Effects of Solubilizer and Magnetic Field during Crystallization Induction of Ammonium Bicarbonate in New Ammonia-Based Carbon Capture Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Chemicals
2.2. Experimental System
2.3. Experimental Steps
2.4. Analytical Methods
3. Results and Discussion
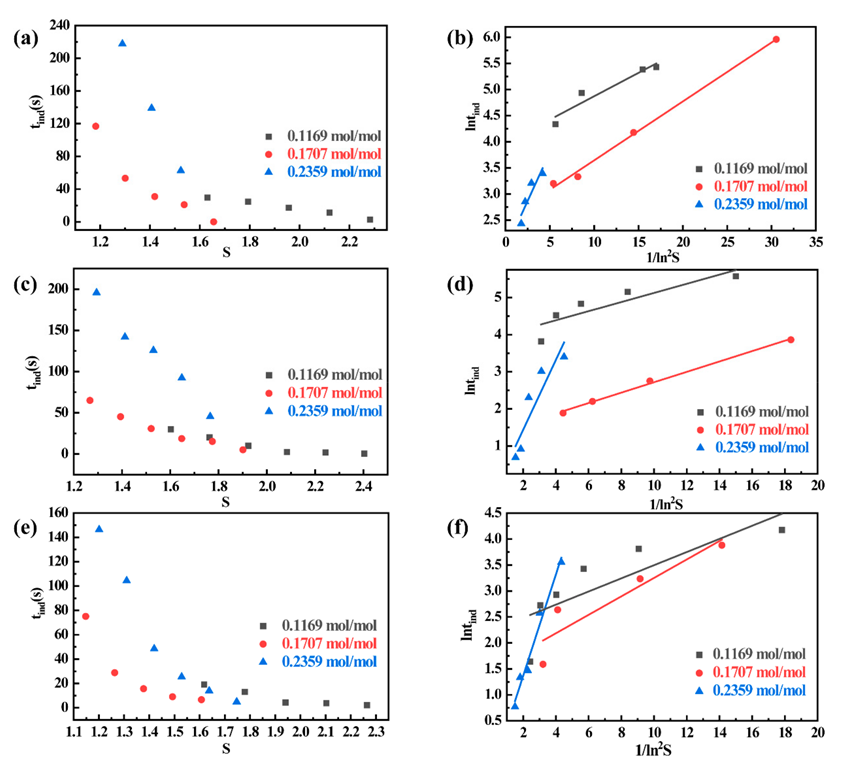
3.1. Effect of Lysis Precipitant Content on Induction Period
3.2. Effect of Temperature on Induction Period
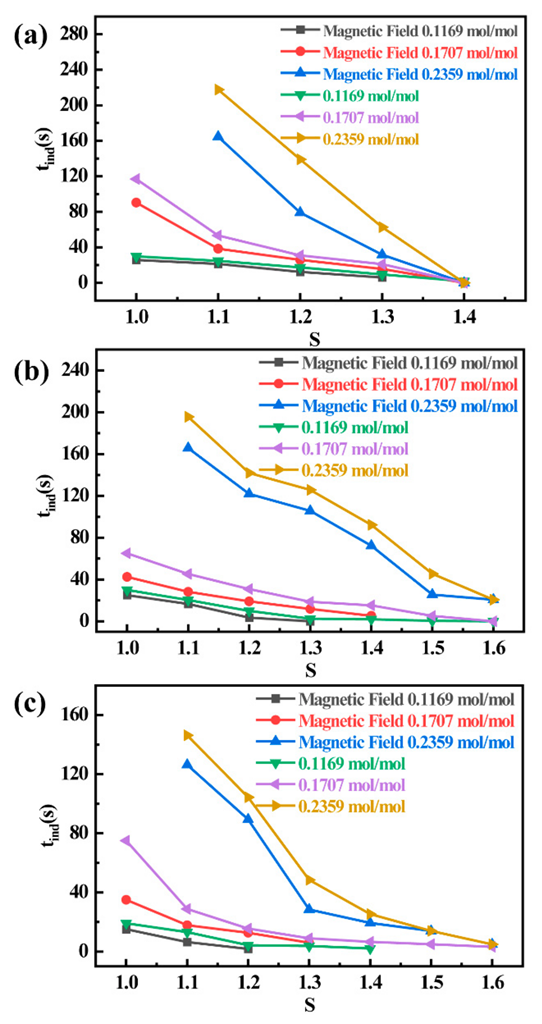
3.3. Effect of Magnetic Field on Induction Period
3.4. Crystal Surface Growth Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Feng, D.; Gao, J.; Zhang, Y.; Li, H.; Du, Q.; Wu, S. Mass transfer in ammonia-based CO2 absorption in bubbling reactor under static magnetic field. Chem. Eng. J. 2018, 338, 450–456. [Google Scholar] [CrossRef]
- Kozak, F.; Petig, A.; Morris, E.B.; Rhudy, R.; Thimsen, D. Chilled ammonia process for CO2 capture. Energy Procedia 2009, 1, 1419–1426. [Google Scholar] [CrossRef]
- Yang, Y.; Liew, R.K.; Tamothran, A.M.; Foong, S.Y.; Yek, P.N.Y.; Chia, P.W.; Van Tran, T.; Peng, W.; Lam, S.S. Gasification of refuse-derived fuel from municipal solid waste for energy production: A review. Environ. Chem. Lett. 2021, 19, 2127–2140. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Guo, Y.; Sun, J.; Li, H.; Liu, W. Progress in MgO sorbents for cyclic CO2 capture: A comprehensive review. J. Mater. Chem. A 2019, 7, 20103–20120. [Google Scholar] [CrossRef]
- Bavarella, S.; Hermassi, M.; Brookes, A.; Moore, A.; Vale, P.; Moon, I.S.; Pidou, M.; McAdam, E.J. Recovery and concentration of ammonia from return liquor to promote enhanced CO2 absorption and simultaneous ammonium bicarbonate crystallisation during biogas upgrading in a hollow fibre membrane contactor. Sep. Purif. Technol. 2020, 241, 116631. [Google Scholar] [CrossRef]
- Al-Hamed, K.H.M.; Dincer, I. A novel multigeneration ammonia-based carbon capturing system powered by a geothermal power plant for cleaner applications. J. Clean. Prod. 2021, 321, 129017. [Google Scholar] [CrossRef]
- Al-Hamed, K.H.M.; Dincer, I. A comparative review of potential ammonia-based carbon capture systems. J. Environ. Manag. 2021, 287, 112357. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, S.-R.; Jung, K.A.; Park, J.M. Process-based life cycle CO2 assessment of an ammonia-based carbon capture and storage system. J. Ind. Eng. Chem. 2019, 76, 223–232. [Google Scholar] [CrossRef]
- Ishaq, H.; Ali, U.; Sher, F.; Anus, M.; Imran, M. Process analysis of improved process modifications for ammonia-based post-combustion CO2 capture. J. Environ. Chem. Eng. 2021, 9, 104928. [Google Scholar] [CrossRef]
- Leung, D.Y.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef] [Green Version]
- Rochelle, G.T. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Yeh, A.C. Removal of CO2 greenhouse gas by ammonia scrubbing. Ind. Eng. Chem. Res. 1997, 36, 2490–2493. [Google Scholar] [CrossRef]
- Zhao, B.; Su, Y.; Tao, W.; Li, L.; Peng, Y. Post-combustion CO2 capture by aqueous ammonia: A state-of-the-art review. Int. J. Greenh. Gas Control 2012, 9, 355–371. [Google Scholar] [CrossRef]
- Mclarnon, C.R.; Duncan, J.L. Testing of Ammonia Based CO2 Capture with Multi-Pollutant Control Technology. Energy Procedia 2009, 1, 1027–1034. [Google Scholar] [CrossRef]
- Yu, H.; Qi, G.; Wang, S.; Morgan, S.; Allport, A.; Cottrell, A.; Do, T.; McGregor, J.; Wardhaugh, L.; Feron, P. Results from trialling aqueous ammonia-based post-combustion capture in a pilot plant at Munmorah Power Station: Gas purity and solid precipitation in the stripper. Int. J. Greenh. Gas Control 2012, 10, 15–25. [Google Scholar] [CrossRef]
- Di Profio, G.; Tucci, S.; Curcio, E.; Drioli, E. Selective Glycine Polymorph Crystallization by Using Microporous Membranes. Cryst. Growth Des. 2007, 7, 526–530. [Google Scholar] [CrossRef]
- Li, L.; Zhao, S.; Xin, Z.; Zhou, S. Nucleation kinetics of clopidogrel hydrogen sulfate polymorphs in reactive crystallization: Induction period and interfacial tension measurements. J. Cryst. Growth 2020, 538, 125610. [Google Scholar] [CrossRef]
- Feng, D.; Guo, D.; Zhang, Y.; Sun, S.; Zhao, Y.; Chang, G.; Guo, Q.; Qin, Y. Adsorption-enrichment characterization of CO2 and dynamic retention of free NH3 in functionalized biochar with H2O/NH3·H2O activation for promotion of new ammonia-based carbon capture. Chem. Eng. J. 2021, 409, 128193. [Google Scholar] [CrossRef]
- Lenka, M.; Sarkar, D. Determination of metastable zone width, induction period and primary nucleation kinetics for cooling crystallization of l-asparaginenohydrate. J. Cryst. Growth 2014, 408, 85–90. [Google Scholar] [CrossRef]
- Prasad, R.; Dalvi, S.V. Sonocrystallization: Monitoring and controlling crystallization using ultrasound. Chem. Eng. Sci. 2020, 226, 115911. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, J.; Zhang, S.; You, G.; Wu, S. Influencing factors for vulcanization induction period of accelerator / natural rubber composites: Molecular simulation and experimental study. Polym. Test. 2019, 80, 106145. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Z.; Zhang, M.; Guo, M.; Xu, S.; Yin, Q. Determination of metastable zone and induction time of analgin for cooling crystallization. Chin. J. Chem. Eng. 2017, 25, 313–318. [Google Scholar] [CrossRef]
- Šimon, P.; Nemčeková, K.; Jóna, E.; Plško, A.; Ondrušová, D. Thermal stability of glass evaluated by the induction period of crystallization. Thermochim. Acta 2005, 428, 11–14. [Google Scholar] [CrossRef]
- He, D.; Ou, Z.; Qin, C.; Deng, T.; Yin, J.; Pu, G. Understanding the catalytic acceleration effect of steam on CaCO3 decomposition by density function theory. Chem. Eng. J. 2020, 379, 122348. [Google Scholar] [CrossRef]
- Söhnel, O.; Mullin, J.W. A method for the determination of precipitation induction periods. J. Cryst. Growth 1978, 44, 377–382. [Google Scholar] [CrossRef]
- Yadav, A.; Labhasetwar, P.K.; Shahi, V.K. Membrane distillation crystallization technology for zero liquid discharge and resource recovery: Opportunities, challenges and futuristic perspectives. Sci. Total Environ. 2022, 806, 150692. [Google Scholar] [CrossRef] [PubMed]
- Chabanon, E.; Mangin, D.; Charcosset, C. Membranes and crystallization processes: State of the art and prospects. J. Membr. Sci. 2016, 509, 57–67. [Google Scholar] [CrossRef]
- Di Profio, G.; Curcio, E.; Drioli, E. Supersaturation Control and Heterogeneous Nucleation in Membrane Crystallizers: Facts and Perspectives. Ind. Eng. Chem. Res. 2010, 49, 11878–11889. [Google Scholar] [CrossRef]
- You, S.; Zhang, Y.; Zhang, Y. Nucleation of ammonium aluminum sulfate dodecahydrate from unseeded aqueous solution. J. Cryst. Growth 2015, 411, 24–29. [Google Scholar] [CrossRef]
- Wang, S.; Feng, M.; Du, H.; Weigand, J.J.; Zhang, Y.; Wang, X. Determination of metastable zone width, induction time and primary nucleation kinetics for cooling crystallization of sodium orthovanadate from NaOH solution. J. Cryst. Growth 2020, 545, 125721. [Google Scholar] [CrossRef]
- Neumann, A.W.; Good, R.J.; Hope, C.J.; Sejpal, M. An equation-of-state approach to determine surface tensions of low-energy solids from contact angles. J. Colloid Interface Sci. 1974, 49, 291–304. [Google Scholar] [CrossRef]
- Neumann, A.W. Haftfestigkeit an Grenzflächen. Mater. Corros. 1969, 20, 19–22. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, W.; Hu, Y.; Sun, J.; Tong, X.; Chen, Q.; Li, Q. One-step synthesis of porous Li4SiO4-based adsorbent pellets via graphite moulding method for cyclic CO2 capture. Chem. Eng. J. 2018, 353, 92–99. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, H.P.; Chang, J.; Wei, B. Experimental study of surface tension, specific heat and thermal diffusivity of liquid and solid titanium. Chem. Phys. Lett. 2015, 639, 105–108. [Google Scholar] [CrossRef]
- Correia, N.T.; Ramos, J.J.M.; Saramago, B.J.V.; Calado, J.C.G. Estimation of the Surface Tension of a Solid: Application to a Liquid Crystalline Polymer. J. Colloid Interface Sci. 1997, 189, 361–369. [Google Scholar] [CrossRef]
- Sangwal, K. Novel Approach to Analyze Metastable Zone Width Determined by the Polythermal Method: Physical Interpretation of Various Parameters. Cryst. Growth Des. 2009, 9, 942–950. [Google Scholar] [CrossRef]
- Gu, C.-H.; Young, V.; Grant, D.J.W. Polymorph screening: Influence of solvents on the rate of solvent-mediated polymorphic transformation. J. Pharm. Sci. 2001, 90, 1878–1890. [Google Scholar] [CrossRef]
- Torkian, M.; Manteghian, M.; Safari, M. Caffeine metastable zone width and induction time in anti-solvent crystallization. J. Cryst. Growth 2022, 594, 126790. [Google Scholar] [CrossRef]
- Ma, L.; Qin, C.; Pi, S.; Cui, H. Fabrication of efficient and stable Li4SiO4-based sorbent pellets via extrusion-spheronization for cyclic CO2 capture. Chem. Eng. J. 2020, 379, 122385. [Google Scholar] [CrossRef]
- Sangwal, K. Some features of metastable zone width of various systems determined by polythermal method. CrystEngComm 2011, 13, 489–501. [Google Scholar] [CrossRef]
- Kashchiev, D.; Verdoes, D.; van Rosmalen, G.M. Induction time and metastability limit in new phase formation. J. Cryst. Growth 1991, 110, 373–380. [Google Scholar] [CrossRef]
- Su, W.; Hao, H.; Glennon, B.; Barrett, M. Spontaneous Polymorphic Nucleation of d-Mannitol in Aqueous Solution Monitored with Raman Spectroscopy and FBRM. Cryst. Growth Des. 2013, 13, 5179–5187. [Google Scholar] [CrossRef]
- Marichev, V.A. General thermodynamic equations for the surface tension of liquids and solids. Surf. Sci. 2010, 604, 458–463. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, X.; Feng, X. An accurate and parallel method with post-processing boundedness control for solving the anisotropic phase-field dendritic crystal growth model. Commun. Nonlinear Sci. Numer. Simul. 2022, 115, 106717. [Google Scholar] [CrossRef]
- Zhao, H.; Li, T.; Li, J.; Li, Q.; Wang, S.; Zheng, C.; Li, J.; Li, M.; Zhang, Y.; Yao, J. Excess polymer-assisted crystal growth method for high-performance perovskite photodetectors. J. Alloys Compd. 2022, 908, 164482. [Google Scholar] [CrossRef]
- Wang, P.F.; Li, M.W.; Zhou, C.; Hu, Z.T.; Yin, H.W. Numerical simulations of flow and mass transfer during large-scale potassium dihydrogen phosphate crystal growth via three-dimensional motion growth method. Int. J. Heat Mass Transf. 2018, 127, 901–907. [Google Scholar] [CrossRef]
- Sangwal, K. On the estimation of surface entropy factor, interfacial tension, dissolution enthalpy and metastable zone-width for substances crystallizing from solution. J. Cryst. Growth 1989, 97, 393–405. [Google Scholar] [CrossRef]
- Jáger, G.; Tomán, J.J.; Juhász, L.; Vecsei, G.; Erdélyi, Z.; Cserháti, C. Nucleation and growth kinetics of ZnAl2O4 spinel in crystalline ZnO–amorphous Al2O3 bilayers prepared by atomic layer deposition. Scr. Mater. 2022, 219, 114857. [Google Scholar] [CrossRef]
- Barata, P.A.; Serrano, M.L. Salting-out precipitation of potassium dihydrogen phosphate (KDP). I. Precipitation mechanism. J. Cryst. Growth 1996, 160, 361–369. [Google Scholar] [CrossRef]
- Guo, K.; Lv, Y.; He, L.; Luo, X.; Zhao, J. Experimental study on the effect of spatial distribution and action order of electric field and magnetic field on oil-water separation. Chem. Eng. Process. 2019, 145, 107658. [Google Scholar] [CrossRef]
- Ren, J.; Zhu, Z.; Qiu, Y.; Yu, F.; Ma, J.; Zhao, J. Magnetic field assisted adsorption of pollutants from an aqueous solution: A review. J. Hazard. Mater. 2021, 408, 124846. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Guo, R.; Shuai, S.; Wang, J.; Xia, M.; Li, J.; Ren, Z.; Li, J.; Wang, Q. Influence of static magnetic field on the heterogeneous nucleation behavior of Al on single crystal Al2O3 substrate. Materialia 2020, 13, 100847. [Google Scholar] [CrossRef]
- Taheri, M.H.; Mohammadpourfard, M.; Sadaghiani, A.K.; Kosar, A. Wettability alterations and magnetic field effects on the nucleation of magnetic nanofluids: A molecular dynamics simulation. J. Mol. Liq. 2018, 260, 209–220. [Google Scholar] [CrossRef]
- Koza, J.A.; Uhlemann, M.; Gebert, A.; Schultz, L. Nucleation and growth of the electrodeposited iron layers in the presence of an external magnetic field. Electrochim. Acta 2008, 53, 7972–7980. [Google Scholar] [CrossRef]
- Kadota, K.; Shirakawa, Y.; Wada, M.; Shimosaka, A.; Hidaka, J. Influence of clusters on the crystal surface of NaCl at initial growth stage investigated by molecular dynamics simulations. J. Mol. Liq. 2012, 166, 31–39. [Google Scholar] [CrossRef]
- Galmarini, S.; Bowen, P. Atomistic simulation of the adsorption of calcium and hydroxyl ions onto portlandite surfaces—towards crystal growth mechanisms. Cem. Concr. Res. 2016, 81, 16–23. [Google Scholar] [CrossRef]
- Separdar, L.; Rino, J.P.; Zanotto, E.D. Decoding crystal growth kinetics and structural evolution in supercooled ZnSe by molecular dynamics simulation. Comput. Mater. Sci. 2022, 212, 111598. [Google Scholar] [CrossRef]
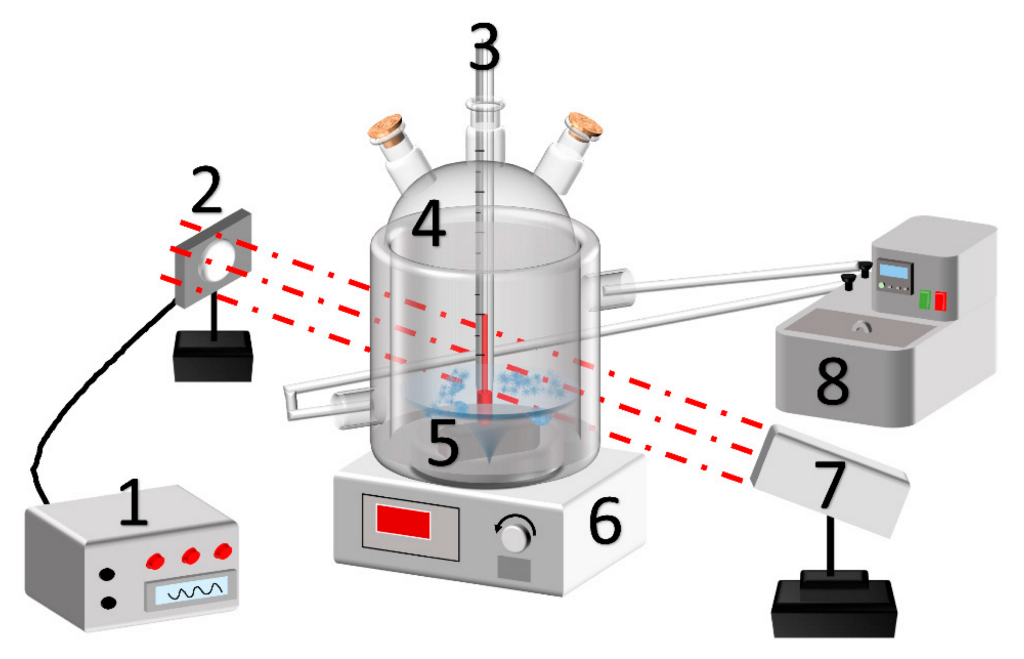

| Name | Chemical Formula | Purity/Concentration |
|---|---|---|
| Ammonia | NH3·H2O | 25~28 wt.% |
| Ammonium bicarbonate | NH4HCO3 | Analysis of pure |
| Ammonium carbamate | NH2COONH4 | Analysis of pure |
| Anhydrous ethanol | CH3CH2OH | Analysis of pure |
| Sulfuric acid | H2SO4 | 0.05 mol/L |
| T/K | Solvent Composition/(mol·mol−1) | /(J·m−2) | |
|---|---|---|---|
| 283.15 | 0.1169 | 0.004187 | 0.752 |
| 283.15 | 0.1707 | 0.004683 | 0.8612 |
| 283.15 | 0.2359 | 0.008404 | 1.499 |
| 288.15 | 0.1169 | 0.00414 | 0.777 |
| 288.15 | 0.1707 | 0.00433 | 0.818 |
| 288.15 | 0.2359 | 0.008167 | 1.403 |
| 293.15 | 0.1169 | 0.003664 | 0.804 |
| 293.15 | 0.1707 | 0.003957 | 0.876 |
| 293.15 | 0.2359 | 0.00589 | 1.127 |
| Surface Entropy Factor | Growing Position | Roughness of Crystal Surface | Growth Type |
|---|---|---|---|
| f < 3 | A bumpy ride into the crystal plane | Rougher | Continuous Growth |
| 3 < f < 5 | Direct growth onto the crystal surface, and also diffusion to the crystal surface of the step | Smoother | Transmissive growth |
| f > 5 | Dislocations through the surface into the lattice | Very smooth | Spiral dislocation growth |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, L.; Feng, D.; Zhang, Y.; Dong, H.; Zhao, Z.; Gao, J.; Zhang, F.; Zhao, Y.; Sun, S.; Huang, Y. Effects of Solubilizer and Magnetic Field during Crystallization Induction of Ammonium Bicarbonate in New Ammonia-Based Carbon Capture Process. Energies 2022, 15, 6231. https://doi.org/10.3390/en15176231
Dong L, Feng D, Zhang Y, Dong H, Zhao Z, Gao J, Zhang F, Zhao Y, Sun S, Huang Y. Effects of Solubilizer and Magnetic Field during Crystallization Induction of Ammonium Bicarbonate in New Ammonia-Based Carbon Capture Process. Energies. 2022; 15(17):6231. https://doi.org/10.3390/en15176231
Chicago/Turabian StyleDong, Linhan, Dongdong Feng, Yu Zhang, Heming Dong, Zhiqi Zhao, Jianmin Gao, Feng Zhang, Yijun Zhao, Shaozeng Sun, and Yudong Huang. 2022. "Effects of Solubilizer and Magnetic Field during Crystallization Induction of Ammonium Bicarbonate in New Ammonia-Based Carbon Capture Process" Energies 15, no. 17: 6231. https://doi.org/10.3390/en15176231
APA StyleDong, L., Feng, D., Zhang, Y., Dong, H., Zhao, Z., Gao, J., Zhang, F., Zhao, Y., Sun, S., & Huang, Y. (2022). Effects of Solubilizer and Magnetic Field during Crystallization Induction of Ammonium Bicarbonate in New Ammonia-Based Carbon Capture Process. Energies, 15(17), 6231. https://doi.org/10.3390/en15176231









