Visualisation and Thermovision of Fuel Combustion Affecting Heat Release to Reduce NOx and PM Diesel Engine Emissions
Abstract
:1. Introduction
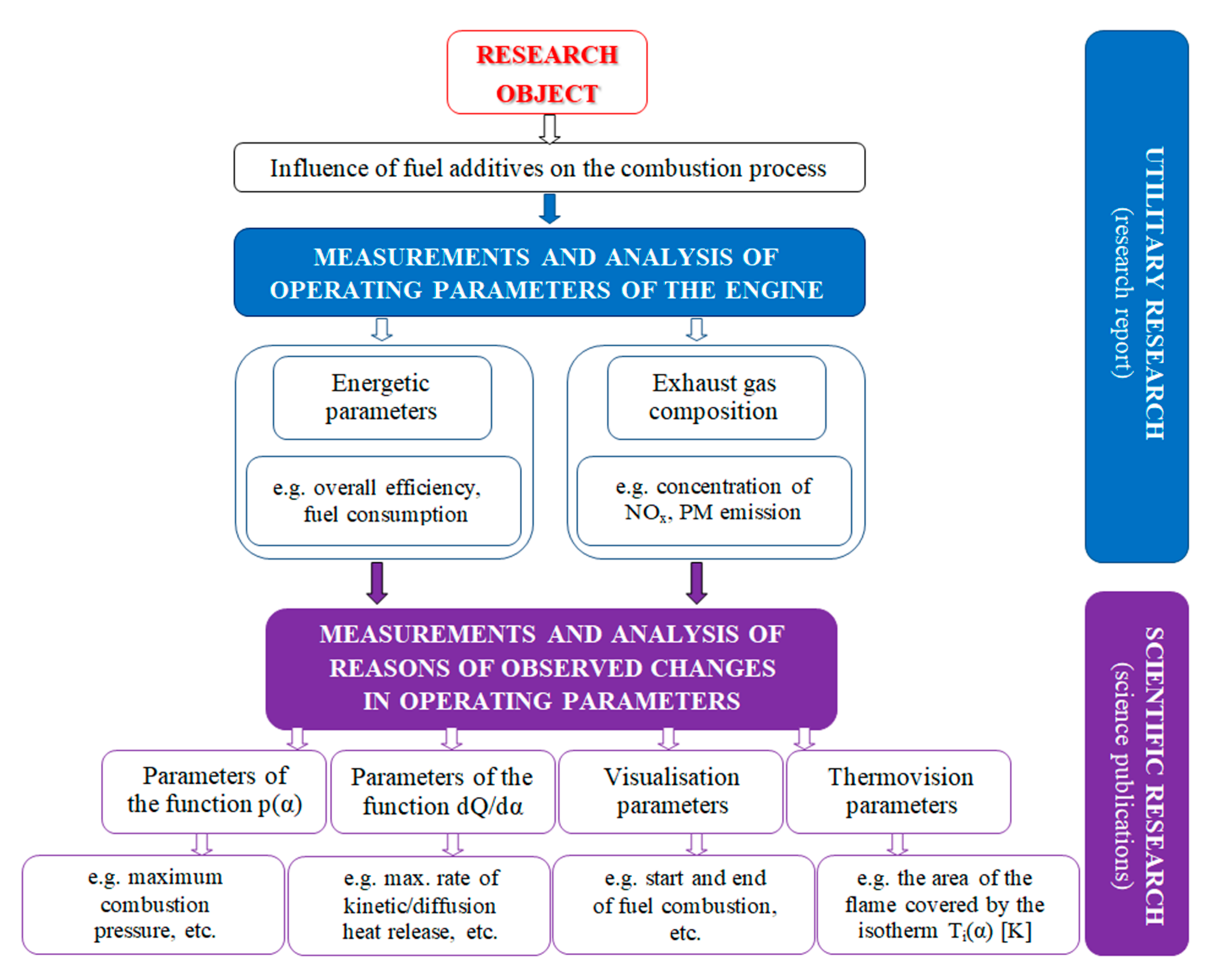
2. The Purpose and Scope of the Research
- Visualisation of images, including injection, self-ignition and combustion of the tested fuels, in order to deepen the scientific (cause-and-effect) analysis of the studied phenomena,
- Thermovision, including the distribution of isotherms in the flame of the tested fuels, also in order to deepen the scientific analysis of the studied phenomena,
- Tests with the use of a different generation engine in order to generalise the analysis of the influence of the tested additives on a larger group of engines.
3. Research Methodology
3.1. Fuel Characteristics
- 2-EHN—2-ethylhexyl nitrate with detergent-dispersant additives. 2-EHN was added mainly to increase the cetane number (CN) of the DFKA fuel. A concentration of 1500 ppm (v/v) of the additive increased the CN from 52.3 to 58 for the DFKA fuel.
- Reduxco®—a liquid catalyst resulting from the reaction of acetic acid, iron, n-butanol, n-propanol, and isopropanol. The chemical composition of Reduxco complies with the Worldwide Fuel Charter, sixth edition, gasoline and diesel fuel, from 28 October 2019 [37]. Reduxco causes a reduction in fuel oxidation activation energy, which results in a selective increase in the rate of diffusion combustion, which enhances afterburning of previously formed PM in the engine cylinder [36,38,39]. DFB fuel with 1500 ppm (v/v) of Reduxco was denoted as DFDA fuel.
- Base fuel with 1500 ppm 2-EHN and 1500 ppm Reduxco was denoted as DFS fuel.
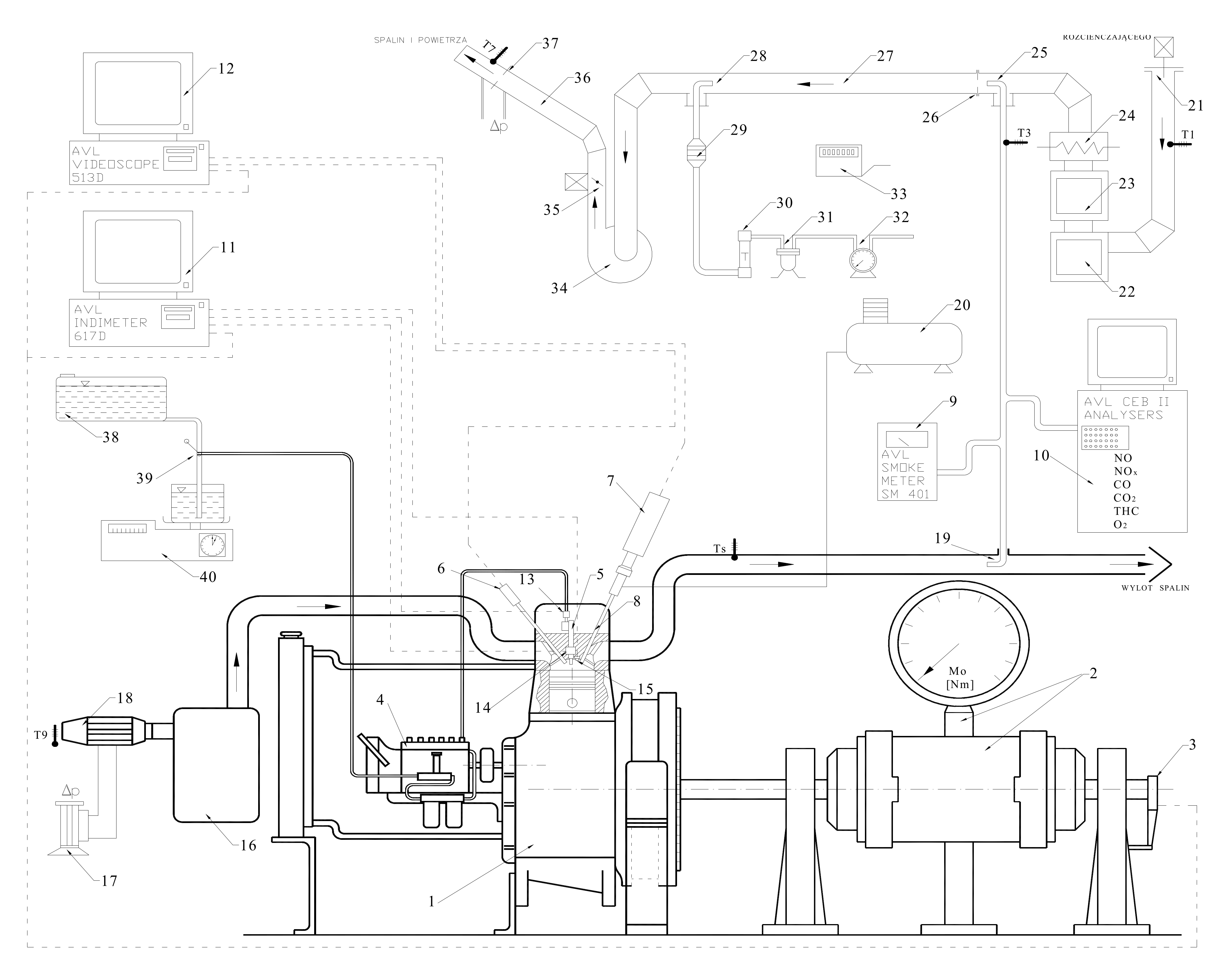
3.2. Measurement Stand
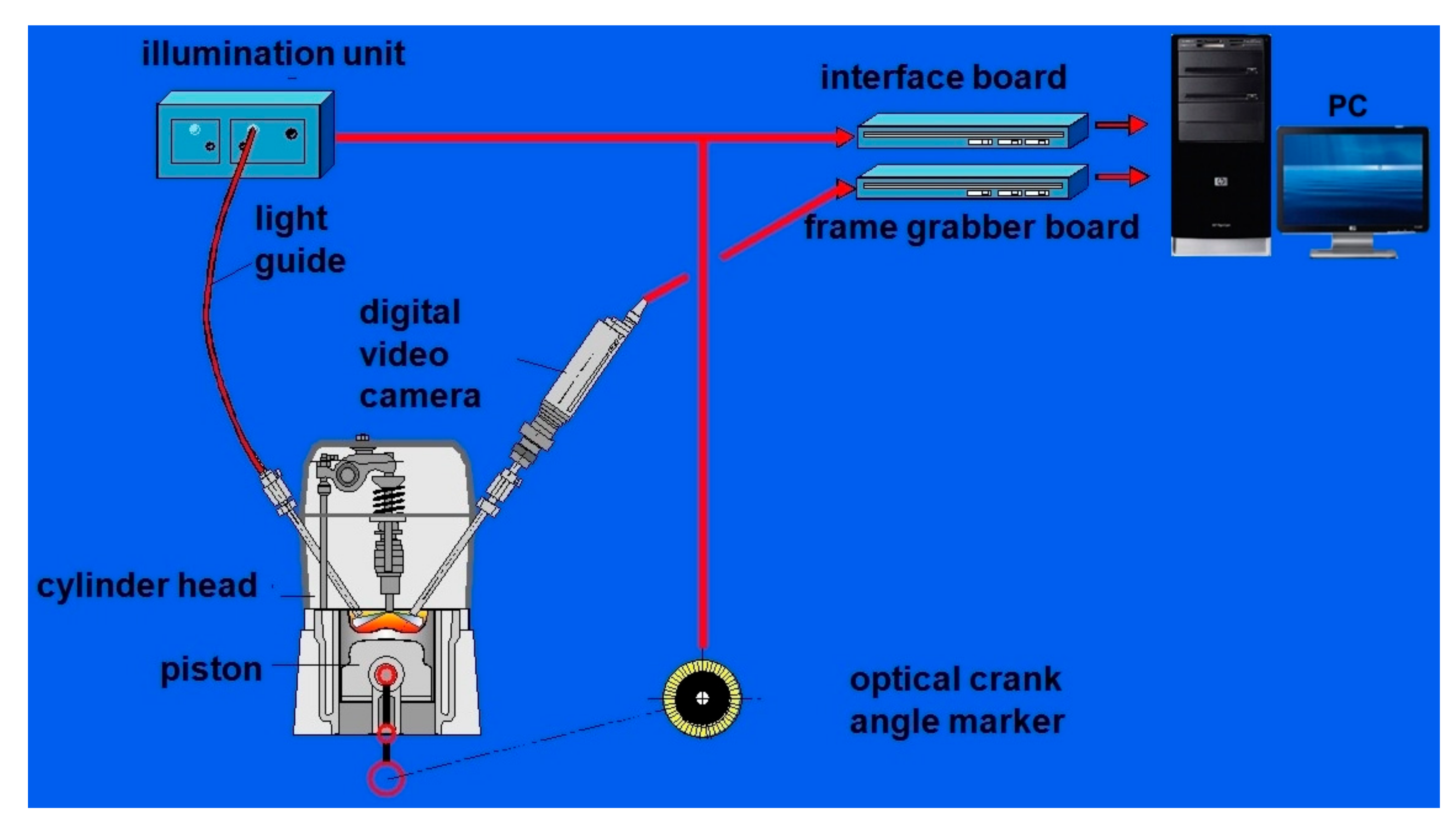
3.3. Optical Measurement of Fast-Changing Processes in Engine Cylinder
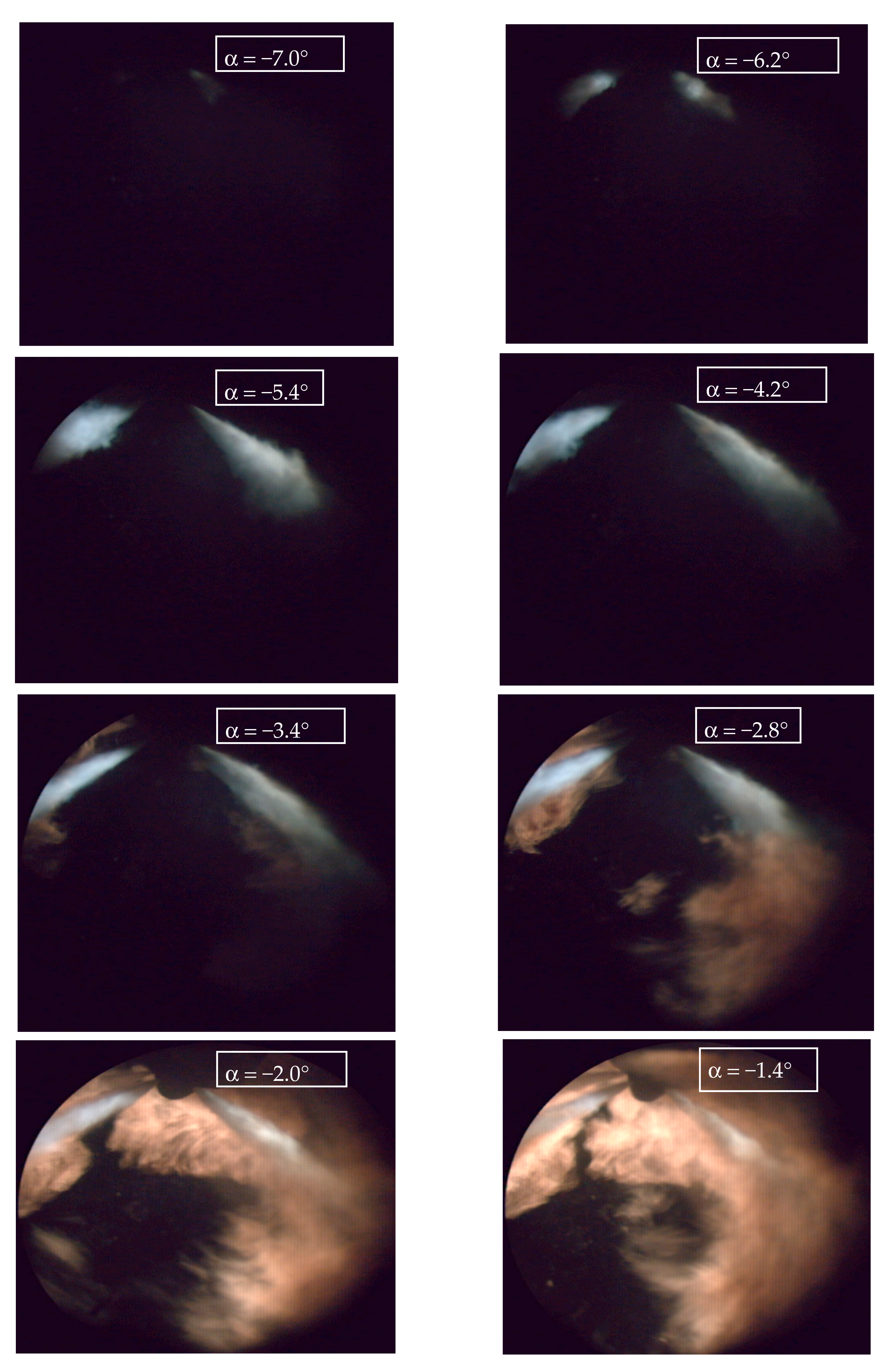
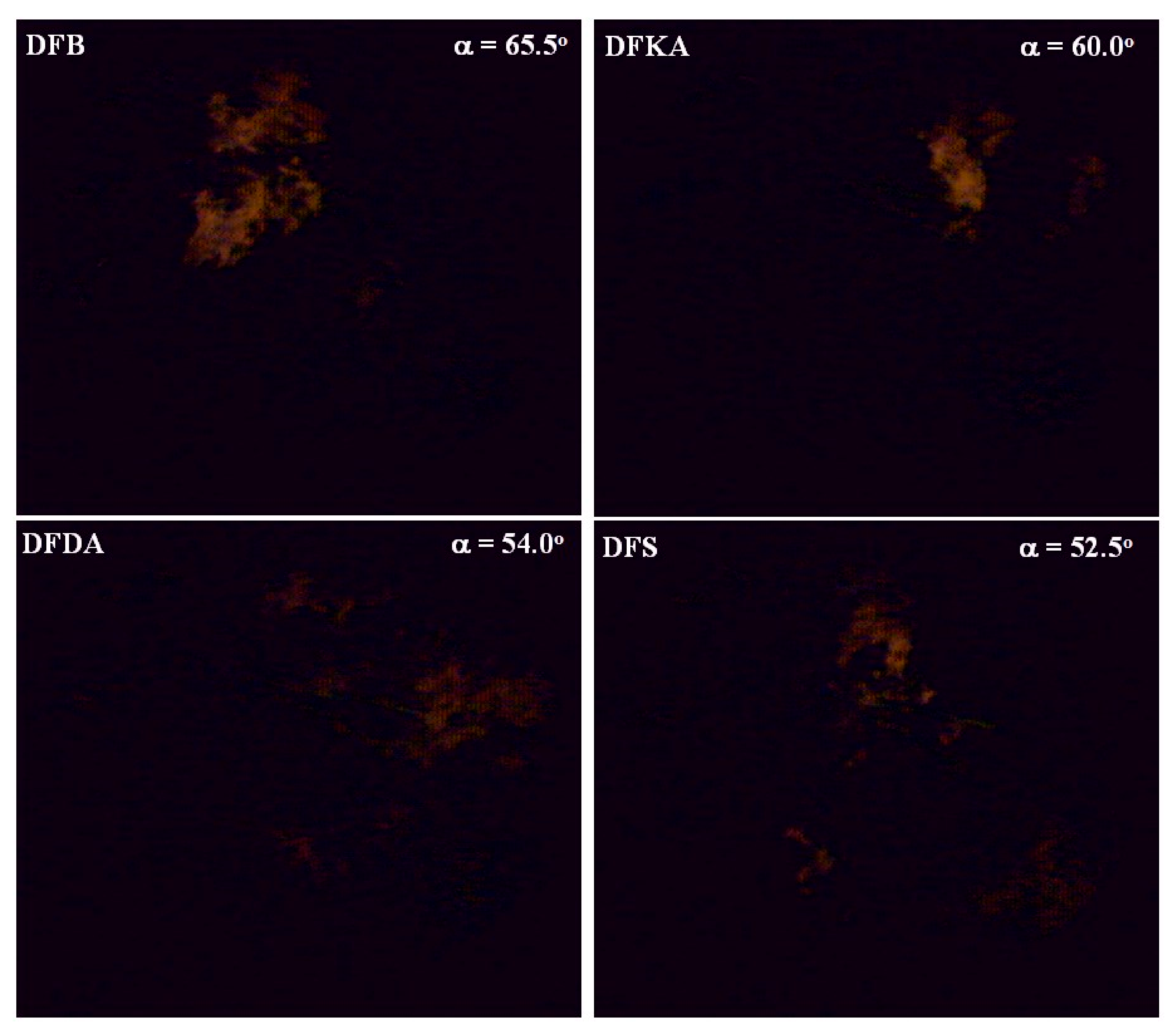
3.3.1. Visualisation of Self-Ignition and Fuel Combustion
- Recording images from different engine cycles for the same crankshaft rotation angle;
- Recording images from different engine work cycles for successive crankshaft rotation angles shifted by a determined interval (the maximum sampling “resolution” is 0.1° CA).
3.3.2. Flame Thermovision (Two-Colour Method)
4. Research Results
- 4.
- Engine energy parameters,
- 5.
- Concentration of gaseous components of exhaust gases,
- 6.
- Particulate emissions and exhaust smoke,
- 7.
- Indicator chart parameters,
- 8.
- Combustion temperature graph parameters (calculated using p(α)),
- 9.
- Graph parameters for rate of heat release in engine cylinder,
- 10.
- Optical image analysis parameters in engine cylinder,
- 11.
- Flame thermovision parameters for tested fuels (for kinetic and diffusion phases of combustion).
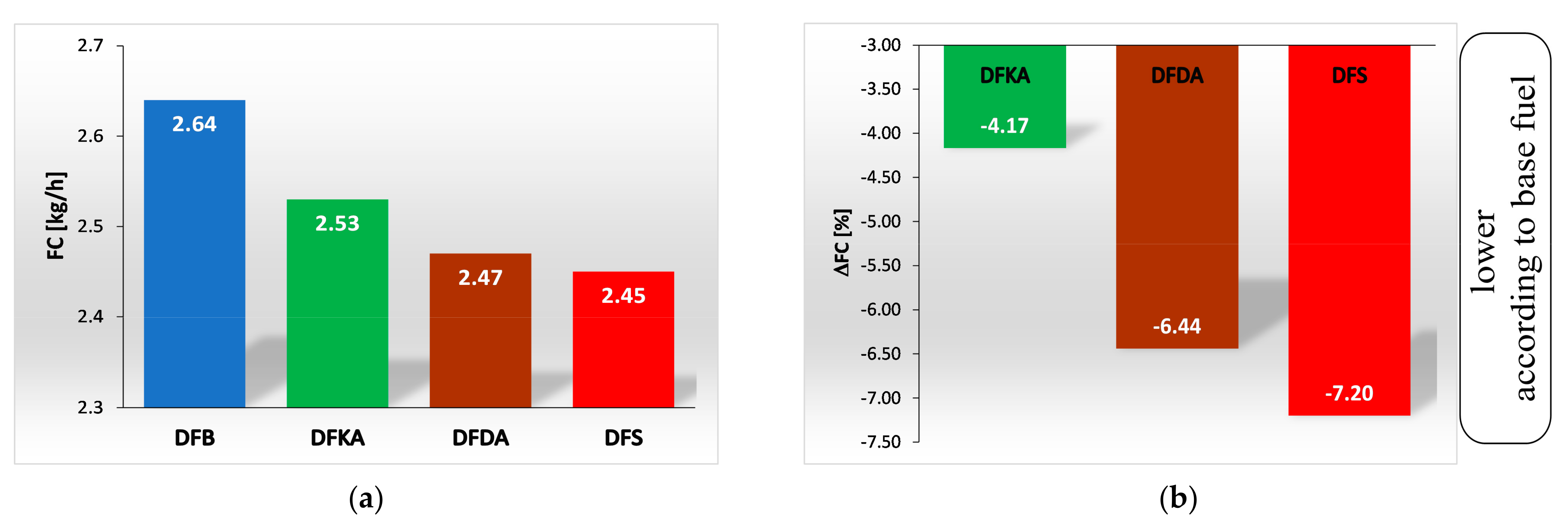
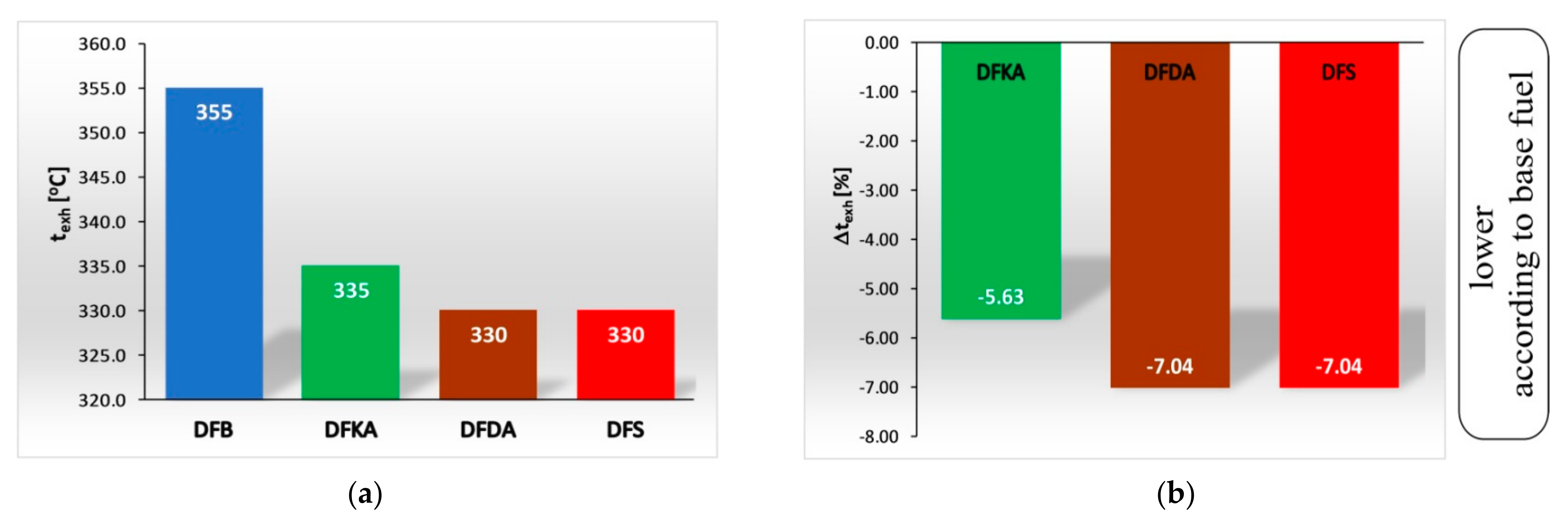
4.1. Energy Parameters
- Approximately 4% for DFKA fuel,
- Approximately 6% for DFDA fuel,
- Approximately 7% for DFS fuel.
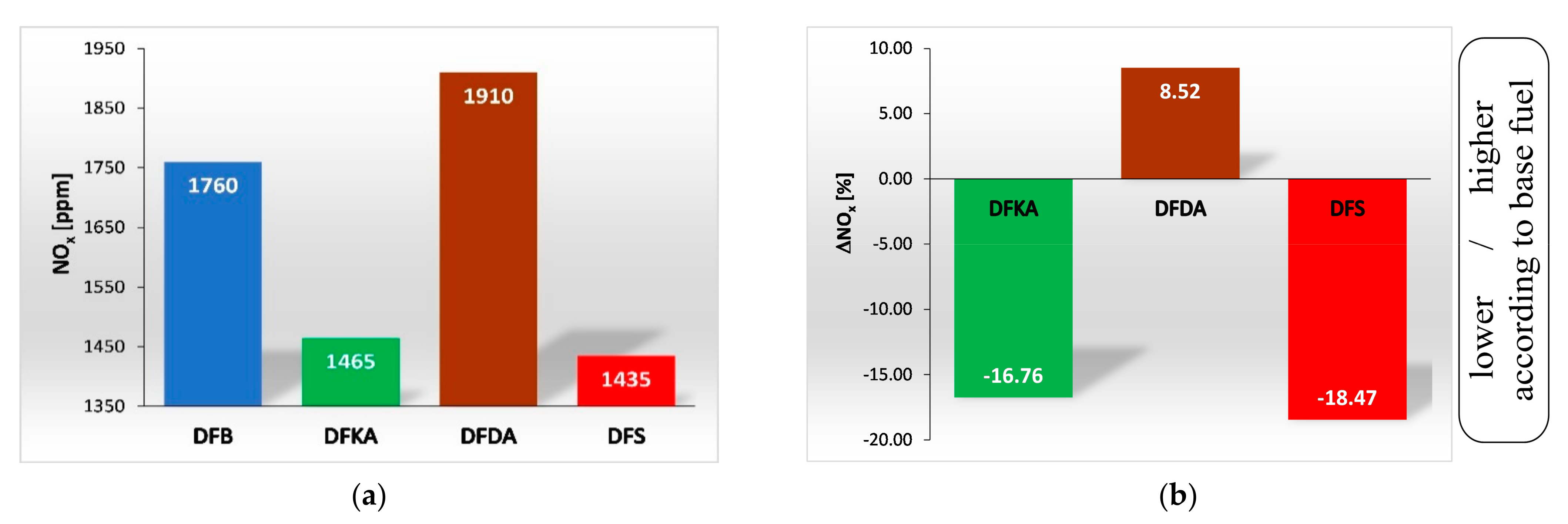
4.2. Exhaust Gas Composition
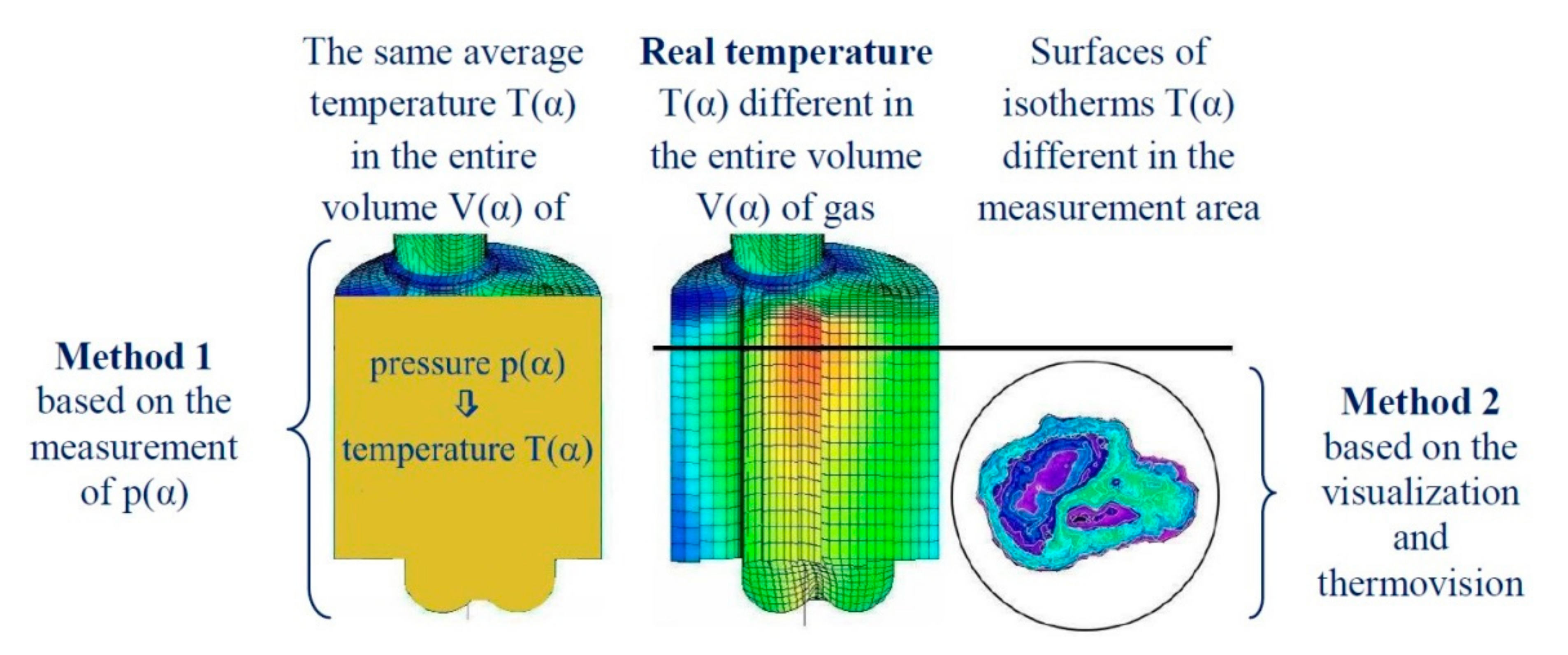
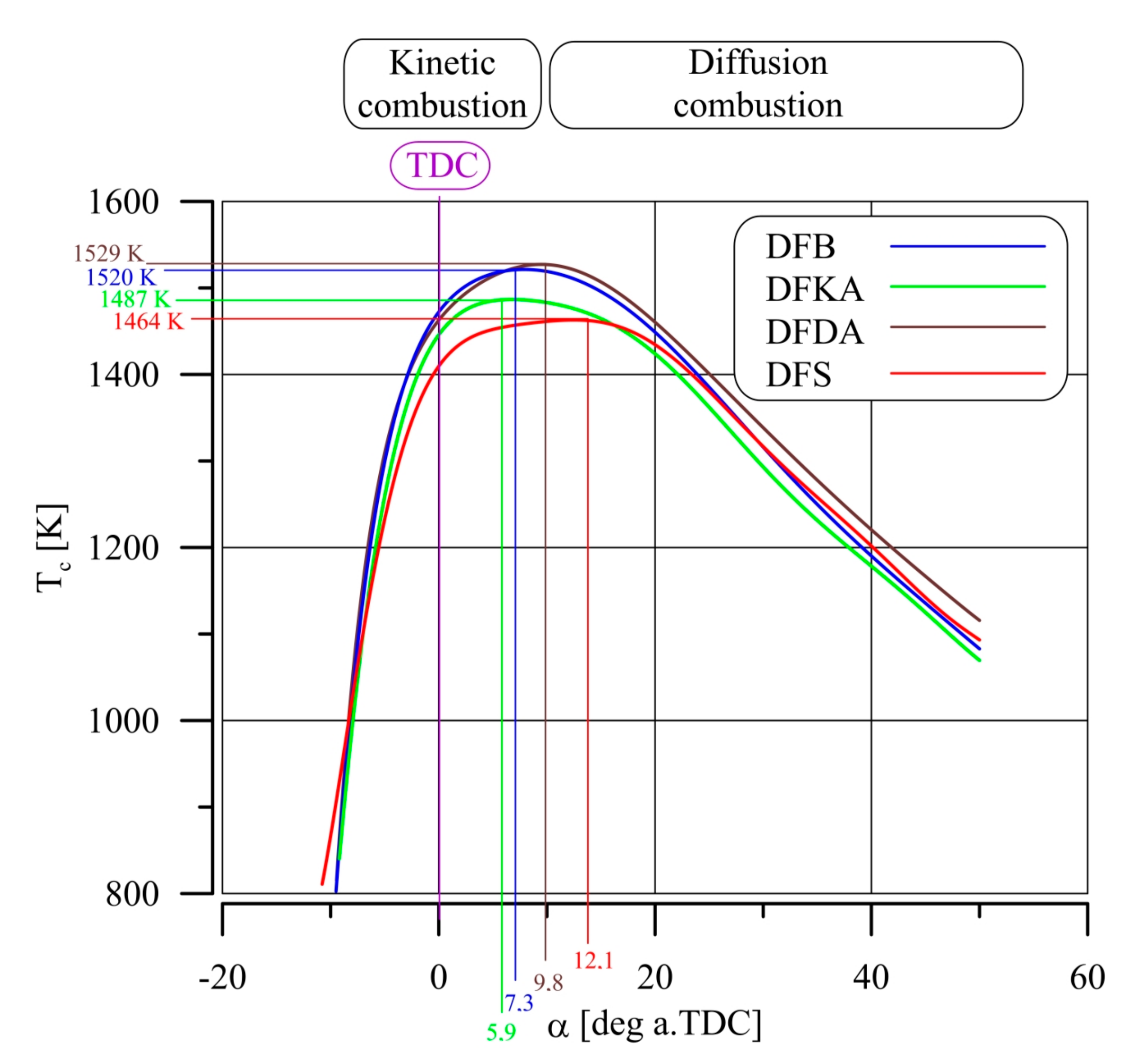
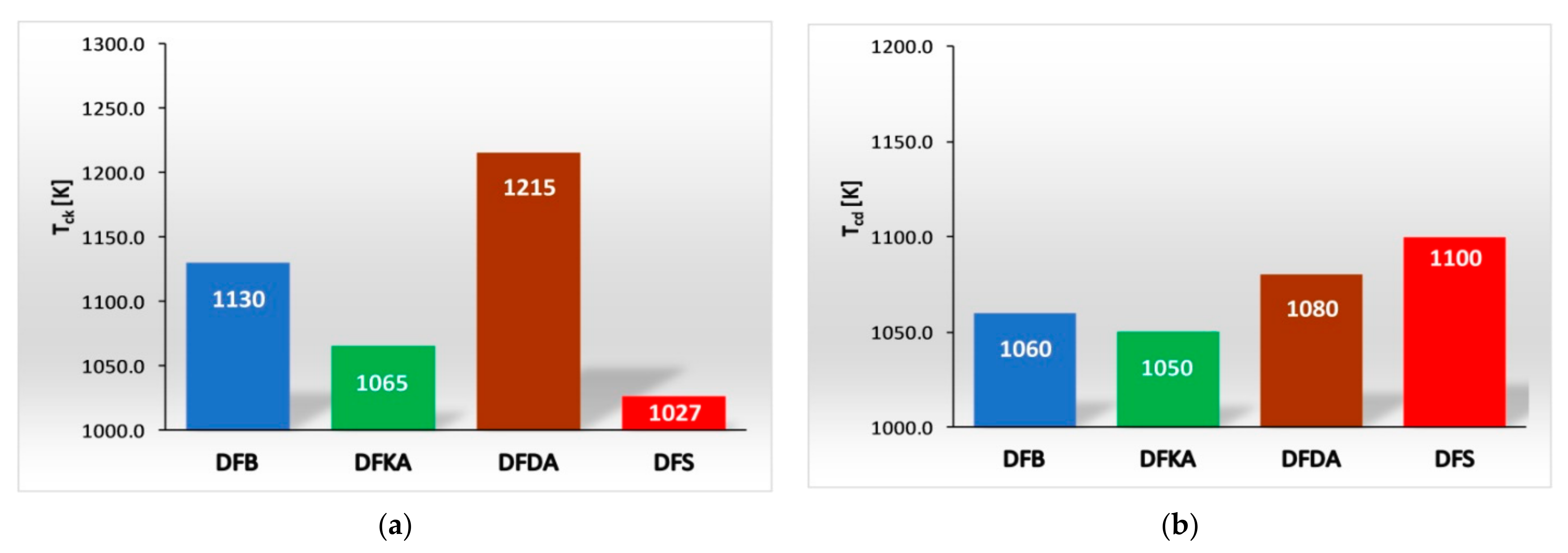
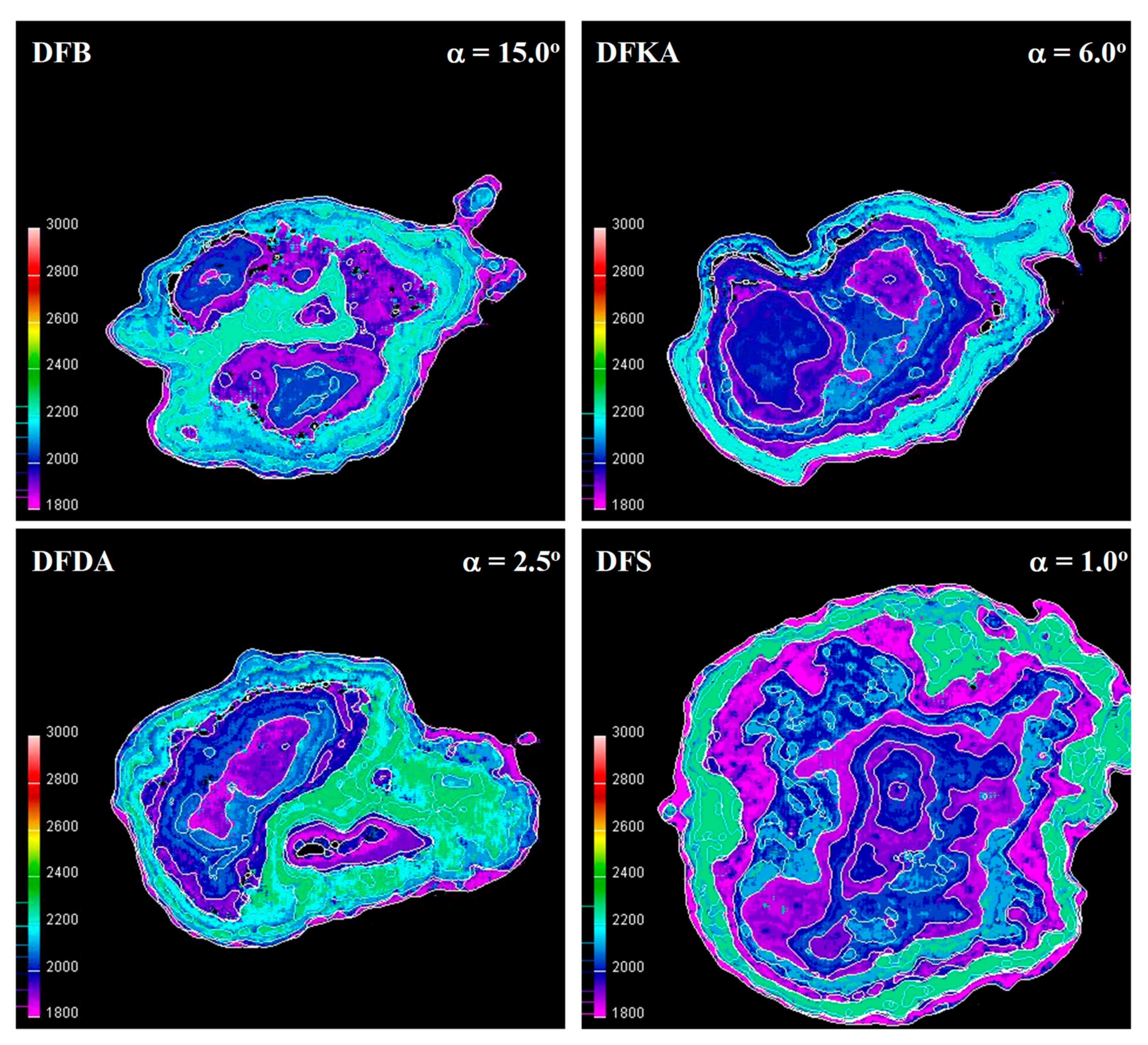
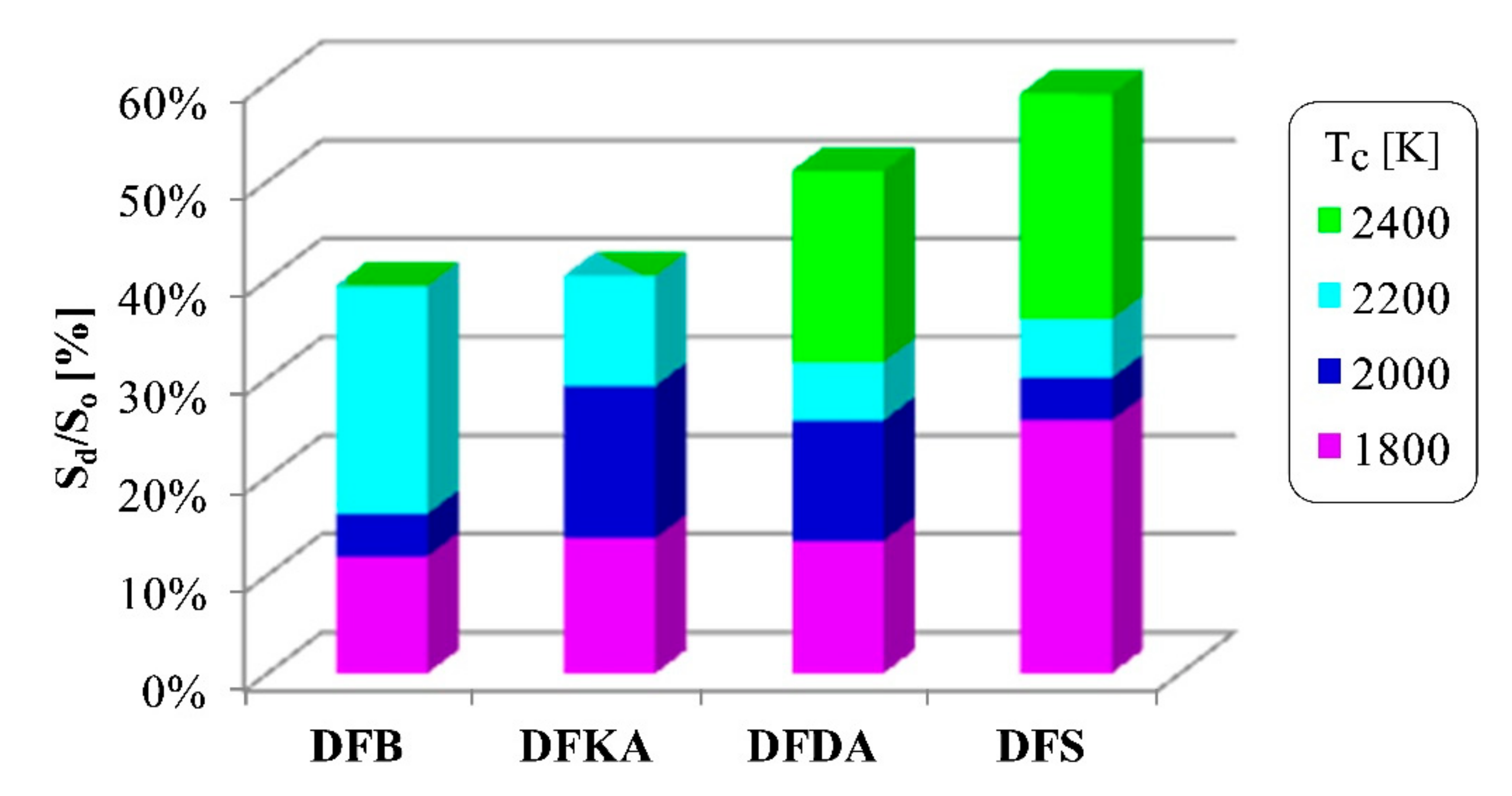
- The combustion process involves a heterogeneous fuel–air mixture, resulting in an uneven temperature distribution in the volume of the working medium for a given engine crankshaft angle of rotation. Thus, the combustion temperature (the main cause of NOx formation) is a function of several variables (even at a single point in engine operation), including the crankshaft angle of rotation and the location in the working medium. From the perspective of NOx formation, the average combustion temperature in the volume of the working medium is as important as the size of the working medium area with high-temperature isotherms. The larger the working medium area with isotherms with temperatures above 1000 K, the more NOx is generated in the engine cylinder,
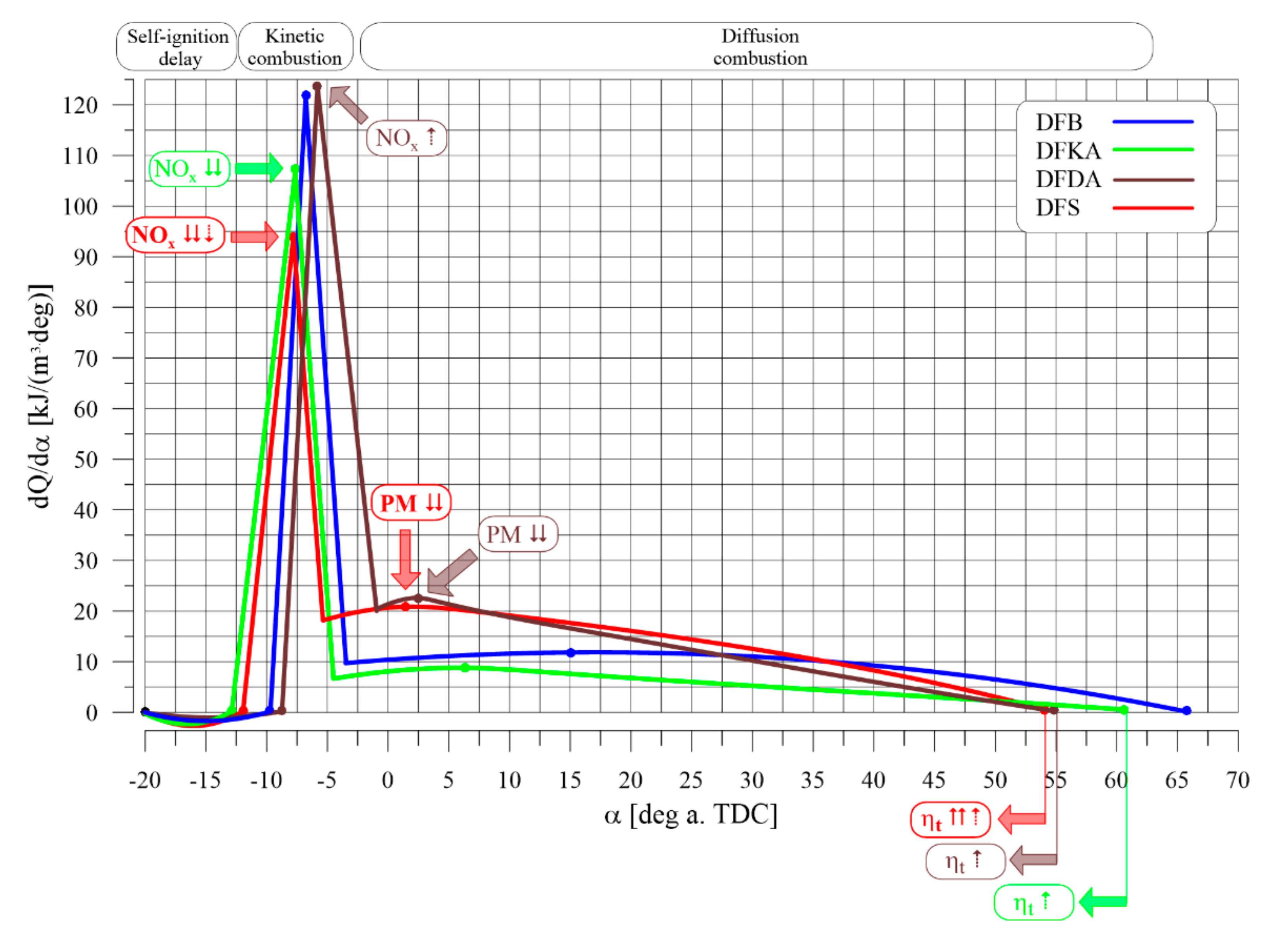
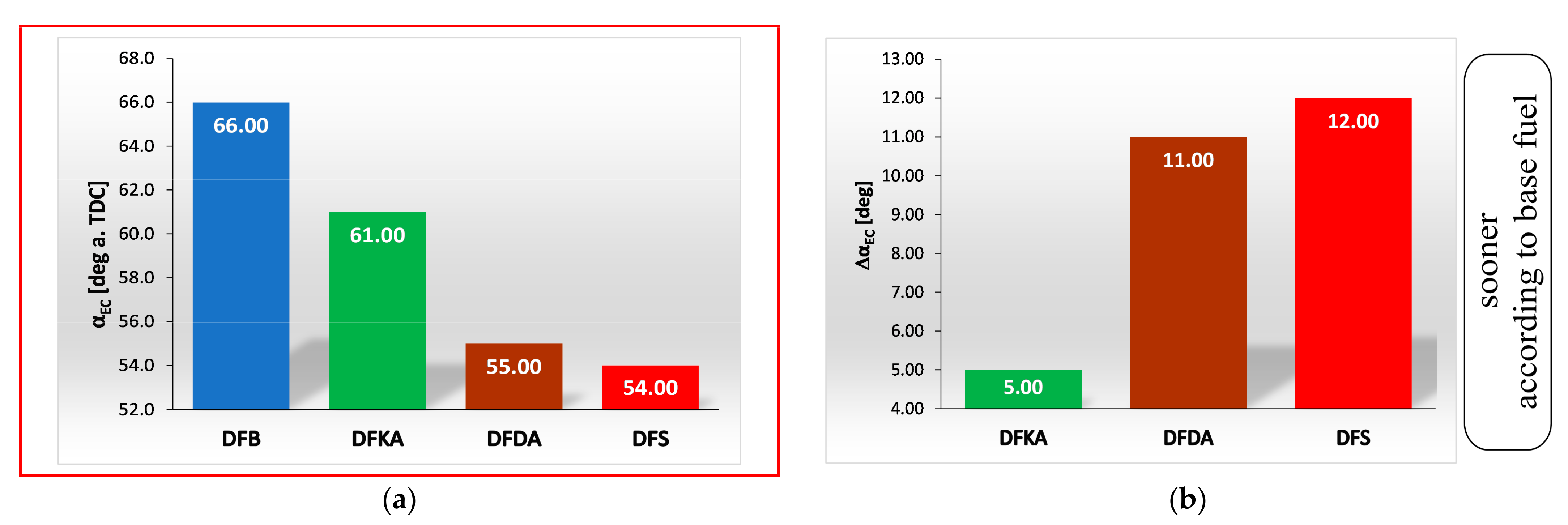
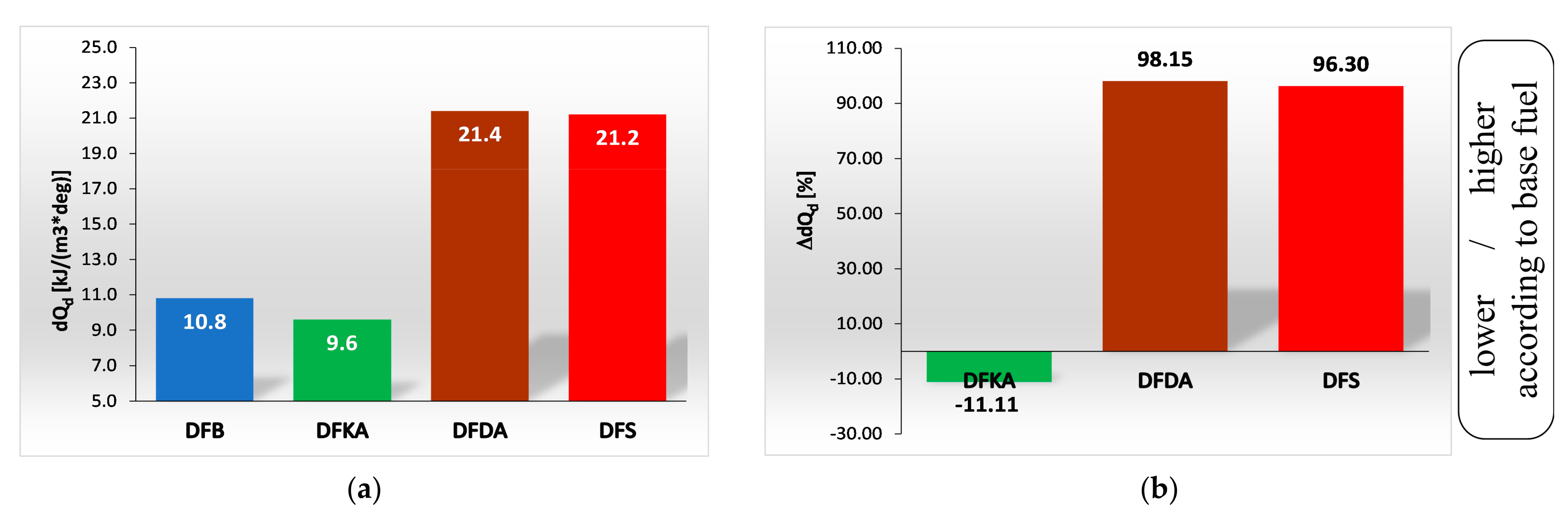
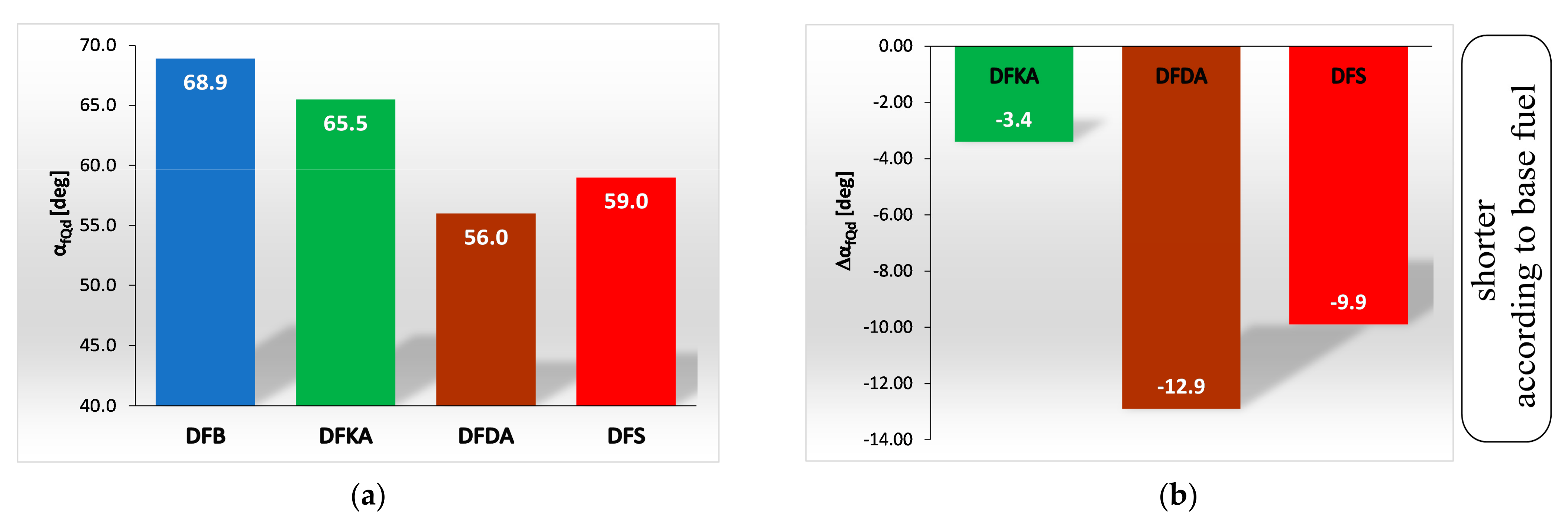
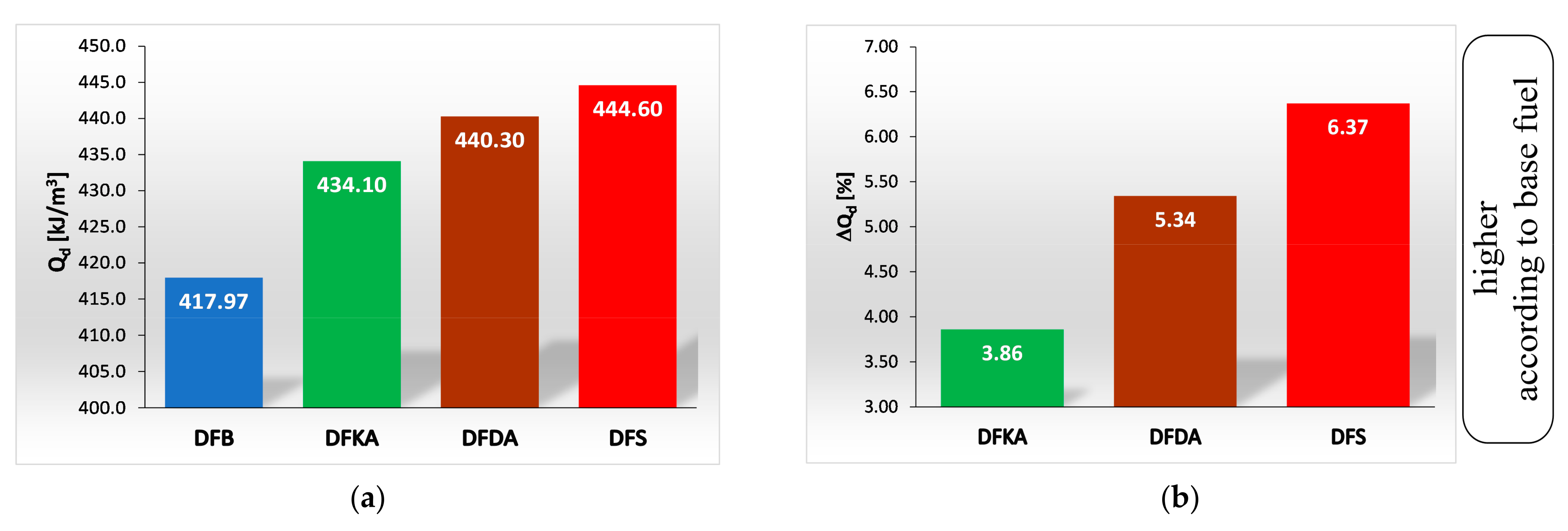
- The combustion process in a compression ignition engine is a bimodal process; it consists of a kinetic combustion phase (auto-ignition and combustion of fuel accumulated in the combustion chamber during auto-ignition delay period τc) and a diffusion combustion phase (combustion rate is dependent on injection speed fuel, as the diffusion mixing of fuel with air at high temperature is such a fast process that it does not limit the combustion rate of the injected fuel). Thus, it is most often assumed that the maximum kinetic combustion velocity (dQk) is responsible for the NOx produced [49]. The mechanism of NOx formation indicates that the kinetic phase duration (αlQk) must also be important. The longer the kinetic combustion phase lasts, the more NOx is formed. Thus, the heat generated in the kinetic phase of combustion (Qk) is also important. In the end, the duration of high-temperature combustion and the size of the working medium area with these isotherms are significant to the amount of NOx produced (Figure 10). This is similarly true for the diffusion combustion phase. In the literature, it is most often assumed that a higher maximum rate of diffusion combustion produces a lower solid particle emission, as the high rate of heat release in this combustion phase causes afterburning of much of the previously formed solid particles. Thus, in the general balance, the exhaust emission of solid particles is lower. However, for the kinetic phase and NOx, the amount of afterburned PM also depends on the diffusion phase duration (αlQd), because together with the rate of diffusion combustion, it determines the amount of heat (Qd) released in this phase, which is related to the combustion temperature responsible for the combustion of solid particles.

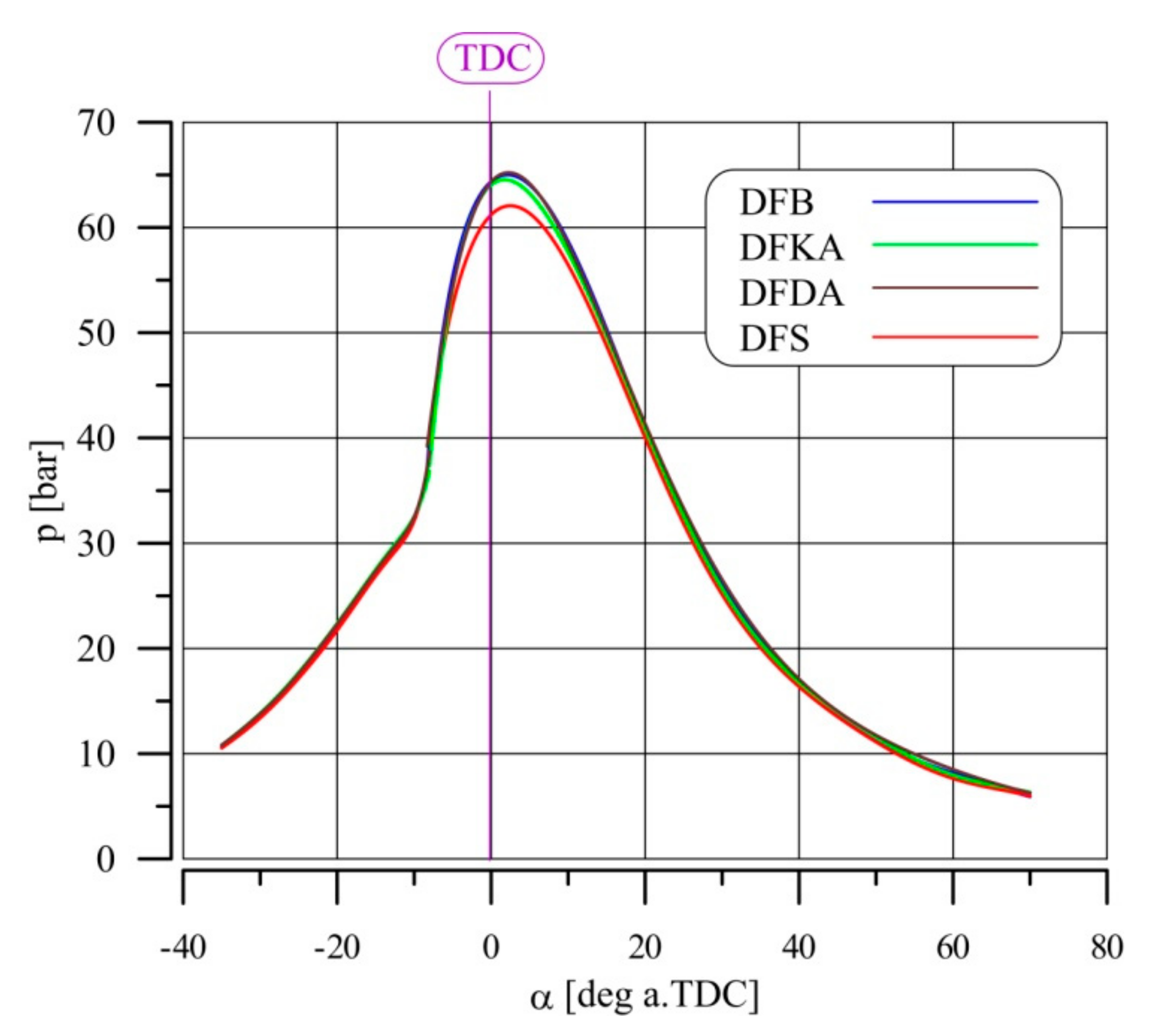
4.3. Indicator Charts
4.4. Heat Release Rate
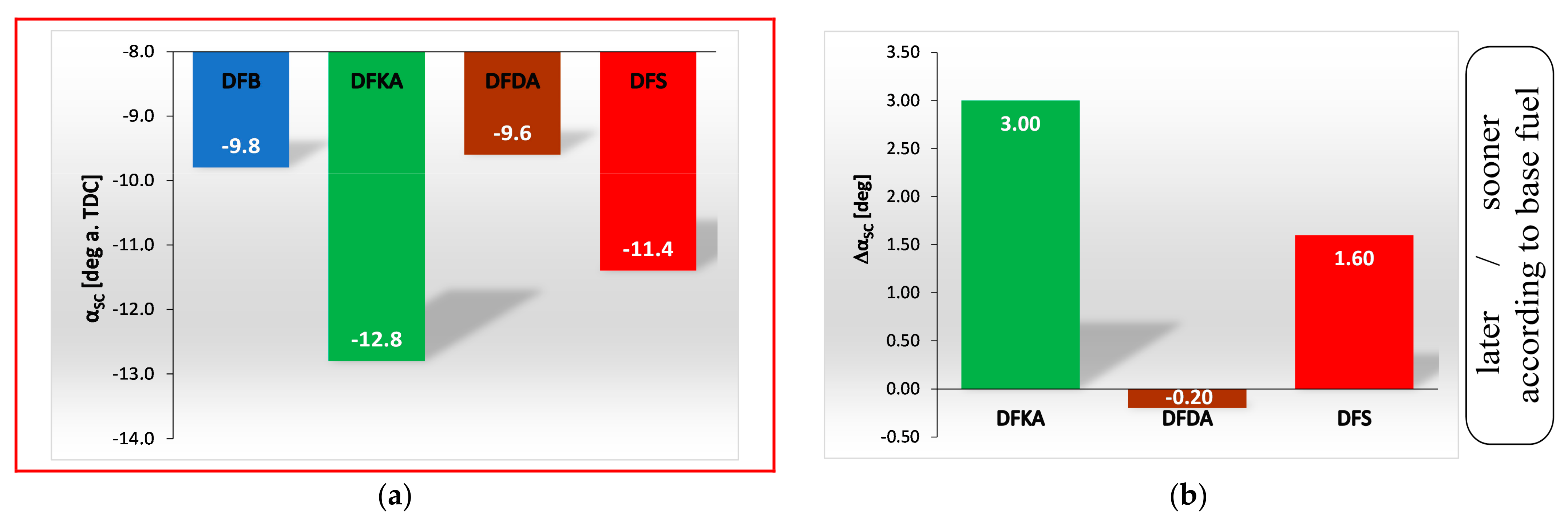
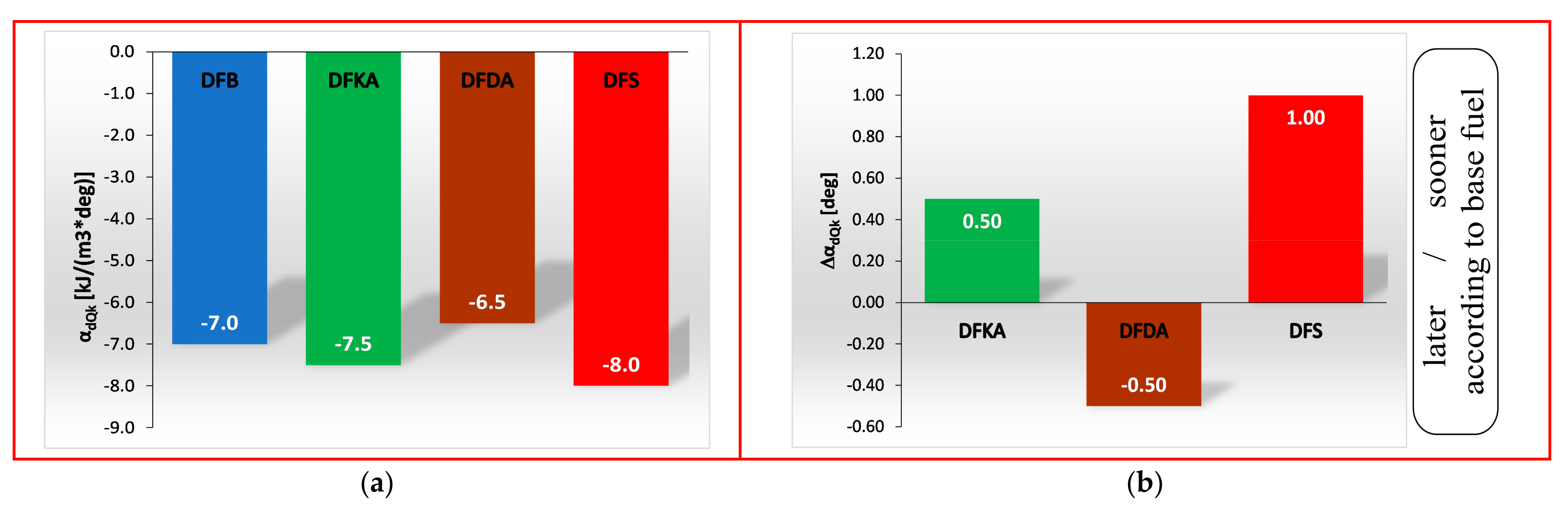
- Occurrence of the maximum rate of kinetic combustion (αdQk) occurs the latest (closest to the piston TDC). The heat released in a small volume (mass) of the working medium produces a large increase in temperature, facilitating NOx formation (Figure 20a),
- The heat released in the engine cylinder in the kinetic combustion phase (Qk) is the greatest for this fuel, favouring NOx formation (Figure 21),
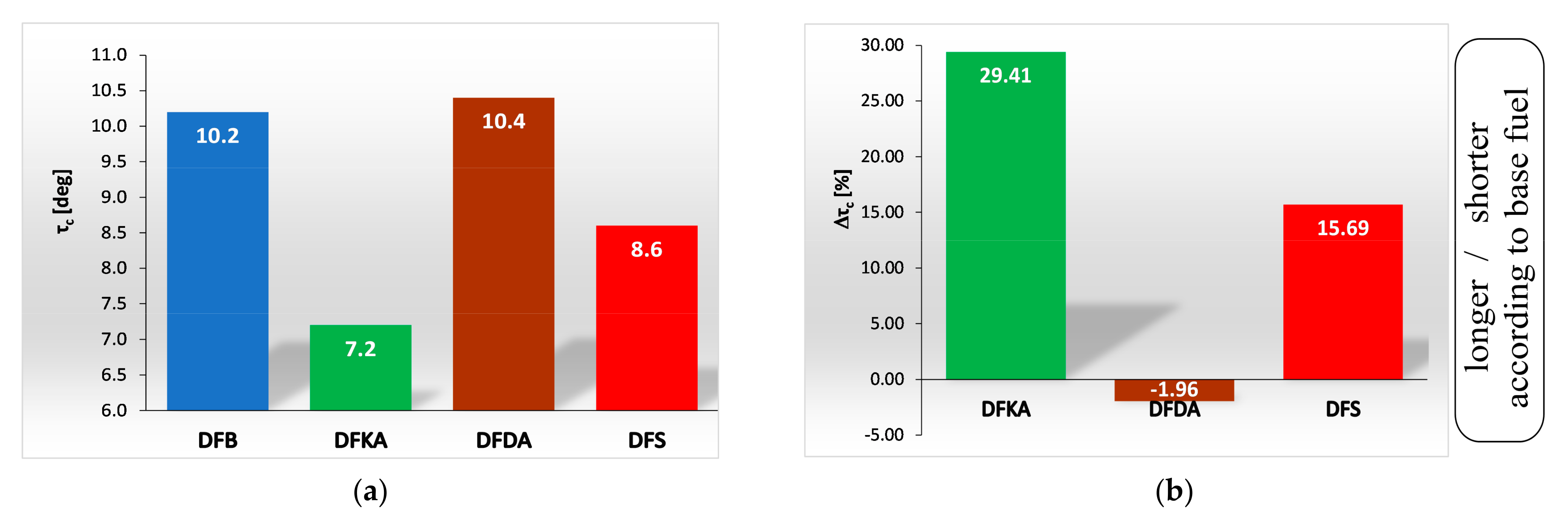
- the duration of the kinetic phase (αfQk) for DFDA fuel is the greatest, which increases the amount of NOx produced in the engine cylinder (Figure 22).
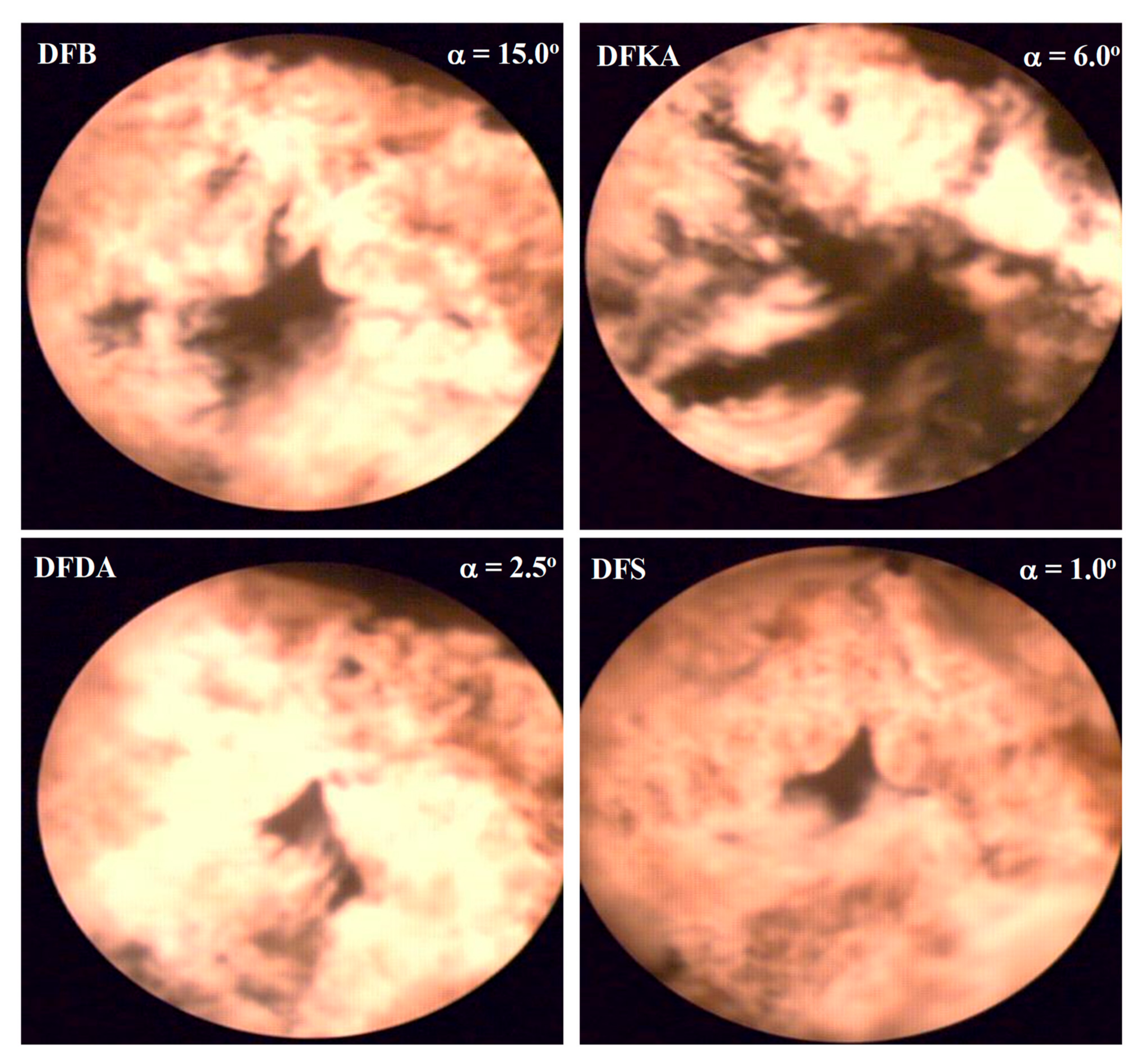
4.5. Visualisation and Thermovision of Combustion Process
5. Summary and Conclusions
- The 2-EHN additive (selectively reducing the kinetic combustion rate) and the Reduxco additive (selectively increasing the diffusion combustion rate) resulted in the same qualitative changes in the combustion process for the series VW 1.9 TDI engine and the SB 3.1 previous generation test engine.
- Relative to the base fuel, hourly fuel consumption (FC) was reduced by the following amounts:
- A total of 4% for DFKA fuel,
- A total of 6% for DFDA fuel,
- A total of 7% for DFS fuel.
- Relative to the base fuel, the NOx concentration in the exhaust gas changed in the following ways:
- A 16% reduction for DFKA fuel,
- An 8% increase for DFDA fuel,
- An 18% reduction for DFS fuel.
- Relative to the base fuel, PM emission in the exhaust gas changed in the following ways:
- A 2% increase for DFKA fuel,
- A 24% reduction for DFDA fuel,
- A 27% reduction for DFS fuel.
- Joint use of the 2-EHN additive and the Reduxco additive produced a beneficial synergy effect that influenced the course of the combustion process, and reduced fuel consumption, NOx concentration, and PM emissions in the engine exhaust.
- In the kinetic combustion phase, the fuel molecules bound to the 2-EHN ignited much earlier than the fuel molecules bound to the Reduxco. After self-ignition of DFS fuel, the heat of combustion of some of the fuel (related to 2-EHN) is reduced by the heat of vaporisation of the fuel, reducing the maximum kinetic combustion speed (dQk) and the combustion temperature, resulting in the smallest amount of NOx in the engine cylinder.
- In the diffusion combustion phase, the combustion of fuel particles bound to the Reduxco is characterised by a high diffusion phase velocity, superimposed on the pre-existing combustion rate of particles bound to the 2-EHN, increasing the duration of high-temperature diffusion combustion and the amount of heat generated, which in turn increases the amount of afterburned solid particles in the engine cylinder. In the overall balance, the greatest reduction in PM emissions is observed in the exhaust gas of the engine powered by DFS fuel.
- Combustion temperature measurements (Tc(α)) based on indicator charts and on visualisation and the two-colour method produce a better understanding of the synergy mechanism (both additives in the fuel) for the simultaneous reduction in NOx concentration and PM emissions in the exhaust gas.
Author Contributions
Funding
Conflicts of Interest
References
- Hill, L. Global trends in emissions legislation. In Proceedings of the VIII PTNSS International Congress on Combustion Engines, Krakow, Poland, 17–19 June 2019. [Google Scholar]
- Bielaczyc, P. Global trends in vehicular emissions regulations, current “hot topics” and their impact on powertrain technology—Introduction to the subject. In Proceedings of the VIII International PTNSS Congress on Combustion Engines, Krakow, Poland, 17–19 June 2019. [Google Scholar]
- Joshi, A. Overview of technologies for near-zero tailpipe emissions from light- and heavy-duty vehicles. In Proceedings of the VIII PTNSS International Congress on Combustion Engines, Krakow, Poland, 17–19 June 2019. [Google Scholar]
- Merkisz, J. Development trends in road transport powertrains. In Proceedings of the VIII PTNSS International Congress on Combustion Engines, Krakow, Poland, 17–19 June 2019. [Google Scholar]
- Engeljehringer, K. LD vehicle emission challenge from legislation to testbed. In Proceedings of the VIII PTNSS International Congress on Combustion Engines, Krakow, Poland, 17–19 June 2019. [Google Scholar]
- Bielaczyc, P.; Woodburn, J. Global trends in vehicular emissions legislation with a special focus on further stages (EU 7/VII and China 7/VII)—Associated development of ICE/hybrid/EV powertrain technologies and testing methodologies for LD, HD and NRMM sectors. In Proceedings of the IX International PTNSS Congress on Combustion Engines, Lublin, Poland, 27–28 June 2021. [Google Scholar]
- Neugebauer, S. Technological scenarios for the decarbonization of road transport. “Connectivity–key to future emission and consumption reduction? Proceedings of the vehicle and powertrain”. In Proceedings of the 30th International AVL Conference “Engine & Environment”, Graz, Austria, 7–8 June 2018. [Google Scholar]
- Winkler, S.L.; Anderson, J.E.; Garza, L. Vehicle criteria pollutant (PM, NOx, CO, HCs) emissions: How low should we go? Npj Clim. Atmos. Sci. 2018, 1, 26. [Google Scholar] [CrossRef]
- Bielaczyc, P. Global development of emissions reduction strategies from light-duty vehicles. IOP Conf. Ser. Earth Environ. Sci. 2019, 214, 012139. [Google Scholar] [CrossRef]
- Kim, M.H.; Chung, W.I.; Chyun, I.B. Three-Dimensional Flow Characteristics and Engine Performance for the Geometry Modification of Intake Manifold in Multi-Cylinder Diesel Engine. In SAE Technical Paper 2000-05-0020, Proceedings of the Seoul 2000 FISITA World Automotive Congress, Seoul, Korea, 12–15 June 2000; SAE: Warrendale, PA, USA, 2000. [Google Scholar]
- Montajir, R.M.; Tsunemoto, H.; Ishitani, H.; Minami, T. Fuel Spray Behavior in A Small DI Diesel Engine: Effect of Combustion Chamber Geometry; SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2000. [Google Scholar] [CrossRef]
- Zeng, K.; Huang, Z.; Liu, B.; Liu, L.; Jiang, D.; Ren, Y.; Wang, J. Combustion characteristics of a direct-injection natural gas engine under various fuel injection timings. Appl. Therm. Eng. 2006, 26, 806–813. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Srivastava, D.K.; Dhar, A.; Maurya, R.K.; Shukla, P.C.; Singh, A.P. Effect of fuel injection timing and pressure on combustion, emissions and performance characteristics of a single cylinder diesel engine. Fuel 2013, 111, 374–383. [Google Scholar] [CrossRef]
- Imtenan, S.; Rahman, S.A.; Masjuki, H.; Varman, M.; Kalam, M. Effect of dynamic injection pressure on performance, emission and combustion characteristics of a compression ignition engine. Renew. Sustain. Energy Rev. 2015, 52, 1205–1211. [Google Scholar] [CrossRef]
- Vedharaj, S.; Vallinayagam, R.; Yang, W.; Saravanan, C.; Lee, P. Optimization of combustion bowl geometry for the operation of kapok biodiesel—Diesel blends in a stationary diesel engine. Fuel 2015, 139, 561–567. [Google Scholar] [CrossRef]
- Millo, F.; Giacominetto, P.F.; Bernardi, M.G. Analysis of different exhaust gas recirculation architectures for passenger car Diesel engines. Appl. Energy 2012, 98, 79–91. [Google Scholar] [CrossRef]
- Asad, U.; Zheng, M. Exhaust gas recirculation for advanced diesel combustion cycles. Appl. Energy 2014, 123, 242–252. [Google Scholar] [CrossRef]
- Sayin Kul, B.; Kahraman, A. Energy and Exergy Analyses of a Diesel Engine Fuelled with Biodiesel-Diesel Blends Containing 5% Bioethanol. Entropy 2016, 18, 387. [Google Scholar] [CrossRef]
- Monsalve-Serrano, J.; Belgiorno, G.; Di Blasio, G.; Guzmán-Mendoza, M. 1D Simulation and Experimental Analysis on the Effects of the Injection Parameters in Methane–Diesel Dual-Fuel Combustion. Energies 2020, 13, 3734. [Google Scholar] [CrossRef]
- Belgiorno, G.; Dimitrakopoulos, N.; Di Blasio, G.; Beatrice, C.; Tuner, M.; Tunestal, P. Parametric Analysis of the Effect of Pilot Quantity, Combustion Phasing and EGR on Efficiencies of a Gasoline PPC Light-Duty Engine. In Proceedings of the 13th International Conference on Engines & Vehicles, Napoli, Italy, 10–14 September 2017; Volume 1, p. 1. [Google Scholar] [CrossRef]
- Rashedul, H.; Masjuki, H.; Kalam, M.; Ashraful, A.; Rahman, S.A.; Shahir, S. The effect of additives on properties, performance and emission of biodiesel fuelled compression ignition engine. Energy Convers. Manag. 2014, 88, 348–364. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Das, L.M. Biodiesel Development and Characterization for Use as a Fuel in Compression Ignition Engines. J. Eng. Gas Turbines Power 2001, 123, 440–447. [Google Scholar] [CrossRef]
- Aldhaidhawi, M.; Chiriac, R.; Badescu, V. Ignition delay, combustion and emission characteristics of Diesel engine fueled with rapeseed biodiesel—A literature review. Renew. Sustain. Energy Rev. 2017, 73, 178–186. [Google Scholar] [CrossRef]
- Hwang, J.; Qi, D.; Jung, Y.; Bae, C. Effect of injection parameters on the combustion and emission characteristics in a common-rail direct injection diesel engine fueled with waste cooking oil biodiesel. Renew. Energy 2014, 63, 9–17. [Google Scholar] [CrossRef]
- Chadwell, C.J.; Dingle, P.J.G. Effect of Diesel and Water Co-injection with Real-Time Control on Diesel Engine Performance and Emissions; SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2008. [Google Scholar] [CrossRef]
- Pidol, L.; Lecointe, B.; Starck, L.; Jeuland, N. Ethanol–biodiesel–diesel fuel blends: Performances and emissions in conventional diesel and advanced low temperature combustions. Fuel 2012, 93, 329–338. [Google Scholar] [CrossRef]
- Bose, P.K.; Maji, D. An experimental investigation on engine performance and emissions of a single cylinder diesel engine using hydrogen as inducted fuel and diesel as injected fuel with exhaust gas recirculation. Int. J. Hydrogen Energy 2009, 34, 4847–4854. [Google Scholar] [CrossRef]
- Choi, C.; Reitz, R. An experimental study on the effects of oxygenated fuel blends and multiple injection strategies on DI diesel engine emissions. Fuel 1999, 78, 1303–1317. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Mujtaba, M.; RezaSafaeibcd, M.; Afzale, A.; V Raju, D.; Ahmeda, W.; Banapurmath, N.; Hossainh, N.; Bashiri, S.; AnjumBadruddinjk, I.; et al. Effect of Sr@ZnO nanoparticles and Ricinus communis biodiesel-diesel fuel blends on modified CRDI diesel engine characteristics. Energy 2021, 215, 119094. [Google Scholar] [CrossRef]
- Gavhane, R.S.; Kate, A.M.; Pawar, A.; Safaei, M.R.; Soudagar, M.E.M.; Abbas, M.M.; Ali, H.M.; Banapurmath, N.R.; Goodarzi, M.; Badruddin, I.A.; et al. Effect of Zinc Oxide Nano-Additives and Soybean Biodiesel at Varying Loads and Compression Ratios on VCR Diesel Engine Characteristics. Symmetry 2020, 12, 1042. [Google Scholar] [CrossRef]
- Khan, H.; Soudagar, M.E.M.; Kumar, R.H.; Safaei, M.R.; Farooq, M.; Khidmatgar, A.; Banapurmath, N.R.; Farade, R.A.; Abbas, M.M.; Afzal, A.; et al. Effect of Nano-Graphene Oxide and n-Butanol Fuel Additives Blended with Diesel—Nigella sativa Biodiesel Fuel Emulsion on Diesel Engine Characteristics. Symmetry 2020, 12, 961. [Google Scholar] [CrossRef]
- Di Blasio, G.; Bonura, G.; Frusteri, F.; Beatrice, C.; Cannilla, C.; Viscardi, M. Experimental Characterization of Diesel Combustion Using Glycerol Derived Ethers Mixtures. SAE Int. J. Fuels Lubr. 2013, 6, 940–950. [Google Scholar] [CrossRef]
- Di Blasio, G.; Beatrice, C.; Molina, S. Effect of Port Injected Ethanol on Combustion Characteristics in a Dual-Fuel Light Duty Diesel Engine; SAE Tech. Pap. 2013-01-1692; SAE International: Warrendale, PA, USA, 2013. [Google Scholar] [CrossRef]
- Napolitano, P.; Guido, C.; Beatrice, C.; Di Blasio, G. “Study of the Effect of the Engine Parameters Calibration to Optimize the Use of Bio-Ethanol/RME/Diesel Blend in a Euro5 Light Duty Diesel Engine”. SAE Int. J. Fuels Lubr. 2013, 6, 263–275. [Google Scholar] [CrossRef]
- Di Blasio, G.; Beatrice, C.; Dijkstra, R.; Boot, M. Low Cetane Number Renewable Oxy-fuels for Premixed Combustion Concept Application: Experimental Investigation on a Light Duty Diesel Engine; SAE Tech. Pap. 2012-01-1310; SAE International: Warrendale, PA, USA, 2012. [Google Scholar] [CrossRef]
- Cisek, J.; Lesniak, S.; Przybylski, W. The synergy of two biofuel additives on combustion process to simultaneously reduce NOx and PM emissions. Energies 2021, 14, 2784. [Google Scholar] [CrossRef]
- Worldwide Fuel Charter. Gasoline and Diesel Fuel, 6th ed.; Worldwide Fuel Charter: Brussels, Belgium, 2019. [Google Scholar]
- Myung, C.L.; Park, S. Exhaust nanoparticle emissions from internal combustion engines: A review. Int. J. Automot. Technol. 2012, 9, 13. [Google Scholar] [CrossRef]
- Benajes, J.; Novella, R.; Arthozoul, S.; Kolodziej, C. Particle size distribution measurements from early to late injection timing low-temperature combustion in a heavy-duty diesel engine. SAE Int. J. Fuels Lubr. 2010, 3, 567–581. [Google Scholar] [CrossRef]
- AVL LIST Gmbh. AVL 513D Engine VideoScope. System Description and Examples; AVL: Graz, Australia, 2000. [Google Scholar]
- Cisek, J. Optical research on the fuel injection into a diesel engine for diesel fuel, RME and rape oil. J. KONES. Powertrain Transp. 2012, 19, 65–73. [Google Scholar] [CrossRef]
- Cisek, J. Injection and combustion of RME with water emulsions in a diesel engine. Acta Polytech. 2010, 50. [Google Scholar] [CrossRef]
- Cisek, J. Options for the analysis of fuel injectors function using visual digitized methods. J. Middle Eur. Constr. Des. Cars 2004, 2, 24–31. [Google Scholar]
- DAGAS Limited Company. Wizualizacja Spalania i Termowizja Płomienia w Badawczym Silniku z Zapłonem Samoczynnym Zasilanym Paliwem Bazowym i Trzema Paliwami z Dodatkami; Research Report, Contract No: M-04/331/2021/P; Cracow University of Technology: Cracow, Poland, 2022. [Google Scholar]
- Ma, B. Development of Quantitative Optical Techniques for Microgravity Combustion and Sooty Flame Characterization. Ph.D. thesis, Faculty of the Graduate School of Yale University, New Haven, CT, USA, September 2013. [Google Scholar]
- Levendis, Y.A.; Estrada, K.R.; Hottel, H.C. Development of multicolor pyrometers to monitor the transient response of burning carbonaceous particles. Rev. Sci. Instrum. 1992, 63, 3608–3622. [Google Scholar] [CrossRef]
- Matsui, Y.; Kamimoto, T.; Matsuoka, S. A Study on the Application of the Two–Color Method to the Measurement of Flame Temperature and Soot Concentration in Diesel Engines; SAE International: Warrendale, PA, USA, 1980. [Google Scholar] [CrossRef]
- AVL LIST Gmbh. Spectral Flame Temperature Measurement Using the Two Colour Method; AVL LIST Gmbh: Graz, Austria, 2000. [Google Scholar]
- Merkisz, J. Ekologiczne Problemy Silników Spalinowych; Wydawnictwo PP: Poznań, Poland, 1999; ISBN 83-7143-039-6. [Google Scholar]
- Bornemann, H.; Scheidt, F.; Sander, W. Thermal decomposition of 2-ethylhexyl nitrate (2-EHN). Int. J. Chem. Kinet. 2002, 34, 34–38. [Google Scholar] [CrossRef]
- Lu, G.B.; Yang, T.; Chen, L.P. Thermal decomposition kinetics of 2-ethylhexyl nitrate under non-isothermal and isothermal conditions. J. Therm. Anal. Calorim. 2015, 124, 471–478. [Google Scholar] [CrossRef]
- Almodovar, C.A.; Goldsmith, C.F. Laser schlieren study of the thermal decomposition of 2-ethylhexyl-nitrate. Proc. Combust. Inst. 2021, 38, 997–1005. [Google Scholar] [CrossRef]
- Cisek, J.; Lesniak, S.; Mokretskyy, V.; Przybylski, W. The Influence of Reduxco Catalyst in the Diesel Fuel on Energy Parameters, Exhaust Gas Composition and Rate of Heat Release of A Diesel Engine; SAE Technical Paper Series; SAE Technical Paper 2020-01-2061; SAE International: Warrendale, PA, USA, 2020. [Google Scholar]










| Type of Engine | SB 3.1 (One-Cylinder Research Engine) Primary Unit: SW 680 (Leyland) |
|---|---|
| combustion system | direct fuel injection to open combustion chamber in piston |
| displacement | 1.85 dm3 |
| number of cylinders | 1 |
| cylinder diameter | 127 mm |
| piston stroke | 146 mm |
| compression ratio | 15.75 |
| rated power Ne | 23 kW |
| nominal rotational speed | 2200 rpm |
| maximum torque Momax | 110 Nm |
| rotation speed nMomax | 1600 rpm |
| direction of crankshaft rotation | left |
| lubrication | circulating, pressurised |
| Cooling | liquid, forced |
| geometric start of fuel injection | 27° CA before TDC |
| static injector opening pressure | 17 MPa |
| injection pump | piston, type P56-01A |
| regulator | type R 14V-20-110/12M |
| injector | type W1B-01 |
| sprayer | 4-hole, φ = 0.35 mm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cisek, J.; Leśniak, S.; Borowski, A.; Przybylski, W.; Mokretskyy, V. Visualisation and Thermovision of Fuel Combustion Affecting Heat Release to Reduce NOx and PM Diesel Engine Emissions. Energies 2022, 15, 4882. https://doi.org/10.3390/en15134882
Cisek J, Leśniak S, Borowski A, Przybylski W, Mokretskyy V. Visualisation and Thermovision of Fuel Combustion Affecting Heat Release to Reduce NOx and PM Diesel Engine Emissions. Energies. 2022; 15(13):4882. https://doi.org/10.3390/en15134882
Chicago/Turabian StyleCisek, Jerzy, Szymon Leśniak, Andrzej Borowski, Włodzimierz Przybylski, and Vitaliy Mokretskyy. 2022. "Visualisation and Thermovision of Fuel Combustion Affecting Heat Release to Reduce NOx and PM Diesel Engine Emissions" Energies 15, no. 13: 4882. https://doi.org/10.3390/en15134882





