Solid Digestate—Mathematical Modeling of Combustion Process
Abstract
1. Introduction
1.1. Digestate Form Methane Fermentation
1.2. Modeling of Combustion
2. Materials and Methods
3. Results
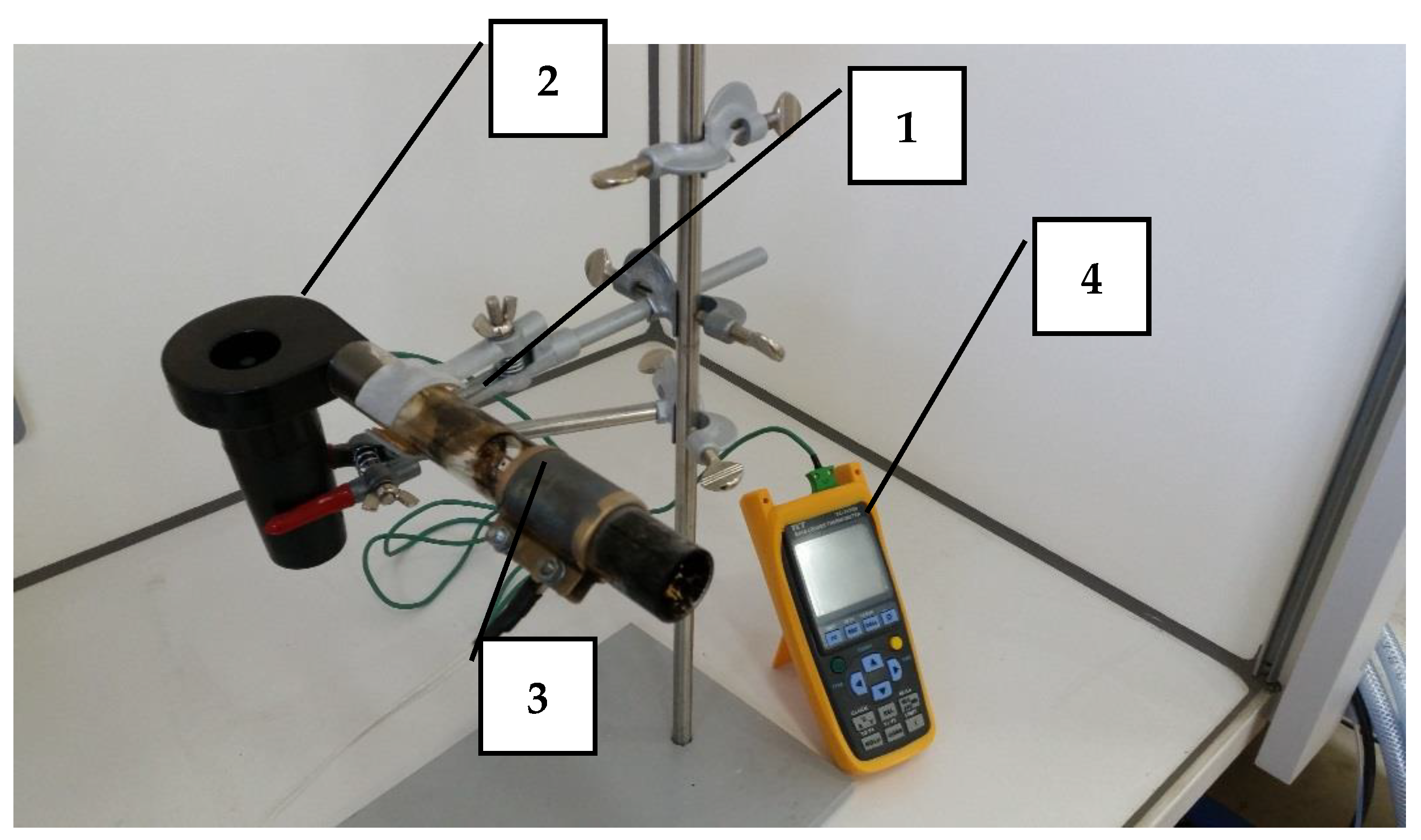
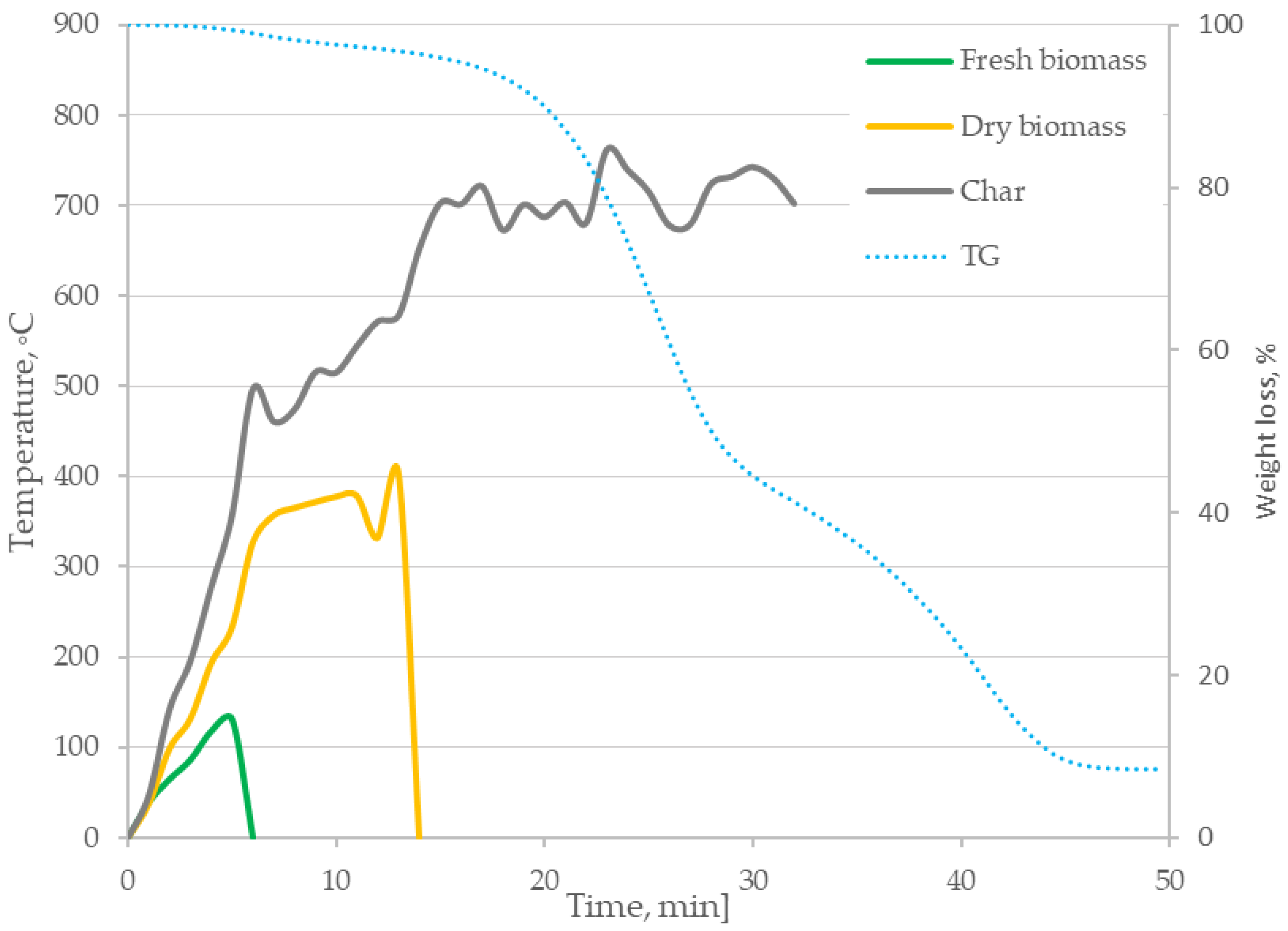
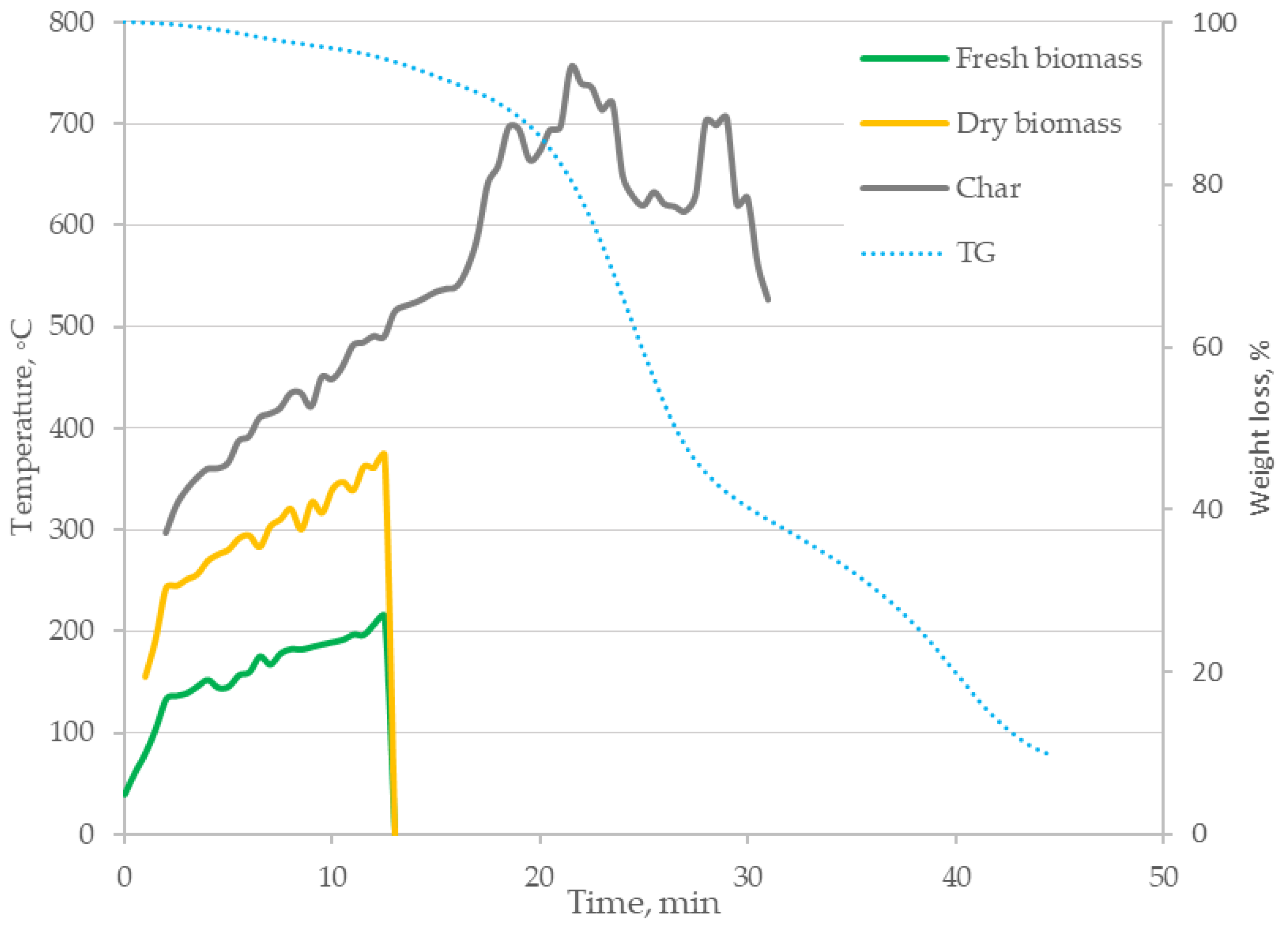
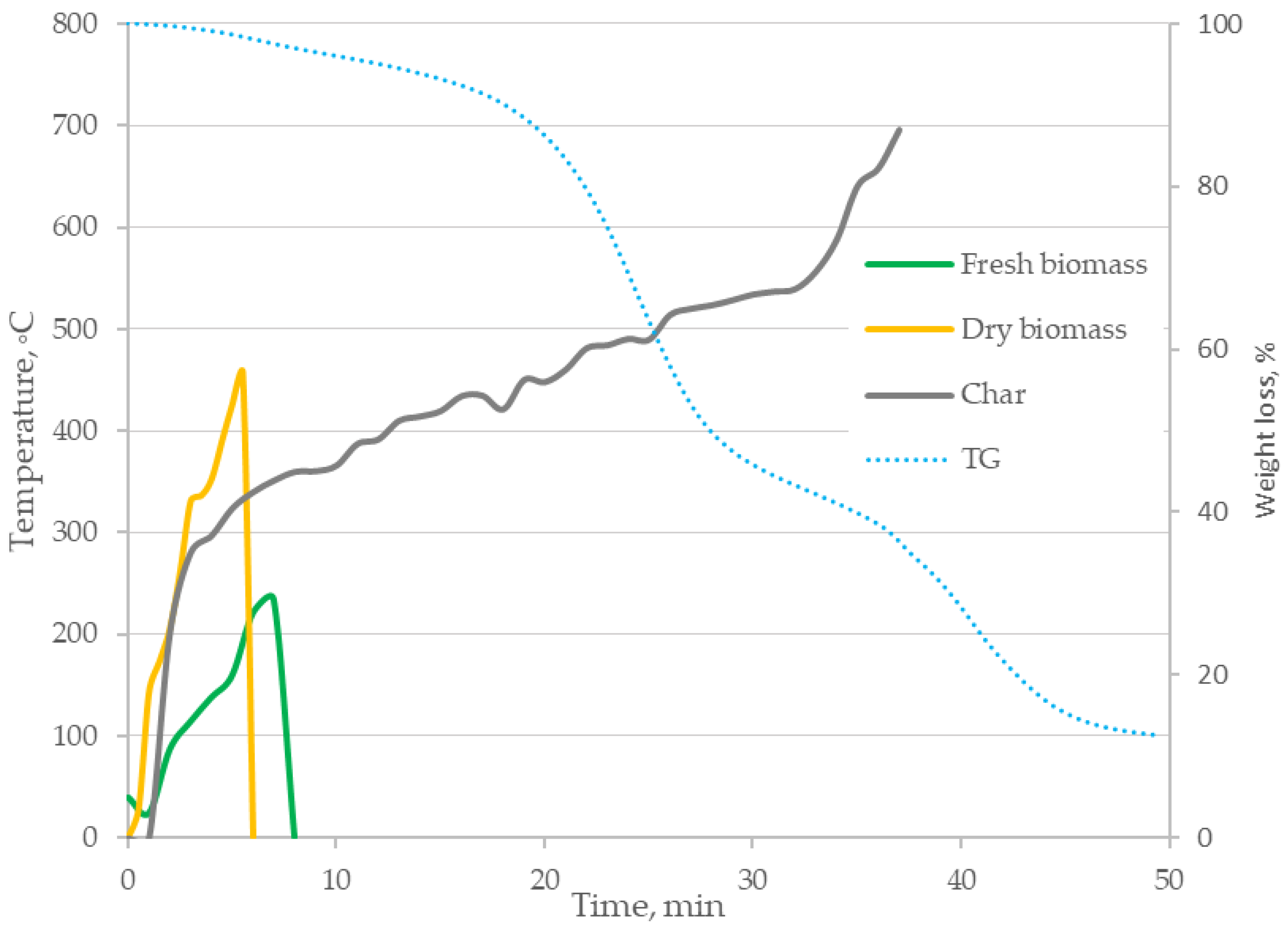
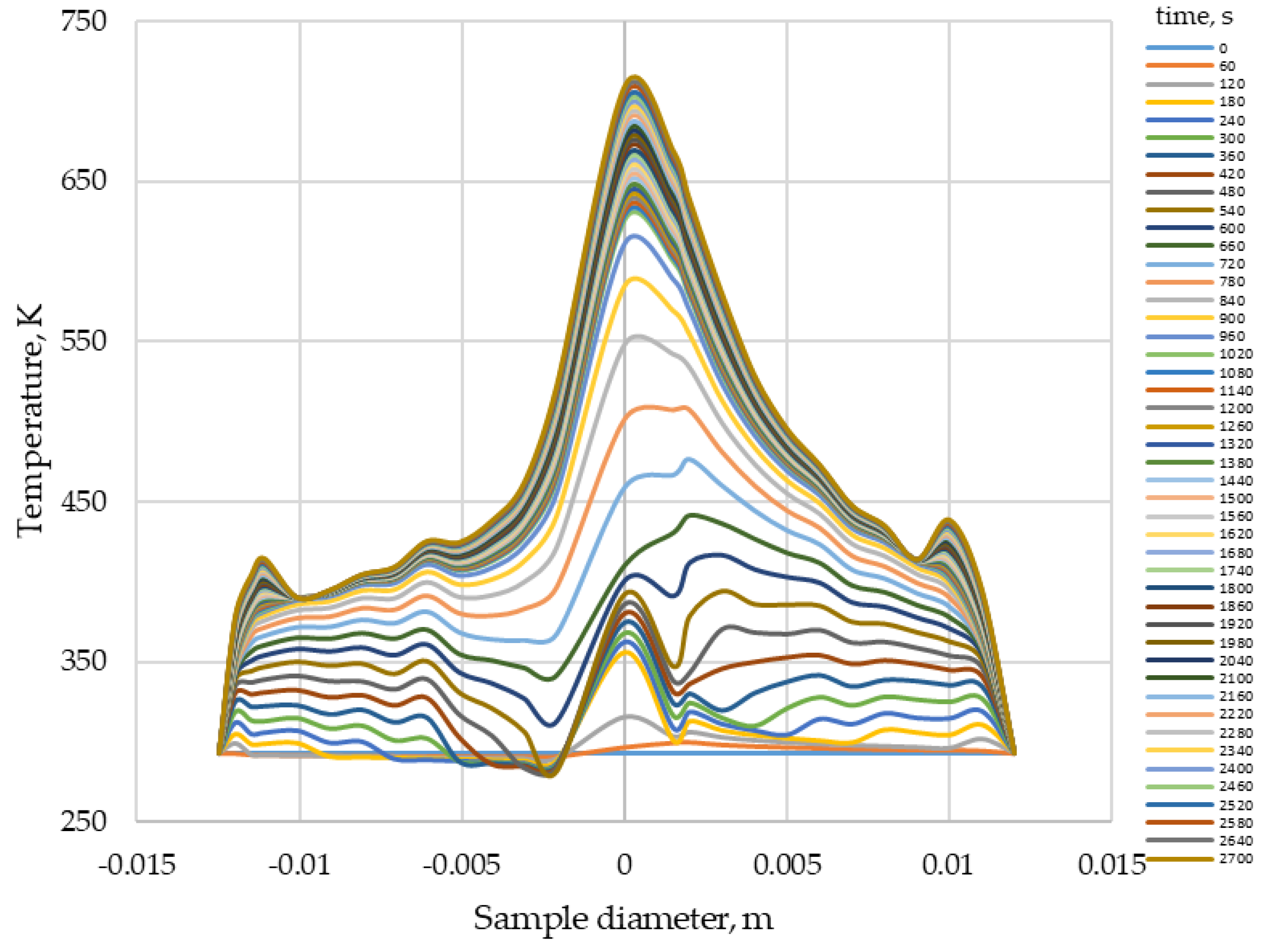
3.1. Thermal Imaging Analysis of the Combustion Process
3.2. Mathematical Model of Biomass Particles Combustion
- The biomass particle is a continuous medium in the shape of a limited cylinder, with isotropic properties;
- The model describes the process of heat and mass exchange in two phases of the combustion process: drying to 100 °C and degassing and combustion of char (degassing and gasification of biomass);
- Physical properties for successively arising phases: density, specific heat and thermal conductivity coefficient are constant;
- The combustion kinetic constant is a function of the activation energy and temperature;
- The model does not take into account a separate burning stage of the char.
- -
- Initial:
- 1st combustion phase τ = 0 s: mp = mp0, u = u0, T = T0 = Tot;
- 2nd phase of combustion τ = end of drying: u = uks, T = Tks = 100 °C, mp = mss, mchr = 0, mv = 0, ma = 0;
- -
- Boundary conditions:
- For mass exchange during drying, the boundary condition of the first type of Dirichlet:
- For heat transfer during the entire combustion process, the Fourier boundary condition of the type:
- 1st combustion phase drying:
- 2nd phase of combustion—degassing and combustion of char:
- P→O, L→O—heat flux supplied to the sample at point O, from the left
- and the right element, W.
- N—power, W.
- r0—the length of the ray in the center of the discrete element O, m.
- —sum of internal sources, Jm−3.
- L—point in the left discrete element.
- P—point in the right discrete element.
- O—point in the main central discrete element.
- i—time step number.
- j—spatial step number.
- s—substance type: “char”, dry substance “s”, volatile matter “v”, ash “a”.
4. Conclusions
- The physicochemical properties of the tested mixtures changed with the change of composition—an increase in the proportion of maize silage in the analysed mixtures resulted in an increase in ash content and a simultaneous decrease in calorific value and heat of combustion, while in the case of an increase in the share of apple pomace, an increase in volatile matter was observed;
- Regardless of the composition of the analysed mixtures, their elemental composition was similar to each other;
- The formulated research hypothesis has been verified correctly—based on thermogravimetric tests, differential scanning calorimetry and the proposed method of thermogram analysis, it was found that the digestate combustion process takes place in the following stages: drying, degassing and gasification as well as char combustion. This was confirmed by temperature measurements inside the sample during the process and mass loss rate tests, enabling identification of phase changes during the process;
- The heating rate of the samples influences the course of the TG curves, shifting the speed maxima towards higher temperatures;
- The compared mixtures differ significantly in terms of the combustion kinetics (p < 0.05);
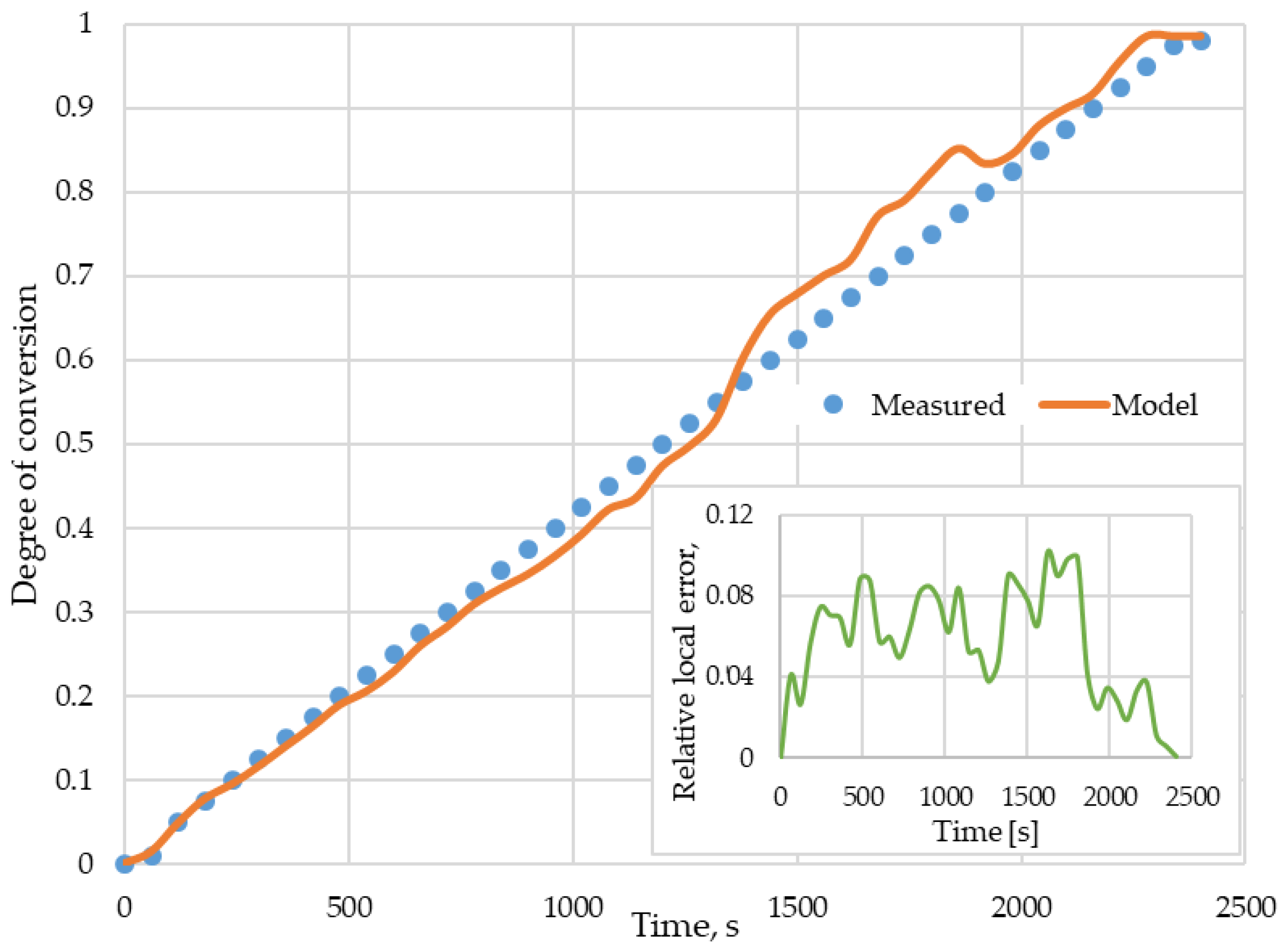
- The mathematical model of digestate combustion, derived from the laws of heat and mass transfer and thermodynamics of chemical processes, has been logically correctly verified and verified by validation with test results;
- The assumption, which simplifies the model, that the combustion process consists of two stages, drying and degassing as well as char combustion, has been confirmed by the validation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mason, P.E.; Darvell, L.I.; Jones, J.M.; Pourkashanian, M.; Williams, A. Single particle flame-combustion studies on solid biomass fuels. Fuel 2015, 151, 21–30. [Google Scholar] [CrossRef]
- Fagerström, J.; Steinvall, E.; Boström, D.; Boman, C. Alkali transformation during single pellet combustion of soft wood and wheat straw. Fuel Processing Technol. 2016, 143, 204–212. [Google Scholar] [CrossRef]
- Mian, I.; Li, X.; Dacres, O.D.; Wang, J.; Wei, B.; Jian, Y.; Zhong, M.; Liu, J.; Ma, F.; Rahman, N. Combustion kinetics and mechanism of biomass pellet. Energy 2020, 205, 117909. [Google Scholar] [CrossRef]
- Fournel, S.; Palacios, J.H.; Morissette, R.; Villeneuve, J.; Godbout, S.; Heitz, M.; Savoie, P. Influence of biomass properties on technical and environmental performance of a multi-fuel boiler during on-farm combustion of energy crops. Appl. Energy 2015, 141, 247–259. [Google Scholar] [CrossRef]
- Chen, W.H.; Lin, B.J.; Lin, Y.Y.; Chu, Y.S.; Ubando, A.T.; Show, P.L.; Ong, H.C.; Chang, J.S.; Ho, S.H.; Culaba, A.B.; et al. Progress in biomass torrefaction: Principles, applications and challenges. Prog. Energy Combust. Sci. 2021, 82, 100887. [Google Scholar] [CrossRef]
- Hustad, J.E.; Skreiberg, O.; Sonju, O.K. Biomass Combustion Research and Utilization in IEA Countries. Biomass Bioenergy 1995, 9, 235–255. [Google Scholar] [CrossRef]
- Fletcher, D.F.; Haynes, B.S.; Christo, F.C.; Joseph, S.D. A CFD based combustion model of an entrained flow biomass gasifier. Appl. Math. Model. 2000, 24, 165–182. [Google Scholar] [CrossRef]
- Saidur, R.; Abdelaziz, E.A.; Demirbas, A.; Hossain, M.S.; Mekhilef, S. A review on biomass as a fuel for boilers. Renew Sustain. Energy Rev. 2011, 15, 2262–2289. [Google Scholar] [CrossRef]
- Nipattummakul, N.; Ahmed, I.I.; Kerdsuwan, S.; Gupta, A.K. Steam gasification of oil palm trunk waste for clean syngas production. Apply Energy 2012, 92, 778–782. [Google Scholar] [CrossRef]
- Kubica, K.; Jewiarz, M.; Kubica, R.; Szlȩk, A. Straw Combustion: Pilot and Laboratory Studies on a Straw-Fired Grate Boiler. Energy Fuels 2016, 30, 4405–4410. [Google Scholar] [CrossRef]
- Bavutti, M.; Guidetti, L.; Allesina, G.; Libbra, A.; Muscio, A.; Pedrazzi, S. Thermal stabilization of digesters of biogas plants by means of optimization of the surface radiative properties of the gasometer domes. Energy Procedia 2014, 45, 1344–1353. [Google Scholar] [CrossRef][Green Version]
- Grinzi, G.; Guidetti, L.; Allesina, G.; Libbra, A.; Martini, P.; Muscio, A. Increase of net power generation of biogas plants by reduction of heat loss. In Proceedings of the 20th European Biomass Conference and Exhibition, Milan, Italy, 18–22 June 2012; pp. 1444–1449. [Google Scholar]
- Stürmer, B.; Pfundtner, E.; Kirchmeyr, F.; Uschnig, S. Legal requirements for digestate as fertilizer in Austria and the European Union compared to actual technical parameters. J. Environ. Manag. 2020, 253, 109756. [Google Scholar] [CrossRef] [PubMed]
- Alburquerque, J.A.; de la Fuente, C.; Ferrer-Costa, A.; Carrasco, L.; Cegarra, J.; Abad, M.; Bernal, M.P. Assessment of the fertiliser potential of digestates from farm and agroindustrial residues. Biomass Bioenergy 2012, 40, 181–189. [Google Scholar] [CrossRef]
- Available online: https://bip.kowr.gov.pl/informacje-publiczne/odnawialne-zrodla-energii/biogaz-rolniczy/dane-dotyczace-dzialalnosci-wytworcow-biogazu-rolniczego-w-latach-2011-2021 (accessed on 1 June 2022).
- Available online: https://bip.kowr.gov.pl/uploads/pliki/oze/biogaz/BIP%20Dane%20dotycz%C4%85ce%20dzia%C5%82%C4%85no%C5%9Bci%20wytw%C3%B3rc%C3%B3w%20biogazu%20rolniczego%20surowce%202021%20r..pdf (accessed on 1 June 2022).
- Herbes, C.; Roth, U.; Wulf, S.; Dahlin, J. Economic assessment of different biogas digestate processing technologies: A scenario-based analysis. J. Clean. Prod. 2020, 255, 120282. [Google Scholar] [CrossRef]
- Available online: https://eur-lex.europa.eu/legal-content/PL/TXT/?uri=celex:31991L0676 (accessed on 1 June 2022).
- Koszel, M.; Lorencowicz, E. Agricultural Use of Biogas Digestate as a Replacement Fertilizers. Agric. Agric. Sci. Procedia 2015, 7, 119–124. [Google Scholar] [CrossRef]
- Nagy, D.; Balogh, P.; Gabnai, Z.; Popp, J.; Oláh, J.; Bai, A. Economic analysis of pellet production in co-digestion biogas plants. Energies 2018, 11, 135. [Google Scholar] [CrossRef]
- Szufa, S.; Piersa, P.; Adrian, Ł.; Sielski, J.; Grzesik, M.; Romanowska-Duda, Z.; Piotrowski, K.; Lewandowska, W. Acquisition of torrefied biomass from Jerusalem artichoke grown in a closed circular system using biogas plant waste. Molecules 2020, 25, 3862. [Google Scholar] [CrossRef]
- Jewiarz, M.; Wróbel, M.; Fraczek, J.; Mudryk, K.; Dziedzic, K. Digestate, ash and Trichoderm based fertilizer-production line concept design. MATEC Web Conf. 2018, 168, 04004. [Google Scholar] [CrossRef]
- Cathcart, A.; Smyth, B.M.; Lyons, G.; Murray, S.T.; Rooney, D.; Johnston, C.R. An economic analysis of anaerobic digestate fuel pellet production: Can digestate fuel pellets add value to existing operations? Clean. Eng. Technol. 2021, 3, 100098. [Google Scholar] [CrossRef]
- Roman, M.; Bobasu, E.; Selisteanu, D. Modelling of biomass combustion process. Energy Procedia 2011, 6, 432–440. [Google Scholar] [CrossRef]
- ISO 17225-6:2014; Solid Biofuels—Fuel Specifications and Classes—Part 6: Graded Non-Woody Pellets. European Committee for Standardization: Geneva, Switzerland, 2014.
- ISO 17225-7:2014; Solid Biofuels—Fuel Specifications and Classes—Part 7: Graded Non-Woody Briquettes. European Committee for Standardization: Geneva, Switzerland, 2015.
- Barnetoa, A.G.; Carmonaa, J.A.; Martín Alfonsoa, J.; Serranob, R.S. Simulation of the thermogravimetry analysis of three non-wood pulps. Bioresour. Technol. 2010, 101, 3220–3229. [Google Scholar] [CrossRef] [PubMed]
- Mansaray, K.G.; Ghaly, A.E. Determination of reaction kinetics of rice husks in air using thermogravimetric analysis. Energy Sources 1999, 21, 899–911. [Google Scholar]
- Senneca, O. Kinetics of pyrolysis, combustion and gasification of three biomass fuels. Fuel Processing Technol. 2007, 88, 87–97. [Google Scholar] [CrossRef]
- Xie, Z.; Ma, X. The thermal behaviour of the co-combustion between paper sludge and rice straw. Bioresour. Technol. 2013, 146, 611–618. [Google Scholar] [CrossRef]
- Akor, C.I.; Osman, A.I.; Farrell, C.; McCallum, C.S.; John Doran, W.; Morgan, K.; Harrison, J.; Walsh, P.J.; Sheldrake, G.N. Thermokinetic study of residual solid digestate from anaerobic digestion. Chem. Eng. J. 2021, 406, 127039. [Google Scholar] [CrossRef]
- Dziedzic, K.; Łapczyńska-Kordon, B.; Jurczyk, M.; Arczewska, M.; Wróbel, M.; Jewiarz, M.; Mudryk, K.; Pająk, T. Solid Digestate—Physicochemical and Thermal Study. Energies 2021, 14, 7224. [Google Scholar] [CrossRef]
- Mudryk, K.; Jewiarz, M.; Wróbel, M.; Niemiec, M.; Dyjakon, A. Evaluation of urban tree leaf biomass-potential, physico-mechanical and chemical parameters of raw material and solid biofuel. Energies 2021, 14, 818. [Google Scholar] [CrossRef]
- Porteiro, J. Mathematical modelling of the combustion of a single wood particle. Fuel Processing Technol. 2006, 87, 169–175. [Google Scholar] [CrossRef]
- Nagórski, Z. Modelowanie Przepływu Ciepła Metodą KM3R: MBE + Excel = KM3R; Oficyna Wydawnicza Politechniki Warszawskiej: Warszawa, Poland, 2014. [Google Scholar]
- Szargut, J. Termodynamika Techniczna; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2011. [Google Scholar]
- Li, H.; Lindmark, J.; Nordlander, E.; Thorin, E.; Dahlquist, E.; Zhao, L. Using the solid digestate from a wet anaerobic digestion process as an energy resource. Energy Technol. 2013, 1, 94–101. [Google Scholar] [CrossRef]
- Souza Costa, F.; Sandberg, D. Mathematical model of a smoldering log. Combust. Flame 2004, 139, 227–238. [Google Scholar] [CrossRef]
- Janse, A.M.C.; Westerhout, R.W.J.; Prins, W. Modelling of flash pyrolysis of a single wood particle. Chem. Eng. Processing Process Intensif. 2000, 39, 239–252. [Google Scholar] [CrossRef]
















| Sample Code | Corn Silage (%) | Apple Pomace (%) |
|---|---|---|
| 10K90J | 10 | 90 |
| 25K75J | 25 | 75 |
| 50K50J | 50 | 50 |
| 75K25J | 75 | 25 |
| Parameter | Unit | Digestate Type | |||
|---|---|---|---|---|---|
| 10K90J | 25K75J | 50K50J | 75K25J | ||
| Mad | % | 12.10 | 12.20 | 11.90 | 11.90 |
| Ad | % | 8.20 | 8.40 | 10.10 | 11.60 |
| Vd | % | 83.10 | 89.80 | 79.70 | 78.80 |
| HHV | J g−1 | 19,600 | 18,550 | 18,310 | 16,420 |
| LHV | J g−1 | 18,410 | 17,140 | 17,110 | 15,340 |
| SD | g cm−3 | 0.88 | 0.88 | 0.82 | 0.89 |
| AD | g cm−3 | 1.53 | 1.55 | 1.56 | 1.53 |
| P | % | 42.50 | 43.20 | 47.10 | 41.80 |
| Parameter | Unit | Value | Source |
|---|---|---|---|
| Water vaporization heat | J·kg−1 | 2,500,000 | [36] |
| Heat of combustion of the dry digestate | J·kg−1 | 18,310,000 | own research |
| Heat of char combustion | J·kg−1 | 32,800 | [37] |
| Specific heat of biomass | J·kg−1 K−1 | 1269 | own research |
| Specific heat of char | J·kg−1 K−1 | 3854 | own research |
| Specific heat of ash | J·kg−1 K−1 | 4012 | own research |
| Heat of volatile components release | J·kg−1 | −418,000 | [37] |
| Sample length | m | 0.025 | own research |
| Density—biomass | kg·m−3 | 880 | own research |
| Density—char | kg·m−3 | 294 | own research |
| Density—ash | kg·m−3 | 602 | own research |
| Time step | s | 60 | - |
| Spatial step | m | 0.001 | - |
| Initial sample weight—Neumann boundary condition | kg | 0.005 | own research |
| Heat flux—Neumann boundary condition | W | 160 | own research |
| Sample diameter | m | 0.025 | own research |
| Evaporation temperature | K | 373 | [36] |
| Universal gas constant | J·kmol−1 K−1 | 8315 | [36] |
| Sample moisture | kg·kg−1 | 0.122 | own research |
| Mass diffusion coefficient | m2·s−1 | 0.000001 | own research |
| Thermal conductivity—biomass | W·m−1·K−1 | 0.58 | [38] |
| Thermal conductivity—char | W·m−1·K−1 | 0.20 | [39] |
| Thermal conductivity—ash | W·m−1·K−1 | 0.10 | [38] |
| Volatile matter content | kg | 0.7301 | own research |
| Ash content | kg | 0.0721 | own research |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziedzic, K.; Łapczyńska-Kordon, B.; Jurczyk, M.; Wróbel, M.; Jewiarz, M.; Mudryk, K.; Pająk, T. Solid Digestate—Mathematical Modeling of Combustion Process. Energies 2022, 15, 4402. https://doi.org/10.3390/en15124402
Dziedzic K, Łapczyńska-Kordon B, Jurczyk M, Wróbel M, Jewiarz M, Mudryk K, Pająk T. Solid Digestate—Mathematical Modeling of Combustion Process. Energies. 2022; 15(12):4402. https://doi.org/10.3390/en15124402
Chicago/Turabian StyleDziedzic, Krzysztof, Bogusława Łapczyńska-Kordon, Michał Jurczyk, Marek Wróbel, Marcin Jewiarz, Krzysztof Mudryk, and Tadeusz Pająk. 2022. "Solid Digestate—Mathematical Modeling of Combustion Process" Energies 15, no. 12: 4402. https://doi.org/10.3390/en15124402
APA StyleDziedzic, K., Łapczyńska-Kordon, B., Jurczyk, M., Wróbel, M., Jewiarz, M., Mudryk, K., & Pająk, T. (2022). Solid Digestate—Mathematical Modeling of Combustion Process. Energies, 15(12), 4402. https://doi.org/10.3390/en15124402







