An Analytical TOOLBOX for the Characterization of Chalks and Other Fine-Grained Rock Types within Enhanced Oil Recovery Research and Its Application—A Guideline
Abstract
:1. Introduction
2. Results—The Toolbox Content
- Field emission gun scanning electron microscopy (FEG-SEM) with energy dispersive X-ray spectroscopy (EDS),
- SEM-based mineral liberation analyzer (MLA),
- Focused ion beam (FIB)-SEM,
- Transmission electron microscopy (TEM),
- Electron microprobe analysis (EMPA),
- Nano secondary ion mass spectrometry (nanoSIMS),
- Specific surface area (SSA),
- Raman spectroscopy,
- Tip enhanced Raman spectroscopy (TERS) with atomic force microscopy (AFM).
3. Electron and Ion Microscopy
3.1. Sample Preparation
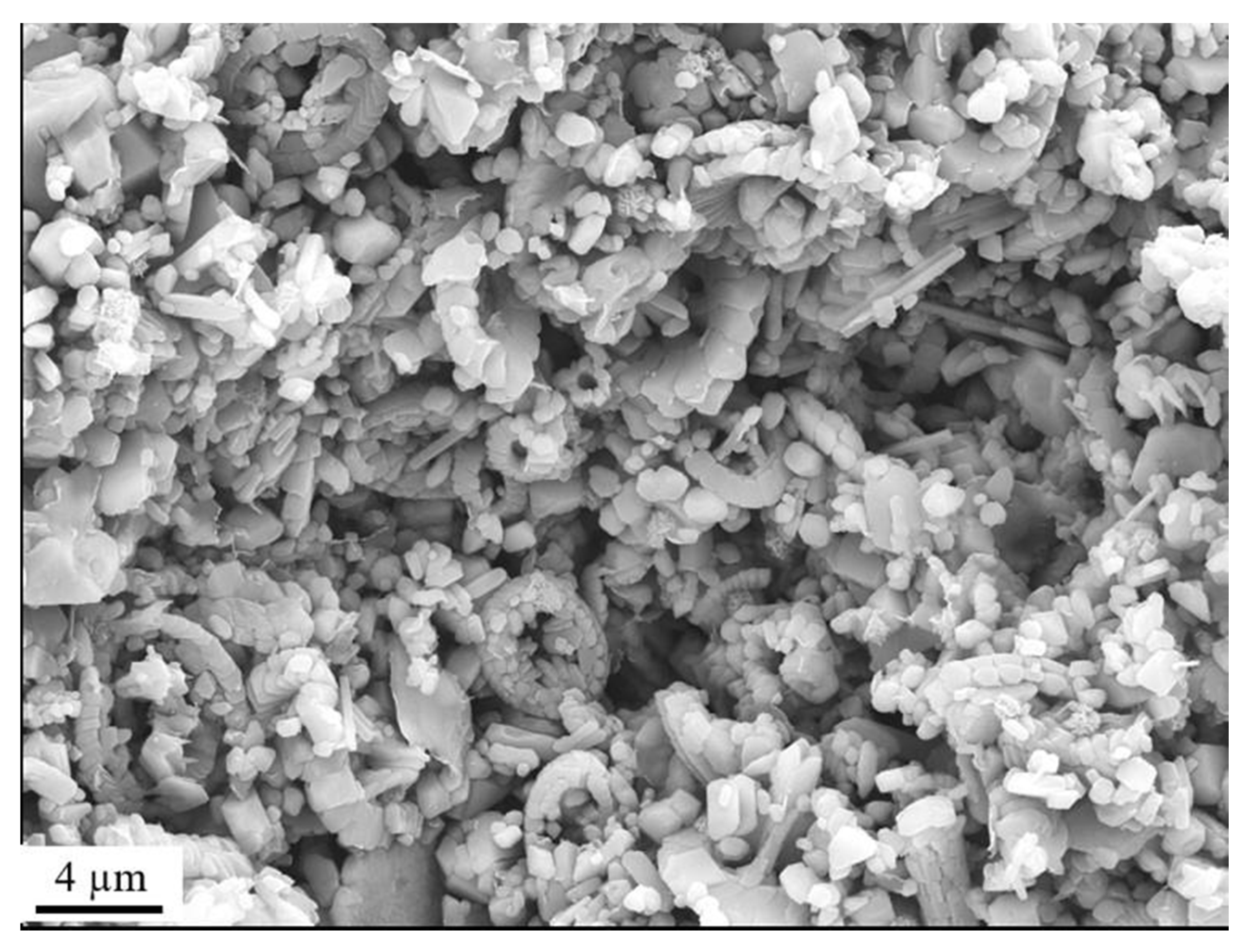
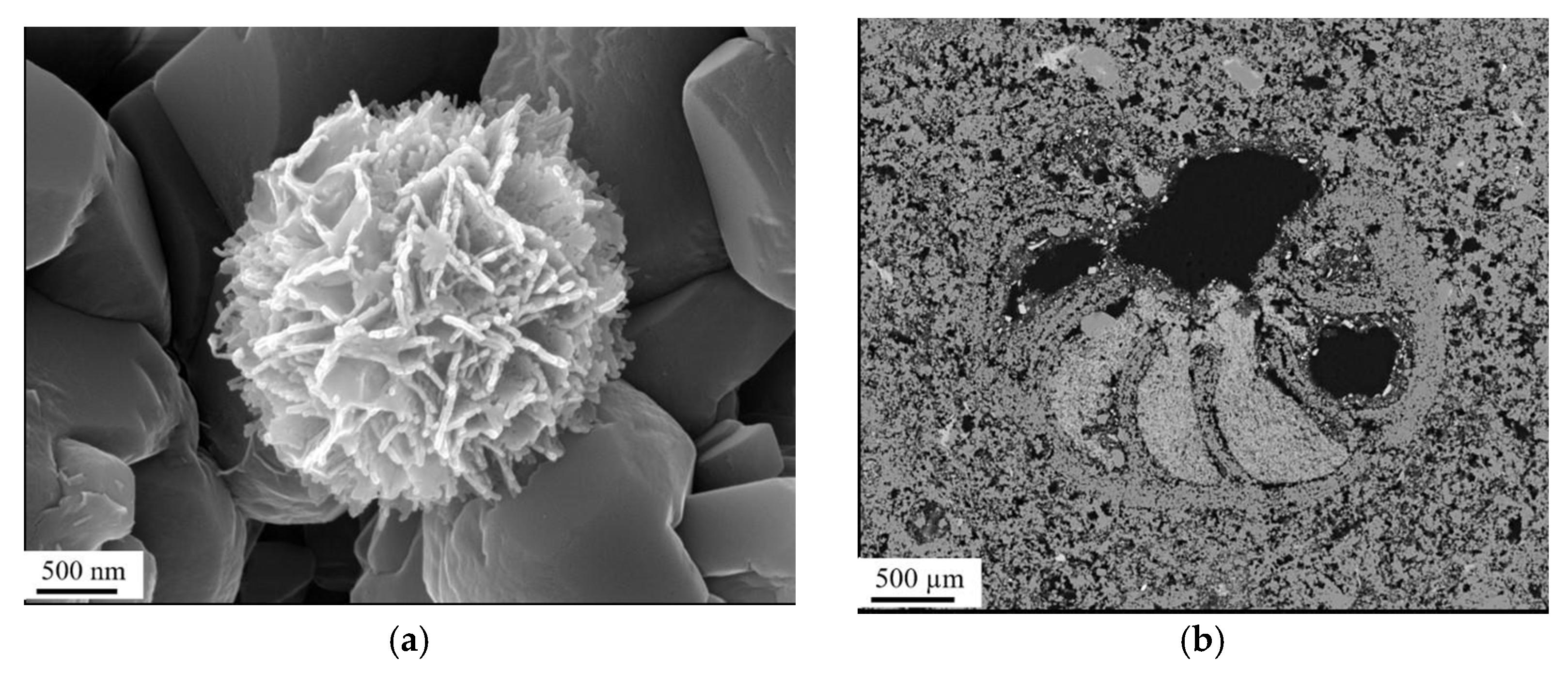
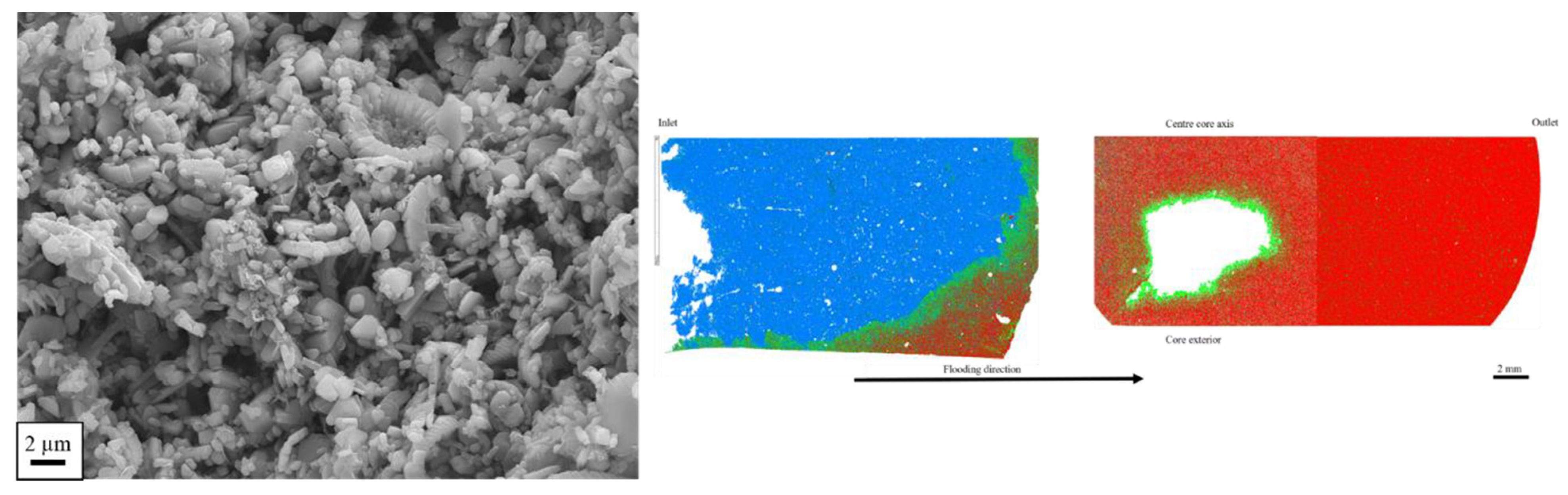
3.1.1. Field Emission Gun Scanning Electron Microscope (FEG-SEM)
3.1.2. Mineral Liberation Analyzer (MLA)
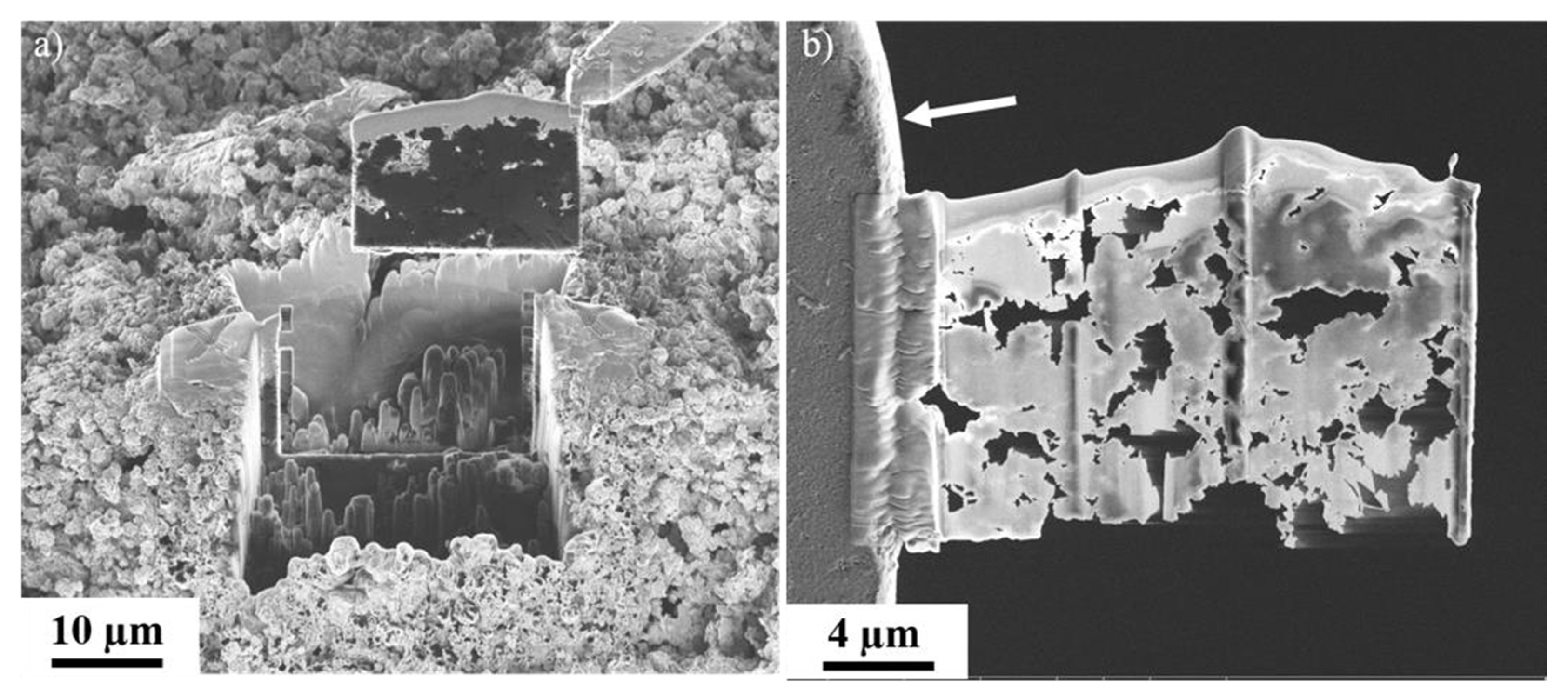
3.1.3. Focused Ion Beam Scanning Electron Microscopy (FIB-SEM)
3.1.4. Transmission Electron Microscope (TEM)
3.1.5. Electron Microprobe Analysis (EMPA)
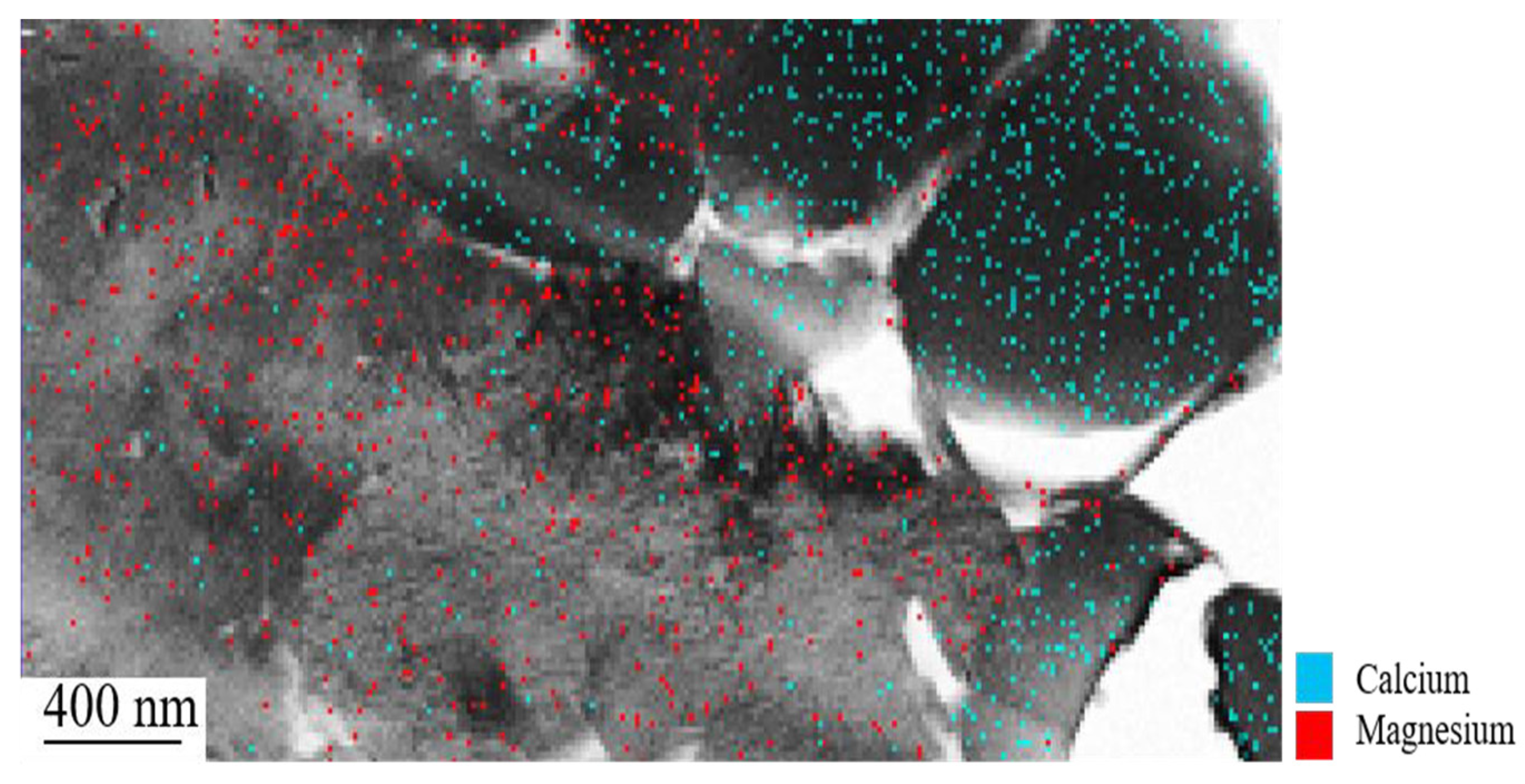
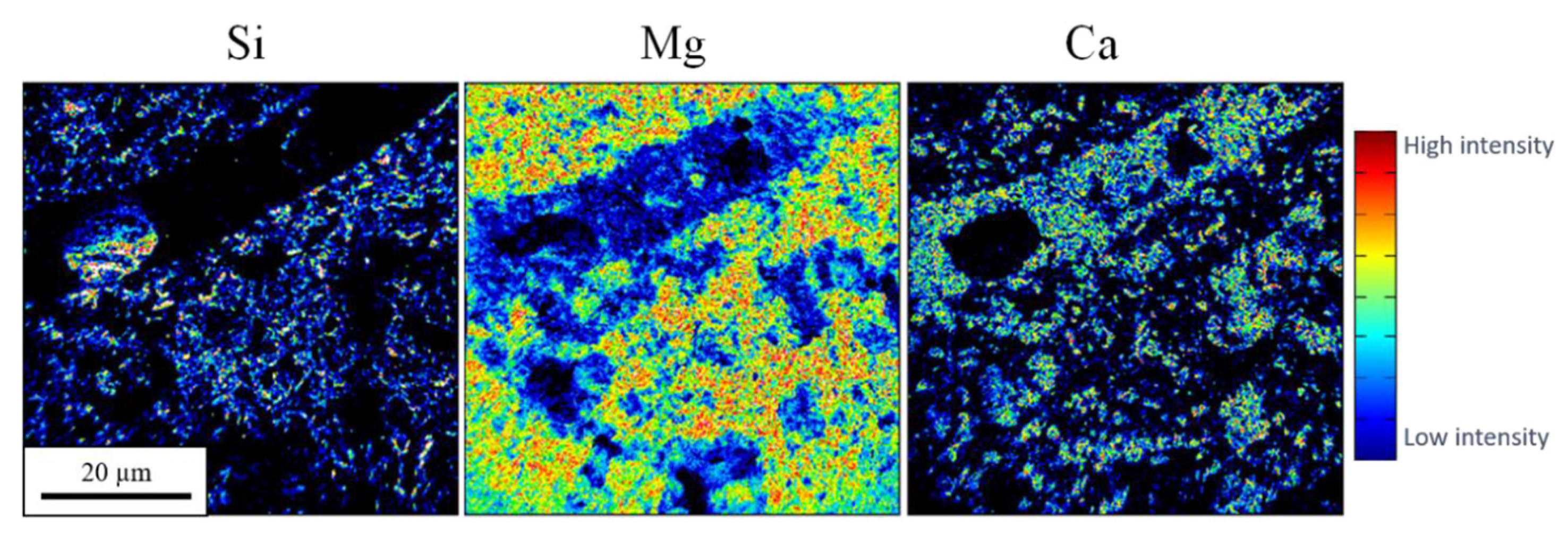
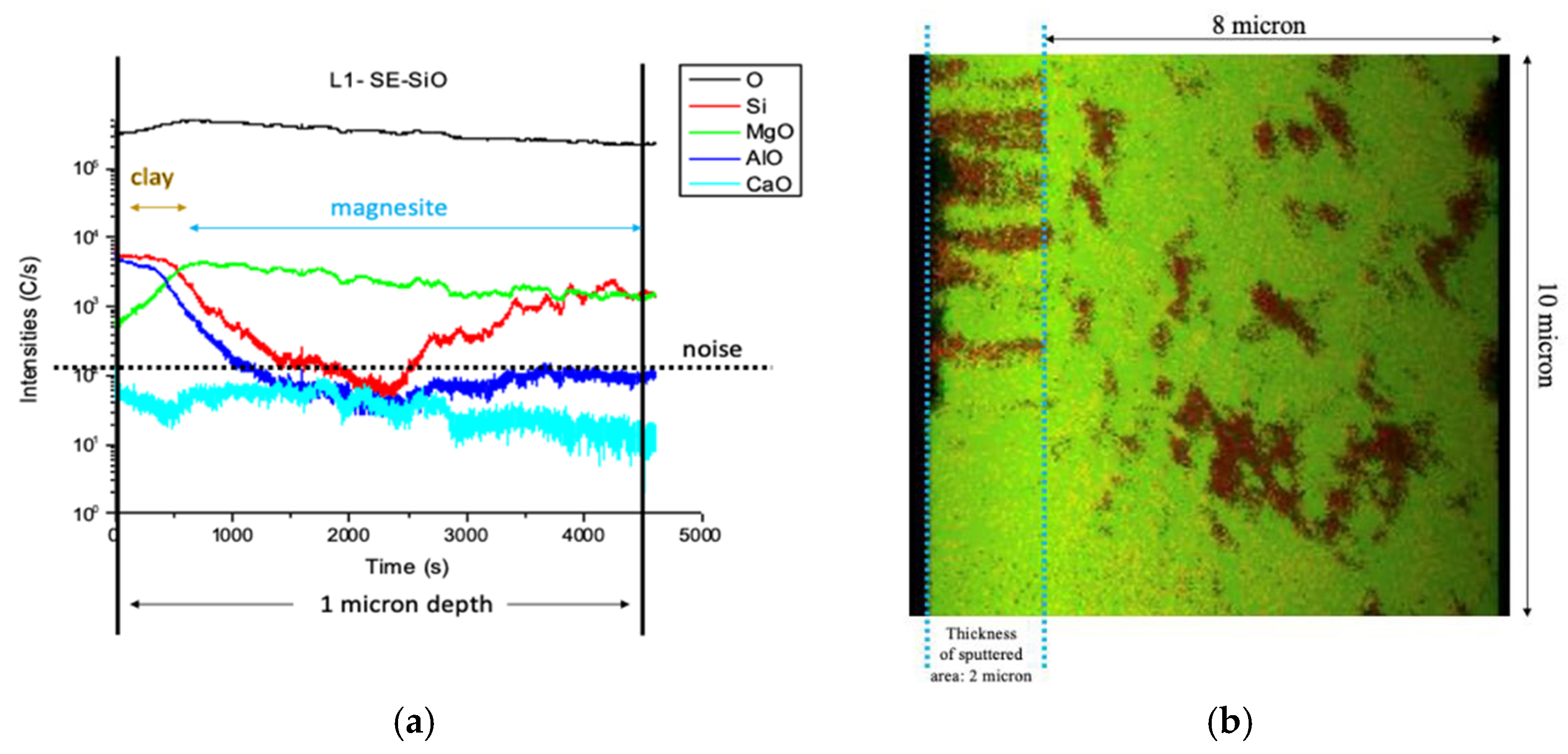
3.1.6. Nano Secondary Ion Mass Spectrometry (NanoSIMS)
3.2. Specific Surface Area (SSA)
3.3. Raman Spectroscopy
4. Discussion
5. Conclusions
- FEG-SEM-EDS,
- XRD,
- Whole-rock and stable isotope geochemistry,
- Traditional stable isotope geochemistry.
- MLA,
- SSA,
- Pycnometry: depending on the specific focus of the IOR/EOR research and refined by focussed work on representative areas to understand key processes causing mineralogical changes,
- TEM analyses on FIB-SEM samples,
- TERS analyses coupled with AFM imaging,
- NanoSIMS analyses.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doornhof, D.; Kristiansen, T.G.; Nagel, N.B.; Pattillo, P.D.; Sayers, C.J.O.R. Compaction and subsidence. Oilfield Rev. 2006, 18, 50–68. [Google Scholar]
- Hermansen, H.; Landa, G.H.; Sylte, J.E.; Thomas, L.K. Experiences after 10 years of waterflooding the Ekofisk Field, Norway. J. Pet. Sci. Eng. 2000, 26, 11–18. [Google Scholar] [CrossRef]
- Nagel, N.B. Ekofisk field overburden modelling. In SPE/ISRM Rock Mechanics in Petroleum Engineering; OnePetro: Trondheim, Norway, 1998. [Google Scholar] [CrossRef]
- Teufel, L.W.; Rhett, D.W.; Farrell, H.E. Effect of Reservoir Depletion and Pore Pressure Drawdown on In Situ Stress and Deformation in the Ekofisk Field, North Sea; American Rock Mechanics Association: Norman, OK, USA, 1991. [Google Scholar]
- Andersen, P.Ø.; Wang, W.; Madland, M.V.; Zimmermann, U.; Korsnes, R.I.; Bertolino, S.R.A.; Minde, M.; Schulz, B.; Gilbricht, S. Comparative Study of Five Outcrop Chalks Flooded at Reservoir Conditions: Chemo-mechanical Behaviour and Profiles of Compositional Alteration. Transp. Porous Media 2017, 121, 135–181. [Google Scholar] [CrossRef]
- Minde, M.W.; Wang, W.; Madland, M.V.; Zimmermann, U.; Korsnes, R.I.; Bertolino, S.R.A.; Andersen, P.Ø. Temperature effects on rock engineering properties and rock-fluid chemistry in opal-CT-bearing chalk. J. Pet. Sci. Eng. 2018, 169, 454–470. [Google Scholar] [CrossRef]
- Hjuler, M.L.; Fabricius, I.L. Engineering properties of chalk related to diagenetic variations of Upper Cretaceous onshore and offshore chalk in the North Sea area. J. Pet. Sci. Eng. 2009, 68, 151–170. [Google Scholar] [CrossRef]
- Kallesten, E.; Zimmermann, U.; Madland, M.V.; Bertolino, S.R.A.; Omdal, E.; Andersen, P.Ø. Mineralogy and geochemistry of reservoir and non-reservoir chalk from the Norwegian continental shelf. J. Pet. Sci. Eng. 2021, 205, 108914. [Google Scholar] [CrossRef]
- Scholle, P.A.; Kinsman, D.J.J. Diagenesis of Upper Cretaceous chalks from North Sea, England and Northern Ireland. Am. Assoc. Pet. Geol. Bull. 1973, 57, 803–804. [Google Scholar]
- Minde, M.W.; Zimmermann, U.; Madland, M.V.; Korsnes, R.I.; Schultz, B.; Gilbrich, S. Mineral Replacement in Long-Term Flooded Porous Carbonate Rocks. Geochim. Cosmochim. Acta 2020, 268, 485–508. [Google Scholar] [CrossRef]
- Skovbjerg, L.L.; Hassenkam, T.; Makovicky, E.; Hem, C.P.; Yang, M.; Bovet, N.; Stipp, S.L.S. Nano sized clay detected on chalk particle surfaces. Geochim. Cosmochim. Acta 2012, 99, 57–70. [Google Scholar] [CrossRef]
- Zimmermann, U.; Madland, M.V.; Nermoen, A.; Hildebrand-Habel, T.; Bertolino, S.A.R.; Hiorth, A.; Korsnes, R.I.; Audinot, J.-N.; Grysan, P. Evaluation of the compositional changes during flooding of reactive fluids using scanning electron microscopy, nano-secondary ion mass spectrometry, X-ray diffraction, and whole-rock geochemistry. Am. Assoc. Pet. Geol. Bulletin 2015, 99, 791–805. [Google Scholar] [CrossRef] [Green Version]
- Hrstka, T.; Gottlieb, P.; Skala, R.; Breiter, K.; Motl, D. Automated mineralogy and petrology—Applications of TESCAN Integrated Mineral Analyzer (TIMA). J. Geosci. 2018, 63, 47–63. [Google Scholar] [CrossRef] [Green Version]
- Pirrie, D.; Rollinson, G.K. Unlocking the applications of automated mineral analysis. Geol. Today 2011, 27, 226–235. [Google Scholar] [CrossRef]
- Tsikouras, B.; Pe-Piper, G.; Piper, D.J.W.; Schaffer, M. Varietal heavy mineral analysis of sediment provenance, Lower Cretaceous Scotian Basin, eastern Canada. Sediment. Geol. 2011, 237, 150–165. [Google Scholar] [CrossRef]
- Ball, M.R.; Taylor, R.J.M.; Einsle, J.F.; Khanom, F.; Guillermier, C.; Harrison, R. Helium ion microscope—Secondary ion mass spectrometry for geological materials. J. Nanotechnol. 2020, 11, 1504–1515. [Google Scholar] [CrossRef] [PubMed]
- Ost, A.D.; Wu, T.; Höschen, C.; Mueller, C.W.; Wirtz, T.; Audinot, J.-N. 4D Surface Reconstructions to Study Microscale Structures and Functions in Soil Biogeochemistry. Environ. Sci. Technol. 2021, 55, 9384–9393. [Google Scholar] [CrossRef] [PubMed]
- Jiruse, J.; Haničinec, M.; Havelka, M. Integrating focused ion beam–scanning electron microscope with confocal Raman microscope into a single instrument. J. Vac. Sci. Technol. 2014, 32, 06FC03. [Google Scholar] [CrossRef]
- Wille, G.; Bourrat, X.; Maubec, N.; Lahfid, A. Raman-in-SEM, a multimodal and multiscale analytical tool: Performance for materials and expertise. Micron 2014, 67, 50–64. [Google Scholar] [CrossRef]
- Borromeo, L.; Zimmermann, U.; Andò, S.; Coletti, G.; Bersani, D.; Basso, D.; Gentile, P.; Schulz, B.; Garzanti, E. Raman Spectroscopy as a tool for magnesium estimation in Mg-calcite. J. Raman Spectrosc. 2017, 48, 983–992. [Google Scholar] [CrossRef]
- Fandrich, R.; Gu, Y.; Burrows, D.; Moeller, K. Modern SEM-based mineral liberation analysis. Int. J. Miner. Processing 2007, 84, 310–320. [Google Scholar] [CrossRef]
- Guhl, A.C.; Greb, V.G.; Schulz, B.; Bertau, M. An Improved Evaluation Strategy for Ash Analysis Using Scanning Electron Microscope Automated Mineralogy. Minerals 2020, 10, 484. [Google Scholar] [CrossRef]
- Keulen, N.; Næsby Malkki, S.; Graham, S. Automated Quantitative Mineralogy Applied to Metamorphic Rocks. Minerals 2020, 10, 47. [Google Scholar] [CrossRef] [Green Version]
- Andrew, M. A quantified study of segmentation techniques on synthetic geological XRM and FIB-SEM images. Comput. Geosci. 2018, 22, 1503–1512. [Google Scholar] [CrossRef] [Green Version]
- Hjuler, M.L.; Hansen, V.F.; Fabricius, I.L. Interpretational challenges related to studies of chalk particle surfaces in scanning and transmission electron microscopy. Bull. Geol. Soc. Den. 2018, 66, 151–165. [Google Scholar] [CrossRef]
- Ohnuki, T.; Yoshida, T.; Ozaki, T.; Samadfam, M.; Kozai, N.; Yubuta, K.; Mitsugashira, T.; Kasama, T.; Francis, A.J. Interactions of uranium with bacteria and kaolinite clay. Chem. Geol. 2005, 220, 237–243. [Google Scholar] [CrossRef]
- Yuan, P.; Liu, D.; Tian, Q.; Song, Y.; Wei, H.; Wang, S.; Zhou, J.; Deng, L.; Du, P. Identification of the occurrence of minor elements in the structure of diatomaceous opal using FIB and TEM-EDS. Am. Mineral. 2019, 104, 1323–1335. [Google Scholar] [CrossRef]
- Hirata, K.; Saitoh, Y.; Chiba, A.; Yamada, K.; Takahashi, Y.; Narumi, K. Secondary ion counting for surface-sensitive chemical analysis of organic compounds using time-of-flight secondary ion mass spectroscopy with cluster ion impact ionization. Rev. Sci. Instrum. 2011, 82, 033101. [Google Scholar] [CrossRef]
- Griffiths, J. Secondary Ion Mass Spectrometry. Anal. Chem. 2008, 80, 7194–7197. [Google Scholar] [CrossRef]
- Handley, J. Product Review: Secondary Ion Mass Spectrometry. Anal. Chem. 2002, 74, 335A–341A. [Google Scholar] [CrossRef] [Green Version]
- Minde, M.W.; Zimmermann, U.; Madland, M.V.; Korsnes, R.I.; Schulz, B.; Audinot, J.N. Fluid-flow During EOR Experiments in Chalk: Insights using SEM-MLA, EMPA and NanoISMS Applications. In Proceedings of the International Symposium of the Society of Core Analysts, Snowmass, CO, USA, 21–26 August 2016; p. 7. [Google Scholar]
- Minde, M.W.; Madland, M.V.; Zimmermann, U.; Egeland, N.; Korsnes, R.I.; Nakamura, E.; Kobayashi, K.; Ota, T. Mineralogical alterations in calcite powder flooded with MgCl2 to study Enhanced Oil Recovery (EOR) mechanisms at pore scale. Microporous Mesoporous Mater. 2019, 304, 109402. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Kuila, U.; Prasad, M. Specific surface area and pore-size distribution in clays and shales. Geophys. Prospect. 2013, 61, 341–362. [Google Scholar] [CrossRef]
- Macht, M.; Eusterhues, K.; Pronk, G.J.; Totsche, K.-U. Specific surface area of clay minerals: Comparison between atomic force microscopy measurements and bulk-gas (N2) and -liquid (EGME) adsorption methods. Appl. Clay Sci. 2011, 53, 20–26. [Google Scholar]
- Raman, C.V. A new radiation. Indian J. Phys. 1928, 2, 387–398. [Google Scholar] [CrossRef]
- Borromeo, L.; Egeland, N.; Minde, M.W.; Zimmermann, U.; Andò, S.; Madland, M.V.; Korsnes, R.I. Quick, Easy, and Economic Mineralogical Studies of Flooded Chalk for EOR Experiments Using Raman Spectroscopy. Minerals 2018, 8, 221. [Google Scholar] [CrossRef] [Green Version]
- Aksenov, Y. Raman Microscopy in an Electron Microscope: Combining Chemical and Morphological Analyses. Ph.D. Thesis, University of Twente, Enschede, Switzerland, 2003. [Google Scholar]
- Borromeo, L.; Toccafondi, C.; Minde, M.W.; Zimmermann, U.; Andò, S.; Madland, M.V.; Korsnes, R.I.; Ossikovski, R. Application of Tip-Enhanced Raman Spectroscopy for the nanoscale characterization of flooded chalk. J. Appl. Phys. 2018, 124, 173101. [Google Scholar] [CrossRef]
- Lünsdorf, N.K.; Kalies, J.; Ahlers, P.; Dunkl, I.; von Eynatten, H. Semi-Automated Heavy-Mineral Analysis by Raman Spectroscopy. Minerals 2019, 9, 385. [Google Scholar] [CrossRef] [Green Version]
- Bredal, T.V.; Zimmermann, U.; Madland, M.V.; Minde, M.W.; Ost, A.D.; Wirtz, T.; Audinot, J.-N.; Korsnes, R.I. High-Resolution Topographic and Chemical Surface Imaging of Chalk for Oil Recovery Improvement Applications. Minerals 2022, 12, 356. [Google Scholar] [CrossRef]
- Kallesten, E.; Andersen, P.Ø.; Berawala, D.S.; Korsnes, R.I.; Madland, M.V.; Omdal, E.; Zimmermann, U. SPE Modelling of Permeability and Strain Evolution in Chemical Creep Compaction Experiments with Fractured and Unfractured Chalk Cores Conducted at Reservoir Conditions. SPE Reserv. Eval. Eng. Reserv. Eng. 2020, 25, 2710–2728. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmermann, U.; Minde, M.W.; Madland, M.V.; Bredal, T.V. An Analytical TOOLBOX for the Characterization of Chalks and Other Fine-Grained Rock Types within Enhanced Oil Recovery Research and Its Application—A Guideline. Energies 2022, 15, 4060. https://doi.org/10.3390/en15114060
Zimmermann U, Minde MW, Madland MV, Bredal TV. An Analytical TOOLBOX for the Characterization of Chalks and Other Fine-Grained Rock Types within Enhanced Oil Recovery Research and Its Application—A Guideline. Energies. 2022; 15(11):4060. https://doi.org/10.3390/en15114060
Chicago/Turabian StyleZimmermann, Udo, Mona Wetrhus Minde, Merete Vadla Madland, and Tine Vigdal Bredal. 2022. "An Analytical TOOLBOX for the Characterization of Chalks and Other Fine-Grained Rock Types within Enhanced Oil Recovery Research and Its Application—A Guideline" Energies 15, no. 11: 4060. https://doi.org/10.3390/en15114060




