Assessment of Hydrogen and Volatile Fatty Acid Production from Fruit and Vegetable Waste: A Case Study of Mediterranean Markets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate Preparation
2.2. Source and Pretreatment of the Inoculum
2.3. Set-Up of the Batch Biochemical Hydrogen Potential Tests
2.4. Analytical Methods
2.5. Kinetic Modeling and Statistical Analysis
3. Results and Discussion
3.1. Physical–Chemical Characteristics of the Fruit and Vegetable Waste
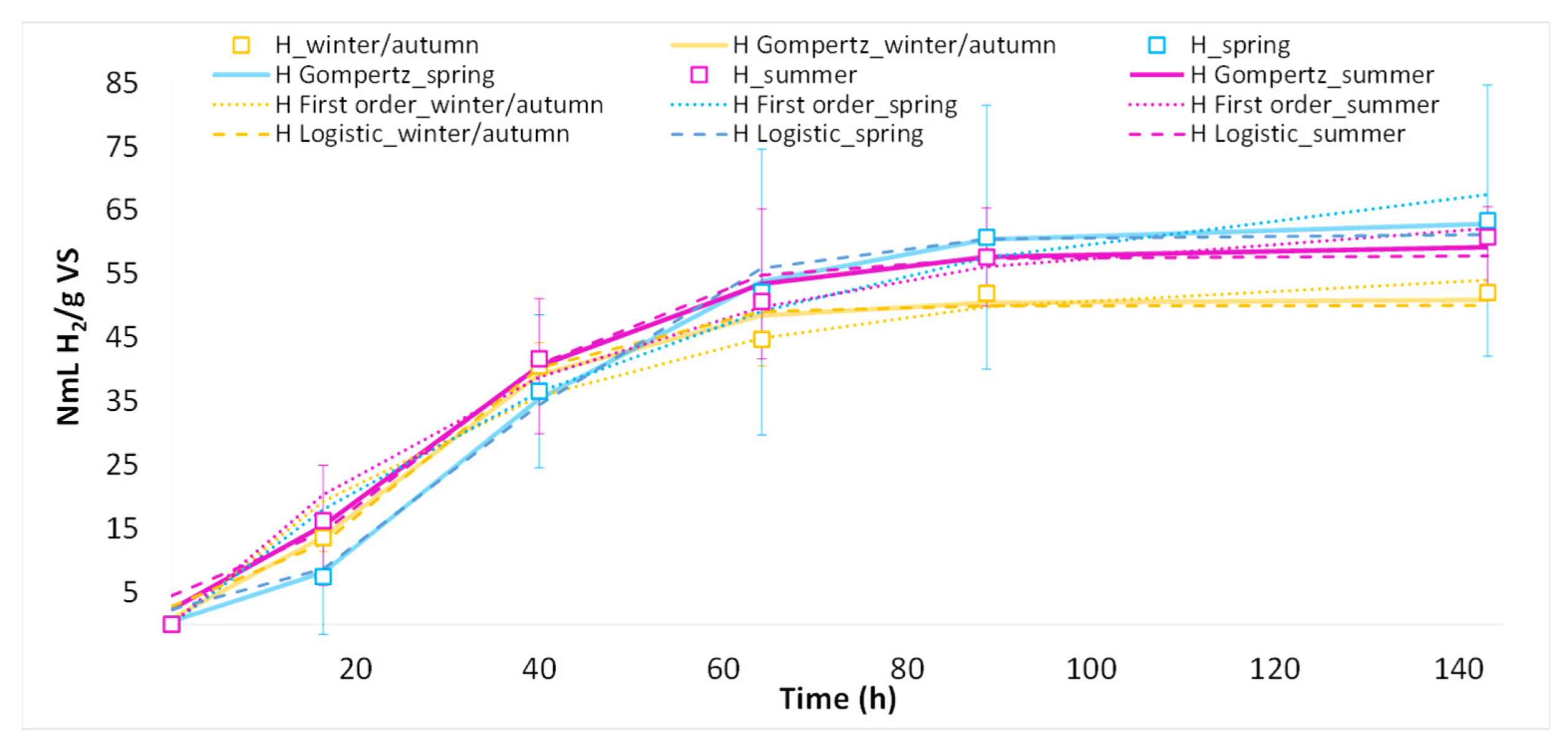
3.2. Effect of Seasonal Variation on H2 Yield and VFA
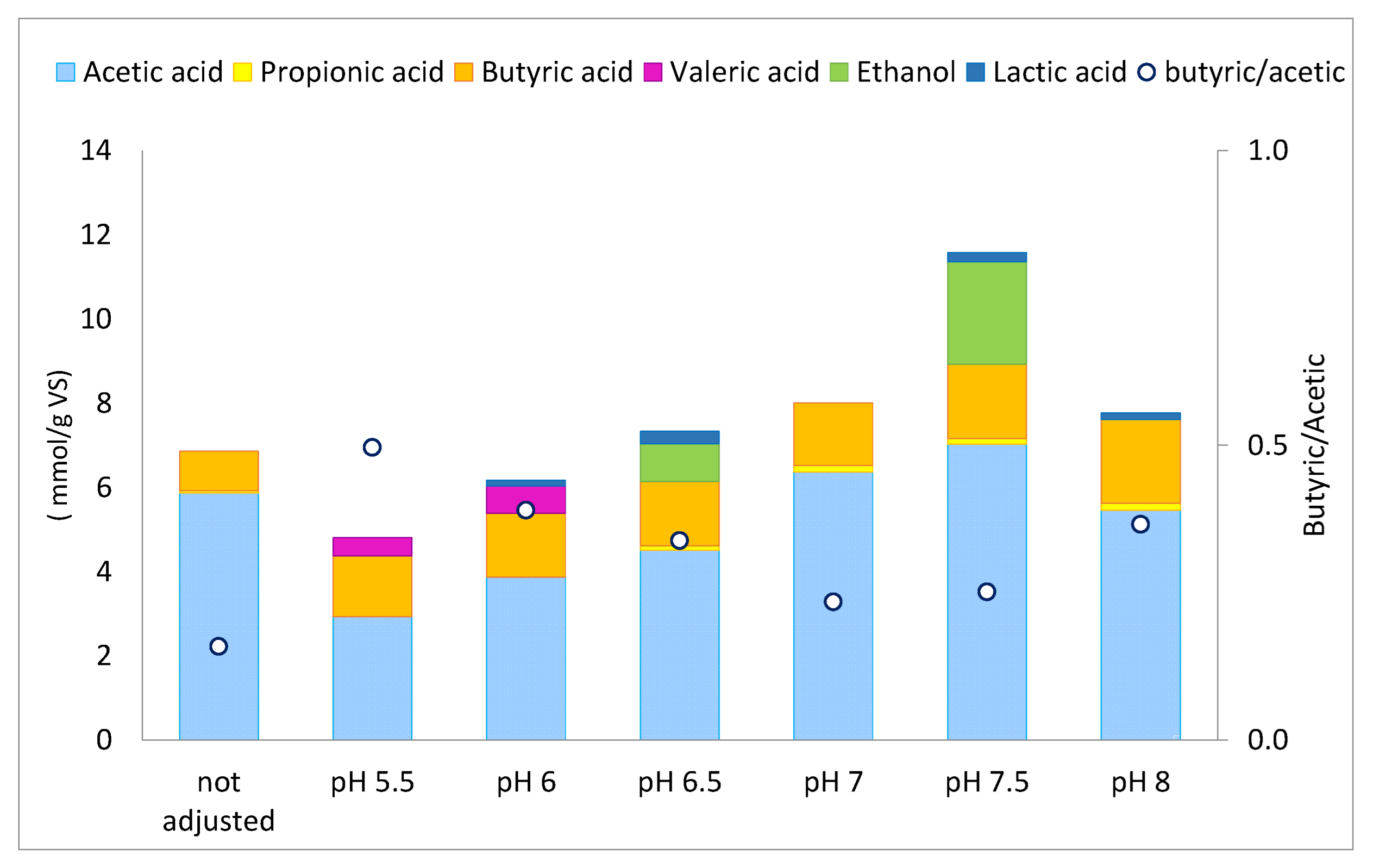
3.3. Effect of Initial pH on H2 Yield and VFA Production and Composition
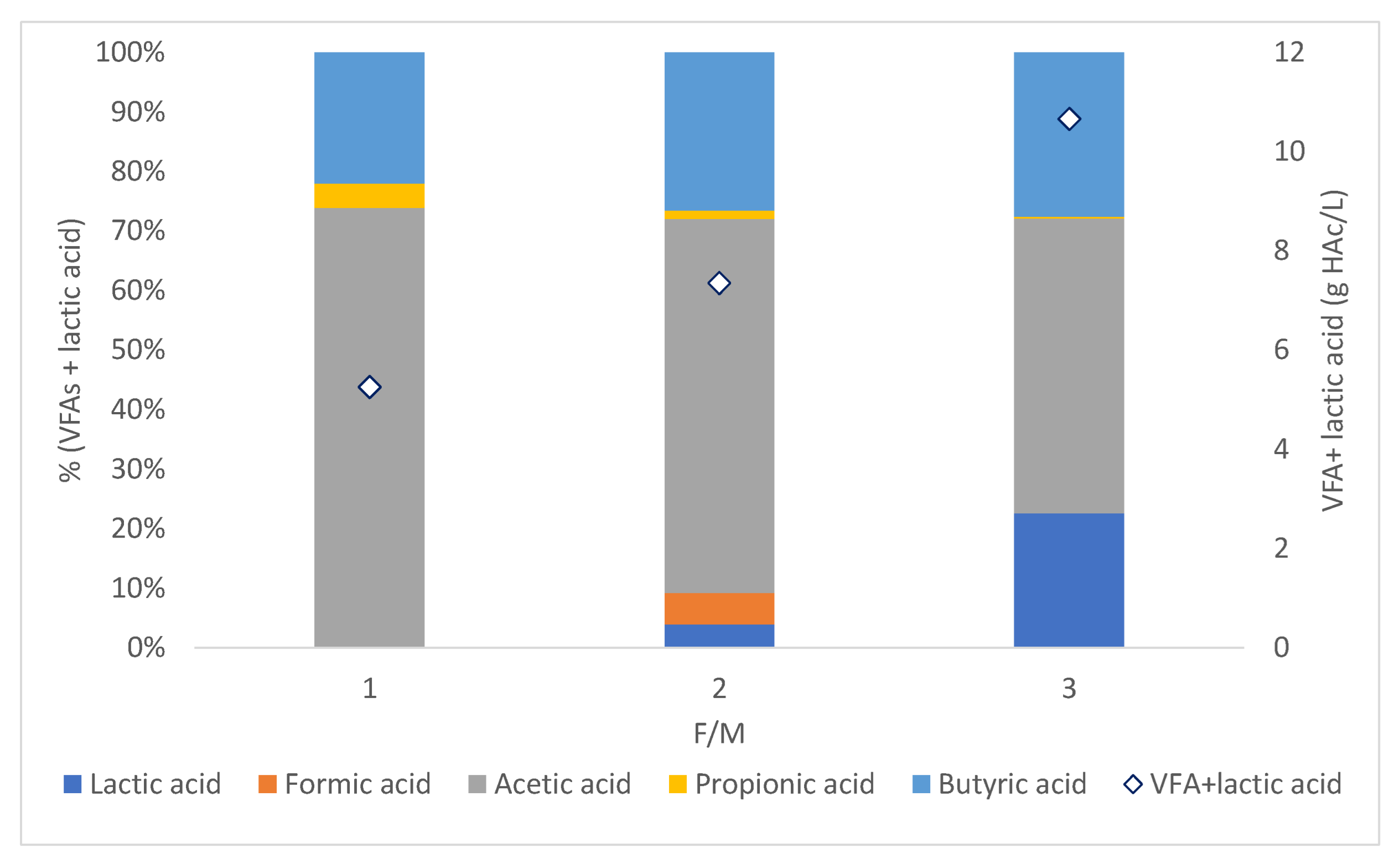
3.4. Effect of the S/I Ratio on H2 Yield and Volatile Fatty Acids Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Acronyms
| FVW | fruit and vegetable waste |
| VFAs | volatile fatty acids |
| S/I | substrate/inoculum |
| TS | total solids |
| VS | volatile solids |
| tCOD | total chemical oxygen demand |
| sCOD | soluble chemical oxygen demand |
| TAN | total ammonia nitrogen |
| TA | total alkalinity |
| B/A | butyric/acetic ratio |
| R2 | determination coefficient |
| RMSE | root mean squared error |
| RSS | residual sum of squares |
| wb | wet basis |
References
- Papirio, S.; Trujillo-Reyes, Á.; Scotto Di Perta, Ε.; Kalogiannis, A.; Kassab, G.; Khoufi, S.; Sayadi, S.; Frunzo, L.; Esposito, G.; Fermoso, F.G.; et al. Exploring the Biochemical Methane Potential of Wholesale Market Waste from Jordan and Tunisia for a Future Scale-Up of Anaerobic Digestion in Amman and Sfax. Waste Biomass Valorization 2022, 1, 1–11. [Google Scholar] [CrossRef]
- Edwiges, T.; Frare, L.; Mayer, B.; Lins, L.; Mi Triolo, J.; Flotats, X.; de Mendonça Costa, M.S.S. Influence of Chemical Composition on Biochemical Methane Potential of Fruit and Vegetable Waste. Waste Manag. 2018, 71, 618–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bräutigam, K.R.; Jörissen, J.; Priefer, C. The Extent of Food Waste Generation across EU-27: Different Calculation Methods and the Reliability of Their Results. Waste Manag. Res. 2014, 32, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Morales-Polo, C.; del MarCledera-Castro, M.; Hueso-Kortekaas, K.; Revuelta-Aramburu, M. Anaerobic Digestion in Wastewater Reactors of Separated Organic Fractions from Wholesale Markets Waste. Compositional and Batch Characterization. Energy and Environmental Feasibility. Sci. Total Environ. 2020, 726, 138567. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, B.; Mazumder, D. New Approach of Characterizing Fruit and Vegetable Waste (FVW) to Ascertain Its Biological Stabilization via Two-Stage Anaerobic Digestion (AD). Biomass Bioenergy 2020, 139, 105594. [Google Scholar] [CrossRef]
- Dwivedi, A.H.; Gedam, V.V.; Kumar, M.S. Sustainable Hydrogen Production from Fruit and Vegetable Waste (FVW) Using Mixed Anaerobic Cultures via Dark Fermentation: Kinetic Aspects Graphic Abstract Keywords Hydrogen (h 2) Fruit and Vegetable Waste (FVW) Waste Management Dark Fermentation. Int. J. Energy Environ. Eng. 2020, 11, 341–349. [Google Scholar] [CrossRef]
- Ravi, P.P.; Lindner, J.; Oechsner, H.; Lemmer, A. Effects of Target PH-Value on Organic Acids and Methane Production in Two-Stage Anaerobic Digestion of Vegetable Waste. Bioresour. Technol. 2018, 247, 96–102. [Google Scholar] [CrossRef]
- Morales-Polo, C.; del MarCledera-Castro, M.; Soria, B.Y.M. Biogas Production from Vegetable and Fruit Markets Waste—Compositional and Batch Characterizations. Sustainability 2019, 11, 6790. [Google Scholar] [CrossRef] [Green Version]
- Scotto di Perta, E.; Cervelli, E.; Pironti di Campagna, M.; Pindozzi, S. From Biogas to Biomethane: Techno-Economic Analysis of an Anaerobic Digestion Power Plant in a Cattle/Buffalo Farm in Central Italy. J. Agric. Eng. 2019, 50, 127–133. [Google Scholar] [CrossRef]
- Moscariello, C.; Matassa, S.; Pirozzi, F.; Esposito, G.; Papirio, S. Valorisation of Industrial Hemp (Cannabis sativa L.) Biomass Residues through Acidogenic Fermentation and Co-Fermentation for Volatile Fatty Acids Production. Bioresour. Technol. 2022, 355, 127289. [Google Scholar] [CrossRef] [PubMed]
- Tsigkou, K.; Tsafrakidou, P.; Athanasopoulou, S.; Zafiri, C.; Kornaros, M. Effect of PH on the Anaerobic Fermentation of Fruit/Vegetables and Disposable Nappies Hydrolysate for Bio-Hydrogen Production. Waste Biomass Valorization 2020, 11, 539–551. [Google Scholar] [CrossRef]
- Sabater, C.; Ruiz, L.; Delgado, S.; Ruas-Madiedo, P.; Margolles, A. Valorization of Vegetable Food Waste and By-Products Through Fermentation Processes. Front. Microbiol. 2020, 11, 2604. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, A.; Sposito, F.; Frunzo, L.; Trably, E.; Escudié, R.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Effects of Operational Parameters on Dark Fermentative Hydrogen Production from Biodegradable Complex Waste Biomass. Waste Manag. 2016, 50, 55–64. [Google Scholar] [CrossRef]
- Guo, X.M.; Trably, E.; Latrille, E.; Carrre, H.; Steyer, J.P. Hydrogen Production from Agricultural Waste by Dark Fermentation: A Review. Int. J. Hydrog. Energy 2010, 35, 10660–10673. [Google Scholar] [CrossRef]
- Zheng, M.; Zheng, M.; Wu, Y.; Ma, H.; Wang, K. Effect of PH on Types of Acidogenic Fermentation of Fruit and Vegetable Wastes. Biotechnol. Bioprocess Eng. 2015, 20, 298–303. [Google Scholar] [CrossRef]
- Morales-Polo, C.; del MarCledera-Castro, M.; Revuelta-Aramburu, M.; Hueso-Kortekaas, K. Enhancing Energy Recovery in Form of Biogas, from Vegetable and Fruit Wholesale Markets By-Products and Wastes, with Pretreatments. Plants 2021, 10, 1298. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.L.; Esposito, G. A Review on Dark Fermentative Biohydrogen Production from Organic Biomass: Process Parameters and Use of by-Products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A Review of the Production and Applications of Waste-Derived Volatile Fatty Acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Holliger, C.; Astals, S.; de Laclos, H.F.; Hafner, S.D.; Koch, K.; Weinrich, S. Towards a Standardization of Biomethane Potential Tests: A Commentary. Water Sci. Technol. 2021, 83, 247–250. [Google Scholar] [CrossRef]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; Van Lier, J.B.; Angelidaki, A.I. Defining the Biomethane Potential (BMP) of Solid Organic Wastes and Energy Crops: A Proposed Protocol for Batch Assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [Green Version]
- Ghimire, A.; Valentino, S.; Frunzo, L.; Trably, E.; Escudié, R.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Biohydrogen Production from Food Waste by Coupling Semi-Continuous Dark-Photofermentation and Residue Post-Treatment to Anaerobic Digestion: A Synergy for Energy Recovery. Int. J. Hydrog. Energy 2015, 40, 16045–16055. [Google Scholar] [CrossRef]
- Esposito, G. Bio-Methane Potential Tests To Measure The Biogas Production From The Digestion and Co-Digestion of Complex Organic Substrates. Open Environ. Eng. J. 2012, 5, 1–8. [Google Scholar] [CrossRef]
- Apha, A. Standard Methods for the Examination of Water and Wastewater; American Public Health Association/American Water Works Association/Water Environment Federation, Ed.; American Public Health Association: Washington, DC, USA, 2005; Volume 21. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Noguerol-Arias, J.; Rodríguez-Abalde, A.; Romero-Merino, E.; Flotats, X. Determination of Chemical Oxygen Demand in Heterogeneous Solid or Semisolid Samples Using a Novel Method Combining Solid Dilutions as a Preparation Step Followed by Optimized Closed Reflux and Colorimetric Measurement. Anal. Chem. 2012, 84, 5548–5555. [Google Scholar] [CrossRef]
- Wainaina, S.; Awasthi, M.K.; Horváth, I.S.; Taherzadeh, M.J. Anaerobic Digestion of Food Waste to Volatile Fatty Acids and Hydrogen at High Organic Loading Rates in Immersed Membrane Bioreactors. Renew. Energy 2020, 152, 1140–1148. [Google Scholar] [CrossRef]
- Cappai, G.; De Gioannis, G.; Friargiu, M.; Massi, E.; Muntoni, A.; Polettini, A.; Pomi, R.; Spiga, D. An Experimental Study on Fermentative H2 Production from Food Waste as Affected by PH. Waste Manag. 2014, 34, 1510–1519. [Google Scholar] [CrossRef]
- Boshagh, F.; Rostami, K.; van Niel, E.W.J. Application of Kinetic Models in Dark Fermentative Hydrogen Production–A Critical Review. Int. J. Hydrog. Energy 2022, 47, 21952–21968. [Google Scholar] [CrossRef]
- Wang, J.; Wan, W. Kinetic Models for Fermentative Hydrogen Production: A Review. Int. J. Hydrog. Energy 2009, 34, 3313–3323. [Google Scholar] [CrossRef]
- Tena, M.; Luque, B.; Perez, M.; Solera, R. Enhanced Hydrogen Production from Sewage Sludge by Cofermentation with Wine Vinasse. Int. J. Hydrog. Energy 2020, 45, 15977–15984. [Google Scholar] [CrossRef]
- Bouallagui, H.; Lahdheb, H.; Ben Romdan, E.; Rachdi, B.; Hamdi, M. Improvement of Fruit and Vegetable Waste Anaerobic Digestion Performance and Stability with Co-Substrates Addition. J. Environ. Manag. 2009, 90, 1844–1849. [Google Scholar] [CrossRef]
- Yu, J.; Huang, Z.; Wu, P.; Zhao, M.; Miao, H.; Liu, C.; Ruan, W. Performance and Microbial Characterization of Two-Stage Caproate Fermentation from Fruit and Vegetable Waste via Anaerobic Microbial Consortia. Bioresour. Technol. 2019, 284, 398–405. [Google Scholar] [CrossRef]
- Sadeghifar, T.; Lama, G.F.C.; Sihag, P.; Bayram, A.; Kisi, O. Wave Height Predictions in Complex Sea Flows through Soft-Computing Models: Case Study of Persian Gulf. Ocean Eng. 2022, 245, 110467. [Google Scholar] [CrossRef]
- Guo, X.M.; Trably, E.; Latrille, E.; Carrere, H.; Steyer, J.P. Predictive and Explicative Models of Fermentative Hydrogen Production from Solid Organic Waste: Role of Butyrate and Lactate Pathways. Int. J. Hydrog. Energy 2014, 39, 7476–7485. [Google Scholar] [CrossRef]
- Ariunbaatar, J.; Scotto Di Perta, E.; Panico, A.; Frunzo, L.; Esposito, G.; Lens, P.N.L.; Pirozzi, F. Effect of Ammoniacal Nitrogen on One-Stage and Two-Stage Anaerobic Digestion of Food Waste. Waste Manag. 2015, 38, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Vazquez, I.; Poggi-Varaldo, H.M. Hydrogen Production by Fermentative Consortia. Renew. Sustain. Energy Rev. 2009, 13, 1000–1013. [Google Scholar] [CrossRef]
- Dreschke, G.; Papirio, S.; Sisinni, D.M.G.; Lens, P.N.L.; Esposito, G. Effect of Feed Glucose and Acetic Acid on Continuous Biohydrogen Production by Thermotoga Neapolitana. Bioresour. Technol. 2019, 273, 416–424. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, R.; El-Mashad, H.M.; Sun, H.; Ying, Y. Effect of Food to Microorganism Ratio on Biohydrogen Production from Food Waste via Anaerobic Fermentation. Int. J. Hydrog. Energy 2008, 33, 6968–6975. [Google Scholar] [CrossRef]
- Pastor-Poquet, V.; Papirio, S.; Trably, E.; Rintala, J.; Escudié, R.; Esposito, G. High-Solids Anaerobic Digestion Requires a Trade-off between Total Solids, Inoculum-to-Substrate Ratio and Ammonia Inhibition. Int. J. Environ. Sci. Technol. 2019, 16, 7011–7024. [Google Scholar] [CrossRef]



| Parameter | Inoculum |
|---|---|
| TS [g/L] | 25.29 |
| VS [g/L] | 16.58 |
| tCOD [mg O2/L] | 23,800 |
| sCOD [mg O2/L] | 4607 |
| N-NH₄⁺ [mg/L] | 331 |
| Alkalinity [mg CaCO3/L] | 1039 |
| pH | 8.5 |
| Test | Aim | Substrate | Initial pH | S/I (g VS/g VS) | Initial Substrate Concentration (g VS/L) | Mixture Concentration (g VS/L) |
|---|---|---|---|---|---|---|
| Set 1 | Effect of seasonal variation | winter–autumn, spring, summer | Not adjusted (9.2) | 1 | 5 | 10 |
| Set 2 | Effect of initial pH | winter–autumn | Not adjusted (7.8), 5.5, 6.0, 6.5, 7.0, 7.5 and 8.0 | 1 | 10 | 20 |
| Set 3 | Effect of S/I ratio | winter–autumn | Not adjusted (7.8, 7.1, 6.6) | 1, 2, 3 | 10, 20, 30 | 20, 30, 40 |
| Parameter | Winter–Autumn | Spring | Summer |
|---|---|---|---|
| Moisture (%) | 90.28 ± 0.14 | 90.07 ± 0.33 | 90.52 ± 0.49 |
| TS [g/kgwb] | 97.19 ± 1.37 | 99.29 ± 3.28 | 94.77 ± 4.90 |
| VS [g/kgwb] | 88.71 ± 1.73 | 91.99 ± 3.23 | 88.10 ± 4.83 |
| tCOD [g/kg VS] | 1294.10 ± 214.69 | 951.76 ± 176.80 | 983.96 ± 4.01 |
| sCOD [g/kg VS] | 604.97 ± 37.20 | 679.42 ± 30.75 | 865.49 ± 12.04 |
| sCOD/tCOD (%) | 46.75% | 71.39% | 87.96% |
| Total carbohydrates [g/kg VS] | 916.64 ± 87.68 | 719.97 ± 89.41 | 804.71 ± 38.86 |
| Soluble carbohydrates [g/kg VS] | 529.19 ± 3.69 | 559.41 ± 25.69 | 727.79 ± 58.51 |
| Model | Winter/Autumn | Spring | Summer | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | RSS | RMSE | R2 | RSS | RMSE | R2 | RSS | RMSE | |
| Gompertz | 0.99 | 20.01 | 1.83 | 1.00 | 5.34 | 0.94 | 0.99 | 17.29 | 1.70 |
| First order kinetic | 0.97 | 63.19 | 3.25 | 0.96 | 148.48 | 4.97 | 0.99 | 30.01 | 2.24 |
| Modified Logistic model | 0.98 | 36.07 | 2.45 | 0.99 | 30.42 | 2.25 | 0.98 | 49.89 | 2.88 |
| Model | pH5.5 | pH6 | pH6.5 | pH7 | pH7.5 | pH8 | Not Adjusted | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | RSS | RMSE | R2 | RSS | RMSE | R2 | RSS | RMSE | R2 | RSS | RMSE | R2 | RSS | RMSE | R2 | RSS | RMSE | R2 | RSS | RMSE | |
| Gompertz | 0.98 | 483.56 | 8.98 | 0.99 | 135.01 | 4.74 | 1.00 | 72.68 | 3.48 | 0.94 | 303.85 | 7.12 | 0.99 | 50.76 | 2.91 | 0.98 | 128.29 | 4.62 | 1.00 | 32.78 | 2.34 |
| First-order kinetic | 0.96 | 1277.35 | 14.59 | 1.00 | 47.24 | 2.81 | 1.00 | 9.97 | 1.29 | 0.97 | 150.11 | 5.00 | 0.99 | 46.87 | 2.79 | 1.00 | 23.00 | 1.96 | 0.99 | 94.81 | 3.98 |
| Modified Logistic model | 0.98 | 518.45 | 9.30 | 0.98 | 399.68 | 8.16 | 0.99 | 232.52 | 6.23 | 0.91 | 424.13 | 8.41 | 0.99 | 76.39 | 3.57 | 0.96 | 242.19 | 6.35 | 0.99 | 48.09 | 2.83 |
| Model | S/I = 1 | S/I = 2 | S/I = 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | RSS | RMSE | R2 | RSS | RMSE | R2 | RSS | RMSE | |
| Gompertz | 0.98 | 304.17 | 7.12 | 1.00 | 45.23 | 2.75 | 1.00 | 0.96 | 0.40 |
| First-order kinetic | 1.00 | 56.87 | 3.08 | 0.99 | 186.77 | 5.58 | 0.95 | 557.51 | 9.64 |
| Modified Logistic model | 0.98 | 450.78 | 8.67 | 0.99 | 196.21 | 5.72 | 1.00 | 21.21 | 1.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scotto di Perta, E.; Cesaro, A.; Pindozzi, S.; Frunzo, L.; Esposito, G.; Papirio, S. Assessment of Hydrogen and Volatile Fatty Acid Production from Fruit and Vegetable Waste: A Case Study of Mediterranean Markets. Energies 2022, 15, 5032. https://doi.org/10.3390/en15145032
Scotto di Perta E, Cesaro A, Pindozzi S, Frunzo L, Esposito G, Papirio S. Assessment of Hydrogen and Volatile Fatty Acid Production from Fruit and Vegetable Waste: A Case Study of Mediterranean Markets. Energies. 2022; 15(14):5032. https://doi.org/10.3390/en15145032
Chicago/Turabian StyleScotto di Perta, Ester, Alessandra Cesaro, Stefania Pindozzi, Luigi Frunzo, Giovanni Esposito, and Stefano Papirio. 2022. "Assessment of Hydrogen and Volatile Fatty Acid Production from Fruit and Vegetable Waste: A Case Study of Mediterranean Markets" Energies 15, no. 14: 5032. https://doi.org/10.3390/en15145032
APA StyleScotto di Perta, E., Cesaro, A., Pindozzi, S., Frunzo, L., Esposito, G., & Papirio, S. (2022). Assessment of Hydrogen and Volatile Fatty Acid Production from Fruit and Vegetable Waste: A Case Study of Mediterranean Markets. Energies, 15(14), 5032. https://doi.org/10.3390/en15145032











