Cross-Comparison of the Impact of Grass Silage Pulsed Electric Field and Microwave-Induced Disintegration on Biogas Production Efficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate
2.2. Equipment
2.3. Pretreatment
2.4. Biochemical Methane Potential
2.5. Analytical Methods
2.6. Statistical Analyses
3. Results and Discussion
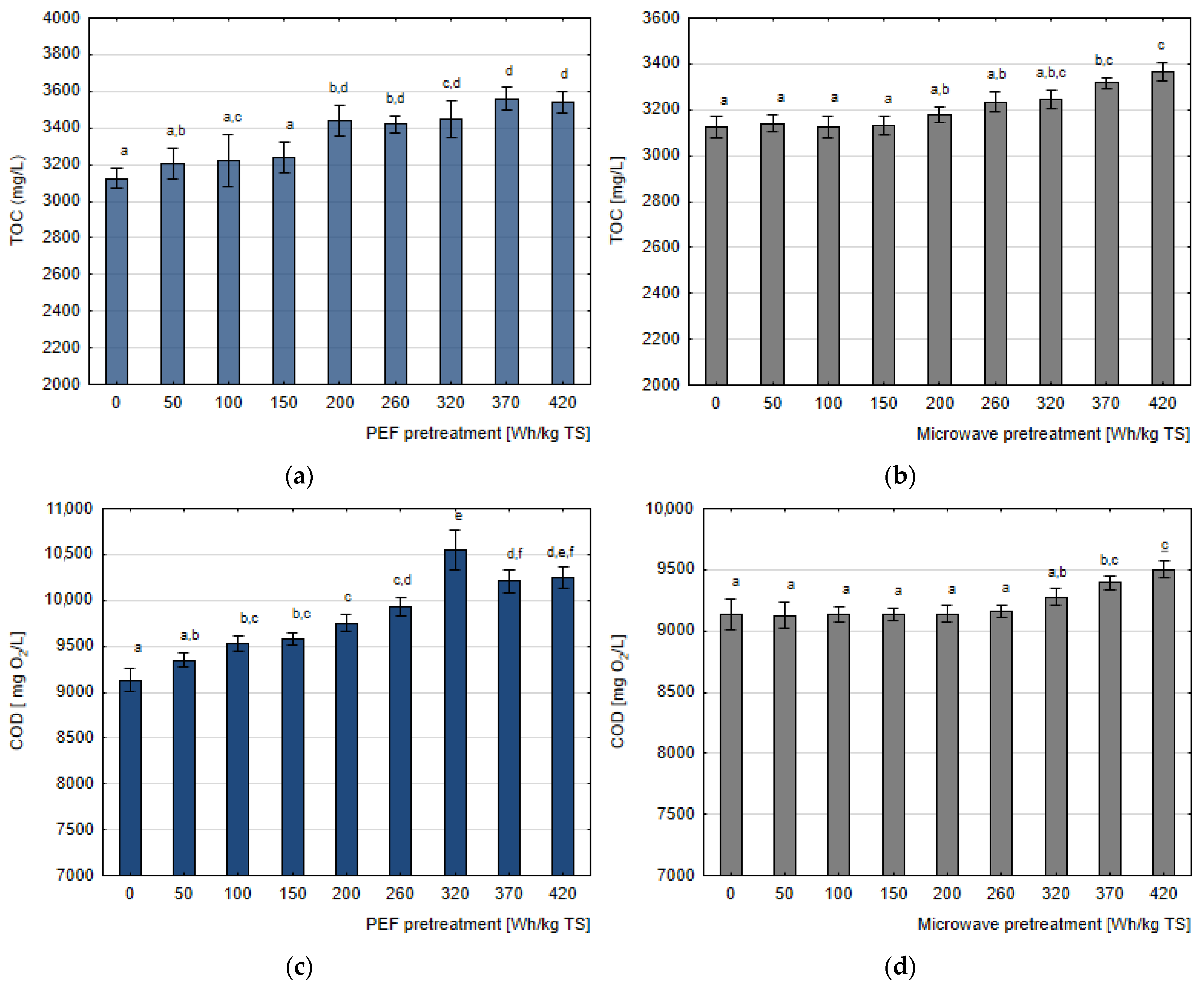
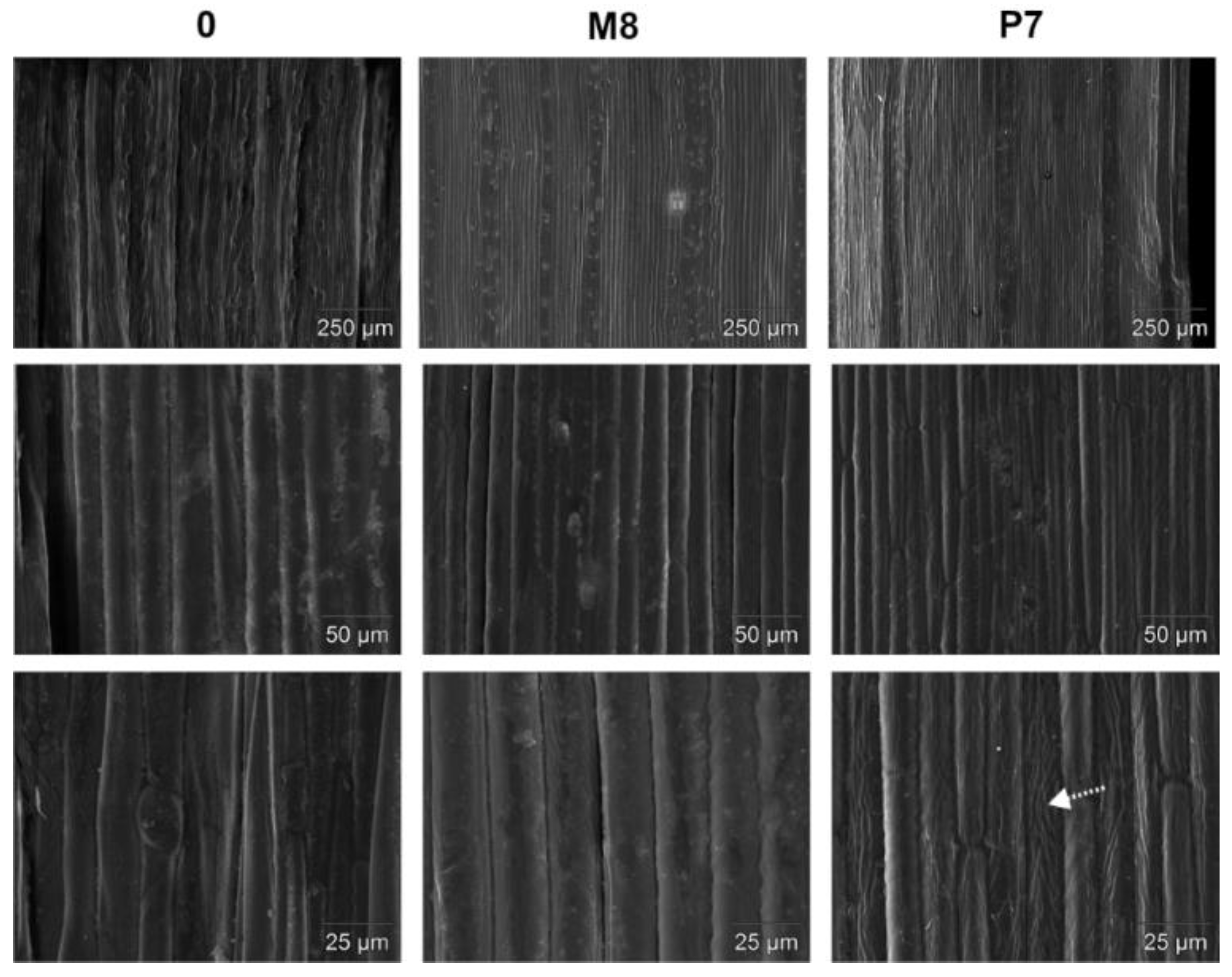
3.1. Pretreatment Efficiency
3.2. Biogas and Methane Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dziennik Ustaw. Available online: Https://Www.Dziennikustaw.Gov.Pl/M2021000026401.Pdf (accessed on 17 November 2021).
- Nowicka, A.; Zieliński, M.; Dębowski, M.; Dudek, M. Progress in the Production of Biogas from Maize Silage after Acid-Heat Pretreatment. Energies 2021, 14, 8018. [Google Scholar] [CrossRef]
- Boro, M.; Verma, A.K.; Chettri, D.; Yata, V.K.; Verma, A.K. Strategies Involved in Biofuel Production from Agro-Based Lignocellulose Biomass. Environ. Technol. Innov. 2022, 28, 102679. [Google Scholar] [CrossRef]
- Naik, G.P.; Poonia, A.K.; Chaudhari, P.K. Pretreatment of Lignocellulosic Agricultural Waste for Delignification, Rapid Hydrolysis, and Enhanced Biogas Production: A Review. J. Indian Chem. Soc. 2021, 98, 100147. [Google Scholar] [CrossRef]
- Aditiya, H.B.; Mahlia, T.M.I.; Chong, W.T.; Nur, H.; Sebayang, A.H. Second Generation Bioethanol Production: A Critical Review. Renew. Sustain. Energy Rev. 2016, 66, 631–653. [Google Scholar] [CrossRef]
- Haghighi Mood, S.; Hossein Golfeshan, A.; Tabatabaei, M.; Salehi Jouzani, G.; Najafi, G.H.; Gholami, M.; Ardjmand, M. Lignocellulosic Biomass to Bioethanol, a Comprehensive Review with a Focus on Pretreatment. Renew. Sustain. Energy Rev. 2013, 27, 77–93. [Google Scholar] [CrossRef]
- Gomes, M.G.; Gomes De Oliveira Paranhos, A.; Camargos, A.B.; Lobo, B.E.; Eta, B.; Alves Baffi, M.; Vinícius, L.; Gurgel, A.; Pasquini, D. Pretreatment of Sugarcane Bagasse with Dilute Citric Acid and Enzymatic Hydrolysis: Use of Black Liquor and Solid Fraction for Biogas Production. Renew. Energy 2022, 191, 428–438. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Cho, J.; Talaiekhozani, A.; Sabbagh, F.; Hashemi, B.; Rupani, P.F.; Mohammadi, A.A. Different Pretreatment Technologies of Lignocellulosic Biomass for Bioethanol Production: An Overview. Energy 2020, 199, 117457. [Google Scholar] [CrossRef]
- Kumari, D.; Jain, Y.; Singh, R. A Study on Green Pretreatment of Rice Straw Using Petha Wastewater and Mausami Waste Assisted with Microwave for Production of Ethanol and Methane. Energy Convers. Manag. X 2021, 10, 100067. [Google Scholar]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of Lignocellulosic Biomass for Enhanced Biogas Production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Kumari, D.; Singh, R. Pretreatment of Lignocellulosic Wastes for Biofuel Production: A Critical Review. Renew. Sustain. Energy Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Al Afif, R.; Pfeifer, C. Enhancement of Methane Yield from Cotton Stalks by Mechanical Pre-Treatment. Carbon Resour. Convers. 2021, 4, 164–168. [Google Scholar] [CrossRef]
- Sumardiono, S.; Matin, H.H.A.; Hartono, I.I.; Choiruly, L. Biogas Production from Corn Stalk as Agricultural Waste Containing High Cellulose Material by Anaerobic Process. Mater. Today Proc. 2022, 63, S477–S483. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar]
- Luengo, E.; Martínez, J.M.; Coustets, M.; Álvarez, I.; Teissié, J.; Rols, M.P.; Raso, J. A Comparative Study on the Effects of Millisecond- and Microsecond-Pulsed Electric Field Treatments on the Permeabilization and Extraction of Pigments from Chlorella Vulgaris. J. Membr. Biol. 2015, 248, 883–891. [Google Scholar] [CrossRef]
- Szwarc, D.; Głowacka, K. Increasing the Biogas Potential of Rapeseed Straw Using Pulsed Electric Field Pre-Treatment. Energies 2021, 14, 8307. [Google Scholar] [CrossRef]
- Naliyadhara, N.; Kumar, A.; Girisa, S.; Daimary, U.D.; Hegde, M.; Kunnumakkara, A.B. Pulsed Electric Field (PEF): Avant-Garde Extraction Escalation Technology in Food Industry. Trends Food Sci. Technol. 2022, 122, 238–255. [Google Scholar] [CrossRef]
- Yan, D.; Ji, Q.; Yu, X.; Li, M.; Abiola Fakayode, O.; Yagoub, A.E.G.A.; Chen, L.; Zhou, C. Multimode-Ultrasound and Microwave Assisted Natural Ternary Deep Eutectic Solvent Sequential Pretreatments for Corn Straw Biomass Deconstruction under Mild Conditions. Ultrason. Sonochem. 2021, 72, 105414. [Google Scholar]
- Zhu, Z.; Rezende, C.A.; Simister, R.; McQueen-Mason, S.J.; Macquarrie, D.J.; Polikarpov, I.; Gomez, L.D. Efficient Sugar Production from Sugarcane Bagasse by Microwave Assisted Acid and Alkali Pretreatment. Biomass Bioenergy 2016, 93, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; He, W.Q.; Aguedo, M.; Xia, X.; Bai, W.B.; Dong, Y.Y.; Song, J.Q.; Richel, A.; Goffin, D. Microwave-Assisted Alkali Hydrolysis for Cellulose Isolation from Wheat Straw: Influence of Reaction Conditions and Non-Thermal Effects of Microwave. Carbohydr. Polym. 2021, 253, 117170. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nižetić, S.; Ong, H.C.; Mofijur, M.; Ahmed, S.F.; Ashok, B.; Bui, V.T.V.; Chau, M.Q. Insight into the Recent Advances of Microwave Pretreatment Technologies for the Conversion of Lignocellulosic Biomass into Sustainable Biofuel. Chemosphere 2021, 281, 130878. [Google Scholar] [CrossRef]
- Rana, M.S.; Prajapati, S.K. Microwave-Assisted Pretreatment of Wet Microalgal Biomass for Recovery of Biofuel Precursors. Fuel 2021, 305, 121610. [Google Scholar]
- Zhou, J.; Wang, M.; Berrada, H.; Zhu, Z.; Grimi, N.; Barba, F.J. Pulsed Electric Fields (PEF), Pressurized Liquid Extraction (PLE) and Combined PEF + PLE Process Evaluation: Effects on Spirulina Microstructure, Biomolecules Recovery and Triple TOF-LC-MS-MS Polyphenol Composition. Innov. Food Sci. Emerg. Technol. 2022, 77, 102989. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Kuşçu, Ö.S.; Çömlekçi, S.; Çört, N. Disintegration of Sewage Sludge Using Pulsed Electrical Field Technique: PEF Optimization, Simulation, and Anaerobic Digestion. Environ. Technol. 2022, 43, 2809–2824. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.D.; Gao, Y.; Men, Y.K.; Du, B.X.; Wang, Y.N.; Liu, C.H. Effect of DC Corona on Performance of Pulsed Electric Field Pretreatment on Waste Activated Sludge. In Proceedings of the Annual Report Conference on Electrical Insulation and Dielectric Phenomena, CEIDP, Toronto, ON, Canada, 16–19 October 2016; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2016; Volume 2016, pp. 747–750. [Google Scholar]
- El Achkar, J.H.; Lendormi, T.; Salameh, D.; Louka, N.; Maroun, R.G.; Lanoisellé, J.L.; Hobaika, Z. Influence of Pretreatment Conditions on Lignocellulosic Fractions and Methane Production from Grape Pomace. Bioresour. Technol. 2018, 247, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, M.; Rusanowska, P.; Krzywik, A.; Dudek, M.; Nowicka, A.; Dębowski, M. Application of Hydrodynamic Cavitation for Improving Methane Fermentation of Sida Hermaphrodita Silage. Energies 2019, 12, 526. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Esveld, E.; Vincken, J.P.; Bruins, M.E. Pulsed Electric Field as an Alternative Pre-Treatment for Drying to Enhance Polyphenol Extraction from Fresh Tea Leaves. Food Bioprocess Technol. 2019, 12, 183–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Shao, Z.; Qiu, L.; Hao, W.; Qu, Q.; Sun, G. The Solid-State Physicochemical Properties and Biogas Production of the Anaerobic Digestion of Corn Straw Pretreated by Microwave Irradiation. RSC Adv. 2021, 11, 3575–3584. [Google Scholar] [CrossRef]
- Kovačić, Đ.; Kralik, D.; Rupčić, S.; Jovičić, D.; Spajić, R.; Tišma, M. Electroporation of Harvest Residues for Enhanced Biogas Production in Anaerobic Co-Digestion with Dairy Cow Manure. Bioresour. Technol. 2019, 274, 215–224. [Google Scholar] [CrossRef]
- Wang, B.; Chen, T.; Qin, X.; Wu, Q.; Zhao, Y.; Bai, S.; Peng, W.; Feng, B. Effect of High-Voltage Pulsed Electric Field (HPEF) Pretreatment on Biogas Production Rates of Hybrid Pennisetum by Anaerobic Fermentation. Nat. Gas Ind. B 2018, 5, 48–53. [Google Scholar] [CrossRef]
- Budiyono, I.; Sumardiono, S.; Mardiani, D.T. Microwave Pretreatment of Fresh Water Hyacinth (Eichhornia Crassipes) in Batch Anaerobic Digestion Tank. Int. J. Eng. 2015, 28, 832–840. [Google Scholar]
- Szwarc, D.; Szwarc, K. Use of a Pulsed Electric Field to Improve the Biogas Potential of Maize Silage. Energies 2020, 14, 119. [Google Scholar] [CrossRef]
- Zou, L.; Ma, C.; Liu, J.; Li, M.; Ye, M.; Qian, G. Pretreatment of Food Waste with High Voltage Pulse Discharge towards Methane Production Enhancement. Bioresour. Technol. 2016, 222, 82–88. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Value |
|---|---|
| Hydration [%] | 52.47 ± 1.06 |
| Dry weight [%] | 47.53 ± 1.06 |
| Dry organic weight [% TS] | 89.62 ± 1.05 |
| Total carbon (TC) [mg C/g TS] | 478.99 ± 18.99 |
| Total organic carbon (TOC) [mg C/g TS] | 390.54 ± 11.67 |
| Total nitrogen (TN) [mg N/g TS] | 20.94 ± 3.89 |
| C/N | 22.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szwarc, D.; Nowicka, A.; Głowacka, K. Cross-Comparison of the Impact of Grass Silage Pulsed Electric Field and Microwave-Induced Disintegration on Biogas Production Efficiency. Energies 2022, 15, 5122. https://doi.org/10.3390/en15145122
Szwarc D, Nowicka A, Głowacka K. Cross-Comparison of the Impact of Grass Silage Pulsed Electric Field and Microwave-Induced Disintegration on Biogas Production Efficiency. Energies. 2022; 15(14):5122. https://doi.org/10.3390/en15145122
Chicago/Turabian StyleSzwarc, Dawid, Anna Nowicka, and Katarzyna Głowacka. 2022. "Cross-Comparison of the Impact of Grass Silage Pulsed Electric Field and Microwave-Induced Disintegration on Biogas Production Efficiency" Energies 15, no. 14: 5122. https://doi.org/10.3390/en15145122





