Study on the Reaction Path of -CH3 and -CHO Functional Groups during Coal Spontaneous Combustion: Quantum Chemistry and Experimental Research
Abstract
:1. Introduction
2. Methods and Experiments
2.1. Establishment of Molecular Model and Calculate Content
2.2. Experimental
2.2.1. Preparation of Coal Samples
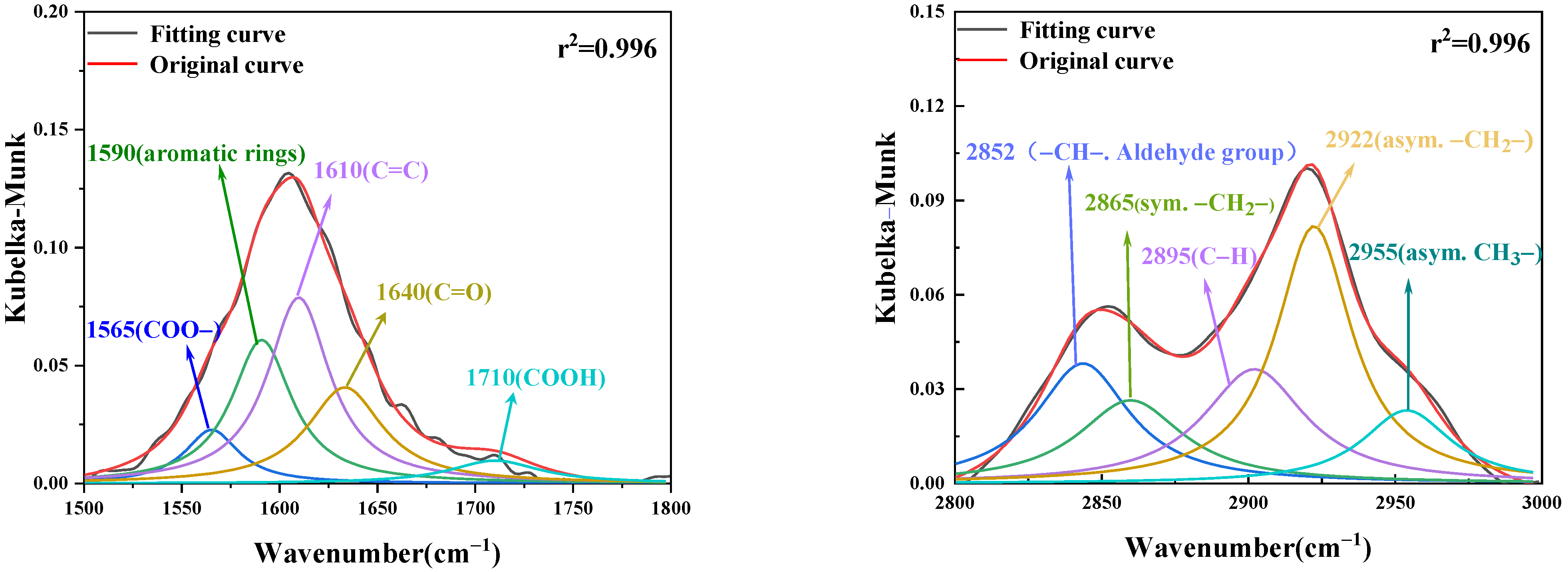
2.2.2. Fourier-Transform Infrared Spectroscopy (FTIR) Experiment
2.2.3. Low-Temperature Oxidation Experiment
3. Free-Radical Reaction Processes of ph-CH3 and ph-CH2CHO with O2
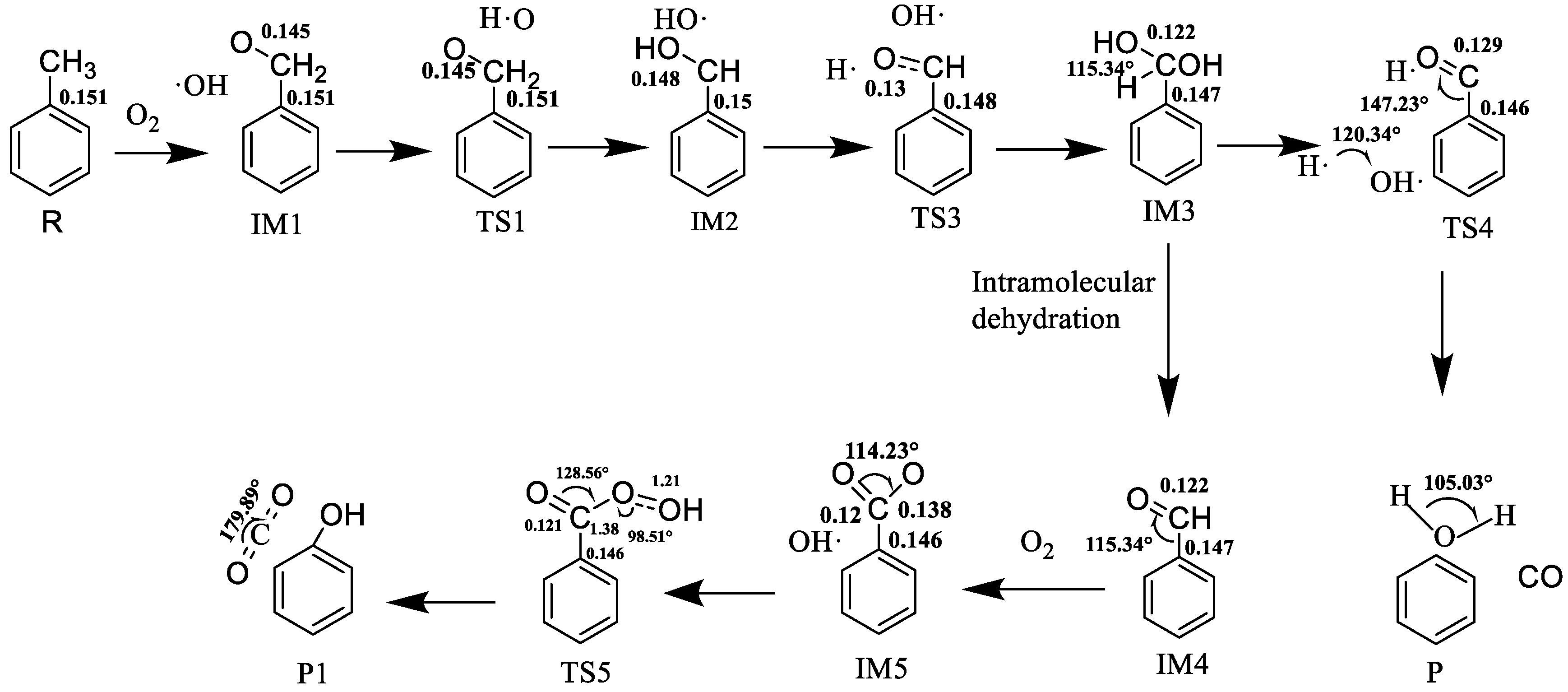
3.1. Reaction of ph-CH3 with O2
3.2. Reaction of ph-CH2CHO with O2
4. Results and Discussion
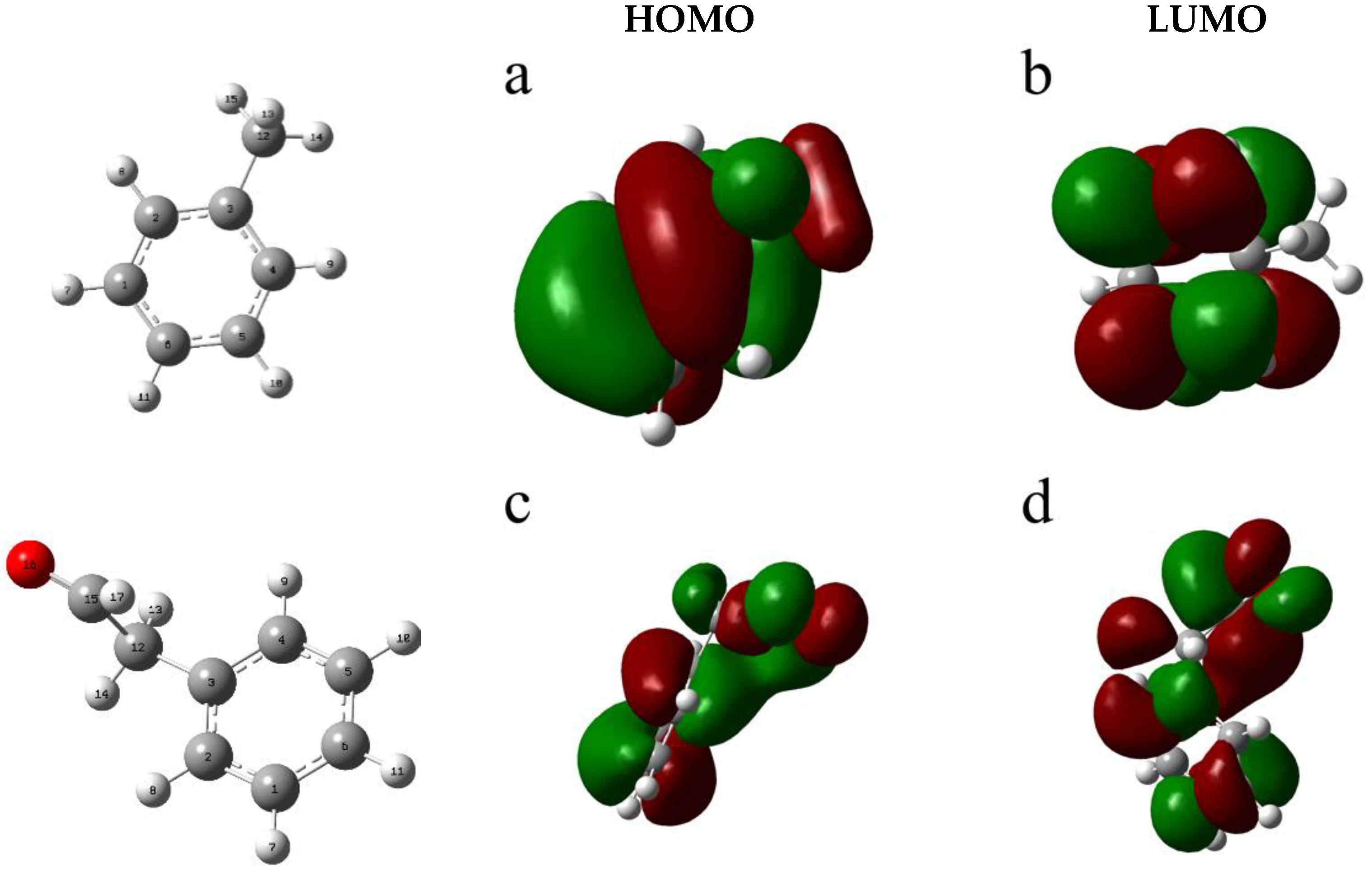
4.1. Frontier Orbital Theory
4.2. Reaction of ph-CH3 with O2
4.2.1. Analysis of Reaction Mechanism and Energy
4.2.2. Analysis of Transition States and Activation Energy
4.3. Reaction of ph-CH2CHO with O2
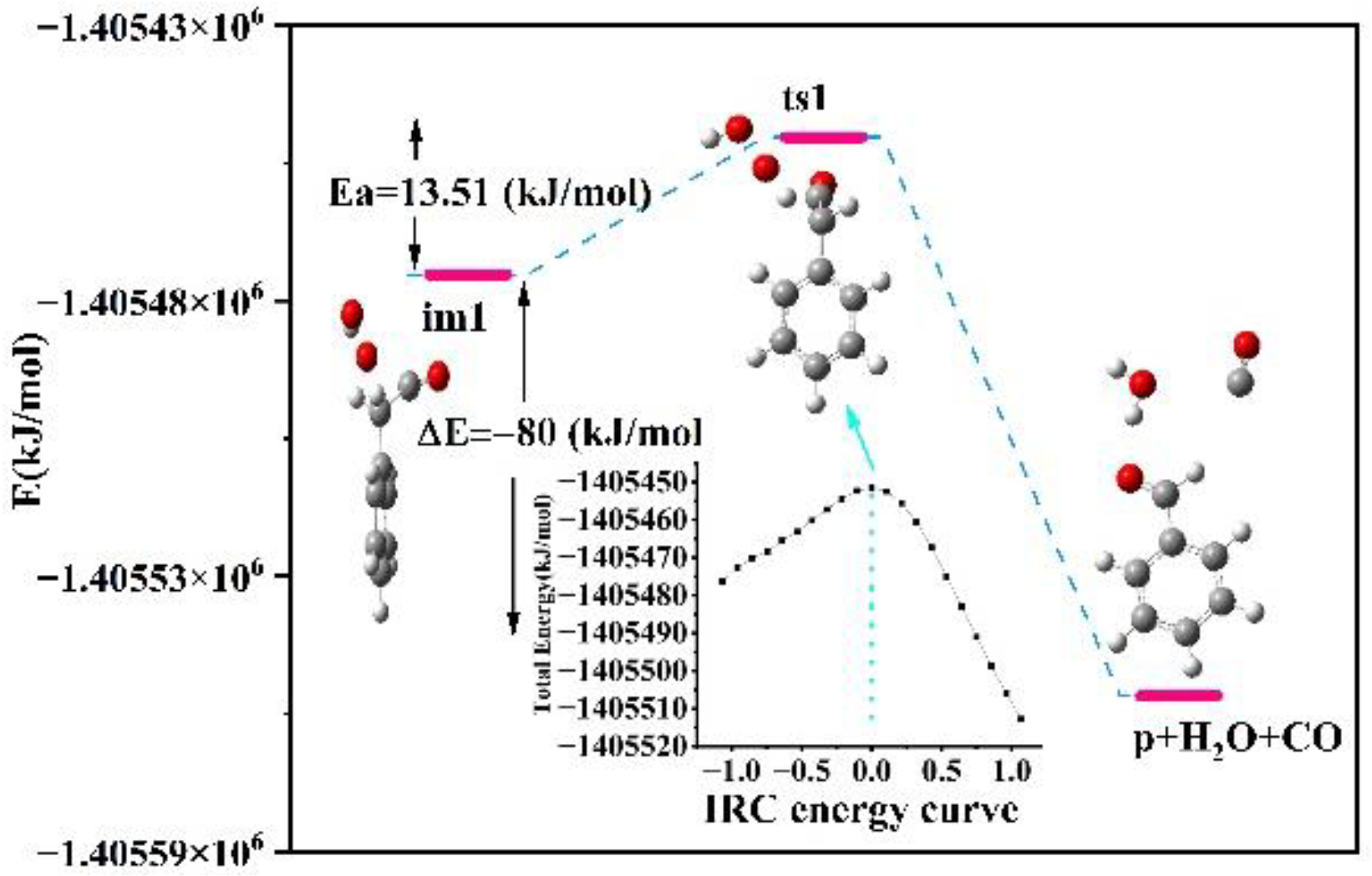
4.3.1. Analysis on Reaction Mechanism and Energy
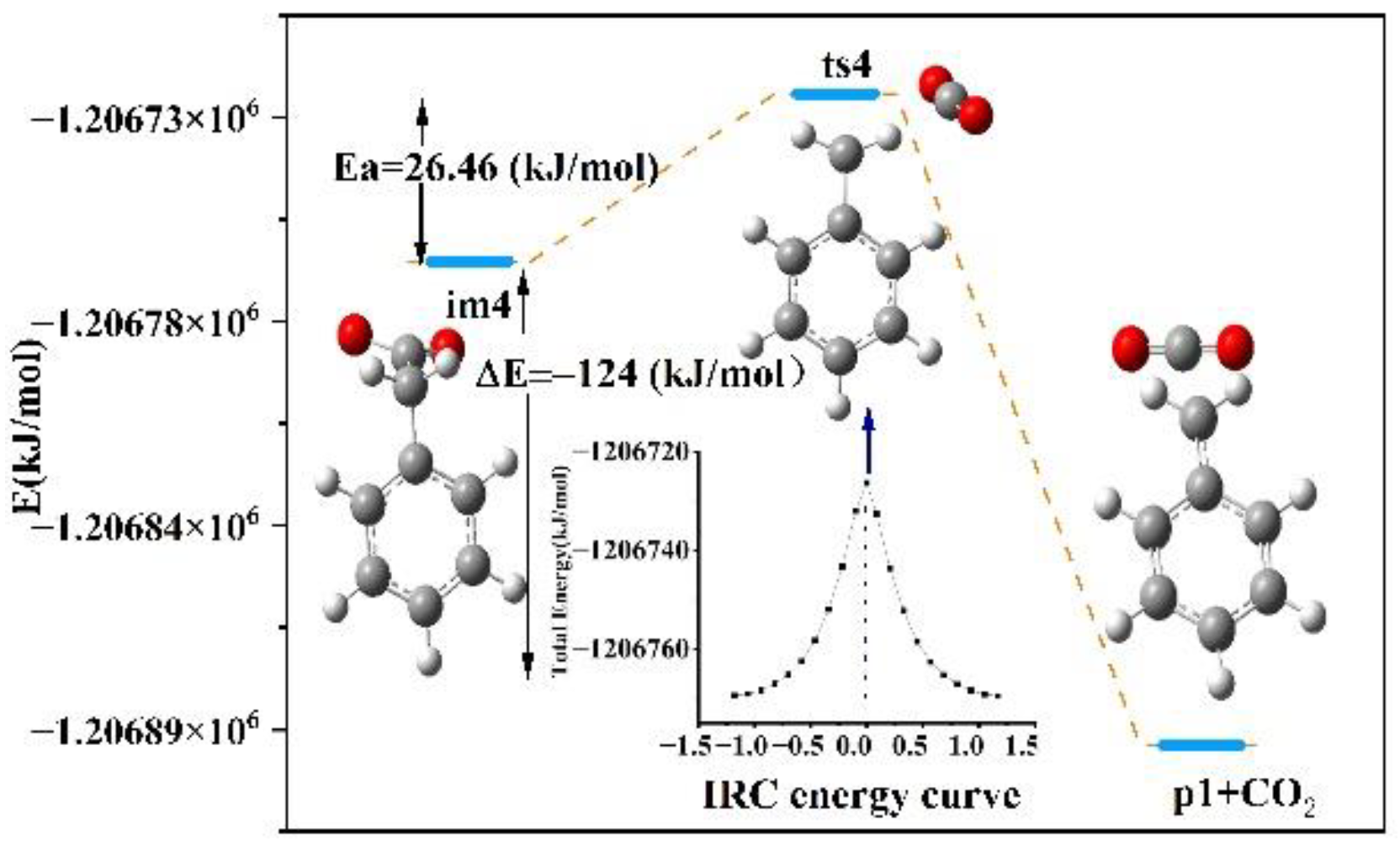
4.3.2. Analysis of Transition States and Activation Energy
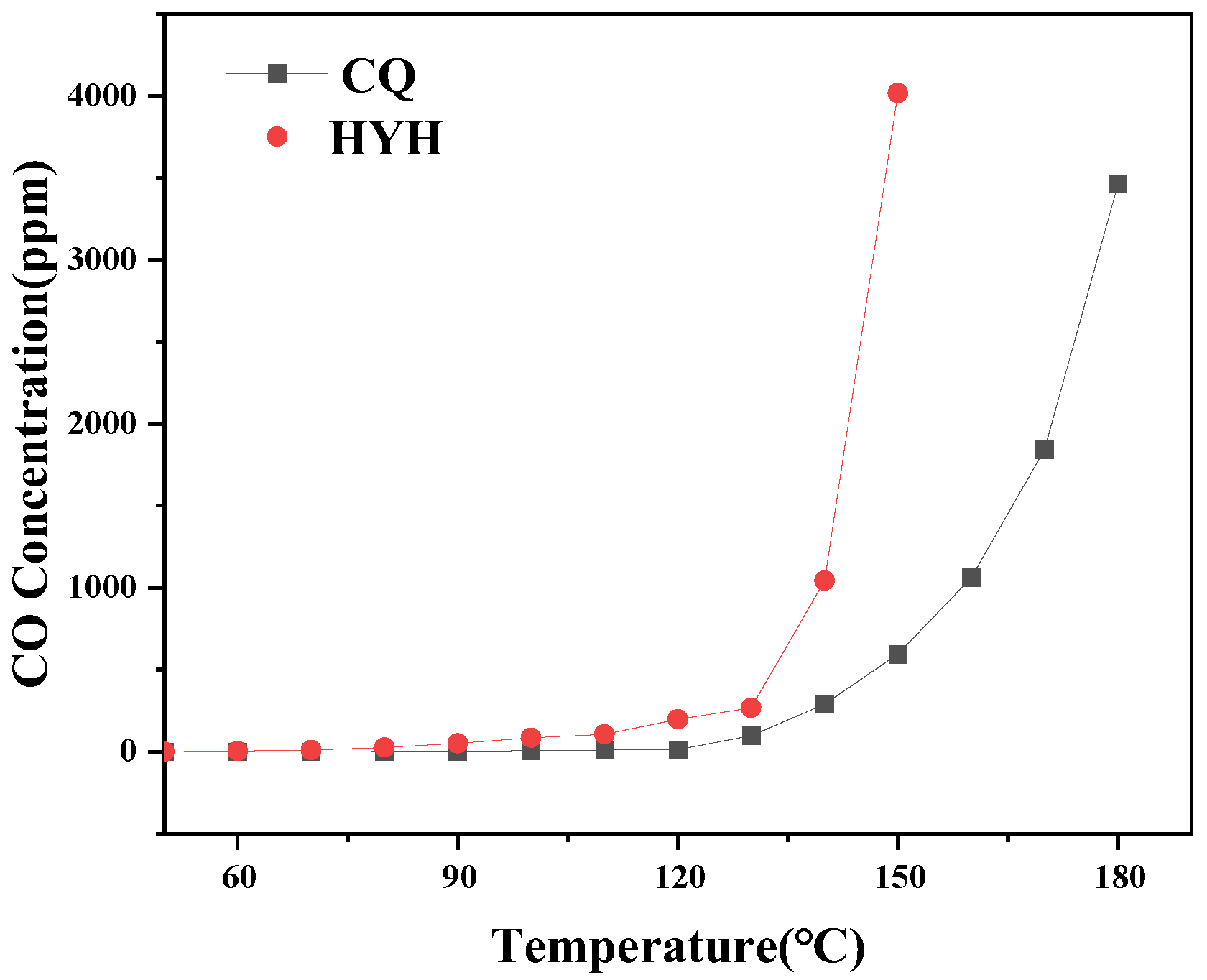
4.4. Changes in CO and CO2 Concentrations during Low-Temperature Oxidation
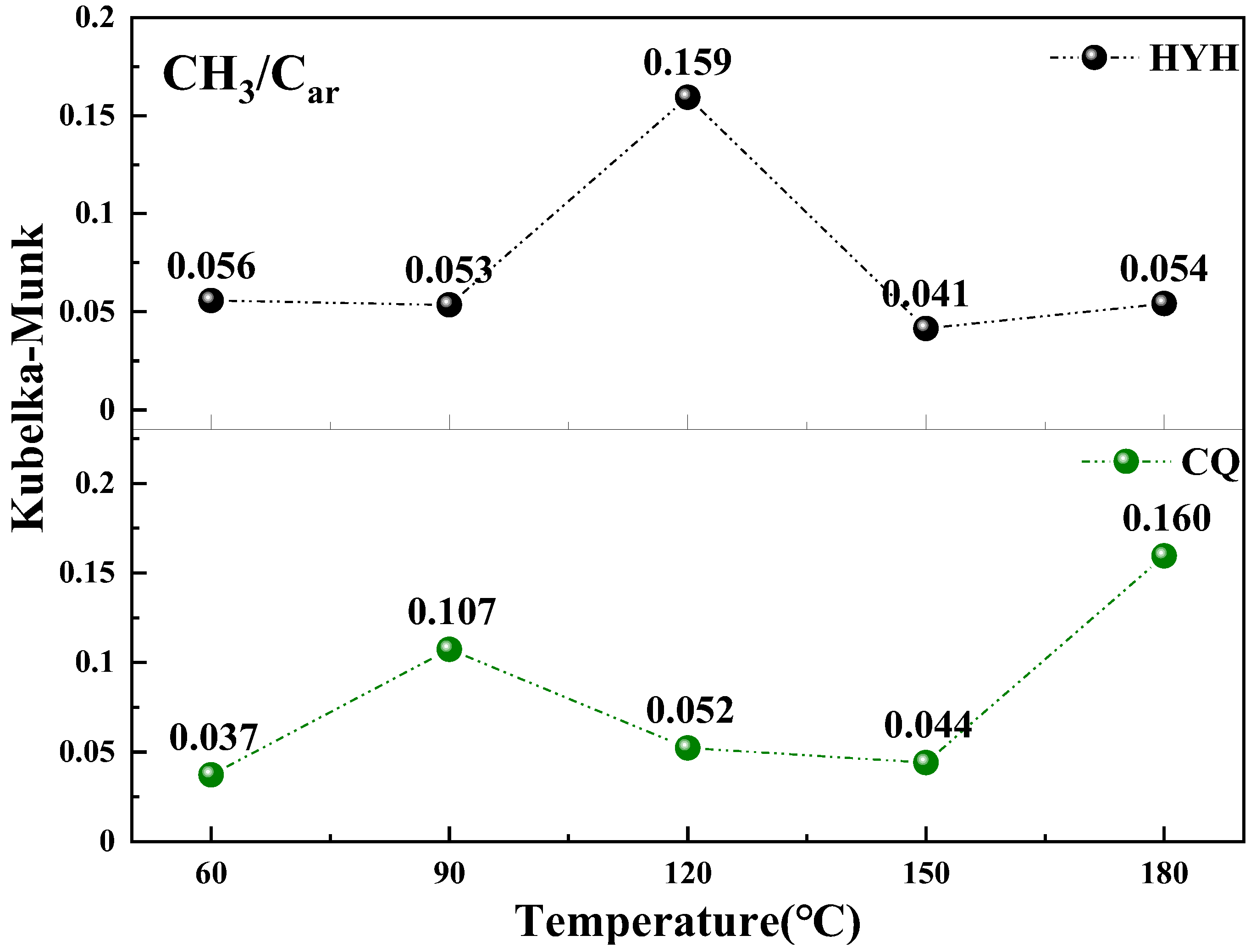
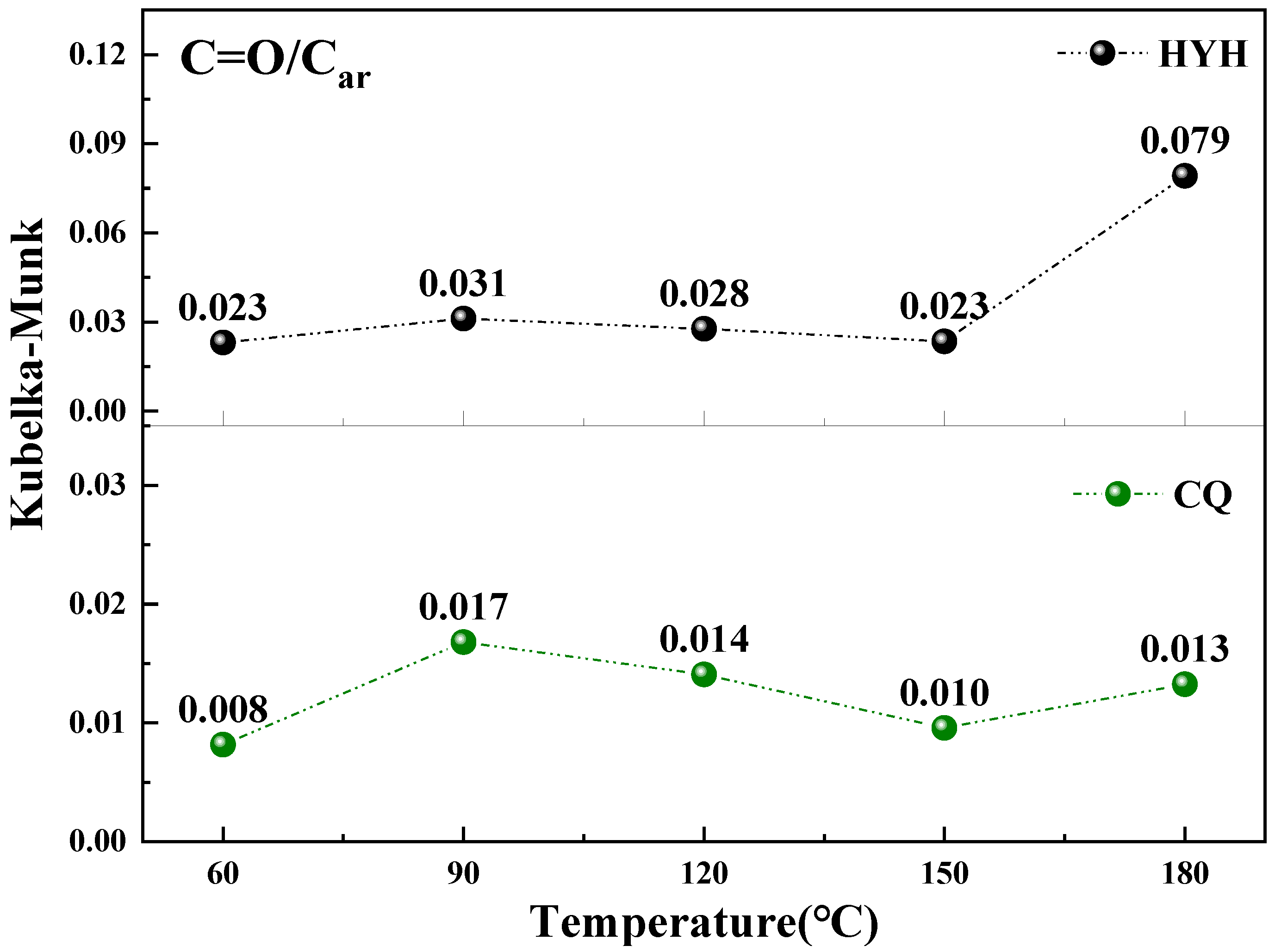
4.5. Changes in Active Groups during Low-Temperature Oxidation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, X.; Zhang, Y.; Chen, X.; Zhang, Y. Effects of thermal boundary conditions on spontaneous combustion of coal under temperature-programmed conditions. Fuel 2021, 295, 120591. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, Y.; Chen, X.; Zhang, Y.; Rui, L.; Guo, R.; Zhao, T.; Deng, Y. Numerical simulation on response characteristics of coal ignition under the disturbance of fluctuating heat. Combust. Flame 2022, 237, 111870. [Google Scholar] [CrossRef]
- Li, J.; Lu, W.; Kong, B.; Cao, Y.; Qi, G.; Qin, C. Mechanism of Gas Generation during Low-Temperature Oxidation of Coal and Model Compounds. Energy Fuel 2019, 33, 1527–1539. [Google Scholar] [CrossRef]
- Wang, D.; Zhong, X.; Gu, J.; Qi, X. Changes in active functional groups during low-temperature oxidation of coal. Min. Sci. Technol. 2010, 20, 35–40. [Google Scholar] [CrossRef]
- Mathews, J.P.; van Duin, A.C.T.; Chaffee, A.L. The utility of coal molecular models. Fuel Process. Technol. 2011, 92, 718–728. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Y.; Wang, J.; Xue, S.; Wu, J.; Chang, L. Study on the relationship between microscopic functional group and coal mass changes during low-temperature oxidation of coal. Int. J. Coal Geol. 2017, 171, 212–222. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Wu, J.; Xue, S.; Li, Z.; Chang, L. Modes and kinetics of CO2 and CO production from low-temperature oxidation of coal. Int. J. Coal Geol. 2015, 140, 1–8. [Google Scholar] [CrossRef]
- Wang, D.M.; Xin, H.H.; Qi, X.Y.; Dou, G.L.; Zhong, X.X. Mechanism and relationships of elementary reactions in spontaneous combustionof coal: The coal oxidation kinetics theory and application. J. China Coal Soc. 2014, 39, 1667–1674. [Google Scholar]
- Zhu, H.; Huo, Y.; Fang, S.; He, X.; Wang, W.; Zhang, Y. Quantum Chemical Calculation of Original Aldehyde Groups Reaction Mechanism in Coal Spontaneous Combustion. Energy Fuel 2020, 34, 14776–14785. [Google Scholar] [CrossRef]
- Qi, X.; Li, Y.; Chen, L.; Tang, J.; Xin, H.; Liang, Z. Reaction Mechanism of Aldehyde Groups during Coal Self-Heating. ACS Omega 2020, 5, 23184–23192. [Google Scholar] [CrossRef]
- Risthaus, T.; Grimme, S. Benchmarking of London Dispersion-Accounting Density Functional Theory Methods on Very Large Molecular Complexes. J. Chem. Theory Comput. 2013, 9, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Darvishnejad, M.H.; Reisi-Vanani, A. DFT-D3 calculations of the charge-modulated CO2 capture of N/Sc-embedded graphyne: Compilation of some factors. J. CO2 Util. 2021, 46, 101469. [Google Scholar] [CrossRef]
- Moellmann, J.; Grimme, S. DFT-D3 Study of Some Molecular Crystals. J. Phys. Chem. C 2014, 118, 7615–7621. [Google Scholar] [CrossRef]
- Lin, Y.; Li, G.; Mao, S.; Chai, J. Long-Range Corrected Hybrid Density Functionals with Improved Dispersion Corrections. J. Chem. Theory Comput. 2013, 9, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Kubba, R.M. Aromatic C-H Bond Rupture;a Density Functional, B3LYP, Study. Z. Für Nat. A 2005, 60, 861–862. [Google Scholar] [CrossRef]
- Paul Winget, T.C. Enthalpies of Formation from B3LYP Calculations. J. Comput. Chem. 2004, 25, 725–733. [Google Scholar] [CrossRef]
- Zhang, I.Y.; Wu, J.; Xu, X. Extending the reliability and applicability of B3LYP. Chem. Commun. 2010, 46, 3057. [Google Scholar] [CrossRef] [Green Version]
- Caldeira, M.T.; Custodio, R. Partial combination of composite strategy and the B3LYP functional for the calculation of enthalpies of formation. J. Mol. Model. 2019, 25, 62–69. [Google Scholar] [CrossRef]
- Smith, D.G.A.; Burns, L.A.; Patkowski, K.; Sherrill, C.D. Revised Damping Parameters for the D3 Dispersion Correction to Density Functional Theory. J. Phys. Chem. Lett. 2016, 7, 2197–2203. [Google Scholar] [CrossRef]
- Qi, X.; Wei, C.; Li, Q.; Zhang, L. Controlled-release inhibitor for preventing the spontaneous combustion of coal. Nat. Hazards 2016, 82, 891–901. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Li, J.; Zhou, Y.; Yang, Y.; Tang, Y. Studies on the Low-Temp Oxidation of Coal Containing Organic Sulfur and the Corresponding Model Compounds. Molecules 2015, 20, 22241–22256. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Li, Y.; Zhang, L.; He, W.; Han, Y.; Xu, D. Study on the effect of organic sulfur on coal spontaneous combustion based on model compounds. Fuel 2021, 289, 119846. [Google Scholar] [CrossRef]
- Shi, T.; Deng, J.; Wang, X. Study on the Reaction Mechanism of Coal Spontaneous Combustion in the Early Stage. J. Fuel Chem. Technol. 2004, 6, 652–657. [Google Scholar]
- Pandey, S.; Karakoti, M.; Dhali, S.; Karki, N.; SanthiBhushan, B.; Tewari, C.; Rana, S.; Srivastava, A.; Melkani, A.B.; Sahoo, N.G. Bulk synthesis of graphene nanosheets from plastic waste: An invincible method of solid waste management for better tomorrow. Waste Manag. 2019, 88, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Kolya, H.; Kuila, T.; Kim, N.H.; Lee, J.H. Colorimetric/naked eye detection of arsenic ions in aqueous medium by mango flower extract: A facile and novel approach. Appl. Surf. Sci. 2020, 513, 145760. [Google Scholar] [CrossRef]
- Katouah, H.A.; Al-Fahemi, J.H.; Elghalban, M.G.; Saad, F.A.; Althagafi, I.A.; El-Metwaly, N.M.; Khedr, A.M. Synthesis of new Cu(II)-benzohydrazide nanometer complexes, spectral, modeling, CT-DNA binding with potential anti-inflammatory and anti-allergic theoretical features. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 740–756. [Google Scholar] [CrossRef]
- Zhu, H.; Huo, Y.; Wang, W.; He, X.; Fang, S.; Zhang, Y. Quantum chemical calculation of reaction characteristics of hydroxyl at different positions during coal spontaneous combustion. Process Saf. Environ. Prot. 2021, 148, 624–635. [Google Scholar] [CrossRef]
- Belhachemi, M.H.M.; Benmohammed, A.; Saiah, H.; Boukabcha, N.; Saidj, M.; Dege, N.; Djafri, A.; Chouaih, A. Synthesis, structural determination, molecular docking and biological activity of 1-(4-fluorobenzyl)-5-bromolindolin-2,3-dione. J. Mol. Struct. 2022, 1265, 133342. [Google Scholar] [CrossRef]
- He, W.; Xu, S.; Liu, M.; Zhao, Y.; Zhang, L.; Huang, T.; Liu, Z. Interactions between Coal and Solvent during the Solvent Extraction of Coal in View of Free Radicals. ACS Omega 2021, 6, 31058–31065. [Google Scholar] [CrossRef]
- Blount, M.C.; Falconer, J.L. Characterization of Adsorbed Species on TiO2 after Photocatalytic Oxidation of Toluene. J. Catal. 2001, 200, 21–33. [Google Scholar] [CrossRef]
- Molina, M.J.; Zhang, R.; Broekhuizen, K.; Lei, W.; Navarro, R.; Molina, L.T. Experimental Study of Intermediates from OH-Initiated Reactions of Toluene. J. Am. Chem. Soc. 1999, 121, 10225–10226. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Yang, Y.; Kong, B.; Wang, C. Laboratory study on the inhibitory effect of free radical scavenger on coal spontaneous combustion. Fuel Process. Technol. 2018, 171, 350–360. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Z.; Ma, D.; Liu, Z. Low-Temperature Oxidation of Aldehyde and Alcohol by Model Compounds on Spontaneous Combustion of Coal. Asian J. Chem. 2013, 25, 8677–8680. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Z.; Ma, D.; Ji, H. Low-Temperature Oxidation Properties of Carboxyl in Coal Based on Model Compound of Spontaneous Combustion of Coal. Asian J. Chem. 2013, 25, 8660–8662. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Z.; Yang, Y.; Song, N.; Ma, D. Oxidation Experiment of Coal Spontaneous Combustion Model Compounds. Asian J. Chem. 2013, 25, 441–446. [Google Scholar] [CrossRef]
- Joseph, W.; Ochterski, P.D. Thermochemistry in Gaussian; Gaussian Inc.: Wallingford, CT, USA, 2000. [Google Scholar]
- Songhang, Z.; Shouren, Z.; Shuheng, T. Adsorption and transport of methane and carbon dioxide mixture in anthracite. J. China Coal Soc. 2021, 46, 544–555. [Google Scholar]
- Jianhua, X.; Le, L. Study on influence of coal surface functional groups on methane and carbon dioxide adsorption propertie. Coal Sci. Technol. 2021, 49, 145–151. [Google Scholar]
- Li, J.; Li, Z.; Yang, Y.; Niu, J.; Meng, Q. Insight into the chemical reaction process of coal self-heating after N2 drying. Fuel 2019, 255, 115780. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Song, J.; Zhang, T.; Ming, H.; Lu, S.; Deng, J.; Shu, C. Microstructure of coal spontaneous combustion in low-oxygen atmospheres at characteristic temperatures. Fuel 2022, 309, 122132. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Yang, Y.; Wang, C. Study on oxidation and gas release of active sites after low-temperature pyrolysis of coal. Fuel 2018, 233, 237–246. [Google Scholar] [CrossRef]







| Coal Sample | Proximate (w/%) | Ultimate (wdaf/%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vdaf | FCd | Odaf | Cdaf | Hdaf | Ndaf | |
| CQ | 2.57 | 53.52 | 22.76 | 35.90 | 5.87 | 79.38 | 2.91 | 1.32 |
| HYH | 6.59 | 15.58 | 37.14 | 53.07 | 15.66 | 74.49 | 4.36 | 1.31 |
| Stagnation Point | Corrected E (kJ/mol) | Enthalpy H (kJ/mol) | Gibbs Free Energy G (kJ/mol) | Enthalpy Change (kJ/mol) | Gibbs Free Energy Change G (kJ/mol) | Activation Energy Ea (kJ/mol) |
|---|---|---|---|---|---|---|
| R | −1,107,488.4 | −1,107,451.38 | −1,107,596.72 | 0 | 0 | |
| IM1 | −1,107,934.7 | −1,107,654.65 | −1,107,770.27 | −203.27 | −173.55 | 105.02 |
| TS1 | −1,107,829.7 | −1,107,452.17 | −1,107,596.87 | −0.79 | −0.15 | |
| IM2 | −1,107,514.7 | −1,107,481.18 | −1,107,603.45 | −29.8 | −6.73 | 131.27 |
| TS2 | −1,107,383.4 | −1,107,906.13 | −1,108,017.46 | −454.75 | −420.74 | |
| IM3 | −1,107,934.7 | −1,107,906.13 | −1,108,017.45 | −454.75 | −420.73 | 157.53 |
| TS3 | −1,107,777.2 | −1,107,762.36 | −1,107,871.98 | −310.98 | −275.26 | |
| IM4 | −1,108,197.3 | −1,107,732.20 | −1,107,844.49 | −280.82 | −247.77 | 105.02 |
| TS4 | −1,108,092.3 | −1,107,940.63 | −1,108,069.98 | −489.25 | −473.26 | |
| P + CO + H2O | −1,108,118.5 | −1,107,910.61 | −1,108,075.84 | −459.23 | −479.12 | |
| IM5 | −1,302,353 | −1,301,871.87 | −1,302,003.05 | 0 | 0 | 236.39 |
| TS5 | −1,302,116.7 | −1,301,871.87 | −1,302,003.07 | −0.021 | −0.015 | |
| P1 + CO2 | −1,302,589.3 | −1,302,461.24 | −1,302,594.85 | −589.34 | −591.79 |
| Stagnation Point | Corrected E (kJ/mol) | Enthalpy H (kJ/mol) | Gibbs Free Energy G (kJ/mol) | Enthalpy Change (kJ/mol) | Gibbs Free Energy Change G (kJ/mol) | Activation Energy Ea (kJ/mol) |
|---|---|---|---|---|---|---|
| r1 | −1,405,085.25 | −1,405,018.3 | −1,405,174.1 | 0 | 0 | |
| im1 | −1,405,521.21 | −1,405,323.1 | −1,405,449.9 | −304.8 | −275.8 | 13.51 |
| ts2 | −1,405,507.70 | −1,405,323.1 | −1,405,449.9 | −304.8 | −275.8 | |
| im2 | −1,405,291.92 | −1,405,204.7 | −1,405,350.7 | −186.4 | −176.6 | |
| p + CO + H2O | −1,405,564.02 | −1,405,518.6 | −1,405,680.2 | −500.3 | −506.1 | |
| im4 | −1,206,760.51 | −1,206,291.2 | −1,206,411.4 | 0 | 0 | 26.46 |
| ts4 | −1,206,734.05 | −1,206,410.9 | −1,206,554.9 | −119.7 | −143.5 | |
| im5 | −1,206,890.46 | −1,206,410.8 | −1,206,554.9 | −119.6 | −143.5 | |
| p1 + CO2 | −1,405,640.19 | −1,405,611.6 | −1,405,757.9 | −593.3 | −583.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Han, Y.; Xu, D.; Jiang, Q.; Xin, H.; Fu, C.; He, W. Study on the Reaction Path of -CH3 and -CHO Functional Groups during Coal Spontaneous Combustion: Quantum Chemistry and Experimental Research. Energies 2022, 15, 4891. https://doi.org/10.3390/en15134891
Zhang L, Han Y, Xu D, Jiang Q, Xin H, Fu C, He W. Study on the Reaction Path of -CH3 and -CHO Functional Groups during Coal Spontaneous Combustion: Quantum Chemistry and Experimental Research. Energies. 2022; 15(13):4891. https://doi.org/10.3390/en15134891
Chicago/Turabian StyleZhang, Lanjun, Yujia Han, Dexin Xu, Qin Jiang, Haihui Xin, Chenhui Fu, and Wenjing He. 2022. "Study on the Reaction Path of -CH3 and -CHO Functional Groups during Coal Spontaneous Combustion: Quantum Chemistry and Experimental Research" Energies 15, no. 13: 4891. https://doi.org/10.3390/en15134891
APA StyleZhang, L., Han, Y., Xu, D., Jiang, Q., Xin, H., Fu, C., & He, W. (2022). Study on the Reaction Path of -CH3 and -CHO Functional Groups during Coal Spontaneous Combustion: Quantum Chemistry and Experimental Research. Energies, 15(13), 4891. https://doi.org/10.3390/en15134891






