Low Heat Capacity 3D Hollow Microarchitected Reactors for Thermal and Fluid Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. 3D Printing of the Polymer Microlattice
2.2. Ni-P Metallic Thin Film Deposition
2.3. Burn-Out of the Polymer and Conversion of Ni-P to Nickel Oxide (NiO)
2.4. Measurement of the Pressure Drop
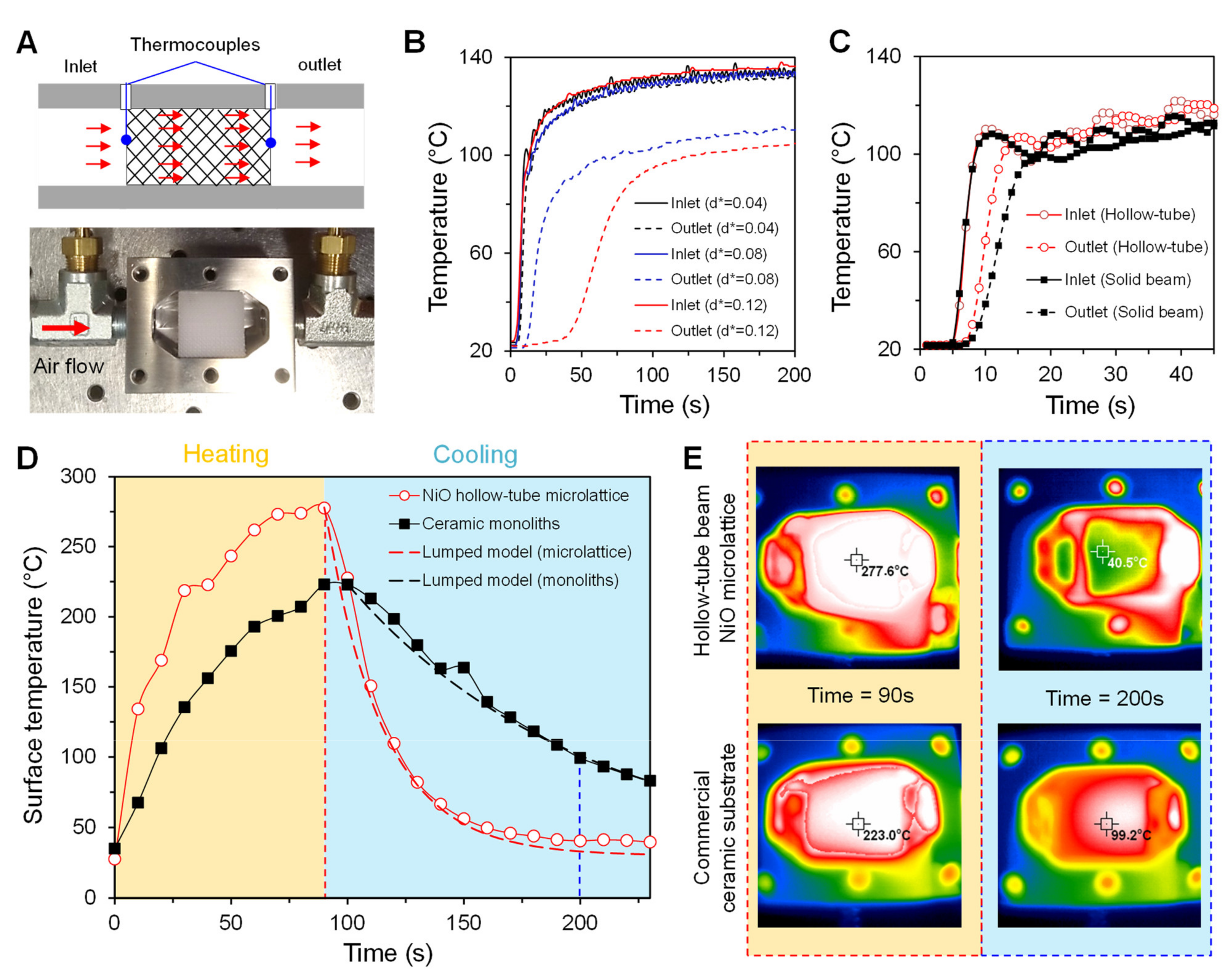
2.5. Measurement of the Thermal Response
2.6. CFD Simulation
3. Results
3.1. Design Concept
3.2. Fabrication of Hollow Microlattices
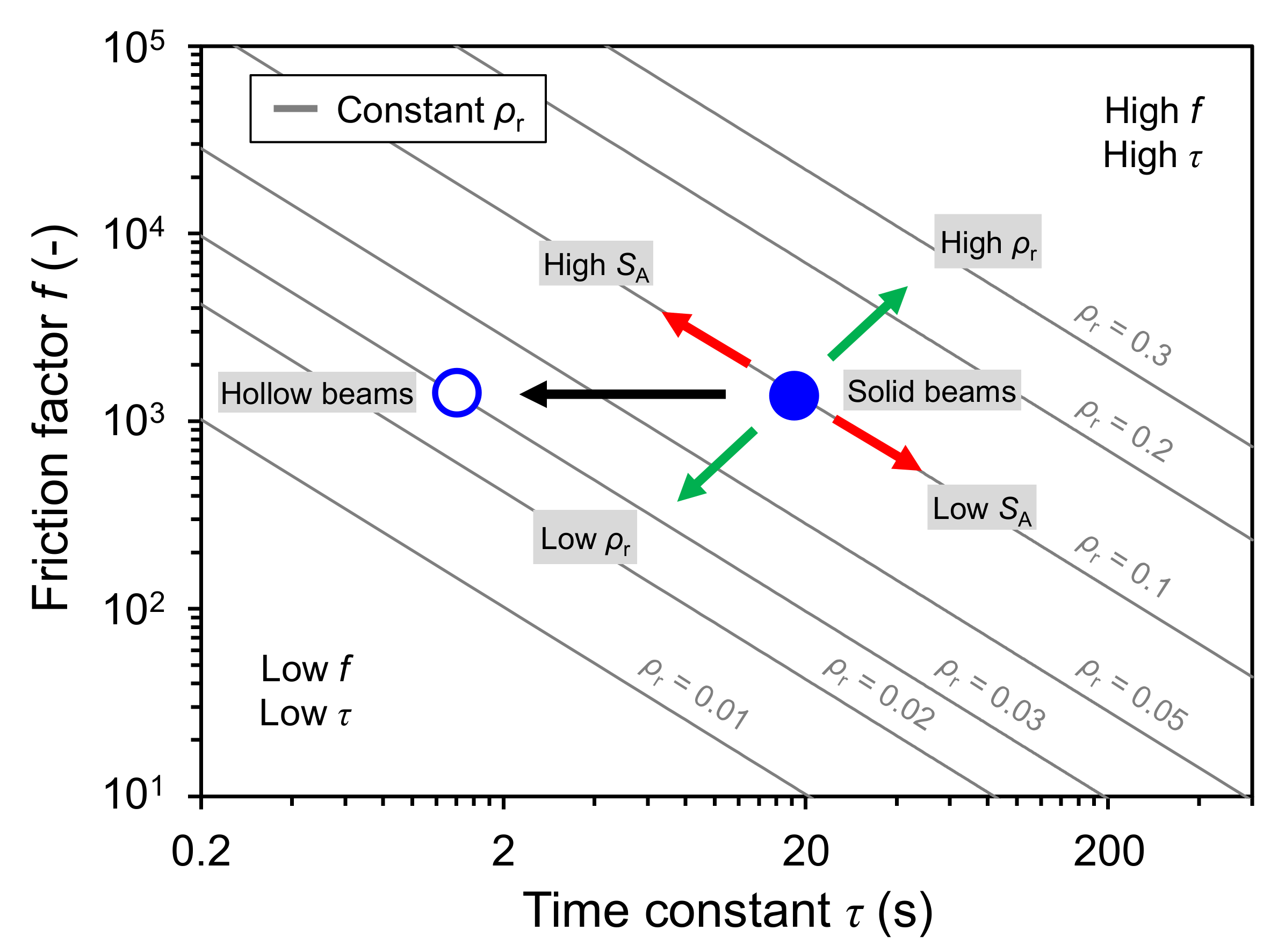
3.3. Fluidic Properties
3.4. Thermal Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| ρr | The relative density |
| d | The diameter |
| t | The wall thickness |
| l | The unit cell length |
| d* | The dimensionless diameter (= d/l) |
| t* | The wall thickness (= t/d) |
| SA | The specific surface area |
| SA* | The dimensionless form surface area (= SA·l) |
| SA*/ρr | The specific surface area-to-relative-density ratio |
| Δp | The pressure drop |
| L | The overall sample length |
| μ | The dynamic viscosity of the fluid |
| u | The flow velocity |
| ρf | The density of the fluid |
| K1 | The Darcian permeability |
| K2 | The non-Darcian permeability |
| ε | The porosity (= 1 − ρr) |
| α | The Ergun parameter of the viscous term |
| β | The Ergun parameter of the inertial term |
| Bi | The Biot number |
| hfluid | The heat transfer coefficient of the working fluid |
| kwall | The thermal conductivity of the hollow tube wall material |
| kfluid | The thermal conductivity of the working fluid |
| Nu | The Nusselt number |
| cp | The specific heat capacity and density of the constituent materials |
| ρs | The density of the constituent materials |
| Tsurr | The ambient temperature |
| f | The dimensionless friction factor |
| τ | The thermal time constant |
| Dh | The hydraulic diameter of the 3D microlattice |
| CA | The dimensionless coefficient depends on the shape of the unit cell |
| C | The thermophysical constant corresponding to unknown numerical coefficients |
References
- Schaedler, T.A.; Carter, W.B. Architected Cellular Materials. Annu. Rev. Mater. Res. 2016, 46, 187–210. [Google Scholar] [CrossRef]
- Thakkar, H.; Eastman, S.; Hajari, A.; Rownaghi, A.A.; Knox, J.C.; Rezaei, F. 3D-Printed Zeolite Monoliths for CO2 Removal from Enclosed Environments. ACS Appl. Mater. Interfaces 2016, 8, 27753–27761. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Morales, J.C.; Tarancon, A.; Canales-Vazquez, J.; Mendez-Ramos, J.; Hernandez-Afonso, L.; Acosta-Mora, P.; Marin Ruedac, J.R.; Fernandez-Gonzalez, R. Three dimensional printing of components and functional devices for energy and environmental applications. Energy Environ. Sci. 2017, 10, 846–859. [Google Scholar] [CrossRef] [Green Version]
- Weng, J.; Lu, X.; Gao, P.X. Nano-Array Integrated Structured Catalysts: A New Paradigm upon Conventional Wash-Coated Monolithic Catalysts? Catalysts 2017, 7, 253. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Qi, Z.; Beck, V.A.; Luneau, M.; Lattimer, J.; Chen, W.; Biener, J. Toward digitally controlled catalyst architectures: Hierarchical nanoporous gold via 3D printing. Sci. Adv. 2018, 4, eaas9459. [Google Scholar] [CrossRef] [Green Version]
- Blanchette, C.D.; Knipe, J.M.; Stolaroff, J.K.; DeOtte, J.R.; Oakdale, J.S.; Maiti, A.; Baker, S.E. Printable enzyme-embedded materials for methane to methanol conversion. Nat. Commun. 2016, 7, 11900. [Google Scholar] [CrossRef] [Green Version]
- Beck, V.A.; Ivanovskaya, A.N.; Chandrasekaran, S.; Forien, J.B.; Baker, S.E.; Duoss, E.B.; Worsley, M.A. Inertially enhanced mass transport using 3D-printed porous flow-through electrodes with periodic lattice structures. Proc. Natl. Acad. Sci. USA 2021, 118, 32. [Google Scholar] [CrossRef]
- Kim, S.; Kim, D.H.; Kim, W.; Cho, Y.T.; Fang, N.X. Additive manufacturing of functional microarchitected reactors for energy, environmental, and biological applications. Int. J. Precis. Eng. Manuf.-Green Technol. 2021, 8, 303–326. [Google Scholar] [CrossRef]
- Ma, C.; Kim, S.; Fang, N.X. Far-field acoustic subwavelength imaging and edge detection based on spatial filtering and wave vector conversion. Nat. Commun. 2019, 10, 204. [Google Scholar] [CrossRef]
- Steiner, T.; Neurauter, D.; Moewius, P.; Pfeifer, C.; Schallhart, V.; Moeltner, L. Heat-Up Performance of Catalyst Carriers—A Parameter Study and Thermodynamic Analysis. Energies 2021, 14, 964. [Google Scholar] [CrossRef]
- Kim, S.; Handler, J.J.; Cho, Y.T.; Barbastathis, G.; Fang, N.X. Scalable 3D printing of aperiodic cellular structures by rotational stacking of integral image formation. Sci. Adv. 2021, 7, eabh1200. [Google Scholar] [CrossRef] [PubMed]
- Roper, C.S.; Schubert, R.C.; Maloney, K.J.; Page, D.; Ro, C.J.; Yang, S.S.; Jacobsen, A.J. Scalable 3D bicontinuous fluid networks: Polymer heat exchangers toward artificial organs. Adv. Mater. 2015, 27, 2479–2484. [Google Scholar] [CrossRef] [PubMed]
- Woodward, I.R.; Attia, L.; Patel, P.; Fromen, C.A. Scalable 3D-printed lattices for pressure control in fluid applications. AIChE J. 2021, 67, e17452. [Google Scholar] [CrossRef] [PubMed]
- Sinn, C.; Wentrup, J.; Pesch, G.R.; Thöming, J.; Kiewidt, L. Structure-heat transport analysis of periodic open-cell foams to be used as catalyst carriers. Chem. Eng. Res. Des. 2021, 166, 209–219. [Google Scholar] [CrossRef]
- Takarazawa, S.; Ushijima, K.; Fleischhauer, R.; Kato, J.; Terada, K.; Cantwell, W.J.; Hasumoto, S. Heat-transfer and pressure drop characteristics of micro-lattice materials fabricated by selective laser metal melting technology. Heat Mass Transf. 2022, 58, 125–141. [Google Scholar] [CrossRef]
- García-Vázquez, M.; Zhang, G.; Hong, Z.; Gu, X.; Garcia-Garcia, F.R. Micro-structured catalytic converter for residual methane emission abatement. Chem. Eng. J. 2020, 396, 125379. [Google Scholar] [CrossRef]
- Narkhede, S.; Sur, A. Performance prediction of hollow micro-lattice cross-flow heat exchanger using a numerical approach. Int. J. Ambient. Energy 2021, 1–8. [Google Scholar] [CrossRef]
- Mazzone, S.; Campbell, A.; Zhang, G.; García-García, F.R. Ammonia cracking hollow fibre converter for on-board hydrogen production. Int. J. Hydrog. Energy 2021, 46, 37697–37704. [Google Scholar] [CrossRef]
- Xu, W.; Liu, L.; Chen, J.; Lv, X.; Yao, Y. A hollow microlattice based ultralight active thermal control device and its fabrication techniques and thermal performances. J. Micromech. Microeng. 2021, 32, 015010. [Google Scholar] [CrossRef]
- Oskooei, A.B.; Koohsorkhi, J.; Mehrpooya, M. Simulation of plasma-assisted catalytic reduction of NOx, CO, and HC from diesel engine exhaust with COMSOL. Chem. Eng. Sci. 2019, 197, 135–149. [Google Scholar] [CrossRef]
- Deshpande, V.S.; Fleck, N.A.; Ashby, M.F. Effective properties of the octet-truss lattice material. J. Mech. Phys. Solids 2001, 49, 1747–1769. [Google Scholar] [CrossRef] [Green Version]
- Tancogne-Dejean, T.; Spierings, A.B.; Mohr, D. Additively-manufactured metallic micro-lattice materials for high specific energy absorption under static and dynamic loading. Acta Mater. 2016, 116, 14–28. [Google Scholar] [CrossRef]
- Meza, L.R.; Phlipot, G.P.; Portela, C.M.; Maggi, A.; Montemayor, L.C.; Comella, A.; Kochmann, D.M.; Greer, J.R. Reexamining the mechanical property space of three-dimensional lattice architectures. Acta Mater. 2017, 140, 424–432. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Lee, H.; Weisgraber, T.H.; Shusteff, M.; DeOtte, J.; Duoss, E.B.; Kuntz, J.D.; Biener, M.M.; Ge, Q.; Jackson, J.A.; et al. Ultralight, ultrastiff mechanical metamaterials. Science 2014, 344, 1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fink, K.D.; Kolodziejska, J.A.; Jacobsen, A.J.; Roper, C.S. Fluid dynamics of flow through microscale lattice structures formed from self-propagating photopolymer waveguides. AICHE J. 2011, 57, 2636–2646. [Google Scholar] [CrossRef]
- Roper, C.S.; Fink, K.D.; Lee, S.T.; Kolodziejska, J.A.; Jacobsen, A.J. Anisotropic convective heat transfer in microlattice materials. AIChE J. 2013, 59, 622–629. [Google Scholar] [CrossRef]
- Maloney, K.J.; Fink, K.D.; Schaedler, T.A.; Kolodziejska, J.A.; Jacobsen, A.J.; Roper, C.S. Multifunctional heat exchangers derived from three-dimensional micro-lattice structures. Int. J. Heat Mass Transf. 2012, 55, 2486. [Google Scholar] [CrossRef]
- Edouard, D.; Lacroix, M.; Huu, C.P.; Luck, F. Pressure drop modeling on SOLID foam: State-of-the art correlation. Chem. Eng. J. 2008, 144, 299–311. [Google Scholar] [CrossRef]
- Chaudhari, A.; Ekade, P.; Krishnan, S. Experimental investigation of heat transfer and fluid flow in octet-truss lattice geometry. Int. J. Therm. Sci. 2019, 143, 64–75. [Google Scholar] [CrossRef]
- Inayat, A.; Klumpp, M.; Lämmermann, M.; Freund, H.; Schwieger, W. Development of a new pressure drop correlation for open-cell foams based completely on theoretical grounds: Taking into account strut shape and geometric tortuosity. Chem. Eng. J. 2016, 287, 704–719. [Google Scholar] [CrossRef]
- Lu, T.J.; Valdevit, L.; Evans, A.G. Active cooling by metallic sandwich structures with periodic cores. Prog. Mater. Sci. 2005, 50, 789–815. [Google Scholar] [CrossRef]
- Lucci, F.; Della Torre, A.; Montenegro, G.; Eggenschwiler, P.D. On the catalytic performance of open cell structures versus honeycombs. Chem. Eng. J. 2015, 264, 514–521. [Google Scholar] [CrossRef]
- Dewitt, D.; Bergman, T.; Lavine, A. Fundamentals of Heat and Mass Transfer; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Pranati Sahoo, P.; Misra, D.K.; Salvador, J.; Makongo, J.P.A.; Chaubey, G.S.; Takas, N.J.; Wiley, J.B.; Poudeu, P.F.P. Microstructure and thermal conductivity of surfactant-free NiO nanostructures. J. Solid State Chem. 2012, 190, 29–35. [Google Scholar] [CrossRef]
- Vollmer, M. Newton’s law of cooling revisited. Eur. J. Phys. 2009, 30, 1063. [Google Scholar] [CrossRef]
- Gómez-Gómez, A.; Moyano, J.J.; Román-Manso, B.; Belmonte, M.; Miranzo, P.; Osendi, M.I. Highly-porous hierarchical SiC structures obtained by filament printing and partial sintering. J. Eur. Ceram. Soc. 2019, 39, 688–695. [Google Scholar] [CrossRef]
- Gu, S.; Lu, T.J.; Evans, A.G. On the design of two-dimensional cellular metals for combined heat dissipation and structural load capacity. Int. J. Heat Mass Transf. 2001, 44, 2163–2175. [Google Scholar] [CrossRef]
- Pires, J.C.; Alvim-Ferraz, M.C.; Martins, F.G. Photobioreactor design for microalgae production through computational fluid dynamics: A review. Renew. Sustain. Energy Rev. 2017, 79, 248–254. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Nam, S.-H.; Kim, S.; Cho, Y.T.; Fang, N.X. Low Heat Capacity 3D Hollow Microarchitected Reactors for Thermal and Fluid Applications. Energies 2022, 15, 4073. https://doi.org/10.3390/en15114073
Kim S, Nam S-H, Kim S, Cho YT, Fang NX. Low Heat Capacity 3D Hollow Microarchitected Reactors for Thermal and Fluid Applications. Energies. 2022; 15(11):4073. https://doi.org/10.3390/en15114073
Chicago/Turabian StyleKim, Seok, Sang-Hoon Nam, Seokho Kim, Young Tae Cho, and Nicholas X. Fang. 2022. "Low Heat Capacity 3D Hollow Microarchitected Reactors for Thermal and Fluid Applications" Energies 15, no. 11: 4073. https://doi.org/10.3390/en15114073





