Overpotential and High Efficiency. Energy Technol. 2019, 7, 1800741. [Google Scholar] [CrossRef]
- Li, K.; Zhang, J.; Wu, R.; Yu, Y.; Zhang, B. Anchoring CoO domains on CoSe2 nanobelts as bifunctional electrocatalysts for overall water splitting in neutral media. Adv. Sci. 2015, 3, 1500426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Yao, H.; Yu, Z.; Islam, S.M.; He, H.; Yuan, M.; Yue, Y.; Xu, K.; Hao, W.; Sun, G.; et al. Hierarchical Nanoassembly of MoS2/Co9S8/Ni3S2/Ni as a Highly Efficient Electrocatalyst for Overall Water Splitting in a Wide pH Range. J. Am. Chem. Soc. 2019, 141, 10417–10430. [Google Scholar] [CrossRef]
- Zhang, C.; Bhoyate, S.; Kahol, P.K.; Siam, K.; Poudel, T.P.; Mishra, S.R.; Perez, F.; Gupta, A.; Gupta, G.; Gupta, R.K. Highly Efficient and Durable Electrocatalyst Based on Nanowires of Cobalt Sulfide for Overall Water Splitting. ChemNanoMat 2018, 4, 1240–1246. [Google Scholar] [CrossRef]
- Gu, W.; Hu, L.; Zhu, X.; Shang, C.; Li, J.; Wang, E. Rapid synthesis of Co3O4 nanosheet arrays on Ni foam by in situ electrochemical oxidization of air-plasma engraved Co(OH)2 for efficient oxygen evolution. Chem. Commun. 2018, 54, 12698–12701. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, J.; Zou, Z.; Wang, X. Folded nanosheet-like Co0.85Se array for overall water splitting. J. Solid State Electrochem. 2018, 22, 1785–1794. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, Y.; Yang, M.; Zhang, M.; Guo, Q.; Shen, W.; He, R.; Li, M. Highly conductive and metallic cobalt-nickel selenide nanorods supported on Ni foam as an efficient electrocatalyst for alkaline water splitting. Nanoscale 2019, 11, 7959–7966. [Google Scholar] [CrossRef]
- Yang, W.Q.; Hua, Y.X.; Zhang, Q.B.; Lei, H.; Xu, C.Y. Electrochemical fabrication of 3D quasi-amorphous pompon-like Co-O and Co-Se hybrid films from choline chloride/urea deep eutectic solvent for efficient overall water splitting. Electrochim. Acta 2018, 273, 71–79. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, X.; Shi, Z.; Ye, Z.; Wang, W.; Zhang, N.; Hong, Z.; Zhi, M. Synthesis of porous NiCo2S4 aerogel for supercapacitor electrode and oxygen evolution reaction electrocatalyst. Chem. Eng. J. 2018, 331, 185–193. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Guo, X.; Wu, X.; Pang, H. Copper sulfides and their composites for high-performance rechargeable batteries. Mater. Today Chem. 2022, 23, 100675. [Google Scholar] [CrossRef]
- Li, Y.; Wu, F.; Qian, J.; Zhang, M.; Yuan, Y.; Bai, Y.; Wu, C. Metal Chalcogenides with Heterostructures for High-Performance Rechargeable Batteries. Small Sci. 2021, 1, 2100012. [Google Scholar] [CrossRef]
- Fang, Y.; Luan, D.; Lou, X.W. Recent Advances on Mixed Metal Sulfides for Advanced Sodium-Ion Batteries. Adv. Mater. 2020, 32, 2002976. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hui, K.S.; Hui, K.N.; Shen, J.; Zhou, G.; Liu, J.; Sun, Y. Engineering strategies for low-cost and high-power density aluminum-ion batteries. Chem. Eng. J. 2021, 418, 129385. [Google Scholar] [CrossRef]
- Yu, J.; Tang, T.; Cheng, F.; Huang, D.; Martin, J.L.; Brewer, C.E.; Grimm, R.L.; Zhou, M.; Luo, H. Exploring spent biomass-derived adsorbents as anodes for lithium ion batteries. Mater. Today Energy 2021, 19, 100580. [Google Scholar] [CrossRef]
- Li, Z.; Liu, H.; Huang, J.; Zhang, L. MOF-derived α-MnSe/C composites as anode materials for Li-ion batteries. Ceram. Int. 2019, 45, 23765–23771. [Google Scholar] [CrossRef]
- Han, X.; Ai, F.; Wang, X.; Chen, B.; Wang, L.; Bi, Y. Thiacalixarene-supported Co24 nanocluster derived octahedral Co9S8 nanoparticles in N-doped carbon for superior Li-ion storage. Polyhedron 2019, 171, 279–284. [Google Scholar] [CrossRef]
- Tran Huu, H.; Le, H.T.T.; Huong Nguyen, T.; Nguyen Thi, L.; Vo, V.; Bin Im, W. Facile synthesis of SnS2@g-C3N4 composites as high performance anodes for lithium ion batteries. Appl. Surf. Sci. 2021, 549, 149312. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.; Gim, J.; Alfaruqi, M.H.; Kim, S.; Mathew, V.; Sambandam, B.; Hwang, J.Y.; Kim, J. Ultra-small ZnS quantum dots embedded in N-doped carbon matrix for high-performance Li-ion battery anode. Compos. Part B Eng. 2022, 231, 109548. [Google Scholar] [CrossRef]
- Zhou, Y.; Tian, R.; Duan, H.; Wang, K.; Guo, Y.; Li, H.; Liu, H. CoSe/Co nanoparticles wrapped by in situ grown N-doped graphitic carbon nanosheets as anode material for advanced lithium ion batteries. J. Power Source 2018, 399, 223–230. [Google Scholar] [CrossRef]
- Wang, S.; Yang, X.; Lee, P.; Yu, D.Y.W. Mechanically and structurally stable Sb2Se3/carbon nanocomposite as anode for the lithium-ion batteries. J. Alloys Compd. 2021, 874, 159859. [Google Scholar] [CrossRef]
- Chen, H.; Liu, R.; Wu, Y.; Cao, J.; Chen, J.; Hou, Y.; Guo, Y.; Khatoon, R.; Chen, L.; Zhang, Q.; et al. Interface coupling 2D/2D SnSe2/graphene heterostructure as long-cycle anode for all-climate lithium-ion battery. Chem. Eng. J. 2021, 407, 126973. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, W.; Shi, C.; Li, L.; Fan, K.; Huang, X.; Wu, X.; Du, K. Misfit layer SnTiS3: An assemble-free van der Waals heterostructure SnS/TiS2 for lithium ion battery anode. J. Power Source 2021, 494, 229712. [Google Scholar] [CrossRef]
- Xu, H.; Sun, L.; Li, W.; Gao, M.; Zhou, Q.; Li, P.; Yang, S.; Lin, J. Facile synthesis of hierarchical g-C3N4@WS2 composite as Lithium-ion battery anode. Chem. Eng. J. 2022, 435, 135129. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, J.; Li, L.; Mao, J.; Xu, J.; Jin, J. One-step solvothermal synthesis of BiSbTe3/N-doped reduced graphene oxide composite as lithium-ion batteries anode materials. Chem. Eng. Sci. 2020, 225, 115829. [Google Scholar] [CrossRef]
- So, S.; Ko, J.; Ahn, Y.N.; Kim, I.T.; Hur, J. Unraveling improved electrochemical kinetics of In2Te3-based anodes embedded in hybrid matrix for Li-ion batteries. Chem. Eng. J. 2022, 429, 132395. [Google Scholar] [CrossRef]
- Liang, Z.; Tu, H.; Zhang, K.; Kong, Z.; Huang, M.; Xu, D. Self-supporting NiSe2 @ BCNNTs electrode for High-Performance sodium ion batteries. Chem. Eng. J. 2022, 437, 135421. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, Y.; Shi, X.L.; Suo, G.; Chen, H.; Noman, M.; Tao, X.; Chen, Z.G. Hierarchical SnS2/carbon nanotube@reduced graphene oxide composite as an anode for ultra-stable sodium-ion batteries. Chem. Eng. J. Adv. 2020, 4, 100053. [Google Scholar] [CrossRef]
- Zheng, F.; Zhong, W.; Deng, Q.; Pan, Q.; Ou, X.; Liu, Y.; Xiong, X.; Yang, C.; Chen, Y.; Liu, M. Three-dimensional (3D) flower-like MoSe2/N-doped carbon composite as a long-life and high-rate anode material for sodium-ion batteries. Chem. Eng. J. 2019, 357, 226–236. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, W.; Zhou, J.; Bai, Z. FeS2@TiO2 nanorods as high-performance anode for sodium ion battery. Chin. J. Chem. Eng. 2020, 28, 2699–2706. [Google Scholar] [CrossRef]
- Li, W.; Huang, J.; Li, R.; Cao, L.; Li, X.; Feng, L. (1 1 0)-Bridged nanoblocks self-assembled VS4 hollow microspheres as sodium-ion battery anode with superior rate capability and long cycling life. Chem. Eng. J. 2020, 384, 123385. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, P.; Xiong, X.; Fu, H. Self-assembled MoS2/C nanoflowers with expanded interlayer spacing as a high-performance anode for sodium ion batteries. Chin. J. Chem. Eng. 2021, 39, 240–246. [Google Scholar] [CrossRef]
- Ye, L.; Wang, W.; Zhang, B.; Li, D.; Xiao, H.; Xiao, Z.; Ming, L.; Ou, X. Regeneration of well-performed anode material for sodium ion battery from waste lithium cobalt oxide via a facile sulfuration process. Mater. Today Energy 2022, 25, 100957. [Google Scholar] [CrossRef]
- Shao, L.; Wang, S.; Qi, J.; Sun, Z.; Shi, X.; Shi, Y.; Lu, X. Highly infiltrative micro-sized Cu2Se as advanced material with excellent rate performance and ultralong cycle-life for sodium ion half/full batteries. Mater. Today Phys. 2021, 19, 100422. [Google Scholar] [CrossRef]
- Young Jeong, S.; Ghosh, S.; Kim, J.K.; Kang, D.W.; Mun Jeong, S.; Chan Kang, Y.; Cho, J.S. Multi-channel-contained few-layered MoSe2 nanosheet/N-doped carbon hybrid nanofibers prepared using diethylenetriamine as anodes for high-performance sodium-ion batteries. J. Ind. Eng. Chem. 2019, 75, 100–107. [Google Scholar] [CrossRef]
- He, X.; Liu, J.; Kang, B.; Li, X.; Zeng, L.; Liu, Y.; Qiu, J.; Qian, Q.; Wei, M.; Chen, Q. Preparation of SnS2/enteromorpha prolifera derived carbon composite and its performance of sodium-ion batteries. J. Phys. Chem. Solids 2021, 152, 109976. [Google Scholar] [CrossRef]
- Wang, L.; Lin, C.; Liang, T.; Wang, N.; Feng, J.; Yan, W. NiSe2 nanoparticles encapsulated in CNTs covered and N-doped TiN/carbon nanofibers as a binder-free anode for advanced sodium-ion batteries. Mater. Today Chem. 2022, 24, 100849. [Google Scholar] [CrossRef]
- Shao, L.; Hong, J.; Wang, S.; Wu, F.; Yang, F.; Shi, X.; Sun, Z. Urchin-like FeS2 hierarchitectures wrapped with N-doped multi-wall carbon nanotubes @ rGO as high-rate anode for sodium ion batteries. J. Power Source 2021, 491, 229627. [Google Scholar] [CrossRef]
- Wang, J.; Wu, N.; Han, L.; Liao, C.; Mu, X.; Kan, Y.; Hu, Y. Polyacrylonitrile @ metal organic frameworks composite-derived heteroatoms doped carbon @ encapsulated cobalt sulfide as superb sodium ion batteries anode. J. Colloid Interface Sci. 2021, 581, 552–565. [Google Scholar] [CrossRef]
- Chen, H.; Tian, P.; Fu, L.; Wan, S.; Liu, Q. Hollow spheres of solid solution Fe7Ni3S11/CN as advanced anode materials for sodium ion batteries. Chem. Eng. J. 2022, 430, 132688. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, K.; Zhang, S.; Miao, F.; Xiao, W.; Shen, Y.; Zhang, P.; Wang, Z.; Shao, G. Enabling remarkable cycling performance of high-loading MoS2@Graphene anode for sodium ion batteries with tunable cut-off voltage. J. Power Source 2020, 458, 228040. [Google Scholar] [CrossRef]
- Duan, M.; Meng, Y.; Xiao, M.; Ahmed, W.H.Z.; Wang, X.; Gao, H.; Zhang, Y.; Zhu, F. Facile synthesis of WS2/Ni3S2 encapsulated in N-doped carbon hybrid electrode with high rate performance as anode for sodium-ion batteries. J. Electroanal. Chem. 2021, 899, 115681. [Google Scholar] [CrossRef]
- Park, G.D.; Park, J.; Kim, J.K.; Kang, Y.C. Metal sulfoselenide solid solution embedded in porous hollow carbon nanospheres as effective anode material for potassium-ion batteries with long cycle life and enhanced rate performance. Chem. Eng. J. 2022, 428, 131051. [Google Scholar] [CrossRef]
- Li, J.; Rui, B.; Wei, W.; Nie, P.; Chang, L.; Le, Z. Nanosheets assembled layered MoS2/MXene as high performance anode materials for potassium ion batteries. J. Power Source 2020, 449, 227481. [Google Scholar] [CrossRef]
- Ho Na, J.; Chan Kang, Y.; Park, S.K. Electrospun MOF-based ZnSe nanocrystals confined in N-doped mesoporous carbon fibers as anode materials for potassium ion batteries with long-term cycling stability. Chem. Eng. J. 2021, 425, 131651. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, C.; Li, Y.Y.; Zheng, F.; Li, Y.Y.; Deng, Q.; Zhong, W.; Wang, G.; Liu, T. FeSe2/nitrogen-doped carbon as anode material for Potassium-ion batteries. Chem. Eng. J. 2020, 393, 124590. [Google Scholar] [CrossRef]
- Ma, G.; Li, C.; Liu, F.; Majeed, M.K.; Feng, Z. Metal-organic framework-derived Co0.85 Se nanoparticles in N-doped carbon as a high-rate and long-lifespan anode material for potassium ion batteries. Mater. Today Energy 2018, 10, 241–248. [Google Scholar] [CrossRef]
- He, Y.; Wang, L.; Dong, C.; Li, C.; Ding, X.; Qian, Y.; Xu, L. In-situ rooting ZnSe/N-doped hollow carbon architectures as high-rate and long-life anode materials for half/full sodium-ion and potassium-ion batteries. Energy Storage Mater. 2019, 23, 35–45. [Google Scholar] [CrossRef]
- Kang, B.; Chen, X.; Zeng, L.; Luo, F.; Li, X.; Xu, L.; Yang, M.Q.; Chen, Q.; Wei, M.; Qian, Q. In situ fabrication of ultrathin few-layered WSe2 anchored on N, P dual-doped carbon by bioreactor for half/full sodium/potassium-ion batteries with ultralong cycling lifespan. J. Colloid Interface Sci. 2020, 574, 217–228. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, L.; Wang, Y.; Qin, M.; Wu, R.; Huang, Z.; Yang, H.J.; Li, Y.; Zhou, T.; Hu, J. Rational design of MoSe2 nanosheet-coated MOF-derived N-doped porous carbon polyhedron for potassium storage. J. Colloid Interface Sci. 2021, 600, 430–439. [Google Scholar] [CrossRef]
- Lei, Z.; Li, X.; Liu, Y.; Wu, J.; Wang, Y.; Luo, Y.; Chen, Q.; Wei, M.; Zeng, L.; Qian, Q. Two-dimentional MoSe2/chitosan-derived nitrogen-doped carbon composite enabling stable sodium/potassium storage. J. Phys. Chem. Solids 2022, 163, 110573. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Suo, G.; Yu, Q.; Wang, W.; Feng, L.; Hou, X.; Ye, X.; Zhang, L.; Yang, Y. Hollow Co0.85Se cubes encapsulated in graphene for enhanced potassium storage. J. Electroanal. Chem. 2020, 864, 114100. [Google Scholar] [CrossRef]
- Suo, G.; Zhang, J.; Li, D.; Yu, Q.; Wang, W.; He, M.; Feng, L.; Hou, X.; Yang, Y.; Ye, X.; et al. N-doped carbon/ultrathin 2D metallic cobalt selenide core/sheath flexible framework bridged by chemical bonds for high-performance potassium storage. Chem. Eng. J. 2020, 388, 124396. [Google Scholar] [CrossRef]
- Xing, W.; Du, D.; Cai, T.; Li, X.; Zhou, J.; Chai, Y.; Xue, Q.; Yan, Z. Carbon-encapsulated CoSe nanoparticles derived from metal-organic frameworks as advanced cathode material for Al-ion battery. J. Power Source 2018, 401, 6–12. [Google Scholar] [CrossRef]
- Li, J.; Liu, W.; Yu, Z.; Deng, J.; Zhong, S.; Xiao, Q.; Chen, F.; Yan, D. N-doped C@ZnSe as a low cost positive electrode for aluminum-ion batteries: Better electrochemical performance with high voltage platform of ~1.8 V and new reaction mechanism. Electrochim. Acta 2021, 370, 137790. [Google Scholar] [CrossRef]
- Yang, W.; Lu, H.; Cao, Y.; Jing, P. Single-/few-layered ultrasmall WS2 nanoplates embedded in nitrogen-doped carbon nanofibers as a cathode for rechargeable aluminum batteries. J. Power Source 2019, 441, 227173. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, D.; Lian, Y.; Hou, S.; Ban, C.; Wang, Z.; Zhao, J.; Zhang, H. Rechargeable aluminum-ion battery with sheet-like MoSe2@C nanocomposites cathode. Electrochim. Acta 2020, 354, 136677. [Google Scholar] [CrossRef]
- Zheng, J.; Ju, S.; Yao, L.; Xia, G.; Yu, X. Construction of solid solution sulfide embedded in MXene@N-doped carbon dual protection matrix for advanced aluminum ion batteries. J. Power Source 2021, 511, 230450. [Google Scholar] [CrossRef]
- Xing, L.; Asare, K.; Liu, X.; Meng, J.; Wang, K.; An, Q.; Mai, L. Nano Energy Insights into the storage mechanism of vs. 4 nanowire clusters in aluminum-ion battery. Nano Energy 2021, 79, 105384. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Yang, L.; Zhao, L.; Dong, X.; Wang, H.; Li, Y.; Sun, T.; Li, Q.; Li, H. Evidence for dual anions co-insertion in a transition metal chalcogenide cathode material NiSe2 for high-performance rechargeable aluminum-ion batteries. Energy Storage Mater. 2022, 47, 336–344. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, B.; Zhang, W.; Jin, H.; Qin, J.; Wan, J.; Zhang, J.; Chen, G. Interfacial engineering of Bi2Te3/Sb2Te3 heterojunction enables high–energy cathode for aluminum batteries. Energy Storage Mater. 2021, 38, 231–240. [Google Scholar] [CrossRef]
- Lu, H.; Li, Y.; Zheng, Y.; Dong, C.; Li, Y.; Meng, F.; Wang, Y.; Teng, C.; Wang, X.; Zhou, D.; et al. Layered double hydroxide-derived Fe-doped NiSe cathode toward stable and high-energy aluminum storage. Mater. Today Energy 2022, 24, 100940. [Google Scholar] [CrossRef]
- Gao, Y.; Zhai, Z.; Dong, Y.; Pang, Y.; Chen, J.; Li, G. 1 T-VSe2 Nanoparticles cooperated with reduced graphene oxide as a superior cathode material for rechargeable Mg-ion batteries. Appl. Surf. Sci. 2022, 592, 153141. [Google Scholar] [CrossRef]
- Tong, Y.; Gao, A.; Zhang, Q.; Gao, T.; Yue, J.; Meng, F.; Gong, Y.; Xi, S.; Lin, Z.; Mao, M.; et al. Cation-synergy stabilizing anion redox of Chevrel phase Mo6S8 in aluminum ion battery. Energy Storage Mater. 2021, 37, 87–93. [Google Scholar] [CrossRef]
- Tang, B.; Tian, N.; Jiang, J.; Li, Y.; Yang, J.; Zhu, Q. Investigation of zinc storage capacity of WS2 nanosheets for rechargeable aqueous Zn-ion batteries. J. Alloys Compd. 2022, 894, 162391. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, M.; Liu, F.; Huang, Q.; Liu, L.; Fu, L.; Wu, Y. Layered TiS2 as a Promising Host Material for Aqueous Rechargeable Zn Ion Battery. Batter. Energy Storage 2020, 34, 11590–11596. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Nuli, Y.; Zhou, J.; Yang, J.; Wang, J. Metal Organic Framework (MOF)-Derived carbon-encapsulated cuprous sulfide cathode based on displacement reaction for Hybrid Mg2þ/Liþ batteries. J. Power Source 2020, 445, 227325. [Google Scholar] [CrossRef]
- de la Parra-Arciniega, S.M.; González-Juárez, E.; Hernández-Carrillo, R.A.; Briones-Martínez, R.; Jiménez-Barrera, R.M.; Garcia-Gómez, N.A.; Sánchez, E.M. A Mg2+/Li+ hybrid-ion battery based on MoS2 prepared by solvothermal synthesis with ionic liquid assistance. J. Mater. Sci. Mater. Electron. 2020, 31, 14702–14713. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Yi, X.; Hu, Y.; Wang, L.; Ma, L.; Zhu, G.; Chen, T.; Jin, Z. Hybrid Mg/Li-ion batteries enabled by Mg2+/Li+ co-intercalation in VS4 nanodendrites. Energy Storage Mater. 2019, 23, 741–748. [Google Scholar] [CrossRef]
- Chen, X.; Wang, S.; Wang, H. High performance hybrid Mg-Li ion batteries with conversion cathodes for low cost energy storage. Electrochim. Acta 2018, 265, 175–183. [Google Scholar] [CrossRef]
- Truong, Q.D.; Kempaiah Devaraju, M.; Nakayasu, Y.; Tamura, N.; Sasaki, Y.; Tomai, T.; Honma, I. Exfoliated MoS2 and MoSe2 Nanosheets by a Supercritical Fluid Process for a Hybrid Mg-Li-Ion Battery. ACS Omega 2017, 2, 2360–2367. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Yu, L.; Dong, J.; Cen, Y.; Zhu, T.; Yu, D.; Chen, C.; Zhang, D.; Liu, Y.; Pan, F. Revealing the electrochemical mechanism of the conversion-type Co3S4 in a novel high-capacity Mg-Li hybrid battery. Electrochim. Acta 2022, 401, 139403. [Google Scholar] [CrossRef]
- Jing, P.; Lu, H.; Yang, W.; Cao, Y. Interlayer-expanded and binder-free VS2 nanosheets assemblies for enhanced Mg2+ and Li+/Mg2+ hybrid ion storage. Electrochim. Acta 2020, 330, 135263. [Google Scholar] [CrossRef]
- Bian, X.; Gao, Y.; Fu, Q.; Indris, S.; Ju, Y.; Meng, Y.; Du, F.; Bramnik, N.; Ehrenberg, H.; Wei, Y. A long cycle-life and high safety Na+/Mg2+ hybrid-ion battery built by using a TiS2 derived titanium sulfide cathode. J. Mater. Chem. A 2017, 5, 600–608. [Google Scholar] [CrossRef]
- Shi, C.; Xia, Q.; Xue, X.; Liu, Q.; Liu, H.J. Synthesis of cobalt-based layered coordination polymer nanosheets and their application in lithium-ion batteries as anode materials. RSC Adv. 2016, 6, 4442–4447. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, X.; Legut, D.; Zhang, Q. Modeling and theoretical design of next-generation lithium metal batteries. Energy Storage Mater. 2019, 16, 169–193. [Google Scholar] [CrossRef]
- Xu, M.; Liang, T.; Shi, M.; Chen, H. Graphene-like two-dimensional materials. Chem. Rev. 2013, 113, 3766–3798. [Google Scholar] [CrossRef]
- Chen, L.J. Nanoscale Copper and Copper Compounds for Advanced Device Applications. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2016, 47, 5845–5851. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Ren, M.; Xu, H.; Liu, W.; Hei, J.; Su, L.; Wang, L. Cu2S@ N, S Dual-Doped Carbon Matrix Hybrid as Superior Anode Materials for Lithium/Sodium ion Batteries. ChemElectroChem 2018, 5, 2135–2141. [Google Scholar] [CrossRef]
- Wu, N.; Miao, D.; Zhou, X.; Zhang, L.; Liu, G.; Guo, D. V3S4 Nanosheets Anchored on N, S Co-Doped Graphene with Pseudocapacitive Effect for Fast and Durable Lithium Storage. Nanomaterials 2019, 9, 1638. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Ni, Y.; Hou, X.; Chen, L.; Li, F.; Chen, J. A Two-Dimensional Metal–Organic Polymer Enabled by Robust Nickel–Nitrogen and Hydrogen Bonds for Exceptional Sodium-Ion Storage. Angew. Chem.-Int. Ed. 2020, 59, 22126–22131. [Google Scholar] [CrossRef]
- Xiao, Y.; Su, D.; Wang, X.; Wu, S.; Zhou, L.; Shi, Y.; Fang, S.; Cheng, H.M.; Li, F. CuS Microspheres with Tunable Interlayer Space and Micropore as a High-Rate and Long-Life Anode for Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1800930. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, P.; Yin, Y.; Zhang, X.; Fan, L.; Zhang, N.; Sun, K. Heterostructured SnS-ZnS@C hollow nanoboxes embedded in graphene for high performance lithium and sodium ion batteries. Chem. Eng. J. 2019, 356, 1042–1051. [Google Scholar] [CrossRef]
- Peng, Q.; Hu, X.; Zeng, T.; Shang, B.; Mao, M.; Jiao, X.; Xi, G. FeSb2S4 anchored on amine-modified graphene towards high-performance anode material for sodium ion batteries. Chem. Eng. J. 2020, 385, 123857. [Google Scholar] [CrossRef]
- Chang, X.; Ma, Y.; Yang, M.; Xing, T.; Tang, L.; Chen, T.; Guo, Q.; Zhu, X.; Liu, J.; Xia, H. In-situ solid-state growth of N, S codoped carbon nanotubes encapsulating metal sulfides for high-efficient-stable sodium ion storage. Energy Storage Mater. 2019, 23, 358–366. [Google Scholar] [CrossRef]
- Cui, Y.; Feng, W.; Wang, D.; Wang, Y.; Liu, W.; Wang, H.; Jin, Y.; Yan, Y.; Hu, H.; Wu, M.; et al. Water-Soluble Salt Template-Assisted Anchor of Hollow FeS2 Nanoparticle Inside 3D Carbon Skeleton to Achieve Fast Potassium-Ion Storage. Adv. Energy Mater. 2021, 11, 2101343. [Google Scholar] [CrossRef]
- Liu, Q.; Ye, J.; Chen, Z.; Hao, Q.; Xu, C.; Hou, J. Double conductivity-improved porous Sn/Sn4P3@carbon nanocomposite as high performance anode in Lithium-ion batteries. J. Colloid Interface Sci. 2019, 537, 588–596. [Google Scholar] [CrossRef]
- Fang, G.; Wu, Z.; Zhou, J.; Zhu, C.; Cao, X.; Lin, T.; Chen, Y.; Wang, C.; Pan, A.; Liang, S. Observation of Pseudocapacitive Effect and Fast Ion Diffusion in Bimetallic Sulfides as an Advanced Sodium-Ion Battery Anode. Adv. Energy Mater. 2018, 8, 1703155. [Google Scholar] [CrossRef]
- Liu, Q.; Hou, J.; Hao, Q.; Huang, P.; Xu, C.; Zhou, Q.; Zhou, J.; Liu, H. Nitrogen-doped carbon encapsulated hollow ZnSe/CoSe2 nanospheres as high performance anodes for lithium-ion batteries. Nanoscale 2020, 12, 22778–22786. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.; Guo, X.; Zhu, S.; Zhao, Y.; Iikubo, S. Bimetallic Sulfide SnS2/FeS2 Nanosheets as High-Performance Anode Materials for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 39248–39256. [Google Scholar] [CrossRef]
- Goin, D.E.; Pearson, R.M.; Craske, M.G.; Stein, A.; Pettifor, A.; Sheri, A.; Kahn, K.; Neilands, T.B.; Hamilton, E.L.; Selin, A.; et al. Cell-like-carbon-micro-spheres for robust potassium anode. Natl. Sci. Rev. 2019, 8, 94720. [Google Scholar]
- Park, G.D.; Kim, J.H.; Park, S.K.; Kang, Y.C. MoSe2 Embedded CNT-Reduced Graphene Oxide Composite Microsphere with Superior Sodium Ion Storage and Electrocatalytic Hydrogen Evolution Performances. ACS Appl. Mater. Interfaces 2017, 9, 10673–10683. [Google Scholar] [CrossRef]
- Yang, S.; Park, G.D.; Kang, Y.C. Conversion reaction mechanism of cobalt telluride-carbon composite microspheres synthesized by spray pyrolysis process for K-ion storage. Appl. Surf. Sci. 2020, 529, 147140. [Google Scholar] [CrossRef]
- Wu, Y.; Nie, P.; Wu, L.; Dou, H.; Zhang, X. 2D MXene/SnS2 composites as high-performance anodes for sodium ion batteries. Chem. Eng. J. 2018, 334, 932–938. [Google Scholar] [CrossRef]
- Yang, Z.; Li, W.; Zhang, G.; Wang, J.; Zuo, J.; Xu, Q.; Shan, H.; He, X.; Lv, M.; Hu, J.; et al. Constructing Sb[sbnd]O[sbnd]C bond to improve the alloying reaction reversibility of free-standing Sb2Se3 nanorods for potassium-ion batteries. Nano Energy 2022, 93, 106764. [Google Scholar] [CrossRef]
- Park, G.D.; Kang, Y.C. Conversion Reaction Mechanism for Yolk-Shell-Structured Iron Telluride-C Nanospheres and Exploration of Their Electrochemical Performance as an Anode Material for Potassium-Ion Batteries. Small Methods 2020, 4, 200055. [Google Scholar] [CrossRef]
- Yang, R.; Yao, W.; Tang, B.; Zhang, F.; Lei, X.; Lee, C.; Tang, Y. Development and challenges of electrode materials for rechargeable Mg batteries. Energy Storage Mater. 2021, 42, 687–704. [Google Scholar] [CrossRef]
- Zhang, Y.; Geng, H.; Wei, W.; Ma, J.; Chen, L.; Li, C.C. Challenges and recent progress in the design of advanced electrode materials for rechargeable Mg batteries. Energy Storage Mater. 2019, 20, 118–138. [Google Scholar] [CrossRef]
- Michail, A.; Silván, B.; Tapia-Ruiz, N. Progress in high-voltage MgMn2O4 oxyspinel cathode materials for Mg batteries. Curr. Opin. Electrochem. 2022, 31, 100817. [Google Scholar] [CrossRef]
- Luo, J.; He, S.; Liu, T.L. Tertiary Mg/MgCl2/AlCl3 Inorganic Mg2+ Electrolytes with Unprecedented Electrochemical Performance for Reversible Mg Deposition. ACS Energy Lett. 2017, 2, 1197–1202. [Google Scholar] [CrossRef]
- Deivanayagam, R.; Ingram, B.J.; Shahbazian-Yassar, R. Progress in development of electrolytes for magnesium batteries. Energy Storage Mater. 2019, 21, 136–153. [Google Scholar] [CrossRef]
- Li, Z.; Han, L.; Wang, Y.; Li, X.; Lu, J.; Hu, X. Microstructure Characteristics of Cathode Materials for Rechargeable Magnesium Batteries. Small 2019, 15, 1900105. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Dong, X.; Zhu, L.; Hou, D.; Zeng, W.; Wang, J. The potential application of VS2 as an electrode material for Mg ion battery: A DFT study. Appl. Surf. Sci. 2021, 544, 148775. [Google Scholar] [CrossRef]
- Wang, Z.; Li, W.; Zheng, Y.; Yang, Z.; Zhang, J. How does the active site in the MoSe2 surface affect its electrochemical performance as anode material for metal-ion batteries? Appl. Surf. Sci. 2020, 526, 146637. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, D.; Yang, D.; Wei, L.; Liu, B.; Wang, X.; Chen, G.; Wei, Y. Superior Mg2+ storage properties of VS2 nanosheets by using an APC-PP14Cl/THF electrolyte. Energy Storage Mater. 2019, 23, 749–756. [Google Scholar] [CrossRef]
- Faegh, E.; Ng, B.; Hayman, D.; Mustain, W.E. Practical assessment of the performance of aluminium battery technologies. Nat. Energy 2021, 6, 450. [Google Scholar] [CrossRef]
- Yao, L.; Ju, S.; Xu, T.; Yu, X. Spatial Isolation-Inspired Ultrafine CoSe2 for High-Energy Aluminum Batteries with Improved Rate Cyclability. ACS Nano 2021, 15, 13662–13673. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Sun, C.; Zhao, K.; Cheng, X.; Rawal, S.; Xu, Y.; Wang, Y. Defect engineering activating (Boosting) zinc storage capacity of MoS2. Energy Storage Mater. 2019, 16, 527–534. [Google Scholar] [CrossRef]
- Wu, T.H.; Zhang, Y.; Althouse, Z.D.; Liu, N. Nanoscale design of zinc anodes for high-energy aqueous rechargeable batteries. Mater. Today Nano 2019, 6, 100032. [Google Scholar] [CrossRef]
- Xiong, F.; Fan, Y.; Tan, S.; Zhou, L.; Xu, Y.; Pei, C.; An, Q.; Mai, L. Magnesium storage performance and mechanism of CuS cathode. Nano Energy 2018, 47, 210–216. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Wang, Y.; Guo, R.; Liu, W.; Pei, H.; Yin, G.; Ye, D.; Yu, S.; Xie, J. A flexible copper sulfide @ multi-walled carbon nanotubes cathode for advanced magnesium-lithium-ion batteries. J. Colloid Interface Sci. 2019, 553, 239–246. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, N.; NuLi, Y.; Yang, J.; Wang, J. Hybrid Mg2+/Li+ batteries with Cu2Se cathode based on displacement reaction. Electrochim. Acta 2018, 261, 503–512. [Google Scholar] [CrossRef]
- Raguzin, I.; Choudhury, S.; Simon, F.; Stamm, M.; Ionov, L. Effect of Current Collector on Performance of Li-S Batteries. Adv. Mater. Interfaces 2017, 4, 1600811. [Google Scholar] [CrossRef]
- Chen, L.; Guo, X.; Lu, W.; Chen, M.; Li, Q.; Xue, H.; Pang, H. Manganese monoxide-based materials for advanced batteries. Coord. Chem. Rev. 2018, 368, 13–34. [Google Scholar] [CrossRef]
- Li, F.; Liu, Q.; Hu, J.; Feng, Y.; He, P.; Ma, J. Recent advances in cathode materials for rechargeable lithium-sulfur batteries. Nanoscale 2019, 11, 15418–15439. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Manthiram, A. A review on the status and challenges of electrocatalysts in lithium-sulfur batteries. Energy Storage Mater. 2019, 20, 55–70. [Google Scholar] [CrossRef]
- Li, P.; Ma, L.; Wu, T.; Ye, H.; Zhou, J.; Zhao, F.; Han, N.; Wang, Y.; Wu, Y.; Li, Y.; et al. Chemical Immobilization and Conversion of Active Polysulfides Directly by Copper Current Collector: A New Approach to Enabling Stable Room-Temperature Li-S and Na-S Batteries. Adv. Energy Mater. 2018, 8, 1800624. [Google Scholar] [CrossRef]
- Guo, Q.; Zheng, Z. Rational Design of Binders for Stable Li-S and Na-S Batteries. Adv. Funct. Mater. 2020, 30, 1907931. [Google Scholar] [CrossRef]
- Deng, X.; Li, J.; Ma, L.; Sha, J.; Zhao, N. Three-dimensional porous carbon materials and their composites as electrodes for electrochemical energy storage systems. Mater. Chem. Front. 2019, 3, 2221–2245. [Google Scholar] [CrossRef]
- Chen, P.; Wu, Z.; Guo, T.; Zhou, Y.; Liu, M.; Xia, X.; Sun, J.; Lu, L.; Ouyang, X.; Wang, X.; et al. Strong Chemical Interaction between Lithium Polysulfides and Flame-Retardant Polyphosphazene for Lithium–Sulfur Batteries with Enhanced Safety and Electrochemical Performance. Adv. Mater. 2021, 33, 2007549. [Google Scholar] [CrossRef]
- Jin, X.; Gao, S.; Wu, A.; Zhao, J.; Huang, H.; Cao, G. Dual-Constrained Sulfur in FeS2@C Nanostructured Lithium-Sulfide Batteries. ACS Appl. Energy Mater. 2020, 3, 10950–10960. [Google Scholar] [CrossRef]
- Xu, W.; Wu, Q.; Che, Z.; Fan, B.; Zhao, D.; Wang, S.; Han, A.; Li, L. A Novel Synthesizing Strategy of 3D Cose2 Porous Hollow Flowers for High Performance Lithium–Sulfur Batteries. Catalysts 2021, 11, 273. [Google Scholar] [CrossRef]
- Steudel, R.; Chivers, T. The role of polysulfide dianions and radical anions in the chemical, physical and biological sciences, including sulfur-based batteries. Chem. Soc. Rev. 2019, 48, 3279–3319. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, W.; Li, X.; Han, L.; Wei, M. Co9S8 embedded into N/S doped carbon composites: In situ derivation from a sulfonate-based metal-organic framework and its electrochemical properties. J. Mater. Chem. A 2019, 7, 10331–10337. [Google Scholar] [CrossRef]
- Ye, C.; Jiao, Y.; Chao, D.; Ling, T.; Shan, J.; Zhang, B.; Gu, Q.; Davey, K.; Wang, H.; Qiao, S.Z. Electron-State Confinement of Polysulfides for Highly Stable Sodium–Sulfur Batteries. Adv. Mater. 2020, 32, 1907557. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tang, A.; Huang, S.; Li, J.; Zeng, L.; Wei, M. In Situ Confined Co5Ge3Alloy Nanoparticles in Nitrogen-Doped Carbon Nanotubes for Boosting Lithium Storage. ACS Appl. Mater. Interfaces 2020, 12, 46247–46253. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, Y.; Bai, Z.; Fang, Z.; Zhang, X.; Xu, Z.; Ding, Y.; Xu, X.; Du, Y.; Dou, S.; et al. High-performance room-temperature sodium-sulfur battery enabled by electrocatalytic sodium polysulfides full conversion. Energy Environ. Sci. 2020, 13, 562–570. [Google Scholar] [CrossRef]
- Yan, Z.; Xiao, J.; Lai, W.; Wang, L.; Gebert, F.; Wang, Y.; Gu, Q.; Liu, H.; Chou, S.L.; Liu, H.; et al. Nickel sulfide nanocrystals on nitrogen-doped porous carbon nanotubes with high-efficiency electrocatalysis for room-temperature sodium-sulfur batteries. Nat. Commun. 2019, 10, 4793. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.W.; Sheng, T.; Wang, Y.X.; Chou, S.; Davey, K.; Dou, S.X.; Qiao, S.Z. Long-Life Room-Temperature Sodium–Sulfur Batteries by Virtue of Transition-Metal-Nanocluster–Sulfur Interactions. Angew. Chem.-Int. Ed. 2019, 58, 1484–1488. [Google Scholar] [CrossRef] [Green Version]
- Xiao, F.; Wang, H.; Yao, T.; Zhao, X.; Yang, X.; Yu, D.Y.W.; Rogach, A.L. MOF-Derived CoS2/N-Doped Carbon Composite to Induce Short-Chain Sulfur Molecule Generation for Enhanced Sodium−Sulfur Battery Performance. ACS Appl. Mater. Interfaces 2021, 13, 18010–18020. [Google Scholar] [CrossRef]
- Wu, D.; Ren, W.; Nuli, Y.; Yang, J.; Wang, J. Recent progress on selenium-based cathode materials for rechargeable magnesium batteries: A mini review. J. Mater. Sci. Technol. 2021, 91, 168–177. [Google Scholar] [CrossRef]
- Yoo, H.D.; Shterenberg, I.; Gofer, Y.; Gershinsky, G.; Pour, N.; Aurbach, D. Mg rechargeable batteries: An on-going challenge. Energy Environ. Sci. 2013, 6, 2265–2279. [Google Scholar] [CrossRef]
- Gao, T.; Noked, M.; Pearse, A.J.; Gillette, E.; Fan, X.; Zhu, Y.; Luo, C.; Suo, L.; Schroeder, M.A.; Xu, K.; et al. Enhancing the Reversibility of Mg/S Battery Chemistry through Li+ Mediation. J. Am. Chem. Soc. 2015, 137, 12388–12393. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Bonnick, P.; Nazar, L.F. Layered TiS2 Positive Electrode for Mg Batteries. ACS Energy Lett. 2016, 1, 297–301. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, R.; Zhang, Y.; Huang, G.; Jiang, B.; Xu, C.; Pan, F. The design of Co3S4@MXene heterostructure as sulfur host to promote the electrochemical kinetics for reversible magnesium-sulfur batteries. J. Magnes. Alloys 2021, 9, 78–89. [Google Scholar] [CrossRef]
- Yoon, K.R.; Lee, G.Y.; Jung, J.W.; Kim, N.H.; Kim, S.O.; Kim, I.D. One-Dimensional RuO2/Mn2O3 Hollow Architectures as Efficient Bifunctional Catalysts for Lithium-Oxygen Batteries. Nano Lett. 2016, 16, 2076–2083. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Long, J.; Shu, C.; Liang, R.; Li, J. Three-Dimensional Interconnected Network Architecture with Homogeneously Dispersed Carbon Nanotubes and Layered MoS2 as a Highly Efficient Cathode Catalyst for Lithium-Oxygen Battery. ACS Appl. Mater. Interfaces 2018, 10, 34077–34086. [Google Scholar] [CrossRef]
- Gao, J.; Cai, X.; Wang, J.; Hou, M.; Lai, L.; Zhang, L. Recent progress in hierarchically structured O2-cathodes for Li-O2 batteries. Chem. Eng. J. 2018, 352, 972–995. [Google Scholar] [CrossRef]
- Liu, B.; Sun, Y.; Liu, L.; Xu, S.; Yan, X. Advances in Manganese-Based Oxides Cathodic Electrocatalysts for Li–Air Batteries. Adv. Funct. Mater. 2018, 28, 1704973. [Google Scholar] [CrossRef]
- Jung, J.W.; Jang, J.S.; Yun, T.G.; Yoon, K.R.; Kim, I.D. Three-Dimensional Nanofibrous Air Electrode Assembled with Carbon Nanotubes-Bridged Hollow Fe2O3 Nanoparticles for High-Performance Lithium-Oxygen Batteries. ACS Appl. Mater. Interfaces 2018, 10, 6531–6540. [Google Scholar] [CrossRef]
- Huang, Z.; Qin, X.; Gu, X.; Li, G.; Mu, Y.; Wang, N.; Ithisuphalap, K.; Wang, H.; Guo, Z.; Shi, Z.; et al. Mn3O4 Quantum Dots Supported on Nitrogen-Doped Partially Exfoliated Multiwall Carbon Nanotubes as Oxygen Reduction Electrocatalysts for High-Performance Zn-Air Batteries. ACS Appl. Mater. Interfaces 2018, 10, 23900–23909. [Google Scholar] [CrossRef]
- Cai, P.; Huang, J.; Chen, J.; Wen, Z. Oxygen-Containing Amorphous Cobalt Sulfide Porous Nanocubes as High-Activity Electrocatalysts for the Oxygen Evolution Reaction in an Alkaline/Neutral Medium. Angew. Chem.-Int. Ed. 2017, 56, 4858–4861. [Google Scholar] [CrossRef]
- Zhu, Q.C.; Xu, S.M.; Harris, M.M.; Ma, C.; Liu, Y.S.; Wei, X.; Xu, H.S.; Zhou, Y.X.; Cao, Y.C.; Wang, K.X.; et al. A Composite of Carbon-Wrapped Mo2C Nanoparticle and Carbon Nanotube Formed Directly on Ni Foam as a High-Performance Binder-Free Cathode for Li-O2 Batteries. Adv. Funct. Mater. 2016, 26, 8514–8520. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, F.; Li, Z.; Yang, Y.; Tamirat, A.G.; Qi, H.; Han, J.; Li, W.; Wang, L.; Feng, S. Lithiophilic Co/Co4N nanoparticles embedded in hollow N-doped carbon nanocubes stabilizing lithium metal anodes for Li-air batteries. J. Mater. Chem. A 2018, 6, 22096–22105. [Google Scholar] [CrossRef]
- Dai, L.; Sun, Q.; Guo, J.; Cheng, J.; Xu, X.; Guo, H.; Li, D.; Chen, L.; Si, P.; Lou, J.; et al. Mesoporous Mn2O3 rods as a highly efficient catalyst for Li-O2 battery. J. Power Source 2019, 435, 226833. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, T.; Chu, C.; Li, Z.; Liu, R.; Li, P.; Li, Y.; Huang, Z. Synthesis of ZIF-Derived CoS2 Nanocages Interconnected by CNTs for Rechargeable Li−O2 Batteries. ACS Sustain. Chem. Eng. 2020, 8, 7581–7587. [Google Scholar] [CrossRef]
- Li, M.; Shu, C.; Hu, A.; Li, J.; Liang, R.; Long, J. Invigorating the Catalytic Activity of Cobalt Selenide via Structural Phase Transition Engineering for Lithium−Oxygen Batteries. ACS Sustain. Chem. Eng. 2020, 8, 5018–5027. [Google Scholar] [CrossRef]
- Li, Y.; Fu, J.; Zhong, C.; Wu, T.; Chen, Z.; Hu, W.; Amine, K.; Lu, J. Recent Advances in Flexible Zinc-Based Rechargeable Batteries. Adv. Energy Mater. 2019, 9, 1802605. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Ma, L.; Han, C.; Wang, Z.; Liu, Z.; Tang, Z.; Zhi, C. Advanced rechargeable zinc-based batteries: Recent progress and future perspectives. Nano Energy 2019, 62, 550–587. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Cui, M.; Xiong, J.; Mi, L.; Chen, S. Heterostructured MoO2@MoS2@Co9S8 nanorods as high efficiency bifunctional electrocatalyst for overall water splitting. Appl. Surf. Sci. 2021, 543, 148804. [Google Scholar] [CrossRef]
- Song, J.; Qiu, S.; Hu, F.; Ding, Y.; Han, S.; Li, L.; Chen, H.Y.; Han, X.; Sun, C.; Peng, S. Sub-2 nm Thiophosphate Nanosheets with Heteroatom Doping for Enhanced Oxygen Electrocatalysis. Adv. Funct. Mater. 2021, 31, 2100618. [Google Scholar] [CrossRef]
- Kaneti, Y.V.; Guo, Y.; Septiani, N.L.W.; Iqbal, M.; Jiang, X.; Takei, T.; Yuliarto, B.; Alothman, Z.A.; Golberg, D.; Yamauchi, Y. Self-templated fabrication of hierarchical hollow manganese-cobalt phosphide yolk-shell spheres for enhanced oxygen evolution reaction. Chem. Eng. J. 2021, 405, 126580. [Google Scholar] [CrossRef]
- Xu, W.; Zhu, S.; Liang, Y.; Cui, Z.; Yang, X.; Inoue, A.; Wang, H. A highly efficient electrocatalyst based on amorphous Pd-Cu-S material for hydrogen evolution reaction. J. Mater. Chem. A 2017, 5, 18793–18800. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, J.; Zhao, H.; Jiang, R.; Tian, F.; Zhang, R. Tremella-like Ni3S2/MnS with ultrathin nanosheets and abundant oxygen vacancies directly used for high speed overall water splitting. Appl. Catal. B Environ. 2019, 257, 117899. [Google Scholar] [CrossRef]
- Wang, J.Y.; Liu, W.T.; Li, X.P.; Ouyang, T.; Liu, Z.Q. Strong hydrophilicity NiS2/Fe7S8 heterojunctions encapsulated in N-doped carbon nanotubes for enhanced oxygen evolution reaction. Chem. Commun. 2020, 56, 1489–1492. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yin, Z.; Cui, M.; Gao, L.; Liu, A.; Su, W.N.; Chen, S.; Ma, T.; Li, Y. Double shelled hollow CoS2@MoS2@NiS2 polyhedron as advanced trifunctional electrocatalyst for zinc-air battery and self-powered overall water splitting. J. Colloid Interface Sci. 2022, 610, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, Z.; He, X.; Zhang, W.; Asefa, T. Co8FeS8/N,S-Doped Carbons Derived from Fe-Co/S-Bridged Polyphthalocyanine: Efficient Dual-Function Air-Electrode Catalysts for Rechargeable Zn-Air Batteries. ACS Sustain. Chem. Eng. 2020, 8, 13147–13158. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Z.; Liu, Y.; Liu, J.; Cui, Z.; Zhang, X.; Ciucci, F.; Tang, Z. Tailoring the interfacial active center of MnSxO2−x/MnCo2S4 heterostructure to boost the performance for oxygen evolution reaction and Zn-Air batteries in neutral electrolyte. Chem. Eng. J. 2022, 427, 131966. [Google Scholar] [CrossRef]
- Li, J.; Wan, T.; Li, J.; Zhang, Z.; Wang, Y.; Liu, G. Three-dimensionally ordered mesoporous trimetal sulfide as efficient electrocatalyst for rechargeable zinc-air batteries. Appl. Surf. Sci. 2022, 575, 151728. [Google Scholar] [CrossRef]
- Cao, L.; Tao, P.; Li, M.; Lyu, F.; Wang, Z.; Wu, S.; Wang, W.; Huo, Y.; Huang, L.; Lu, Z. Synergistic Effects of C/α-MoC and Ag for Efficient Oxygen Reduction Reaction. J. Phys. Chem. Lett. 2018, 9, 779–784. [Google Scholar] [CrossRef]
- Lee, D.U.; Xu, P.; Cano, Z.P.; Kashkooli, A.G.; Park, M.G.; Chen, Z. Recent progress and perspectives on bi-functional oxygen electrocatalysts for advanced rechargeable metal-air batteries. J. Mater. Chem. A 2016, 4, 7107–7134. [Google Scholar] [CrossRef]
- Cao, Y.; Lu, H.; Xu, B.; Yang, W.; Hong, Q. Nitrogen/sulfur dual-doped porous carbon nanofibers with Co9S8 nanoparticles encapsulated by graphitic shells: A highly active stable free- standing air electrode for rechargeable non-aqueous Li-O2 batteries and primary alkaline Al-air batteries. Chem. Eng. J. 2019, 378, 122247. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, B.; Hong, J.; Choi, H.; Jang, H.; Hou, B.; Pak, S.; Lee, J.; Lee, S.; Morris, S.M.; et al. Hierarchically assembled tubular shell-core-shell heterostructure of hybrid transition metal chalcogenides for high-performance supercapacitors with ultrahigh cyclability. Nano Energy 2017, 37, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Nagaraju, G.; Sekhar, S.C.; Ramulu, B.; Yu, J.S. High-performance hybrid supercapacitors based on MOF-derived hollow ternary chalcogenides. Energy Storage Mater. 2021, 35, 750–760. [Google Scholar] [CrossRef]
- Kumbhar, V.S.; Lokhande, A.C.; Gaikwad, N.S.; Lokhande, C.D. One-step chemical synthesis of samarium telluride thin films and their supercapacitive properties. Chem. Phys. Lett. 2016, 645, 112–117. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Z.; Liu, J.; Wei, G.; Du, J.; Li, Y.; An, C.; Zhang, J. Synthesis of Few-layer 1T’-MoTe2 Ultrathin Nanosheets for High- performance Pseudocapacitor. J. Mater. Chem. A 2017, 5, 1035–1042. [Google Scholar] [CrossRef]
- Xiao, M.; Su, Y.; Zhao, M.; Du, B. Synthesis of CoTe nanowires: A new electrode material for supercapacitor with high-stability and high-performance. Nanotechnology 2019, 31, 055706. [Google Scholar] [CrossRef]
- Bhat, K.S.; Shenoy, S.; Nagaraja, H.S.; Sridharan, K. Porous cobalt chalcogenide nanostructures as high performance pseudo-capacitor electrodes. Electrochim. Acta 2017, 248, 188–196. [Google Scholar] [CrossRef]
- Shivasharma, T.K.; Bommineedi, L.K.; Sankapal, B.R. Pseudocapacitive nanostructured silver selenide thin film through room temperature chemical route: First approach towards supercapacitive application. Inorg. Chem. Commun. 2022, 135, 109083. [Google Scholar] [CrossRef]
- Hou, L.; Shi, Y.; Wu, C.; Zhang, Y.; Ma, Y.; Sun, X.; Sun, J.; Zhang, X.; Yuan, C. Monodisperse Metallic NiCoSe2 Hollow Sub-Microspheres: Formation Process, Intrinsic Charge-Storage Mechanism, and Appealing Pseudocapacitance as Highly Conductive Electrode for Electrochemical Supercapacitors. Adv. Funct. Mater. 2018, 28, 1705921. [Google Scholar] [CrossRef]
- Banerjee, A.; Bhatnagar, S.; Upadhyay, K.K.; Yadav, P.; Ogale, S. Hollow Co0.85Se nanowire array on carbon fiber paper for high rate pseudocapacitor. ACS Appl. Mater. Interfaces 2014, 6, 18844–18852. [Google Scholar] [CrossRef]
- Song, Y.; Li, H.; Yang, L.; Bai, D.; Zhang, F.; Xu, S. Solid-Solution Sulfides Derived from Tunable Layered Double Hydroxide Precursors/Graphene Aerogel for Pseudocapacitors and Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 42742–42750. [Google Scholar] [CrossRef]
- Shaikh, S.; Rabinal, M.K. Facile one-step growth of nickel sulfide nano-architecture as binder less electrodes for efficient supercapacitor applications. Mater. Sci. Semicond. Process. 2022, 142, 106524. [Google Scholar] [CrossRef]
- Hong, W.; Wang, J.; Li, Z.; Yang, S. Fabrication of Co3O4@Co-Ni sulfides core/shell nanowire arrays as binder-free electrode for electrochemical energy storage. Energy 2015, 93, 435–441. [Google Scholar] [CrossRef]
- Li, S.; He, W.; Liu, B.; Cui, J.; Wang, X.; Peng, D.; Liu, B.; Qu, B. One-step construction of three-dimensional nickel sulfide-embedded carbon matrix for sodium-ion batteries and hybrid capacitors. Energy Storage Mater. 2020, 25, 636–643. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Atchudan, R.; Karthik, N.; Ganesh, K.; Xiong, D.; Lee, Y.R. A novel binder-free electro-synthesis of hierarchical nickel sulfide nanostructures on nickel foam as a battery-type electrode for hybrid-capacitors. Fuel 2020, 276, 118077. [Google Scholar] [CrossRef]
- Kaliaraj, G.S.; Ramadoss, A. Nickel-zinc sulfide nanocomposite thin film as an efficient cathode material for high-performance hybrid supercapacitors. Mater. Sci. Semicond. Process. 2020, 105, 104709. [Google Scholar] [CrossRef]
- Meyer, E.; Bede, A.; Mutukwa, D.; Taziwa, R.; Zingwe, N. Optimization, and analysis of carbon supported VS2 nanocomposites as potential electrodes in supercapacitors. J. Energy Storage 2020, 27, 101074. [Google Scholar] [CrossRef]
- Fayed, M.G.; Attia, S.Y.; Barakat, Y.F.; El-shereafy, E.E.; Rashad, M.M.; Mohamed, S.G. Carbon and nitrogen co-doped MoS2 nanoflakes as an electrode material for lithium-ion batteries and supercapacitors. Sustain. Mater. Technol. 2021, 29, e00306. [Google Scholar] [CrossRef]
- Liu, B.; Ma, Y.; Wang, J.; Kang, X.; He, L.; Lei, L.; Ran, F. Fabrication of nickel cobalt bimetallic sulfide doped graphite carbon nanohybrids as electrode materials for supercapacitors. Diam. Relat. Mater. 2022, 124, 108955. [Google Scholar] [CrossRef]
- Chen, W.; Xia, C.; Alshareef, H.N. One-step electrodeposited nickel cobalt sulfide nanosheet arrays for high-performance asymmetric supercapacitors. ACS Nano 2014, 8, 9531–9541. [Google Scholar] [CrossRef]
- Chebrolu, V.T.; Balakrishnan, B.; Chinnadurai, D.; Kim, H.J. Selective Growth of Zn–Co–Se Nanostructures on Various Conductive Substrates for Asymmetric Flexible Hybrid Supercapacitor with Enhanced Performance. Adv. Mater. Technol. 2020, 5, 1900873. [Google Scholar] [CrossRef]
- Li, S.; Wang, S.; Wang, J. Optimal surface/diffusion-controlled kinetics of bimetallic selenide nanotubes for hybrid supercapacitors. J. Colloid Interface Sci. 2022, 617, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Li, Z.; Zhao, G.; Zhang, H.; Aslan, H.; Li, J.; Sun, F.; Zhu, L.; Du, B.; Yang, B.; et al. Porous Ultrathin NiSe Nanosheet Networks on Nickel Foam for High-Performance Hybrid Supercapacitors. ChemSusChem 2020, 13, 260–266. [Google Scholar] [CrossRef] [Green Version]
- Qu, Z.; Li, J.; Guo, M.; Zhao, L.; Duan, L.; Ding, S. Design tremella-like Ni-Co selenide with wonderful electrochemical performances as supercapacitor cathode material. Electrochim. Acta 2021, 393, 139049. [Google Scholar] [CrossRef]
- Bhol, P.; Swain, S.; Jena, S.; Bhatte, K.; Rout, C.S.; Saxena, M.; Jadhav, A.H.; Samal, A.K. Co-Decorated Tellurium Nanotubes for Energy Storage Applications. ACS Appl. Nano Mater. 2021, 9, 9008–9021. [Google Scholar] [CrossRef]
- Manikandan, M.; Subramani, K.; Dhanuskodi, S.; Sathish, M. One-Pot Hydrothermal Synthesis of Nickel Cobalt Telluride Nanorods for Hybrid Energy Storage Systems. ACS Energy Fuels 2021, 35, 12527–12537. [Google Scholar] [CrossRef]
- Li, Y.; Gong, J.; Xing, X.; Du, J.; Xu, P.; Cheng, K.; Ye, K.; Zhu, K.; Yan, J.; Cao, D.; et al. High-performance all-solid-state supercapacitor with binder-free binary transition metal sulfide array as cathode. Int. J. Energy Res. 2021, 45, 5517–5526. [Google Scholar] [CrossRef]
- Mu, C.; Sun, X.; Chang, Y.; Wen, F.; Nie, A.; Wang, B.; Xiang, J.; Zhai, K.; Xue, T.; Liu, Z. High-performance flexible all-solid-state micro-supercapacitors based on two-dimensional InSe nanosheets. J. Power Source 2021, 482, 228987. [Google Scholar] [CrossRef]
- Hu, X.; Shao, W.; Hang, X.; Xiaodong, Z.; Zhu, W.; Xie, Y. Superior Electrical Conductivity in Hydrogenated Layered Ternary Chalcogenide Nanosheets for Flexible All-Solid-State Supercapacitors. Angew. Chem. 2016, 128, 5827–5832. [Google Scholar] [CrossRef]
- Yan, S.X.; Luo, S.H.; Wang, Q.; Zhang, Y.H.; Liu, X. Rational design of hierarchically sulfide and MXene-reinforced porous carbon nanofibers as advanced electrode for high energy density flexible supercapacitors. Compos. Part B Eng. 2021, 224, 109246. [Google Scholar] [CrossRef]
- Harish, K.; Balamurugan, J.; Nguyen, T.T.; Kim, N.H.; Lee, J.H. Advanced interfacial engineering of oxygen-enriched FexSn1−xOSe nanostructures for efficient overall water splitting and flexible zinc-air batteries. Appl. Catal. B Environ. 2022, 305, 120924. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, X.; Cao, Y.; Li, L.; Zhong, C.; Deng, Y.; Han, X.; Hu, W. Developing Indium-based Ternary Spinel Selenides for Efficient Solid Flexible Zn-Air Batteries and Water Splitting. ACS Appl. Mater. Interfaces 2020, 12, 8115–8123. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Luo, C.; Zhang, H.; Yang, L.; Huang, N.; Zhang, M.; Yu, H.; Sun, P.; Wang, L.; Lv, X.; et al. In-Situ derived Co1-xS@nitrogen-doped carbon nanoneedle array as a bifunctional electrocatalyst for flexible Zinc-air battery. J. Electroanal. Chem. 2021, 900, 115711. [Google Scholar] [CrossRef]
- Feng, T.; Zhao, T.; Zhu, S.; Zhang, N.; Wei, Z.; Wang, K.; Li, L.; Wu, F.; Chen, R. Anion-Doped Cobalt Selenide with Porous Architecture for High-Rate and Flexible Lithium-Sulfur Batteries. Small Methods 2021, 5, 2100649. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Miao, H.; Chen, B.; Hu, R.; Xia, L.; Zhang, C.; Wang, F.; Zhang, H.; Yuan, J. Self-supported metal sulfide electrode for flexible quasi-solid-state zinc-air batteries. J. Alloys Compd. 2021, 878, 160434. [Google Scholar] [CrossRef]
- Xie, M.; Zhou, Y.; Wang, Z.; Wang, H.; Yan, D.; Han, B.; Zhang, S.; Deng, C. Heterostructured hollow fibers stitched together from nickel sulfides capped S,N-codoped carbon nanotubes as a trifunctional electrode for flexible hybrid Zn batteries. Chem. Eng. J. 2022, 431, 133920. [Google Scholar] [CrossRef]
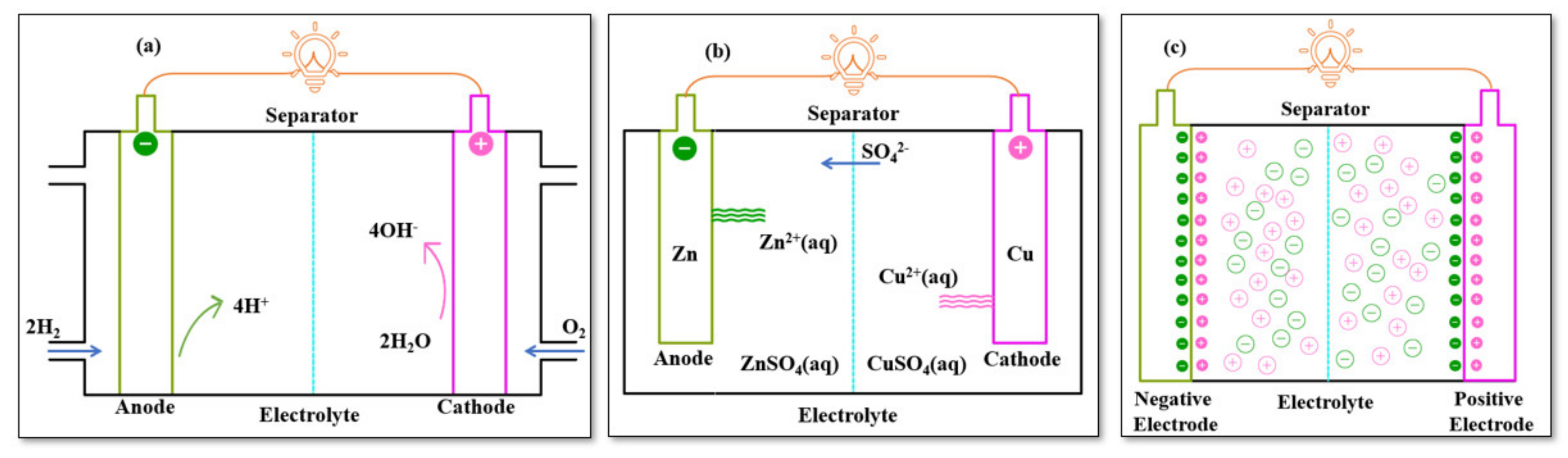
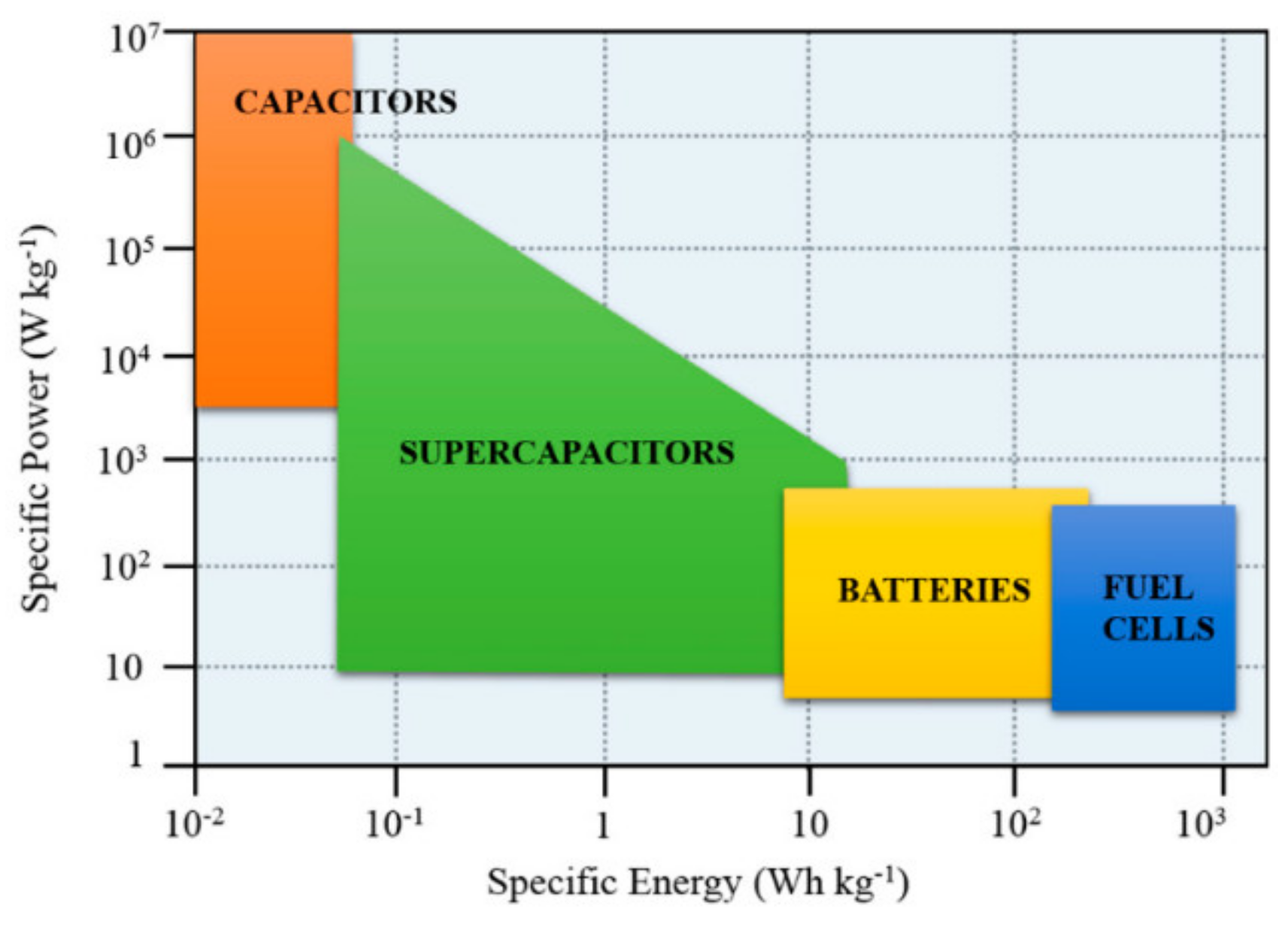
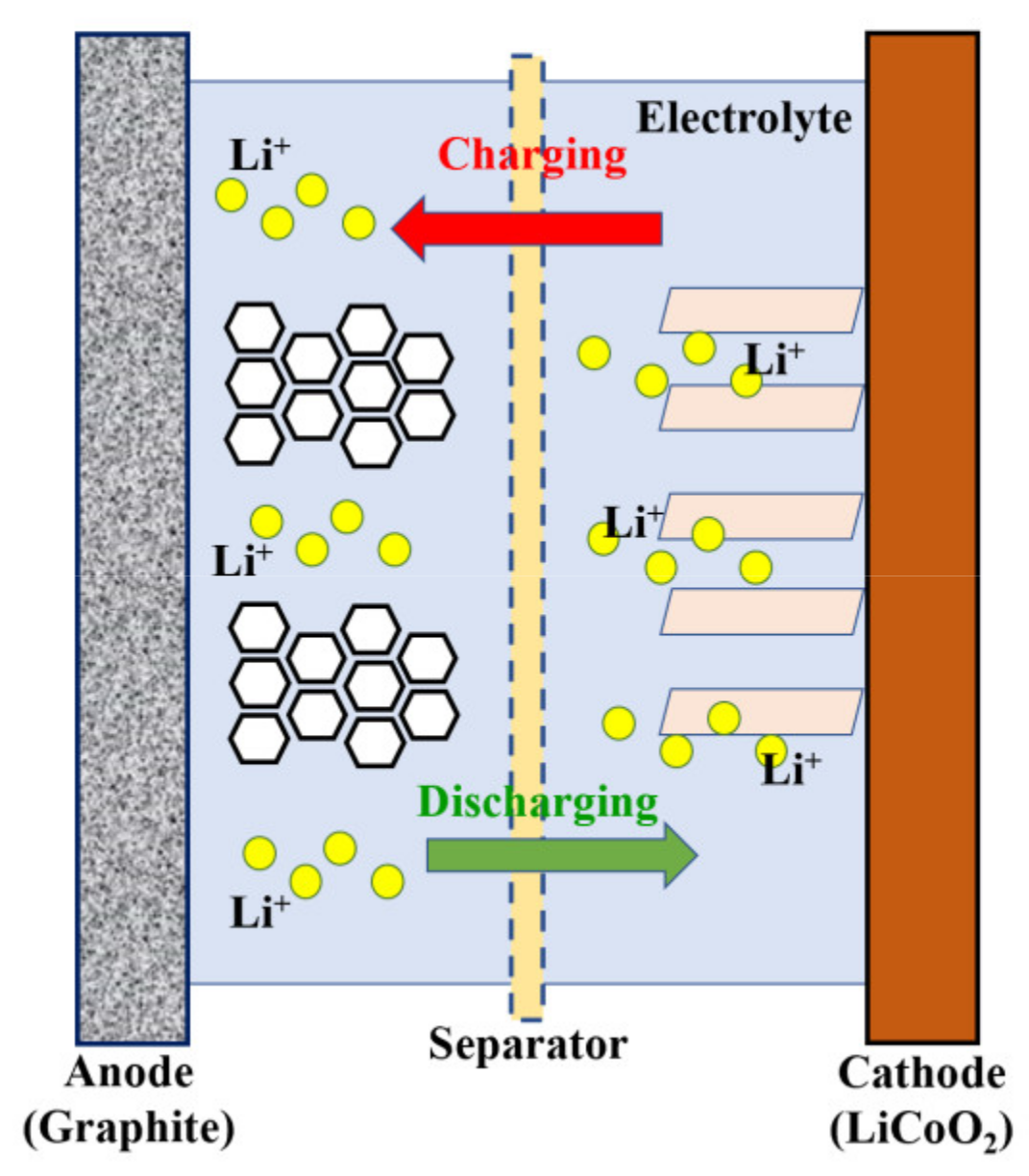
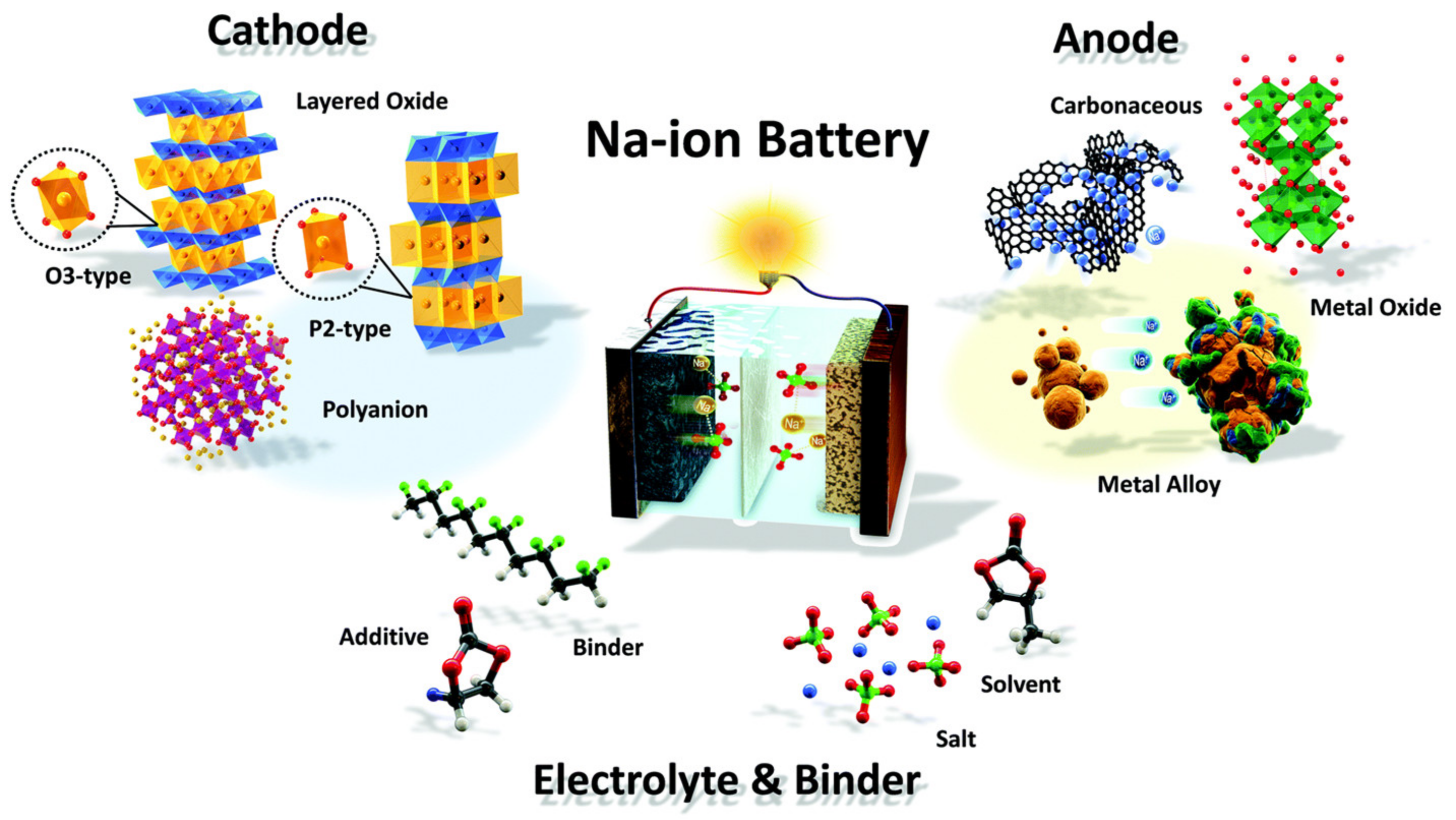
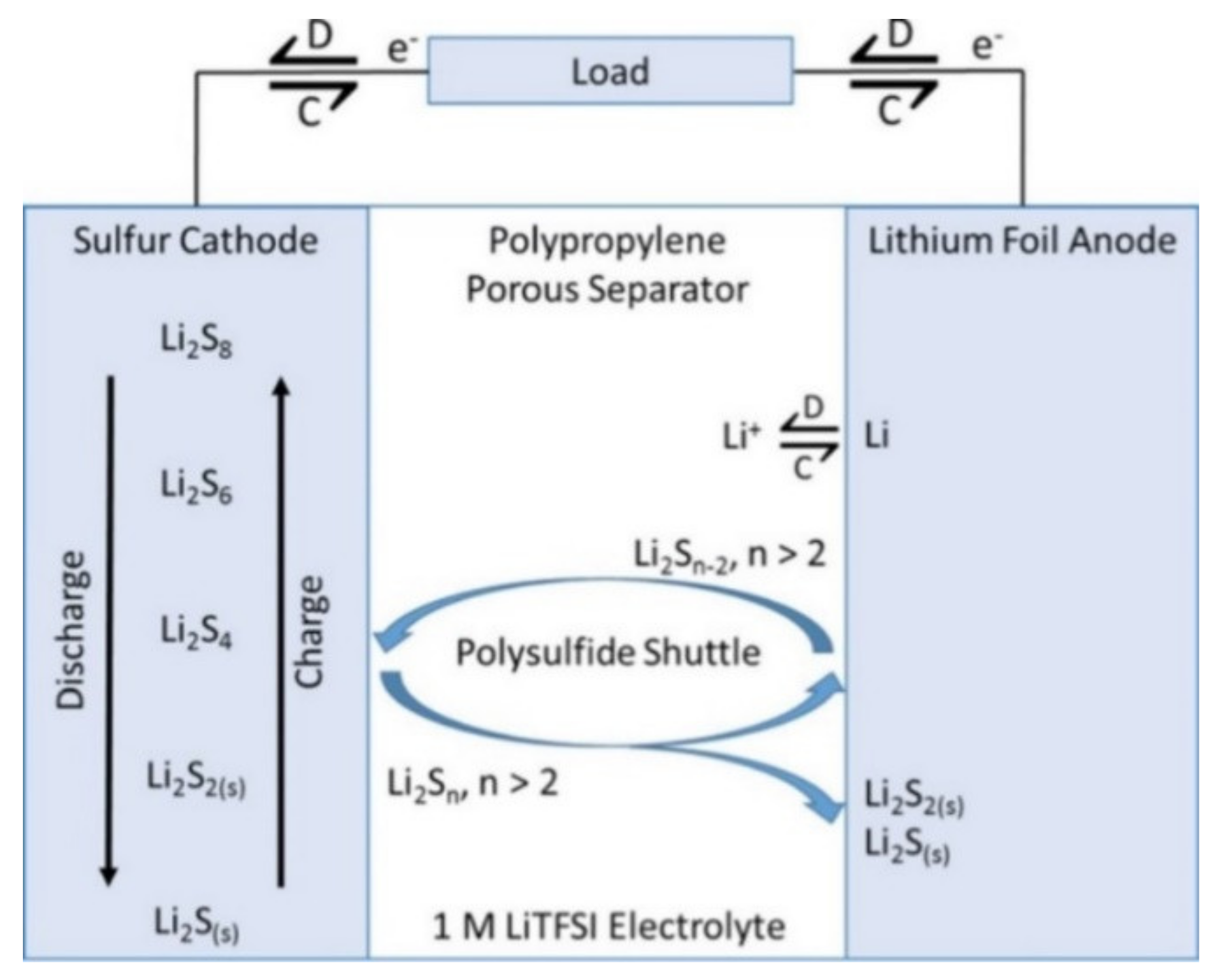
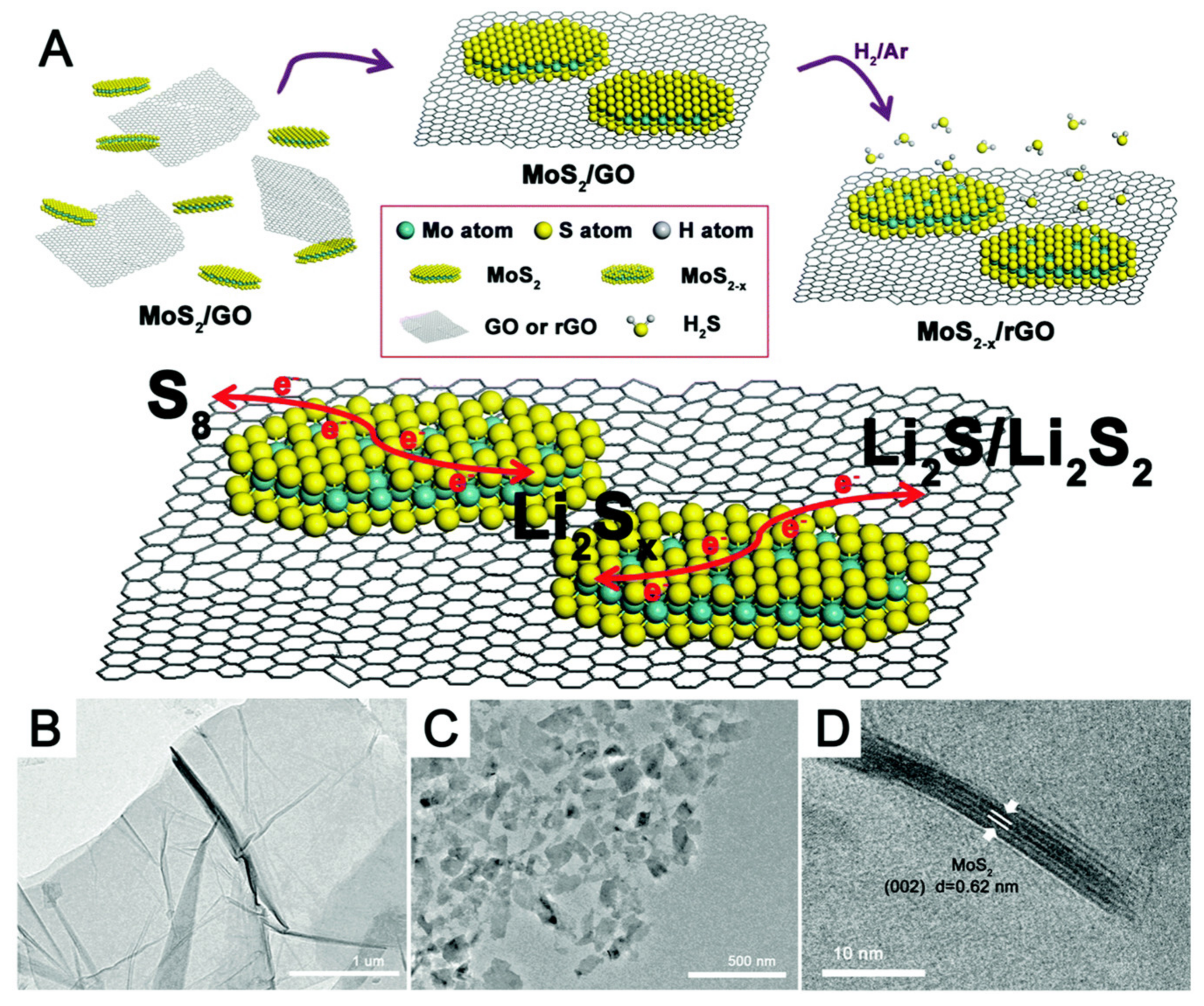
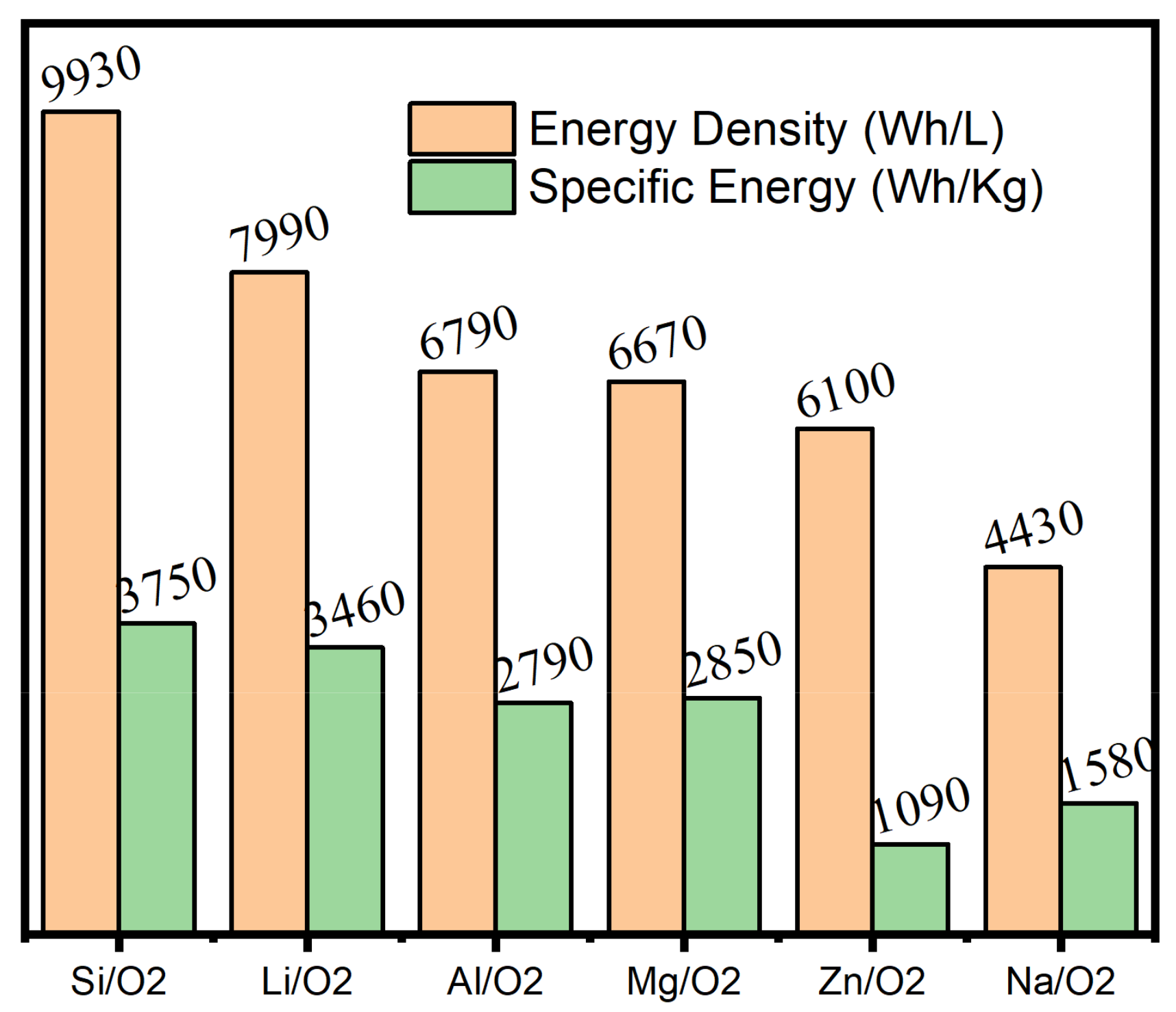
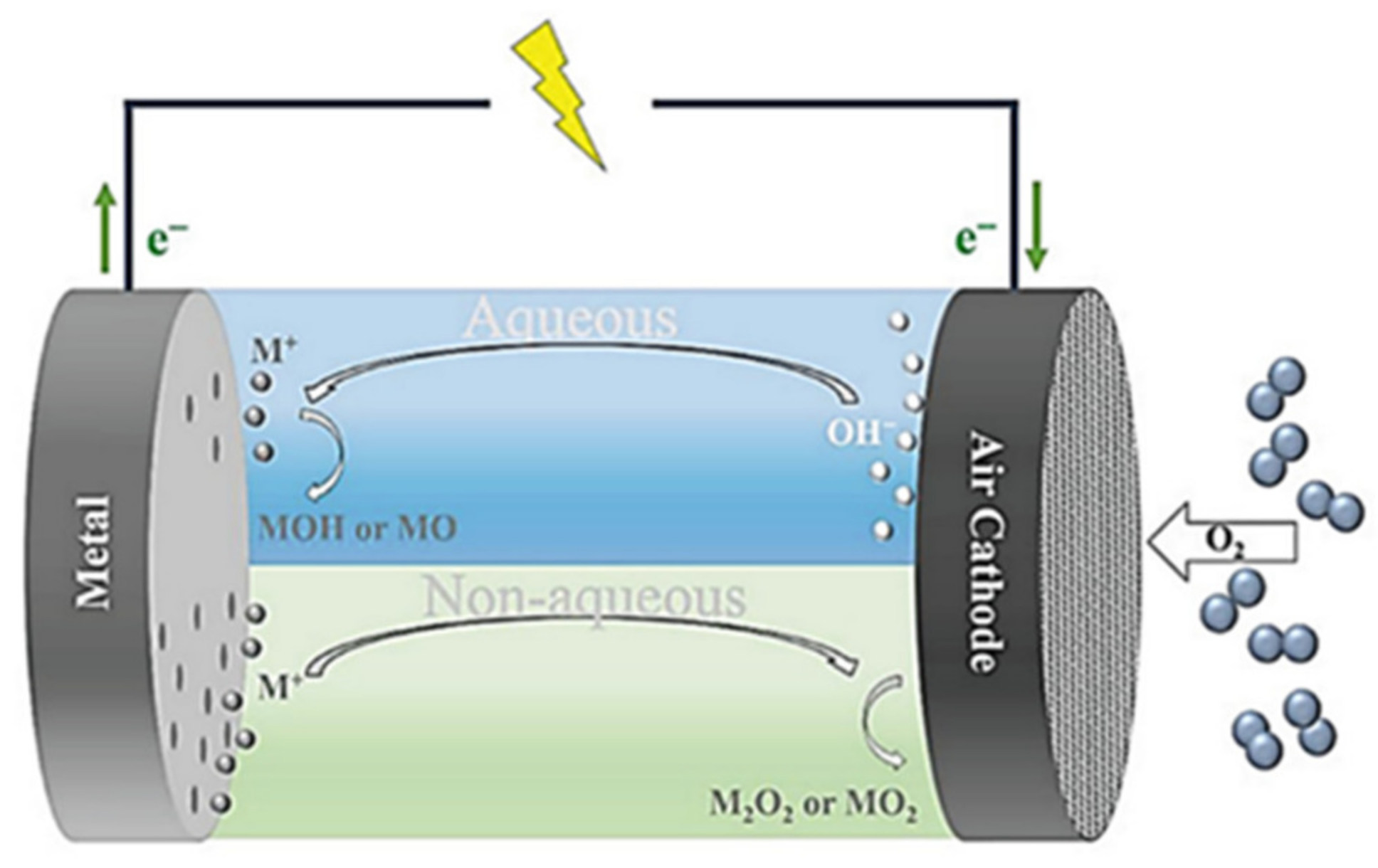
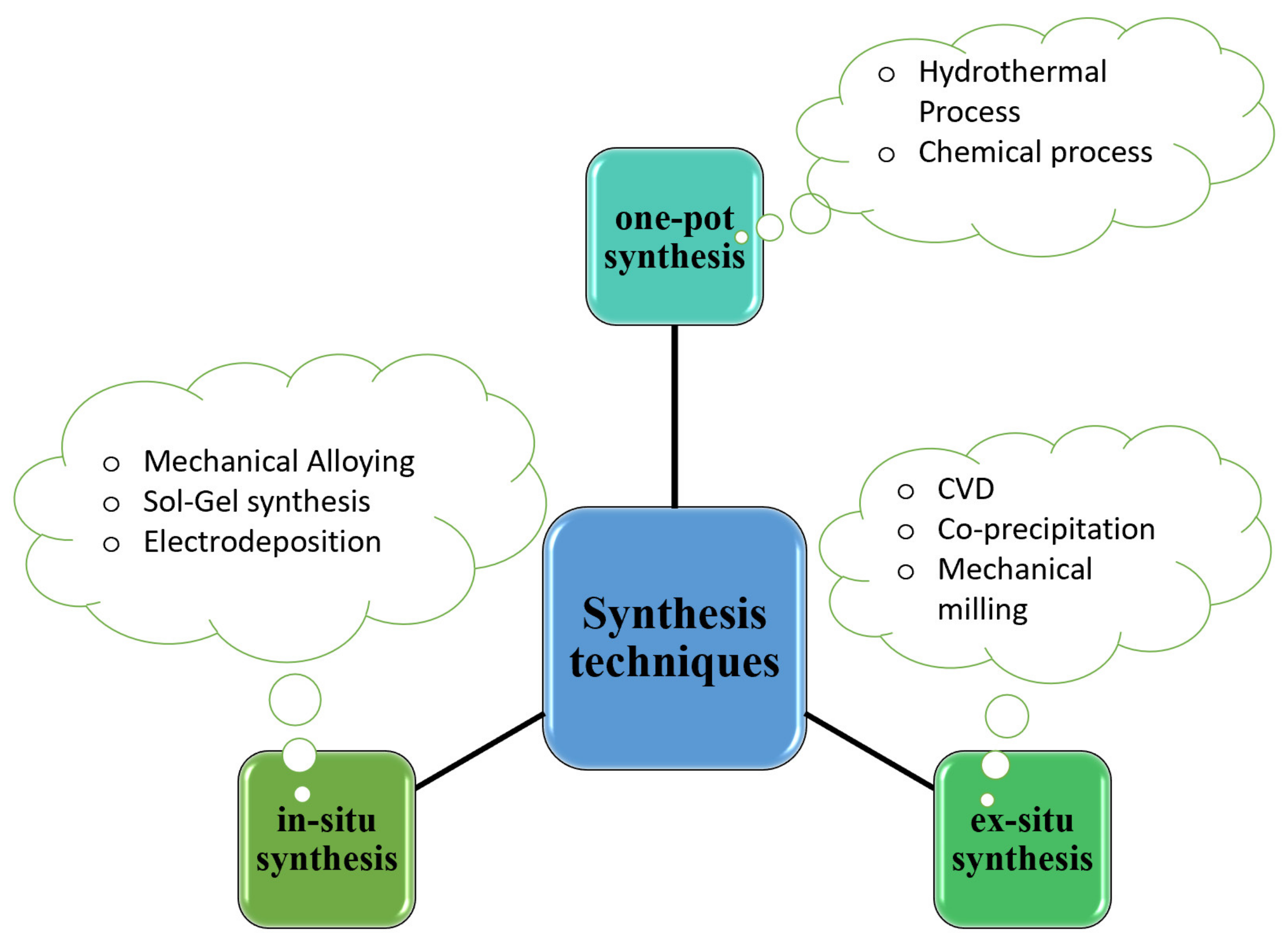
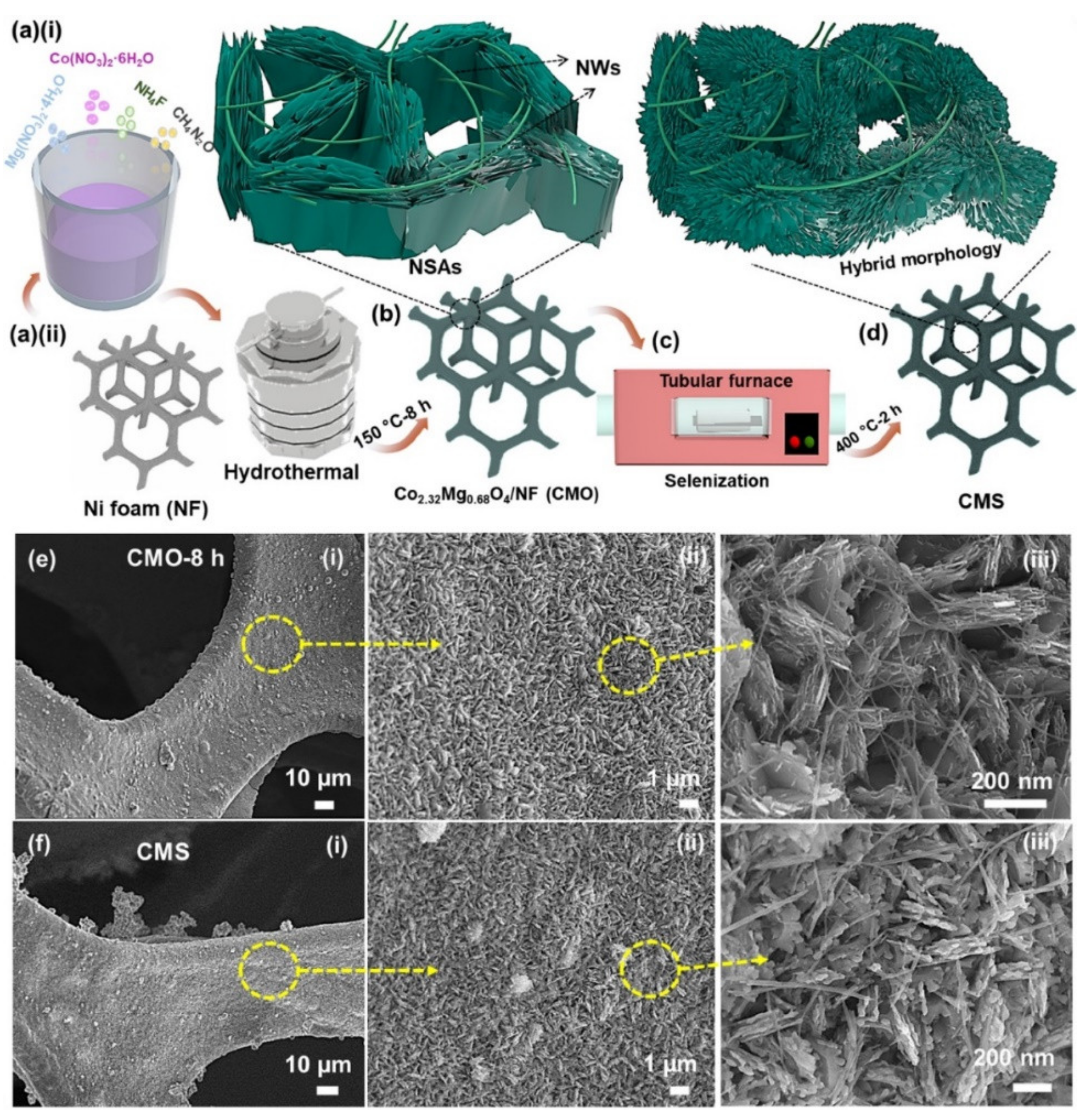


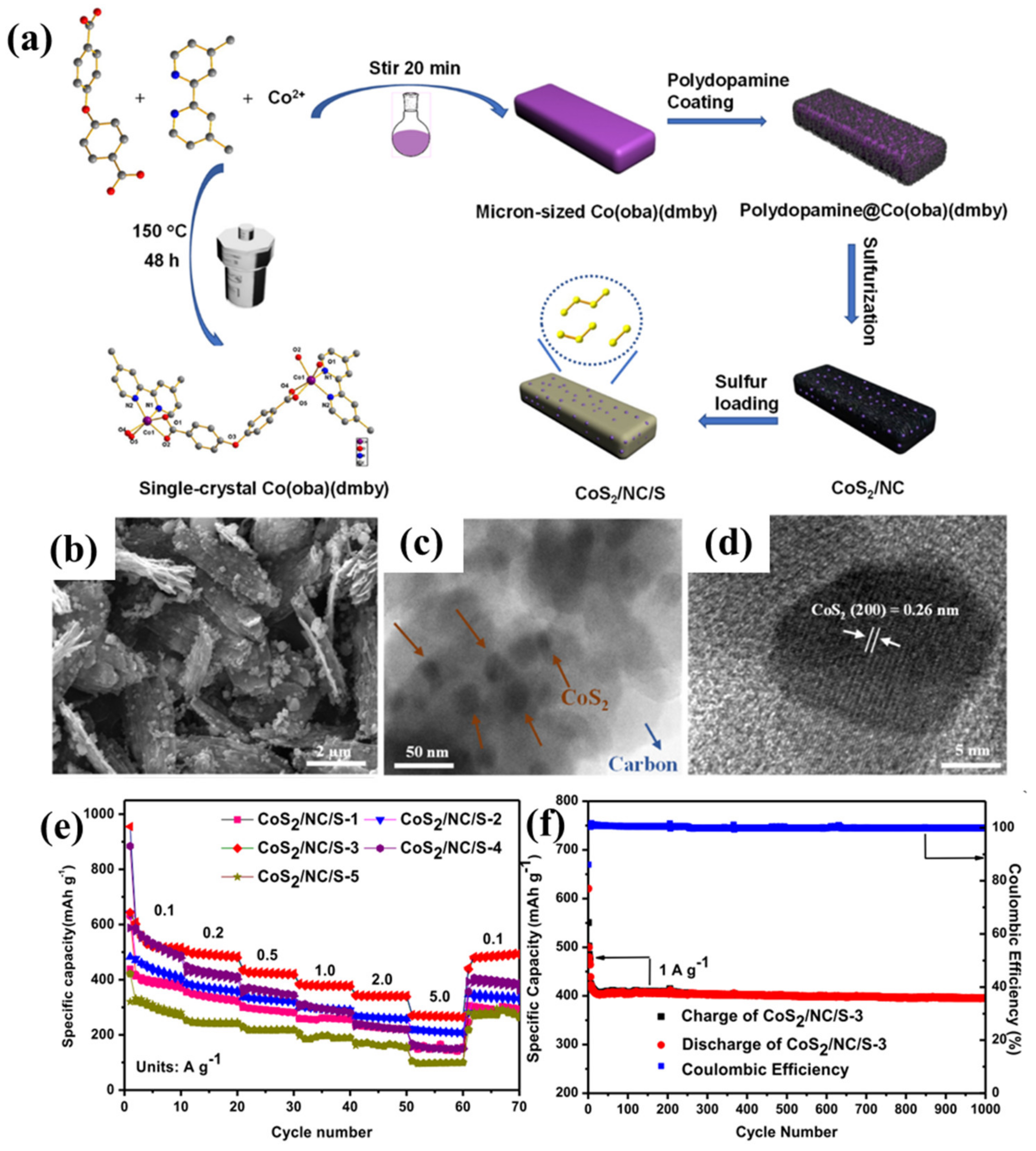
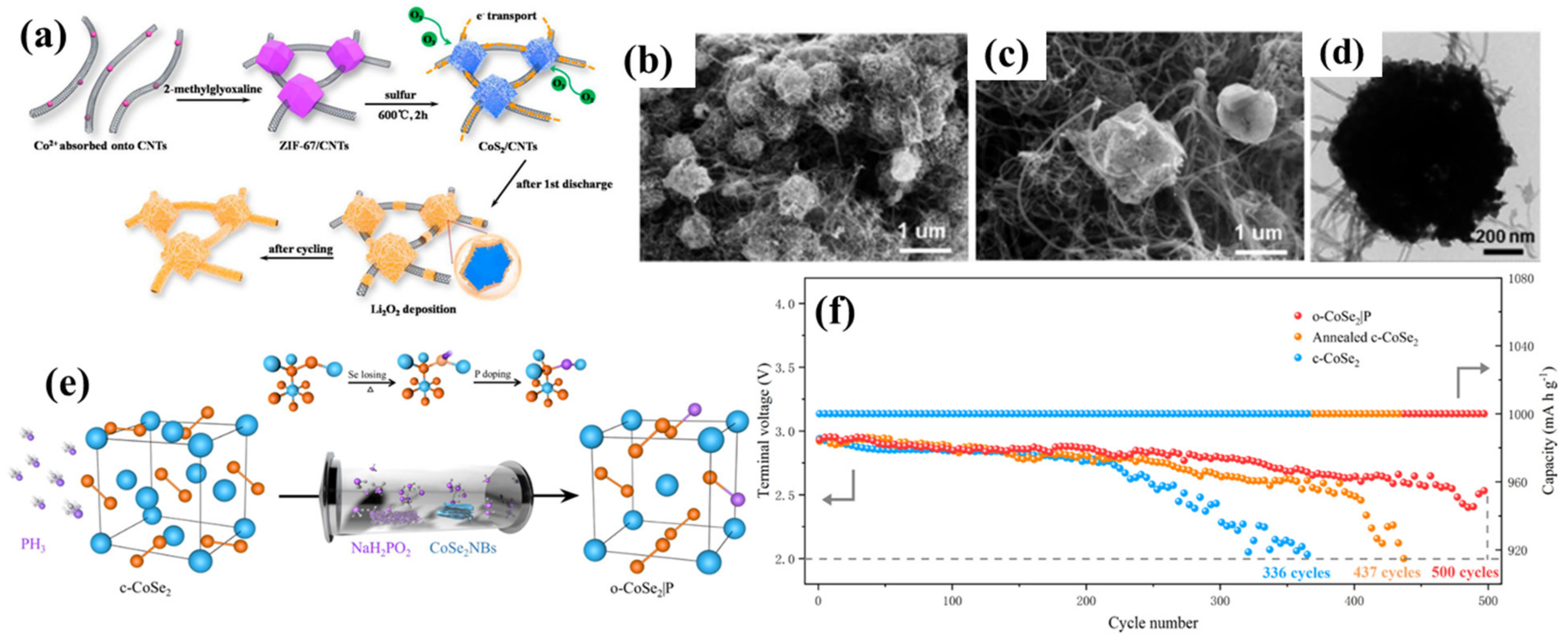





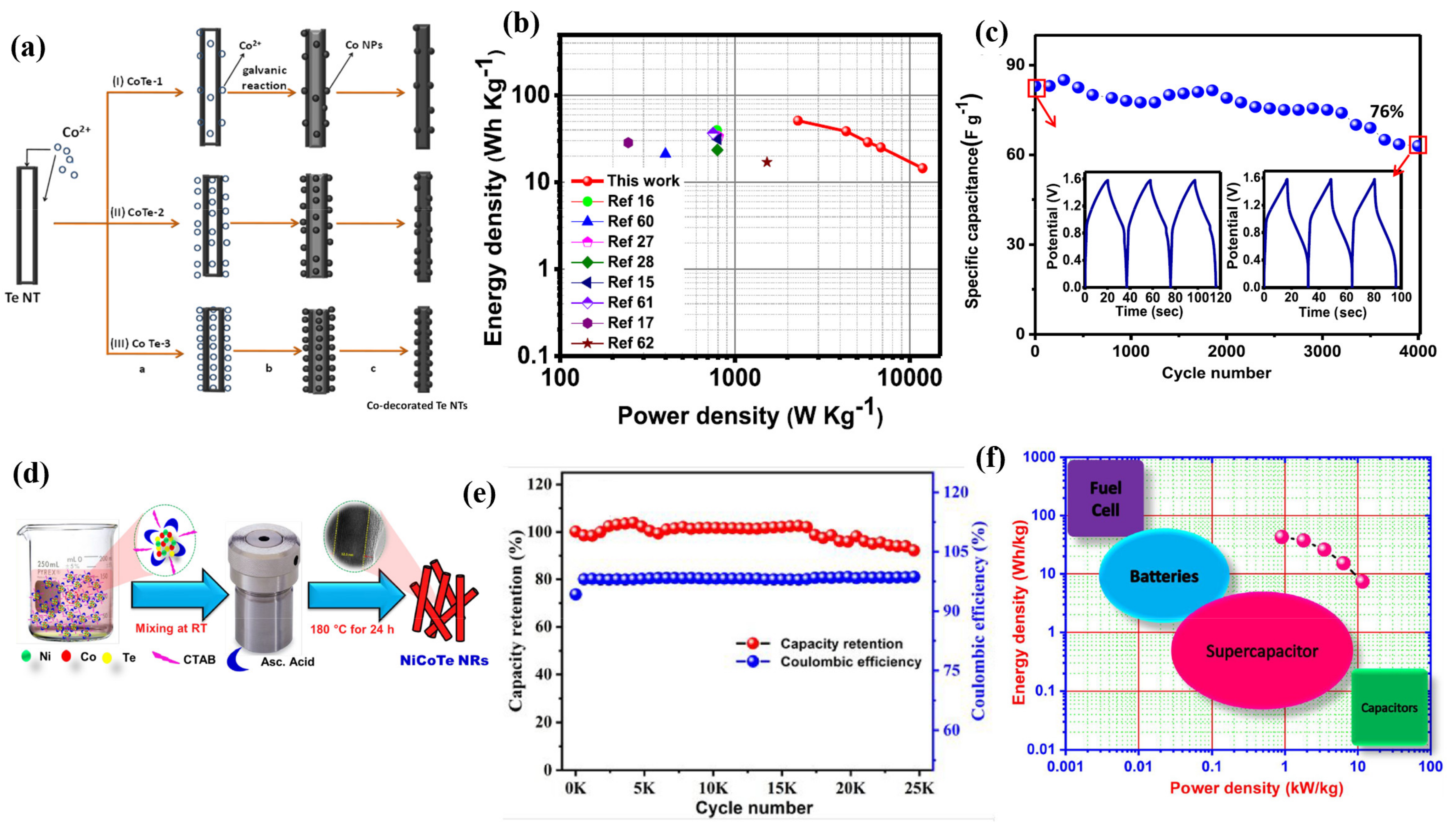

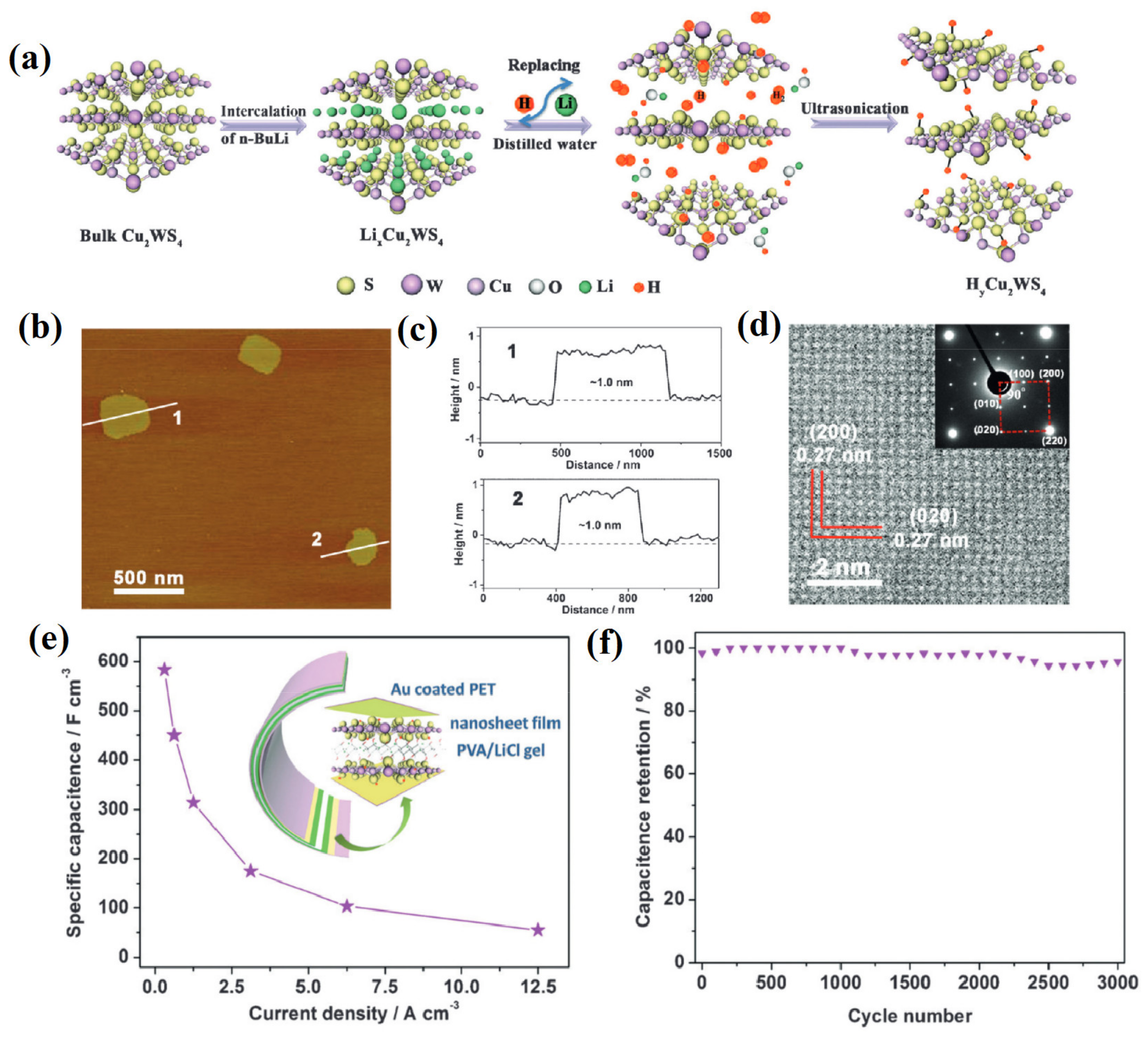

| Material | Synthesis | Potential Window (V) | Electrolyte (M) | Scan Rate (mV/s) | Specific Capacitance (F/g/C/cm2/mF cm−2/Cg−1) | Current Density (A/g/mA/cm2/mAcm−2) | % Retention Capacity/Cycles | Energy Density (Wh/kg mWh/cm3/μWhcm−2) | Power Density (W/kg/mW/cm3/mWcm−2) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| NiS/rGO hybrid | Microwave-assisted hydrothermal | 0.46 | 2 M KOH | 5–50 | 1188.9 | 5 | 68.1 | 7.1 | 1836 | [68] |
| NiS nanostructures | Microwave-hydrothermal | 0.46 | 2 M KOH | 100 | 119 | 1 | 100/2000 | 16.5 | 250 | [69] |
| CuFeS2 nanomaterial | Solvothermal | 0.55 | 6 M KOH | 1–50 | 589 | 4 | 82/2000 | - | - | [70] |
| NiSe2@rGO nanocomposite | Hydrothermal | 1.7 | 2 M KOH | 2-50 | 467 | 93/6500 | 34.3 | - | [71] | |
| MoSSe/rGO composite | Solvothermal | 0.35 | 3 M KOH | 5–50 | 373 | 1 | 89.5/4600 | - | - | [72] |
| MoS2/Ni3S2 | Hydrothermal | 0.58 | 1 M KOH | 10–100 | 1.033 | 1 | 62.5/10,000 | 35.93 | 1064.76 | [73] |
| NiSe2@Fe3Se4 | chemical bath deposition | 1.7 | 6 M KOH | 2–50 | 147 | 5 | 92/10,000 | 52 | 398 | [74] |
| NiGa2S4 nanosheets | Hydrothermal | 1.1 | 6 M KOH | 10–100 | 488 | 0.5 | 109/20,000 | 40 | 5.5 | [75] |
| CF@CoS | tube furnace | 0.7 | 2 M KOH | 10–100 | 3576.0 | 5.0 | 80/4000 | 149.4 | 4.3 | [76] |
| copper sulfide | Chemical reaction | 0.8 | 2 M KOH | 1–50 | 1173 | 2 | 99/1500 | 301.4 | 1400 | [77] |
| LaDySn2O7-SnSe nanocomposite | Hydrothermal | 0.2–0.8 | 0.1 M KOH | 10–100 | 1780 | 0.3 | 127/2000 | 67.46 | 6157.411 | [78] |
| Zinc cobalt sulfide nano hybrid | hydrothermal | 1.8 V | 1MTEABF4acetonitrile (ACN) | 10 | 785 | 2 | 106/10,000 | 49.6 | 1799.6 | [79] |
| NiCo2Se4/RGO (NCSG) | two-step hydrothermal | 0.4 | 6 M KOH | 30 | 1776 | 2 | 93.5/5000 | 66.2 | 1500 | [80] |
| MoS2/graphene oxide/meso-MnO2 nanocomposite | two stage ultra-sonication | - | 1 M H2SO4 and KI | 1–500 | 980 | 0.5 | - | - | - | [65] |
| MoS2@3DG//NPC | solution-based process | 0.0–4.0 | 1 M LiPF6 | 0.5 | 88.3 | 1.0 | 78/2000 | 156 | 197 | [81] |
| MoS2-C-RGO//PDPC | hydrothermal | 0.01–3.0 | 1 M LiPF6 | 0.1 | 759 | 2 | 80/10,000 | 188 | 200 | [82] |
| MoS2/CP//AC | ball-milling and pyrolysis | 1.5–4.5 | 1 M LiPF6 | 10 | 530 | 1 | 79.61/1000 | 87.74 | 200 | [83] |
| (P-MoS2/PANI/rGO HNSs)//rGO | hydrothermal | 0.3–1.1 | 1 M Li2SO4 | 5–20 | 221.9 | 5 | 93.5/30,000 | 56 | 0.45 | [84] |
| MoS2 @C//AC | hydrothermal | 0.0–4.5 | 1 M LiPF6 | 1–20 | 1083.4 | 1 | 72.12/3000 | 119.68 | 11.25 | [85] |
| MoS2-C//PDPC | hydrothermal | 0.4–3.0 | 1 M NaClO4 | 5 | 200 | 2 | 77.3/10,000 | 111.4 | 12000 | [86] |
| TiO2/MoS2 @NC//AC | Solid phase reaction | 2.0–4.5 | 1 M NaClO4 | 0.2 | 66.3 | 2 | 70/3000 | 148 | 200 | [87] |
| VSe2/rGO//AC | hydrothermal | 1–4 | 1 M NaClO4 | 0.5 | 365 | 1 | 68/1000 | 106 | 125 | [88] |
| TiS2 @Cpvp//AC | in situ conversion | 2.0–4.0 | 1 M NaPF6 | 0.2–1.2 | 340 | 2 | 87/5000 | 101.7 | 200 | [89] |
| MoS2/CoS2-RGO//Na3V2(PO4)3/C | facile hydrothermal | 0.01–4.5 | 1 M NaClO4 | 0.1 | 596.3 | 2 | 92.3/1000 | 152.98 | 562.5 | [90] |
| CNF@VS2//CNF@GS | Hydrothermal | 0.01–3.0 | 1 M NaClO4 | 0.5 | 52.3 | 5 | 82/4500 | 166 | 0.4 | [91] |
| MoS2/SnS2-RGO//CC | - | 0.01–3.00 | 1 M NaClO4 | - | 550.3 | 2 | 100/3000 | 113.9 | 561.25 | [92] |
| MoSe2/TiO2−x/graphene//AC | hydrothermal | 0.5–3.5 | 1M NaPF6 | - | 415.2 | 2 | 88/3000 | 109 | 100 | [93] |
| MoSe2/C//AC | hydrothermal | 0.01–3.0 | 0.8 M KPF6 | 0.1 | 320 | 1 | 81.58/1000 | 169 | 106 | [94] |
| WS2@NCNs//NCHS | Hydrothermal | 2.6–4.2 | 0.8 M KPF6 | 0.1 | 44 | 2 | 92.2/2000 | 103.4 | 235 | [95] |
| MoS2/graphene | L-cysteine-assisted hydrothermal process | −0.1–0 (vs. SCE) | 1 M Na2SO4 | 20 | 243 | 1 | 92.7/1000 | 73.5 | 19.8 | [96] |
| MoS2/CA (Carbon Aerogel) | Hydrothermal | −0.8–0.2 (vs. Hg/HgO) | 1 M Na2SO4 | 2–50 | 260 | 1 | 92.4/1500 | - | - | [97] |
| MoS2/graphene aerogel | Hydrothermal | - | 1 M Na2SO4 | - | 268 | 0.5 | 93/1000 | - | - | [98] |
| 1T-2H MoS2/GA (Graphene Aerogel) | Hydrothermal | −0.8–0.4 | 1 M H2SO4 | - | 416 | - | - | - | - | [99] |
| NiCo2S4/CS (Carbon Sponge) | Direct carbonization | 0–0.9 | 3 M KOH | 5–20 | 1093 | 0.5 | 90/500 | - | - | [100] |
| CoS2/rGO | Solvothermal | −0.3–0.65 | 6 M KOH | 5 | 331 | 0.5 | 97/2000 | 30 | 4750 | [101] |
| CuS/rGO | Solvothermal | −0.4–0.6 | 6 M KOH | 50 | 2317.8 | 1 | 96.2/1200 | - | - | [102] |
| CuS/rGO | Solvothermal | 0–0.5 | 6 M KOH | 10 | 906 | 1 | 89/5000 | 105.5 | 2.5 | [103] |
| CuS/rGO | Solvothermal | −1–−0.1 | 2 M KOH | 5–100 | 368.3 | 1 | 88.4/1000 | - | - | [104] |
| CuS/rGO | Hydrothermal | 0–0.5 | 3 M KOH | 5–80 | 1604 | 2 | 97/5000 | 21 | 315 | [105] |
| NiCo2.1Se3.3 NSs/3D G/NF | CVD + Hydrothermal | 0–0.6 | 6 M KOH | 3–20 | 742.4 | 1 | 83.8/1000 | - | - | [106] |
| NiCo2Se4-rGO | Hydrothermal | −0.2–0.5 | 1 M KOH | 10–80 | 2038.55 | 1 | 90/1000 | 67.01 | 903.61 | [107] |
| NiCo2Se4/rGO | Hydrothermal | −1–0 | 6 M KOH | 30 | 1776 | 2 | 93.5/5000 | 66.2 | 1500 | [80] |
| NiCo2Se4/rGO | Hydrothermal | −0.1–0.4 | 6 M KOH | 30 | 139 | 2 | 87.27/5000 | 37.83 | 1433.55 | [108] |
| CoNi2S4/rGO | One step pyrolysis | 0–1.6 | 6 M KOH | 50 | - | - | 93.7/8000 | 54.8 | 798 | [109] |
| (Cu2NiSnS4)/rGO | Hydrothermal | 0–0.6 | 6 M KOH | - | 16.38 | 5 | 89.2/2000 | 5.68 | 98.8 | [110] |
| MoS2/MWCNT | Hydrothermal | −0.8–0.2 | 1 M Na2SO4 | 2–50 | 452.7 | 1 | 95.8/1000 | - | - | [111] |
| MoS2@CNTs/Ni core | PLD | −1–0 | 1 M Na2SO4 | 200 | 512 | 1 | 100/2000 | 25.5 | 44.2 | [112] |
| CuS/MWCNTs | Hydrothermal | −0.4–0.6 | 6 M KOH | - | 2831 | 1 | 90/600 | - | - | [113] |
| CuS/CNT | PVP assisted refluxing | 0–0.6 | 2 M KOH | 20 | 2204 | 10 | 89/10,000 | - | - | [114] |
| CuS/CNT | Hydrothermal | −0.2–0.6 (vs. Hg/HgO) | 2 M KOH | 1–50 | 566.4 | 1 | 94.5/5000 | 50.4 | - | [115] |
| NiS/CNT | CBD + SILAR | −0.2–0.6 | 1 M Na2S, 2 M S, and 0.1 M KCl in methanol: water (7:3) | 100 | 398.16 | 1 | 98.39/1000 | 35.39 | 145.45 | [116] |
| CNT/CoS | CBD + SILAR | −0.2–0.6 | 1 M Na2S, 2 M S, and 0.1 M KCl in methanol: water (7:3) | 100 | 288.43 | 1 | 95.3/1000 | 25.63 | 145.45 | [116] |
| CNT/CuS | CBD + SILAR | −0.2–0.6 | 1 M Na2S, 2 M S, and 0.1 M KCl in methanol:water (7:3) | 100 | 176.2 | 1 | 78.57/1000 | 15.66 | 145.45 | [116] |
| CNT/PbS | CBD + SILAR | −0.2–0.6 | 1 M Na2S, 2 M S, and 0.1 M KCl in methanol:water (7:3) | 100 | 101.94 | 1 | 69.49/1000 | 9.06 | 145.45 | [116] |
| NiS2/MWCNTs | Solid state method | −0.2–0.5 | 2 M KOH | 2–20 | 2054.28 | 2 | 99.99/10,000 | - | - | [117] |
| Ni3S2/CNT | Electrodeposition + ion exchange | −0.1–0.6 | 3 M KOH | 30 | 1643 | 1 | 91.5/2000 | - | - | [118] |
| VS2/MWCNT | SILAR | −0.6–0.2 | 2 M KCl | 100 | 182 | 2 | 95.9/1000 | 42 | 2.8 | [119] |
| TiS2/CNTs | Atomic layer deposition/sulfurization | −1.5–1.5 | 21 m LiTFSI/5% PVA | 1–50 | 195 | 1 | 95/10,000 | 60.9 | 1250 | [120] |
| NiS/graphene/CNT | Hydrothermal | −0.1–0.35 | 6 M KOH | 100 | 2377 | 1 | 68/1000 | 14 | 16 | [121] |
| MoS2@rGO-CNT | Micropatterning + pyrolysis | 0–1 | H2SO4/PVA gel | - | 13.7 | 1 | 96.6/10,000 | 5.6 | - | [122] |
| Hollow MoS2/Carbon | Sonogashira coupling | −0.5–0.5 | 0.5 M Na2SO4 | - | 418 | 0.5 | - | - | - | [123] |
| MnS/MoS2/Carbon | Calcination+ sulphuration | 0–1.55 | 2 M KOH | - | 1162 | 0.5 | 81/5000 | 31 | 388.3 | [124] |
| CoSe2/N-doped Carbon | Selenylation | 0–0.4 | 6 M KOH | 120.2 | 1 | 92/10,000 | 40.9 | 980 | [125] | |
| MoS2/NiCo2S4@Carbon | Self-templating | 0–0.5 | 6 M KOH | 10 | 250 | 2 | 90.1/10,000 | 36.46 | 73.75 | [126] |
| CoTe/MOF | Hydrothermal | 0–0.5 | 2 M KOH | 5–100 | 297.27 | 2 | 83.33/10,000 | 43.84 | 738.88 | [127] |
| Ni3S2/AC | Electrodeposition | 0–1.4 | PVA-KOH gel | 10 | 2797.43 | 1 | 83.47/10,000 | 55.32 | 1053.71 | [128] |
| ZnS/CuS/AC | SILAD | 0–1.2 | PVA-KOH gel | - | 1925 | 4 | 79/2000 | - | - | [129] |
| MoS2/GA | Hydrothermal | −0.3–0.7 | 1 M Na2SO4 | 50 | 268 | 0.5 | 93/1000 | - | - | [130] |
| Material | Synthesis | Voltage Range | Cycling Performance (mAh g−1/No. of Cycles | Maximum Rate Capability (mAh g−1/) | Current Density (A/g/mA g−1) | Ref. |
|---|---|---|---|---|---|---|
| MoS2@N-doped carbon nanowall @carbon cloth | Solvothermal | 0.01–3 | 619.2/100 | 235 | 2 | [131] |
| MoS2/carbon fiber @MoS2@C | Electrospinning | 0.01–2.5 | 332.6/1000 | 233.6 | 10 | [132] |
| MoS2@CF | Vacuum infiltration | 0.05–3 | 240/500 | 171 | 5 | [133] |
| MoS2/single-wall carbon nanotubes | Liquid Phase Exfoliation | 0.1–3 | 390/100 | 192 | 20 | [134] |
| N-doped hollow MoS2/C nanospheres | Hydrothermal | 0.01–3 | 128/5000 | 242 | 5 | [135] |
| MoS2/graphene | Ball milling/exfoliation | 0.01–2.7 | 421/250 | 201 | 50 | [136] |
| MoS2/S-doped graphene | Hydrothermal | 0.005–3 | 309/1000 | 264 | 5 | [137] |
| Fe3O4@MoS2 graphite paper | Modified hydrothermal | 0.01–3 | 388/300 | 231 | 3.2 | [138] |
| 1T MoS2/graphene tube | Solvothermal | 0.01–3 | 313/200 | 175 | 2 | [139] |
| MoS2/cotton-derived carbon fibers | hydrothermal | 0.01–3 | 323.1/150 | 355.6 | 2 | [140] |
| NBT/C@MoS2 NFs | electrospinning | 0.01–3.0 | 448.2/600 | 2000 | 200 | [141] |
| N-doped amorphous micron-sized carbon ribbons @MoS2 | Hydrothermal | 0.01–3 | 305/300 | 302 | 2 | [142] |
| Hollow flower-like MoS2@C-RGO | one-pot hydrothermal | 0.01–3 | 637/50 | 467 | 1 | [143] |
| amorphous MoS3@carbon nanotube | Facile acid precipitation method | 0.05–2.8 | 565/100 | 2350 | 20 | [144] |
| MoSe2/N, P-rGO | solvothermal | 0.01–3 | 378/1000 | 216 | 15 | [145] |
| MoSe2@MWCNT | one-step hydrothermal | 0.01–3 | 459/90 | 385 | 2 | [146] |
| VS4-rGO | in situ hydrothermal | 0.01–3 | 540/50 | 123 | 20 | [147] |
| VS4/rGO | hydrothermal | 0.01–2.2 | 241/50 | 219.9 | 0.5 | [148] |
| Co9S8@C nanospheres | facile hydrothermal | 0.01–3 | 305/1000 | 297 | 5 | [149] |
| CoS2@ multichannel carbon nanofibers | facile solvothermal | 0.4–2.9 | 315.7/1000 | 201.9 | 10 | [150] |
| CoS2eMWCNT | simple hydrothermal | 1–2.9 | 568/100 | 550.5 | 0.8 | [151] |
| Sb2S3 nanorods/C | facile hydrothermal | 0–2 | 570/100 | 337 | 2 | [152] |
| Sb2S3/S-doped graphene sheets | ultrasonication | 0.01–2.5 | 524.4/900 | 591.6 | 5 | [153] |
| ZnSeSb2S3@C | sulfurization reaction | 0.01–1.8 | 630/120 | 390.6 | 0.8 | [154] |
| Sb2Se3/C rods | self-assembly reaction | 0.01–2.5 | 485.2/100 | 311.5 | 2 | [155] |
| Sb2Se3 nanowire-based membrane | Hydrothermal/vacuum filtration | 0.01–2 | 296/50 | 153 | 1.6 | [156] |
| SnS/3D N-doped graphene | Facile, self-assembly method | 0.01–2.5 | 509.9/1000 | 404.8 | 6 | [157] |
| NiS2@CoS2@N-doped carbon coreshell nanocubes | co-precipitation method | 0.01–3 | 600/250 | 560 | 5 | [158] |
| SnS2/EDA-RGO | Hydrothermal | 0.01–3 | 480/1000 | 250 | 11.2 | [159] |
| MnS@CNF film | Electrospinning/thermal treatment | 0.01–3 | 220/200 | 107 | 0.5 | [160] |
| Material | Synthesis | Electrolyte | Current Density (mA cm−2/Ag−1) | Potential (mV/V) | Ref. |
|---|---|---|---|---|---|
| β-NiS | Facile Hydrothermal | 1 M NaOH | 40 | 598 | [161] |
| Ni3S2@NF | Hydrothermal | 1 M NaOH | 275 | 531 | [162] |
| Ni–Co9S8 | Hydrothermal | 1 M KOH | 75 | 478 | [163] |
| NiCo2S4/C | Pyrolysis/hydrothermal | 1 M KOH | 100 | 180 | [164] |
| NiS@C2-550 | Hydrothermal | 1 M KOH | 360 | ~577 | [165] |
| MoS2/Ni3S2/NiFe-LDH/NF | two-step hydrothermal | 1 M KOH | 280 | ~548 | [166] |
| NiSe2 | Hydrothermal | 1 M KOH NG | 105 | 502 | [167] |
| CoSe2 | one-step selenylation | 1.0 M KOH | 10 | 330 | [168] |
| Ni–Co–S | electrodeposition | 1.0 M KOH | 100 | 363 | [169] |
| (CoMn)Se2 | selenation | 1.0 M KOH | 10 | 270 | [170] |
| NiCoSe2 | electrodeposition | 1.0 M KOH | 10 | 256 | [171] |
| NiFeCoSex | electrodeposition | 1.0 M KOH | 10 | 150 | [172] |
| NiCoFe-PS | hydrothermal electrodeposition | 1.0 M KOH | 10 | 195 | [173] |
| CoOSeP/Co foil | thermal selenization | 1.0 M KOH | 10 | 155 | [174] |
| CoP/CN@MoS2 | solvothermal | 1.0 M KOH | 10 | 289 | [175] |
| Co9S8/Co3O4 | hydrothermal-sulfurization method | 1.0 M KOH | 20 | 260 | [176] |
| Co3O4/MoS2 | liquid-phase deposition | 1.0 M KOH | 20 | 230 | [177] |
| Ni3S2/Co(OH)2 | facile hydrothermal | 1.0 M KOH | 10 | 257 | [178] |
| Co9S8/NiCo LDH | two-step hydrothermal | 1.0 M KOH | 30 | 278 | [179] |
| NiFe/Co9S8 | chemical bath deposition | 1.0 M KOH | 10 | 219 | [180] |
| CoCO3/CoSe | two-step hydrothermal | 1.0 M KOH | 10 | 255 | [181] |
| CoO/CoSe2 | Hydrothermal | 1.0 M KOH | 10 | 510 | [182] |
| CoMoNiSeNF | one-pot hydrothermal | 1.0 M KOH | 10 | 405 | [183] |
| Co9S8 | hydrothermal | 1.0 M KOH | 10 | 1.66 | [184] |
| p-CoSe2/CC | electrodeposition | 1.0 M KOH | 10 | 1.62 | [185] |
| Co0.85Se | liquid-phase chemical conversion | 1.0 M KOH | 10 | 1.60 | [186] |
| Co0.75Ni0.25Se/NF | thermal conversion | 1.0 M KOH | 10 | 1.60 | [187] |
| CoMoNiSeNF | hydrothermal | 1.0 M KOH | 10 | 1.45 | [183] |
| CoeO/CoeSe/Cu | electrodeposition | 1.0 M KOH | 10 | 1.65 | [188] |
| Co9S8/NiCo LDH/NF | hydrothermal | 1.0 M KOH | 10 | 1.63 | [179] |
| Material | Structure | Preparation Method | Rate Performance [Capacity (mAh g−1) @ Current Density (Ag−1)] | Cycle Performance [Capacity (mAh g−1) @ Current Density (Ag−1) @ Cycles] | Ref. |
|---|---|---|---|---|---|
| LIB anode | |||||
| α-MnSe/CNF | Microspheres | Selenization | 1226.75@0.1 | 845.54@0.1@100 | [195] |
| α-MnSe/C | Microspheres | Selenization | 945.78@0.1 | 784.82@1@100 | [195] |
| Co9S8@CN | Nanoparticles | Direct annealing | 618.1@ 0.1 | 406.5@1@100 | [196] |
| SnS2@g-C3N4 | Porous nanosheets | Sintering | 1592.8@0.5 | 1305.7@0.5@600 | [197] |
| ZnS-QD@NC | Polymorph structures | Pyro-synthesis technique | 805@0.1 | 620@1@500 | [198] |
| CoSe/Co–NCNS | Layered structure | Selenization + annealing | 401@0.2 | 640@1@500 | [199] |
| Sb2Se3/C | Helical nanoparticles | Selenization | 548@0.25 | 545@0.25@1000 | [200] |
| SnSe2/graphene | Heterostructure nanosheets | Hydrothermal | 490.9@0.1 | 291.1@0.1@1500 | [201] |
| SnS/TiS2/GO | Misfit-layered heterostructure | Solvent-free hand-grinding | 1147@0.5 | 614@2.0@1000 | [202] |
| g-C3N4@WS2 | Hierarchical | Solvothermal | 1136.1@0.1 | 433.8@0.1@1000 | [203] |
| BiSbTe3/N-rGO | Nanocomposite | Solvothermal | 641.2@0.1 | 388.7@0.1@80 | [204] |
| In2Te3–TiO2–C | Hierarchical layered | High-energy ball-milling | ~450@10 | ~435@0.5@500 | [205] |
| SIB anode | |||||
| NiSe2@BCNNTs | 3D nanotubes | Pyrolysis | 787@0.1 | 382.4@2@2000 | [206] |
| SnS2/CNT@rGO | Hierarchical powdery | Solvothermal | 528@0.05 | 301@1@1000 | [207] |
| MoSe2/CN | 3D flower-like | Hydrothermal | 523.6@0.1 | 328.7@1@500 | [208] |
| FeS2@TiO2 | Core-shell structure | Hydrothermal | 222.2@10 | 374.9@5.0@600 | [209] |
| PNBH-VS4 | Hollow microspheres | Hydrothermal | 866@0.2 | 629@0.2@250 | [210] |
| MoS2/CNF | Nanoflower | Hydrothermal | 318.2@5.0 | 314@1@300 | [211] |
| CoS2 | Aggregated nanoparticles | Facile-sulfuration process | 580@0.1 | 500@2@500 | [212] |
| Cu2Se | Micro-sized monoclinic | Selenization | 264@0.1 | ~260@1@1000 | [213] |
| MC-NCNF/MoSe2 | Multi-channeled | Electrospinning+heat-treatment | 285@10 | 386@0.5@300 | [214] |
| SnS2/EPC | Graphene-like | Hydrothermal | 443@0.1 | 340@2@450 | [215] |
| NiSe2@N-TCF/CNTs | Nanofibers | Electrospinning + pyrolysis | 428.0@0.05 | 392.1@0.2 @1000 | [216] |
| FeS2@N/CNTs@rGO | Urchin-like hierarchitecture | Hydrothermal | 578@0.1 | 570@2@>750 | [217] |
| NSPCFS@CoS2 | Nanoparticles | Sulfuration | 540.7@4 | 546.3@1@2095 | [218] |
| Fe7Ni3S11/CN | Hollow-spheres | One-pot hydrothermal + post-annealing | 567@0.2 | 477@2@900 | [219] |
| MoS2@RGO | Few-layer nano-roses | One-step solvothermal | 513.8@0.1 | 223.2@0.5@500 | [220] |
| WS2/Ni3S2@NC | Layered-heterostructure | Solvothermal + annealing | 329.6@0.1 | 219.7@0.1@80 | [221] |
| PIB Anode | |||||
| CoSSe–C | Porous-shell nanospheres | Sulfo-selenization + calcination | 185@5.0 | 286@1.5@3000 | [222] |
| MoS2/MXene | Layered nanosheets | Hydrothermal | 290.7@0.05 | 145.5@0.2@50 | [223] |
| ZnSe@N-PCNF | Ultrafine nanocrystals | Electrospinning + thermal-treatment | 270@0.5 | 139@2.0@1000 | [224] |
| FeSe2/NC | Nanosheets | Pyrolysis + annealing + carbonizing | 203@10 | 301@1@250 | [225] |
| Co0.85Se@NC | Mesoporous structure | Annealing + selenidation | 600.9@0.1 | 114.7@1@250 | [226] |
| ZnSeNP@NHC | Hollow polyhedron | Pyrolysis + selenization | 310.5@0.5 | ~132.9@0.1@1200 | [227] |
| WSe2/N,P-C-2 | Ultrathin nanosheets | One-pot calcination | 659@0.1 | 333 @0.1@100 | [228] |
| NPCP@MoSe2 | Hollow-interlayered nanosheets | Solvothermal | 362@0.1 | 128@0.5@800 | [229] |
| MoSe2/N–C | Ultra-thin nanosheets | Selenization + calcination | 914@0.1 | 240@0.1@100 | [230] |
| Co0.85Se/G | Hollow structure | Hydrothermal | 430@0.05 | 324@0.05@200 | [231] |
| CoSe2@NCF | Flexible core/sheath | Solvothermal | 351@0.05 | 335@0.05@200 | [232] |
| AIB cathode | |||||
| CoSe@C | 3D nanoparticles | Pyrolysis + annealing | 427@1 | 62.4@5@100 | [233] |
| NC@ZnSe | Dispersed porous | Pyrolysis + selenization | 172.7@0.3 | 70.4@0.5@250 | [234] |
| WS2@NCNFs | Layered nanoplates | Electrospinning + annealing | 314.07@0.1 | 195.81@0.1@100 | [235] |
| MoSe2@C | Sheet nanocomposite | Hydrothermal | 294.97@0.1 | 110.3@0.1@3000 | [236] |
| Ni0.6Co0.4S@MXene@NC | Layered sheet | Sulfurization + carbonization | 481.2@ 0.4 | 125.2@1@300 | [237] |
| VS4 | Nanowire clusters | Amine ions-assisted method | 252.51@0.1 | 129.24@0.4@120 | [238] |
| NiSe2@GO | 3D sponge | Selenidation + annealing | ~281@0.5 | ~164@1@250 | [239] |
| Bi2Te3/Sb2Te3 | Nanoflakes | Solvothermal | 230@1 | 203@1@300 | [240] |
| Fe-NiSe | Nanoflake | Hydrothermal | 304@1 | 197@1@13.500 | [241] |
| MIB cathode | |||||
| 1T-VSe2@rGO | Thin-layered nanoparticles | Hydrothermal | 235.5@0.05 | 147.66@0.05@500 | [242] |
| Cu7.2S4 | Nanotubes | Microwave-induced selective-etching | 314@0.1 | 59.1@1@1600 | [243] |
| ZIB cathode | |||||
| 1T-WS2 | Nanosheet | Hydrothermal | 233.26@0.5 | 85.11@0.5@1000 | [244] |
| TiS2 | Layered | Hydrothermal | 76@1 | ~53.2@1@500 | [245] |
| HMIB cathode | |||||
| Cu2S@C/MLIB | Monodisperse | Sulfurization + carbonization | 399.2@0.05 | 150@0.05@50 | [246] |
| MoS2/MLIB | Flower-like architectures | Solvothermal | 220@0.07 | 160@0.07@65 | [247] |
| VS4/MLIB | Nanodendrites | Solution-phase approach | ~300@0.5 | 110@1@1500 | [248] |
| FeS-CNFs/MLIB | 1D network | Electrospinning + thermal treatment | 463@0.07 | 200@0.257@800 | [249] |
| MoS2&MoSe2/MLIB | Nanosheets | Exfoliation processing | 135@0.02&75@0.02 | 81@0.02@10&82@.02@3 | [250] |
| Co3S4-F/MLIB | Particle-like | Solvothermal | 779.8@0.1 | 399.5@0.1@100 | [251] |
| VS2/MLIB | Nanosheets | One-pot solvothermal | ~234@0.1 | ~222@0.1@200 | [252] |
| TiS2/SMIB | Layered structure | As-received | 200@1 | 67.5@20@3000 | [253] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mensah-Darkwa, K.; Nframah Ampong, D.; Agyekum, E.; de Souza, F.M.; Gupta, R.K. Recent Advancements in Chalcogenides for Electrochemical Energy Storage Applications. Energies 2022, 15, 4052. https://doi.org/10.3390/en15114052
Mensah-Darkwa K, Nframah Ampong D, Agyekum E, de Souza FM, Gupta RK. Recent Advancements in Chalcogenides for Electrochemical Energy Storage Applications. Energies. 2022; 15(11):4052. https://doi.org/10.3390/en15114052
Chicago/Turabian StyleMensah-Darkwa, Kwadwo, Daniel Nframah Ampong, Emmanuel Agyekum, Felipe M. de Souza, and Ram K. Gupta. 2022. "Recent Advancements in Chalcogenides for Electrochemical Energy Storage Applications" Energies 15, no. 11: 4052. https://doi.org/10.3390/en15114052
APA StyleMensah-Darkwa, K., Nframah Ampong, D., Agyekum, E., de Souza, F. M., & Gupta, R. K. (2022). Recent Advancements in Chalcogenides for Electrochemical Energy Storage Applications. Energies, 15(11), 4052. https://doi.org/10.3390/en15114052







