Assessment of Biomass and Biochar of Maritime Pine as a Porous Medium for Water Retention in Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biochar Production
2.2. Characterization of Biomass, Biochar and Activated Biochar
2.2.1. Proximate Analysis
2.2.2. Thermogravimetric Analysis
2.2.3. SEM
2.2.4. Kripton and N2 Adsorption at 77 K
2.2.5. Mercury Porosimetry
2.2.6. FTIR Spectroscopy
2.3. Water Retention Curves (pF curves)
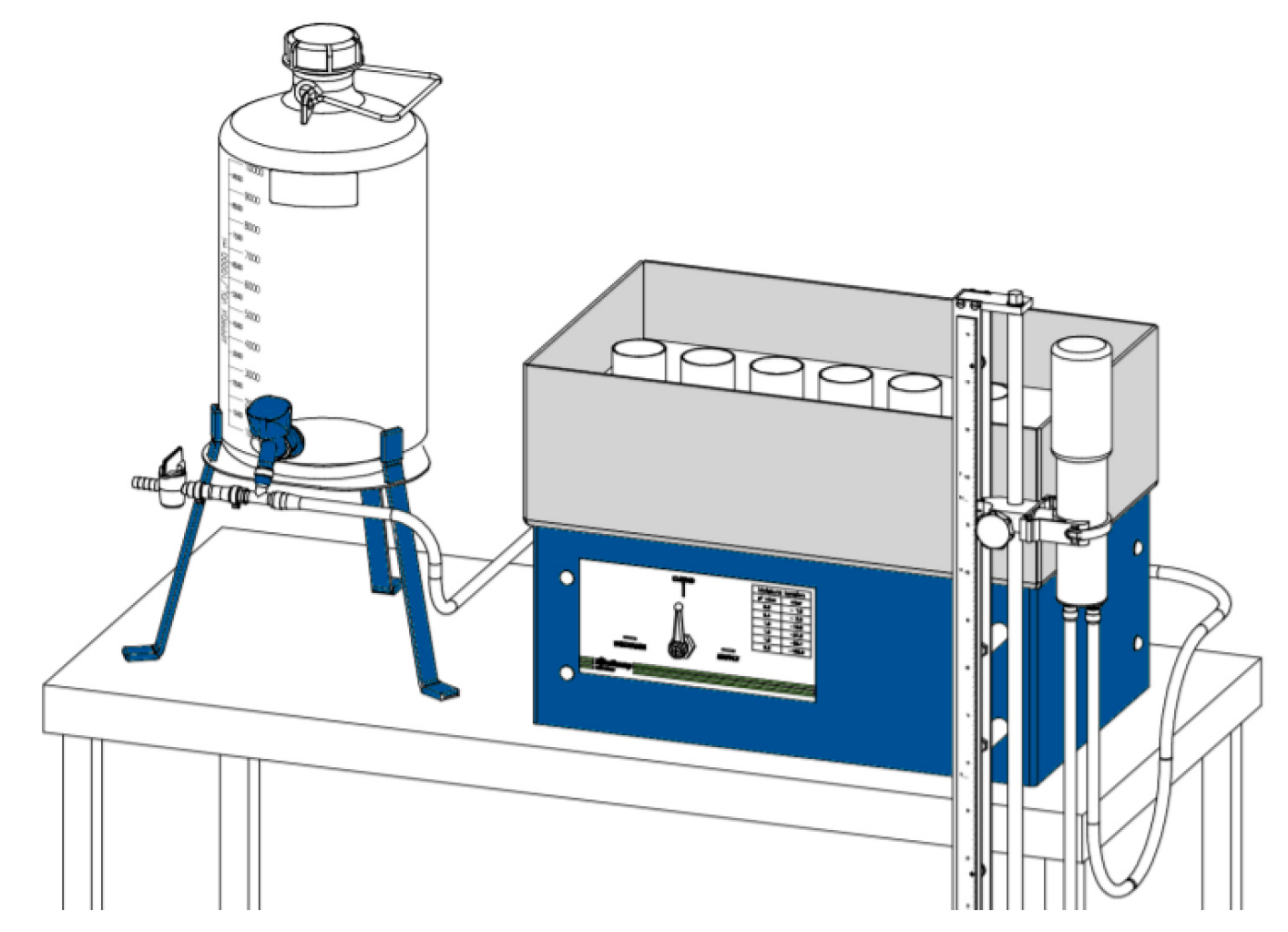
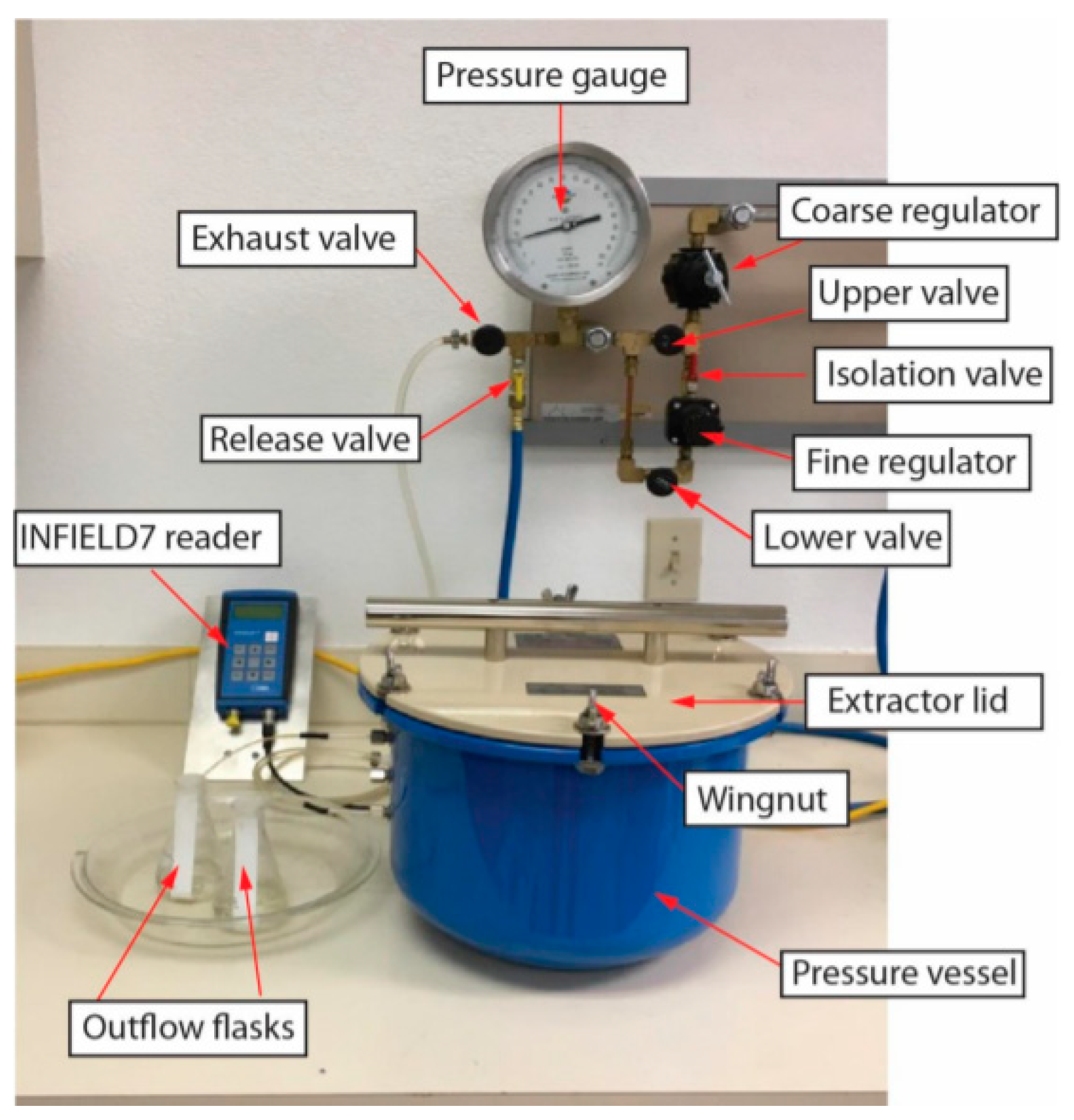
2.3.1. The Experimental Approach
2.3.2. Plant Available Water
2.3.3. Van Genutchen Model
2.3.4. Water Holding Capacity (Field Capacity)
3. Results and Discussion
3.1. Biochar Production
3.2. Characterization of Biomass and Biochar Samples
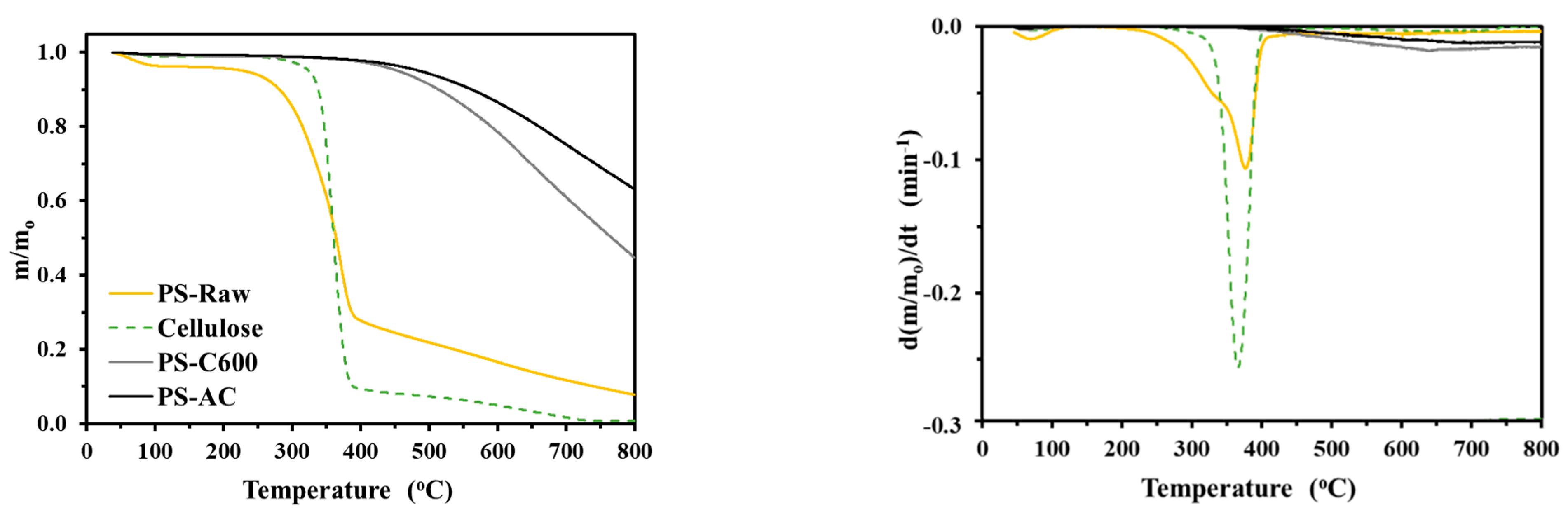
3.2.1. Proximate, Thermogravimetric and FTIR Analysis
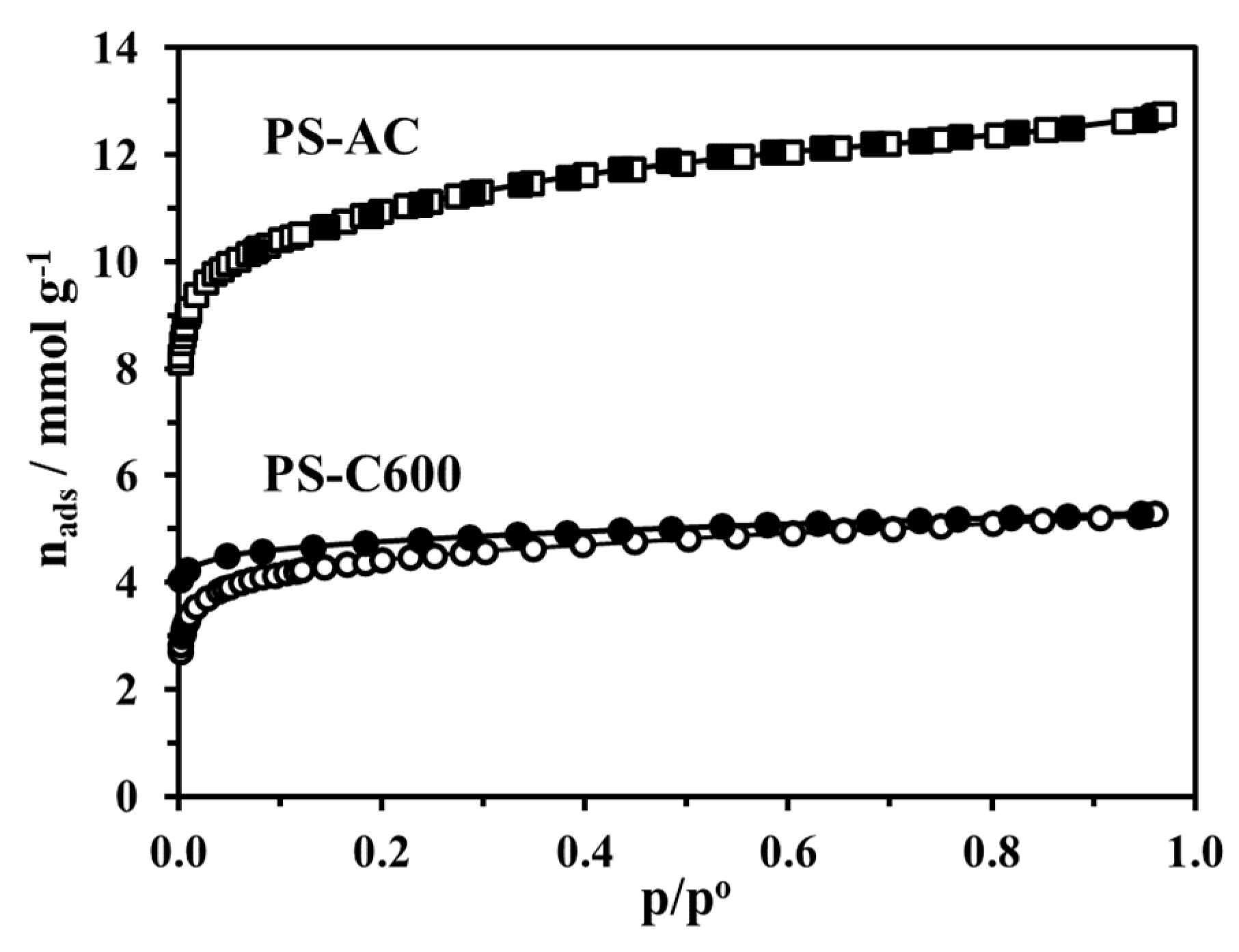
3.2.2. Adsorption Equilibrium and Porous Structure
3.3. Water Holding Capacity (WHC)
3.4. Water Retention Curves
3.4.1. Sand and Biochars
3.4.2. Plant Available Water
4. Conclusions
- (i)
- The pyrogenic nanoporosity was revealed by IUPAC Type I isotherms indicative of microporosity, more developed in the activated biochar;
- (ii)
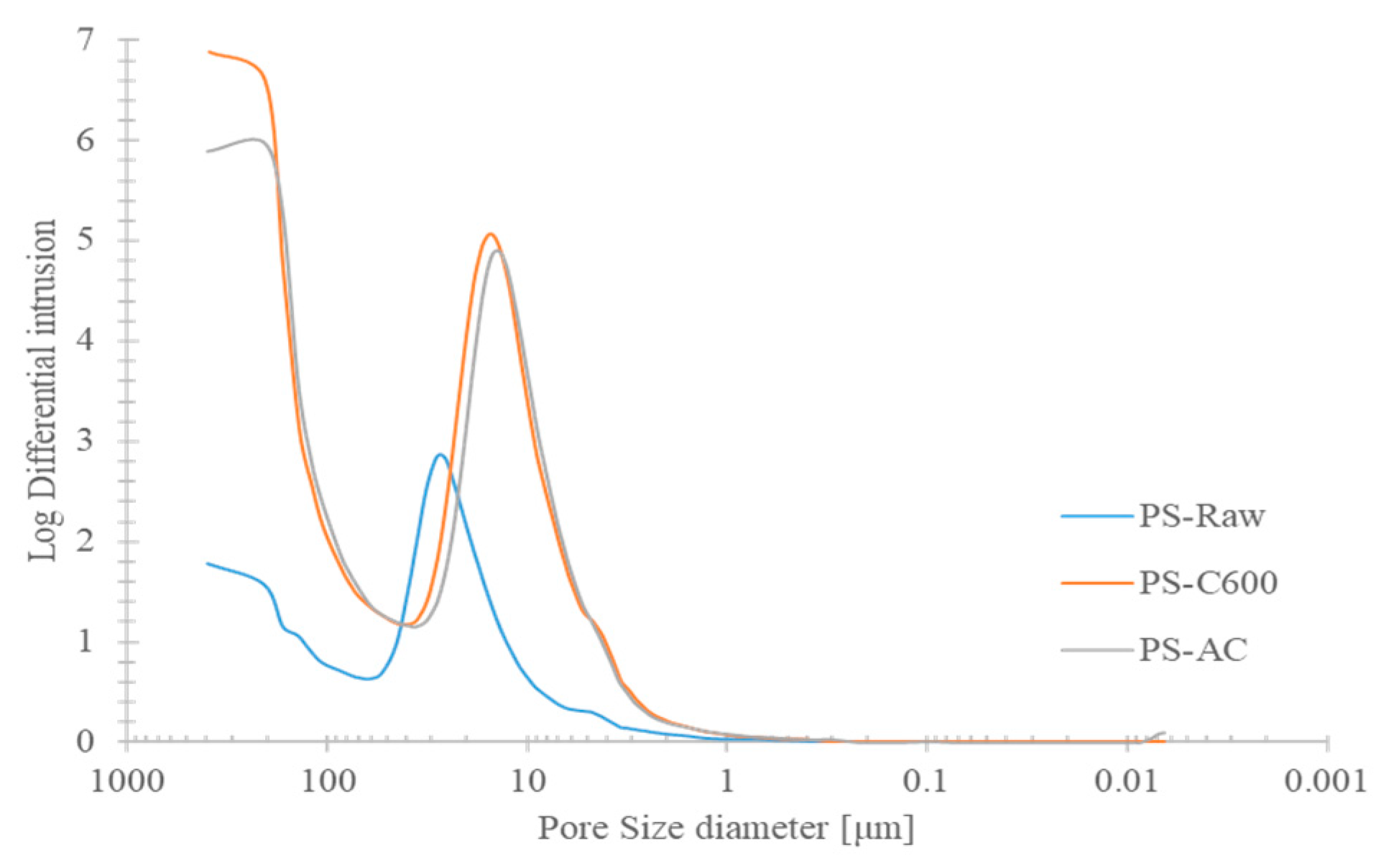
- From mercury porosimetry, it was shown that pores between 392 and 250 μm corresponding to a biochar delivered the highest pore volume for water retention. For activated biochar, the largest correspondent porosities respected pore dimensions between 32 μm to 6 μm. These results reflected a major macroporosity increase under carbonization stage by comparison with subsequent physical activation.
- (iii)
- SEM evaluation showed an enlargement of biological tracheid, safeguarding the whole cellular structure of raw biomass;
- (iv)
- The Van Genutchen model proved useful to simulate water dynamics in biochar-sand mixtures;
- (v)
- The biochar-sand mixtures showed a significant improvement in plant available water indicating the potential of biochar as feedstock for the betterment of hydric availability for agricultural purposes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lei, O.; Zhang, R. Effects of biochars derived from different feedstocks and pyrolysis temperatures on soil physical and hydraulic properties. J. Soils Sediments 2013, 13, 1561–1572. [Google Scholar] [CrossRef]
- Masiello, C.; Dugan, B.; Brewer, C.; Spokas, K.; Novak, J.; Liu, Z. Biochar Effects on Soil Hydrology. In Biochar for Environmental Management, Science, Technology and Implementation; Routledge: London, UK, 2021; pp. 543–562. [Google Scholar]
- Zhang, J.; You, C. Water Holding Capacity and Absorption Properties of Wood Chars. Energy Fuels 2013, 27, 2643–2648. [Google Scholar] [CrossRef]
- Hafeez, A.; Pan, T.; Tian, J.; Cai, K. Modified Biochars and Their Effects on Soil Quality: A Review. Environments 2022, 9, 60. [Google Scholar] [CrossRef]
- Ferreira, S.; Monteiro, E.; Brito, P.; Vilarinho, C. Biomass resources in Portugal: Current status and prospects. Renew. Sustain. Energy Rev. 2017, 78, 1221–1235. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Lopez-Capel, E.; Zwart, K.; Shackley, S.; Postma, R.; Strenstrom, J.; Rasse, D.; Budai, A.; Glaser, B. Biochar Properties, Ch.3 in Biochar in European Soils and Agriculture, Science and Practiceo Title; Shackley, S., Ruysschaert, G., Zwart, K., Glaser, B., Eds.; Earthscan from Routledge: London, UK, 2020; ISBN 978-0-367-60604-6. [Google Scholar]
- Chia, C.; Downie, A.; Munroe, P. Characteristics of Biochar: Physical and Structural Properties. In Biochar for Environmental Management; Lehmann, J., Stephen, J., Eds.; Routledge: London, UK, 2021; pp. 89–111. ISBN 13:978-0-367-77918-4. [Google Scholar]
- Irfan, M. Potential value of biochar as a soil amendment: A review. Pure Appl. Biol. 2017, 6, 1494–1502. [Google Scholar] [CrossRef]
- Troeh, F.; Thompson, L. Soils and Soil Fertility; Blackwell Publishing: Iowa, IA, USA, 2005; ISBN 978-0-813-80955-7. [Google Scholar]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Using poultry litter biochars as soil amendments. Aust. J. Soil Res. 2008, 46, 437–444. [Google Scholar] [CrossRef]
- Downie, A.; Crosky, A.; Munroe, P. Physical Properties of Biochar, Chapter 2. In Biochar for Environmental Management; Lehmann, J., Joseph, S., Eds.; Routledge: London, UK, 2009; pp. 13–30. [Google Scholar]
- Nelissen, V.; Ruysschaert, G.; Manka’Abusi, D.; D’Hose, T.; De Beuf, K.; Al-Barri, B.; Cornelis, W.; Boeckx, P. Impact of a woody biochar on properties of a sandy loam soil and spring barley during a two-year field experiment. Eur. J. Agron. 2015, 62, 65–78. [Google Scholar] [CrossRef]
- Cross, A.; Zwart, K.; Shackley, S.; Ruysschaert, G. The Role of Biochar in Agricultural Soils. In Biochar in European Soils and Agriculture: Science and Practice; Routledge: London, UK, 2016; pp. 73–98. ISBN 9781134654949. [Google Scholar]
- Kinney, T.J.; Masiello, C.A.; Dugan, B.; Hockaday, W.C.; Dean, M.R.; Zygourakis, K.; Barnes, R.T. Hydrologic properties of biochars produced at different temperatures. Biomass Bioenergy 2012, 41, 34–43. [Google Scholar] [CrossRef]
- Novak, J.M.; Busscher, W.J.; Watts, D.W.; Amonette, J.E.; Ippolito, J.A.; Lima, I.M.; Gaskin, J.; Das, K.C.; Steiner, C.; Ahmedna, M.; et al. Biochars Impact on Soil-Moisture Storage in an Ultisol and Two Aridisols. Soil Sci. 2012, 177, 310–320. [Google Scholar] [CrossRef]
- Gray, M.; Johnson, M.G.; Dragila, M.I.; Kleber, M. Water uptake in biochars: The roles of porosity and hydrophobicity. Biomass Bioenergy 2014, 61, 196–205. [Google Scholar] [CrossRef]
- Wildman, J.; Derbyshire, F. Origins and functions of macroporosity in activated carbons from coal and wood precursors. Fuel 1991, 70, 655–661. [Google Scholar] [CrossRef]
- Sun, H.; Hockaday, W.; Masiello, C.; Zygourakis, K. Multiple Controls on the Chemical and Physical Structure of Biochars. Ind. Eng. Chem. Res. 2012, 51, 3587–3597. [Google Scholar] [CrossRef]
- Chun, Y.; Sheng, G.; Chiou, C.T.; Xing, B. Compositions and Sorptive Properties of Crop Residue-Derived Chars. Environ. Sci. Technol. 2004, 38, 4649–4655. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Hu, S.; Xiang, J.; Sun, L.; Su, S.; Wang, J. Evaluation of the porous structure development of chars from pyrolysis of rice straw: Effects of pyrolysis temperature and heating rate. J. Anal. Appl. Pyrolysis 2012, 98, 177–183. [Google Scholar] [CrossRef]
- Li, W.; Yang, K.; Peng, J.; Zhang, L.; Guo, S.; Xia, H. Effects of carbonization temperatures on characteristics of porosity in coconut shell chars and activated carbons derived from carbonized coconut shell chars. Ind. Crop. Prod. 2008, 28, 190–198. [Google Scholar] [CrossRef]
- Marsh, H.; Rodríguez-Reinoso, F. (Eds.) Chapter 4-Characterization of Activated Carbon. In Activated Carbon; Elsevier Science Ltd.: Oxford, UK, 2006; pp. 143–242. ISBN 978-0-08-044463-5. [Google Scholar] [CrossRef]
- Novak, J.M.; Lima, I.; Xing, B.; Gaskin, J.W.; Steiner, C.; Das, K.C.; Ahmedna, M.; Rehrah, D.; Watts1, D.W.; Busscher1, W.J.; et al. Characterization of Designer Biochar Produced at Different Temperatures and Their Effects on a Loamy Sand. Ann. Environ. Sci. 2009, 3, 195–206. [Google Scholar]
- Ferreira, A.F.; Ribau, J.P.; Costa, M. A decision support method for biochars characterization from carbonization of grape pomace. Biomass Bioenergy 2021, 145, 105946. [Google Scholar] [CrossRef]
- Basu, P. (Ed.) Chapter 5-Pyrolysis. In Biomass Gasification, Pyrolysis and Torrefaction, 2nd ed.; Academic Press: Boston, MA, USA, 2013; pp. 147–176. ISBN 978-0-12-396488-5. [Google Scholar]
- Sakhiya, A.K.; Anand, A.; Kaushal, P. Production, activation, and applications of biochar in recent times. Biochar 2020, 2, 253–285. [Google Scholar] [CrossRef]
- Grima-Olmedo, C.; Ramírez-Gómez, Á.; Gómez-Limón, D.; Clemente-Jul, C. Activated carbon from flash pyrolysis of eucalyptus residue. Heliyon 2016, 2, e00155. [Google Scholar] [CrossRef]
- Zhang, T.; Walawender, W.P.; Fan, L.; Fan, M.; Daugaard, D.; Brown, R. Preparation of activated carbon from forest and agricultural residues through CO2 activation. Chem. Eng. J. 2004, 105, 53–59. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S.W.; Llewellyn, P.; Maurin, G. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-08-097035-6. [Google Scholar]
- Fong, A.; Tuli, A. Determination of Soil Water Characteristic Curve Using 5 Bar Ceramic Pressure Plate Extractor. Standard Operating Procedure Number: METH016.00; California Department of Pesticide Regulation, Environmental Monitoring Branch: Sacramento, CA, USA, 2018. [Google Scholar]
- van Genuchten, M.T. A Closed-form Equation for Predicting the Hydraulic Conductivity of Unsaturated Soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Bikbulatova, S.; Tahmasebi, A.; Zhang, Z.; Rish, S.K.; Yu, J. Understanding water retention behavior and mechanism in bio-char. Fuel Process. Technol. 2018, 169, 101–111. [Google Scholar] [CrossRef]
- Carrott, P.J.M.; Carrott, M.R.; Guerrero, C.I.; Delgado, L.A. Reactivity and porosity development during pyrolysis and physical activation in CO2 or steam of kraft and hydrolytic lignins. J. Anal. Appl. Pyrolysis 2008, 82, 264–271. [Google Scholar] [CrossRef]
- Stehfest, K.; Toepel, J.; Wilhelm, C. The application of micro-FTIR spectroscopy to analyze nutrient stress-related changes in biomass composition of phytoplankton algae. Plant Physiol. Biochem. 2005, 43, 717–726. [Google Scholar] [CrossRef]
- Mecozzi, M.; Pietroletti, M.; Tornambè, A. Molecular and structural characteristics in toxic algae cultures of Ostreopsis ovata and Ostreopsis spp. evidenced by FTIR and FTNIR spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 78, 1572–1580. [Google Scholar] [CrossRef]
- Ferreira, A.F.; Dias, A.S.; Silva, C.M.; Costa, M. Evaluation of thermochemical properties of raw and extracted microalgae. Energy 2015, 92, 365–372. [Google Scholar] [CrossRef]
- Paulo, I.; Costa, L.; Rodrigues, A.; Orišková, S.; Matos, S.; Gonçalves, D.; Gonçalves, A.R.; Silva, L.; Vieira, S.; Bordado, J.C.; et al. Acid-Catalyzed Liquefaction of Biomasses from Poplar Clones for Short Rotation Coppice Cultivations. Molecules 2022, 27, 304. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, D.; Orišková, S.; Matos, S.; Machado, H.; Vieira, S.; Bastos, D.; Gaspar, D.; Paiva, R.; Bordado, J.C.; Rodrigues, A.; et al. Thermochemical Liquefaction as a Cleaner and Efficient Route for Valuing Pinewood Residues from Forest Fires. Molecules 2021, 26, 7156. [Google Scholar] [CrossRef]
- Liu, Y.; He, Z.; Uchimiya, M. Comparison of Biochar Formation from Various Agricultural By-Products Using FTIR Spectroscopy. Mod. Appl. Sci. 2015, 9, 246–253. [Google Scholar] [CrossRef]
- Slaný, M.; Jankovič, L’.; Madejová, J. Structural characterization of organo-montmorillonites prepared from a series of primary alkylamines salts: Mid-IR and near-IR study. Appl. Clay Sci. 2019, 176, 11–20. [Google Scholar] [CrossRef]
- Yi, S.; Chang, N.Y.; Imhoff, P.T. Predicting water retention of biochar-amended soil from independent measurements of biochar and soil properties. Adv. Water Resour. 2020, 142, 103638. [Google Scholar] [CrossRef]
- Liu, Z.; Dugan, B.; Masiello, C.; Gonnermann, H.M. Biochar particle size, shape, and porosity act together to influence soil water properties. PLoS ONE 2017, 12, e0179079. [Google Scholar] [CrossRef] [PubMed]








| Test | Biomass/Biochar [% Volume] | Sand [% Volume] |
|---|---|---|
| Water holding Capacity Water Retention curves | - | 100% |
| 100% | - | |
| 50% | 50% | |
| 10% | 90% |
| Sample Type | Volatile Matter (%) | Ash (%) | Fixed Carbon (%) | Calorific Value [MJ/kg] |
|---|---|---|---|---|
| PS-Raw | 86.85 | 0.49 | 12.66 | 18.01 |
| PS-C600 | 15.16 | 1.39 | 83.46 | 31.86 |
| Wavenumber (cm−1) | Band Assignment | Compounds/Groups |
|---|---|---|
| 3338 | OH stretching | Hydroxyls groups |
| 2889–2981 | CH2−, CH3− stretching | methylene and methyl groups from holocellulose and lignin |
| 1731 | C=O stretching | ketones and esters |
| 1720 | C=O carbonyls in ester groups and acetyl groups in xylan | Ketones, esters, hemicellulose, and carboxylic acids and esters |
| 1646–1633 | OH bending | water |
| 1604 | C=C aromatic ring vibration | Lignin |
| 1599 | C=C stretching | aromatic ring, lignin |
| 1514 | C=C aromatic ring stretching | Lignin |
| 1461–1444 | OCH3−, -CH2-, and C-H stretching | Carbohydrates, Cellulose, hemicellulose |
| 1369 | aromatic C–H deformation | syringyl rings (from lignin) |
| 1330 | C-O syringyl ring | Lignin |
| 1264–1246 | Aromatic ring vibration | Guaicyl lignin |
| 1096 | C-O-C stretching | Cellulose, hemicellulose |
| 1020 | C-O, C=C, and C-C-O stretching | Cellulose, hemicellulose, lignin |
| 906 | Glycosidic linkage | Cellulose, hemicellulose |
| 896 | C−O, C=C, and C−C−O | hemicellulose |
| Sample | ABET [m2g−1] | V0.95 [cm3g−1] |
|---|---|---|
| PS-Raw | 0.34 | - |
| PS-C600 | 375 | 0.184 |
| PS-AC | 937 | 0.443 |
| Sample | Total Pore Volume [mL·g−1] | Porosity [%] | Macropores (>50 nm) [mL/g] | Mesopores (2–50 nm) [mL/g] | Cryptopores (<0.1µm) [mL/g] * | Bulk Density [g/mL] |
|---|---|---|---|---|---|---|
| PS-Raw | 2.47 | 76.94 | 2.47 | 0.000 | 0.00 | 0.31 |
| PS-C600 | 6.63 | 88.67 | 6.63 | 0.000 | 0.00 | 0.13 |
| PS-AC | 6.37 | 88.89 | 6.37 | 0.006 | 7.00e-03 | 0.14 |
| PS-C600 0% Biochar Amount | PS-C600 10% Biochar Amount | PS-C600 50% Biochar Amount | PS-C600 100% Biochar Amount | |||||
|---|---|---|---|---|---|---|---|---|
| θ * | % θ Reduction ** | θ * | % θ Reduction ** | θ * | % θ Reduction ** | θ * | % θ Reduction ** | |
| θ-pF 0 | 0.39 | - | 0.43 | - | 0.62 | - | 0.88 | - |
| θ-pF 2 | 0.08 | 20.51 | 0.50 | 116.28 | 0.17 | 27.42 | 0.21 | 23.86 |
| θ-pF 2.5 | 0.04 | 10.26 | 0.12 | 27.91 | 0.13 | 20.97 | 0.17 | 19.32 |
| θ-pF 4.2 | 0.02 | 5.13 | 0.08 | 18.60 | 0.08 | 12.90 | 0.10 | 11.36 |
| Category | Biochar Amount | Sample | θpaw(cm3/cm3) | % θpaw Increases |
|---|---|---|---|---|
| Mixtures | 0% | Sand | 0.063 | - |
| Mixtures | 10% | PS-C600 | 0.074 | 117 |
| Mixtures | 50% | PS-C600 | 0.095 | 151 |
| Biochar | 100% | PS-C600 | 0.108 | 171 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, R.V.; Mendes, M.A.A.; Alexandre, C.; Carrott, M.R.; Rodrigues, A.; Ferreira, A.F. Assessment of Biomass and Biochar of Maritime Pine as a Porous Medium for Water Retention in Soils. Energies 2022, 15, 5882. https://doi.org/10.3390/en15165882
Santos RV, Mendes MAA, Alexandre C, Carrott MR, Rodrigues A, Ferreira AF. Assessment of Biomass and Biochar of Maritime Pine as a Porous Medium for Water Retention in Soils. Energies. 2022; 15(16):5882. https://doi.org/10.3390/en15165882
Chicago/Turabian StyleSantos, Rodrigo V., Miguel A. A. Mendes, Carlos Alexandre, Manuela Ribeiro Carrott, Abel Rodrigues, and Ana F. Ferreira. 2022. "Assessment of Biomass and Biochar of Maritime Pine as a Porous Medium for Water Retention in Soils" Energies 15, no. 16: 5882. https://doi.org/10.3390/en15165882






