Physical Properties of Ti45Zr38Fe17 Alloy and Its Amorphous Hydride
Abstract
1. Introduction
2. Experimental
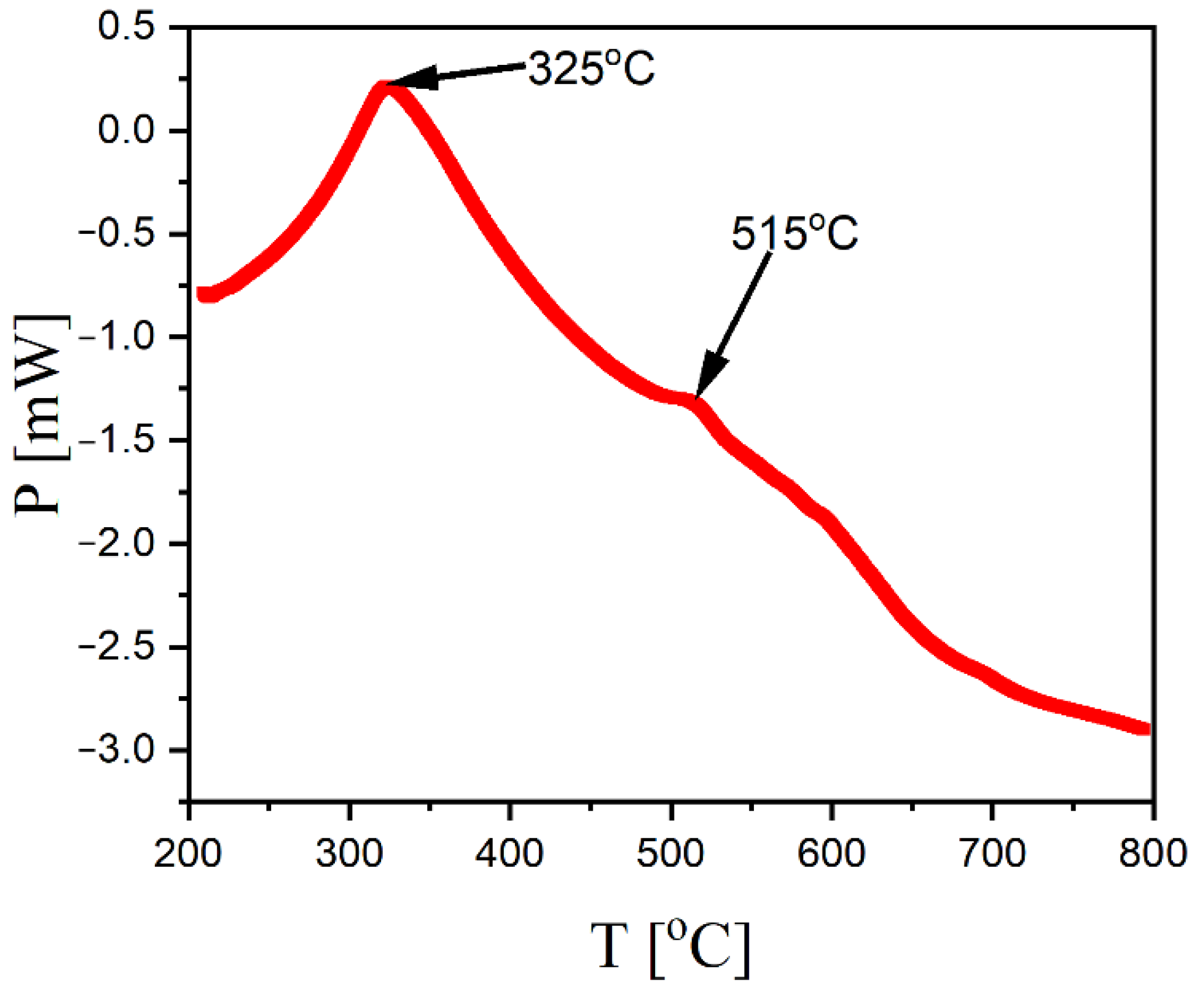
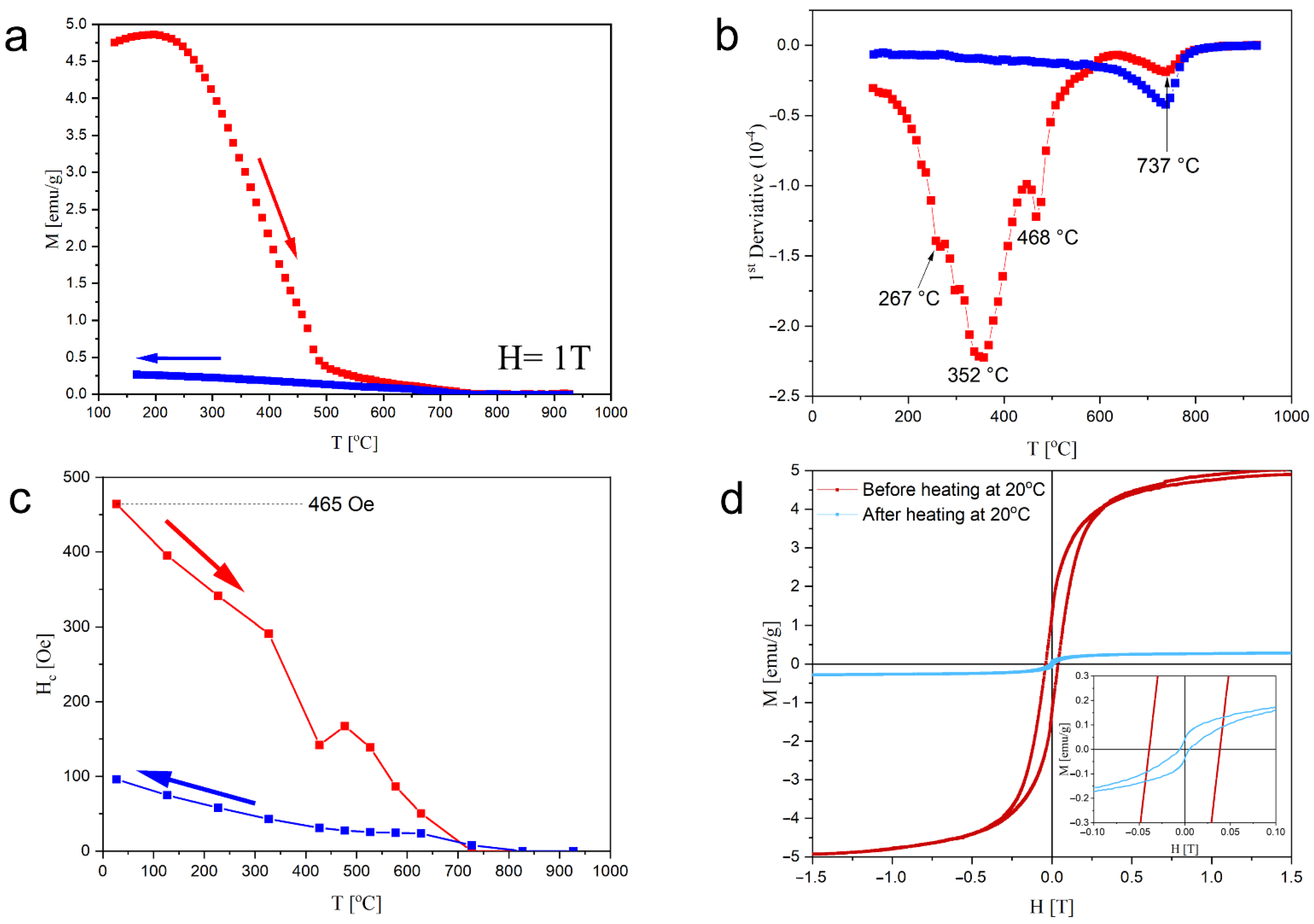
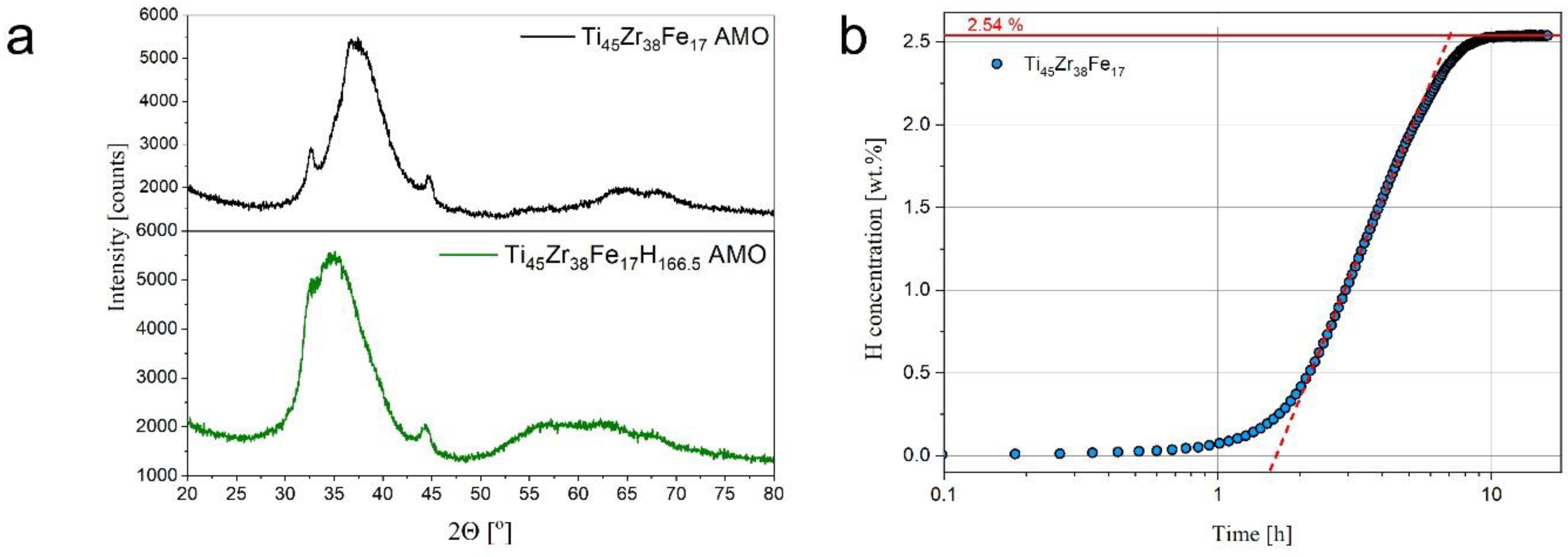
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reuß, M.; Dimos, P.; Léon, A.; Grube, T.; Robinius, M.; Stolten, D. Hydrogen Road Transport Analysis in the Energy System: A Case Study for Germany through 2050. Energies 2021, 14, 3166. [Google Scholar] [CrossRef]
- Capurso, T.; Stefanizzi, M.; Torresi, M.; Camporeale, S. Perspective of the role of hydrogen in the 21st century energy transition. Energy Convers. Manag. 2022, 251, 114898. [Google Scholar] [CrossRef]
- Clees, T.; Baldin, A.; Klaassen, B.; Nikitina, L.; Nikitin, I.; Spelten, P. Efficient method for simulation of long-distance gas transport networks with large amounts of hydrogen injection. Energy Convers. Manag. 2021, 234, 113984. [Google Scholar] [CrossRef]
- Kuczyński, S.; Łaciak, M.; Olijnyk, A.; Szurlej, A.; Włodek, T. Thermodynamic and Technical Issues of Hydrogen and Methane-Hydrogen Mixtures Pipeline Transmission. Energies 2019, 12, 569. [Google Scholar] [CrossRef]
- Oliva, D.; Fuentes, M.; Borzone, E.; Meyer, G.; Aguirre, P. Hydrogen storage on LaNi5−xSnx. Experimental and phenomenological Model-based analysis. Energy Convers. Manag. 2018, 173, 113–122. [Google Scholar] [CrossRef]
- Dematteis, E.M.; Barale, J.; Corno, M.; Sciullo, A.; Baricco, M.; Rizzi, P. Solid-State Hydrogen Storage Systems and the Relevance of a Gender Perspective. Energies 2021, 14, 6158. [Google Scholar] [CrossRef]
- Takasaki, A.; Kelton, K. High-pressure hydrogen loading in Ti45Zr38Ni17 amorphous and quasicrystal powders synthesized by mechanical alloying. J. Alloys Compd. 2002, 347, 295–300. [Google Scholar] [CrossRef]
- Kim, W.J.; Kelton, K.F. Icosahedral and related phase formation in rapidly quenched Ti-Zr-Fe alloys. Philos. Mag. A 1995, 72, 1397–1408. [Google Scholar] [CrossRef]
- Kim, W.J.; Gibbons, P.C.; Kelton, K.F.; Yelon, W.B. Structural refinement of1/1bcc approximants to quasicrystals: Bergman-typeW(TiZrNi)and Mackay-typeM(TiZrFe). Phys. Rev. B 1998, 58, 2578–2585. [Google Scholar] [CrossRef]
- Żywczak, A.; Gondek, Ł.; Figiel, H.; Żukrowski, J.; Czub, J.; Takasaki, A. Structural and hyperfine properties of Ti48Zr7Fe18 nano-compounds and its hydrides. J. Alloys Compd. 2011, 509, 3952–3957. [Google Scholar] [CrossRef]
- Ji, P.; Chen, B.; Li, B.; Tang, Y.; Zhang, G.; Zhang, X.; Ma, M.; Liu, R. Influence of Nb addition on microstructural evolution and compression mechanical properties of Ti-Zr alloys. J. Mater. Sci. Technol. 2020, 69, 7–14. [Google Scholar] [CrossRef]
- Ivanova, A.; Surmeneva, M.; Shugurov, V.; Koval, N.; Shulepov, I.; Surmenev, R. Physico-mechanical properties of Ti-Zr coatings fabricated via ion-assisted arc-plasma deposition. Vacuum 2018, 149, 129–133. [Google Scholar] [CrossRef]
- Jhou, W.-T.; Wang, C.; Ii, S.; Chiang, H.-S.; Hsueh, C.-H. TiNiCuAg shape memory alloy films for biomedical applications. J. Alloys Compd. 2018, 738, 336–344. [Google Scholar] [CrossRef]
- Liu, S.; Miao, J.; Zhang, W.; Wei, R.; Chen, C.; Wang, T.; Zhao, W.; Jiang, Z.; Li, F. Interfacial microstructure and shear strength of TC4 alloy joints vacuum brazed with Ti–Zr–Ni–Cu filler metal. Mater. Sci. Eng. A 2020, 775, 138990. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Qian, M.; Shi, Z.; Song, T.; Huang, L.; Zou, J. A novel quaternary equiatomic Ti-Zr-Nb-Ta medium entropy alloy (MEA). Intermetallics 2018, 101, 39–43. [Google Scholar] [CrossRef]
- Liens, A.; Ter-Ovanessian, B.; Courtois, N.; Fabregue, D.; Wada, T.; Kato, H.; Chevalier, J. Effect of alloying elements on the microstructure and corrosion behavior of TiZr-based bulk metallic glasses. Corros. Sci. 2020, 177, 108854. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Faverani, L.P.; Grandini, C.R.; Rangel, E.C.; da Cruz, N.C.; Junior, F.H.N.; Almeida, A.B.; Vicente, F.B.; Morais, B.R.; Barão, V.A.; et al. Characterization of chemically treated Ti-Zr system alloys for dental implant application. Mater. Sci. Eng. C 2018, 92, 849–861. [Google Scholar] [CrossRef]
- Zuo, S.; Wu, R.; Pang, G.; Yang, Y.; Jin, M. High temperature internal friction in Ni50.3Ti29.7Zr20 shape memory alloy. Intermetallics 2019, 109, 174–178. [Google Scholar] [CrossRef]
- Mishra, S.; Yadav, T.; Srivastava, O.; Mukhopadhyay, N.; Biswas, K. Formation and stability of C14 type Laves phase in multi component high-entropy alloys. J. Alloys Compd. 2020, 832, 153764. [Google Scholar] [CrossRef]
- Lee, S.-H.; Huq, A.; Yang, W.; Kim, J. Analysis of local sites of deuterium in Ti53Zr27Ni20 alloys. Phys. B Condens. Matter 2018, 551, 33–36. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Z.; Liu, F.; Xu, J. Nonlinear dynamic characteristics and bifurcation analysis of Ti–Zr–Ni quasicrystal as hydrogen storage material. Int. J. Hydrogen Energy 2021, 46, 16667–16675. [Google Scholar] [CrossRef]
- Lee, S.-H.; Park, T.; Yi, W.; Kim, J. Structure and Electrical Conductivity of Ag-Doped TiZrNi Quasicrystals. J. Nanosci. Nanotechnol. 2016, 16, 10532–10534. [Google Scholar] [CrossRef]
- Jo, Y.; Lee, S.-H.; Shin, H.S.; Kim, J. Analysis of Structure and P–c–T Curve of Hydrogenated Ti53Zr27−xNi20Pdx Quasicrystals. J. Nanosci. Nanotechnol. 2013, 13, 7959–7962. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Li, Z.; Zhou, X.; Liu, H.; Sun, J.; Su, Z.; Liu, W.; Zhao, J. Improved electrochemical hydrogen storage properties of Ti49Zr26Ni25 quasicrystal alloy by doping with Pd and MWCNTs. Int. J. Hydrogen Energy 2019, 44, 29356–29364. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Mi, G.; Zhang, Z.; Wang, L. Crystallographic and electrochemical characteristics of Ti–Zr–Ni–Pd quasicrystalline alloys. Int. J. Hydrogen Energy 2009, 34, 6925–6929. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, J. Structure and hydrogen absorption properties of Ti53Zr27Ni20(Pd,V) quasicrystals. Int. J. Hydrogen Energy 2018, 43, 19130–19140. [Google Scholar] [CrossRef]
- Żywczak, A.; Rusinek, D.; Czub, J.; Sikora, M.; Stępień, J.; Gondek, Ł.; Takasaki, A.; Hoser, A. Amorphous hydrides of the Ti45Zr38Ni17−xCox nano-powders. Int. J. Hydrogen Energy 2015, 40, 15534–15539. [Google Scholar] [CrossRef]
- Liu, B.; Wu, Y.; Wang, L. Kinetic and electrochemical properties of icosahedral quasicrystalline Ti45Zr35Ni17Cu3 powder. Int. J. Hydrogen Energy 2006, 31, 1394–1400. [Google Scholar] [CrossRef]
- Liu, C.; Duan, Y.; Ouyang, Z.; Shang, J.; Liu, K.; Xing, C.; Fu, Y.; Liu, W.; Wang, L. Effect of Li on structure and electrochemical hydrogen storage properties of Ti55V10Ni35 quasicrystal alloy. Int. J. Hydrogen Energy 2015, 40, 3015–3022. [Google Scholar] [CrossRef]
- Liu, W.; Duan, Q.; Liang, F.; Lin, J.; Jiang, D.; Wang, L. Effect of Ce on electrochemical properties of the TiVNi quasicrystal material as an anode for Ni/MH batteries. Int. J. Hydrogen Energy 2013, 38, 14810–14815. [Google Scholar] [CrossRef]
- Lin, J.; Liang, F.; Wu, Y.; Liu, W.; Wang, L. Hydrogen storage properties of Ti1.4V0.6Ni + x Mg (x = 1–3, wt.%) alloys. Int. J. Hydrogen Energy 2014, 39, 3313–3319. [Google Scholar] [CrossRef]
- Żywczak, A.; Shinya, D.; Gondek, Ł.; Takasaki, A.; Figiel, H. Hydriding of Ti45Zr38Ni17−xFex nanocompounds. Solid State Commun. 2010, 150, 1–4. [Google Scholar] [CrossRef]
- Ibuka, S.; Iida, K.; Sato, T.J. Magnetic properties of the Ag–In–rare-earth 1/1 approximants. J. Phys. Condens. Matter 2011, 23, 056001. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hiroto, T.; Gebresenbut, G.H.; Gómez, C.P.; Muro, Y.; Isobe, M.; Ueda, Y.; Tokiwa, K.; Tamura, R. Ferromagnetism and re-entrant spin-glass transition in quasicrystal approximants Au–SM–Gd (SM = Si, Ge). J. Phys. Condens. Matter 2013, 25, 426004. [Google Scholar] [CrossRef]
- Tamura, R.; Muro, Y.; Hiroto, T.; Nishimoto, K.; Takabatake, T. Long-range magnetic order in the quasicrystalline approximant Cd6Tb. Phys. Rev. B 2010, 82, 220201. [Google Scholar] [CrossRef]
- Ishikawa, A.; Fujii, T.; Takeuchi, T.; Yamada, T.; Matsushita, Y.; Tamura, R. Antiferromagnetic order is possible in ternary quasicrystal approximants. Phys. Rev. B 2018, 98, 220403. [Google Scholar] [CrossRef]
- Inagaki, K.; Suzuki, S.; Ishikawa, A.; Tsugawa, T.; Aya, F.; Yamada, T.; Tokiwa, K.; Takeuchi, T.; Tamura, R. Ferromagnetic 2/1 quasicrystal approximants. Phys. Rev. B 2020, 101, 180405. [Google Scholar] [CrossRef]
- Goldman, A.I.; Kong, T.; Kreyssig, A.; Jesche, A.; Ramazanoglu, M.; Dennis, K.W.; Bud’Ko, S.L.; Canfield, P.C. A family of binary magnetic icosahedral quasicrystals based on rare earths and cadmium. Nat. Mater. 2013, 12, 714–718. [Google Scholar] [CrossRef]
- Shin, H.; Lee, S.-H.; Jo, Y.; Kim, J. Effects of hydrogen on the magnetic properties of TiZrNi quasicrystals. J. Korean Phys. Soc. 2012, 61, 1541–1544. [Google Scholar] [CrossRef]
- Czub, J.; Przewoźnik, J.; Żywczak, A.; Takasaki, A.; Hoser, A. On magnetism in the quasicrystalline Ti 45 Zr 38 Ni 17 alloy. J. Non-Cryst. Solids 2017, 470, 108–111. [Google Scholar] [CrossRef]
- Liu, W.; Feya, O.D.; Debela, T.T.; Hester, J.R.; Webb, C.J.; Gray, E.M. Experimental and computational modelling study of Ni substitution for Fe in Zr3Fe and its hydride. J. Alloys Compd. 2018, 781, 131–139. [Google Scholar] [CrossRef]
- Chun, Y.; Kang, S.; Lee, D.; Cho, S.; Jeong, Y.; Żywczak, A.; Rhee, C. Development of Zr-containing advanced reduced-activation alloy (ARAA) as structural material for fusion reactors. Fusion Eng. Des. 2016, 109–111, 629–633. [Google Scholar] [CrossRef]
- Liu, Y.; Chabane, D.; Elkedim, O. Intermetallic Compounds Synthesized by Mechanical Alloying for Solid-State Hydrogen Storage: A Review. Energies 2021, 14, 5758. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żywczak, A.; Gondek, Ł.; Czub, J.; Janusz, P.; Selvaraj, N.B.; Takasaki, A. Physical Properties of Ti45Zr38Fe17 Alloy and Its Amorphous Hydride. Energies 2022, 15, 4236. https://doi.org/10.3390/en15124236
Żywczak A, Gondek Ł, Czub J, Janusz P, Selvaraj NB, Takasaki A. Physical Properties of Ti45Zr38Fe17 Alloy and Its Amorphous Hydride. Energies. 2022; 15(12):4236. https://doi.org/10.3390/en15124236
Chicago/Turabian StyleŻywczak, Antoni, Łukasz Gondek, Joanna Czub, Piotr Janusz, Nivas Babu Selvaraj, and Akito Takasaki. 2022. "Physical Properties of Ti45Zr38Fe17 Alloy and Its Amorphous Hydride" Energies 15, no. 12: 4236. https://doi.org/10.3390/en15124236
APA StyleŻywczak, A., Gondek, Ł., Czub, J., Janusz, P., Selvaraj, N. B., & Takasaki, A. (2022). Physical Properties of Ti45Zr38Fe17 Alloy and Its Amorphous Hydride. Energies, 15(12), 4236. https://doi.org/10.3390/en15124236






