Energy and Exergy-Based Screening of Various Refrigerants, Hydrocarbons and Siloxanes for the Optimization of Biomass Boiler–Organic Rankine Cycle (BB–ORC) Heat and Power Cogeneration Plants
Abstract
:1. Introduction
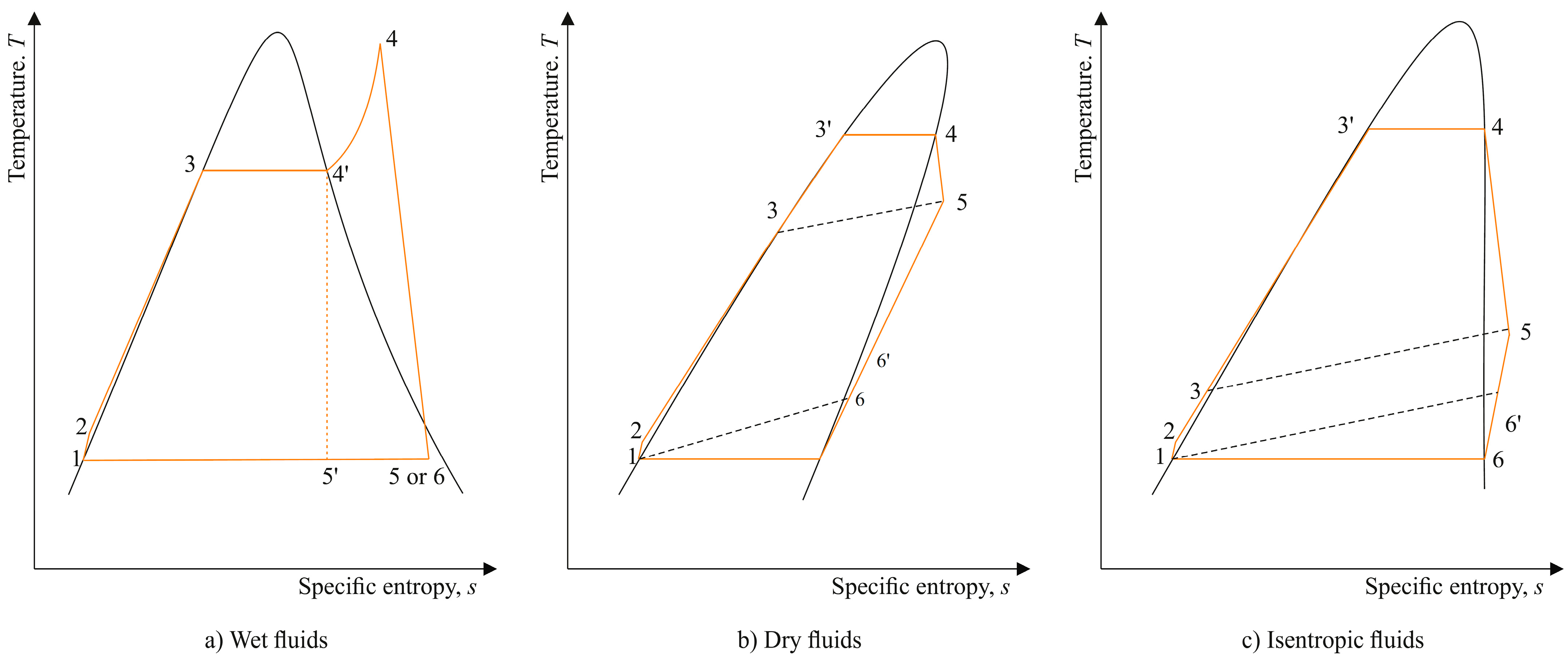
2. The ORC–CHP System and the Working Fluids
- i.
- Process 1–2: compression of liquid organic fluid in a pump.
- ii.
- Process 2–3: isobaric preheating of the organic fluid in a vapor to liquid heat exchanger termed as recuperator or regenerator.
- iii.
- Process 3–4: isobaric heat addition and evaporation of the organic fluid in an oil heat recovery exchanger. Evaporation may take place at a pressure higher or lower than the critical pressure of the working fluid, but in the present study, only subcritical pressures will be considered.
- iv.
- Process 4–5: expansion of the organic fluid in a turbine (expander).
- v.
- Process 5–6: isobaric cooling of the organic fluid in the regenerator.
- vi.
- Process 6–1: isobaric cooling of the organic fluid and condensation to the state of saturated liquid 1 (also heating of the process water flow).
2.1. Classification of the Working Fluids according to Chemical Structure
- (a)
- hydrocarbons can provide high efficiencies when they have a high critical temperature and high molar mass;
- (b)
- they provide a higher enthalpy drop in the expander in comparison to siloxanes of the same critical temperature;
- (c)
- cycloalkanes are more efficient than linear hydrocarbons of the same critical temperature;
- (d)
- siloxanes can provide high efficiencies with high expander inlet temperatures in cycles equipped with a regenerator;
- (e)
- they have a similar performance independent of their cyclic or linear molecular structure if they have the same critical pressure;
- (f)
- fluorocarbons have lower cycle efficiencies compared to siloxanes and hydrocarbons, especially in high temperature processes;
- (g)
- they provide lower enthalpy drop in the expander compared to hydrocarbons.
2.2. Classification of Working Fluids according to the Slope of the Saturation Vapor Curve
2.3. Environmental and Safety Selection Criteria
- Ozone Depletion Potential (ODP) quantifies the effect of each fluid on the degradation of the atmospheric ozone layer in comparison to the same effect of the specific fluid R11 for which it is considered that ODP equals unity (ODP = 1). The higher the ODP index of a fluid, the higher its impact on the ozone layer. Fluids with ODP below unity are considered to have an impact of medium intensity, and fluids with ODP above unity are considered highly detrimental to the ozone layer.
- Global Warming Potential (GWP) quantifies the solar heat absorbed by the gases of each fluid when released into the atmosphere in comparison to the solar heat absorbed by an equal mass of atmospheric carbon dioxide. Usually, GWP values are calculated over the time interval of 100 years and are considered low when GWP < 150, medium when 150 < GWP < 2500, and high when GWP > 2500.
- Health group A indicates that the substance does not show any evidence of toxicity below 400 ppm.
- Health group B indicates that the substance shows evidence of toxicity below 400 ppm.
- Flammability group 1 indicates a fluid that does not propagate a flame in open air under normal conditions.
- Flammability group 2 indicates a fluid that may propagate a flame in open air under specific conditions.
- Flammability group 3 indicates a fluid of high flammability.
- Health and flammability classification groups are combined into a single safety classification index, which can be either A1, A2, A3, B1, B2, or B3. Recently, two new safety classes, A2L and B2L, were introduced for fluids that are mildly flammable. Table 2 provides the safety classification index for each specific working fluid.
3. Modeling of the ORC–CHP Plant
4. Results and Discussion
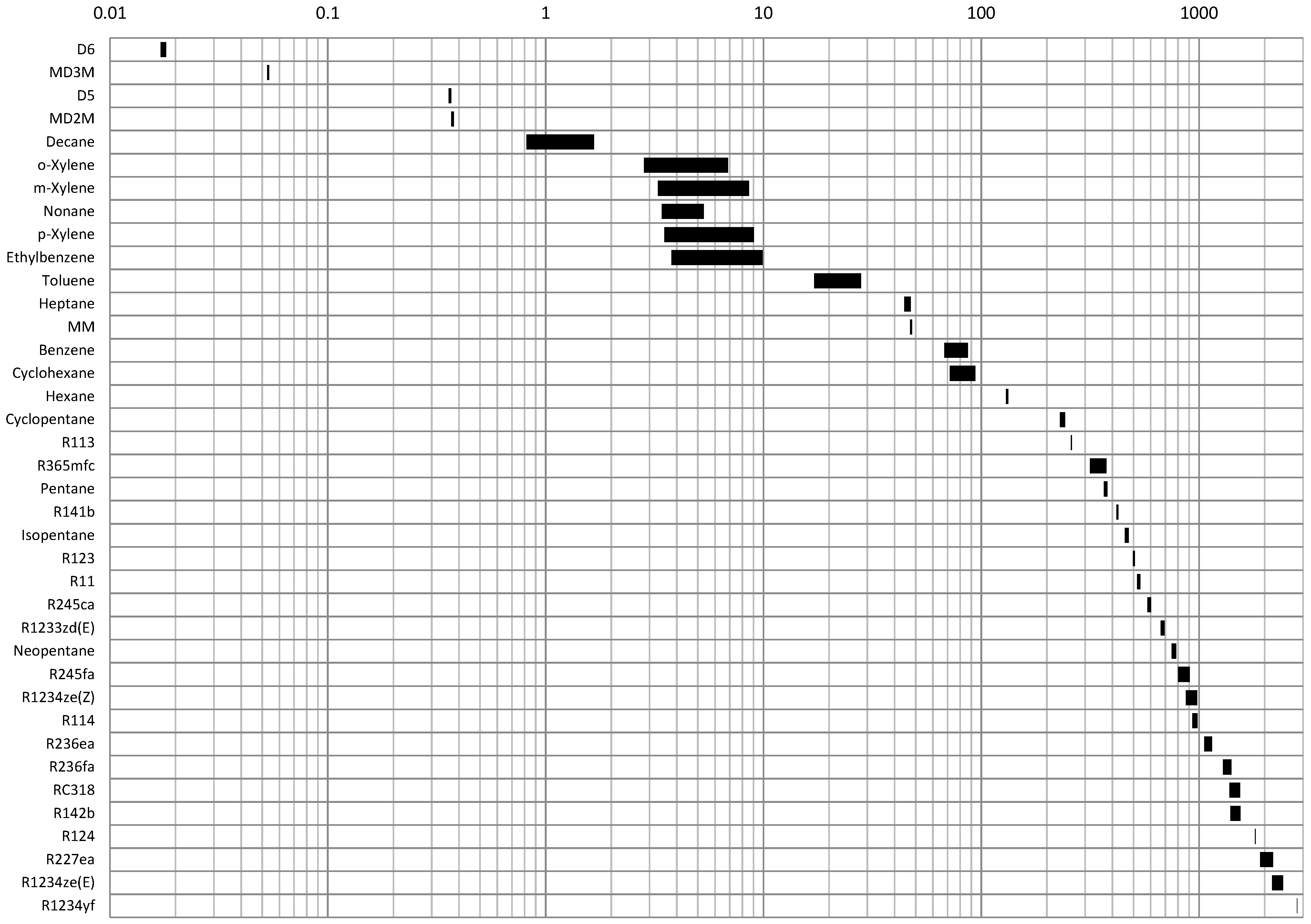
4.1. Optimal Condensation Pressure
- Low condensation pressure fluids with a condensation pressure below 10 kPa.
- Medium condensation pressure fluids with a condensation pressure between 10 and 1000 kPa.
- High condensation pressure fluids with a condensation pressure above 1000 kPa.
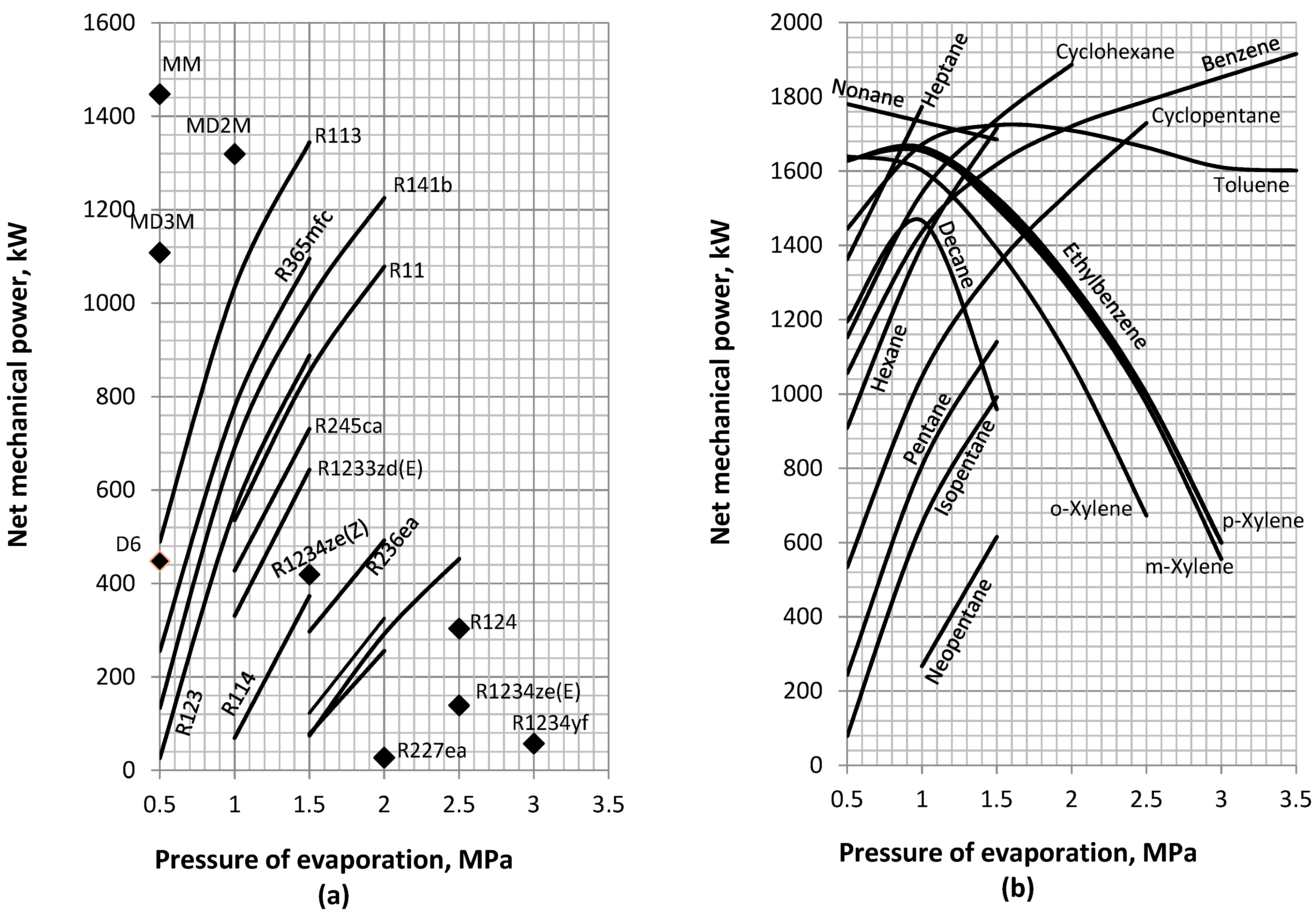
4.2. Net Mechanical Power
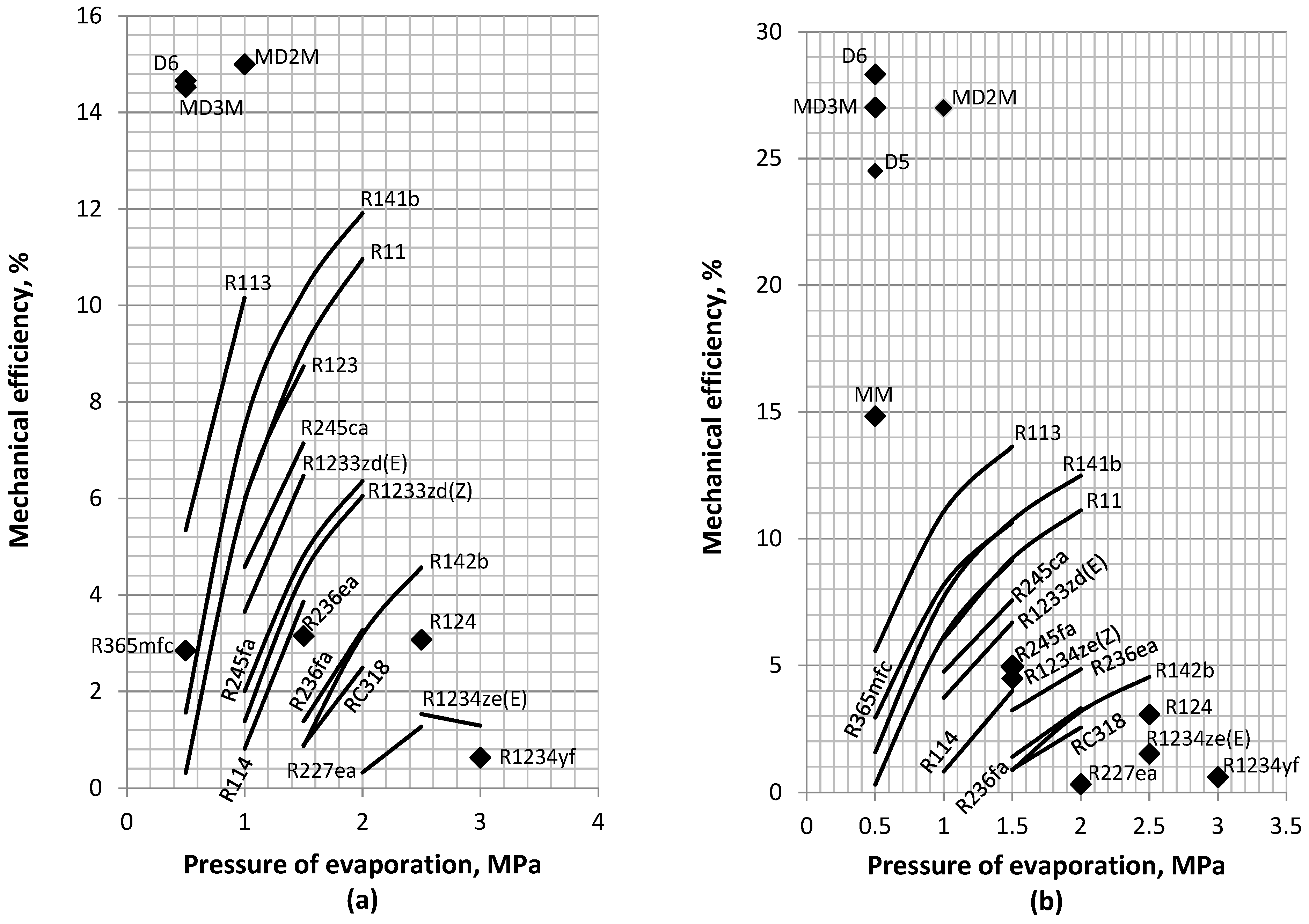
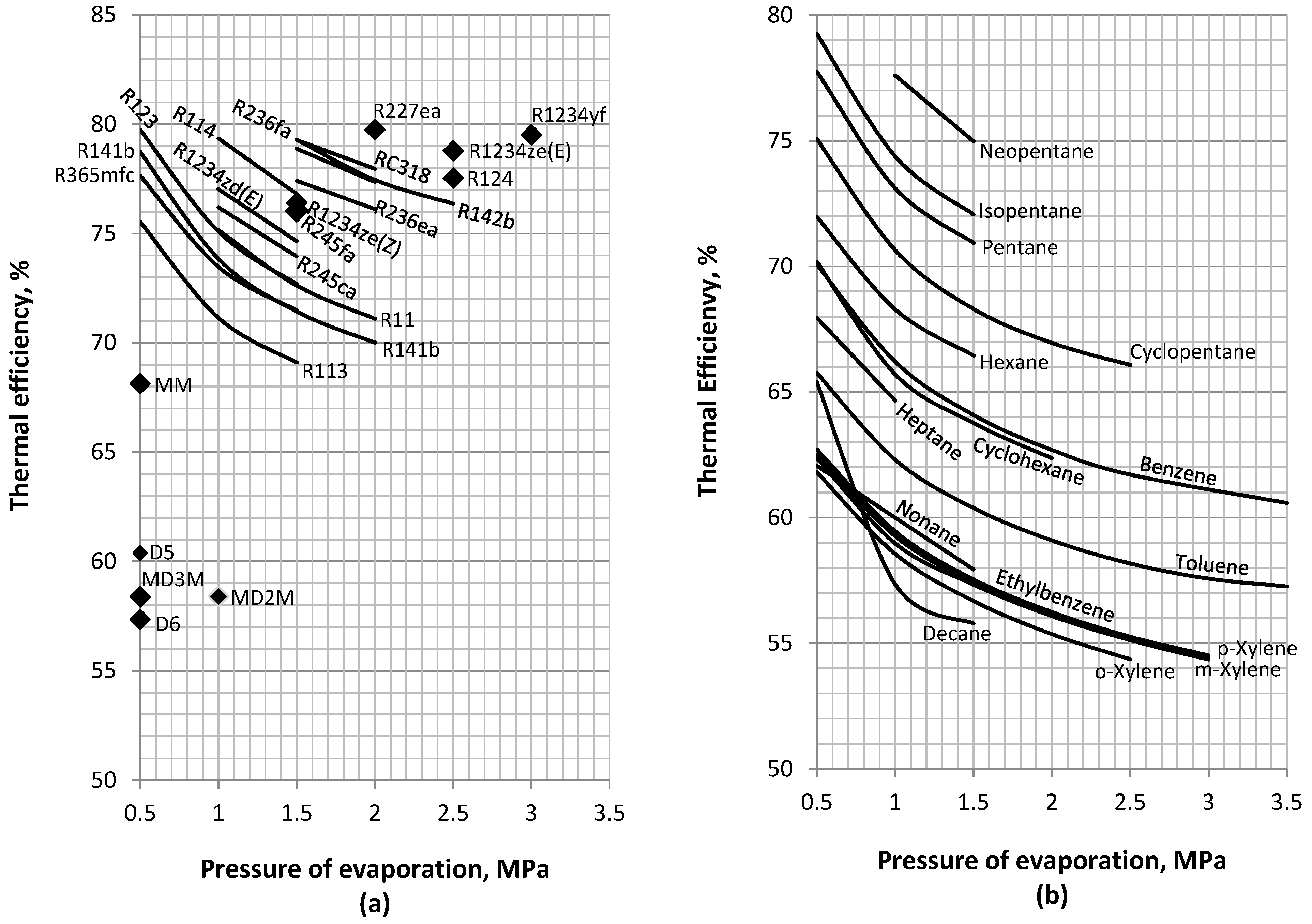
4.3. Mechanical and Thermal Efficiency
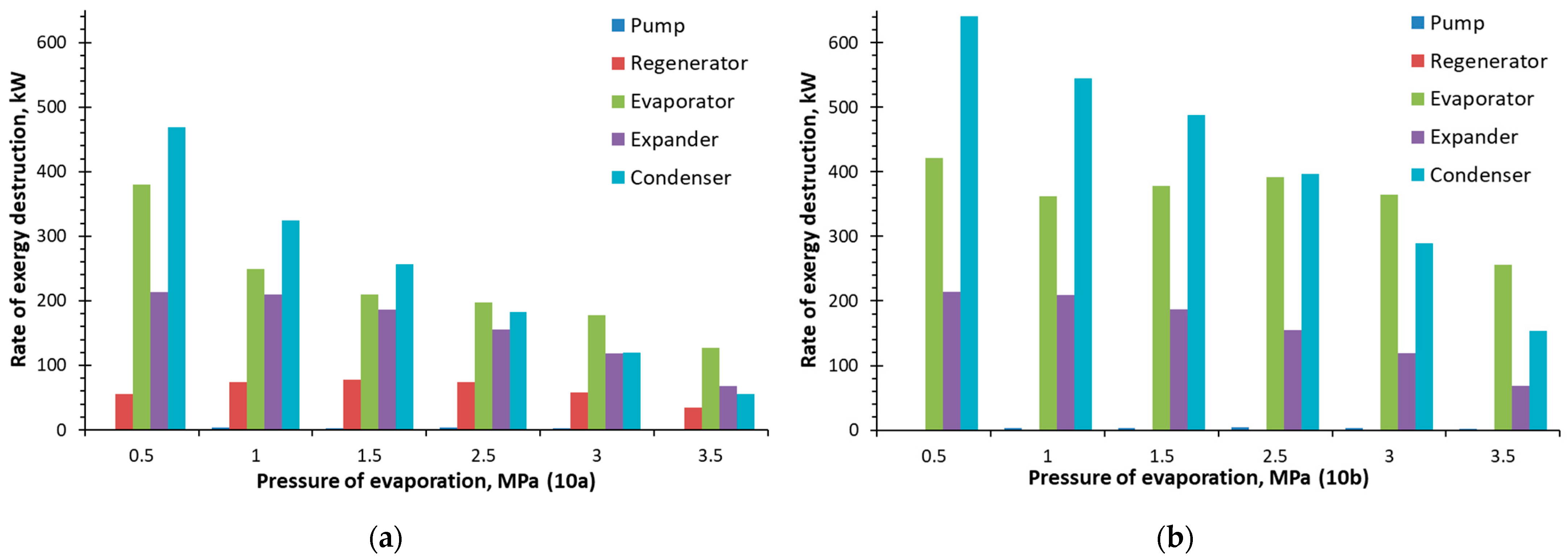
4.4. Exergy Analysis
4.5. Selection of the Working Fluid
- Dry and isentropic hydrocarbons have shown high thermodynamic performance in the ORC equipped with internal regeneration and also have minimal ozone and global warming impact. They are also nontoxic, but due to their high flammability, they need special safety measures for handling and operation. Despite this drawback, hydrocarbons are generally considered rational fluid options in organic Rankine cycles.
- Chlorofluorocarbons (CFCs) have been banned, and therefore fluids such as R11, R113 and R114 that have shown high thermodynamic performance, must be excluded from the final selection list of working fluids.
- The Kigali Amendment of the Montreal Protocol on Substances that Deplete the Ozone Layer has called for the phase-out of hydrochlorofluorocarbons (HCFCs) after 2016. Therefore, various countries have already planned the gradual substitution of HCFCs in the near future. As a result, refrigerants, such as R123, R124, R141b, and R142b must be excluded from the final selection.
- Fluids with an ODP value above 1 and a GWP value above 2500 will be excluded from the final selection.
- Refrigerants are not appropriate options for BB–ORC plants since they demonstrate extremely low mechanical efficiencies or they are banned for environmental issues.
- Siloxanes demonstrate extremely high mechanical efficiencies at low evaporation pressures but very low thermal efficiencies and hot water temperatures. Moreover, they operate at extremely low and often impractical condensing pressures.
- Hydrocarbons are found to lie in the optimum middle range of the fluid spectrum, between the siloxanes that maximize the production of mechanical power and the refrigerants that maximize the production of heat. Most of them also operate at the practical medium range of condensing pressures between 10 and 1000 kPa.
- When the maximization of mechanical power output is of paramount importance, specific hydrocarbons, such as ethylbenzene, the xylene m-, o-, and p- isomers; and nonane are appropriate options if condensation pressures below 10 kPa can be attained. These fluids can operate at relative low evaporation pressures (0.5–1 MPa) and may be further classified depending on their mass flow rate during operation on the order o-xylene (10.38–13.59 kg/s), m-xylene (11.35–14.36 kg/s), p-xylene (11.59–14.64 kg/s), ethylbenzene (11.84–14.93 kg/s), and nonane (10.87–16.17 kg/s). At higher condensation pressures, cyclopentane, hexane, cyclohexane, heptane, benzene, and toluene are found to be the most appropriate fluids. These can be used at moderate evaporation pressures of 1–2 MPa. In this group, toluene has the lowest mass flow rate (9.58–16.78 kg/s) followed by benzene (14.26–18.53 kg/s), cyclohexane (17.69–22.03 kg/s), heptane (20.45–21.7 kg/s), cyclopentane (20.45–21.7 kg/s), and, finally, hexane (23.47–24.03 kg/s).
- For efficient CHP applications with adequately high mechanical performance and high temperature water production, cyclopentane, hexane, and MM are the most appropriate fluids. According to their mass flow rates, these are classified in the order cyclopentane (20.45–21.7 kg/s), hexane (23.47–24.03 kg/s), and MM (36.77 kg/s).
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Biomass Boiler–Organic Rankine Cycle | BB–ORC |
| Chlorofluorocarbons | CFCs |
| Combined Heat and Power | CHP |
| Decamethylcyclopentasiloxane | D5 |
| Decamethyltetrasiloxane | MD2M |
| Dodecamethylcyclohexasiloxane | D6 |
| Dodecamethylpentasiloxane | MD3M |
| Global Warming Potential | GWP |
| Hexamethyldisiloxane | MM |
| Hydrocarbons | HCs |
| Hydrochlorofluorocarbons | HCFCs |
| Hydrofluorocarbons | HFCs |
| Hydrolfluoroolefins | HFOs |
| Medium Density Fiberboards | MDFs |
| Octamethyltrisiloxane | MDM |
| Organic Rankine Cycles | ORCs |
| Ozone Depletion Potential | ODP |
| Perfluorocarbons | PFCs |
References
- Zalasiewicz, J.; Waters, C.N.; Summerhayes, C.P.; Wolfe, A.P.; Barnosky, A.D.; Cearreta, A.; Crutzen, P.; Ellis, E.; Fairchild, I.J.; Gałuszka, A.; et al. The Working Group on the Anthropocene: Summary of Evidence and Interim Recommendations. Anthropocene 2017, 19, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Liu, P.; Li, Z. Low Carbon Transition Pathway of Power Sector with High Penetration of Renewable Energy. Renew. Sustain. Energy Rev. 2020, 130, 109985. [Google Scholar] [CrossRef]
- Genus, A.; Iskandarova, M. Transforming the Energy System? Technology and Organisational Legitimacy and the Institutionalisation of Community Renewable Energy. Renew. Sustain. Energy Rev. 2020, 125, 109795. [Google Scholar] [CrossRef]
- Inayat, A.; Raza, M. District Cooling System via Renewable Energy Sources: A Review. Renew. Sustain. Energy Rev. 2019, 107, 360–373. [Google Scholar] [CrossRef]
- Douvartzides, S.L.; Charisiou, N.D.; Papageridis, K.N.; Goula, M.A. Green Diesel: Biomass Feedstocks, Production Technologies, Catalytic Research, Fuel Properties and Performance in Compression Ignition Internal Combustion Engines. Energies 2019, 12, 809. [Google Scholar] [CrossRef] [Green Version]
- Charisiou, N.D.; Polychronopoulou, K.; Asif, A.; Goula, M.A. The Potential of Glycerol and Phenol towards H2 Production Using Steam Reforming Reaction: A Review. Surf. Coat. Technol. 2018, 352, 92–111. [Google Scholar] [CrossRef]
- Sharma, H.B.; Sarmah, A.K.; Dubey, B. Hydrothermal Carbonization of Renewable Waste Biomass for Solid Biofuel Production: A Discussion on Process Mechanism, the Influence of Process Parameters, Environmental Performance and Fuel Properties of Hydrochar. Renew. Sustain. Energy Rev. 2020, 123, 109761. [Google Scholar] [CrossRef]
- Ong, H.C.; Chen, W.-H.; Singh, Y.; Gan, Y.Y.; Chen, C.-Y.; Show, P.L. A State-of-the-Art Review on Thermochemical Conversion of Biomass for Biofuel Production: A TG-FTIR Approach. Energy Convers. Manag. 2020, 209, 112634. [Google Scholar] [CrossRef]
- IEA Bioenergy. Electricity from Biomass: From Small to Large Scale. In Summary and Conclusions from the IEA Bioenergy ExCo72 Workshop; IEA Bioenergy: Göteborg, Sweden, 2015. [Google Scholar]
- Oyekale, J.; Petrollese, M.; Heberle, F.; Brüggemann, D.; Cau, G. Exergetic and Integrated Exergoeconomic Assessments of a Hybrid Solar-Biomass Organic Rankine Cycle Cogeneration Plant. Energy Convers. Manag. 2020, 215, 112905. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, W.; He, Z. Thermodynamic Analysis and Optimization of a Novel Organic Rankine Cycle-Based Micro-Scale Cogeneration System Using Biomass Fuel. Energy Convers. Manag. 2019, 198, 111803. [Google Scholar] [CrossRef]
- Modi, A.; Bühler, F.; Andreasen, J.G.; Haglind, F. A Review of Solar Energy Based Heat and Power Generation Systems. Renew. Sustain. Energy Rev. 2017, 67, 1047–1064. [Google Scholar] [CrossRef] [Green Version]
- Rayegan, R.; Tao, Y.X. A Procedure to Select Working Fluids for Solar Organic Rankine Cycles (ORCs). Renew. Energy 2011, 36, 659–670. [Google Scholar] [CrossRef]
- Tchanche, B.F.; Lambrinos, G.; Frangoudakis, A.; Papadakis, G. Low-Grade Heat Conversion into Power Using Organic Rankine Cycles—A Review of Various Applications. Renew. Sustain. Energy Rev. 2011, 15, 3963–3979. [Google Scholar] [CrossRef]
- Tchanche, B.F.; Pétrissans, M.; Papadakis, G. Heat Resources and Organic Rankine Cycle Machines. Renew. Sustain. Energy Rev. 2014, 39, 1185–1199. [Google Scholar] [CrossRef]
- Vélez, F.; Segovia, J.J.; Martín, M.C.; Antolín, G.; Chejne, F.; Quijano, A. A Technical, Economical and Market Review of Organic Rankine Cycles for the Conversion of Low-Grade Heat for Power Generation. Renew. Sustain. Energy Rev. 2012, 16, 4175–4189. [Google Scholar] [CrossRef]
- Tzivanidis, C.; Bellos, E.; Antonopoulos, K.A. Energetic and Financial Investigation of a Stand-Alone Solar-Thermal Organic Rankine Cycle Power Plant. Energy Convers. Manag. 2016, 126, 421–433. [Google Scholar] [CrossRef]
- Bellos, E.; Tzivanidis, C. Parametric Analysis and Optimization of an Organic Rankine Cycle with Nanofluid Based Solar Parabolic Trough Collectors. Renew. Energy 2017, 114, 1376–1393. [Google Scholar] [CrossRef]
- Heberle, F.; Brüggemann, D. Exergy Based Fluid Selection for a Geothermal Organic Rankine Cycle for Combined Heat and Power Generation. Appl. Therm. Eng. 2010, 30, 1326–1332. [Google Scholar] [CrossRef]
- Javanshir, A.; Sarunac, N.; Razzaghpanah, Z. Thermodynamic Analysis of ORC and Its Application for Waste Heat Recovery. Sustainability 2017, 9, 1974. [Google Scholar] [CrossRef] [Green Version]
- Jouhara, H.; Khordehgah, N.; Almahmoud, S.; Delpech, B.; Chauhan, A.; Tassou, S.A. Waste Heat Recovery Technologies and Applications. Therm. Sci. Eng. Prog. 2018, 6, 268–289. [Google Scholar] [CrossRef]
- Xu, B.; Rathod, D.; Yebi, A.; Filipi, Z.; Onori, S.; Hoffman, M. A Comprehensive Review of Organic Rankine Cycle Waste Heat Recovery Systems in Heavy-Duty Diesel Engine Applications. Renew. Sustain. Energy Rev. 2019, 107, 145–170. [Google Scholar] [CrossRef]
- Pantaleo, A.M.; Camporeale, S.; Fortunato, B. Small Scale Biomass CHP: Techno-Economic Performance of Steam vs Gas Turbines with Bottoming ORC. Energy Procedia 2015, 82, 825–832. [Google Scholar] [CrossRef] [Green Version]
- Qiu, G.; Shao, Y.; Li, J.; Liu, H.; Riffat, S.B. Experimental Investigation of a Biomass-Fired ORC-Based Micro-CHP for Domestic Applications. Fuel 2012, 96, 374–382. [Google Scholar] [CrossRef] [Green Version]
- Nur, T.B.; Syahputra, A.W. Integrated Biomass Pyrolysis with Organic Rankine Cycle for Power Generation. In Proceedings of the 10th International Conference Numerical Analysis in Engineering, Banda Aceh, Indonesia, 24–25 August 2017; Volume 308, p. 012030. [Google Scholar] [CrossRef]
- Nur, T.B.; Pane, Z.; Amin, M.N. Application of Biomass from Palm Oil Mill for Organic Rankine Cycle to Generate Power in North Sumatera Indonesia. In Proceedings of the 1st Annual Applied Science and Engineering Conference (AASEC), in Conjuction with The International Conference on Sport Science, Health, Bandung, Indonesia, 16–18 November 2016, and Physical Education (ICSSHPE); Volume 180, p. 012035. [CrossRef]
- Algieri, A.; Morrone, P. Techno-Economic Analysis of Biomass-Fired ORC Systems for Single-Family Combined Heat and Power (CHP) Applications. Energy Procedia 2014, 45, 1285–1294. [Google Scholar] [CrossRef] [Green Version]
- Eyidogan, M.; Canka Kilic, F.; Kaya, D.; Coban, V.; Cagman, S. Investigation of Organic Rankine Cycle (ORC) Technologies in Turkey from the Technical and Economic Point of View. Renew. Sustain. Energy Rev. 2016, 58, 885–895. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, W.; Li, J.; Li, H.; Wang, Y.; Li, S. Thermodynamic Analysis and Economic Assessment of Biomass-Fired Organic Rankine Cycle Combined Heat and Power System Integrated with CO2 Capture. Energy Convers. Manag. 2020, 204, 112310. [Google Scholar] [CrossRef]
- Imran, M.; Haglind, F.; Asim, M.; Zeb Alvi, J. Recent Research Trends in Organic Rankine Cycle Technology: A Bibliometric Approach. Renew. Sustain. Energy Rev. 2018, 81, 552–562. [Google Scholar] [CrossRef] [Green Version]
- Tartière, T.; Astolfi, M. A World Overview of the Organic Rankine Cycle Market. Energy Procedia 2017, 129, 2–9. [Google Scholar] [CrossRef]
- Available online: https://www.turboden.com/products/2463/orc-system (accessed on 30 November 2021).
- Morrone, P.; Algieri, A. Biomass Exploitation in Efficient ORC Systems. Appl. Mech. Mater. 2012, 260–261, 77–82. [Google Scholar] [CrossRef]
- Schuster, A.; Karellas, S.; Kakaras, E.; Spliethoff, H. Energetic and Economic Investigation of Organic Rankine Cycle Applications. Appl. Therm. Eng. 2009, 29, 1809–1817. [Google Scholar] [CrossRef] [Green Version]
- Zhai, H.; An, Q.; Shi, L.; Lemort, V.; Quoilin, S. Categorization and Analysis of Heat Sources for Organic Rankine Cycle Systems. Renew. Sustain. Energy Rev. 2016, 64, 790–805. [Google Scholar] [CrossRef]
- Drescher, U.; Brüggemann, D. Fluid Selection for the Organic Rankine Cycle (ORC) in Biomass Power and Heat Plants. Appl. Therm. Eng. 2007, 27, 223–228. [Google Scholar] [CrossRef]
- Ismail, H.; Aziz, A.A.; Rasih, R.A.; Jenal, N.; Michael, Z.; Roslan, A. Performance of Organic Rankine Cycle Using Biomass as Source of Fuel. J. Adv. Res. Appl. Sci. Eng. Technol. 2016, 4, 29–46. [Google Scholar]
- Algieri, A. Comparative Investigation of the Performances of Subcritical and Transcritical Biomass-Fired ORC Systems for Micro-Scale CHP Applications. Procedia Comput. Sci. 2016, 83, 855–862. [Google Scholar] [CrossRef] [Green Version]
- Pezzuolo, A.; Benato, A.; Stoppato, A.; Mirandola, A. Fluid Selection and Plant Configuration of an ORC-Biomass Fed System Generating Heat and/or Power. Energy Procedia 2016, 101, 822–829. [Google Scholar] [CrossRef]
- Jang, Y.; Lee, J. Optimizations of the Organic Rankine Cycle-Based Domestic CHP Using Biomass Fuel. Energy Convers. Manag. 2018, 160, 31–47. [Google Scholar] [CrossRef]
- Mikielewicz, D.; Mikielewicz, J. Criteria for Selection of Working Fluid in Low-Temperature ORC. Chem. Process Eng. 2016, 37, 429–440. [Google Scholar] [CrossRef] [Green Version]
- Moharamian, A.; Soltani, S.; Rosen, M.A.; Mahmoudi, S.M.S.; Morosuk, T. A Comparative Thermoeconomic Evaluation of Three Biomass and Biomass-Natural Gas Fired Combined Cycles Using Organic Rankine Cycles. J. Clean. Prod. 2017, 161, 524–544. [Google Scholar] [CrossRef]
- Fernández, F.J.; Prieto, M.M.; Suárez, I. Thermodynamic Analysis of High-Temperature Regenerative Organic Rankine Cycles Using Siloxanes as Working Fluids. Energy 2011, 36, 5239–5249. [Google Scholar] [CrossRef]
- Méndez-Cruz, L.E.; Gutiérrez-Limón, M.Á.; Lugo-Méndez, H.; Lugo-Leyte, R.; Lopez-Arenas, T.; Sales-Cruz, M. Comparative Thermodynamic Analysis of the Performance of an Organic Rankine Cycle Using Different Working Fluids. Energies 2022, 15, 2588. [Google Scholar] [CrossRef]
- Thurairaja, K.; Wijewardane, A.; Jayasekara, S.; Ranasinghe, C. Working Fluid Selection and Performance Evaluation of ORC. Energy Procedia 2019, 156, 244–248. [Google Scholar] [CrossRef]
- Babatunde, A.F.; Sunday, O.O. A Review of Working Fluids for Organic Rankine Cycle (ORC) Applications. In Proceedings of the 2nd International Conference on Engineering for Sustainable World (ICESW 2018), Ota, Nigeria, 9–13 July 2018; Volume 413, p. 012019. [Google Scholar] [CrossRef]
- Arjunan, P.; Gnana Muthu, J.H.; Somanasari Radha, S.L.; Suryan, A. Selection of Working Fluids for Solar Organic Rankine Cycle—A Review. Int. J. Energy Res. 2022, 1–27. [Google Scholar] [CrossRef]
- Imre, A.R.; Kustán, R.; Groniewsky, A. Thermodynamic Selection of the Optimal Working Fluid for Organic Rankine Cycles. Energies 2019, 12, 2028. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, Y.; Cao, M.; Wang, J.; Wu, Y.; Ma, C. Working Fluid Selection for Organic Rankine Cycle Using Single-Screw Expander. Energies 2019, 12, 3197. [Google Scholar] [CrossRef] [Green Version]
- Invernizzi, C.M.; Ayub, A.; Di Marcoberardino, G.; Iora, P. Pure and Hydrocarbon Binary Mixtures as Possible Alternatives Working Fluids to the Usual Organic Rankine Cycles Biomass Conversion Systems. Energies 2019, 12, 4140. [Google Scholar] [CrossRef] [Green Version]
- Uusitalo, A.; Honkatukia, J.; Turunen-Saaresti, T.; Grönman, A. Thermodynamic Evaluation on the Effect of Working Fluid Type and Fluids Critical Properties on Design and Performance of Organic Rankine Cycles. J. Clean. Prod. 2018, 188, 253–263. [Google Scholar] [CrossRef]
- Chen, H.; Goswami, D.Y.; Stefanakos, E.K. A Review of Thermodynamic Cycles and Working Fluids for the Conversion of Low-Grade Heat. Renew. Sustain. Energy Rev. 2010, 14, 3059–3067. [Google Scholar] [CrossRef]
- Poling, B.E.; Prausnitz, J.M.; O’Connell, J.P. The Properties of Gases and Liquids; McGraw-Hill Professional: New York, NY, USA, 2000. [Google Scholar]
- Calm, J.M.; Didion, D.A. Trade-Offs in Refrigerant Selections: Past, Present, and Future. Int. J. Refrig. 1998, 21, 308–321. [Google Scholar] [CrossRef]
- Bell, I.H.; Wronski, J.; Quoilin, S.; Lemort, V. Pure and Pseudo-Pure Fluid Thermophysical Property Evaluation and the Open-Source Thermophysical Property Library CoolProp. Ind. Eng. Chem. Res. 2014, 53, 2498–2508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Xu, J.; Chen, H. A New Design Method for Organic Rankine Cycles with Constraint of Inlet and Outlet Heat Carrier Fluid Temperatures Coupling with the Heat Source. Appl. Energy 2012, 98, 562–573. [Google Scholar] [CrossRef]
- Wang, J.; Yan, Z.; Wang, M.; Ma, S.; Dai, Y. Thermodynamic Analysis and Optimization of an (Organic Rankine Cycle) ORC Using Low Grade Heat Source. Energy 2013, 49, 356–365. [Google Scholar] [CrossRef]
- Sarkar, J. A Novel Pinch Point Design Methodology Based Energy and Economic Analyses of Organic Rankine Cycle. J. Energy Resour. Technol. 2018, 140, 052004. [Google Scholar] [CrossRef]
- Li, Y.-R.; Wang, J.-N.; Du, M.-T.; Wu, S.-Y.; Liu, C.; Xu, J.-L. Effect of Pinch Point Temperature Difference on Cost-Effective Performance of Organic Rankine Cycle: Effect of PPTD on Cost-Effective Performance of Organic Rankine Cycle. Int. J. Energy Res. 2013, 37, 1952–1962. [Google Scholar] [CrossRef]
- Zhang, X.; He, M.; Wang, J. A New Method Used to Evaluate Organic Working Fluids. Energy 2014, 67, 363–369. [Google Scholar] [CrossRef]
- Darvish, K.; Ehyaei, M.; Atabi, F.; Rosen, M. Selection of Optimum Working Fluid for Organic Rankine Cycles by Exergy and Exergy-Economic Analyses. Sustainability 2015, 7, 15362–15383. [Google Scholar] [CrossRef] [Green Version]



| Overall thermal power input | |
| Biomass consumption 1 | 2471 kg/h |
| Gross active electric power (turbine power) | |
| Thermal power to hot water circuit | 4081 kW |
| Gross electric efficiency | 19.8% |
| Thermal efficiency for water heating | 79.4% |
| Thermal oil inlet temperature | |
| Thermal oil outlet temperature | |
| Cooling water inlet temperature | |
| Cooling water outlet temperature |
| Code | Substance Name | MW (kg/kmol) | Latent heat of Vaporization ΔHvap at 0.1 Mpa (kJ/kg) | Critical Temperature Tcr (K) | Critical Pressure pcr (Mpa) | Type of Fluid | Ozone Depletion Potential (ODP) | Global Warming Potential (GWP) 100 y | ASHRAE 34 Safety Group |
|---|---|---|---|---|---|---|---|---|---|
| Chlorofluorocarbons (CFCs) | |||||||||
| R11 | Trichloromonofluoromethane | 137.37 | 181.49 | 471.11 | 4.4076 | Isentropic | 1 | 4750 | A1 |
| R113 | 1,1,2-Trichloro-1,2,2-Trifluoroethane | 187.38 | 144.45 | 487.21 | 3.3922 | Dry | 1 | 6130 | A1 |
| R114 | 1,2-Dichloro-1,1,2,2-Tetrafluoroethane | 170.92 | 136.06 | 418.83 | 3.257 | Dry | 1 | 10,000 | A1 |
| Hydrochlorofluorocarbons (HCFCs) | |||||||||
| R123 | 2,2-Dichloro-1,1,1-Trifluoroethane | 152.93 | 170.35 | 456.831 | 3.6618 | Dry | 0.02 | 77 | B1 |
| R124 | 1-Chloro-1,2,2,2-Tetrafluoroethane | 136.5 | 165.99 | 395.425 | 3.6242 | Isentropic | 0.022 | 609 | A1 |
| R141b | 1,1-Dichloro-1-Fluoroethane | 116.9 | 222.88 | 477.5 | 4.212 | Isentropic | 0.12 | 725 | A2 |
| R142b | 1-Chloro-1,1-Difluoroethane | 100.5 | 223.43 | 410.26 | 4.055 | Isentropic | 0.12 | 2310 | A2 |
| Hydrofluorocarbons (HFCs) | |||||||||
| R125 | Pentafluoroethane | 120 | 164.25 | 339.173 | 3.6177 | Isentropic | 0 | 3500 | A1 |
| R227ea | 1,1,1,2,3,3,3-Heptafluoropropane | 170.02 | 131.90 | 374.9 | 2.92 | Dry | 0 | 3220 | A1 |
| R236ea | 1,1,1,2,3,3-Hexafluoropropane | 152 | 165.32 | 412.44 | 3.5019 | Dry | 0 | 1370 | n.a. |
| R236fa | 1,1,1,3,3,3-Hexafluoropropane | 152 | 160.48 | 398.07 | 3.2 | Dry | 0 | 9810 | A1 |
| R245ca | 1,1,2,2,3-Pentafluoropropane | 134 | 201.15 | 447.57 | 3.925 | Dry | 0 | 693 | n.a. |
| R245fa | 1,1,1,3,3-Pentafluoropropane | 134 | 196.88 | 427.2 | 3.64 | Dry | 0 | 1030 | B1 |
| R365mfc | 1,1,1,3,3-Pentafluorobutane | 148.07 | 188.36 | 460.0 | 3.26 | Dry | 0 | 796 | n.a. |
| Perfluorocarbons (PFCs) | |||||||||
| R116 | Hexafluoroethane | 138.02 | 117.09 | 293.03 | 3.048 | Isentropic | 0 | 12,200 | A1 |
| RC318 | Octafluorocyclobutane | 200.03 | 116.87 | 388.38 | 2.77 | Dry | 0 | 10,300 | A1 |
| Hydrofluoroolefins (HFOs) | |||||||||
| R1233zd(E) | trans-1-chloro-3,3,3-trifluoro-1-propene | 130.49 | 195.52 | 439.6 | 3.62 | Isentropic | 0 | 74 | A1 |
| R1234yf | 2,3,3,3-Tetrafluoropropene | 114.04 | 180.40 | 367.85 | 3.38 | Dry | 0 | 4 | A2L |
| R1234ze(E) | trans-1,3,3,3-Tetrafluoropropene | 114.04 | 195.60 | 382.52 | 3.63 | Isentropic | n.a. | 6 | n.a. |
| R1234ze(Z) | 1,3,3,3-Tetrafluoropropene | 114.04 | 215.11 | 423.27 | 3.53 | Isentropic | n.a. | 1.4 | n.a. |
| Hydrocarbons (HCs) | |||||||||
| R601 | n-Pentane | 72.1 | 357.89 | 469.7 | 3.37 | Dry | 0 | 4 | A3 |
| R601a | Isopentane | 72.14 | 343.57 | 460.35 | 3.37 | Dry | 0 | 4 | A3 |
| Cyclopentane | 70.13 | 389.45 | 511.72 | 4.57 | Dry | 0 | 11 | n.a. | |
| Neo-Pentane | 72.14 | 315.95 | 433.74 | 3.19 | Dry | 0 | n.a. | A3 | |
| n-Hexane | 86.17 | 335.24 | 507.82 | 3.03 | Dry | n.a. | n.a. | n.a. | |
| Cyclohexane | 84.15 | 356.66 | 553.64 | 4.07 | Dry | n.a. | n.a. | A3 | |
| n-Heptane | 100.20 | 317.20 | 540.13 | 2.73 | Dry | n.a. | n.a. | n.a. | |
| n-Nonane | 128.25 | 288.91 | 594.55 | 2.28 | Dry | n.a. | n.a. | n.a. | |
| n-Decane | 142.28 | 276.72 | 617.7 | 2.10 | Dry | n.a. | n.a. | n.a. | |
| Benzene | 78.11 | 394.96 | 562.02 | 4.89 | Dry | n.a. | n.a. | B2 | |
| Ethyl Benzene | 106.16 | 335.60 | 617.12 | 3.62 | Dry | n.a. | n.a. | n.a. | |
| Toluene | 92.13 | 361.00 | 591.75 | 4.12 | Dry | n.a. | n.a. | A3 | |
| m-Hylene | 106.16 | 340.44 | 616.89 | 3.53 | Dry | n.a. | n.a. | n.a. | |
| o-Hylene | 160.16 | 343.01 | 630.25 | 3.73 | Dry | n.a. | n.a. | n.a. | |
| p-Hylene | 106.16 | 336.66 | 616.16 | 3.53 | Dry | n.a. | n.a. | n.a. | |
| Siloxanes | |||||||||
| D4 | 296.61 | 131.23 | 586.5 | 1.34 | Dry | n.a. | n.a. | A3 | |
| D5 | 370.76 | 111.72 | 619.15 | 1.16 | Dry | n.a. | n.a. | n.a. | |
| D6 | 444.92 | 99.23 | 645.78 | 0.96 | Dry | n.a. | n.a. | n.a. | |
| MD2M | 310.68 | 129.22 | 599.4 | 1.22 | Dry | n.a. | n.a. | A3 | |
| MD3M | 384.83 | 110.84 | 628.3 | 0.94 | Dry | n.a. | n.a. | n.a. | |
| MDM (or OMTS) | 236.65 | 152.78 | 564.09 | 1.41 | Dry | n.a. | n.a. | n.a. | |
| MM | 162.37 | 193.58 | 518.75 | 1.93 | Dry | n.a. | n.a. | n.a. | |
| Operation Parameter | Value |
|---|---|
| Thermal oil inlet temperature: | |
| Cooling water inlet temperature: | |
| Thermal oil flow rate: | |
| Cooling water flow rate: | |
| Thermal oil specific heat capacity: | |
| Cooling water specific heat capacity: | |
| Regenerator efficiency: | |
| Condenser efficiency: | |
| Isentropic efficiency of pump: | |
| Isentropic efficiency of turbine (expander): | |
| Pinch point temperature difference in the evaporator: | |
| Pinch point temperature difference in the condenser: |
| Working Fluid | With Regeneration | Without Regeneration | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R11 | 1 | 52.48 | 535.47 | 6.06 | 75.15 | 81.21 | 61.7 | 75.4 | 6 | 75.2 | 81.2 | 62.1 | 72.7 |
| 1.5 | 53.03 | 853.14 | 9.22 | 72.62 | 81.84 | 62.1 | 62.7 | 9.09 | 72.73 | 81.82 | 62.6 | 58.5 | |
| 2 | 54.62 | 1078.19 | 11.12 | 71.1 | 82.22 | 62.9 | 48.8 | 10.96 | 71.23 | 82.19 | 63.5 | 44.5 | |
| R113 | 0.5 | 61.32 | 489.33 | 5.57 | 75.54 | 81.11 | 61.7 | 77.4 | 5.34 | 75.73 | 81.07 | 63.2 | 65.2 |
| 1 | 60.52 | 1033.02 | 11.07 | 71.14 | 82.21 | 61.7 | 60.2 | 10.16 | 71.88 | 82.03 | 64.9 | 34 | |
| 1.5 | 61.74 | 1344.24 | 13.63 | 69.1 | 82.73 | 62.6 | 43.6 | - | - | - | - | - | |
| R114 | 1 | 82.88 | 69.34 | 0.81 | 79.35 | 80.16 | 62.3 | 85.5 | 0.81 | 79.35 | 80.16 | 62.5 | 84 |
| 1.5 | 88.87 | 373.25 | 3.99 | 76.81 | 80.8 | 64.3 | 59.3 | 3.86 | 76.91 | 80.77 | 65.6 | 49.4 | |
| R123 | 0.5 | 57.52 | 26.08 | 0.31 | 79.75 | 80.06 | 62 | 89.2 | 0.31 | 79.75 | 80.06 | 62 | 89.1 |
| 1 | 58.12 | 556.97 | 6.14 | 75.09 | 81.23 | 62.6 | 68.1 | 5.94 | 75.24 | 81.19 | 63.7 | 59 | |
| 1.5 | 60.21 | 888.19 | 9.14 | 72.69 | 81.83 | 63.7 | 48.3 | 8.74 | 73.01 | 81.75 | 65.4 | 34.3 | |
| R124 | 2.5 | 98.24 | 303.83 | - | - | - | - | - | 3.07 | 77.54 | 80.61 | 66.7 | 42.6 |
| R141b | 0.5 | 42.19 | 133.55 | 1.57 | 78.74 | 80.31 | 61.9 | 86.6 | 1.56 | 78.75 | 80.31 | 62.1 | 84.9 |
| 1 | 41.54 | 690.42 | 7.7 | 73.84 | 81.54 | 61.6 | 71.5 | 7.49 | 74.01 | 81.5 | 62.6 | 63.7 | |
| 1.5 | 41.84 | 1005.78 | 10.72 | 71.43 | 82.14 | 62 | 58.5 | 10.29 | 71.76 | 82.06 | 63.5 | 46.5 | |
| 2 | 42.98 | 1225.11 | 12.49 | 70.01 | 82.5 | 62.8 | 45.2 | 11.91 | 70.47 | 82.38 | 64.6 | 30.6 | |
| R142b | 1.5 | 54.35 | 74.56 | 0.87 | 79.3 | 80.17 | 62.3 | 85 | 0.87 | 79.3 | 80.17 | 62.3 | 85 |
| 2 | 57.99 | 291.98 | 3.18 | 77.45 | 80.64 | 63.9 | 65.2 | 3.18 | 77.45 | 80.64 | 64 | 65 | |
| 2.5 | 63.52 | 452.81 | 4.54 | 76.37 | 80.91 | 66.4 | 40.4 | 4.57 | 76.35 | 80.91 | 66.2 | 42.2 | |
| R227ea | 2 | 130.31 | 27.49 | 0.32 | 79.75 | 80.06 | 63 | 80.6 | 0.32 | 79.75 | 80.06 | 63.1 | 80 |
| 2.5 | 174.33 | 124.51 | - | - | - | - | - | 1.27 | 78.99 | 80.25 | 67 | 45.1 | |
| R236ea | 1.5 | 74.51 | 297.26 | 3.23 | 77.41 | 80.65 | 64 | 64.4 | 3.15 | 77.48 | 80.63 | 65 | 56.6 |
| 2 | 82.58 | 492.28 | 4.85 | 76.12 | 80.97 | 66.9 | 34.9 | - | - | - | - | - | |
| R236fa | 1.5 | 82.92 | 122.89 | 1.39 | 78.88 | 80.28 | 63.2 | 76.4 | 1.38 | 78.89 | 80.28 | 63.5 | 73.7 |
| 2 | 94.19 | 325.23 | 3.31 | 77.36 | 80.66 | 66.4 | 44.4 | 3.27 | 77.39 | 80.65 | 66.8 | 40.8 | |
| R245ca | 1 | 50.15 | 427.55 | 4.75 | 76.2 | 80.95 | 62.8 | 70.3 | 4.58 | 76.34 | 80.92 | 64 | 60.1 |
| 1.5 | 52.15 | 730.93 | 7.57 | 73.95 | 81.51 | 64.1 | 50 | 7.14 | 74.29 | 81.43 | 66.3 | 32 | |
| 1 | 50.15 | 427.55 | 4.58 | 76.34 | 80.92 | 64 | 60.1 | - | - | - | - | - | |
| 1.5 | 52.15 | 730.93 | 7.14 | 74.29 | 81.43 | 66.3 | 32 | - | - | - | - | - | |
| R245fa | 1 | 55.41 | 177.11 | - | - | - | - | - | 2.01 | 78.39 | 80.4 | 63 | 76.6 |
| 1.5 | 58.76 | 467.91 | 4.95 | 76.04 | 80.99 | 64.3 | 56.5 | 4.81 | 76.16 | 80.96 | 65.4 | 47.6 | |
| R365mfc | 0.5 | 50.12 | 255.39 | 2.94 | 77.65 | 80.59 | 62.2 | 80.2 | 2.85 | 77.72 | 80.57 | 63.3 | 71.7 |
| 1 | 51.07 | 777.39 | 8.17 | 73.47 | 81.63 | 63.4 | 54.3 | - | - | - | - | - | |
| 1.5 | 53.64 | 1095.21 | 10.64 | 71.49 | 82.13 | 65.2 | 30.3 | - | - | - | - | - | |
| RC318 | 1.5 | 121.32 | 78.1 | 0.9 | 79.28 | 80.18 | 63 | 79.4 | 0.89 | 79.29 | 80.18 | 63.4 | 76.2 |
| 2 | 146.08 | 255.89 | 2.55 | 77.96 | 80.51 | 67.3 | 38.7 | 2.49 | 78.01 | 80.5 | 68.3 | 30.7 | |
| R1233zd(E) | 1 | 54.16 | 330.85 | 3.72 | 77.03 | 80.74 | 62.7 | 73.6 | 3.65 | 77.08 | 80.73 | 63.4 | 68.4 |
| 1.5 | 56.95 | 643.6 | 6.69 | 74.65 | 81.34 | 64.3 | 51 | 6.47 | 74.82 | 81.29 | 65.6 | 41.1 | |
| R1234yf | 3 | 145.82 | 57.35 | 0.6 | 79.52 | 80.12 | 66.1 | 55.2 | 0.63 | 79.5 | 80.13 | 64.72 | 66.4 |
| R1234ze(E) | 2.5 | 86.86 | 139.41 | 1.51 | 78.79 | 80.3 | 64.7 | 63.8 | 1.53 | 78.77 | 80.31 | 64.2 | 67.4 |
| 3 | 108.13 | 128.44 | - | - | - | - | - | 1.29 | 78.97 | 80.26 | 67.5 | 40.9 | |
| R1234ze(Z) | - | - | - | - | - | - | - | - | 1.38 | 78.89 | 80.28 | 62.5 | 82.5 |
| 1.5 | 54.64 | 419.06 | 4.49 | 76.41 | 80.9 | 64.1 | 60.1 | 4.44 | 76.45 | 80.89 | 64.5 | 56.9 | |
| - | - | - | - | - | - | - | - | 6.05 | 75.16 | 81.21 | 66.9 | 30.9 | |
| Working Fluid | With Regeneration | Without Regeneration | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pentane | 0.5 | 26.14 | 243.43 | 2.82 | 77.74 | 80.56 | 62 | 82.3 | 2.75 | 77.8 | 80.55 | 62.9 | 75.2 |
| 1 | 26.21 | 806.47 | 8.62 | 73.1 | 81.72 | 62.7 | 59.6 | 7.94 | 73.65 | 81.59 | 65.8 | 34.3 | |
| 1.5 | 27.18 | 1140.32 | 11.34 | 70.93 | 82.27 | 64.1 | 37.6 | - | - | - | - | - | |
| IsoPentane | 0.5 | 28.15 | 78.9 | 0.93 | 79.25 | 80.19 | 62 | 87.2 | 0.92 | 79.26 | 80.18 | 62.3 | 85 |
| 1 | 28.37 | 651.11 | 7.04 | 74.37 | 81.41 | 62.8 | 62.8 | 6.55 | 74.76 | 81.31 | 65.5 | 41.2 | |
| 1.5 | 29.54 | 991.67 | 9.92 | 72.06 | 81.98 | 64.4 | 39.5 | - | - | - | - | - | |
| Cyclopentane | 0.5 | 22.03 | 533.51 | 6.17 | 75.07 | 81.23 | 61 | 81.3 | 6.01 | 75.19 | 81.2 | 61.9 | 74.2 |
| 1 | 21.14 | 1046.23 | 11.7 | 70.64 | 82.34 | 60.2 | 72.2 | 11.03 | 71.18 | 82.21 | 62.3 | 55.3 | |
| 1.5 | 20.84 | 1345.52 | 14.62 | 68.3 | 82.92 | 60 | 64.2 | 13.51 | 69.19 | 82.7 | 62.9 | 40.6 | |
| 2 | 20.98 | 1550.21 | 16.32 | 66.95 | 83.26 | 60.4 | 54.9 | - | - | - | - | - | |
| 2.5 | 21.66 | 1729.53 | 17.42 | 66.07 | 83.48 | 61.3 | 41.5 | - | - | - | - | - | |
| NeoPentane | 1 | 34.23 | 267.2 | 3.02 | 77.59 | 80.6 | 62.8 | 74.8 | 2.91 | 77.67 | 80.58 | 64.1 | 64.7 |
| 1.5 | 36.43 | 615.1 | 6.29 | 74.97 | 81.26 | 65 | 46.4 | — | — | — | — | — | |
| Hexane | 0.5 | 24.03 | 908.19 | 10.04 | 71.97 | 82.01 | 61.1 | 69 | 9.02 | 72.78 | 81.8 | 65 | 37.2 |
| 1 | 23.47 | 1398.71 | 14.65 | 68.28 | 82.93 | 61.2 | 53.5 | - | - | - | - | - | |
| 1.5 | 23.94 | 1715.48 | 16.93 | 66.45 | 83.39 | 62.2 | 35.4 | - | - | - | - | - | |
| Cyclohexane | 0.5 | 22.39 | 1152.51 | 12.27 | 70.18 | 82.45 | 61.5 | 58.2 | 11.2 | 71.04 | 82.24 | 64.9 | 30.3 |
| 1 | 18.65 | 1541.17 | 17.87 | 65.7 | 83.57 | 57.1 | 82.1 | 15.5 | 67.6 | 83.1 | 62.1 | 41.2 | |
| 1.5 | 17.69 | 1738.7 | 20.3 | 63.76 | 84.06 | 56.1 | 84 | 17.09 | 66.33 | 83.42 | 62.2 | 34 | |
| 2 | 17.09 | 1886.55 | 22.05 | 62.36 | 84.41 | 55.5 | 84.2 | - | - | - | - | - | |
| Heptane | 0.5 | 21.7 | 1362.57 | 15.07 | 67.95 | 83.01 | 59.4 | 69.1 | - | - | - | - | - |
| 1 | 20.45 | 1772.89 | 19.19 | 64.65 | 83.84 | 58.5 | 63 | - | - | - | - | - | |
| Nonane | 0.5 | 16.17 | 1780.78 | 22.43 | 62.06 | 84.49 | 53.5 | 103.4 | - | - | - | - | - |
| 1.5 | 10.87 | 1685.07 | 27.58 | 57.93 | 85.52 | 46.9 | 160.2 | 19.04 | 64.77 | 83.81 | 57.4 | 75.1 | |
| 2 | 10.23 | 1672.53 | - | - | - | - | - | 19.44 | 64.45 | 83.89 | 56.5 | 82.7 | |
| Decane | 0.5 | 13.32 | 1195.17 | 18.28 | 65.38 | 83.66 | 50.4 | 146.9 | - | - | - | - | - |
| 1 | 9.03 | 1466.69 | 28.33 | 57.34 | 85.67 | 44.2 | 189.1 | 19.35 | 64.52 | 83.87 | 53.4 | 114.5 | |
| 1.5 | 5.22 | 958.95 | 30.27 | 55.78 | 86.05 | 38.4 | 251.6 | 20.26 | 63.79 | 84.05 | 44.4 | 203 | |
| Benzene | 0.5 | 18.53 | 1055.77 | 12.42 | 70.07 | 82.48 | 58.5 | 85.9 | 11.92 | 70.47 | 82.38 | 59.8 | 74.9 |
| 1 | 16.89 | 1435.87 | 17.25 | 66.2 | 83.45 | 56.3 | 91.4 | 16.12 | 67.1 | 83.22 | 58.5 | 73.4 | |
| 1.5 | 15.8 | 1618.02 | 19.91 | 64.08 | 83.98 | 54.9 | 97.5 | 18.3 | 65.36 | 83.66 | 57.6 | 75.4 | |
| 2 | 15.01 | 1720.53 | 21.64 | 62.69 | 84.33 | 53.8 | 103 | 19.67 | 64.26 | 83.93 | 56.8 | 78.3 | |
| 2.5 | 14.46 | 1788.82 | 22.86 | 61.71 | 84.57 | 53.1 | 107 | 20.63 | 63.5 | 84.13 | 56.3 | 80.6 | |
| 3.5 | 14.26 | 1915.6 | 24.28 | 60.58 | 84.86 | 52.8 | 104.9 | 21.75 | 62.6 | 84.35 | 56.3 | 76.4 | |
| EthylBenzene | 0.5 | 14.93 | 1628.79 | 21.61 | 62.71 | 84.32 | 52.6 | 115.9 | 18.57 | 65.15 | 83.71 | 57.3 | 77.5 |
| 1 | 11.84 | 1664.73 | 25.68 | 59.45 | 85.14 | 48.4 | 148.6 | 21.2 | 63.04 | 84.24 | 53.6 | 106.1 | |
| 1.5 | 9.45 | 1529.72 | 28.07 | 57.54 | 85.61 | 45 | 180.7 | 22.68 | 61.86 | 84.54 | 49.9 | 140.4 | |
| 2 | 7.33 | 1302.39 | 29.71 | 56.24 | 85.94 | 41.7 | 213.8 | 23.67 | 61.06 | 84.73 | 46.1 | 179.1 | |
| 2.5 | 5.25 | 1000.7 | 30.94 | 55.25 | 86.19 | 38.5 | 249.5 | 24.47 | 60.43 | 84.89 | 41.8 | 222.9 | |
| 3 | 2.99 | 601.28 | 31.95 | 54.44 | 86.39 | 34.9 | 291.5 | 25.23 | 59.81 | 85.05 | 36.8 | 276 | |
| Toluene | 0.5 | 16.78 | 1444.9 | 17.81 | 65.75 | 83.56 | 55.5 | 98 | 16.21 | 67.03 | 83.24 | 58.5 | 73.2 |
| 1 | 14.49 | 1671.51 | 22.13 | 62.3 | 84.43 | 52.5 | 115.4 | 19.47 | 64.43 | 83.89 | 56.4 | 83.3 | |
| 1.5 | 12.89 | 1723.94 | 24.53 | 60.38 | 84.91 | 50.3 | 131.7 | 21.15 | 63.08 | 84.23 | 54.5 | 96.9 | |
| 2 | 11.62 | 1709.19 | 26.14 | 59.09 | 85.23 | 48.4 | 146.9 | 22.26 | 62.19 | 84.45 | 52.8 | 111.5 | |
| 2.5 | 10.59 | 1663.63 | 27.28 | 58.17 | 85.46 | 46.9 | 160.6 | 23.06 | 61.55 | 84.61 | 51.2 | 125.9 | |
| 3 | 9.83 | 1610.27 | 28.04 | 57.57 | 85.61 | 45.8 | 171.6 | 23.61 | 61.11 | 84.72 | 49.9 | 138.1 | |
| 3.5 | 9.58 | 1601.61 | 28.43 | 57.26 | 85.69 | 45.4 | 175 | 23.94 | 60.84 | 84.79 | 49.4 | 142.2 | |
| m-Xylene | 0.5 | 14.36 | 1627.59 | 22.04 | 62.37 | 84.41 | 52 | 120.6 | 19.02 | 64.78 | 83.8 | 56.5 | 84.2 |
| 1 | 11.35 | 1664.42 | 26.29 | 58.97 | 85.26 | 47.8 | 153.3 | 21.92 | 62.47 | 84.38 | 52.6 | 114.1 | |
| 1.5 | 9.07 | 1501.43 | 28.33 | 57.33 | 85.67 | 44.5 | 185.4 | 23.09 | 61.53 | 84.62 | 49.1 | 148 | |
| 2 | 7.05 | 1275.27 | 29.91 | 56.08 | 85.98 | 41.4 | 217.5 | 24.08 | 60.74 | 84.82 | 45.3 | 185.5 | |
| 2.5 | 5.04 | 973.81 | 31.09 | 55.12 | 86.22 | 38.2 | 252.7 | 24.88 | 60.1 | 84.98 | 41.2 | 228.4 | |
| 3 | 2.73 | 555.44 | 32.07 | 54.35 | 86.41 | 34.5 | 296.2 | 25.66 | 59.47 | 85.13 | 36.1 | 282.7 | |
| o-Xylene | 0.5 | 13.59 | 1639.73 | 22.73 | 61.81 | 84.55 | 51.3 | 125.9 | 19.46 | 64.44 | 83.89 | 55.9 | 88.2 |
| 1 | 10.38 | 1602.08 | 26.81 | 58.55 | 85.36 | 46.7 | 164.4 | 22.1 | 62.32 | 84.42 | 51.6 | 124.8 | |
| 1.5 | 7.87 | 1390.83 | 29.15 | 56.68 | 85.83 | 42.9 | 201.8 | 23.55 | 61.16 | 84.71 | 47.2 | 166.5 | |
| 2 | 5.59 | 1082.58 | 30.8 | 55.36 | 86.16 | 39.3 | 240.8 | 24.57 | 60.34 | 84.91 | 42.7 | 213.1 | |
| 2.5 | 3.24 | 672.57 | 32.05 | 54.36 | 86.41 | 35.4 | 284.8 | 25.4 | 59.68 | 85.08 | 37.5 | 267.7 | |
| p-Xylene | 0.5 | 14.64 | 1630.87 | 21.89 | 62.49 | 84.38 | 52.2 | 118.6 | 18.89 | 64.89 | 83.78 | 56.7 | 81.9 |
| 1 | 11.59 | 1654.09 | 25.92 | 59.26 | 85.18 | 48 | 151.8 | 21.54 | 62.77 | 84.31 | 53 | 111.5 | |
| 1.5 | 9.27 | 1514.85 | 28.25 | 57.4 | 85.65 | 44.7 | 183.4 | 23.01 | 61.59 | 84.6 | 49.4 | 145.5 | |
| 2 | 7.23 | 1291.12 | 29.81 | 56.15 | 85.96 | 41.6 | 215.4 | 23.99 | 60.81 | 84.8 | 45.6 | 182.8 | |
| 2.5 | 5.24 | 996.83 | 30.94 | 55.25 | 86.19 | 38.5 | 249.9 | 24.76 | 60.2 | 84.95 | 41.5 | 224.9 | |
| 3 | 3 | 599.43 | 31.86 | 54.51 | 86.37 | 34.9 | 291.5 | 25.51 | 59.59 | 85.1 | 36.6 | 277 | |
| Working Fluid | With Regeneration | Without Regeneration | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D5 | 0.5 | 20.22 | 1429.29 | 24.51 | 60.39 | 84.9 | 46.8 | 168.8 | - | - | - | - | - |
| D6 | 0.5 | 5 | 447.79 | 28.32 | 57.35 | 85.66 | 34.3 | 300.8 | 14.66 | 68.27 | 82.93 | 39.9 | 255.1 |
| MD2M | 1 | 14.41 | 1319.54 | 27 | 58.4 | 85.4 | 43.6 | 198.2 | 15 | 68 | 83 | 58.6 | 76.8 |
| MD3M | 0.5 | 12.49 | 1108.29 | 27.02 | 58.38 | 85.4 | 41.4 | 222.6 | 14.53 | 68.38 | 82.91 | 54.9 | 113 |
| MM | 0.5 | 36.77 | 1447.47 | 14.83 | 68.13 | 82.97 | 61.8 | 46.9 | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Douvartzides, S.L.; Tsiolikas, A.; Charisiou, N.D.; Souliotis, M.; Karayannis, V.; Taousanidis, N. Energy and Exergy-Based Screening of Various Refrigerants, Hydrocarbons and Siloxanes for the Optimization of Biomass Boiler–Organic Rankine Cycle (BB–ORC) Heat and Power Cogeneration Plants. Energies 2022, 15, 5513. https://doi.org/10.3390/en15155513
Douvartzides SL, Tsiolikas A, Charisiou ND, Souliotis M, Karayannis V, Taousanidis N. Energy and Exergy-Based Screening of Various Refrigerants, Hydrocarbons and Siloxanes for the Optimization of Biomass Boiler–Organic Rankine Cycle (BB–ORC) Heat and Power Cogeneration Plants. Energies. 2022; 15(15):5513. https://doi.org/10.3390/en15155513
Chicago/Turabian StyleDouvartzides, Savvas L., Aristidis Tsiolikas, Nikolaos D. Charisiou, Manolis Souliotis, Vayos Karayannis, and Nikolaos Taousanidis. 2022. "Energy and Exergy-Based Screening of Various Refrigerants, Hydrocarbons and Siloxanes for the Optimization of Biomass Boiler–Organic Rankine Cycle (BB–ORC) Heat and Power Cogeneration Plants" Energies 15, no. 15: 5513. https://doi.org/10.3390/en15155513
APA StyleDouvartzides, S. L., Tsiolikas, A., Charisiou, N. D., Souliotis, M., Karayannis, V., & Taousanidis, N. (2022). Energy and Exergy-Based Screening of Various Refrigerants, Hydrocarbons and Siloxanes for the Optimization of Biomass Boiler–Organic Rankine Cycle (BB–ORC) Heat and Power Cogeneration Plants. Energies, 15(15), 5513. https://doi.org/10.3390/en15155513










