Mineral-Supported Photocatalysts: A Review of Materials, Mechanisms and Environmental Applications
Abstract
:1. Introduction
| Photocatalyst | Synthesis Method | Organic Pollutant | Light Source | Quantum Efficiency (%) | Refs. |
|---|---|---|---|---|---|
| Chrysotile/SnO2 | A direct precipitation process coupled with calcination treatment | MB | 250 W 450 nm mercury lamp | 99 | [26] |
| Chrysotile @ZnO | Precipitation | MB | 250 W 365 nm mercury lamp | 99.50 | [27] |
| TiO2/micro-meso porous silica nanofibers | Sol-adhesion | RhB | 25 W 254 nm ultraviolet light | 95 | [28] |
| Exfoliated kaolinite nanolayers | Intercalation and delamination | RhB | 150 W 254 nm mercury lamp | over 95 | [29] |
| g-C3N4/TiO2/kaolinite | A mild sol-gel method associated with chemical stripping and self-assembly | CIP and S. aureus | 500 W over 400 nm xenon lamp and 8 W fluorescent lamp | 92 | [5] |
| N-doped TiO2/kaolinite | A modified two-step sol-gel method | CR | 8 W lamp | 99 | [30] |
| Ti-Fe kaolinite composite | A simple precipitation method | Cr(VI) | 300 W over 420 nm dysprosium lamp | 87 at PH = 3.0 | [31] |
| Ag/g-C3N4/kaolinite | A two-step assembly strategy by employing in situ impregnation-calcination and photo-deposition process | IBP | 500 W over 400 nm xenon lamp | 99.90 | [32] |
| Kaolinite/TiO2 | Sol-gel method | MB | 8 W 254 nm high-intensity UV-C radiation lamp | 97 | [33] |
| Mica/TiO2/Fe2O3 | A sol-gel assisted hydrothermal method | G-MeCHO | 400 W xenon lamp | 80 | [34] |
| MoS2/mica | Hydrothermal | TH | 300 W xenon lamp | 90.60 | [35] |
| Ti-Fe-based alkaline muscovite | Impregnation | TBBPA | 150 W xenon lamp | 90 | [36] |
| TiO2/illite | Hydrothermal | MO | 30 W UV light source | 73.4 | [37] |
| KNbO3/vermiculite | In situ hydrothermal method | MB | 300 W xenon lamp | 81 | [38] |
| AgI-Bi2MoP6/vermiculite | Sol-gel and precipitation methods | MG | 65 W lamp | 98.89 | [39] |
| BiOBr/magnetic bentonite | In situ coprecipitation followed by microwave-assisted hydrothermal method | TC and CIP | 500 W over 420 nm xenon lamp | 85 and 95 for TC and CIP | [10] |
| Ag/AgCl/montmorillonite | Dispersion method | MB | 100 W over 400 nm tungsten lamp | 98 | [40] |
| Calcite/TiO2 | Sol-gel method | TC | 24 W 254 nm UV-lamp | 90 | [41] |
| ZnO-halloysite | Ultrasonic treatment | CIP | 160 W mercury lamp | 91 | [42] |
| Fe(III)/montmorillonite | Simple adsorption | Cr(VI) | 300 W xenon lamp | 100 | [43] |
| BiOBr/Ti3C2/exfoliated montmorillonite | In situ co-precipitation coupling with microwave hydrothermal | CIP | 500 W over 400 nm xenon lamp | 96 | [44] |
| γ-Fe2O3/montmorillonite | Hydrothermal | RhB | 40 W xenon lamp | 99 | [45] |
| CdS/MoS2/montmorillonite | Direct coagulation casting | TC | 50 W LED lamp | 90.03 | [46] |
| Coal-bearing strata aolinite/MnFe2O4 | Hydrothermal | CTC•HCl | 300 W over 420 nm xenon lamp | 85.1 | [47] |
| Cu2O/ZnO/kaolinite | Co-precipitation | MB | 150 W halogen lamp | 93 | [48] |
| ZnO-bentonite | Facile synthesis | MB and eriochrome black-T | Solar radiation | Over 95 | [49] |
| ZnO/γ-Fe2O3/bentonite | Facile co-precipitation | CIP | Solar simulator | 95 | [50] |
2. Photocatalyst Modification Methods
2.1. Ion Doping
2.2. Noble-Metal Deposition
2.3. Heterojunction Design
2.4. Support Material Combination
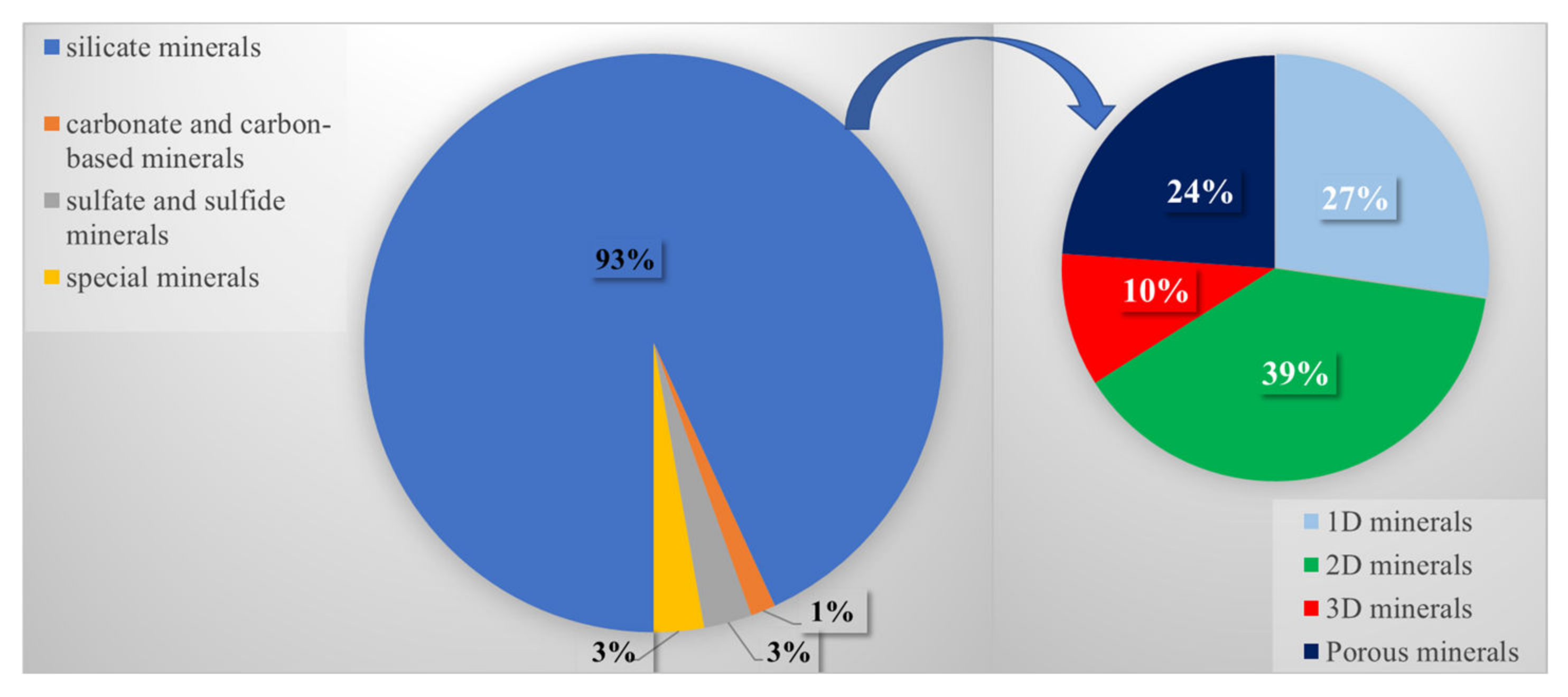
3. Natural Silicate Mineral-Supported Photocatalyst
3.1. 1D Mineral-Supported Photocatalyst
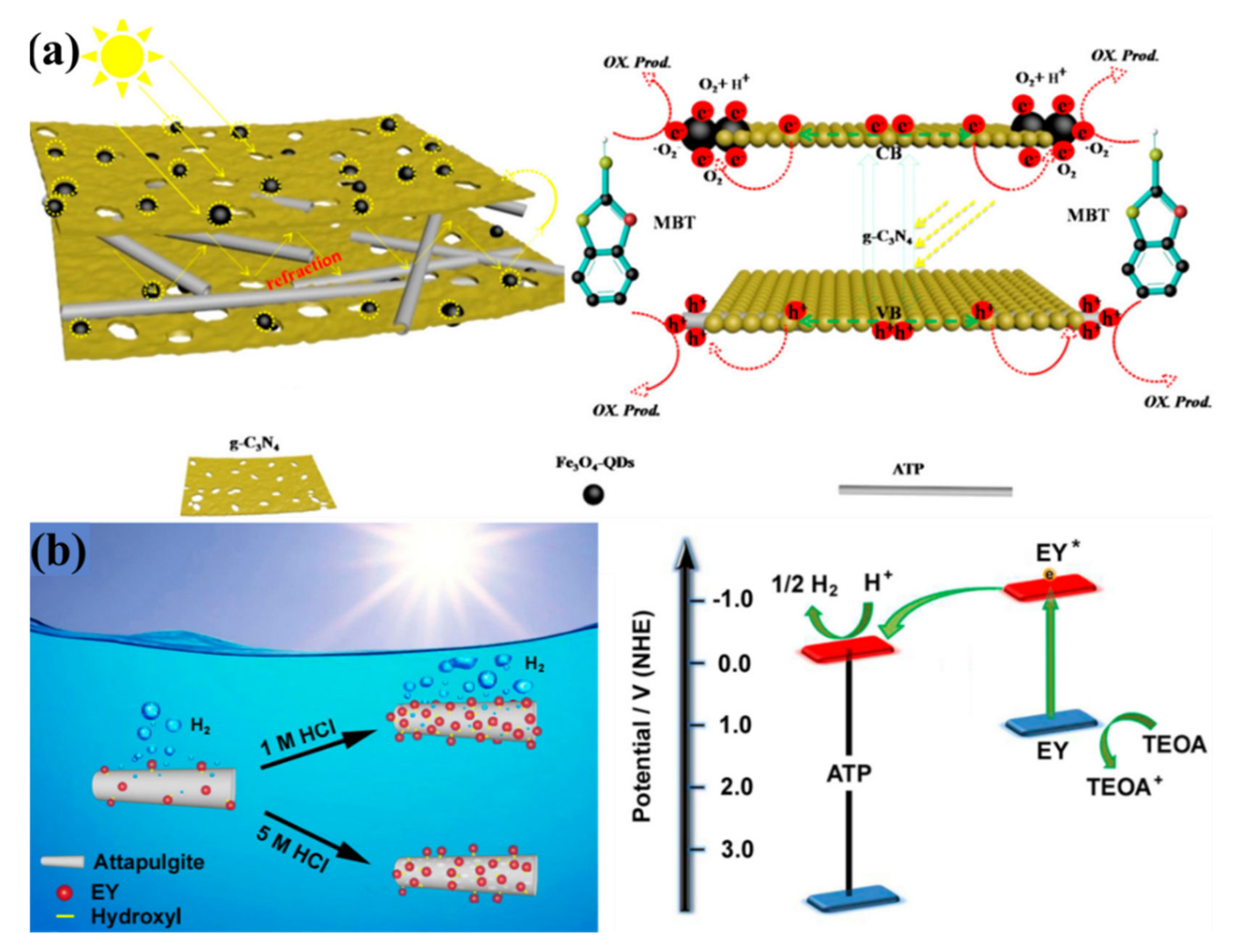
3.1.1. Attapulgite-Supported Photocatalyst
3.1.2. Palygorskite-Supported Photocatalyst
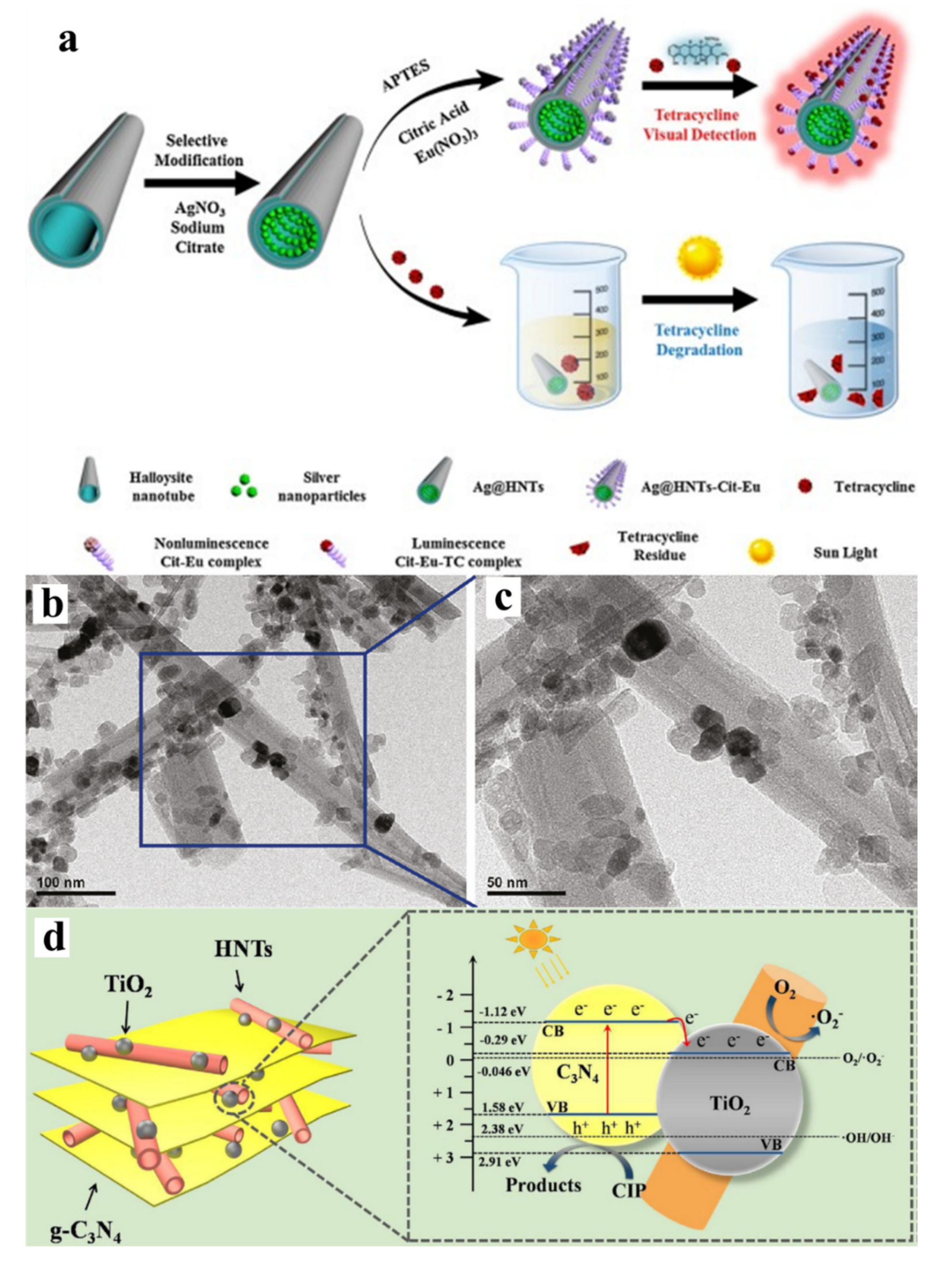
3.1.3. Halloysite-Supported Photocatalyst
3.1.4. Sepiolite-Supported Photocatalyst
3.1.5. Imogolite-Supported Photocatalyst
3.1.6. Wollastonite-Supported Photocatalyst
3.1.7. Basalt-Supported Photocatalyst
PbTiO3: Easy electron transfer and water adsorption
Reduction: CO2 + e− → ∙CO2−
Oxidation: H2O + h+ → ∙OH + H+ → H+ + O2
CH4 production: H+ + e− → ∙H
CO2− + ∙H → ∙CH → ∙CH2 → ∙CH3 → CH4
3.1.8. Tourmaline-Supported Photocatalyst
3.1.9. Chrysotile-Supported Photocatalyst
3.1.10. Talc-Supported Photocatalyst
3.2. 2D Mineral-Supported Photocatalyst
3.2.1. Smectite-Supported Photocatalyst
Bentonite-Supported Photocatalyst
Montmorillonite-Supported Photocatalyst
Nontronite Catalysis
Rectorite-Supported Photocatalyst
Saponite-Supported Photocatalyst
3.2.2. Kaolinite-Supported Photocatalyst
3.2.3. Dickite-Supported Photocatalyst
3.2.4. Mica- and Muscovite-Supported Photocatalysts
3.2.5. Illite-Supported Photocatalyst
3.2.6. Vermiculite-Supported Photocatalyst
3.3. 3D Mineral-Supported Photocatalysts
3.3.1. Allophane-Supported Photocatalyst
3.3.2. Pyrophyllite-Supported Photocatalyst
3.3.3. Perlite-Supported Photocatalyst
3.3.4. Quartz-Supported Photocatalyst
3.3.5. Bauxite-Supported Photocatalyst
3.4. Porous Mineral-Supported Photocatalysts
3.4.1. Diatomite-Supported Photocatalyst
3.4.2. Zeolite-Supported Photocatalyst
3.4.3. Pumice-Supported Photocatalyst
4. Natural Carbonate or Carbon-Based Mineral-Supported Photocatalysts
4.1. Calcite-Supported Photocatalyst
4.2. Other Carbon Material-Supported Photocatalyst
5. Natural Sulfate or Sulfide Mineral—Supported Photocatalyst
5.1. Gypsum-Supported Photocatalyst
5.2. Pyrite-Supported Photocatalyst
6. Natural Special Mineral-Based Photocatalysts
6.1. Ilmenite-Based Photocatalyst
6.2. Monazite-Supported Photocatalyst
7. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| Abbreviation | Name |
| OH | Hydroxide radicals |
| 2,6-DCP | 2,6-dichlorophenol |
| 4-NP | 4-nitrophenol |
| AS | Acidized sepiolite |
| ATP | Attapulgite |
| BF | Basalt fiber |
| BPA | Bisphenol A |
| BPB | Bromophenol blue |
| CB | Conduction band |
| CIP | Ciprofloxacin |
| Cit-Eu | Citrate–europium |
| CR | Congo red |
| CTC•HCl | Chlortetracycline hydrochloride |
| DET | Density functional theory |
| DRM | Dry reforming of methane |
| e− | Electron |
| E. coli | Escherichia coli |
| EDS | Energy dispersive spectrometer |
| ESR | Electron spin resonance spectroscopy |
| EY | Eosin Y dye |
| FESEM | Field emission scanning electron microscope |
| G-MeCHO | Gaseous acetaldehyde |
| h+ | Hole |
| HCHO | Formaldehyde |
| HNTs | Halloysite nanotubes |
| IBP | Ibuprofen |
| IUGS | International Union of Geological Sciences |
| MB | Methylene blue |
| MBT | 2-Mercaptobenzothiazole |
| MG | Malachite green |
| MMT | Montmorillonite |
| MO | Methyl orange |
| O2−. | Superoxide radical |
| OTC | Oxytetracycline |
| P25 | Degussa P25 TiO2 |
| PEG6000 | Polyethylene glycol 6000 |
| PMS | Peroxymonosulfate |
| PNA | Poly-naphthylamine |
| POPD | Poly-o-phenylenediamine |
| PVP | Polyvinyl pyrrolidone |
| RhB | Rhodamine B |
| S. aureus | Staphylococcus aureus |
| SDS | Sodium dodecyl sulfate |
| SMX | Sulfamethoxazole |
| TBBPA | Tetrabromobisphenol A |
| TC | Tetracycline |
| TCS | Triclosan |
| TH | Tetracycline hydrochloride |
| TMP | Trimethoprim |
| UV-vis DRS | UV-vis diffuse reflectance spectrophotometry |
| VB | Valence band |
| XPS | X-ray photoelectron spectroscopy |
References
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, K.; Zhao, Y.; Zhu, S.; Yuan, X.; Huo, M.; Zhang, Y.; Qiu, Y. Bi2WO6/TiO2/Pt nanojunction system: A UV–vis light responsive photocatalyst with high photocatalytic performance. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 252–260. [Google Scholar] [CrossRef]
- Rimoldi, L.; Meroni, D.; Cappelletti, G.; Ardizzone, S. Green and low cost tetracycline degradation processes by nanometric and immobilized TiO2 systems. Catal. Today 2017, 281, 38–44. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, Z.; Duan, Y.; Ma, R.; Zheng, S. Synthesis of nano-TiO2/diatomite composite and its photocatalytic degradation of gaseous formaldehyde. Appl. Surf. Sci. 2017, 412, 105–112. [Google Scholar] [CrossRef]
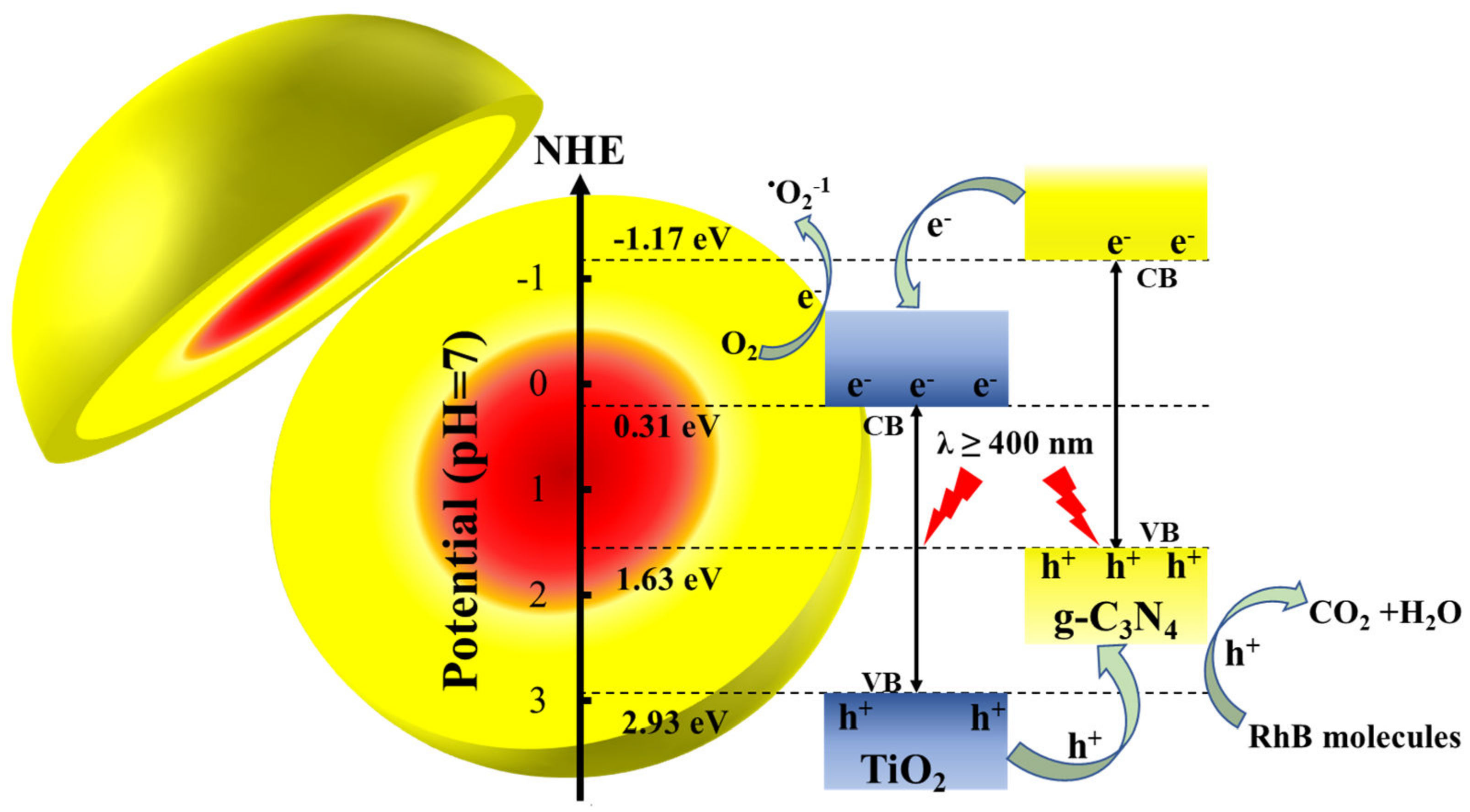
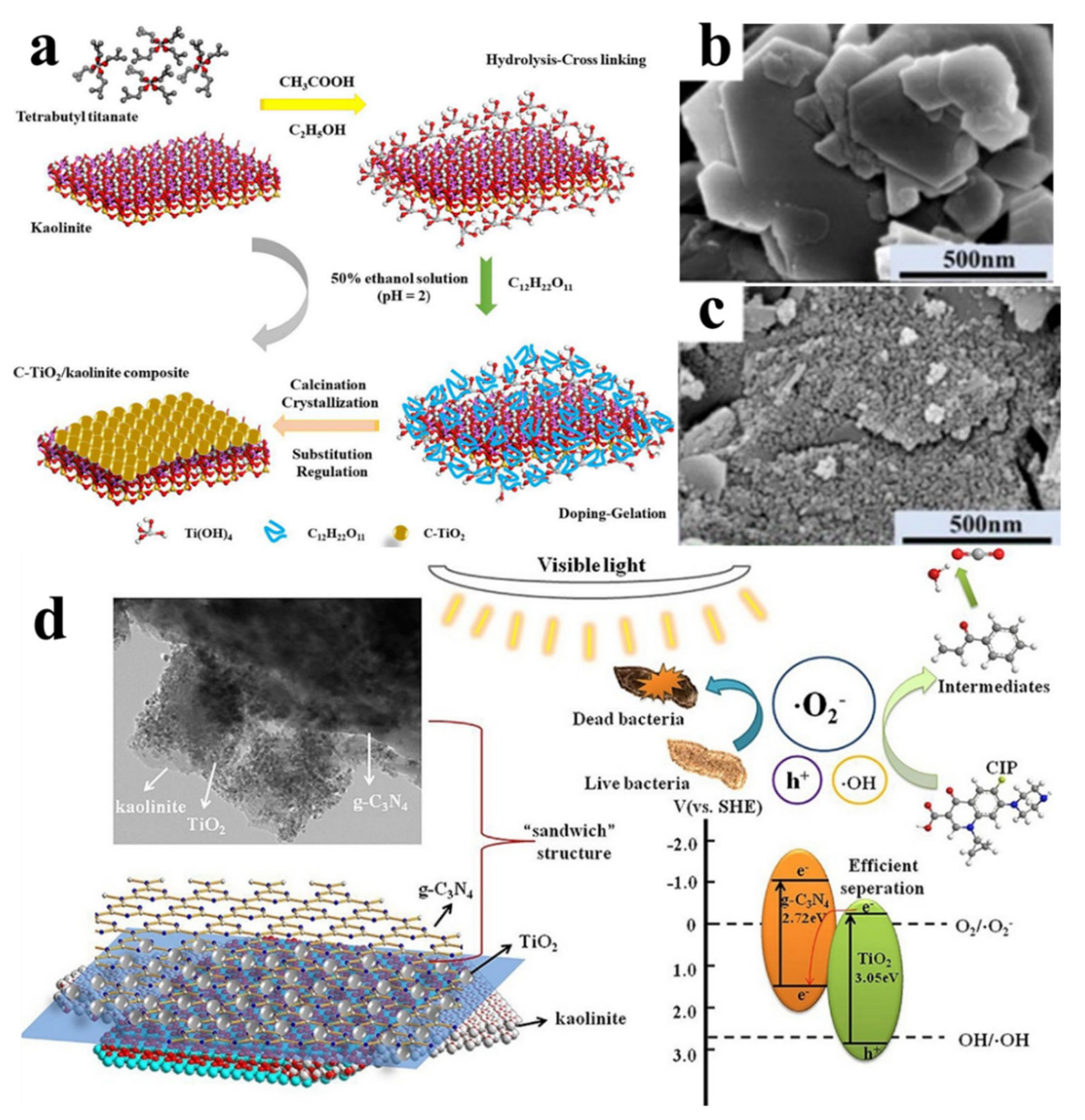
- Li, C.; Sun, Z.; Zhang, W.; Yu, C.; Zheng, S. Highly efficient g-C3N4/TiO2/kaolinite composite with novel three-dimensional structure and enhanced visible light responding ability towards ciprofloxacin and S. aureus. Appl. Catal. B Environ. 2018, 220, 272–282. [Google Scholar] [CrossRef]
- Fu, L.; Guo, Y.; Pan, S.; Huang, J.; Wang, L. Core-shell type Tourmaline@ZnO composites equipped with carbon dots for high efficiency photocatalyst. Surf. Coat. Technol. 2019, 359, 190–196. [Google Scholar] [CrossRef]
- Nada, A.A.; Orimolade, B.O.; El-Maghrabi, H.H.; Koiki, B.A.; Rivallin, M.; Bekheet, M.F.; Viter, R.; Damberga, D.; Lesage, G.; Iatsunskyi, I.; et al. Photoelectrocatalysis of paracetamol on Pd–ZnO/N-doped carbon nanofibers electrode. Appl. Mater. Today 2021, 24, 101129. [Google Scholar] [CrossRef]
- Bu, X.; Wu, B.; Long, T.; Hu, M. Preparation, characterization and enhancement of the visible-light photocatalytic activity of In2O3/rectorite composite. J. Alloys Compd. 2014, 586, 202–207. [Google Scholar] [CrossRef]
- Li, P.; Huang, L.; Li, Y.; Xu, Y.; Huang, S.; Yuan, D.; Xu, H.; Li, H. Synthesis of dark orange montmorillonite/g-C3N4 composites and their applications in the environment. J. Phys. Chem. Solids 2017, 107, 131–139. [Google Scholar] [CrossRef]
- Liu, K.; Tong, Z.; Muhammad, Y.; Huang, G.; Zhang, H.; Wang, Z.; Zhu, Y.; Tang, R. Synthesis of sodium dodecyl sulfate modified BiOBr/magnetic bentonite photocatalyst with Three-dimensional parterre like structure for the enhanced photodegradation of tetracycline and ciprofloxacin. Chem. Eng. J. 2020, 388, 124374. [Google Scholar] [CrossRef]
- Xu, H.-Q.; Yang, S.; Ma, X.; Huang, J.; Jiang, H.-L. Unveiling Charge-Separation Dynamics in CdS/Metal–Organic Framework Composites for Enhanced Photocatalysis. ACS Catal. 2018, 8, 11615–11621. [Google Scholar] [CrossRef]
- Fatimah, I.; Nurillahi, R.; Sahroni, I.; Muraza, O. TiO2-pillared saponite and photosensitization using a ruthenium complex for photocatalytic enhancement of the photodegradation of bromophenol blue. Appl. Clay Sci. 2019, 183, 105302. [Google Scholar] [CrossRef]
- Kumar, S.G.; Devi, L.G. Review on modified TiO2 photocatalysis under UV/visible light: Selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef]
- Wenderich, K.; Mul, G. Methods, mechanism, and applications of photodeposition in photocatalysis: A review. Chem. Rev. 2016, 116, 14587–14619. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Pan, Y.; Liang, J.; Zeng, G.; Wu, Z.; Wang, H. Doping of graphitic carbon nitride for photocatalysis: A reveiw. Appl. Catal. B Environ. 2017, 217, 388–406. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, G. Recent advances in synthesis and applications of clay-based photocatalysts: A review. Phys. Chem. Chem. Phys. 2014, 16, 8178–8192. [Google Scholar] [CrossRef]
- Szczepanik, B. Photocatalytic degradation of organic contaminants over clay-TiO2 nanocomposites: A review. Appl. Clay Sci. 2017, 141, 227–239. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S. Clay supported TiO2 nanoparticles for photocatalytic degradation of environmental pollutants: A review. J. Environ. Chem. Eng. 2018, 6, 6088–6107. [Google Scholar] [CrossRef]
- Serwicka, E.M. Titania-Clay Mineral Composites for Environmental Catalysis and Photocatalysis. Catalysts 2021, 11, 1087. [Google Scholar] [CrossRef]
- Papoulis, D. Halloysite based nanocomposites and photocatalysis: A Review. Appl. Clay Sci. 2019, 168, 164–174. [Google Scholar] [CrossRef]
- Li, C.; Zhu, N.; Yang, S.; He, X.; Zheng, S.; Sun, Z.; Dionysiou, D.D. A review of clay based photocatalysts: Role of phyllosilicate mineral in interfacial assembly, microstructure control and performance regulation. Chemosphere 2021, 273, 129723. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, L.; Cheng, H. Rational design of kaolinite-based photocatalytic materials for environment decontamination. Appl. Clay Sci. 2021, 208, 106098. [Google Scholar] [CrossRef]
- Zou, Y.; Hu, Y.; Shen, Z.; Yao, L.; Tang, D.; Zhang, S.; Wang, S.; Hu, B.; Zhao, G.; Wang, X. Application of aluminosilicate clay mineral-based composites in photocatalysis. J. Environ. Sci. 2022, 115, 190–214. [Google Scholar] [CrossRef]
- Mustapha, S.; Ndamitso, M.M.; Abdulkareem, A.S.; Tijani, J.O.; Shuaib, D.T.; Ajala, A.O.; Mohammed, A.K. Application of TiO2 and ZnO nanoparticles immobilized on clay in wastewater treatment: A review. Appl. Water Sci. 2020, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Tang, R.; Xiong, S.; Zheng, J.; Li, L.; Zhou, Z.; Gong, D.; Deng, Y.; Su, L.; Liao, C. Application of natural minerals in photocatalytic degradation of organic pollutants: A review. Sci. Total Environ. 2022, 812, 152434. [Google Scholar] [CrossRef]
- Luo, Q.; Peng, H.; Tian, X.; Guo, J. Facile synthesis and characterization of Chrysotile/SnO2 nanocomposite for enhanced photocatalytic properties. Appl. Organometal Chem. 2020, 34, e5356. [Google Scholar] [CrossRef]
- Liu, Q.; Peng, H.; Tian, X.; Guo, J. Synthesis of chrysotile based nanocomposites for tuning band gap and photocatalytic property. Appl. Clay Sci. 2020, 199, 105885. [Google Scholar] [CrossRef]
- Tang, X.; Feng, Q.; Liu, K.; Luo, X.; Huang, J.; Li, Z. A simple and innovative route to remarkably enhance the photocatalytic performance of TiO2: Using micro-meso porous silica nanofibers as carrier to support highly-dispersed TiO2 nanoparticles. Microporous Mesoporous Mater. 2018, 258, 251–261. [Google Scholar] [CrossRef]
- Shawky, A.; El-Sheikh, S.M.; Rashed, M.N.; Abdo, S.M.; El-Dosoqy, T.I. Exfoliated kaolinite nanolayers as an alternative photocatalyst with superb activity. J. Environ. Chem. Eng. 2019, 7, 103174. [Google Scholar] [CrossRef]
- Sia, T.H.; Dai, S.; Jin, B.; Biggs, M.; Chong, M.N. Hybridising nitrogen doped titania with kaolinite: A feasible catalyst for a semi-continuous photo-degradation reactor system. Chem. Eng. J. 2015, 279, 939–947. [Google Scholar] [CrossRef] [Green Version]
- Fida, H.; Guo, S.; Zhang, G. Preparation and characterization of bifunctional Ti–Fe kaolinite composite for Cr(VI) removal. J. Colloid Interface Sci. 2015, 442, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, X.; Dong, X.; Liu, X.; Tan, Y.; Yuan, F.; Zheng, S.; Li, C. Hierarchical assembly of highly efficient visible-light-driven Ag/g-C3N4/kaolinite composite photocatalyst for the degradation of ibuprofen. J. Mater. 2020, 6, 582–592. [Google Scholar] [CrossRef]
- De Oliveira, C.R.S.; Batistella, M.A.; de Souza, U.; Augusto, A.; de Arruda Guelli, S.M. Synthesis of superacid sulfated TiO2 prepared by sol-gel method and its use as a titania precursor in obtaining a kaolinite/TiO2 nano-hybrid composite. Powder Technol. 2021, 381, 366–380. [Google Scholar] [CrossRef]
- Fang, X.; Lu, G.; Mahmood, A.; Tang, Z.; Liu, Z.; Zhang, L.; Wang, Y.; Sun, J. A novel ternary Mica/TiO2/Fe2O3 composite pearlescent pigment for the photocatalytic degradation of acetaldehyde. J. Photochem. Photobiol. A Chem. 2020, 400, 112617. [Google Scholar] [CrossRef]
- Zuo, S.; Cao, X.; Wang, P.; Li, X.; Liu, W.; Xu, R.; Yao, C.; Fu, Y.; Liu, X. Synthesis of ultrathin-layered MoS2/conductive mica composites with enhanced visible photocatalytic activity. Mater. Sci. Semicond. Process. 2021, 121, 105457. [Google Scholar] [CrossRef]
- Touaa, N.D.; Bouberka, Z.; Gherdaoui, C.E.; Supiot, P.; Roussel, P.; Pierlot, C.; Maschke, U. Titanium and iron-modified delaminated muscovite as photocatalyst for enhanced degradation of Tetrabromobisphenol A by visible light. Funct. Mater. Lett. 2020, 13, 2051008. [Google Scholar] [CrossRef]
- Dong, Z.; Ling, M.; Jiang, Y.; Han, M.; Ren, G.; Zhang, J.; Ren, X.; Li, F.; Xue, B. Preparation and properties of TiO2/illite composites synthesized at different hydrothermal pH values. Chem. Phys. 2019, 525, 110394. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, X.; Tian, W.; Lei, D.; Lei, X. Three-dimensional potassium niobate nanoarray on vermiculite for high-performance photocatalyst fabricated by an in situ hydrothermal process. RSC Adv. 2016, 6, 58401–58408. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, R.; Duan, M.; Chen, Y.; Hu, M.; Luo, X.; Teng, H. Efficient Adsorption and Photocatalytic Degradation of Dyes by AgI-Bi2MoO6/Vermiculite Composite under Visible Light. ChemistrySelect 2019, 4, 12022–12031. [Google Scholar] [CrossRef]
- Sohrabnezhad, S.; Zanjanchi, M.A.; Razavi, M. Plasmon-assisted degradation of methylene blue with Ag/AgCl/montmorillonite nanocomposite under visible light. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 130, 129–135. [Google Scholar] [CrossRef]
- Belhouchet, N.; Hamdi, B.; Chenchouni, H.; Bessekhouad, Y. Photocatalytic degradation of tetracycline antibiotic using new calcite/titania nanocomposites. J. Photochem. Photobiol. A Chem. 2019, 372, 196–205. [Google Scholar] [CrossRef]
- Freitas, W.A.; Soares, B.E.C.F.; Rodrigues, M.S.; Trigueiro, P.; Honorio, L.M.C.; Peña-Garcia, R.; Alcântara, A.C.S.; Silva-Filho, E.C.; Fonseca, M.G.; Furtini, M.B.; et al. Facile synthesis of ZnO-clay minerals composites using an ultrasonic approach for photocatalytic performance. J. Photochem. Photobiol. A Chem. 2022, 429, 113934. [Google Scholar] [CrossRef]
- Zhang, L.; Chuaicham, C.; Balakumar, V.; Sasaki, K. Effect of ionic Fe(III) doping on montmorillonite for photocatalytic reduction of Cr(VI) in wastewater. J. Photochem. Photobiol. A Chem. 2022, 429, 113909. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, H.; Fu, T.; Wang, L.; Tang, R.; Tong, Z.; Huang, X. Construction of BiOBr/Ti3C2/exfoliated montmorillonite Schottky junction: New insights into exfoliated montmorillonite for inducing MXene oxygen functionalization and enhancing photocatalytic activity. Chem. Eng. J. 2022, 438, 135609. [Google Scholar] [CrossRef]
- Fatimah, I.; Purwiandono, G.; Hidayat, A.; Sagadevan, S.; Kamari, A. Mechanistic insight into the adsorption and photocatalytic activity of a magnetically separable γ-Fe2O3/Montmorillonite nanocomposite for rhodamine B removal. Chem. Phys. Lett. 2022, 792, 139410. [Google Scholar] [CrossRef]
- Yue, Y.; Shen, S.; Cheng, W.; Han, G.; Wu, Q.; Jiang, J. Construction of mechanically robust and recyclable photocatalytic hydrogel based on nanocellulose-supported CdS/MoS2/Montmorillonite hybrid for antibiotic degradation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 636, 128035. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, Z.; Chen, Y.; Jia, Y.; Wang, Q.; Cheng, H. Heterostructure coal-bearing strata kaolinite/MnFe2O4 composite for activation of peroxydisulfate to efficiently degrade chlortetracycline hydrochloride. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128789. [Google Scholar] [CrossRef]
- Fufa, P.A.; Feysia, G.B.; Gultom, N.S.; Kuo, D.-H.; Chen, X.; Kabtamu, D.M.; Zelekew, O.A. Visible light-driven photocatalytic activity of Cu2O/ZnO/Kaolinite-based composite catalyst for the degradation of organic pollutant. Nanotechnology 2022, 33, 315601. [Google Scholar] [CrossRef]
- Golmohammadi, M.; Honarmand, M.; Esmaeili, A. Biosynthesis of ZnO nanoparticles supported on bentonite and the evaluation of its photocatalytic activity. Mater. Res. Bull. 2022, 149, 111714. [Google Scholar] [CrossRef]
- Kamali, M.; Xue, Y.; Khalaj, M.; Laats, B.; Teunckens, R.; Verbist, M.; Costa, M.E.V.; Capela, I.; Appels, L.; Dewil, R. ZnO/γ-Fe2O3/Bentonite: An Efficient Solar-Light Active Magnetic Photocatalyst for the Degradation of Pharmaceutical Active Compounds. Molecules 2022, 27, 3050. [Google Scholar] [CrossRef]
- El Gaidoumi, A.; Doña Rodríguez, J.M.; Pulido Melián, E.; González-Díaz, O.M.; Navío Santos, J.A.; El Bali, B.; Kherbeche, A. Synthesis of sol-gel pyrophyllite/TiO2 heterostructures: Effect of calcination temperature and methanol washing on photocatalytic activity. Surf. Interfaces 2019, 14, 19–25. [Google Scholar] [CrossRef]
- Hao, R.; Wang, G.; Tang, H.; Sun, L.; Xu, C.; Han, D. Template-free preparation of macro/mesoporous g-C3N4/TiO2 heterojunction photocatalysts with enhanced visible light photocatalytic activity. Appl. Catal. B Environ. 2016, 187, 47–58. [Google Scholar] [CrossRef]
- Shayegan, Z.; Lee, C.-S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase—A review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef] [Green Version]
- Nada, A.A.; Bekheet, M.F.; Viter, R.; Miele, P.; Roualdes, S.; Bechelany, M. BN/GdxTi(1-x)O(4-x)/2 nanofibers for enhanced photocatalytic hydrogen production under visible light. Appl. Catal. B Environ. 2019, 251, 76–86. [Google Scholar] [CrossRef]
- Nada, A.A.; Bekheet, M.F.; Roualdes, S.; Gurlo, A.; Ayral, A. Functionalization of MCM-41 with titanium oxynitride deposited via PECVD for enhanced removal of methylene blue. J. Mol. Liq. 2019, 274, 505–515. [Google Scholar] [CrossRef]
- Nada, A.A.; El Rouby, W.M.A.; Bekheet, M.F.; Antuch, M.; Weber, M.; Miele, P.; Viter, R.; Roualdes, S.; Millet, P.; Bechelany, M. Highly textured boron/nitrogen co-doped TiO2 with honeycomb structure showing enhanced visible-light photoelectrocatalytic activity. Appl. Surf. Sci. 2020, 505, 144419. [Google Scholar] [CrossRef]
- Jiang, P.; Xiang, W.; Kuang, J.; Liu, W.; Cao, W. Effect of cobalt doping on the electronic, optical and photocatalytic properties of TiO2. Solid State Sci. 2015, 46, 27–32. [Google Scholar] [CrossRef]
- Jia, T.; Fu, F.; Yu, D.; Cao, J.; Sun, G. Facile synthesis and characterization of N-doped TiO2/C nanocomposites with enhanced visible-light photocatalytic performance. Appl. Surf. Sci. 2018, 430, 438–447. [Google Scholar] [CrossRef]
- Tamai, K.; Hosokawa, S.; Asakura, H.; Teramura, K.; Tanaka, T. Low-temperature NOx trapping on alkali or alkaline earth metal modified TiO2 photocatalyst. Catal. Today 2019, 332, 76–82. [Google Scholar] [CrossRef]
- Ben Saber, N.; Mezni, A.; Alrooqi, A.; Altalhi, T. A review of ternary nanostructures based noble metal/semiconductor for environmental and renewable energy applications. J. Mater. Res. Technol. 2020, 9, 15233–15262. [Google Scholar] [CrossRef]
- Diak, M.; Grabowska, E.; Zaleska, A. Synthesis, characterization and photocatalytic activity of noble metal-modified TiO2 nanosheets with exposed {001} facets. Appl. Surf. Sci. 2015, 347, 275–285. [Google Scholar] [CrossRef]
- Low, J.; Cheng, B.; Yu, J. Surface modification and enhanced photocatalytic CO2 reduction performance of TiO2: A review. Appl. Surf. Sci. 2017, 392, 658–686. [Google Scholar] [CrossRef]
- El-Maghrabi, H.H.; Nada, A.A.; Roualdes, S.; Bekheet, M.F. Design of Ni/NiO–TiO2/rGO nanocomposites on carbon cloth conductors via PECVD for electrocatalytic water splitting. Int. J. Hydrogen Energy 2020, 45, 32000–32011. [Google Scholar] [CrossRef]
- Ghorbanloo, M.; Nada, A.A.; El-Maghrabi, H.H.; Bekheet, M.F.; Riedel, W.; Djamel, B.; Viter, R.; Roualdes, S.; Soliman, F.S.; Moustafa, Y.M.; et al. Superior efficiency of BN/Ce2O3/TiO2 nanofibers for photocatalytic hydrogen generation reactions. Appl. Surf. Sci. 2022, 594, 153438. [Google Scholar] [CrossRef]
- Avcıoğlu, C.; Avcıoğlu, S.; Bekheet, M.F.; Gurlo, A. Solar hydrogen generation using niobium-based photocatalysts: Design strategies, progress, and challenges. Mater. Today Energy 2022, 24, 100936. [Google Scholar] [CrossRef]
- Alcudia-Ramos, M.A.; Fuentez-Torres, M.O.; Ortiz-Chi, F.; Espinosa-González, C.G.; Hernández-Como, N.; García-Zaleta, D.S.; Kesarla, M.K.; Torres-Torres, J.G.; Collins-Martínez, V.; Godavarthi, S. Fabrication of g-C3N4/TiO2 heterojunction composite for enhanced photocatalytic hydrogen production. Ceram. Int. 2020, 46, 38–45. [Google Scholar] [CrossRef]
- Li, B.; Chen, X.; Zhang, T.; Jiang, S.; Zhang, G.; Wu, W.; Ma, X. Photocatalytic selective hydroxylation of phenol to dihydroxybenzene by BiOI/TiO2 p-n heterojunction photocatalysts for enhanced photocatalytic activity. Appl. Surf. Sci. 2018, 439, 1047–1056. [Google Scholar] [CrossRef]
- Carriazo, J.G.; Moreno-Forero, M.; Molina, R.A.; Moreno, S. Incorporation of titanium and titanium–iron species inside a smectite-type mineral for photocatalysis. Appl. Clay Sci. 2010, 50, 401–408. [Google Scholar] [CrossRef]
- Dong, G.; Zhao, L.; Wu, X.; Zhu, M.; Wang, F. Photocatalysis removing of NO based on modified carbon nitride: The effect of celestite mineral particles. Appl. Catal. B Environ. 2019, 245, 459–468. [Google Scholar] [CrossRef]
- Cao, Y.-C.; Fu, Z.; Wei, W.; Zou, L.; Mi, T.; He, D.; Yan, C.; Liu, X.; Zhu, Y.; Chen, L.; et al. Reduced graphene oxide supported titanium dioxide nanomaterials for the photocatalysis with long cycling life. Appl. Surf. Sci. 2015, 355, 1289–1294. [Google Scholar] [CrossRef]
- Padmanabhan, S.K.; Pal, S.; Licciulli, A. Diatomite/silver phosphate composite for efficient degradation of organic dyes under solar radiation. Bull. Mater. Sci. 2020, 43, 295. [Google Scholar] [CrossRef]
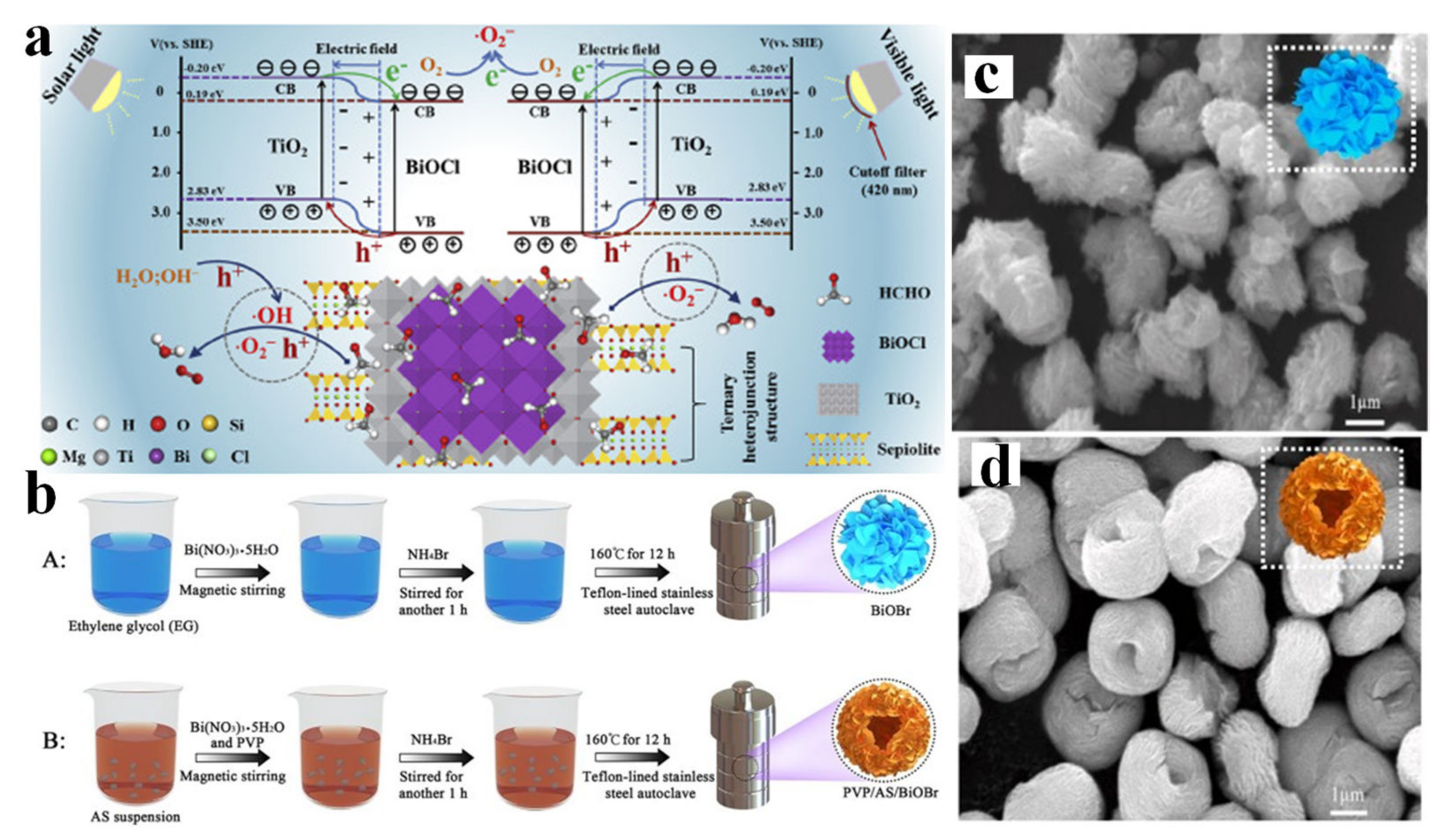
- Zhang, L.; Zhang, J.; Zhang, W.; Liu, J.; Zhong, H.; Zhao, Y. Photocatalytic activity of attapulgite–BiOCl–TiO2 toward degradation of methyl orange under UV and visible light irradiation. Mater. Res. Bull. 2015, 66, 109–114. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Chen, J.; Fu, Y.; Li, Q.; Yin, J.; Cheng, Z.; Kan, W.; Zhao, P.; Zhong, H.; et al. Synthesis of nano-Ag-assisted attapulgite/g-C3N4 composites with superior visible light photocatalytic performance. Mater. Chem. Phys. 2019, 221, 447–456. [Google Scholar] [CrossRef]
- Tan, Y.; Yin, C.; Zheng, S.; Di, Y.; Sun, Z.; Li, C. Design and controllable preparation of Bi2MoO6/attapulgite photocatalyst for the removal of tetracycline and formaldehyde. Appl. Clay Sci. 2021, 215, 106319. [Google Scholar] [CrossRef]
- Zhu, Z.; Yu, Y.; Dong, H.; Liu, Z.; Li, C.; Huo, P.; Yan, Y. Intercalation Effect of Attapulgite in g-C3N4 Modified with Fe3O4 Quantum Dots to Enhance Photocatalytic Activity for Removing 2-Mercaptobenzothiazole under Visible Light. ACS Sustain. Chem. Eng. 2017, 5, 10614–10623. [Google Scholar] [CrossRef]
- Zuo, S.; Chen, Y.; Liu, W.; Yao, C.; Li, X.; Li, Z.; Ni, C.; Liu, X. A facile and novel construction of attapulgite/Cu2O/Cu/g-C3N4 with enhanced photocatalytic activity for antibiotic degradation. Ceram. Int. 2017, 43, 3324–3329. [Google Scholar] [CrossRef]
- Zheng, J.; Zhu, Z.; Gao, G.; Liu, Z.; Wang, Q.; Yan, Y. Construction of spindle structured CeO2 modified with rod-like attapulgite as a high-performance photocatalyst for CO2 reduction. Catal. Sci. Technol. 2019, 9, 3788–3799. [Google Scholar] [CrossRef]
- Guan, J.; Wang, H.; Li, J.; Ma, C.; Huo, P. Enhanced photocatalytic reduction of CO2 by fabricating In2O3/CeO2/HATP hybrid multi-junction photocatalyst. J. Taiwan Inst. Chem. Eng. 2019, 99, 93–103. [Google Scholar] [CrossRef]
- Li, X.; Zhu, W.; Lu, X.; Zuo, S.; Yao, C.; Ni, C. Integrated nanostructures of CeO2/attapulgite/g-C3N4 as efficient catalyst for photocatalytic desulfurization: Mechanism, kinetics and influencing factors. Chem. Eng. J. 2017, 326, 87–98. [Google Scholar] [CrossRef]
- Ma, J.; Zou, J.; Li, L.; Yao, C.; Kong, Y.; Cui, B.; Zhu, R.; Li, D. Nanocomposite of attapulgite–Ag3PO4 for Orange II photodegradation. Appl. Catal. B Environ. 2014, 144, 36–40. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X. Photocatalytic hydrogen production from water under visible light irradiation using a dye-sensitized attapulgite nanocrystal photocatalyst. Phys. Chem. Chem. Phys. 2014, 16, 8655–8660. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, A.; Wang, L.; Li, X.; Huang, W. Striving Toward Visible Light Photocatalytic Water Splitting Based on Natural Silicate Clay Mineral: The Interface Modification of Attapulgite at the Atomic-Molecular Level. ACS Sustain. Chem. Eng. 2016, 4, 4601–4607. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, R.; Zhang, G.; Gao, L.; Zhang, W. Preparation and photocatalytic properties of visible light driven Ag–AgCl–TiO2/palygorskite composite. J. Alloys Compd. 2016, 657, 801–808. [Google Scholar] [CrossRef]
- Bouna, L.; Rhouta, B.; Amjoud, M.; Maury, F.; Lafont, M.-C.; Jada, A.; Senocq, F.; Daoudi, L. Synthesis, characterization and photocatalytic activity of TiO2 supported natural palygorskite microfibers. Appl. Clay Sci. 2011, 52, 301–311. [Google Scholar] [CrossRef] [Green Version]
- Stathatos, E.; Papoulis, D.; Aggelopoulos, C.A.; Panagiotaras, D.; Nikolopoulou, A. TiO2/palygorskite composite nanocrystalline films prepared by surfactant templating route: Synergistic effect to the photocatalytic degradation of an azo-dye in water. J. Hazard. Mater. 2012, 211–212, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Kuang, M.; Zhang, J.; Wang, W.; Chen, J.; Cao, Y.; Wang, J.; Ji, Z. Ternary Ag-deposited TiO2/palygorskite composites with synergistic effect for enhanced photocatalytic activity. Solid State Sci. 2019, 97, 106015. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, N.; Chen, F.; Zhang, T.; Zhang, G.; Wang, D.; Xie, X.; Cai, D.; Ma, X.; Wu, L.; et al. Improvement of Cr (VI) photoreduction under visible-light by g-C3N4 modified by nano-network structured palygorskite. Chem. Eng. J. 2019, 358, 398–407. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, M.; Yin, H.; Yao, J.; Chen, S.-m.; Liu, X. One pot controllable synthesis of palygorskite/bismuth oxyiodide hierarchical microspheres for improved visible-light photocatalytic performance. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123573. [Google Scholar] [CrossRef]
- He, C.; Li, X.; Chen, X.; Ma, S.; Yan, X.; Zhang, Y.; Zuo, S.; Yao, C. Palygorskite supported rare earth fluoride for photocatalytic nitrogen fixation under full spectrum. Appl. Clay Sci. 2020, 184, 105398. [Google Scholar] [CrossRef]
- Yuan, Z.; Lan, Y.; Chen, S.; Chen, D. Preparation of magnetically recyclable palygorskite Fe-octacarboxylic acid phthalocyanine nano-composites and their photocatalytic behavior for degradation of Rhodamine B. Appl. Clay Sci. 2017, 147, 153–159. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, Z.; Li, Y.; Li, S.; Lu, G. Photosensitized reduction of water to hydrogen using novel Maya blue-like organic–inorganic hybrid material. J. Colloid Interface Sci. 2009, 333, 285–293. [Google Scholar] [CrossRef]
- Jiang, L.; Huang, Y.; Liu, T. Enhanced visible-light photocatalytic performance of electrospun carbon-doped TiO2/halloysite nanotube hybrid nanofibers. J. Colloid Interface Sci. 2015, 439, 62–68. [Google Scholar] [CrossRef]
- Wang, R.; Jiang, G.; Ding, Y.; Wang, Y.; Sun, X.; Wang, X.; Chen, W. Photocatalytic Activity of Heterostructures Based on TiO2 and Halloysite Nanotubes. ACS Appl. Mater. Interfaces 2011, 3, 4154–4158. [Google Scholar] [CrossRef]
- Yu, X.; Lu, Z.; Si, N.; Zhou, W.; Chen, T.; Gao, X.; Song, M.; Yan, Y.; Huo, P.; Yan, C. Preparation of rare earth metal ion/TiO2Hal-conducting polymers by ions imprinting technique and its photodegradation property on tetracycline. Appl. Clay Sci. 2014, 99, 125–130. [Google Scholar] [CrossRef]
- Szczepanik, B.; Rogala, P.; Słomkiewicz, P.M.; Banaś, D.; Kubala-Kukuś, A.; Stabrawa, I. Synthesis, characterization and photocatalytic activity of TiO2-halloysite and Fe2O3-halloysite nanocomposites for photodegradation of chloroanilines in water. Appl. Clay Sci. 2017, 149, 118–126. [Google Scholar] [CrossRef]
- Papoulis, D.; Komarneni, S.; Panagiotaras, D.; Stathatos, E.; Toli, D.; Christoforidis, K.C.; Fernández-García, M.; Li, H.; Yin, S.; Sato, T.; et al. Halloysite–TiO2 nanocomposites: Synthesis, characterization and photocatalytic activity. Appl. Catal. B Environ. 2013, 132–133, 416–422. [Google Scholar] [CrossRef]
- Hojamberdiev, M.; Khan, M.M.; Kadirova, Z.; Kawashima, K.; Yubuta, K.; Teshima, K.; Riedel, R.; Hasegawa, M. Synergistic effect of g-C3N4, Ni(OH)2 and halloysite in nanocomposite photocatalyst on efficient photocatalytic hydrogen generation. Renew. Energy 2019, 138, 434–444. [Google Scholar] [CrossRef]
- Wu, D.; Li, J.; Guan, J.; Liu, C.; Zhao, X.; Zhu, Z.; Ma, C.; Huo, P.; Li, C.; Yan, Y. Improved photoelectric performance via fabricated heterojunction g-C3N4/TiO2/HNTs loaded photocatalysts for photodegradation of ciprofloxacin. J. Ind. Eng. Chem. 2018, 64, 206–218. [Google Scholar] [CrossRef]
- Gao, X.; Tang, F.; Jin, Z. Pt–Cu Bimetallic Nanoparticles Loaded in the Lumen of Halloysite Nanotubes. Langmuir 2019, 35, 14651–14658. [Google Scholar] [CrossRef]
- Das, S.; Samanta, A.; Jana, S. Light-Assisted Synthesis of Hierarchical Flower-Like MnO2 Nanocomposites with Solar Light Induced Enhanced Photocatalytic Activity. ACS Sustain. Chem. Eng. 2017, 5, 9086–9094. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, B.; Jia, L.; Bi, N.; Zhao, T. Metal-enhanced fluorescence detection and degradation of tetracycline by silver nanoparticle-encapsulated halloysite nano-lumen. J. Hazard. Mater. 2020, 386, 121630. [Google Scholar] [CrossRef]
- Bielska, D.; Karewicz, A.; Lachowicz, T.; Berent, K.; Szczubiałka, K.; Nowakowska, M. Hybrid photosensitizer based on halloysite nanotubes for phenol-based pesticide photodegradation. Chem. Eng. J. 2015, 262, 125–132. [Google Scholar] [CrossRef]
- Li, F.; Dai, Y.; Gong, M.; Yu, T.; Chen, X. Synthesis, characterization of magnetic-sepiolite supported with TiO2, and the photocatalytic performance over Cr(VI) and 2,4-dichlorophenol co-existed wastewater. J. Alloys Compd. 2015, 638, 435–442. [Google Scholar] [CrossRef]
- Karamanis, D.; Ökte, A.N.; Vardoulakis, E.; Vaimakis, T. Water vapor adsorption and photocatalytic pollutant degradation with TiO2–sepiolite nanocomposites. Appl. Clay Sci. 2011, 53, 181–187. [Google Scholar] [CrossRef]
- Papoulis, D.; Somalakidi, K.; Todorova, N.; Trapalis, C.; Panagiotaras, D.; Sygkridou, D.; Stathatos, E.; Gianni, E.; Mavrikos, A.; Komarneni, S. Sepiolite/TiO2 and metal ion modified sepiolite/TiO2 nanocomposites: Synthesis, characterization and photocatalytic activity in abatement of NOx gases. Appl. Clay Sci. 2019, 179, 105156. [Google Scholar] [CrossRef]
- Hu, X.; Li, C.; Sun, Z.; Song, J.; Zheng, S. Enhanced photocatalytic removal of indoor formaldehyde by ternary heterogeneous BiOCl/TiO2/sepiolite composite under solar and visible light. Build. Environ. 2020, 168, 106481. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Q.; Wang, X.; Yang, J.; Dai, Y.; He, Y.; Chen, W.; Zhang, W. Photocatalytic degradation of rhodamin B and diclofenac sodium on hollow hierarchical microspheres of BiOBr modified with sepiolite and polyvinyl pyrrolidone (PVP). Mater. Sci. Eng. B 2019, 244, 12–22. [Google Scholar] [CrossRef]
- Wang, P.; Qi, C.; Hao, L.; Wen, P.; Xu, X. Sepiolite/Cu2O/Cu photocatalyst: Preparation and high performance for degradation of organic dye. J. Mater. Sci. Technol. 2019, 35, 285–291. [Google Scholar] [CrossRef]
- Akkari, M.; Aranda, P.; Belver, C.; Bedia, J.; Ben Haj Amara, A.; Ruiz-Hitzky, E. Reprint of ZnO/sepiolite heterostructured materials for solar photocatalytic degradation of pharmaceuticals in wastewater. Appl. Clay Sci. 2018, 160, 3–8. [Google Scholar] [CrossRef]
- Akkari, M.; Aranda, P.; Mayoral, A.; García-Hernández, M.; Ben Haj Amara, A.; Ruiz-Hitzky, E. Sepiolite nanoplatform for the simultaneous assembly of magnetite and zinc oxide nanoparticles as photocatalyst for improving removal of organic pollutants. J. Hazard. Mater. 2017, 340, 281–290. [Google Scholar] [CrossRef]
- Chuaicham, C.; Pawar, R.; Sasaki, K. Dye-sensitized Photocatalyst of Sepiolite for Organic Dye Degradation. Catalysts 2019, 9, 235. [Google Scholar] [CrossRef] [Green Version]
- Paineau, E. Imogolite Nanotubes: A Flexible Nanoplatform with Multipurpose Applications. Appl. Sci. 2018, 8, 1921. [Google Scholar] [CrossRef] [Green Version]
- Bottero, I.; Bonelli, B.; Ashbrook, S.E.; Wright, P.A.; Zhou, W.; Tagliabue, M.; Armandi, M.; Garrone, E. Synthesis and characterization of hybrid organic/inorganic nanotubes of the imogolite type and their behaviour towards methane adsorption. Phys. Chem. Chem. Phys. 2011, 13, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, E.; Vaiano, V.; Esposito, S.; Armandi, M.; Sannino, D.; Bonelli, B. Photo-activated degradation of tartrazine by H2O2 as catalyzed by both bare and Fe-doped methyl-imogolite nanotubes. Catal. Today 2018, 304, 199–207. [Google Scholar] [CrossRef]
- Olson, N.; Deshpande, N.; Gunduz, S.; Ozkan, U.S.; Brunelli, N.A. Utilizing imogolite nanotubes as a tunable catalytic material for the selective isomerization of glucose to fructose. Catal. Today 2019, 323, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, Y.; Fukumoto, K.; Kuroda, K. Uniform and high dispersion of gold nanoparticles on imogolite nanotubes and assembly into morphologically controlled materials. Appl. Clay Sci. 2012, 55, 10–17. [Google Scholar] [CrossRef]
- Katsumata, K.-I.; Hou, X.; Sakai, M.; Nakajima, A.; Fujishima, A.; Matsushita, N.; MacKenzie, K.J.D.; Okada, K. Visible-light-driven photodegradation of acetaldehyde gas catalyzed by aluminosilicate nanotubes and Cu(II)-grafted TiO2 composites. Appl. Catal. B Environ. 2013, 138–139, 243–252. [Google Scholar] [CrossRef]
- Yao, G.; Sun, Z.; Zheng, S. Synthesis and enhanced visible-light photocatalytic activity of wollastonite/g-C3N4 composite. Mater. Res. Bull. 2017, 86, 186–193. [Google Scholar] [CrossRef]
- Ren, S.; Zhao, X.; Zhao, L.; Yuan, M.; Yu, Y.; Guo, Y.; Wang, Z. Preparation of porous TiO2/silica composites without any surfactants. J. Solid State Chem. 2009, 182, 312–316. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, Y.; Ding, H.; Chu, P.K. Environmentally friendly wollastonite@TiO2 composite particles prepared by a mechano-chemical method. Particuology 2018, 40, 105–112. [Google Scholar] [CrossRef]
- Kwak, B.S.; Kim, K.M.; Park, S.-M.; Kang, M. Synthesis of basalt fiber@Zn1-xMgxO core/shell nanostructures for selective photoreduction of CO2 to CO. Appl. Surf. Sci. 2017, 407, 109–116. [Google Scholar] [CrossRef]
- Rakitskaya, T.L.; Kiose, T.A.; Oleksenko, L.P.; Lutsenko, L.V.; Dlubovskii, R.M.; Volkova, V.J. Influence of water content in the Pd(II)-Cu(II) catalyst fixed on acid-modified basalt tuff on its activity in the carbon monoxide oxidation by oxygen. Russ. J. Appl. Chem. 2012, 85, 1339–1344. [Google Scholar] [CrossRef]
- Qiu, L.; Chu, Z.; Yang, S.; Chen, Y.; Song, L.; Du, P.; Xiong, J. Microwave Hydrothermal Synthesis of Basalt Fibers/Titanium Dioxide and Their Photocatalytic Activity for the Degradation of Rhodamine B. J. Nanosci. Nanotechnol. 2019, 19, 5723–5728. [Google Scholar] [CrossRef]
- Do, J.Y.; Im, Y.; Kwak, B.S.; Park, S.-M.; Kang, M. Preparation of basalt fiber@perovskite PbTiO3 core–shell composites and their effects on CH4 production from CO2 photoreduction. Ceram. Int. 2016, 42, 5942–5951. [Google Scholar] [CrossRef]
- Wang, J.; Liang, X.; Chen, P.; Zhang, D.; Yang, S.; Liu, Z. Microstructure and photocatalytic properties of Ag/Ce4+/La3+ co-modified TiO2/Basalt fiber for ammonianitrogen removal from synthetic wastewater. J. Sol-Gel Sci. Technol. 2016, 82, 289–298. [Google Scholar] [CrossRef]
- Yu, L.; Wang, C.; Chen, F.; Zhang, J.; Ruan, Y.; Xu, J. Investigating the synergistic effects in tourmaline/TiO2-based heterogeneous photocatalysis: Underlying mechanism insights. J. Mol. Catal. A Chem. 2016, 411, 1–8. [Google Scholar] [CrossRef]
- Zhang, G.; Qin, X. Efficient photocatalytic degradation of gaseous formaldehyde by the TiO2/tourmaline composites. Mater. Res. Bull. 2013, 48, 3743–3749. [Google Scholar] [CrossRef]
- Tzeng, J.-H.; Weng, C.-H.; Lin, Y.-H.; Huang, S.-M.; Yen, L.-T.; Anotai, J.; Lin, Y.-T. Synthesis, characterization, and visible light induced photoactivity of tourmaline-N-TiO2 composite for photooxidation of ethylene. J. Ind. Eng. Chem. 2019, 80, 376–384. [Google Scholar] [CrossRef]
- Baeissa, E.S. Green synthesis of methanol by photocatalytic reduction of CO2 under visible light using a graphene and tourmaline co-doped titania nanocomposites. Ceram. Int. 2014, 40, 12431–12438. [Google Scholar] [CrossRef]
- Li, Y.; Xing, X.; Pei, J.; Li, R.; Wen, Y.; Cui, S.; Liu, T. Automobile exhaust gas purification material based on physical adsorption of tourmaline powder and visible light catalytic decomposition of g-C3N4/BiVO4. Ceram. Int. 2020, 46, 12637–12647. [Google Scholar] [CrossRef]
- Bian, X.; Chen, J.; Ji, R. Degradation of methyl blue using Fe-tourmaline as a novel photocatalyst. Molecules 2013, 18, 1457–1463. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Feng, Q.; Liu, K.; Tan, Y. Synthesis and characterization of a novel nanofibrous TiO2/SiO2 composite with enhanced photocatalytic activity. Mater. Lett. 2016, 183, 175–178. [Google Scholar] [CrossRef]
- Zhang, H.; Duan, T.; Zhu, W.; Yao, W.-T. Natural Chrysotile-Based Nanowires Decorated with Monodispersed Ag Nanoparticles as a Highly Active and Reusable Hydrogenation Catalyst. J. Phys. Chem. C 2015, 119, 21465–21472. [Google Scholar] [CrossRef]
- Lou, J.P.; Xue, G.X.; Li, S.Z.; Wang, J. Particle Coating of Talc with TiO2: Synthesis, Characterization and Photo Catalytic Degradation of Methylene Blue. Adv. Mater. Res. 2013, 781–784, 157–162. [Google Scholar] [CrossRef]
- Ai, M.; Qin, W.; Xia, T.; Ye, Y.; Chen, X.; Zhang, P.; Justin Thomas, K.R. Photocatalytic Degradation of 2,4-Dichlorophenol by TiO2 Intercalated Talc Nanocomposite. Int. J. Photoenergy 2019, 2019, 1540271. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Rhimi, B.; Wang, H.; Wang, C. Efficient photocatalytic degradation of gaseous toluene over F-doped TiO2/exfoliated bentonite. Appl. Surf. Sci. 2020, 530, 147286. [Google Scholar] [CrossRef]
- Krishnan, B.; Mahalingam, S. Ag/TiO2/bentonite nanocomposite for biological applications: Synthesis, characterization, antibacterial and cytotoxic investigations. Adv. Powder Technol. 2017, 28, 2265–2280. [Google Scholar] [CrossRef]
- Li, J.; Wang, W. A study of photodegradation of sulforhodamine B on Au–TiO2/bentonite under UV and visible light irradiation. Solid State Sci. 2009, 11, 2037–2043. [Google Scholar] [CrossRef]
- WANG, S.-q.; LI, J.-m.; LIU, W.-b. Effect of F, V and Mn co-doping on the catalytic performance of TiO2-pillared bentonite in the photocatalytic denitration. J. Fuel Chem. Technol. 2020, 48, 1131–1139. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Kainth, S.; Basu, S. Effect of g-C3N4 loading on TiO2/Bentonite nanocomposites for efficient heterogeneous photocatalytic degradation of industrial dye under visible light. J. Alloys Compd. 2018, 764, 406–415. [Google Scholar] [CrossRef]
- Yang, S.; Liang, G.; Gu, A.; Mao, H. Synthesis of TiO2 pillared montmorillonite with ordered interlayer mesoporous structure and high photocatalytic activity by an intra-gallery templating method. Mater. Res. Bull. 2013, 48, 3948–3954. [Google Scholar] [CrossRef]
- Tahir, M.; Amin, N.S. Photocatalytic reduction of carbon dioxide with water vapors over montmorillonite modified TiO2 nanocomposites. Appl. Catal. B Environ. 2013, 142–143, 512–522. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B.; Zakaria, Z.Y.; Muhammad, A. Enhanced photocatalytic carbon dioxide reforming of methane to fuels over nickel and montmorillonite supported TiO2 nanocomposite under UV-light using monolith photoreactor. J. Clean. Prod. 2019, 213, 451–461. [Google Scholar] [CrossRef]
- Umer, M.; Tahir, M.; Azam, M.U.; Tahir, B.; Jaffar, M.M.; Alias, H. Montmorillonite dispersed single wall carbon nanotubes (SWCNTs)/TiO2 heterojunction composite for enhanced dynamic photocatalytic H2 production under visible light. Appl. Clay Sci. 2019, 174, 110–119. [Google Scholar] [CrossRef]
- Yang, L.; Ma, X.; Zhang, W.; Gen, W. Ag@AgCl-TiO2/organic rectorite/quaternized chitosan microspheres: An efficient and environmental photocatalyst. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, G.; Xu, W. Facile synthesis and photocatalytic properties of AgAgClTiO2/rectorite composite. J. Colloid Interface Sci. 2012, 376, 217–223. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, L.; Zhang, G.; Gan, H. Facile synthesis and photocatalytic property of bicrystalline TiO2/rectorite composites. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 137–144. [Google Scholar] [CrossRef]
- Zhang, Y.; Miao, B.; Chen, Q.; Bai, Z.; Cao, Y.; Davaa, B. Synthesis, Structure, and Photocatalytic Activity of TiO2-Montmorillonite Composites. Catalysts 2022, 12, 486. [Google Scholar] [CrossRef]
- Cardona, Y.; Węgrzyn, A.; Miśkowiec, P.; Korili, S.A.; Gil, A. Catalytic photodegradation of organic compounds using TiO2/pillared clays synthesized using a nonconventional aluminum source. Chem. Eng. J. 2022, 446, 136908. [Google Scholar] [CrossRef]
- Hu, X.; Song, J.; Zheng, S.; Sun, Z.; Li, C. Insight into the defective sites of TiO2/sepiolite composite on formaldehyde removal and H2 evolution. Mater. Today Energy 2022, 24, 100932. [Google Scholar] [CrossRef]
- Hu, X.; Li, C.; Song, J.; Zheng, S.; Sun, Z. Hierarchical assembly of visible-light-driven Bi2MoO6/TiO2/sepiolite composite for effective formaldehyde removal. Appl. Clay Sci. 2022, 227, 106590. [Google Scholar] [CrossRef]
- Dlamini, M.C.; Dlamini, M.L.; Mente, P.; Tlhaole, B.; Erasmus, R.; Maubane-Nkadimeng, M.S.; Moma, J.A. Photocatalytic abatement of phenol on amorphous TiO2-BiOBr-bentonite heterostructures under visible light irradiation. J. Ind. Eng. Chem. 2022, 111, 419–436. [Google Scholar] [CrossRef]
- Venu Sreekala, S.; Vayalveettil, A.; Kazhuthuttil Kochu, J.; Thoppil Ramakrishnan, R.; Puthenveedu Sadasivan Pillai, H. Bentonite-titanium dioxide functional nanocomposites suitable for wastewater treatment: An integrated photocatalyst-adsorbent system. N. J. Chem. 2022, 46, 4772–4783. [Google Scholar] [CrossRef]
- Ardani, M.; Imani, M.; Tadjarodi, A. Core shell Fe3O4@TiO2/silica aerogel nanocomposite; synthesis and study of structural, magnetic and photocatalytic properties. Microporous Mesoporous Mater. 2022, 338, 111757. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Q.; Zhu, L.; Zou, J.; Wang, K.; Yang, M.; Komarneni, S. Visible light photocatalytic activity enhancement of Ag3PO4 dispersed on exfoliated bentonite for degradation of rhodamine B. Appl. Catal. B Environ. 2016, 182, 26–32. [Google Scholar] [CrossRef]
- Nguyen, D.B.; Nguyen, T.D.C.; Dao, T.P.; Tran, H.T.; van Nguyen, N.; Ahn, D.H. Preparation, characterization and evaluation of catalytic activity of titania modified with silver and bentonite. J. Ind. Eng. Chem. 2012, 18, 1764–1767. [Google Scholar] [CrossRef]
- Ma, J.; Huang, D.; Zhang, W.; Zou, J.; Kong, Y.; Zhu, J.; Komarneni, S. Nanocomposite of exfoliated bentonite/g-C3N4/Ag3PO4 for enhanced visible-light photocatalytic decomposition of Rhodamine B. Chemosphere 2016, 162, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Thiruppathi, M.; Selvakumar, K.; Arunpandian, M.; Thirumalai, K.; Ramalingan, C.; Swaminathan, M.; Nagarajan, E.R. An affordable photocatalyst for pharmaceuticals and superior electrocatalyst for methanol oxidation—A dual role by CuWO4 anchored bentonite clay. Colloids Surf. A Physicochem. Eng. Asp. 2019, 563, 148–159. [Google Scholar] [CrossRef]
- Tahir, B.; Tahir, M.; Mohd Nawawi, M.G. Well-Designed 3D/2D/2D WO3/Bt/g-C3N4 Z-Scheme Heterojunction for Tailoring Photocatalytic CO2 Methanation with 2D-Layered Bentonite-Clay as the Electron Moderator under Visible Light. Energy Fuels 2020, 34, 14400–14418. [Google Scholar] [CrossRef]
- Riaz, U.; Ashraf, S.M.; Ruhela, A. Catalytic degradation of orange G under microwave irradiation with a novel nanohybrid catalyst. J. Environ. Chem. Eng. 2015, 3, 20–29. [Google Scholar] [CrossRef]
- Bentouami, A.; Ouali, M.S.; de Menorval, L.-C. Photocatalytic decolourization of indigo carmine on 1,10-phenanthrolinium intercalated bentonite under UV-B and solar irradiation. J. Photochem. Photobiol. A Chem. 2010, 212, 101–106. [Google Scholar] [CrossRef]
- Huang, S.; Lu, X.; Li, Z.; Ravishankar, H.; Wang, J.; Wang, X. A biomimetic approach towards the synthesis of TiO2/carbon-clay as a highly recoverable photocatalyst. J. Photochem. Photobiol. A Chem. 2018, 351, 131–138. [Google Scholar] [CrossRef]
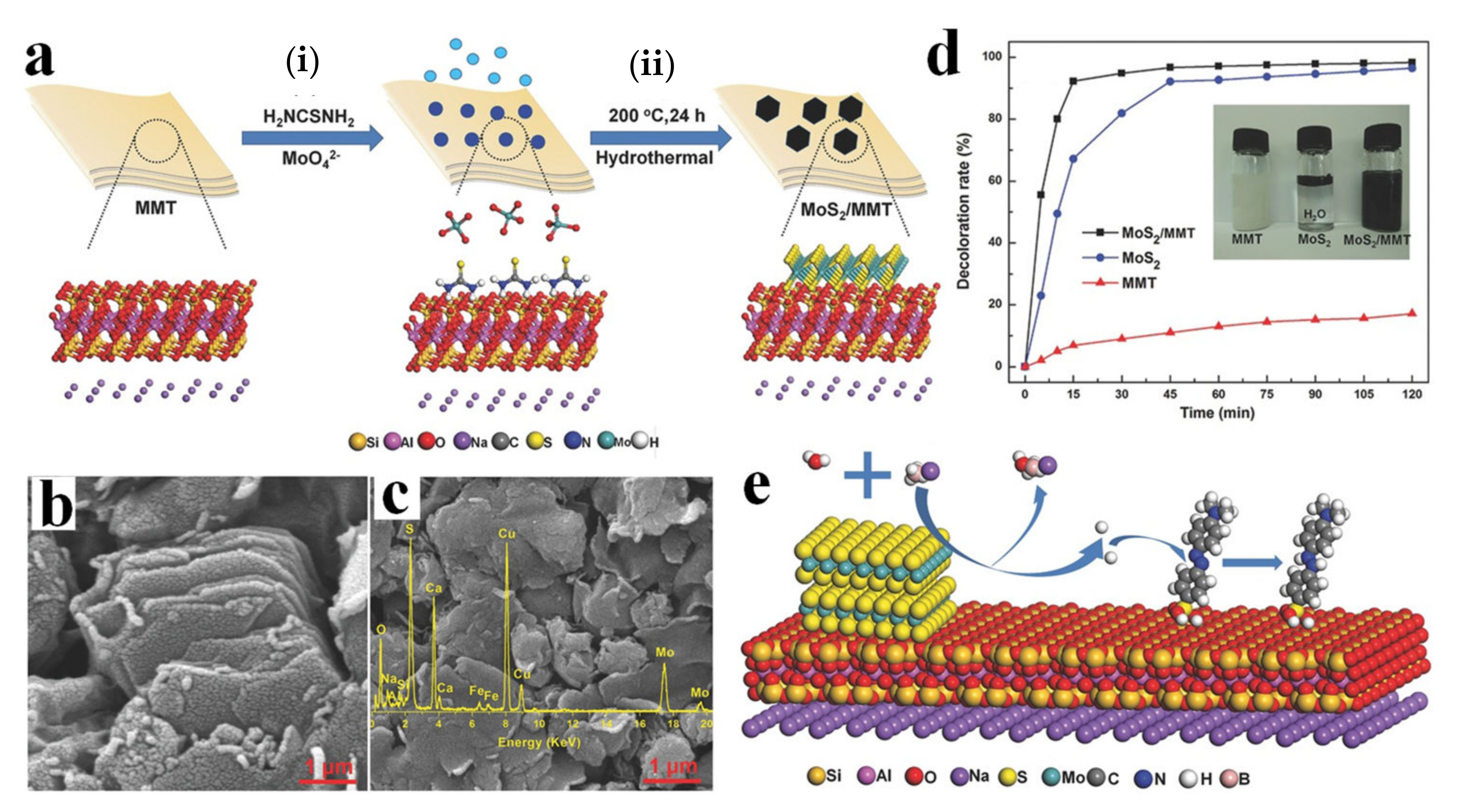
- Peng, K.; Fu, L.; Ouyang, J.; Yang, H. Emerging Parallel Dual 2D Composites: Natural Clay Mineral Hybridizing MoS2 and Interfacial Structure. Adv. Funct. Mater. 2016, 26, 2666–2675. [Google Scholar] [CrossRef]
- Obalová, L.; Šihor, M.; Praus, P.; Reli, M.; Kočí, K. Photocatalytic and photochemical decomposition of N2O on ZnS-MMT catalyst. Catal. Today 2014, 230, 61–66. [Google Scholar] [CrossRef] [Green Version]
- Gu, N.; Gao, J.; Li, H.; Wu, Y.; Ma, Y.; Wang, K. Montmorillonite-supported with Cu2O nanoparticles for damage and removal of Microcystis aeruginosa under visible light. Appl. Clay Sci. 2016, 132–133, 79–89. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, Y.; Wei, H.; Li, K. In situ growth of cube-like AgCl on montmorillonite as an efficient photocatalyst for dye (Acid Red 18) degradation. Appl. Surf. Sci. 2018, 456, 577–585. [Google Scholar] [CrossRef]
- Xu, J.; Qi, Y.; Wang, W.; Wang, L. Montmorillonite-hybridized g-C3N4 composite modified by NiCoP cocatalyst for efficient visible-light-driven photocatalytic hydrogen evolution by dye-sensitization. Int. J. Hydrogen Energy 2019, 44, 4114–4122. [Google Scholar] [CrossRef]
- Pastorková, K.; Jesenák, K.; Kadlečíková, M.; Breza, J.; Kolmačka, M.; Čaplovičová, M.; Lazišťan, F.; Michalka, M. The growth of multi-walled carbon nanotubes on natural clay minerals (kaolinite, nontronite and sepiolite). Appl. Surf. Sci. 2012, 258, 2661–2666. [Google Scholar] [CrossRef]
- Liu, X.; Dong, H.; Zeng, Q.; Yang, X.; Zhang, D. Synergistic Effects of Reduced Nontronite and Organic Ligands on Cr(VI) Reduction. Environ. Sci. Technol. 2019, 53, 13732–13741. [Google Scholar] [CrossRef]
- Xia, Q.; Wang, X.; Zeng, Q.; Guo, D.; Zhu, Z.; Chen, H.; Dong, H. Mechanisms of Enhanced Antibacterial Activity by Reduced Chitosan-Intercalated Nontronite. Environ. Sci. Technol. 2020, 54, 5207–5217. [Google Scholar] [CrossRef]
- Li, S.-Q.; Zhou, P.-J.; Zhang, W.-S.; Chen, S.; Peng, H. Effective photocatalytic decolorization of methylene blue utilizing ZnO/rectorite nanocomposite under simulated solar irradiation. J. Alloys Compd. 2014, 616, 227–234. [Google Scholar] [CrossRef]
- Wu, S.; Fang, J.; Xu, W.; Cen, C. Bismuth-modified rectorite with high visible light photocatalytic activity. J. Mol. Catal. A Chem. 2013, 373, 114–120. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, W.; Chen, J.; Wang, X.; Gao, B.; Wang, G. Ag3PO4/rectorite nanocomposites: Ultrasound-assisted preparation, characterization and enhancement of stability and visible-light photocatalytic activity. Ultrason. Sonochem. 2017, 34, 831–838. [Google Scholar] [CrossRef]
- Naing, H.H.; Wang, K.; Tun, P.P.; Zhang, G. Enhanced broad spectrum (vis-NIR) responsive photocatalytic performance of Ag2O/rectorite nanoarchitectures. Appl. Surf. Sci. 2019, 491, 216–224. [Google Scholar] [CrossRef]
- Chen, Y.; Fang, J.; Lu, S.; Wu, Y.; Chen, D.; Huang, L.; Xu, W.; Zhu, X.; Fang, Z. Fabrication, characterization and photocatalytic properties of Ag/AgI/BiOI heteronanostructures supported on rectorite via a cation-exchange method. Mater. Res. Bull. 2015, 64, 97–105. [Google Scholar] [CrossRef]
- Bu, X.; Wang, Y.; Li, J.; Zhang, C. Improving the visible light photocatalytic activity of TiO2 by combining sulfur doping and rectorite carrier. J. Alloys Compd. 2015, 628, 20–26. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, L.; Zhang, Y.; Yan, J.; Lu, X.; Huang, Y.; Tang, H. Removing organic contaminants with bifunctional iron modified rectorite as efficient adsorbent and visible light photo-Fenton catalyst. J. Hazard. Mater. 2012, 215–216, 57–64. [Google Scholar] [CrossRef]
- Guo, S.; Yang, W.; You, L.; Li, J.; Chen, J.; Zhou, K. Simultaneous reduction of Cr(VI) and degradation of tetracycline hydrochloride by a novel iron-modified rectorite composite through heterogeneous photo-Fenton processes. Chem. Eng. J. 2020, 393, 124758. [Google Scholar] [CrossRef]
- Nikolopoulou, A.; Papoulis, D.; Komarneni, S.; Tsolis-Katagas, P.; Panagiotaras, D.; Kacandes, G.H.; Zhang, P.; Yin, S.; Sato, T. Solvothermal preparation of TiO2/saponite nanocomposites and photocatalytic activity. Appl. Clay Sci. 2009, 46, 363–368. [Google Scholar] [CrossRef]
- David, C.; Arivazhagan, M.; Ibrahim, M. Spent wash decolourization using nano-Al2O3/kaolin photocatalyst: Taguchi and ANN approach. J. Saudi Chem. Soc. 2015, 19, 537–548. [Google Scholar] [CrossRef] [Green Version]
- Kočí, K.; Matějka, V.; Kovář, P.; Lacný, Z.; Obalová, L. Comparison of the pure TiO2 and kaolinite/TiO2 composite as catalyst for CO2 photocatalytic reduction. Catal. Today 2011, 161, 105–109. [Google Scholar] [CrossRef]
- Li, C.; Dong, X.; Zhu, N.; Zhang, X.; Yang, S.; Sun, Z.; Liu, Y.; Zheng, S.; Dionysiou, D.D. Rational design of efficient visible-light driven photocatalyst through 0D/2D structural assembly: Natural kaolinite supported monodispersed TiO2 with carbon regulation. Chem. Eng. J. 2020, 396, 125311. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, L.; Lv, Z.; Chen, Y.; Jin, H.; Hou, S.; Qiu, L.; Duan, L.; Liu, J.; Dai, K. Porous carbon nitride with defect mediated interfacial oxidation for improving visible light photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 232, 384–390. [Google Scholar] [CrossRef]
- Jiang, D.; Liu, Z.; Fu, L.; Yang, H. Interfacial Chemical-Bond-Modulated Charge Transfer of Heterostructures for Improving Photocatalytic Performance. ACS Appl. Mater. Interfaces 2020, 12, 9872–9880. [Google Scholar] [CrossRef]
- Xue, B.; Yang, K.; Wang, X.; Chi, Q.; Jiang, Y. The role of potassium chlorate on expansion of dickite layers and the preparation of a novel TiO2 impregnated dickite photocatalyst using expanded dickite as carrier. RSC Adv. 2016, 6, 9803–9811. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, Z.; Liu, L.; Xing, J. Preparation of muscovite/tungsten-doped TiO2 composites for the efficient photocatalytic degradation of methyl orange under simulated solar light irradiation. Inorg. Chem. Commun. 2022, 138, 109285. [Google Scholar] [CrossRef]
- Shimizu, K.-i.; Murayama, H.; Nagai, A.; Shimada, A.; Hatamachi, T.; Kodama, T.; Kitayama, Y. Degradation of hydrophobic organic pollutants by titania pillared fluorine mica as a substrate specific photocatalyst. Appl. Catal. B Environ. 2005, 55, 141–148. [Google Scholar] [CrossRef]
- Fang, X.; Lu, G.; Mahmood, A.; Wang, Y.; Wang, X.; Xie, X.; Tang, Z.; Sun, J. A novel ternary mica-titania@rGO composite pearlescent pigment for the photocatalytic degradation of gaseous acetaldehyde. Chem. Eng. J. 2020, 396, 125312. [Google Scholar] [CrossRef]
- Zhou, S.; Lv, J.; Guo, L.K.; Xu, G.Q.; Wang, D.M.; Zheng, Z.X.; Wu, Y.C. Preparation and photocatalytic properties of N-doped nano-TiO2/muscovite composites. Appl. Surf. Sci. 2012, 258, 6136–6141. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Peng, T.; You, H.; Qin, Y.; Zeng, L. Effects of muscovite matrix on photocatalytic degradation in TiO2/muscovite nanocomposites. Appl. Clay Sci. 2019, 179, 105155. [Google Scholar] [CrossRef]
- Senthilnathan, A.; Dissanayake, D.M.S.N.; Chandrakumara, G.T.D.; Mantilaka, M.M.M.G.P.G.; Rajapakse, R.M.G.; Pitawala, H.M.T.G.A.; Nalin de Silva, K.M. Akaganeite nanorices deposited muscovite mica surfaces as sunlight active green photocatalyst. R. Soc. Open Sci. 2019, 6, 182212. [Google Scholar] [CrossRef] [Green Version]
- Peng, F.; Ni, Y.; Zhou, Q.; Kou, J.; Lu, C.; Xu, Z. New g-C3N4 based photocatalytic cement with enhanced visible-light photocatalytic activity by constructing muscovite sheet/SnO2 structures. Constr. Build. Mater. 2018, 179, 315–325. [Google Scholar] [CrossRef]
- Bao, T.; Damtie, M.M.; Hosseinzadeh, A.; Frost, R.L.; Yu, Z.M.; Jin, J.; Wu, K. Catalytic degradation of P-chlorophenol by muscovite-supported nano zero valent iron composite: Synthesis, characterization, and mechanism studies. Appl. Clay Sci. 2020, 195, 105735. [Google Scholar] [CrossRef]
- Mantilaka, M.; Senthilnathan, A.; Ekanayake, U.M.; Dissanayake, D. In-Situ Synthesis of Zinc Oxide Nano-Seeds on Muscovite Mica Sheets as A Highly Active Photocatalyst. J. Nanosci. Res. 2020, 1, 5–14. [Google Scholar]
- Sun, Z.; Li, C.; Du, X.; Zheng, S.; Wang, G. Facile synthesis of two clay minerals supported graphitic carbon nitride composites as highly efficient visible-light-driven photocatalysts. J. Colloid Interface Sci. 2018, 511, 268–276. [Google Scholar] [CrossRef]
- Akri, M.; Pronier, S.; Chafik, T.; Achak, O.; Granger, P.; Simon, P.; Trentesaux, M.; Batiot-Dupeyrat, C. Development of nickel supported La and Ce-natural illite clay for autothermal dry reforming of methane: Toward a better resistance to deactivation. Appl. Catal. B Environ. 2017, 205, 519–531. [Google Scholar] [CrossRef]
- Akri, M.; Chafik, T.; Granger, P.; Ayrault, P.; Batiot-Dupeyrat, C. Novel nickel promoted illite clay based catalyst for autothermal dry reforming of methane. Fuel 2016, 178, 139–147. [Google Scholar] [CrossRef]
- Gili, A.; Schlicker, L.; Bekheet, M.F.; Görke, O.; Penner, S.; Grünbacher, M.; Götsch, T.; Littlewood, P.; Marks, T.J.; Stair, P.C.; et al. Surface Carbon as a Reactive Intermediate in Dry Reforming of Methane to Syngas on a 5% Ni/MnO Catalyst. ACS Catal. 2018, 8, 8739–8750. [Google Scholar] [CrossRef]
- Dong, X.; Duan, X.; Sun, Z.; Zhang, X.; Li, C.; Yang, S.; Ren, B.; Zheng, S.; Dionysiou, D.D. Natural illite-based ultrafine cobalt oxide with abundant oxygen-vacancies for highly efficient Fenton-like catalysis. Appl. Catal. B Environ. 2020, 261, 118214. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J.; Zhang, Y.; Dong, H.; Qu, J.; Qi, T. Preparation of nanosized anatase TiO2-coated illite composite pigments by Ti(SO4)2 hydrolysis. Powder Technol. 2015, 271, 262–269. [Google Scholar] [CrossRef]
- Li, X.; Dong, G.; Guo, F.; Zhu, P.; Huang, Y.; Wang, C. Enhancement of photocatalytic NO removal activity of g-C3N4 by modification with illite particles. Environ. Sci. Nano 2020, 7, 1990–1998. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, W.; Liu, X. Stable hydrogen generation from vermiculite sensitized by CdS quantum dot photocatalytic splitting of water under visible-light irradiation. Dalton Trans. 2014, 43, 9296–9302. [Google Scholar] [CrossRef]
- Martínez-Costa, J.I.; Rivera-Utrilla, J.; Leyva-Ramos, R.; Sánchez-Polo, M.; Velo-Gala, I. Individual and simultaneous degradation of antibiotics sulfamethoxazole and trimethoprim by UV and solar radiation in aqueous solution using bentonite and vermiculite as photocatalysts. Appl. Clay Sci. 2018, 160, 217–225. [Google Scholar] [CrossRef]
- Hojamberdiev, M.; Katsumata, K.-i.; Matsushita, N.; Okada, K. Preparation of Bi2WO6– and BiOI–allophane composites for efficient photodegradation of gaseous acetaldehyde under visible light. Appl. Clay Sci. 2014, 101, 38–43. [Google Scholar] [CrossRef]
- Nishikiori, H.; Furuichi, N.; Teshima, K.; Yamashita, H. Reaction Kinetics on Allophane–Titania Nanocomposite Electrodes for Photofuel Cells. Chem. Lett. 2017, 46, 659–661. [Google Scholar] [CrossRef]
- Nishikiori, H.; Furukawa, M.; Fujii, T. Degradation of trichloroethylene using highly adsorptive allophane–TiO2 nanocomposite. Appl. Catal. B Environ. 2011, 102, 470–474. [Google Scholar] [CrossRef] [Green Version]
- Ono, Y.; Katsumata, K.-I. Enhanced photocatalytic activity of titanium dioxide/allophane mixed powder by acid treatment. Appl. Clay Sci. 2014, 90, 61–66. [Google Scholar] [CrossRef]
- Hojamberdiev, M.; Kadirova, Z.C.; Makinose, Y.; Zhu, G.; Emin, S.; Matsushita, N.; Hasegawa, M.; Okada, K. Involving CeVO4 in improving the photocatalytic activity of a Bi2WO6/allophane composite for the degradation of gaseous acetaldehyde under visible light. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 600–612. [Google Scholar] [CrossRef]
- El Gaidoumi, A.; Doña-Rodríguez, J.M.; Pulido Melián, E.; González-Díaz, O.M.; El Bali, B.; Navío, J.A.; Kherbeche, A. Mesoporous pyrophyllite–titania nanocomposites: Synthesis and activity in phenol photocatalytic degradation. Res. Chem. Intermed. 2019, 45, 333–353. [Google Scholar] [CrossRef]
- El Gaidoumi, A.; Doña-Rodríguez, J.M.; Pulido Melián, E.; González-Díaz, O.M.; Navío, J.A.; El Bali, B.; Kherbeche, A. Catalytic Efficiency of Cu-Supported Pyrophyllite in Heterogeneous Catalytic Oxidation of Phenol. Arab. J. Sci. Eng. 2019, 44, 6313–6325. [Google Scholar] [CrossRef]
- Rabbani, M.; Haghverdi, M.; Heidari-Golafzani, M.; Rahimi, R.; Javaheri Kachousangi, M. Preparation of a new adsorbent expanded perlite@ZnO@reduced graphene oxide for the synergistic photocatalytic–adsorption removal of organic pollutants. N. J. Chem. 2017, 41, 8011–8015. [Google Scholar] [CrossRef]
- Długosz, M.; Waś, J.; Szczubiałka, K.; Nowakowska, M. TiO2-coated EP as a floating photocatalyst for water purification. J. Mater. Chem. A 2014, 2, 6931–6938. [Google Scholar] [CrossRef]
- Xue, H.; Jiang, Y.; Yuan, K.; Yang, T.; Hou, J.; Cao, C.; Feng, K.; Wang, X. Floating photocatalyst of B–N–TiO2/expanded perlite: A sol–gel synthesis with optimized mesoporous and high photocatalytic activity. Sci. Rep. 2016, 6, 29902. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, X.; Ma, J.; Wang, X.; Wang, J.; Zhao, J. Visible-light-driven in situ inactivation of Microcystis aeruginosa with the use of floating g-C3N4 heterojunction photocatalyst: Performance, mechanisms and implications. Appl. Catal. B Environ. 2018, 226, 83–92. [Google Scholar] [CrossRef]
- Khan, U.A.; Liu, J.; Pan, J.; Ma, H.; Zuo, S.; Yu, Y.; Ahmad, A.; Iqbal, M.; Ullah, S.; Li, B. One-Pot Fabrication of Hierarchical Floating Bi–Bi2S3–Bi2WO6/Expanded Perlite Photocatalysts for Efficient Photocatalysis of Organic Contaminants Utilized Sunlike Illumination. Ind. Eng. Chem. Res. 2019, 58, 9286–9299. [Google Scholar] [CrossRef]
- Darkhosh, F.; Lashanizadegan, M.; Mahjoub, A.R.; Cheshme Khavar, A.H. One pot synthesis of CuFeO2 @ expanding perlite as a novel efficient floating catalyst for rapid degradation of methylene blue under visible light illumination. Solid State Sci. 2019, 91, 61–72. [Google Scholar] [CrossRef]
- Qiu, H.; Hu, J.; Zhang, R.; Gong, W.; Yu, Y.; Gao, H. The photocatalytic degradation of diesel by solar light-driven floating BiOI/EP composites. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123996. [Google Scholar] [CrossRef]
- Eddy, D.R.; Puri, F.N.; Noviyanti, A.R. Synthesis and Photocatalytic Activity of Silica-based Sand Quartz as the Supporting TiO2 Photocatalyst. Procedia Chem. 2015, 17, 55–58. [Google Scholar] [CrossRef] [Green Version]
- Okemoto, A.; Kishishita, K.; Maeda, S.; Gohda, S.; Misaki, M.; Koshiba, Y.; Ishida, K.; Horie, T.; Taniya, K.; Ichihashi, Y.; et al. Application of picene thin-film semiconductor as a photocatalyst for photocatalytic hydrogen formation from water. Appl. Catal. B Environ. 2016, 192, 88–92. [Google Scholar] [CrossRef]
- Misawa, K.; Sekine, Y.; Kusukubo, Y.; Sohara, K. Photocatalytic degradation of atmospheric fine particulate matter (PM2.5) collected on TiO2 supporting quartz fibre filter. Environ. Technol. 2020, 41, 1266–1274. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Li, C. Photocatalytic degradation of ofloxacin on Gd2Ti2O7 supported on quartz spheres. J. Phys. Chem. Solids 2018, 118, 144–149. [Google Scholar] [CrossRef]
- Li, R.; Wang, X.; Jin, S.; Zhou, X.; Feng, Z.; Li, Z.; Shi, J.; Zhang, Q.; Li, C. Photo-induced H2 production from a CH3OH-H2O solution at insulator surface. Sci. Rep. 2015, 5, 13475. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Tao, Y.; Li, C. Sol-gel synthesize and characterization of χGd2Ti2O7/SiO2 photocatalyst for ofloxacin decomposition. Mater. Res. Bull. 2018, 105, 55–62. [Google Scholar] [CrossRef]
- Li, D.; Zhu, Q.; Han, C.; Yang, Y.; Jiang, W.; Zhang, Z. Photocatalytic degradation of recalcitrant organic pollutants in water using a novel cylindrical multi-column photoreactor packed with TiO2-coated silica gel beads. J. Hazard. Mater. 2015, 285, 398–408. [Google Scholar] [CrossRef]
- Ismail, N.; Othman, M.H.; Kamaludin, R.; Esham, M.; Ali, N.; Rahman, M.; Jaafar, J.; Bakar, S. Characterization of Bauxite as a Potential Natural Photocatalyst for Photodegradation of Textile Dye. Arab. J. Sci. Eng. 2019, 44, 10031–10040. [Google Scholar] [CrossRef]
- Du, Y.; Wang, X.; Wu, J.; Qi, C.; Li, Y. Adsorption and photoreduction of Cr(VI) via diatomite modified by Nb2O5 nanorods. Particuology 2018, 40, 123–130. [Google Scholar] [CrossRef]
- Padmanabhan, S.K.; Pal, S.; Ul Haq, E.; Licciulli, A. Nanocrystalline TiO2–diatomite composite catalysts: Effect of crystallization on the photocatalytic degradation of rhodamine B. Appl. Catal. A Gen. 2014, 485, 157–162. [Google Scholar] [CrossRef]
- Xia, Y.; Li, F.; Jiang, Y.; Xia, M.; Xue, B.; Li, Y. Interface actions between TiO2 and porous diatomite on the structure and photocatalytic activity of TiO2-diatomite. Appl. Surf. Sci. 2014, 303, 290–296. [Google Scholar] [CrossRef]
- Zhang, G.; Peyravi, A.; Hashisho, Z.; Sun, Z.; Liu, Y.; Zheng, S.; Zhong, L. Integrated adsorption and photocatalytic degradation of VOCs using a TiO2/diatomite composite: Effects of relative humidity and reaction atmosphere. Catal. Sci. Technol. 2020, 10, 2378–2388. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, Z.; Hao, X.; Zhou, W.; Han, J.; Tang, X.; Yao, S.; Zhang, X. Enhanced oxidation of naphthalene using plasma activation of TiO2/diatomite catalyst. J. Hazard. Mater. 2018, 347, 48–57. [Google Scholar] [CrossRef]
- Barbosa, I.A.; Zanatta, L.D.; Espimpolo, D.M.; da Silva, D.L.; Nascimento, L.F.; Zanardi, F.B.; de Sousa Filho, P.C.; Serra, O.A.; Iamamoto, Y. Magnetic diatomite(Kieselguhr)/Fe2O3/TiO2 composite as an efficient photo-Fenton system for dye degradation. Solid State Sci. 2017, 72, 14–20. [Google Scholar] [CrossRef]
- He, H.; Luo, Z.; Yu, C. Diatomite-anchored g-C3N4 nanosheets for selective removal of organic dyes. J. Alloys Compd. 2020, 816, 152652. [Google Scholar] [CrossRef]
- Liu, G.; Abukhadra, M.R.; El-Sherbeeny, A.M.; Mostafa, A.M.; Elmeligy, M.A. Insight into the photocatalytic properties of diatomite@Ni/NiO composite for effective photo-degradation of malachite green dye and photo-reduction of Cr (VI) under visible light. J. Environ. Manag. 2020, 254, 109799. [Google Scholar] [CrossRef]
- Tajmiri, S.; Hosseini, M.R.; Azimi, E. Combined photocatalytic-adsorptive removal of water contaminants using a biologically prepared CdS-diatomite nanocomposite. Mater. Chem. Phys. 2021, 258, 123913. [Google Scholar] [CrossRef]
- Hua, C.; Liu, X.; Ren, S.; Zhang, C.; Liu, W. Preparation of visible light-responsive photocatalytic paper containing BiVO4@diatomite/MCC/PVBCFs for degradation of organic pollutants. Ecotoxicol. Environ. Saf. 2020, 202, 110897. [Google Scholar] [CrossRef]
- Liu, M.Y.; Zheng, L.; Lin, G.L.; Ni, L.F.; Song, X.C. Synthesis and photocatalytic activity of BiOCl/diatomite composite photocatalysts: Natural porous diatomite as photocatalyst support and dominant facets regulator. Adv. Powder Technol. 2020, 31, 339–350. [Google Scholar] [CrossRef]
- Jia, Z.; Li, T.; Zheng, Z.; Zhang, J.; Liu, J.; Li, R.; Wang, Y.; Zhang, X.; Wang, Y.; Fan, C. The BiOCl/diatomite composites for rapid photocatalytic degradation of ciprofloxacin: Efficiency, toxicity evaluation, mechanisms and pathways. Chem. Eng. J. 2020, 380, 122422. [Google Scholar] [CrossRef]
- Xiong, C.; Ren, Q.; Liu, X.; Jin, Z.; Ding, Y.; Zhu, H.; Li, J.; Chen, R. Fenton activity on RhB degradation of magnetic g-C3N4/diatomite/Fe3O4 composites. Appl. Surf. Sci. 2021, 543, 148844. [Google Scholar] [CrossRef]
- Xiong, C.; Ren, Q.; Chen, S.; Liu, X.; Jin, Z.; Ding, Y. A multifunctional Ag3PO4/Fe3O4/Diatomite composites: Photocatalysis, adsorption and sterilization. Mater. Today Commun. 2021, 28, 102695. [Google Scholar] [CrossRef]
- Yang, B.; Ma, Z.; Wang, Q.; Yang, J. Synthesis and Photoelectrocatalytic Applications of TiO2/ZnO/Diatomite Composites. Catalysts 2022, 12, 268. [Google Scholar] [CrossRef]
- Yuan, F.; Yang, R.; Li, C.; Zhang, X.; Sun, Z. Enhanced visible-light properties of TiO2/diatomite composite over varied bismuth semiconductors modification for formaldehyde photodegradation: A comparative study. Sep. Purif. Technol. 2022, 297, 121477. [Google Scholar] [CrossRef]
- Xue, L.; Liang, E.; Wang, J. Fabrication of magnetic ZnO/ZnFe2O4/diatomite composites: Improved photocatalytic efficiency under visible light irradiation. J. Mater. Sci. Mater. Electron. 2022, 33, 1405–1424. [Google Scholar] [CrossRef]
- Zhang, X.; Duan, J.; Tan, Y.; Deng, Y.; Li, C.; Sun, Z. Insight into peroxymonosulfate assisted photocatalysis over Fe2O3 modified TiO2/diatomite composite for highly efficient removal of ciprofloxacin. Sep. Purif. Technol. 2022, 293, 121123. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Cui, M.-S.; Guo, Z.; Chen, Y.-H.; Cui, K.-P.; Ding, Z.-G.; Weerasooriya, R. Binding Fe-doped g-C3N4 on the porous diatomite for efficient degradation of tetracycline via photo-Fenton process. J. Environ. Chem. Eng. 2022, 10, 107406. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhu, H.; Guo, W.; Ren, Q.; Ding, Y.; Chen, S.; Chen, J.; Jia, X. Ag3VO4/g-C3N4/diatomite ternary compound reduces Cr(vi) ion in aqueous solution effectively under visible light. RSC Adv. 2022, 12, 7671–7679. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, J.; Sun, H.; Gong, Y.; Zhang, S. Room Temperature Oxidation of Formaldehyde Using TiO2/Recycled Diatomite Composite. JOM 2022, 74, 2716–2723. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Amiri, M. CuO supported Clinoptilolite towards solar photocatalytic degradation of p-aminophenol. Powder Technol. 2013, 235, 279–288. [Google Scholar] [CrossRef]
- Nassar, M.Y.; Abdelrahman, E.A. Hydrothermal tuning of the morphology and crystallite size of zeolite nanostructures for simultaneous adsorption and photocatalytic degradation of methylene blue dye. J. Mol. Liq. 2017, 242, 364–374. [Google Scholar] [CrossRef]
- Takeuchi, M.; Hidaka, M.; Anpo, M. Efficient removal of toluene and benzene in gas phase by the TiO2/Y-zeolite hybrid photocatalyst. J. Hazard. Mater. 2012, 237–238, 133–139. [Google Scholar] [CrossRef]
- Kovalevskiy, N.S.; Lyulyukin, M.N.; Selishchev, D.S.; Kozlov, D.V. Analysis of air photocatalytic purification using a total hazard index: Effect of the composite TiO2/zeolite photocatalyst. J. Hazard. Mater. 2018, 358, 302–309. [Google Scholar] [CrossRef]
- Rahmani-Aliabadi, A.; Nezamzadeh-Ejhieh, A. A visible light FeS/Fe2S3/zeolite photocatalyst towards photodegradation of ciprofloxacin. J. Photochem. Photobiol. A Chem. 2018, 357, 1–10. [Google Scholar] [CrossRef]
- Rajabi, S.K.; Sohrabnezhad, S. Synthesis and characterization of magnetic core with two shells: Mordenite zeolite and CuO to form Fe3O4@MOR@CuO core-shell: As a visible light driven photocatalyst. Microporous Mesoporous Mater. 2017, 242, 136–143. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Taofeek Popoola, L.; Aderibigbe, E.I. Solar photocatalytic degradation of organic pollutants in textile industry wastewater by ZnO/pumice composite photocatalyst. J. Environ. Chem. Eng. 2020, 8, 103907. [Google Scholar] [CrossRef]
- Taheri-Ledari, R.; Valadi, K.; Gharibi, S.; Maleki, A. Synergistic photocatalytic effect between green LED light and Fe3O4/ZnO-modified natural pumice: A novel cleaner product for degradation of methylene blue. Mater. Res. Bull. 2020, 130, 110946. [Google Scholar] [CrossRef]
- Colangiuli, D.; Lettieri, M.; Masieri, M.; Calia, A. Field study in an urban environment of simultaneous self-cleaning and hydrophobic nanosized TiO2-based coatings on stone for the protection of building surface. Sci. Total Environ. 2019, 650, 2919–2930. [Google Scholar] [CrossRef]
- Fadhilah, N.; Etruly, N.; Muharja, M.; Sawitri, D. Self-Cleaning Limestone Paint Modified by Nanoparticles TiO2 Synthesized from TiCl3 as Precursors and PEG6000 as Dispersant. Bull. Chem. React. Eng. Catal. 2017, 12. [Google Scholar] [CrossRef] [Green Version]
- Mostafa, M.M.; El saied, M.; Morshedy, A.S. Novel Calcium Carbonate-titania nanocomposites for enhanced sun light photo catalytic desulfurization process. J. Environ. Manag. 2019, 250, 109462. [Google Scholar] [CrossRef]
- Belarbi, I.; Çoruh, A.; Hamacha, R.; Marouf-Khelifa, K.; Khelifa, A. Development and characterization of a new dolomite-based catalyst: Application to the photocatalytic degradation of pentachlorophenol. Water Sci. Technol. 2019, 79, 741–752. [Google Scholar] [CrossRef]
- Yi, H.; Huang, D.; Qin, L.; Zeng, G.; Lai, C.; Cheng, M.; Ye, S.; Song, B.; Ren, X.; Guo, X. Selective prepared carbon nanomaterials for advanced photocatalytic application in environmental pollutant treatment and hydrogen production. Appl. Catal. B Environ. 2018, 239, 408–424. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Zhuang, Y.; Fu, X. New Insight for Enhanced Photocatalytic Activity of TiO2 by Doping Carbon Nanotubes: A Case Study on Degradation of Benzene and Methyl Orange. J. Phys. Chem. C 2010, 114, 2669–2676. [Google Scholar] [CrossRef]
- Gnayem, H.; Uvarov, V.; Lahad, O.; Sasson, Y. Hybrid bismuth oxyhalides@gypsum as self-cleaning composites: Novel aspects of a sustainable photocatalytic technology for solar environmental cleanup. RSC Adv. 2015, 5, 66650–66656. [Google Scholar] [CrossRef]
- Janus, M.; Zatorska, J.; Zając, K.; Kusiak-Nejman, E.; Czyżewski, A.; Morawski, A.W. The mechanical and photocatalytic properties of modified gypsum materials. Mater. Sci. Eng. B 2018, 236, 1–9. [Google Scholar] [CrossRef]
- Binas, V.; Papadaki, D.; Maggos, T.; Katsanaki, A.; Kiriakidis, G. Study of innovative photocatalytic cement based coatings: The effect of supporting materials. Constr. Build. Mater. 2018, 168, 923–930. [Google Scholar] [CrossRef]
- Diao, Z.-H.; Xu, X.-R.; Liu, F.-M.; Sun, Y.-X.; Zhang, Z.-W.; Sun, K.-F.; Wang, S.-Z.; Cheng, H. Photocatalytic degradation of malachite green by pyrite and its synergism with Cr(VI) reduction: Performance and reaction mechanism. Sep. Purif. Technol. 2015, 154, 168–175. [Google Scholar] [CrossRef]
- Kalantary, R.R.; Moradi, M.; Pirsaheb, M.; Esrafili, A.; Jafari, A.J.; Gholami, M.; Vasseghian, Y.; Antolini, E.; Dragoi, E.-N. Enhanced photocatalytic inactivation of E. coli by natural pyrite in presence of citrate and EDTA as effective chelating agents: Experimental evaluation and kinetic and ANN models. J. Environ. Chem. Eng. 2019, 7, 102906. [Google Scholar] [CrossRef]
- Guo, Q.; Tang, G.; Zhu, W.; Luo, Y.; Gao, X. In situ construction of Z-scheme FeS2/Fe2O3 photocatalyst via structural transformation of pyrite for photocatalytic degradation of carbamazepine and the synergistic reduction of Cr(VI). J. Environ. Sci. 2021, 101, 351–360. [Google Scholar] [CrossRef]
- Puthirath Balan, A.; Radhakrishnan, S.; Kumar, R.; Neupane, R.; Sinha, S.K.; Deng, L.; de los Reyes, C.A.; Apte, A.; Rao, B.M.; Paulose, M.; et al. A Non-van der Waals Two-Dimensional Material from Natural Titanium Mineral Ore Ilmenite. Chem. Mater. 2018, 30, 5923–5931. [Google Scholar] [CrossRef]
- Simpraditpan, A.; Wirunmongkol, T.; Pavasupree, S.; Pecharapa, W. Effect of calcination temperature on structural and photocatalyst properties of nanofibers prepared from low-cost natural ilmenite mineral by simple hydrothermal method. Mater. Res. Bull. 2013, 48, 3211–3217. [Google Scholar] [CrossRef]
- Shao, S.; Yu, J.; Love, J.B.; Fan, X. An economic approach to produce iron doped TiO2 nanorods from ilmenite for photocatalytic applications. J. Alloys Compd. 2021, 858, 158388. [Google Scholar] [CrossRef]
- Hong, T.; Mao, J.; Tao, F.; Lan, M. Recyclable Magnetic Titania Nanocomposite from Ilmenite with Enhanced Photocatalytic Activity. Molecules 2017, 22. [Google Scholar] [CrossRef] [Green Version]
- Xia, D.; He, H.; Liu, H.; Wang, Y.; Zhang, Q.; Li, Y.; Lu, A.; He, C.; Wong, P.K. Persulfate-mediated catalytic and photocatalytic bacterial inactivation by magnetic natural ilmenite. Appl. Catal. B Environ. 2018, 238, 70–81. [Google Scholar] [CrossRef]
- Lee, R.B.; Lee, K.M.; Lai, C.W.; Pan, G.-T.; Yang, T.C.K.; Juan, J.C. The relationship between iron and Ilmenite for photocatalyst degradation. Adv. Powder Technol. 2018, 29, 1779–1786. [Google Scholar] [CrossRef]
- Zazo, J.A.; García-Muñoz, P.; Pliego, G.; Silveira, J.E.; Jaffe, P.; Casas, J.A. Selective reduction of nitrate to N2 using ilmenite as a low cost photo-catalyst. Appl. Catal. B Environ. 2020, 273, 118930. [Google Scholar] [CrossRef]
- Yu, C.; Wang, C.-F.; Chen, T.; Chang, Y. Synthesis and characterization of radiation sensitive TiO2/monazite photocatalyst. J. Radioanal. Nucl. Chem. 2008, 277, 337–345. [Google Scholar] [CrossRef]




| Photocatalyst | TiO2 Precursor | Method | Synthesis Temperature | Specific Surface Area (m2/g) | Application | Refs. |
|---|---|---|---|---|---|---|
| F-doped TiO2/exfoliated bentonite | Tetrabutyl titanate | A liquid exfoliation and facile sol–gel method | 400 °C | 49 | Gaseous toluene | [136] |
| Ag/TiO2/bentonite | Titanium isopropoxide | A facile thermal decomposition method | 500 °C | 80 | S. aureus, E. coli and human embryonic kidney cell line | [137] |
| Au–TiO2/bentonite | Tetra-n-butyl titanate | Deposition–precipitation and calcination method | 400 °C | 178 | Sulforhodamine B photodegradation | [138] |
| F, V and Mn co-doped TiO2-pillared bentonite | Butyl titanate | A sol-gel method | 500 °C | 86 | NO photocatalytic denitration | [139] |
| g-C3N4/TiO2/bentonite | Titanium butoxide | A wet impregnation process | 60 °C | 70 | Reactive brilliant red dye | [140] |
| TiO2 pillared montmorillonite | Tetrabutyl titanate | An intra-gallery template method | 500 °C | 392 | MB | [141] |
| Montmorillonite Modified TiO2 | Tetra-isopropyl orthotitanate | A single sol-gel method | 500 °C | 83 | CO2 reduction | [142] |
| Ni/montmorillonite supported TiO2 | Tetraisopropyl orthotitanate | Sol-gel method | 500 °C | 58 | CO2 reforming of methane | [143] |
| Montmorillonite dispersed single wall carbon nanotubes (SWCNTs)/TiO2 | Tetraisopropyl orthotitanate | A simple sol-gel assisted wet-impregnation method | 180 °C | -- | H2 production | [144] |
| Ag@AgCl-TiO2/organic rectorite/quaternized chitosan | Titanium tetraisopropoxide | Sol–gel combing calcination technique, precipitation and photoreduction combined with emulsification/chemical crosslinking method | 80 °C | 89 | MO | [145] |
| Ag-AgCl-TiO2/rectorite | Titanium tetraisopropoxide | A facile deposition–photoreduction method | Room temperature | -- | Acid orange and 4-NP | [146] |
| Bi-crystalline TiO2/rectorite | Titanium tetraisopropoxide | A facile sol–gel method | 80 °C | 100 | Acid red G and 4-NP | [147] |
| TiO2-pillared saponite | Titanium tetraisopropoxide | Intercalation, neutralization and calcination process | 400 °C | 154 | BPB | [12] |
| TiO2-montmorillonite | Tetrabutyl titanate | Hydrothermal | 160 °C | 77 | MB | [148] |
| TiO2/pillared clays | Titanium isopropoxid | Wet impregnation through stirring followed by calcination | 500 | 29 ± 5 | TCS, 2,6-DCP and BPA | [149] |
| TiO2/sepiolite | Titanyl sulfate | Structural assembly and thermal treatment | 400 | 135 | HCHO removal and H2 evolution | [150] |
| Bi2MoO6/TiO2/sepiolitte | Titanyl sulfate | A facile hydrolysis precipitation and calcination crystallization route | 500 | 100 | HCHO | [151] |
| TiO2-BiOBr-bentonite | Tetrabutyl titanate | Solvothermal | 160 | 125 | Phenol | [152] |
| Bentonite-TiO2 | Titanium isopropoxide | Sol-gel | 500 | 124 | TC | [153] |
| Fe3O4 @TiO2/silica aerogel | Tetraethyl orthotitanate | Sol-gel and ultrasound assisted reflux technique | 400 | 347 | TC | [154] |
| Photocatalyst | Method | Application | Light Source | Photocatalytic Efficiency (%) | Refs. |
|---|---|---|---|---|---|
| Nb2O5@diatomite | Hydrothermal | Photoreduction of Cr(VI) | 500 W mercury lamp | 94.50 | [226] |
| TiO2-diatomite | Thermal hydrolysis and annealing method | RhB | 300 W UV lamp | -- | [227] |
| TiO2-diatomite | Sol-gel and calcination or phosphoric acid pretreatment | MO | 250 W mercury lamp and 300 W white light lamp | 90 and 60 | [228] |
| Diatomite/Fe2O3/TiO2 | Co-precipitation and impregnation method | MB | 300 W xenon lamp | 99 | [231] |
| g-C3N4/diatomite | Calcination | MB | 350 W xenon lamp | 90 | [232] |
| Diatomite @Ni/NiO | Acid leaching and in situ reduction method | MG and Cr(VI) photoreduction | 400 W metal halide lamp | almost 100 | [233] |
| CdS @diatomite | Biological synthesis using Bacillus licheniformis | MB and Cr(VI) photoreduction | 360–450 nm UVA lamp | over 90 | [234] |
| BiVO4 @diatomite/microcrystalline cellulose/PVB composite fibers | Hydrothermal and electrospinning method | MB and HCHO | 250 W high pressure mercury lamp | 66.80 and 56.80 for MB and HCHO | [235] |
| BiOCl/diatomite | Hydrothermal | TH and gaseous HCHO | 300 W over 420 nm xenon lamp | 91.02 and 63.01 for TC and HCHO | [236] |
| BiOCl/diatomite | Hydrolysis | CIP | 300 W 320–780 nm xenon lamp | 94 | [237] |
| Magnetic g-C3N4/diatomite/Fe3O4 | An electrostatic self-assembly method | RhB | 500 W over 420 nm xenon lamp | 98 | [238] |
| Ag3PO4/Fe3O4/diatomite | Hydrothermal | E. coli and RhB | 500 W xenon lamp | 100 and 98 for E. coli and RhB | [239] |
| TiO2/ZnO/diatomite | Two-step precipitation | MB | 30 W xenon lamp | 80.34 | [240] |
| Bi2MoO6 modified TiO2/diatomite | Hydrothermal | HCHO | 300 W over 400 nm xenon lamp | 96.32 | [241] |
| ZnO/ZnFe2O4/diatomite | Hydrothermal-precipitation | OTC | 300 W xenon lamp | 95 | [242] |
| Fe2O3/TiO2/diatomite | Chemical precipitation-calcination | CIP | 300 W over 420 nm xenon lamp | 88 | [243] |
| Fe-g-C3N4/diatomite | Thermal polymerization | TC | 500 W xenon lamp | 98.3 | [244] |
| Ag3VO4/g-C3N4/diatomite | Annealing-precipitation | Cr(VI) | 500 W over 420 nm xenon lamp | 70 | [245] |
| Fe3O4@TiO2/diatomite | Sol-gel and ultrasound assisted reflux technique | TC | 500 W high-pressure mercury-vapor lamp | 97 | [154] |
| TiO2/recycled diatomite | Acid leaching and alkaline treatment | HCHO | 300 W xenon lamp | 95.59 | [246] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Simon, U.; Bekheet, M.F.; Gurlo, A. Mineral-Supported Photocatalysts: A Review of Materials, Mechanisms and Environmental Applications. Energies 2022, 15, 5607. https://doi.org/10.3390/en15155607
Li X, Simon U, Bekheet MF, Gurlo A. Mineral-Supported Photocatalysts: A Review of Materials, Mechanisms and Environmental Applications. Energies. 2022; 15(15):5607. https://doi.org/10.3390/en15155607
Chicago/Turabian StyleLi, Xue, Ulla Simon, Maged F. Bekheet, and Aleksander Gurlo. 2022. "Mineral-Supported Photocatalysts: A Review of Materials, Mechanisms and Environmental Applications" Energies 15, no. 15: 5607. https://doi.org/10.3390/en15155607
APA StyleLi, X., Simon, U., Bekheet, M. F., & Gurlo, A. (2022). Mineral-Supported Photocatalysts: A Review of Materials, Mechanisms and Environmental Applications. Energies, 15(15), 5607. https://doi.org/10.3390/en15155607








