Visualization and Measurement of Swirling Flow of Dry Ice Particles in Cyclone Separator-Sublimator
Abstract
:1. Introduction
2. Materials and Methods
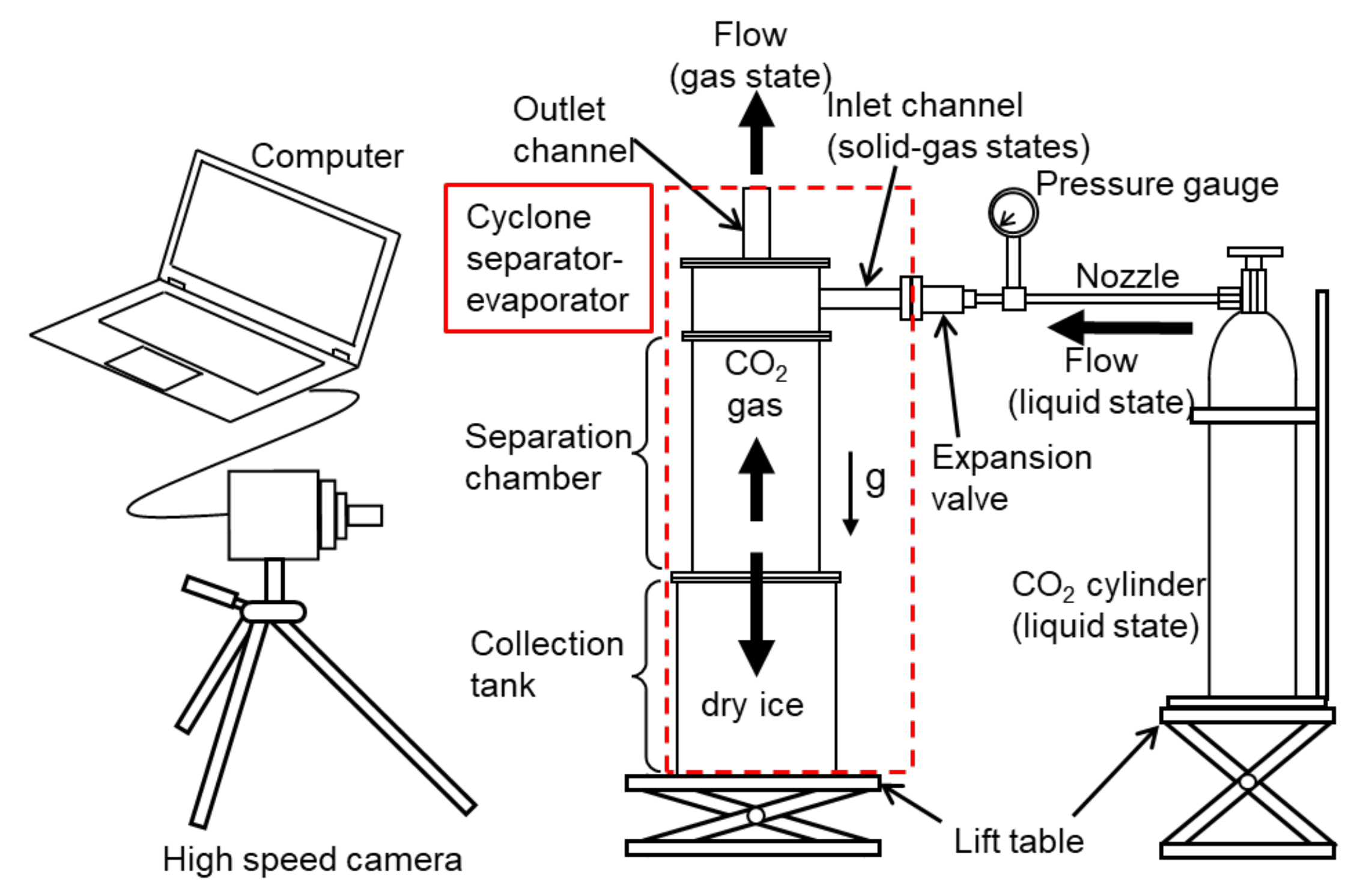
2.1. Experimental Setup
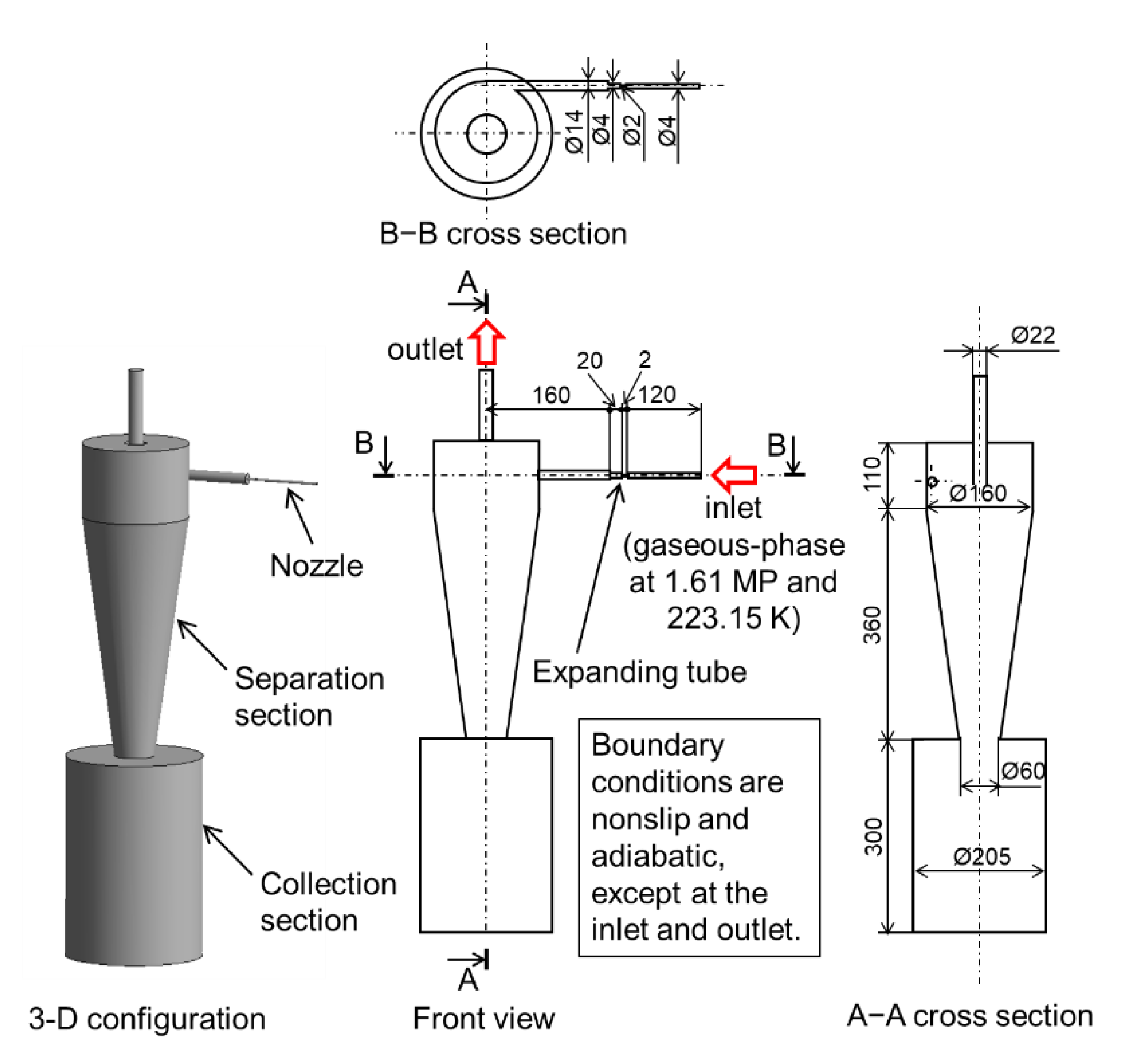
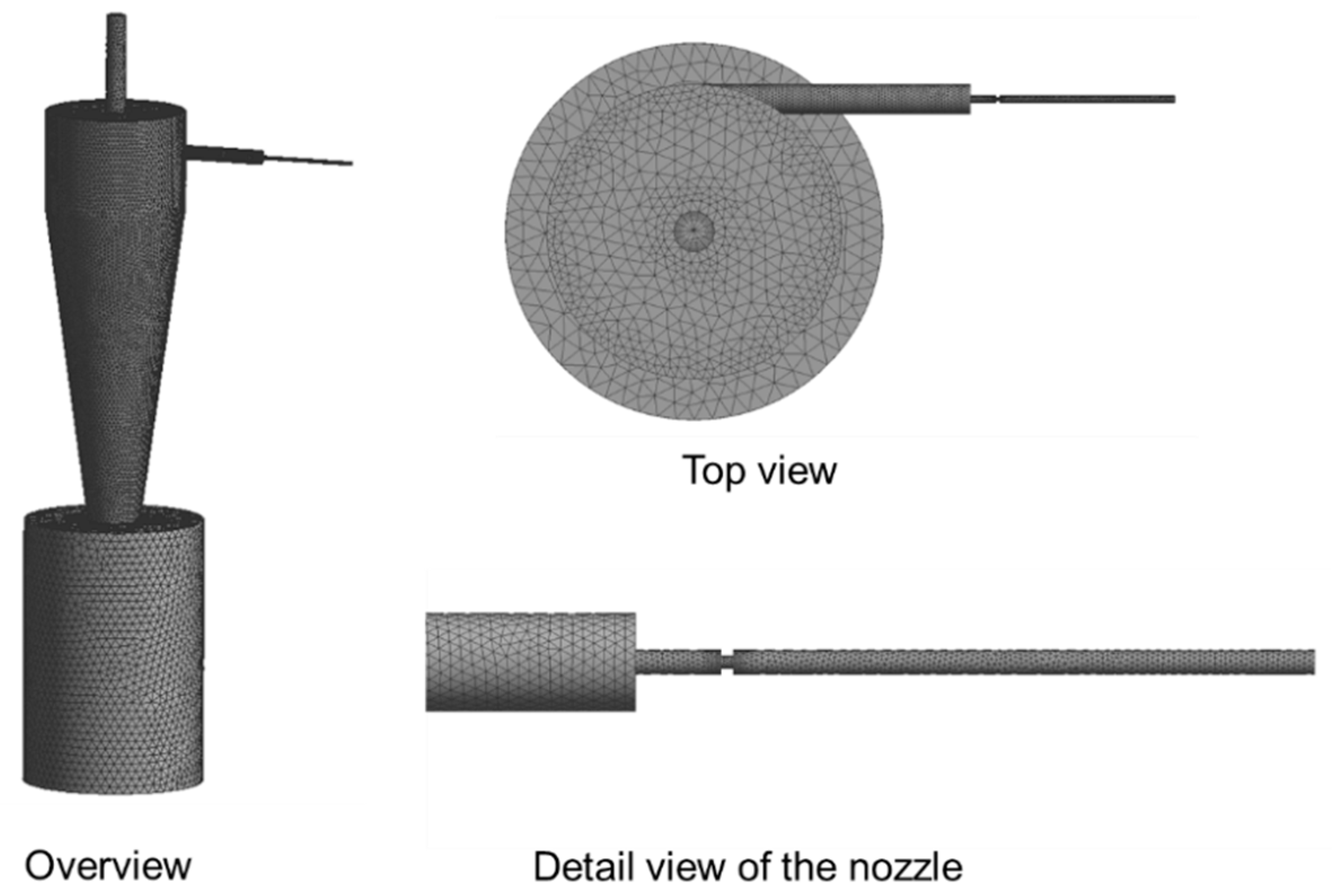
2.2. Simulation Model of CO2 Gaseous Phase Flow in the Separator-Evaporator
3. Results and Discussion
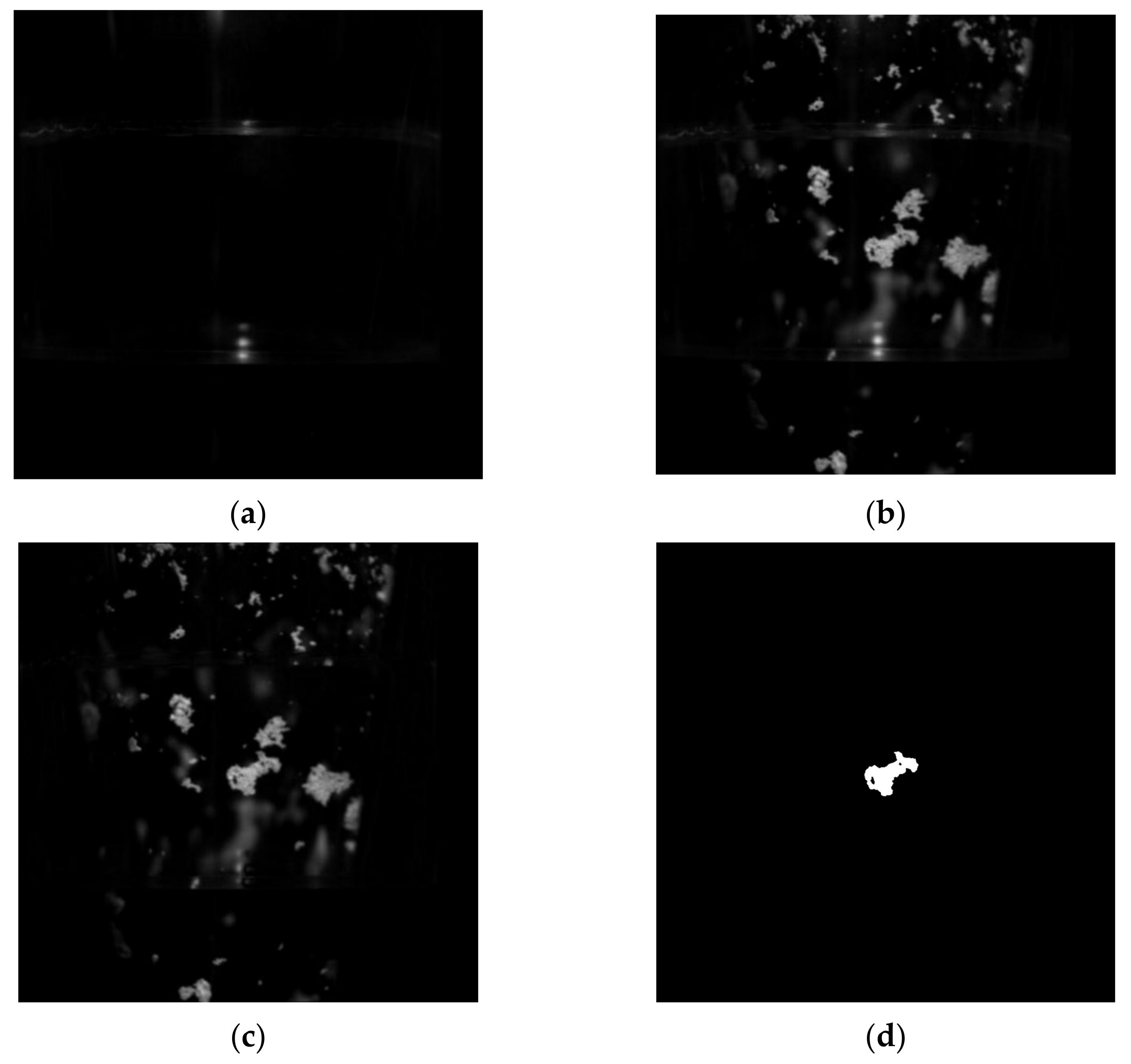
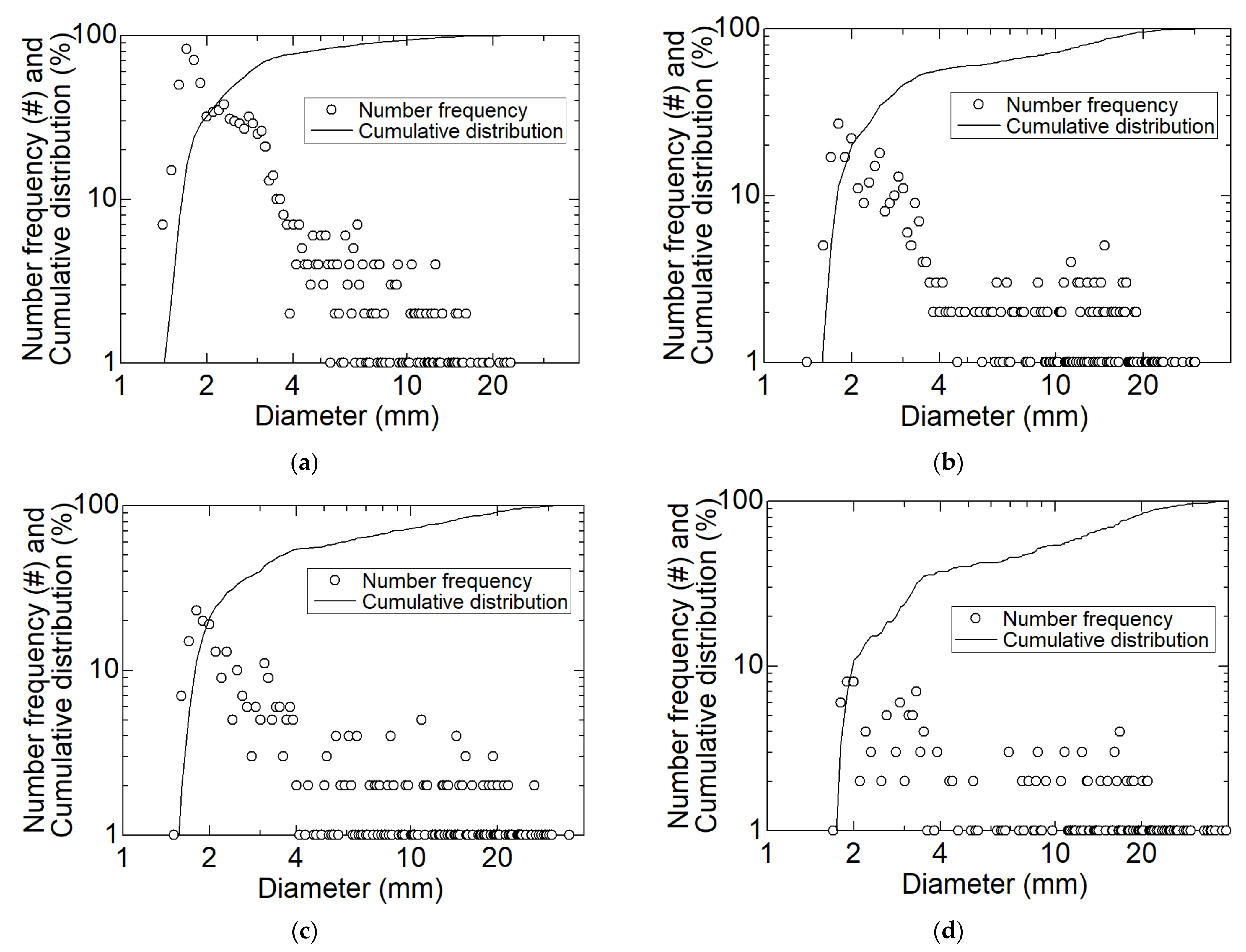
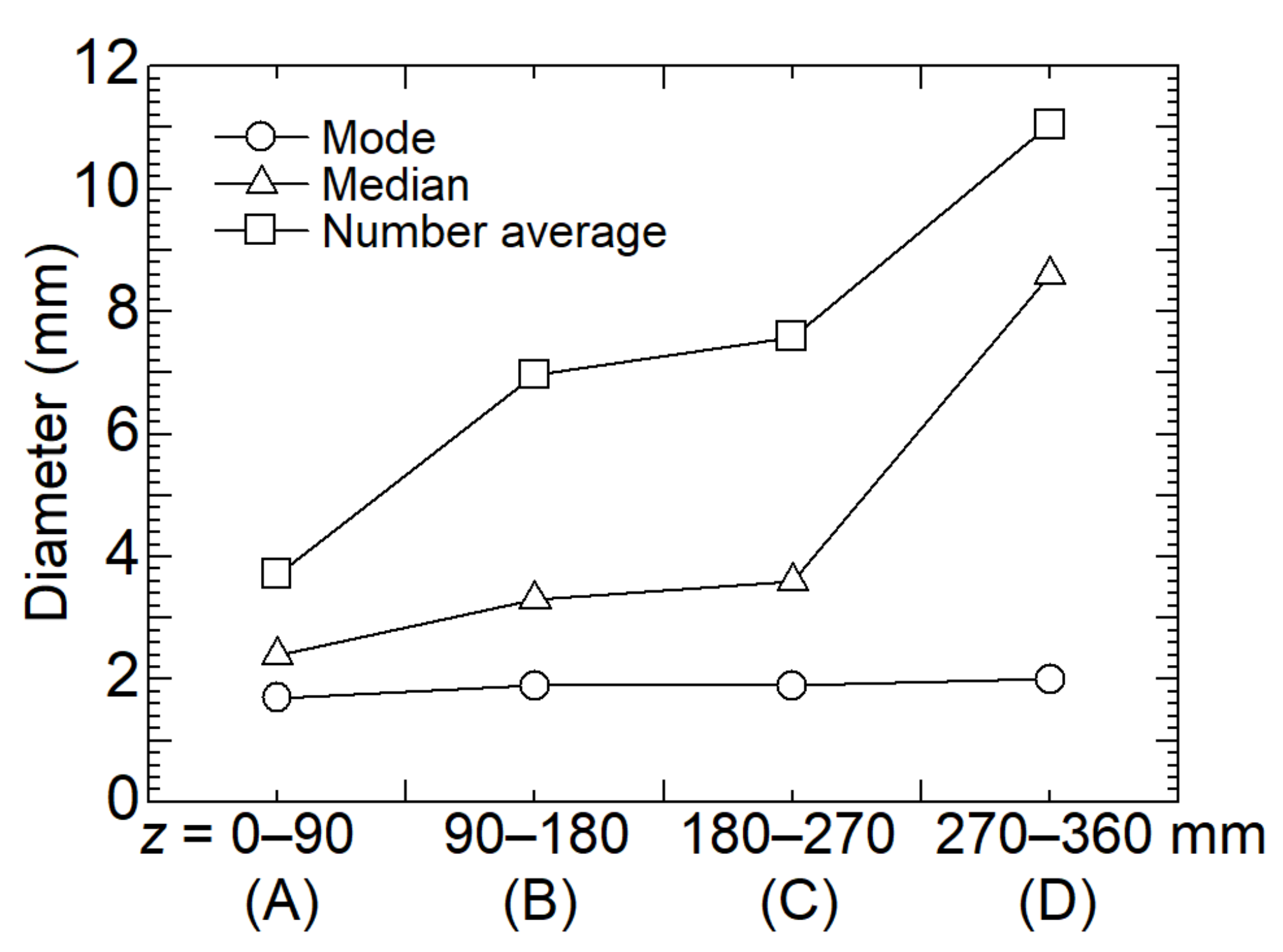
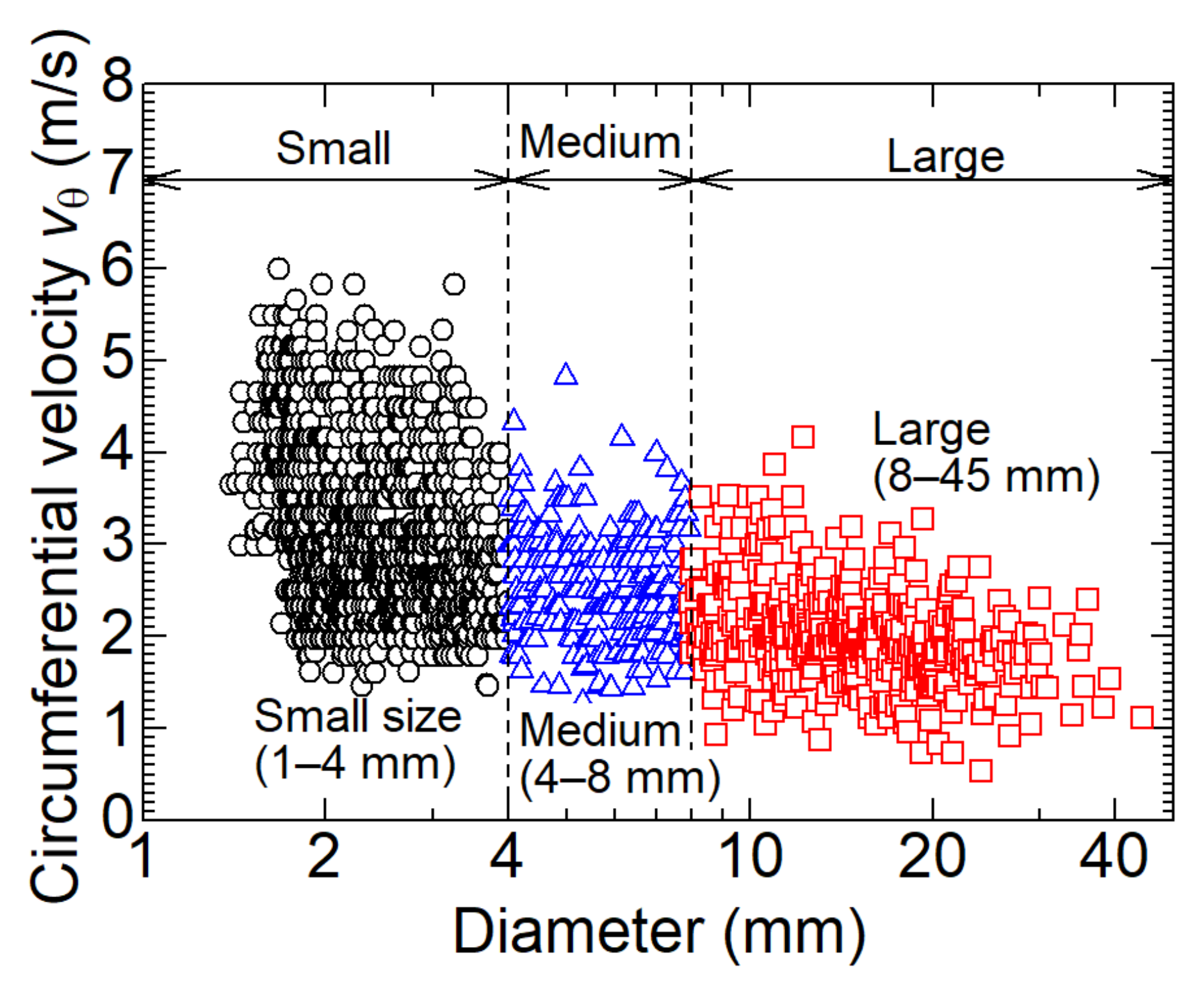
3.1. Size of Dry Ice Particles
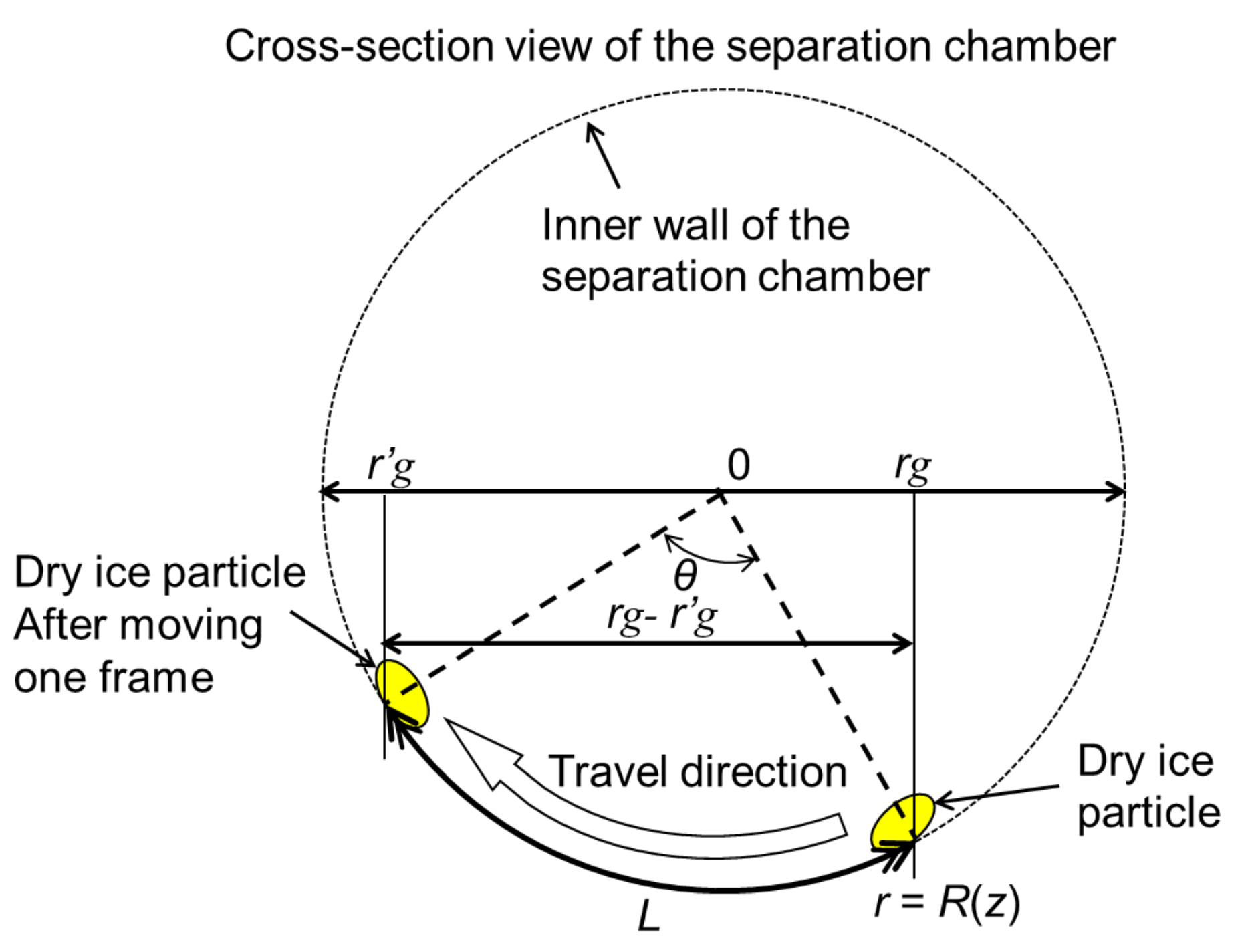
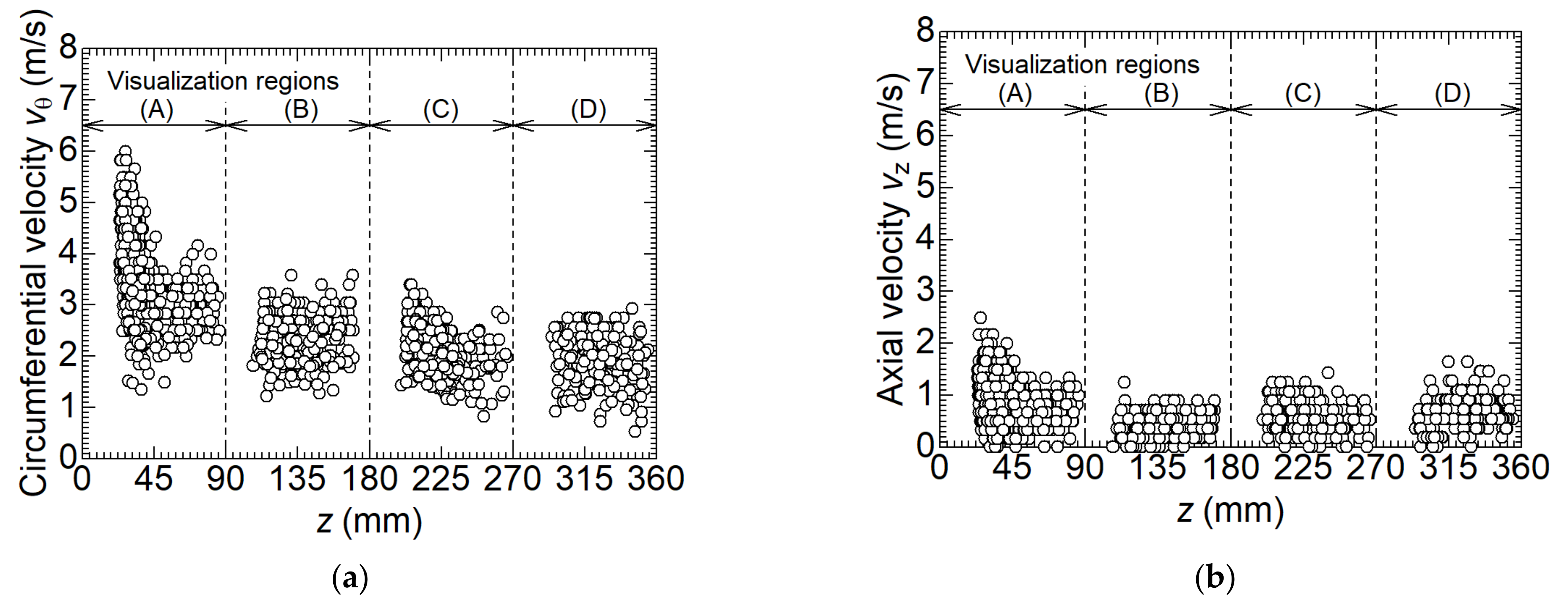
3.2. Dynamic Behavior of Dry Ice Particles
4. Conclusions
- After passing through the expansion valve, the high-pressure CO2 gas becomes a dry ice solid–gas two-phase flow, and the dry ice particles move on the inner wall by centrifugal force in the tapered channel of the cyclone separator-evaporator;
- The largest number of dry ice particles generated at any location in the cyclone separation section had an equivalent diameter of 2.0 mm. It was found that a certain amount of initially formed smaller dry ice particles does not necessarily contribute to coalescence. As the inner diameter of the separation section decreased, the dry ice particles coalesced and grew from 4.0 mm to up to 40 mm in equivalent diameter. However, particles as small as 2.0 mm may flow out of the separator outlet by approximately half;
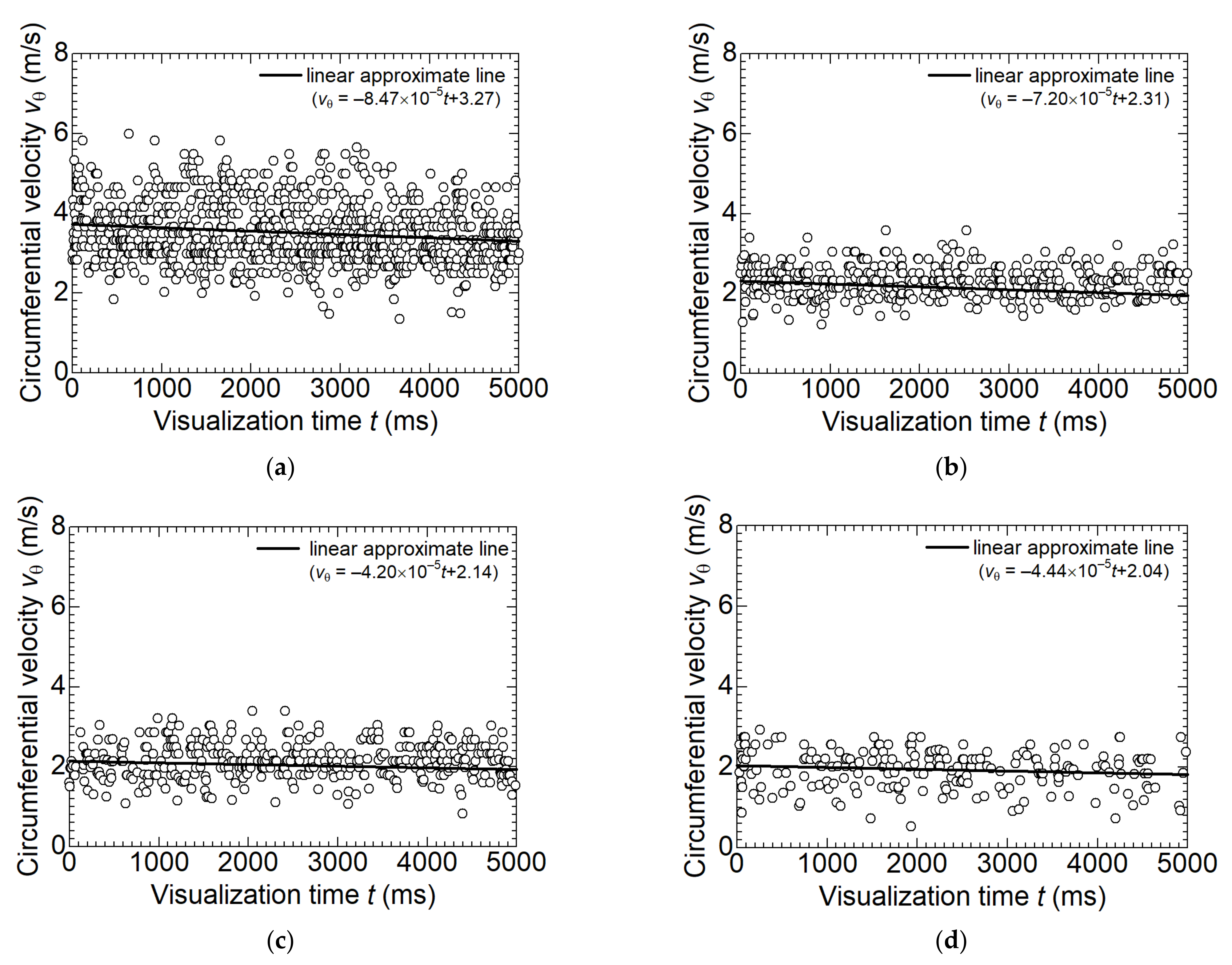
- In the separation chamber, the circumferential velocity decreases with increasing dry ice particle size. This is due to the increase in drag force on the surrounding gas as the particle size increases. The receiving centrifugal force increases the size of particles. The grown dry ice particles split as the circumferential velocity distribution increases. In addition, in the middle of the separation chamber, there are areas of particle retention due to the balance of centrifugal force, the normal reaction force from the wall surface, and gravity acting on the particles, which contribute to an increase in particle size;
- A particle motion model due to the drag force between CO2 gas and dry ice was formulated to compare the experimental and simulation results. The model showed good agreement with the experimental results, indicating that the drag force was dominant in the circumferential velocity of the dry ice particles;
- The kinetic model identified in this study can be used to advanced design a cyclone separator-evaporator for dry ice particles. The heat extracted from the sublimation of dry ice can be estimated from the particle size distribution, which can be realized for a new evaporator design in future heat pump systems.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| CO2 | Carbon dioxide |
| HCFCs | Hydrochlorofluorocarbons |
| HCFs | Hydrofluorocarbons |
| SST | Shear-stress transport |
| c | Number of particles at each particle size |
| Average drag coefficient | |
| Dave | Average particle diameter |
| E | Energy |
| g | Gravitational acceleration |
| i | Horizontal pixel number |
| j | Vertical pixel number |
| L | Circumferential movement distance |
| Average mass of particles | |
| n | Number of particle sizes |
| p | Pressure |
| Pc | Saturated pressure of CO2 |
| R | Gas constant |
| rg | Pixel center position |
| rp | Pixel coordinates |
| Rs | Swirl radius |
| Sp | Projection is of dry ice particles |
| T | Temperature |
| Tc | Saturated temperature of CO2 |
| u | Velocity vector of CO2 gas phase |
| V | Volume |
| vz | Axial velocity of dry ice particles |
| vθ | Circumferential velocity of dry ice particles |
| vθ,gas | Circumferential velocity of gas phase calculated by simulation |
| w | Variable that distinguishes between particles and non-particle |
| θ | Angle between the particle centers of the dry ice particles in each frame |
| λ | Thermal conductivity |
| μ | Viscosity of CO2 gas phase |
| ρ | Density of CO2 gas phase |
| ΨD | Dissipation loss |
References
- Tsai, W.T. An overview of environmental hazards and exposure risk of hydrofluorocarbons (HFCs). Chemosphere 2005, 61, 1539–1547. [Google Scholar] [CrossRef]
- Norman, C.; DeCanio, S.; Fan, L. The Montreal Protocol at 20: Ongoing opportunities for integration with climate protection. Glob. Environ. Change 2008, 18, 330–340. [Google Scholar] [CrossRef]
- Harby, K. Hydrocarbons and their mixtures as alternatives to environmental unfriendly halogenated refrigerants: An updated overview. Renew. Sustain. Energy Rev. 2017, 73, 1247–1264. [Google Scholar] [CrossRef]
- Velders, G.J.M.; Fahey, D.W.; Daniel, J.S.; Andersen, S.O.; McFarland, M. Future atmospheric abundances and climate forcings from scenarios of global and regional hydrofluorocarbon (HFC) emissions. Atmos. Environ. 2015, 123, 200–209. [Google Scholar] [CrossRef]
- Lorentzen, G. The use of natural refrigerants: A complete solution to the CFC/HCFC predicament. Int. J. Refrig. 1995, 18, 190–197. [Google Scholar] [CrossRef]
- Bamigbetan, O.; Eikevik, T.M.; Nekså, P.; Bantle, M. Review of vapour compression heat pumps for high temperature heating using natural working fluid. Int. J. Refrig. 2017, 80, 197–211. [Google Scholar] [CrossRef]
- Lorentzen, G. Revival of carbon dioxide as a refrigerant. Int. J. Refrig. 1994, 17, 292–301. [Google Scholar] [CrossRef]
- Rony, R.U.; Yang, H.; Krishnan, S.; Song, J. Recent advances in transcritical CO2 (R774) heat pump system: A review. Energies 2019, 12, 457. [Google Scholar] [CrossRef] [Green Version]
- Gullo, P.; Hafner, A.; Banasiak, K. Transcritical R744 refrigeration systems for supermarket applications: Current status and future perspectives. Int. J. Refrig. 2018, 93, 269–310. [Google Scholar] [CrossRef]
- Feng, F.; Zhang, Z.; Liu, X.; Liu, C.; Hou, Y. The Influence of internal heat exchanger on the performance of transcritical CO2 water source heat pump water heater. Energies 2020, 13, 1787. [Google Scholar] [CrossRef] [Green Version]
- Flores, J.M.B.; García, V.P.; Yudonago, J.F.I.; Muñoz, J.L.R.; Minguela, J.J.R. General aspects of carbon dioxide as a refrigerant. J. Energy South. Afr. 2014, 25, 96–106. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, B.; Mancuhan, E.; Erdonmez, N. A parametric study on a subcritical CO2/NH3 cascade Refrigeration system for low temperature applications. ASME J. Energy Resour. Technol. 2018, 140, 092004. [Google Scholar] [CrossRef]
- Aminyavari, M.; Najafi, B.; Shirazi, A.; Rinaldi, F. Exergetic, economic and environmental (3E) analyses, and multi-objective optimization of a CO2/NH3 cascade refrigeration system. Appl. Therm. Eng. 2014, 65, 42–50. [Google Scholar] [CrossRef]
- Gholamian, E.; Hanafizadeh, P.; Ahmadi, P. Advanced exergy analysis of a carbon dioxide ammonia cascade refrigeration system. Appl. Therm. Eng. 2018, 137, 689–699. [Google Scholar] [CrossRef]
- Bansal, P. A review—Status of CO2 as a low temperature refrigerant: Fundamentals and R&D opportunities. Appl. Therm. Eng. 2012, 41, 18–29. [Google Scholar]
- Huang, D.; Ding, G.; Quack, H. New refrigeration system using CO2 vapor-solid as refrigerant. Front. Energy Power Eng. China 2008, 2, 494–498. [Google Scholar] [CrossRef]
- Liu, Y.H.; Maruyama, H.; Matsusaka, S. Agglomeration process of dry ice particles produced by expanding liquid carbon dioxide. Adv. Powder Technol. 2010, 21, 652–657. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.H.; Calvert, G.; Hare, C.; Ghadiri, M.; Matsusaka, S. Size measurement of dry ice particles produced from liquid carbon dioxide. J. Aerosol. Sci. 2012, 48, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, H.; Zhang, X.R. A novel CO2 refrigeration system achieved by CO2 solid-gas two-phase fluid and its basic study on system performance. Int. J. Refrig. 2009, 32, 1683–1693. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Niu, X.D.; Sekimoto, K.; Nekså, P. Investigation of dry ice blockage in an ultra-low temperature cascade refrigeration system using CO2 as a working fluid. Int. J. Refri. 2011, 34, 466–475. [Google Scholar] [CrossRef]
- Yamasaki, H.; Yamaguchi, H.; Hattori, K.; Nekså, P. Experimental observation of CO2 dry-ice behavior in an evaporator/sublimator. Energy Procedia 2017, 143, 375–380. [Google Scholar] [CrossRef]
- Yamasaki, H. Boiling flow and heat transfer of CO2 in an evaporator. In Transcritical CO2 Heat Pump: Fundamentals and Applications; Zhang, X.R., Yamaguchi, H., Eds.; John Wiley & Sons Inc.: New York, NY, USA, 2021. [Google Scholar]
- Yamasaki, H.; Önder, K.; Yamaguchi, H.; Kamimura, T.; Hattori, K.; Nekså, P. Experimental investigation of dry ice cyclone separator for ultra-low temperature energy storage using carbon dioxide. Energy Storage 2020, 2, e149. [Google Scholar] [CrossRef]
- OpenCV. Available online: https://opencv.org/ (accessed on 17 May 2022).
- Hoekstra, A.J.; Derksen, J.J.; Van Den Akker, H.E.A. An experimental and numerical study of turbulent swirling fliw in gas cyclones. Chem. Eng. Sci. 1999, 54, 2055–2065. [Google Scholar] [CrossRef] [Green Version]
- Sterczynska, M.; Stachnik, M.; Poreda, A.; Piepiórka-Stepuk, J.; Zdaniewicz, M.; Jakubowski, M. The improvement of flow conditions in a whirlpool with a modified bottom: An experimental study based on particle image velocimetry (PIV). J. Food Eng. 2021, 289, 110164. [Google Scholar] [CrossRef]
- Zhao, B.; Su, Y.; Zhang, J. Simulation of gas flow patter and separation efficiency in cyclone with conventional single and spiral double inlet configuration. Chem. Eng. Res. Des. 2006, 84, 1158–1165. [Google Scholar] [CrossRef]
- Redlich, O.; Kwong, J.N.S. On the thermodynamics of solutions. V. An equation of state. Fugacities of gaseous solutions. Chem. Rev. 1949, 44, 233–244. [Google Scholar] [CrossRef]
- Stachnik, M.; Jalubowski, M. Multiphase model of flow and separation phases in a whirlpool: Advanced simulation and phenomena visualization approach. J. Food Eng. 2020, 274, 109846. [Google Scholar] [CrossRef]
- Chlebnikovas, A.; Kilikevičius, A.; Selech, J.; Matijošius, J.; Kilikevičienė, K.; Vainorius, D.; Passerini, G.; Marcinkiewicz, J. The Numerical Modeling of Gas Movement in a Single Inlet New Generation Multi-Channel Cyclone Separator. Energies 2021, 14, 8092. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamasaki, H.; Wakimoto, H.; Kamimura, T.; Hattori, K.; Nekså, P.; Yamaguchi, H. Visualization and Measurement of Swirling Flow of Dry Ice Particles in Cyclone Separator-Sublimator. Energies 2022, 15, 4128. https://doi.org/10.3390/en15114128
Yamasaki H, Wakimoto H, Kamimura T, Hattori K, Nekså P, Yamaguchi H. Visualization and Measurement of Swirling Flow of Dry Ice Particles in Cyclone Separator-Sublimator. Energies. 2022; 15(11):4128. https://doi.org/10.3390/en15114128
Chicago/Turabian StyleYamasaki, Haruhiko, Hiroyuki Wakimoto, Takeshi Kamimura, Kazuhiro Hattori, Petter Nekså, and Hiroshi Yamaguchi. 2022. "Visualization and Measurement of Swirling Flow of Dry Ice Particles in Cyclone Separator-Sublimator" Energies 15, no. 11: 4128. https://doi.org/10.3390/en15114128




