Improvement in Low-Temperature Properties of Fatty Acid Methyl Esters
Abstract
:1. Introduction
1.1. General Description of Biofuels
1.2. Low-Temperature Properties of Fatty Acid Methyl Esters, Fame
2. Materials and Methods
Experiment Description
3. Results
3.1. Improvement in FAME I Properties
3.2. Improvement in FAME II Properties
3.3. Improvement in FAME III Properties
3.4. Profiles of Fatty Acids
4. Discussion
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ptak, S.; Burnus, Z. Ocena Możliwości Wydzielania Glukozydów Steroli z Estrów Metylowych Kwasów Tłuszczowych (FAME). INIG–PIB Documentation 2014, No. DK-4100-176/2014, unpublished materials.
- EN 14214:2012+A2:2019; Liquid Petroleum Products. Fatty Acid Methyl Esters (FAME) for Use in Diesel Engines and Heating Applications. Requirements and Test Methods. European Committee for Standardization: Brussels, Belgium, 2018.
- EN 590:2013+A1:2017; Automotive Fuels. Diesel. Requirements and Test Methods. Committee European for Standardization: Brussels, Belgium, 2017.
- Żółty, M.; Krasodomski, W. Oxidation Stability of Fatty Acid Methyl Esters as a B100 Fuel or as a Biocomponent of Diesel Fuels. Nafta-Gaz 2018, 74, 399–405. [Google Scholar] [CrossRef]
- Sacha, D. The influence of fatty acid methyl esters on the low-temperature properties of motor fuels. Nafta-Gaz 2018, 74, 148–155. [Google Scholar] [CrossRef]
- Krasodomski, W.; Żółty, M. Comparison of standard methods for determining the oxidative stability of diesel fuels containing FAME. Nafta-Gaz 2017, 74, 422–429. [Google Scholar] [CrossRef]
- Instytut Technologii Nafty. Bio-Fuel for The Compression Ignition Engines. PL Patent 196335, 3 November 2003.
- Krasodomski, M.; Krasodomski, W. Degradation of fatty acid methyl esters during storage and exploitation. Przem. Chem. 2009, 88, 785–788. [Google Scholar]
- Burnus, Z.; Jakóbiec, J. Study on the impact of free steryl glucosides on the low temperature parameters of biofuels for diesel engines. Przemysł Chem. 2016, 95, 1822–1827. [Google Scholar]
- Łaczek, T. Wdrożenie Metody Oceny Przydatności Estrów Metylowych Kwasów Tłuszczowych (FAME) do Komponowania Biopaliw w Oparciu o Test Filtracji po Sezonowaniu w Niskich Temperaturach. INIG–PIB Documentation 2010, No. DK-4100-80/2010, unpublished materials.
- Sacha, D. New research tools for assessing the properties of diesel engine fuels. Nafta-Gaz 2012, 68, 133–137. [Google Scholar]
- Sierra-Cantor, J.F.; Guerrero-Fajardo, C.A. Methods for improving the cold flow properties of biodiesel with high saturated fatty acids content: A review. Renew. Sustain. Energy Rev. 2017, 72, 774–790. [Google Scholar] [CrossRef]
- Mohanan, A.; Bouzidi, L.; Li, S.J.; Narine, S.S. Mitigating crystallization of saturated fames in biodiesel: Lowering crystallization temperatures via addition of metathesized soybean oil. Energy 2016, 96, 335–345. [Google Scholar] [CrossRef]
- Harrow, G. E85 and Biodiesel Deployment; National Renewable Energy Laboratory: Golden, CO, USA, 2007. [Google Scholar]
- Sajjadi, B.; Raman, A.A.A.; Arandiyan, H. A comprehensive review on properties of edible and non-edible vegetable oil-based biodiesel: Composition, specifications and prediction models. Renew. Sustain. Energy Rev. 2016, 63, 62–92. [Google Scholar] [CrossRef]
- Sorate, K.A.; Bhale, P.V. Biodiesel properties and automotive system compatibility issues. Renew. Sustain. Energy Rev. 2015, 41, 777–798. [Google Scholar] [CrossRef]
- Knothe, G.; Razon, L.F. Biodiesel fuels. Prog. Energy Combust. Sci. 2017, 58, 36–59. [Google Scholar] [CrossRef]
- Abe, M.; Hirata, S.; Komatsu, H.; Yamagiwa, K.; Tajima, H. Thermodynamic selection o effective additives to improve the cloud point of biodiesel fuels. Fuel 2016, 171, 94–100. [Google Scholar] [CrossRef]
- Chiu, C.-W.; Schumacher, L.G.; Suppes, G.J. Impact of cold flow improvers on soybean biodiesel blend. Biomass Bioenergy 2004, 27, 485–491. [Google Scholar] [CrossRef]
- Leggieri, P.A.; Senra, M.; Soh, L. Cloud point and crystallization in fatty acid ethyl ester biodiesel mixtures with and without additives. Fuel 2018, 222, 243–249. [Google Scholar] [CrossRef]
- Baranik, M.; Łaczek, T. Właściwości niskotemperaturowe biopaliw zawierających estry metylowe kwasów tłuszczowych, pochodzących z przeróbki tłuszczów zwierzęcych. Nafta-Gaz 2010, 11, 1047–1058. [Google Scholar]
- NUCELIS INC. Fatty Acid Blends and Uses Therefor. U.S. Patent 2,017,051,219, 23 February 2017. [Google Scholar]
- NUCELIS INC. Fatty Acid Blends and Uses Therefor. EP Patent 2,679,687, 1 January 2014. [Google Scholar]
- CIBUS LLC. Fatty Acid Blends and Uses Therefor. WO Patent 2,008,002,643, 3 January 2008. [Google Scholar]
- HENKEL KGAA. HENKEL KGAA. Mixtures of Fatty Alkyl Lower Alkyl Esters. U.S. Patent 5,389,113, 14 February 1995. [Google Scholar]
- VEBA OEL AG. Fuel for High Compression Diesel Engines. EP Patent 716,139, 12 June 1996. [Google Scholar]
- ROHMAX ADDITIVES GMBH. Additive for Biodiesel and Biofuel Oils. EP Patent 1,032,620, 6 September 2000.
- ISO 12966-1:2014; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters–Part 1: Guidelines on Modern Gas Chromatography of Fatty Acid Methyl Esters. ISO: Geneva, Switzerland, 2014.




| No | Property | Unit | FAME I | FAME II | FAME III | Test Method |
|---|---|---|---|---|---|---|
| 1 | Fatty acid methyl esters (FAME) content | % (m/m) | 97.5 | 97.1 | 96.4 | PN-EN 14103:2012 |
| 2 | Density at 15 °C | kg/m3 | 883.0 | 882.8 | 882.7 | PN-EN ISO 12185:2002 |
| 3 | Kinematic viscosity at 40 °C | mm2/s | 4.537 | 4.480 | 4.379 | PN-EN ISO 3104:2021-03 |
| 5 | Sulphur content | mg/kg | <5 | 3.4 | 3.8 | PN-EN ISO 20884:2012 |
| 6 | Sulphate ash | % (m/m) | 0.01 | 0.01 | 0.01 | PN-ISO 3987:2005 |
| 9 | Corroding action Cu/50 °C/3 h | degree of corrosion | 1 | 1 | 1 | PN-EN ISO 2160:2004 |
| 10 | Oxidation stability at 110 °C | h | 9.7 | 10.9 | 9.4 | PN-EN 14112:2004 |
| 11 | Acid number | mg KOH/g | 0.26 | 0.21 | 0.24 | PN-EN 14104:2004 |
| 12 | Iodine number | g of iodine/100 g | 109 | 108 | 101 | PN-EN 14111:2004 |
| 13 | Linolenic acid methyl ester content | % (m/m) | 8.3 | 8.6 | 8.2 | PN-EN 14103:2012 |
| 15 | Monoacylglycerols content | % (m/m) | 0.59 | 0.57 | 0.78 | PN-EN 14105:2012 |
| 16 | Diacylglycerols content | % (m/m) | <0.10 | <0.10 | <0.10 | PN-EN 14105:2012 |
| 17 | Triacylglycerols content | % (m/m) | <0.10 | <0.10 | <0.10 | PN-EN 14105:2012 |
| 23 | Cloud point | °C | −4 | −5 | +1 | PN-ISO 3016:2005 |
| 24 | Pour point | °C | −12 | −12 | 0 | PN-ISO 3016:2005 |
| 25 | Cold filter plugging point (CFPP) | °C | −15.0 | −12.4 | −1.0 | PN-ISO 3016:2005 |
| 26 | Sterol glucosides content | ppm | 32.0 | − | 14.8 | INIG-PIB method |
| Test No. | IA | IB | IC | ID |
|---|---|---|---|---|
| Solvent type | MEK-TOL | MEK-TOL | MEK-TOL | MEK |
| Symbol of obtained biofuel sample | FAME IA | FAME IB | FAME IC | FAME ID |
| Process technological parameters | ||||
| Solvent, mass ratio | 40:60 | 40:60 | 40:60 | - |
| Crystallisation/filtration temperature, °C | −20 | −29 | −29 | −25 |
| Total solvent to material ratio, (m/m) | 4:1 | 2:1 | 0.85:1 | 2:1 |
| Mass balance of processes, averaged results | ||||
| Yield of modified biofuel, % (m/m) | 96.6 | 88.0 | 85.0 | 93.5 |
| Yield of precipitated solids, % (m/m) | − | 8 | 8 | 3.5 |
| Losses, % (m/m) | 3.4 | 4 | 7 | 2 |
| Filtration time | 49 | 40 | 120 | 45 |
| Vacuum conditions, bar | 0.12 | 0.12 | 0.12 | 0.12 |
| Properties of modified biofuel | ||||
| Cloud point, °C | −4 | −8 | −4 | −15 |
| Pour point, °C | −12 | −14 | −12 | −17 |
| Cold filter plugging point (CFPP), °C | −15 | −15 | −15 | −18 |
| Kinematic viscosity at 40 °C, mm2/s | 4.637 | 4.850 | 4.823 | 4.914 |
| Sterol glucosides content, mg/kg | 31 | 25 | 28 | 29 |
| No. of Dewaxing | IIA | IIB | IIC | IID | IIE | IIF | IIG |
|---|---|---|---|---|---|---|---|
| Solvent Type | MEK-TOL | MEK-TOL | MEK-TOL | MEK | MEK | MEK | MEK |
| Viscosity modifier | − | − | Viscoplex 9—327 | − | − | Viscoplex 9—327 | − |
| Symbol of modified biofuel sample | FAME IIA | FAME IIB | FAME IIC | FAME IID | FAME IIE | FAME IIF | FAME IIG |
| Technological parameters of dewaxing processes | |||||||
| Solvent, mass ratio | 60:40 | 50:50 | 40:60 | − | − | − | − |
| Crystallisation/filtration temperature, °C | −28 | −28 | −28 | −21 | −28 | −28 | −28 |
| Total solvent to material ratio, (m/m) | 3.2:1 | 2.9:1 | 5.5:1 | 2.0:1 | 2.9:1 | 2.9:1 | 6.0:1 |
| Washing at filtration temperature, (m/m) | 0.6:1 | 0.2:1 | 0.6:1 | 0.4:1 | 0.2:1 | 0.2:1 | 0.4:1 |
| Mass balance of dewaxing processes, averaged results | |||||||
| Yield of modified biofuel, % (m/m) | 96.6 | 90.0 | 87.0 | 92.8 | 90.0 | 94.6 | 91.6 |
| Yield of precipitated solids, % (m/m) | 3.4 | 8.0 | 9.0 | 4.0 | 5.0 | 3.5 | 5.0 |
| Losses, % (m/m) | 3.0 | 2.0 | 4.0 | 3.2 | 5.0 | 1.9 | 3.4 |
| Filtration time, seconds | 3 min 19 s | 40 s | 2 min 20 s | 1 min 59 s | 2 min 24 s | 1 min 52 s | 1 min 38 s |
| Vacuum conditions, bar | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 |
| Properties of modified biofuel | |||||||
| Cloud point, °C | −9 | −8 | −14 | −7 | −14 | −12 | −12 |
| Pour point, °C | −15 | −14 | −15 | −9 | −15 | −14 | −16 |
| Cold filter plugging point (CFPP), °C | −15.1 | −15.0 | −17.0 | −10.2 | −15.4 | −15 | −16.4 |
| Kinematic viscosity at 40 °C, mm2/s | 4.714 | 4.841 | 4.910 | 4.735 | 4.832 | 4.729 | 4.768 |
| No. of Dewaxing | IIIA | IIIB | IIIC | IIID | IIIE |
|---|---|---|---|---|---|
| Solvent type | MEK-TOL | MEK-TOL | MEK-TOL | MEK | MEK |
| Viscosity modifier | − | − | Viscoplex 9—327 | − | − |
| Symbol of dewaxed material sample | FAME IIIA | FAME IIIB | FAME IIIC | FAME IIID | FAME IIIE |
| Technological parameters of dewaxing processes | |||||
| Solvent, mass ratio | 40:60 | 70:30 | 50:50 | − | − |
| Crystallisation/filtration temperature, °C | −28 | −21 | −21 | −21 | −21 |
| Total solvent to material ratio, (m/m) | 5.5:1 | 5.5:1 | 3.5:1 | 5.5:1 | 3.8:1 |
| Washing at filtration temperature, (m/m) | 0.5:1 | 0.5:1 | 0.5:1 | 0.5:1 | 0.2:1 |
| Mass balance of dewaxing processes, averaged results | |||||
| Yield of dewaxed material, % (m/m) | 96.0 | 80.6 | 85.4 | 89.4 | 90.1 |
| Yield of slack wax, % (m/m) | 1.7 | 13.7 | 9.8 | 4.7 | 4.5 |
| Losses, % (m/m) | 2.3 | 5.7 | 4.8 | 5.9 | 5.4 |
| Filtration time, seconds | 46 s | 62 s | 85 s | 62 s | 63 s |
| Vacuum conditions, bar | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 |
| Properties of modified biofuel | |||||
| Cloud point, °C | +1 | −9 | −10 | −7 | −8 |
| Pour point, °C | 0 | −9 | −11 | −8 | −9 |
| Cold filter plugging point (CFPP), °C | −1.4 | −11.6 | −11.8 | −9.4 | −10.6 |
| Kinematic viscosity at 40 °C, mm2/s | 4.463 | 4.527 | 4.568 | 4.618 | 4.598 |
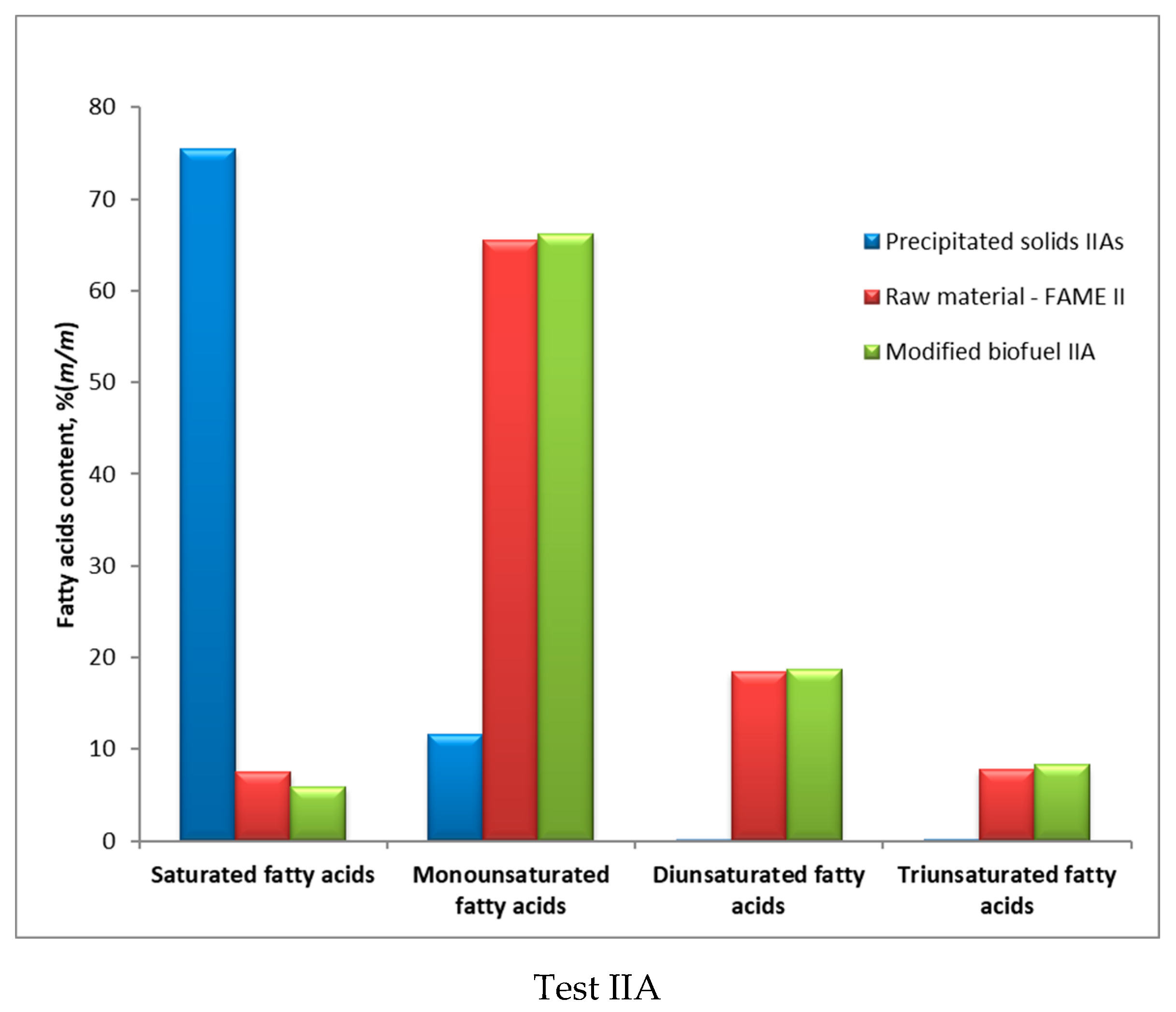
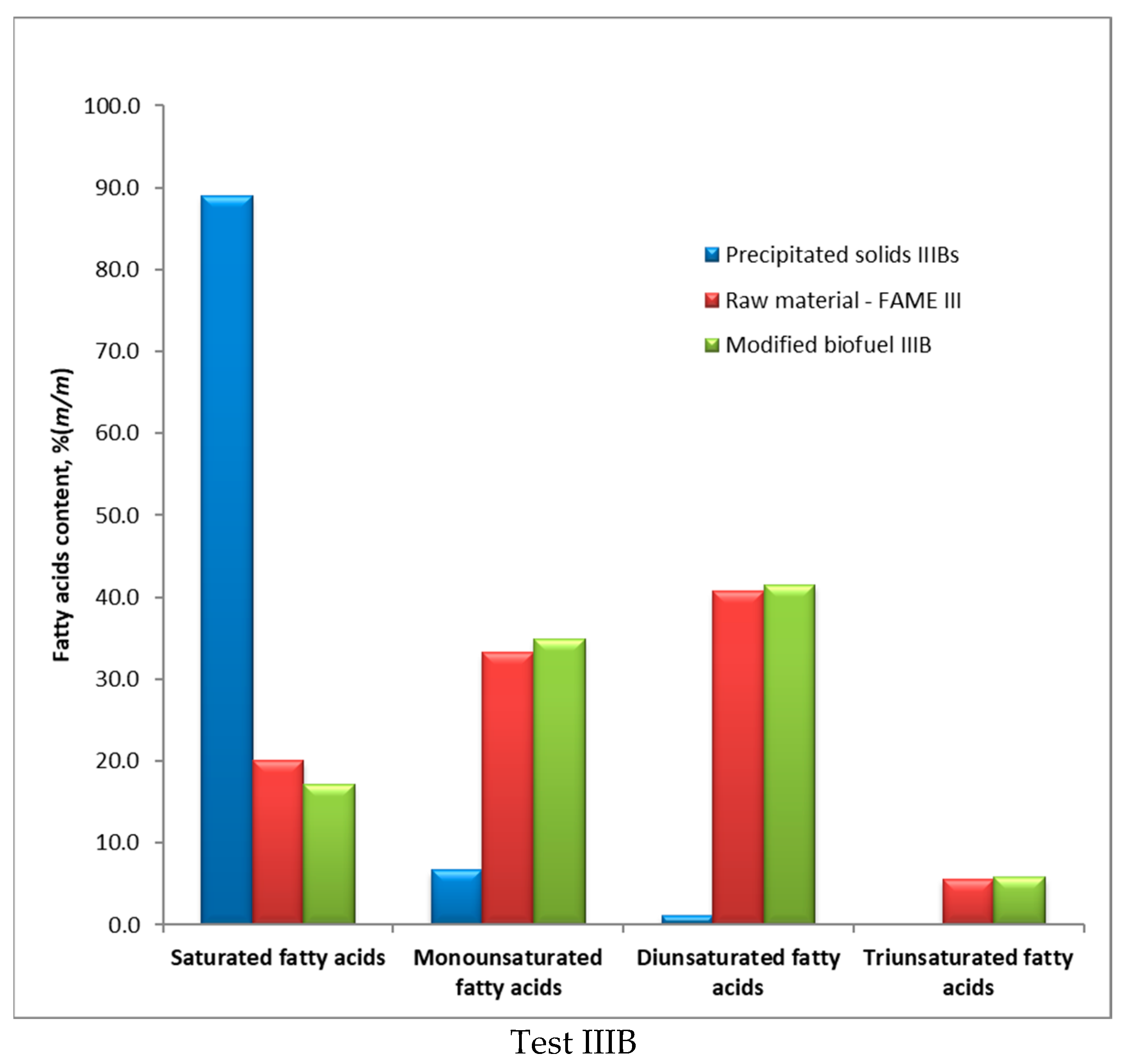
| No. | Fatty Acids | Unit | Raw FAME | Precipitated Solids | Modified FAME | |||
|---|---|---|---|---|---|---|---|---|
| II | III | IIAs | IIIBs | IIA | IIIB | |||
| 1 | <C16 | % (m/m) | − | − | 1.4 | 1.2 | 0.1 | 0.1 |
| 2 | C14:0 | % (m/m) | − | 0.2 | − | − | 0.2 | 0.1 |
| 3 | C16:0 | % (m/m) | 4.5 | 15.5 | 5.2 | 54.4 | 4.3 | 13.6 |
| 4 | C16:1 | % (m/m) | 0.3 | 0.2 | − | 0.2 | 0.3 | 0.2 |
| 5 | C16 unident. | % (m/m) | 0.4 | 0.2 | 0.6 | 0.3 | 0.3 | 0.2 |
| 6 | C18:0 | % (m/m) | 1.7 | 3.4 | 10.9 | 20.7 | 1.2 | 3.0 |
| 7 | C18:1 | % (m/m) | 63.2 | 32.5 | 9.5 | 6.1 | 63.4 | 34.2 |
| 8 | C18:2 | % (m/m) | 18.5 | 40.7 | 0.1 | 1.1 | 18.7 | 41.5 |
| 9 | C18 unident. | % (m/m) | 0.2 | 0.1 | 0.4 | − | 0.1 | 0.2 |
| 10 | C18:3 | % (m/m) | 7.8 | 5.6 | 0.2 | 0.1 | 8.3 | 5.8 |
| 11 | C20:0 | % (m/m) | 0.6 | 0.4 | 20.8 | 9.9 | 0.1 | 0.2 |
| 12 | C20:1 | % (m/m) | 1.3 | 0.4 | 0.3 | 0.2 | 1.5 | 0.4 |
| 13 | C20 unident. | % (m/m) | 0.2 | 0.1 | 1.2 | 1.3 | 0.2 | 0.1 |
| 14 | C22:0 | % (m/m) | 0.3 | 0.4 | 26.1 | 3.4 | 0.1 | 0.2 |
| 15 | C22:1 | % (m/m) | 0.4 | 0.1 | 0.6 | 0.2 | 0.7 | 0.0 |
| 16 | C22 unident. | % (m/m) | 0.1 | 0.1 | 4.3 | 0.3 | 0.1 | 0.1 |
| 17 | C24:0 | % (m/m) | 0.2 | 0.1 | 12.4 | 0.6 | 0.0 | 0.1 |
| 18 | C24:1 | % (m/m) | 0.3 | − | 1.2 | − | 0.3 | − |
| 19 | C24 + | % (m/m) | − | − | 4.8 | − | 0.1 | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ptak, S.; Krasodomski, W.; Żółty, M. Improvement in Low-Temperature Properties of Fatty Acid Methyl Esters. Energies 2022, 15, 4536. https://doi.org/10.3390/en15134536
Ptak S, Krasodomski W, Żółty M. Improvement in Low-Temperature Properties of Fatty Acid Methyl Esters. Energies. 2022; 15(13):4536. https://doi.org/10.3390/en15134536
Chicago/Turabian StylePtak, Stefan, Wojciech Krasodomski, and Magdalena Żółty. 2022. "Improvement in Low-Temperature Properties of Fatty Acid Methyl Esters" Energies 15, no. 13: 4536. https://doi.org/10.3390/en15134536
APA StylePtak, S., Krasodomski, W., & Żółty, M. (2022). Improvement in Low-Temperature Properties of Fatty Acid Methyl Esters. Energies, 15(13), 4536. https://doi.org/10.3390/en15134536






