Recent Advances in the Development of Nanocatalysts for Direct Methanol Fuel Cells
Abstract
:1. Introduction
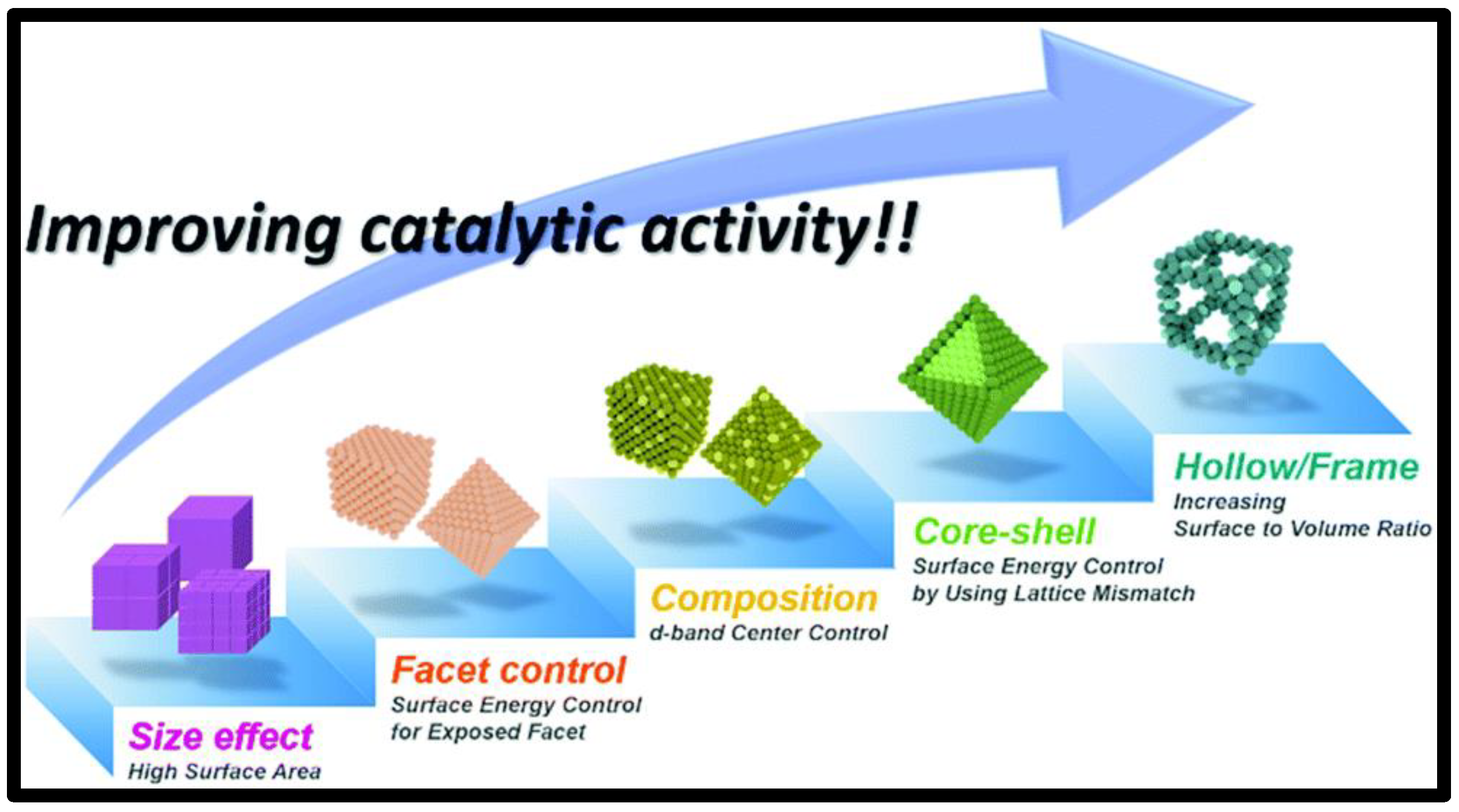
2. Nanocatalysts for the Anode
2.1. Pt-Only
2.2. Pt-Hybrid Metals
2.2.1. Core-Shell Structured Catalysts
2.2.2. Other Supports
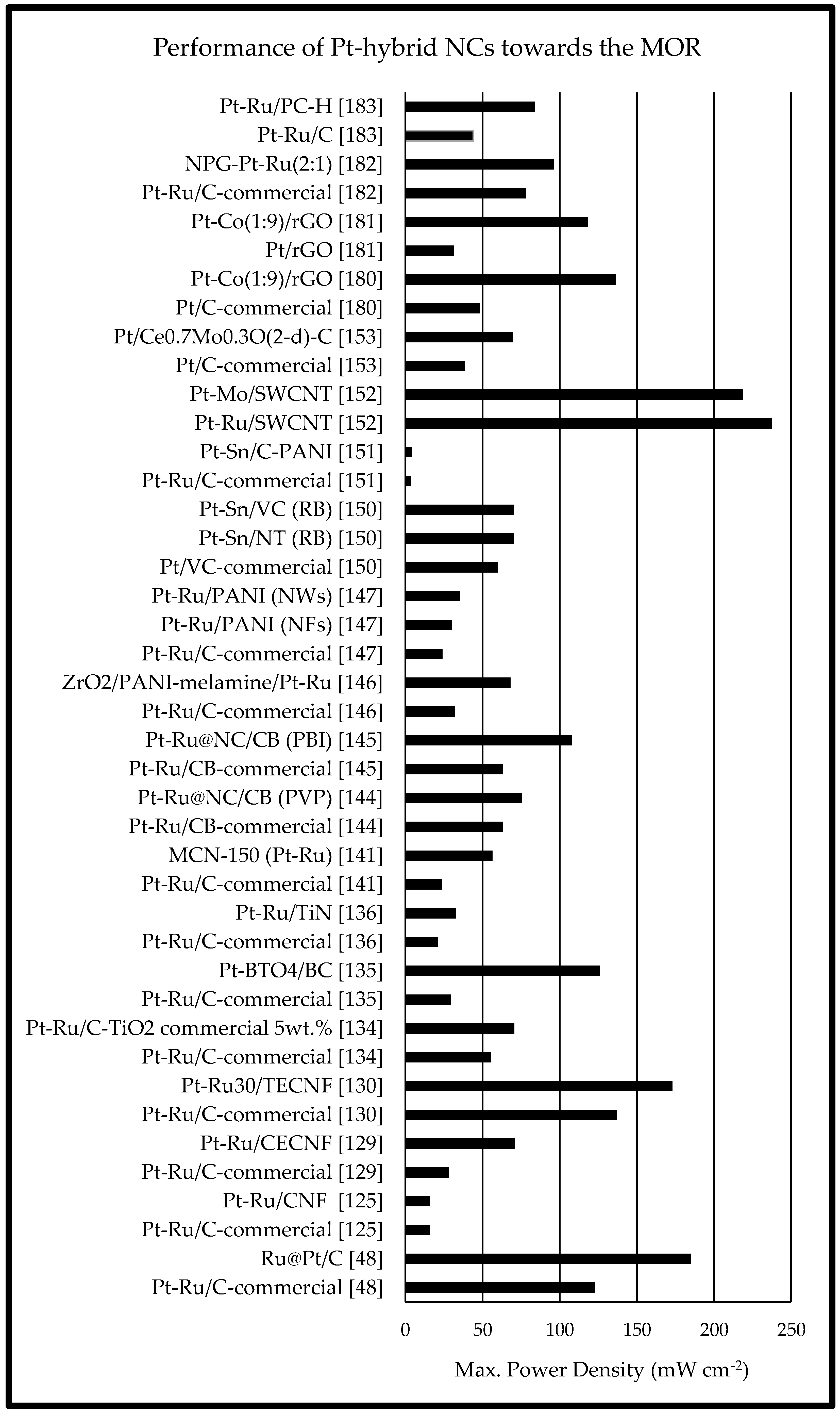
2.3. Pt-Free
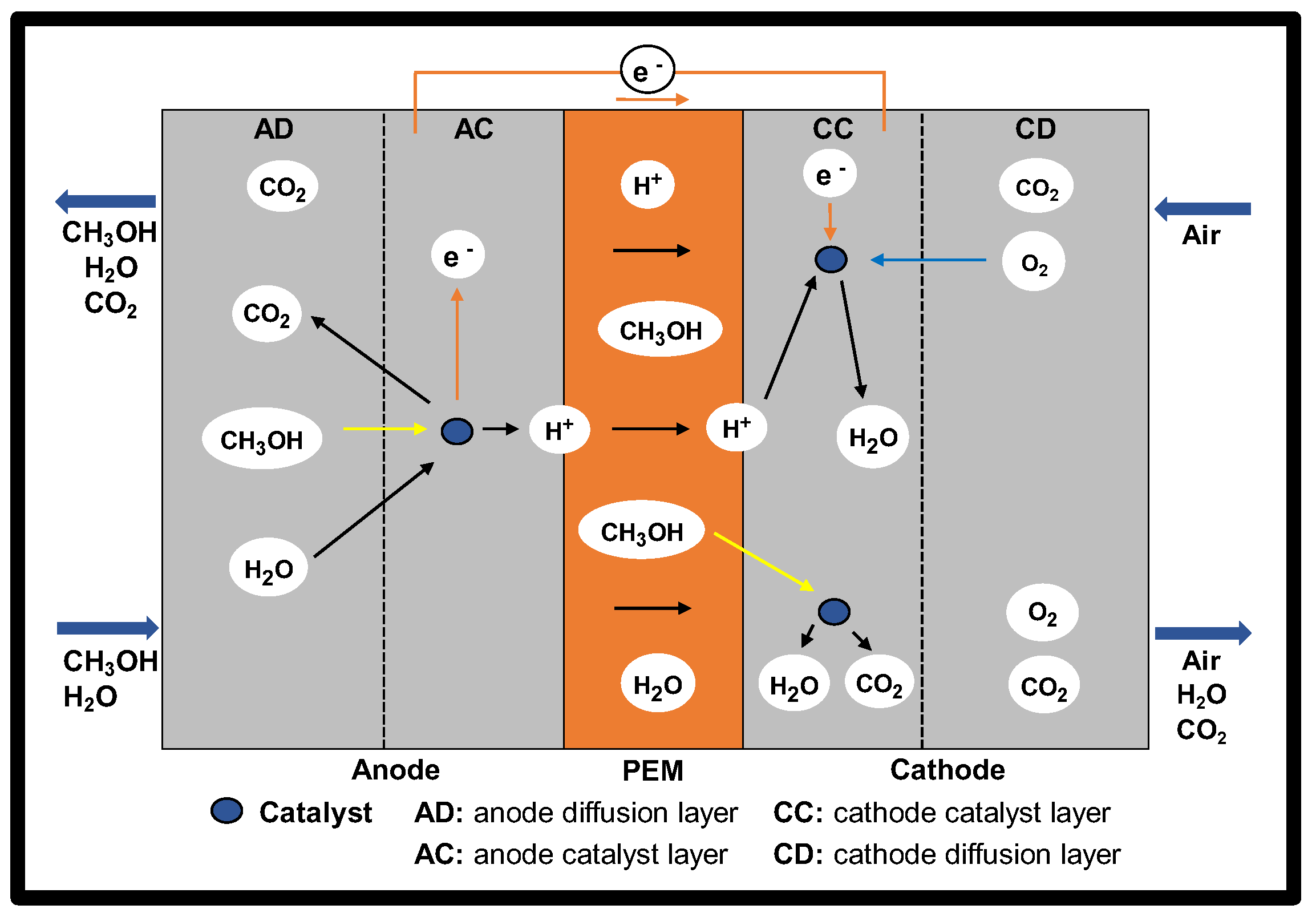
2.4. Passive Feed Systems
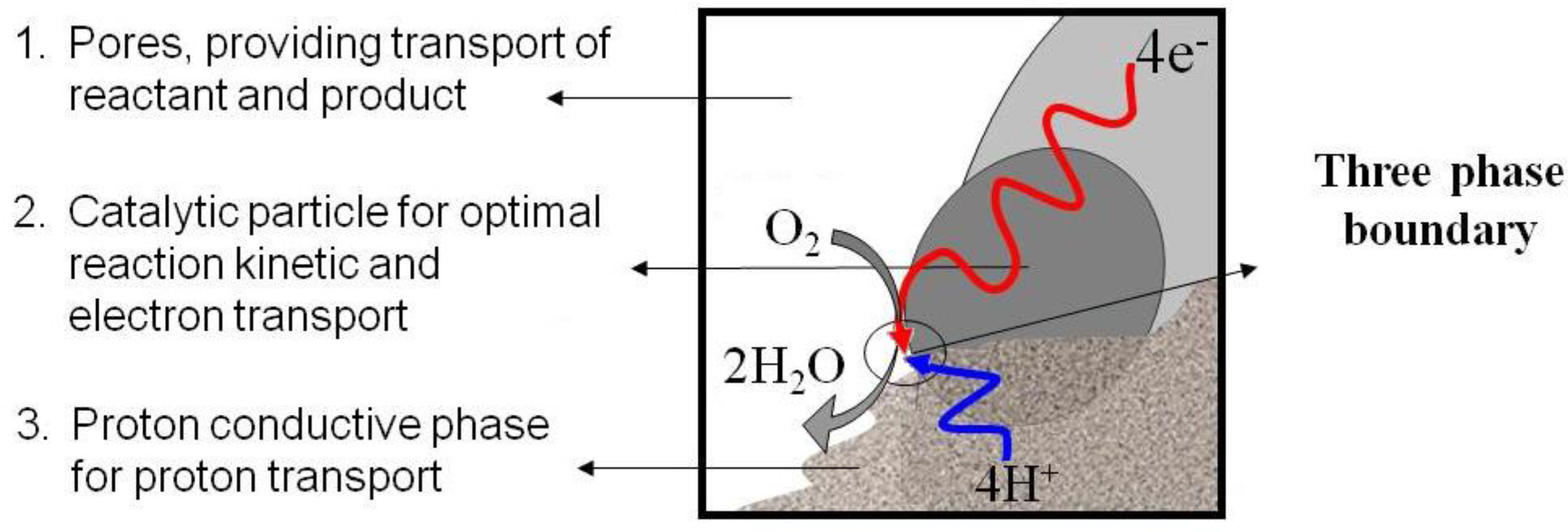
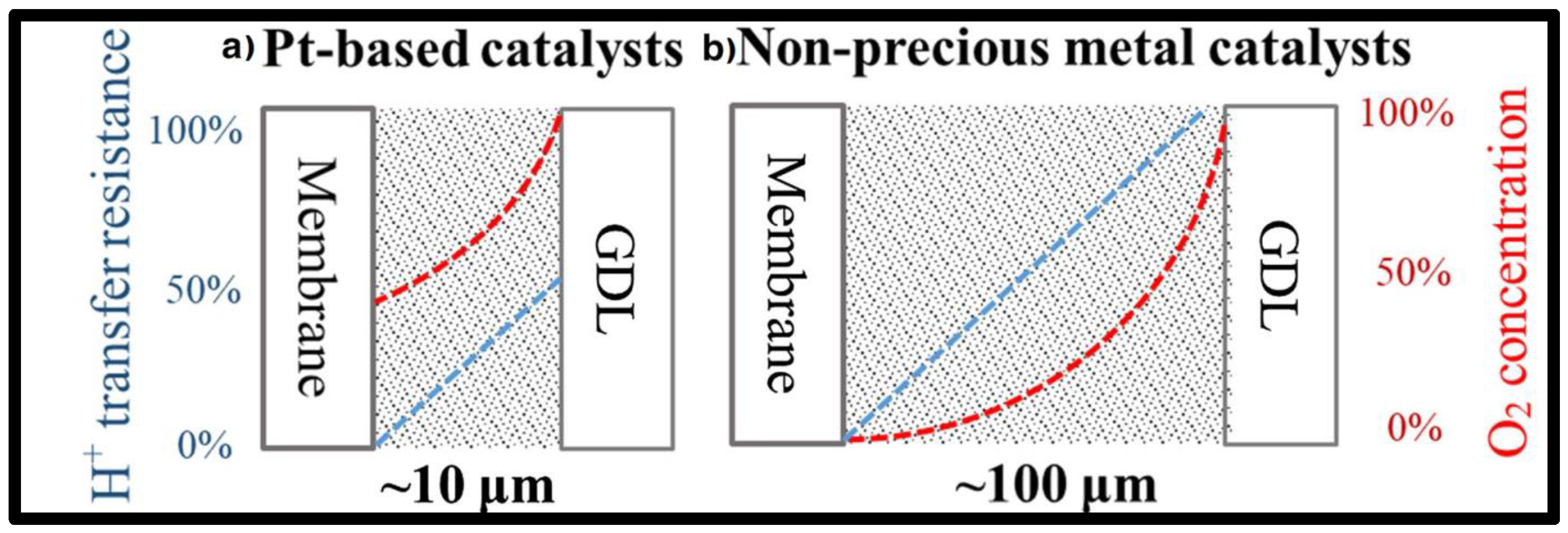
3. Nanocatalysts for the Cathode
3.1. Pt-Only

3.2. Pt-Hybrid Metals
3.3. Pt-Free
3.3.1. Pd-Based
3.3.2. Other Supports
3.3.3. Sacrificial Support Method
3.3.4. PGM-Free Materials
3.4. Passive Feed Systems
4. Theoretical/Modelling Approaches
5. Concluding and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Feng, Y.; Liu, H.; Yang, J. A selective electrocatalyst–based direct methanol fuel cell operated at high concentrations of methanol. Sci. Adv. 2017, 3, e1700580. [Google Scholar] [CrossRef]
- Dias, V.; Pochet, M.; Contino, F.; Jeanmart, H. Energy and Economic Costs of Chemical Storage. Front. Mech. Eng. 2020, 6, 21. [Google Scholar] [CrossRef]
- Zhao, X.; Yin, M.; Ma, L.; Liang, L.; Liu, C.; Liao, J.; Liu, T.; Xing, W. Recent advances in catalysts for direct methanol fuel cells. Energy Environ. Sci. 2011, 4, 2736–2753. [Google Scholar] [CrossRef]
- Falcão, D.S.; Pereira, J.P.; Rangel, C.M.; Pinto, A.M.F.R. Development and performance analysis of a metallic passive micro-direct methanol fuel cell for portable applications. Int. J. Hydrogen Energy 2015, 40, 5408–5415. [Google Scholar] [CrossRef]
- Munjewar, S.S.; Thombre, S.B.; Mallick, R.K. Approaches to overcome the barrier issues of passive direct methanol fuel cell—Review. Renew. Sustain. Energy Rev. 2017, 67, 1087–1104. [Google Scholar] [CrossRef]
- Braz, B.A.; Moreira, C.S.; Oliveira, V.B.; Pinto, A.M.F.R. Effect of the current collector design on the performance of a passive direct methanol fuel cell. Electrochim. Acta 2019, 300, 306–315. [Google Scholar] [CrossRef]
- Siwal, S.S.; Thakur, S.; Zhang, Q.B.; Thakur, V.K. Electrocatalysts for electrooxidation of direct alcohol fuel cell: Chemistry and applications. Mater. Today Chem. 2019, 14, 100182. [Google Scholar] [CrossRef]
- Mo, J.; Kang, Z.; Retterer, S.T.; Cullen, D.A.; Toops, T.J.; Green, J.B., Jr.; Mench, M.M.; Zhang, F.-Y. Discovery of true electrochemical reactions for ultrahigh catalyst mass activity in water splitting. Sci. Adv. 2016, 2, e1600690. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, B. Recent advances in porous Pt-based nanostructures: Synthesis and electrochemical applications. Chem. Soc. Rev. 2014, 43, 2439–2450. [Google Scholar] [CrossRef]
- Wang, Y.J.; Fang, B.; Li, H.; Bi, X.T.; Wang, H. Progress in modified carbon support materials for Pt and Pt-alloy cathode catalysts in polymer electrolyte membrane fuel cells. Prog. Mater. Sci. 2016, 82, 445–498. [Google Scholar] [CrossRef]
- Kamarudin, S.K.; Achmad, F.; Daud, W.R.W. Overview on the application of direct methanol fuel cell (DMFC) for portable electronic devices. Int. J. Hydrogen Energy 2009, 34, 6902–6916. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, X.; Sun, H.; Wang, S.; Sun, G. Recent advances in multi-scale design and construction of materials for direct methanol fuel cells. Nano Energy 2019, 65, 104048. [Google Scholar] [CrossRef]
- Hogarth, M.P.; Hards, G.A. Direct Methanol Fuel Cells: Technological Advances and Further Requirements. Platinum Metals Rev. 1996, 40, 150–159. [Google Scholar]
- Ramli, Z.A.C.; Kamarudin, S.K. Platinum-Based Catalysts on Various Carbon Supports and Conducting Polymers for Direct Methanol Fuel Cell Applications: A Review. Nanoscale Res. Lett. 2018, 13, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Basri, S.; Kamarudin, S.K.; Daud, W.R.W.; Yaakob, Z.; Kadhum, A.A.H. Novel Anode Catalyst for Direct Methanol Fuel Cells. Sci. World J. 2014, 2014, 547604. [Google Scholar] [CrossRef] [PubMed]
- Salgado, J.R.C.; Alcaide, F.; Álvarez, G.; Calvillo, L.; Lázaro, M.J.; Pastor, E. Pt–Ru electrocatalysts supported on ordered mesoporous carbon for direct methanol fuel cell. J. Power Sources 2010, 195, 4022–4029. [Google Scholar] [CrossRef]
- Basri, S.; Kamarudin, S.K.; Daud, W.R.W.; Yaakub, Z. Nanocatalyst for direct methanol fuel cell (DMFC). Int. J. Hydrogen Energy 2010, 35, 7957–7970. [Google Scholar] [CrossRef]
- Ogungbemi, E.; Ijaodola, O.; Khatib, F.N.; Wilberforce, T.; El Hassan, Z.; Thompson, J.; Ramadan, M.; Olabi, A.G. Fuel cell membranes—Pros and cons. Energy 2019, 172, 155–172. [Google Scholar] [CrossRef]
- Farooqui, U.R.; Ahmad, A.L.; Hamid, N.A. Graphene oxide: A promising membrane material for fuel cells. Renew. Sustain. Energy Rev. 2018, 82, 714–733. [Google Scholar] [CrossRef]
- Fadzillah, D.M.; Kamarudin, S.K.; Zainoodin, M.A.; Masdar, M.S. Critical challenges in the system development of direct alcohol fuel cells as portable power supplies: An overview. Int. J. Hydrogen Energy 2019, 44, 3031–3054. [Google Scholar] [CrossRef]
- Munjewar, S.S.; Thombre, S.B.; Mallick, R.K. A comprehensive review on recent material development of passive direct methanol fuel cell. Ionics 2017, 23, 1–18. [Google Scholar] [CrossRef]
- Chen, X.; Li, T.; Shen, J.; Hu, Z. From structures, packaging to application: A system-level review for micro direct methanol fuel cell. Renew. Sustain. Energy Rev. 2017, 80, 669–678. [Google Scholar] [CrossRef]
- Shrivastava, N.K.; Thombre, S.B.; Chadge, R.B. Liquid feed passive direct methanol fuel cell: Challenges and recent advances. Ionics 2016, 22, 1–23. [Google Scholar] [CrossRef]
- Velisala, V.; Srinivasulu, G.N.; Reddy, B.S.; Rao, K.V.K. Review on challenges of direct liquid fuel cells for portable application. World J. Eng. 2015, 12, 591–605. [Google Scholar] [CrossRef]
- Falcão, D.S.; Oliveira, V.B.; Rangel, C.M.; Pinto, A.M.F.R. Review on micro-direct methanol fuel cells. Renew. Sustain. Energy Rev. 2014, 34, 58–70. [Google Scholar] [CrossRef]
- Kumar, P.; Dutta, K.; Das, S.; Kundu, P.P. An overview of unsolved deficiencies of direct methanol fuel cell technology: Factors and parameters affecting its widespread use. Int. J. Energy Res. 2014, 38, 1367–1390. [Google Scholar] [CrossRef]
- Oliveira, V.B.; Falcão, D.S.; Rangel, C.M.; Pinto, A.M.F.R. Water management in a passive direct methanol fuel cell. Int. J. Energy Res. 2013, 37, 991–1001. [Google Scholar] [CrossRef]
- Shao, M.; Chang, Q.; Dodelet, J.P.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef]
- Schoekel, A.; Melke, J.; Bruns, M.; Wippermann, K.; Kuppler, F.; Roth, C. Quantitative study of ruthenium cross-over in direct methanol fuel cells during early operation hours. J. Power Sources 2016, 301, 210–218. [Google Scholar] [CrossRef]
- Meier, J.C.; Galeano, C.; Katsounaros, I.; Witte, J.; Bongard, H.J.; Topalov, A.A.; Baldizzone, C.; Mezzavilla, S.; Schüth, F.; Mayrhofer, K.J.J. Design criteria for stable Pt/C fuel cell catalysts. Beilstein J. Nanotechnol. 2014, 5, 44–67. [Google Scholar] [CrossRef]
- Oh, H.S.; Lim, K.H.; Roh, B.; Hwang, I.; Kim, H. Corrosion resistance and sintering effect of carbon supports in polymer electrolyte membrane fuel cells. Electrochim. Acta 2009, 54, 6515–6521. [Google Scholar] [CrossRef]
- Cherevko, S. Stability and dissolution of electrocatalysts: Building the bridge between model and ‘real world’ systems. Curr. Opin. Electrochem. 2018, 8, 118–125. [Google Scholar] [CrossRef]
- Du, L.; Shao, Y.; Sun, J.; Yin, G.; Liu, J.; Wang, Y. Advanced catalyst supports for PEM fuel cell cathodes. Nano Energy 2016, 29, 314–322. [Google Scholar] [CrossRef]
- Samad, S.; Loh, K.S.; Wong, W.Y.; Lee, T.K.; Sunarso, J.; Chong, S.T.; Daud, W.R.W. Carbon and non-carbon support materials for platinum-based catalysts in fuel cells. Int. J. Hydrogen Energy 2018, 43, 7823–7854. [Google Scholar] [CrossRef]
- Mohanta, P.; Regnet, F.; Jörissen, L. Graphitized Carbon: A Promising Stable Cathode Catalyst Support Material for Long Term PEMFC Applications. Materials 2018, 11, 907. [Google Scholar] [CrossRef]
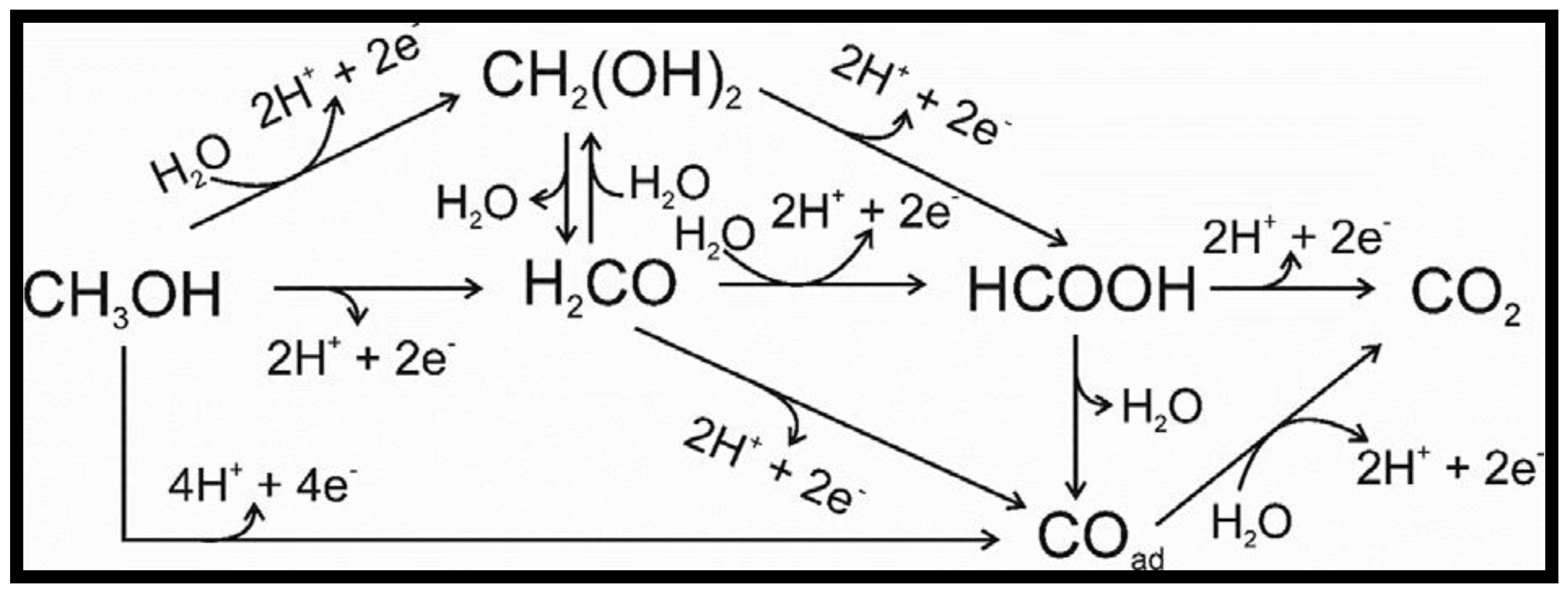
- Cuesta, A. Electrooxidation of C1 organic molecules on Pt electrodes. Curr. Opin. Electrochem. 2017, 4, 32–38. [Google Scholar] [CrossRef]
- Kübler, M.; Jurzinsky, T.; Ziegenbalg, D.; Cremers, C. Methanol oxidation reaction on core-shell structured Ruthenium-Palladium nanoparticles: Relationship between structure and electrochemical behavior. J. Power Sources 2018, 375, 320–334. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Tiwari, R.N.; Singh, G.; Kim, K.S. Recent progress in the development of anode and cathode catalysts for direct methanol fuel cells. Nano Energy 2013, 2, 553–578. [Google Scholar] [CrossRef]
- Liu, H.; Song, C.; Zhang, L.; Zhang, J.; Wang, H.; Wilkinson, D.P. A review of anode catalysis in the direct methanol fuel cell. J. Power Sources 2006, 155, 95–110. [Google Scholar] [CrossRef]
- Falcão, D.S.; Silva, R.A.; Rangel, C.M.; Pinto, A.M.F.R. Performance of an Active Micro Direct Methanol Fuel Cell Using Reduced Catalyst Loading MEAs. Energies 2017, 10, 1683. [Google Scholar] [CrossRef]
- Olu, P.Y.; Ohnishi, T.; Mochizuki, D.; Sugimoto, W. Uncovering the real active sites of ruthenium oxide for the carbon monoxide electro-oxidation reaction on platinum: The catalyst acts as a co-catalyst. J. Electroanal. Chem. 2018, 810, 109–118. [Google Scholar] [CrossRef]
- Sahin, O.; Kivrak, H. A comparative study of electrochemical methods on Pt–Ru DMFC anode catalysts: The effect of Ru addition. Int. J. Hydrogen Energy 2013, 38, 901–909. [Google Scholar] [CrossRef]
- Bresciani, F.; Rabissi, C.; Zago, M.; Gazdzicki, P.; Schulze, M.; Guétaz, L.; Escribano, S.; Bonde, J.L.; Marchesi, R.; Casalegno, A. A combined in-situ and post-mortem investigation on local permanent degradation in a direct methanol fuel cell. J. Power Sources 2016, 306, 49–61. [Google Scholar] [CrossRef]
- Mehmood, A.; Scibioh, M.A.; Prabhuram, J.; An, M.G.; Ha, H.Y. A review on durability issues and restoration techniques in long-term operations of direct methanol fuel cells. J. Power Sources 2015, 297, 224–241. [Google Scholar] [CrossRef]
- Bresciani, F.; Rabissi, C.; Casalegno, A.; Zago, M.; Marchesi, R. Experimental investigation on DMFC temporary degradation. Int. J. Hydrogen Energy 2014, 39, 21647–21656. [Google Scholar] [CrossRef]
- Piela, P.; Eickes, C.; Brosha, E.; Garzon, F.; Zelenay, P. Ruthenium Crossover in Direct Methanol Fuel Cell with Pt–Ru Black Anode. J. Electrochem. Soc. 2004, 151, 2053–2059. [Google Scholar] [CrossRef]
- Antolini, E.; Salgado, J.R.C.; Gonzalez, E.R. Carbon supported Pt75M25 (M = Co, Ni) alloys as anode and cathode electrocatalysts for direct methanol fuel cells. J. Electroanal. Chem. 2005, 580, 145–154. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, Q.; Gu, L.; Xu, S.; Wang, P.; Liu, J.; Ding, Y.; Yao, Y.F.; Nan, C.; Zhao, M.; et al. Ruthenium-platinum core-shell nanocatalysts with substantially enhanced activity and durability towards methanol oxidation. Nano Energy 2016, 21, 247–257. [Google Scholar] [CrossRef]
- Singh, R.N.; Awasthi, R.; Sharma, C.S. Review: An Overview of Recent Development of Platinum-Based Cathode Materials for Direct Methanol Fuel Cells. Int. J. Electrochem. Sci. 2014, 9, 5607–5639. [Google Scholar]
- Gómez-Marín, A.M.; Rizo, R.; Feliu, J.M. Some reflections on the understanding of the oxygen reduction reaction at Pt(111). Beilstein J. Nanotechnol. 2013, 4, 956–967. [Google Scholar] [CrossRef]
- Holton, O.T.; Stevenson, J.W. The Role of Platinum in Proton Exchange Membrane Fuel Cells Evaluation of platinum’s unique properties for use in both the anode and cathode of a proton exchange membrane fuel cell. Platin. Met. Rev 2013, 57, 259–271. [Google Scholar] [CrossRef]
- Chen, C.; Fuller, T.F. Modeling of H2O2 formation in PEMFCs. Electrochim. Acta 2009, 54, 3984–3995. [Google Scholar] [CrossRef]
- Borup, R.; Meyers, J.; Pivovar, B.; Kim, Y.S.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D.; et al. Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar] [CrossRef]
- Zainoodin, A.M.; Kamarudin, S.K.; Daud, W.R.W. Electrode in direct methanol fuel cells. Int. J. Hydrogen Energy 2010, 35, 4606–4621. [Google Scholar] [CrossRef]
- Zhao, T.S.; Xu, C.; Chen, R.; Yang, W.W. Mass transport phenomena in direct methanol fuel cells. Prog. Energy Combust. Sci. 2009, 35, 275–292. [Google Scholar] [CrossRef]
- Knights, S.D.; Colbow, K.M.; St-Pierre, J.; Wilkinson, D.P. Aging mechanisms and lifetime of PEFC and DMFC. J. Power Sources 2004, 127, 127–134. [Google Scholar] [CrossRef]
- Huang, Y.; Babu, D.D.; Wu, M.; Wang, Y. Synergistic Supports Beyond Carbon Black for Polymer Electrolyte Fuel Cell Anodes. ChemCatChem 2018, 10, 4497–4508. [Google Scholar] [CrossRef]
- Moura, A.; Fajín, J.; Mandado, M.; Cordeiro, M. Ruthenium–Platinum Catalysts and Direct Methanol Fuel Cells (DMFC): A Review of Theoretical and Experimental Breakthroughs. Catalysts 2017, 7, 47. [Google Scholar] [CrossRef]
- Lv, H.; Li, D.; Strmcnik, D.; Paulikas, A.P.; Markovic, N.M.; Stamenkovic, V.R. Recent advances in the design of tailored nanomaterials for efficient oxygen reduction reaction. Nano Energy 2016, 29, 149–165. [Google Scholar] [CrossRef]
- Holby, E.F.; Zelenay, P. Linking structure to function: The search for active sites in non-platinum group metal oxygen reduction reaction catalysts. Nano Energy 2016, 29, 54–64. [Google Scholar] [CrossRef]
- Eslamibidgoli, M.J.; Huang, J.; Kadyk, T.; Malek, A.; Eikerling, M. How theory and simulation can drive fuel cell electrocatalysis. Nano Energy 2016, 29, 334–361. [Google Scholar] [CrossRef]
- Spendelow, J.S.; Wieckowski, A. Electrocatalysis of oxygen reduction and small alcohol oxidation in alkaline media. Phys. Chem. Chem. Phys. 2007, 9, 2654–2675. [Google Scholar] [CrossRef] [PubMed]
- Kakati, N.; Maiti, J.; Lee, S.H.; Jee, S.H.; Viswanathan, B.; Yoon, Y.S. Anode catalysts for direct methanol fuel cells in acidic media: Do we have any alternative for Pt or Pt–Ru? Chem. Rev. 2014, 114, 12397–12429. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Yang, Z.; Li, K.; Xing, W.; Liu, C.; Ge, J. Recent development of methanol electrooxidation catalysts for direct methanol fuel cell. J. Energy Chem. 2018, 27, 1618–1628. [Google Scholar] [CrossRef]
- Yang, L.; Ge, J.; Liu, C.; Wang, G.; Xing, W. Approaches to improve the performance of anode methanol oxidation reaction—a short review. Curr. Opin. Electrochem. 2017, 4, 83–88. [Google Scholar] [CrossRef]
- Tolmachev, Y.V.; Petrii, O.A. Pt–Ru electrocatalysts for fuel cells: Developments in the last decade. J. Solid State Electrochem. 2017, 21, 613–639. [Google Scholar] [CrossRef]
- Mansor, M.; Timmiati, S.N.; Lim, K.L.; Wong, W.Y.; Kamarudin, S.K.; Kamarudin, N.H.N. Recent progress of anode catalysts and their support materials for methanol electrooxidation reaction. Int. J. Hydrogen Energy 2019, 44, 14744–14769. [Google Scholar] [CrossRef]
- Shang, C.; Wang, E. Recent progress in Pt and Pd-based hybrid nanocatalysts for methanol electrooxidation. Phys. Chem. Chem. Phys. 2019, 21, 21185–21199. [Google Scholar] [CrossRef]
- Tian, H.; Yu, Y.; Wang, Q.; Li, J.; Rao, P.; Li, R.; Du, Y.; Jia, C.; Luo, J.; Deng, P.; et al. Recent advances in two-dimensional Pt based electrocatalysts for methanol oxidation reaction. Int. J. Hydrogen Energy 2021, 46, 31202–31215. [Google Scholar] [CrossRef]
- Ciapina, E.G.; Santos, S.F.; Gonzalez, E.R. Electrochemical CO stripping on nanosized Pt surfaces in acid media: A review on the issue of peak multiplicity. J. Electroanal. Chem. 2018, 815, 47–60. [Google Scholar] [CrossRef]
- Lai, S.C.S.; Lebedeva, N.P.; Housmans, T.H.M.; Koper, M.T.M. Mechanisms of carbon monoxide and methanol oxidation at single-crystal electrodes. Top. Catal. 2007, 46, 320–333. [Google Scholar] [CrossRef]
- Kamyabi, M.; Martínez-Hincapié, R.; Feliu, J.; Herrero, E. Effects of the Interfacial Structure on the Methanol Oxidation on Platinum Single Crystal Electrodes. Surfaces 2019, 2, 177–192. [Google Scholar] [CrossRef] [Green Version]
- Iwasita, T. Electrocatalysis of methanol oxidation. Electrochim. Acta 2002, 47, 3663–3674. [Google Scholar] [CrossRef]
- Tian, N.; Zhou, Z.Y.; Sun, S.G.; Ding, Y.; Zhong, L.W. Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science 2007, 316, 732–735. [Google Scholar] [CrossRef]
- Sheng, T.; Xu, Y.F.; Jiang, Y.X.; Huang, L.; Tian, N.; Zhou, Z.Y.; Broadwell, I.; Sun, S.G. Structure Design and Performance Tuning of Nanomaterials for Electrochemical Energy Conversion and Storage. Acc. Chem. Res. 2016, 49, 2569–2577. [Google Scholar] [CrossRef] [PubMed]
- Bayati, M.; Abad, J.M.; Nichols, R.J.; Schiffrin, D.J. Substrate structural effects on the synthesis and electrochemical properties of platinum nanoparticles on highly oriented pyrolytic graphite. J. Phys. Chem. C 2010, 114, 18439–18448. [Google Scholar] [CrossRef]
- Andreaus, B.; Maillard, F.; Kocylo, J.; Savinova, E.R.; Eikerling, M. Kinetic modeling of COad monolayer oxidation on carbon-supported platinum nanoparticles. J. Phys. Chem. B 2006, 110, 21028–21040. [Google Scholar] [CrossRef]
- Mukerjee, S.; McBreen, J. Effect of particle size on the electrocatalysis by carbon-supported Pt electrocatalysts: An in situ XAS investigation. J. Electroanal. Chem. 1998, 448, 163–171. [Google Scholar] [CrossRef]
- Pan, D.; Li, X.; Zhang, A. Platinum assisted by carbon quantum dots for methanol electro-oxidation. Appl. Surf. Sci. 2018, 427, 715–723. [Google Scholar] [CrossRef]
- Sha, R.; Solomon Jones, S.; Badhulika, S. Controlled synthesis of platinum nanoflowers supported on carbon quantum dots as a highly effective catalyst for methanol electro-oxidation. Surf. Coat. Technol. 2019, 360, 400–408. [Google Scholar] [CrossRef]
- Liang, L.; Xiao, M.; Zhu, J.; Ge, J.; Liu, C.; Xing, W. Low-temperature synthesis of nitrogen doped carbon nanotubes as promising catalyst support for methanol oxidation. J. Energy Chem. 2019, 28, 118–122. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, J.; Wu, C.; Guan, L. Networks of connected Pt nanoparticles supported on carbon nanotubes as superior catalysts for methanol electrooxidation. J. Power Sources 2017, 342, 273–278. [Google Scholar] [CrossRef]
- Zhu, Z.; Bukowski, B.; Deskins, N.A.; Zhou, H.S. Bamboo shaped carbon nanotube supported platinum electrocatalyst synthesized by high power ultrasonic-assisted impregnation method for methanol electrooxidation and related density functional theory calculations. Int. J. Hydrogen Energy 2015, 40, 2216–2224. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, W.; Duan, J.; Zhang, Y.; Liu, X. Graphene nanosheets modified by nitrogen-doped carbon layer to support Pt nanoparticles for direct methanol fuel cell. Microelectron. Eng. 2015, 141, 234–237. [Google Scholar] [CrossRef]
- Radhakrishnan, T.; Sandhyarani, N. Three dimensional assembly of electrocatalytic platinum nanostructures on reduced graphene oxide—An electrochemical approach for high performance catalyst for methanol oxidation. Int. J. Hydrogen Energy 2017, 42, 7014–7022. [Google Scholar] [CrossRef]
- Yu, M.; Wu, X.; Zhang, J.; Meng, Y.; Ma, Y.; Liu, J.; Li, S. Platinum nanoparticles-loaded holey reduced graphene oxide framework as freestanding counter electrodes of dye sensitized solar cells and methanol oxidation catalysts. Electrochim. Acta 2017, 258, 485–494. [Google Scholar] [CrossRef]
- Li, X.; Lv, Y.; Pan, D. Pt catalysts supported on lignin-based carbon dots for methanol electro-oxidation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 569, 110–118. [Google Scholar] [CrossRef]
- Mu, X.; Xu, Z.; Ma, Y.; Xie, Y.; Mi, H.; Ma, J. Graphene-carbon nanofiber hybrid supported Pt nanoparticles with enhanced catalytic performance for methanol oxidation and oxygen reduction. Electrochim. Acta 2017, 253, 171–177. [Google Scholar] [CrossRef]
- Yang, Z.; Luo, F. Pt nanoparticles deposited on dihydroxy-polybenzimidazole wrapped carbon nanotubes shows a remarkable durability in methanol electro-oxidation. Int. J. Hydrogen Energy 2017, 42, 507–514. [Google Scholar] [CrossRef]
- Hong, T.Z.; Xue, Q.; Yang, Z.Y.; Dong, Y.P. Great-enhanced performance of Pt nanoparticles by the unique carbon quantum dot/reduced graphene oxide hybrid supports towards methanol electrochemical oxidation. J. Power Sources 2016, 303, 109–117. [Google Scholar] [CrossRef]
- An, G.H.; Jo, H.G.; Ahn, H.J. Surface effect of platinum catalyst-decorated mesoporous carbon support using the dissolution of zinc oxide for methanol oxidation. Appl. Surf. Sci. 2019, 473, 511–515. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, G.; Zhang, X.; Zhang, Y. High-loading Pt nanoparticles on mesoporous carbon with large mesopores for highly active methanol electro-oxidation. J. Solid State Electrochem. 2016, 20, 1705–1712. [Google Scholar] [CrossRef]
- Kasturi, P.R.; Selvan, R.K.; Lee, Y.S. Pt decorated: Artocarpus heterophyllus seed derived carbon as an anode catalyst for DMFC application. RSC Adv. 2016, 6, 62680–62694. [Google Scholar] [CrossRef]
- Yang, Z.; Hafez, I.H.; Berber, M.R.; Nakashima, N. An Enhanced Anode based on Polymer-Coated Carbon Black for use as a Direct Methanol Fuel Cell Electrocatalyst. ChemCatChem 2015, 7, 808–813. [Google Scholar] [CrossRef]
- Eris, S.; Daşdelen, Z.; Yıldız, Y.; Sen, F. Nanostructured Polyaniline-rGO decorated platinum catalyst with enhanced activity and durability for methanol oxidation. Int. J. Hydrogen Energy 2018, 43, 1337–1343. [Google Scholar] [CrossRef]
- Li, X.; Wei, J.; Chai, Y.; Zhang, S.; Zhou, M. Different polyaniline/carbon nanotube composites as Pt catalyst supports for methanol electro-oxidation. J. Mater. Sci. 2015, 50, 1159–1168. [Google Scholar] [CrossRef]
- Daşdelen, Z.; Yıldız, Y.; Eriş, S.; Şen, F. Enhanced electrocatalytic activity and durability of Pt nanoparticles decorated on GO-PVP hybride material for methanol oxidation reaction. Appl. Catal. B Environ. 2017, 219, 511–516. [Google Scholar] [CrossRef]
- Sun, J.; Ling, Y.; Zhang, Q.; Yu, X.; Yang, Z. Simultaneous enhancements in stability and CO tolerance of Pt electrocatalyst by double poly(vinyl pyrrolidone) coatings. RSC Adv. 2017, 7, 29839–29843. [Google Scholar] [CrossRef]
- Eris, S.; Daşdelen, Z.; Sen, F. Enhanced electrocatalytic activity and stability of monodisperse Pt nanocomposites for direct methanol fuel cells. J. Colloid Interface Sci. 2018, 513, 767–773. [Google Scholar] [CrossRef]
- Eris, S.; Daşdelen, Z.; Sen, F. Investigation of electrocatalytic activity and stability of Pt@f-VC catalyst prepared by in-situ synthesis for Methanol electrooxidation. Int. J. Hydrogen Energy 2018, 43, 385–390. [Google Scholar] [CrossRef]
- Mondal, S.; Malik, S. Easy synthesis approach of Pt-nanoparticles on polyaniline surface: An efficient electro-catalyst for methanol oxidation reaction. J. Power Sources 2016, 328, 271–279. [Google Scholar] [CrossRef]
- Sha, R.; Badhulika, S. Facile synthesis of three-dimensional platinum nanoflowers on reduced graphene oxide—Tin oxide composite: An ultra-high performance catalyst for methanol electro-oxidation. J. Electroanal. Chem. 2018, 820, 9–17. [Google Scholar] [CrossRef]
- Yu, F.; Xie, Y.; Tang, H.; Yang, N.; Meng, X.; Wang, X.; Tian, X.L.; Yang, X. Platinum decorated hierarchical porous structures composed of ultrathin titanium nitride nanoflakes for efficient methanol oxidation reaction. Electrochim. Acta 2018, 264, 216–224. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, Z.; Yu, K.; Feng, G.; Xiao, J.; Wu, S.; Li, J.; Chen, C.; Lin, Y.; Hu, G.; et al. Titanium vanadium nitride supported Pt nanoparticles as high-performance catalysts for methanol oxidation reaction. J. Solid State Electrochem. 2017, 21, 3065–3070. [Google Scholar] [CrossRef]
- Liu, G.; Pan, Z.; Zhang, B.; Xiao, J.; Xia, G.; Zhao, Q.; Shi, S.; Hu, G.; Xiao, C.; Wei, Z.; et al. A novel TiN coated CNTs nanocomposite CNTs@TiN supported Pt electrocatalyst with enhanced catalytic activity and durability for methanol oxidation reaction. Int. J. Hydrogen Energy 2017, 42, 12467–12476. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, X.; Zheng, Y.; Shen, J.; Yuan, J.; Wang, A.J.; Niu, L.; Huang, S. Uniform Pt Nanoparticles Incorporated into Reduced Graphene Oxides with MoO3 as Advanced Anode Catalysts for Methanol Electro-oxidation. Electrochim. Acta 2016, 198, 127–134. [Google Scholar] [CrossRef]
- Zhan, G.; Fu, Z.; Sun, D.; Pan, Z.; Xiao, C.; Wu, S.; Chen, C.; Hu, G.; Wei, Z. Platinum nanoparticles decorated robust binary transition metal nitride–carbon nanotubes hybrid as an efficient electrocatalyst for the methanol oxidation reaction. J. Power Sources 2016, 326, 84–92. [Google Scholar] [CrossRef]
- Yang, F.; Ma, L.; Gan, M.; Zhang, J.; Yan, J.; Huang, H.; Yu, L.; Li, Y.; Ge, C.; Hu, H. Polyaniline-functionalized TiO2-C supported Pt catalyst for methanol electro-oxidation. Synth. Met. 2015, 205, 23–31. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Zang, J.; Lu, J.; Xu, X. A novel support of nano titania modified graphitized nanodiamond for Pt electrocatalyst in direct methanol fuel cell. Int. J. Hydrogen Energy 2015, 40, 4540–4547. [Google Scholar] [CrossRef]
- Vu, T.H.T.; Tran, T.T.T.; Le, H.N.T.; Tran, L.T.; Nguyen, P.H.T.; Essayem, N. Pt-AlOOH-SiO2/graphene hybrid nanomaterial with very high electrocatalytic performance for methanol oxidation. J. Power Sources 2015, 276, 340–346. [Google Scholar] [CrossRef]
- Vu, T.H.T.; Tran, T.T.T.; Le, H.N.T.; Tran, L.T.; Nguyen, P.H.T.; Nguyen, H.T.; Bui, N.Q. Solvothermal synthesis of Pt-SiO2/graphene nanocomposites as efficient electrocatalyst for methanol oxidation. Electrochim. Acta 2015, 161, 335–342. [Google Scholar] [CrossRef]
- Li, K.; Xiao, M.; Jin, Z.; Zhu, J.; Ge, J.; Liu, C.; Xing, W. Advanced architecture carbon with in-situ embedded ultrafine titanium dioxide as outstanding support material for platinum catalysts towards methanol electrooxidation. Electrochim. Acta 2017, 235, 508–518. [Google Scholar] [CrossRef]
- Sui, X.L.; Wang, Z.B.; Xia, Y.F.; Yang, M.; Zhao, L.; Gu, D.M. A rapid synthesis of TiO2 nanotubes in an ethylene glycol system by anodization as a Pt-based catalyst support for methanol electrooxidation. RSC Adv. 2015, 5, 35518–35523. [Google Scholar] [CrossRef]
- Medina Ramirez, A.; Ruiz Camacho, B.; Villicaña Aguilera, M.; Galindo Esquivel, I.R.; Ramírez-Minguela, J.J. Effect of different zeolite as Pt supports for methanol oxidation reaction. Appl. Surf. Sci. 2018, 456, 204–214. [Google Scholar] [CrossRef]
- Medina Ramírez, A.; Villicaña Aguilera, M.; López-Badillo, C.M.; Ruiz-Camacho, B. Synthesis of FAU zeolite-C composite as catalyst support for methanol electro-oxidation. Int. J. Hydrogen Energy 2017, 42, 30291–30300. [Google Scholar] [CrossRef]
- Daas, B.M.; Ghosh, S. Electro-oxidation of Methanol and Ethanol Catalyzed by Pt/ZSM-5/C. Electroanalysis 2017, 29, 2516–2525. [Google Scholar] [CrossRef]
- Yao, J.; Yao, Y. Investigation of zeolite supported platinum electrocatalyst for electrochemical oxidation of small organic species. Int. J. Hydrogen Energy 2016, 41, 14747–14757. [Google Scholar] [CrossRef]
- Daas, B.M.; Ghosh, S. Fuel cell applications of chemically synthesized zeolite modified electrode (ZME) as catalyst for alcohol electro-oxidation—A review. J. Electroanal. Chem. 2016, 783, 308–315. [Google Scholar] [CrossRef]
- Ekrami-Kakhki, M.S.; Naeimi, A.; Donyagard, F. Pt nanoparticles supported on a novel electrospun polyvinyl alcohol-CuO–Co 3 O 4 /chitosan based on Sesbania sesban plant as an electrocatalyst for direct methanol fuel cells. Int. J. Hydrogen Energy 2019, 44, 1671–1685. [Google Scholar] [CrossRef]
- Li, G.; Yao, S.; Zhu, J.; Liu, C.; Xing, W. The enhancement effect of nitrogen, fluorine-codoped titanium dioxide on the carbon supported platinum nano-catalyst for methanol electrooxidation reaction. J. Power Sources 2015, 278, 9–17. [Google Scholar] [CrossRef]
- Antolini, E. Photo-assisted methanol oxidation on Pt–TiO2 catalysts for direct methanol fuel cells: A short review. Appl. Catal. B Environ. 2018, 237, 491–503. [Google Scholar] [CrossRef]
- Antolini, E. Evaluation of the Optimum Composition of Low-Temperature Fuel Cell Electrocatalysts for Methanol Oxidation by Combinatorial Screening. ACS Comb. Sci. 2017, 19, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Torbina, V.V.; Vodyankin, A.A.; Ten, S.; Mamontov, G.V.; Salaev, M.A.; Sobolev, V.I.; Vodyankina, O.V. Ag-Based Catalysts in Heterogeneous Selective Oxidation of Alcohols: A Review. Catalysts 2018, 8, 447. [Google Scholar] [CrossRef]
- Calderón, J.C.; García, G.; Calvillo, L.; Rodríguez, J.L.; Lázaro, M.J.; Pastor, E. Electrochemical oxidation of CO and methanol on Pt–Ru catalysts supported on carbon nanofibers: The influence of synthesis method. Appl. Catal. B Environ. 2015, 165, 676–686. [Google Scholar] [CrossRef]
- Calderón, J.C.; García, G.; Querejeta, A.; Alcaide, F.; Calvillo, L.; Lázaro, M.J.; Rodríguez, J.L.; Pastor, E. Carbon monoxide and methanol oxidations on carbon nanofibers supported Pt–Ru electrodes at different temperatures. Electrochim. Acta 2015, 186, 359–368. [Google Scholar] [CrossRef]
- Trovarelli, A. Catalytic properties of ceria and CeO2-Containing materials. Catal. Rev. Sci. Eng. 1996, 38, 439–520. [Google Scholar] [CrossRef]
- McFarland, E.W.; Metiu, H. Catalysis by doped oxides. Chem. Rev. 2013, 113, 4391–4427. [Google Scholar] [CrossRef]
- Feng, C.; Takeuchi, T.; Abdelkareem, M.A.; Tsujiguchi, T.; Nakagawa, N. Carbon-CeO2 composite nanofibers as a promising support for a PtRu anode catalyst in a direct methanol fuel cell. J. Power Sources 2013, 242, 57–64. [Google Scholar] [CrossRef]
- Kunitomo, H.; Ishitobi, H.; Nakagawa, N. Optimized CeO2 content of the carbon nanofiber support of PtRu catalyst for direct methanol fuel cells. J. Power Sources 2015, 297, 400–407. [Google Scholar] [CrossRef]
- Tsukagoshi, Y.; Ishitobi, H.; Nakagawa, N. Improved performance of direct methanol fuel cells with the porous catalyst layer using highly-active nanofiber catalyst. Carbon Resour. Convers. 2018, 1, 61–72. [Google Scholar] [CrossRef]
- Abdullah, N.; Kamarudin, S.K.; Shyuan, L.K.; Karim, N.A. Fabrication and Characterization of New Composite TiO2 Carbon Nanofiber Anodic Catalyst Support for Direct Methanol Fuel Cell via Electrospinning Method. Nanoscale Res. Lett. 2017, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, H.; Dai, Y.; Zhang, N.; Zhao, W.; Wang, S.; Lou, Y.; Li, Y.; Sun, Y. Preparation and characterization of Pt/TiO2 nanofibers catalysts for methanol electro-oxidation. Electrochim. Acta 2015, 178, 74–79. [Google Scholar] [CrossRef]
- Ito, Y.; Takeuchi, T.; Tsujiguchi, T.; Abdelkareem, M.A.; Nakagawa, N. Ultrahigh methanol electro-oxidation activity of PtRu nanoparticles prepared on TiO2-embedded carbon nanofiber support. J. Power Sources 2013, 242, 280–288. [Google Scholar] [CrossRef]
- Ercelik, M.; Ozden, A.; Seker, E.; Colpan, C.O. Characterization and performance evaluation of Pt[sbnd]Ru/C[sbnd]TiO2 anode electrocatalyst for DMFC applications. Int. J. Hydrogen Energy 2017, 42, 21518–21529. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, Y.; Wang, X.; Zhang, G.; Cui, P. Boron as a superior activator for Pt anode catalyst in direct alcohol fuel cell. J. Power Sources 2019, 431, 125–134. [Google Scholar] [CrossRef]
- Patel, P.P.; Datta, M.K.; Jampani, P.H.; Hong, D.; Poston, J.A.; Manivannan, A.; Kumta, P.N. High performance and durable nanostructured TiN supported Pt50-Ru50 anode catalyst for direct methanol fuel cell (DMFC). J. Power Sources 2015, 293, 437–446. [Google Scholar] [CrossRef]
- Avasarala, B.; Haldar, P. On the stability of TiN-based electrocatalysts for fuel cell applications. Int. J. Hydrogen Energy 2011, 36, 3965–3974. [Google Scholar] [CrossRef]
- Zhong, Y.; Xia, X.; Shi, F.; Zhan, J.; Tu, J.; Fan, H.J. Transition Metal Carbides and Nitrides in Energy Storage and Conversion. Adv. Sci. 2016, 3, 1500286. [Google Scholar] [CrossRef]
- Ham, D.; Lee, J. Transition Metal Carbides and Nitrides as Electrode Materials for Low Temperature Fuel Cells. Energies 2009, 2, 873–899. [Google Scholar] [CrossRef]
- Goel, J.; Basu, S. Effect of support materials on the performance of direct ethanol fuel cell anode catalyst. Int. J. Hydrogen Energy 2014, 39, 15956–15966. [Google Scholar] [CrossRef]
- Bello, M.; Zaidi, S.M.J.; Al-Ahmed, A.; Basu, S.; Park, D.H.; Lakhi, K.S.; Vinu, A. Pt–Ru nanoparticles functionalized mesoporous carbon nitride with tunable pore diameters for DMFC applications. Microporous Mesoporous Mater. 2017, 252, 50–58. [Google Scholar] [CrossRef]
- Corpuz, A.R.; Olson, T.S.; Joghee, P.; Pylypenko, S.; Dameron, A.A.; Dinh, H.N.; O’Neill, K.J.; Hurst, K.E.; Bender, G.; Gennett, T.; et al. Effect of a nitrogen-doped PtRu/carbon anode catalyst on the durability of a direct methanol fuel cell. J. Power Sources 2012, 217, 142–151. [Google Scholar] [CrossRef]
- Corpuz, A.R.; Wood, K.N.; Pylypenko, S.; Dameron, A.A.; Joghee, P.; Olson, T.S.; Bender, G.; Dinh, H.N.; Gennett, T.; Richards, R.M.; et al. Effect of nitrogen post-doping on a commercial platinum-ruthenium/carbon anode catalyst. J. Power Sources 2014, 248, 296–306. [Google Scholar] [CrossRef]
- Ling, Y.; Yang, Z.; Yang, J.; Zhang, Y.; Zhang, Q.; Yu, X.; Cai, W. PtRu nanoparticles embedded in nitrogen doped carbon with highly stable CO tolerance and durability. Nanotechnology 2018, 29, 055402. [Google Scholar] [CrossRef]
- Yang, Z.; Cai, W.; Zhang, Q.; Ling, Y.; Yu, X.; Zhang, Y. Stabilization of PtRu electrocatalyst by nitrogen doped carbon layer derived from carbonization of poly(vinyl pyrrolidone). Int. J. Hydrogen Energy 2017, 42, 12583–12592. [Google Scholar] [CrossRef]
- Ganesan, A.; Narayanasamy, M.; Shunmugavel, K.; Jayanthi Chinnappa, I. Ultra low loading of anode catalyst for direct methanol fuel cells with ZrO2/ pyrolysed (PANI-melamine) as catalyst support. Int. J. Hydrogen Energy 2016, 41, 8963–8977. [Google Scholar] [CrossRef]
- Das, S.; Dutta, K.; Kundu, P.P.; Bhattacharya, S.K. Nanostructured Polyaniline: An Efficient Support Matrix for Platinum-Ruthenium Anode Catalyst in Direct Methanol Fuel Cell. Fuel Cells 2018, 18, 369–378. [Google Scholar] [CrossRef]
- El Rhazi, M.; Majid, S.; Elbasri, M.; Salih, F.E.; Oularbi, L.; Lafdi, K. Recent progress in nanocomposites based on conducting polymer: Application as electrochemical sensors. Int. Nano Lett. 2018, 8, 79–99. [Google Scholar] [CrossRef]
- Pašti, I.A.; Janošević Ležaić, A.; Gavrilov, N.M.; Ćirić-Marjanović, G.; Mentus, S.V. Nanocarbons derived from polymers for electrochemical energy conversion and storage—A review. Synth. Met. 2018, 246, 267–281. [Google Scholar] [CrossRef]
- Veizaga, N.S.; Rodriguez, V.I.; Rocha, T.A.; Bruno, M.; Scelza, O.A.; de Miguel, S.R.; Gonzalez, E.R. Promoting Effect of Tin in Platinum Electrocatalysts for Direct Methanol Fuel Cells (DMFC). J. Electrochem. Soc. 2015, 162, F243–F249. [Google Scholar] [CrossRef]
- Amani, M.; Kazemeini, M.; Hamedanian, M.; Pahlavanzadeh, H.; Gharibi, H. Investigation of methanol oxidation on a highly active and stable Pt–Sn electrocatalyst supported on carbon-polyaniline composite for application in a passive direct methanol fuel cell. Mater. Res. Bull. 2015, 68, 166–178. [Google Scholar] [CrossRef]
- Maya-Cornejo, J.; Garcia-Bernabé, A.; Compañ, V. Bimetallic Pt-M electrocatalysts supported on single-wall carbon nanotubes for hydrogen and methanol electrooxidation in fuel cells applications. Int. J. Hydrogen Energy 2018, 43, 872–884. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, Z.; Zhang, W.; Wang, Y. Nanosized Mo-doped CeO2 enhances the electrocatalytic properties of the Pt anode catalyst in direct methanol fuel cells. J. Mater. Chem. A 2017, 5, 1481–1487. [Google Scholar] [CrossRef]
- Du, H.; Wan, T.; Qu, B.; Scott, J.; Lin, X.; Younis, A.; Chu, D. Tailoring the multi-functionalities of one-dimensional ceria nanostructures via oxygen vacancy modulation. J. Colloid Interface Sci. 2017, 504, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Puigdollers, A.R.; Schlexer, P.; Tosoni, S.; Pacchioni, G. Increasing oxide reducibility: The role of metal/oxide interfaces in the formation of oxygen vacancies. ACS Catalysis 2017, 7, 6493–6513. [Google Scholar] [CrossRef]
- Figueroba, A.; Bruix, A.; Kovács, G.; Neyman, K.M. Metal-doped ceria nanoparticles: Stability and redox processes. Phys. Chem. Chem. Phys. 2017, 19, 21729–21738. [Google Scholar] [CrossRef]
- Chu, Y.-Y.; Wang, Z.-B.; Jiang, Z.-Z.; Gu, D.-M.; Yin, G.-P. A Novel Structural Design of a Pt/C-CeO2 Catalyst with Improved Performance for Methanol Electro-Oxidation by β-Cyclodextrin Carbonization. Adv. Mater. 2011, 23, 3100–3104. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef]
- Lykhach, Y.; Bruix, A.; Fabris, S.; Potin, V.; Matolínová, I.; Matolín, V.; Libuda, J.; Neyman, K.M. Oxide-based nanomaterials for fuel cell catalysis: The interplay between supported single Pt atoms and particles. Catal. Sci. Technol. 2017, 7, 4315–4345. [Google Scholar] [CrossRef]
- Du, H.; Wang, Y.; Arandiyan, H.; Younis, A.; Scott, J.; Qu, B.; Wan, T.; Lin, X.; Chen, J.; Chu, D. Design and synthesis of CeO2 nanowire/MnO2 nanosheet heterogeneous structure for enhanced catalytic properties. Mater. Today Commun. 2017, 11, 103–111. [Google Scholar] [CrossRef]
- Videla, A.; Osmieri, L.; Esfahani, R.; Zeng, J.; Francia, C.; Specchia, S. The Use of C-MnO2 as Hybrid Precursor Support for a Pt/C-MnxO1+x Catalyst with Enhanced Activity for the Methanol Oxidation Reaction (MOR). Catalysts 2015, 5, 1399–1416. [Google Scholar] [CrossRef]
- Abdel Hameed, R.M.; Fetohi, A.E.; Amin, R.S.; El-Khatib, K.M. Promotion effect of manganese oxide on the electrocatalytic activity of Pt/C for methanol oxidation in acid medium. Appl. Surf. Sci. 2015, 359, 651–663. [Google Scholar] [CrossRef]
- Sun, Q.; Park, S.J.; Kim, S. Preparation and electrocatalytic oxidation performance of Pt/MnO2-graphene oxide nanocomposites. J. Ind. Eng. Chem. 2015, 26, 265–269. [Google Scholar] [CrossRef]
- Jin, H.; Guo, C.; Liu, X.; Liu, J.; Vasileff, A.; Jiao, Y.; Zheng, Y.; Qiao, S.Z. Emerging Two-Dimensional Nanomaterials for Electrocatalysis. Chem. Rev. 2018, 118, 6337–6408. [Google Scholar] [CrossRef]
- Yadav, R.; Subhash, A.; Chemmenchery, N.; Kandasubramanian, B. Graphene and Graphene Oxide for Fuel Cell Technology. Ind. Eng. Chem. Res. 2018, 57, 9333–9350. [Google Scholar] [CrossRef]
- Wang, C.; Astruc, D. Recent developments of metallic nanoparticle-graphene nanocatalysts. Prog. Mater. Sci. 2018, 94, 306–383. [Google Scholar] [CrossRef]
- Ambrosi, A.; Chua, C.K.; Latiff, N.M.; Loo, A.H.; Wong, C.H.A.; Eng, A.Y.S.; Bonanni, A.; Pumera, M. Graphene and its electrochemistry-an update. Chem. Soc. Rev. 2016, 45, 2458–2493. [Google Scholar] [CrossRef]
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Metal nanoparticles supported on two-dimensional graphenes as heterogeneous catalysts. Coord. Chem. Rev. 2016, 312, 99–148. [Google Scholar] [CrossRef]
- Lawal, A.T. Graphene-based nano composites and their applications. A review. Biosens. Bioelectron. 2019, 141, 111384. [Google Scholar] [CrossRef]
- Guo, M.; Tu, Q.; Wang, L.; Tang, Y.; Song, H.; Zhou, J.; Zhang, Z.; Wang, Y.; Liu, C. Hollow Pt skim-sandwiched Cu spheres supported on reduced graphene oxide-carbon nanotube architecture for efficient methanol electrooxidation. Int. J. Hydrogen Energy 2019, 44, 6886–6895. [Google Scholar] [CrossRef]
- Salarizadeh, P.; Askari, M.B.; Beheshti-Marnani, A.; Seifi, M.; Rozati, S.M.; Rohani, T.; Askari, N.; Salarizadeh, N.; Mohammadi, S.Z. Synthesis and characterization of (Co, Fe, Ni) 9 S 8 nanocomposite supported on reduced graphene oxide as an efficient and stable electrocatalyst for methanol electrooxidation toward DMFC. J. Mater. Sci. Mater. Electron. 2019, 30, 3521–3529. [Google Scholar] [CrossRef]
- Org, W.E.; Lu, F.; Zhang, C.; Cheng, J.; Zhu, C.; Zhang, H.; Cheng, X. Electrochemical Science Ball-like Pt Nanoparticles on GO-modified Carbon Fiber Cloth with High Electrocatalytic Activity for Methanol Oxidation. Int. J. Electrochem. Sci. 2018, 13, 9007–9016. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Huang, H.; Jiang, Q.; Wu, Y. Platinum nanoparticles anchored on graphene oxide-dispersed pristine carbon nanotube supports: High-performance electrocatalysts toward methanol electrooxidation. Electrochim. Acta 2017, 258, 919–926. [Google Scholar] [CrossRef]
- Jovanovic, Z.; Bajuk-Bogdanović, D.; Jovanović, S.; Mravik, Z.; Kovač, J.; Holclajtner-Antunović, I.; Vujković, M. The role of surface chemistry in the charge storage properties of graphene oxide. Electrochim. Acta 2017, 258, 1228–1243. [Google Scholar] [CrossRef]
- Wang, H.; Xue, Y.; Zhu, B.; Yang, J.; Wang, L.; Tan, X.; Wang, Z.; Chu, Y. CeO2 nanowires stretch-embedded in reduced graphite oxide nanocomposite support for Pt nanoparticles as potential electrocatalyst for methanol oxidation reaction. Int. J. Hydrogen Energy 2017, 42, 20549–20559. [Google Scholar] [CrossRef]
- Ji, Y.; Hou, M.; Zheng, Y.; Chen, W.; Wang, Z. A 3D network structured reduced graphene oxide/PtRu alloyed composite catalyst in-situ assembled via particle-constructing method. Colloids Surf. A Physicochem. Eng. Asp. 2019, 561, 292–300. [Google Scholar] [CrossRef]
- Iqbal, M.; Dinh, D.K.; Abbas, Q.; Imran, M.; Sattar, H.; Ul Ahmad, A. Controlled Surface Wettability by Plasma Polymer Surface Modification. Surfaces 2019, 2, 349–371. [Google Scholar] [CrossRef]
- Qian, W.; Hao, R.; Zhou, J.; Eastman, M.; Manhat, B.A.; Sun, Q.; Goforth, A.M.; Jiao, J. Exfoliated graphene-supported Pt and Pt-based alloys as electrocatalysts for direct methanol fuel cells. Carbon N. Y. 2013, 52, 595–604. [Google Scholar] [CrossRef]
- Antolini, E.; Salgado, J.R.C.; Gonzalez, E.R. The stability of Pt-M (M = first row transition metal) alloy catalysts and its effect on the activity in low temperature fuel cells. A literature review and tests on a Pt-Co catalyst. J. Power Sources 2006, 160, 957–968. [Google Scholar] [CrossRef]
- Baronia, R.; Goel, J.; Kaswan, J.; Shukla, A.; Singhal, S.K.; Singh, S.P. PtCo/rGO nano-anode catalyst: Enhanced power density with reduced methanol crossover in direct methanol fuel cell. Mater. Renew. Sustain. Energy 2018, 7, 1–13. [Google Scholar] [CrossRef]
- Baronia, R.; Goel, J.; Tiwari, S.; Singh, P.; Singh, D.; Singh, S.P.; Singhal, S.K. Efficient electro-oxidation of methanol using PtCo nanocatalysts supported reduced graphene oxide matrix as anode for DMFC. Int. J. Hydrogen Energy 2017, 42, 10238–10247. [Google Scholar] [CrossRef]
- Tian, M.; Shi, S.; Shen, Y.; Yin, H. PtRu alloy nanoparticles supported on nanoporous gold as an efficient anode catalyst for direct methanol fuel cell. Electrochim. Acta 2019, 293, 390–398. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, X.; Han, Y.; Shen, L.; Yin, S.; Li, G.; Jiang, Y.; Sun, S. Engineering PtRu bimetallic nanoparticles with adjustable alloying degree for methanol electrooxidation: Enhanced catalytic performance. Appl. Catal. B Environ. 2020, 263, 118345. [Google Scholar] [CrossRef]
- Banham, D.; Choi, J.; Kishimoto, T.; Ye, S. Integrating PGM-Free Catalysts into Catalyst Layers and Proton Exchange Membrane Fuel Cell Devices. Adv. Mater. 2019, 31, 1804846. [Google Scholar] [CrossRef]
- Yang, L.; Shui, J.; Du, L.; Shao, Y.; Liu, J.; Dai, L.; Hu, Z. Carbon-Based Metal-Free ORR Electrocatalysts for Fuel Cells: Past, Present, and Future. Adv. Mater. 2019, 31, 1804799. [Google Scholar] [CrossRef]
- Lo Vecchio, C.; Sebastián, D.; Lázaro, M.; Aricò, A.; Baglio, V. Methanol-Tolerant M–N–C Catalysts for Oxygen Reduction Reactions in Acidic Media and Their Application in Direct Methanol Fuel Cells. Catalysts 2018, 8, 650. [Google Scholar] [CrossRef]
- Sial, M.A.Z.G.; Din, M.A.U.; Wang, X. Multimetallic nanosheets: Synthesis and applications in fuel cells. Chem. Soc. Rev. 2018, 47, 6175–6200. [Google Scholar] [CrossRef]
- Banham, D.; Kishimoto, T.; Zhou, Y.; Sato, T.; Bai, K.; Ozaki, J.I.; Imashiro, Y.; Ye, S. Critical advancements in achieving high power and stable nonprecious metal catalyst-based MEAs for real-world proton exchange membrane fuel cell applications. Sci. Adv. 2018, 4, eaar7180. [Google Scholar] [CrossRef]
- Pattanayak, P.; Pramanik, N.; Kumar, P.; Kundu, P.P. Fabrication of cost-effective non-noble metal supported on conducting polymer composite such as copper/polypyrrole graphene oxide (Cu2O/PPy–GO) as an anode catalyst for methanol oxidation in DMFC. Int. J. Hydrogen Energy 2018, 43, 11505–11519. [Google Scholar] [CrossRef]
- Das, S.; Dutta, K.; Kundu, P.P. Sulfonated polypyrrole matrix induced enhanced efficiency of Ni nanocatalyst for application as an anode material for DMFCs. Mater. Chem. Phys. 2016, 176, 143–151. [Google Scholar] [CrossRef]
- Park, K.W.; Choi, J.H.; Kwon, B.K.; Lee, S.A.; Sung, Y.E.; Ha, H.Y.; Hong, S.A.; Kim, H.; Wieckowski, A. Chemical and electronic effects of Ni in Pt/Ni and Pt/Ru/Ni alloy nanoparticles in methanol electrooxidation. J. Phys. Chem. B 2002, 106, 1869–1877. [Google Scholar] [CrossRef]
- Das, S.; Dutta, K.; Kundu, P.P. Nickel nanocatalysts supported on sulfonated polyaniline: Potential toward methanol oxidation and as anode materials for DMFCs. J. Mater. Chem. A 2015, 3, 11349–11357. [Google Scholar] [CrossRef]
- Li, K.; Jin, Z.; Ge, J.; Liu, C.; Xing, W. Platinum nanoparticles partially-embedded into carbon sphere surfaces: A low metal-loading anode catalyst with superior performance for direct methanol fuel cells. J. Mater. Chem. A 2017, 5, 19857–19865. [Google Scholar] [CrossRef]
- Yuan, W.; Zhang, Y.; Zhang, N.; Yin, C.; Zhang, X.; Liu, X. Carbon riveted Pt–MnO2/reduced graphene oxide anode catalyst for DMFC. Catal. Commun. 2017, 100, 66–70. [Google Scholar] [CrossRef]
- Rethinasabapathy, M.; Kang, S.M.; Haldorai, Y.; Jankiraman, M.; Jonna, N.; Choe, S.R.; Huh, Y.S.; Natesan, B. Ternary PtRuFe nanoparticles supported N-doped graphene as an efficient bifunctional catalyst for methanol oxidation and oxygen reduction reactions. Int. J. Hydrogen Energy 2017, 42, 30738–30749. [Google Scholar] [CrossRef]
- Rethinasabapathy, M.; Kang, S.M.; Haldorai, Y.; Jonna, N.; Jankiraman, M.; Lee, G.W.; Jang, S.C.; Natesan, B.; Roh, C.; Huh, Y.S. Quaternary PtRuFeCo nanoparticles supported N-doped graphene as an efficient bifunctional electrocatalyst for low-temperature fuel cells. J. Ind. Eng. Chem. 2019, 69, 285–294. [Google Scholar] [CrossRef]
- Zhao, S.; Yin, H.; Du, L.; Yin, G.; Tang, Z.; Liu, S. Three dimensional N-doped graphene/PtRu nanoparticle hybrids as high performance anode for direct methanol fuel cells. J. Mater. Chem. A 2014, 2, 3719–3724. [Google Scholar] [CrossRef]
- Thiagarajan, V.; Karthikeyan, P.; Thanarajan, K.; Neelakrishnan, S.; Manoharan, R.; Chen, R.; Fly, A.; Anand, R.; Karuppa Raj, T.R.; Sendhil Kumar, N. Experimental investigation on DMFCs using reduced noble metal loading with NiTiO3 as supportive material to enhance cell performances. Int. J. Hydrogen Energy 2019, 44, 13415–13423. [Google Scholar] [CrossRef]
- Fard, H.F.; Khodaverdi, M.; Pourfayaz, F.; Ahmadi, M.H. Application of N-doped carbon nanotube-supported Pt–Ru as electrocatalyst layer in passive direct methanol fuel cell. Int. J. Hydrogen Energy 2021, 45, 25307–25316. [Google Scholar] [CrossRef]
- Kulkarni, A.; Siahrostami, S.; Patel, A.; Nørskov, J.K. Understanding Catalytic Activity Trends in the Oxygen Reduction Reaction. Chem. Rev. 2018, 118, 2302–2312. [Google Scholar] [CrossRef]
- Kang, S.Y.; Kim, H.J.; Chung, Y.H. Recent developments of nano-structured materials as the catalysts for oxygen reduction reaction. Nano Converg. 2018, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Stacy, J.; Regmi, Y.N.; Leonard, B.; Fan, M. The recent progress and future of oxygen reduction reaction catalysis: A review. Renew. Sustain. Energy Rev. 2017, 69, 401–414. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.J.; Baek, J.B. Metal-Free Catalysts for Oxygen Reduction Reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Holewinski, A.; Wang, C. Prospects of Platinum-Based Nanostructures for the Electrocatalytic Reduction of Oxygen. ACS Catal. 2018, 8, 9388–9398. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, X.; Pan, Y.; Peng, Z. A review of Pt-based electrocatalysts for oxygen reduction reaction. Front. Energy 2017, 11, 268–285. [Google Scholar] [CrossRef]
- Sakthivel, M.; Drillet, J.F. An extensive study about influence of the carbon support morphology on Pt activity and stability for oxygen reduction reaction. Appl. Catal. B Environ. 2018, 231, 62–72. [Google Scholar] [CrossRef]
- Yarlagadda, V.; Carpenter, M.K.; Moylan, T.E.; Kukreja, R.S.; Koestner, R.; Gu, W.; Thompson, L.; Kongkanand, A.A.A. Boosting Fuel Cell Performance with Accessible Carbon Mesopores. ACS Energy Lett. 2018, 3, 618–621. [Google Scholar] [CrossRef]
- Mora-Hernández, J.M.; Luo, Y.; Alonso-Vante, N. What can we learn in electrocatalysis, from nanoparticulated precious and/or non-precious catalytic centers interacting with their support? Catalysts 2016, 6, 145. [Google Scholar] [CrossRef]
- Shahgaldi, S.; Hamelin, J. Improved carbon nanostructures as a novel catalyst support in the cathode side of PEMFC: A critical review. Carbon 2015, 94, 705–728. [Google Scholar] [CrossRef]
- Zhao, G.; Zhao, T.S.; Xu, J.; Lin, Z.; Yan, X. Impact of pore size of ordered mesoporous carbon FDU-15-supported platinum catalysts on oxygen reduction reaction. Int. J. Hydrogen Energy 2017, 42, 3325–3334. [Google Scholar] [CrossRef]
- Tesfu-Zeru, T.; Sakthivel, M.; Drillet, J.F. Investigation of mesoporous carbon hollow spheres as catalyst support in DMFC cathode. Appl. Catal. B Environ. 2017, 204, 173–184. [Google Scholar] [CrossRef]
- Yang, Z.; Ling, Y.; Zhang, Y.; Yang, M. Highly methanol-tolerant platinum electrocatalyst derived from poly(vinylpoyrrolidone) coating. Nanotechnology 2017, 28, 055404. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Nakashima, N. A simple preparation of very high methanol tolerant cathode electrocatalyst for direct methanol fuel cell based on polymer-coated carbon nanotube/platinum. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, C.T.; Zhao, L.; Zhang, J.J.; Zhang, L.M.; Wang, Z.B. Effect of different structures of carbon supports for cathode catalyst on performance of direct methanol fuel cell. Int. J. Hydrogen Energy 2016, 41, 1859–1870. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, S.; Sun, S. Tuning Nanoparticle Catalysis for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2013, 52, 8526–8544. [Google Scholar] [CrossRef]
- Mistry, H.; Varela, A.S.; Kühl, S.; Strasser, P.; Cuenya, B.R. Nanostructured electrocatalysts with tunable activity and selectivity. Nat. Rev. Mater. 2016, 1, 1–14. [Google Scholar] [CrossRef]
- Lu, Y.; Du, S.; Steinberger-Wilckens, R. One-dimensional nanostructured electrocatalysts for polymer electrolyte membrane fuel cells—A review. Appl. Catal. B Environ. 2016, 199, 292–314. [Google Scholar] [CrossRef]
- Calle-Vallejo, F.; Pohl, M.D.; Reinisch, D.; Loffreda, D.; Sautet, P.; Bandarenka, A.S. Why conclusions from platinum model surfaces do not necessarily lead to enhanced nanoparticle catalysts for the oxygen reduction reaction. Chem. Sci. 2017, 8, 2283–2289. [Google Scholar] [CrossRef]
- Shao, M.; Peles, A.; Shoemaker, K. Electrocatalysis on platinum nanoparticles: Particle size effect on oxygen reduction reaction activity. Nano Lett. 2011, 11, 3714–3719. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Z.; Cheng, T.; Fortunelli, A.; Chen, C.Y.; Yu, R.; Zhang, Q.; Gu, L.; Merinov, B.V.; Lin, Z.; et al. Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science 2016, 354, 1414–1419. [Google Scholar] [CrossRef]
- Stephens, I.E.L.; Rossmeisl, J.; Chorkendorff, I. Toward sustainable fuel cells. Science 2016, 354, 1378–1379. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kwon, T.; Kim, J.; Jin, H.; Kim, H.Y.; Kim, B.; Joo, S.H.; Lee, K. Hollow nanoparticles as emerging electrocatalysts for renewable energy conversion reactions. Chem. Soc. Rev. 2018, 47, 8173–8202. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, J.E.; Hwang, W.; Cho, Y.H.; Sung, Y.E. A hierarchical cathode catalyst layer architecture for improving the performance of direct methanol fuel cell. Appl. Catal. B Environ. 2017, 209, 91–97. [Google Scholar] [CrossRef]
- Glass, D.E.; Prakash, G.K.S. Effect of the Cathode Catalyst Layer Thickness on the Performance in Direct Methanol Fuel Cells. Electroanalysis 2019, 31, 718–725. [Google Scholar] [CrossRef]
- Hunt, S.T.; Román-Leshkov, Y. Principles and Methods for the Rational Design of Core-Shell Nanoparticle Catalysts with Ultralow Noble Metal Loadings. Acc. Chem. Res. 2018, 51, 1054–1062. [Google Scholar] [CrossRef]
- Stamenkovic, V.; Mun, B.S.; Mayrhofer, K.J.J.; Ross, P.N.; Markovic, N.M.; Rossmeisl, J.; Greeley, J.; Nørskov, J.K. Changing the Activity of Electrocatalysts for Oxygen Reduction by Tuning the Surface Electronic Structure. Angew. Chemie Int. Ed. 2006, 45, 2897–2901. [Google Scholar] [CrossRef]
- Calle-Vallejo, F.; Martínez, J.I.; Rossmeisl, J. Density functional studies of functionalized graphitic materials with late transition metals for oxygen reduction reactions. Phys. Chem. Chem. Phys. 2011, 13, 15639–15643. [Google Scholar] [CrossRef]
- Li, J.; Alsudairi, A.; Ma, Z.F.; Mukerjee, S.; Jia, Q. Asymmetric volcano trend in oxygen reduction activity of Pt and non-Pt catalysts: In situ identification of the site-blocking effect. J. Am. Chem. Soc. 2017, 139, 1384–1387. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Mun, B.S.; Arenz, M.; Mayrhofer, K.J.J.; Lucas, C.A.; Wang, G.; Ross, P.N.; Markovic, N.M. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 2007, 6, 241–247. [Google Scholar] [CrossRef]
- Greeley, J.; Stephens, I.E.L.; Bondarenko, A.S.; Johansson, T.P.; Hansen, H.A.; Jaramillo, T.F.; Rossmeisl, J.; Chorkendorff, I.; Nørskov, J.K. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 2009, 1, 552–556. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Fowler, B.; Mun, B.S.; Wang, G.; Ross, P.N.; Lucas, C.A.; Markovic, N.M. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 2007, 315, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Rudi, S.; Cui, C.; Heggen, M.; Strasser, P. Size-Controlled Synthesis of Sub-10 nm PtNi 3 Alloy Nanoparticles and their Unusual Volcano-Shaped Size Effect on ORR Electrocatalysis. Small 2016, 12, 3189–3196. [Google Scholar] [CrossRef] [PubMed]
- Strasser, P.; Koh, S.; Anniyev, T.; Greeley, J.; More, K.; Yu, C.; Liu, Z.; Kaya, S.; Nordlund, D.; Ogasawara, H.; et al. Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts. Nat. Chem. 2010, 2, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Escribano, M.; Malacrida, P.; Hansen, M.H.; Vej-Hansen, U.G.; Velázquez-Palenzuela, A.; Tripkovic, V.; Schiøtz, J.; Rossmeisl, J.; Stephens, I.E.L.; Chorkendorff, I. Tuning the activity of Pt alloy electrocatalysts by means of the lanthanide contraction. Science 2016, 352, 73–76. [Google Scholar] [CrossRef]
- Chen, C.; Kang, Y.; Huo, Z.; Zhu, Z.; Huang, W.; Xin, H.L.; Snyder, J.D.; Li, D.; Herron, J.A.; Mavrikakis, M.; et al. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science 2014, 343, 1339–1343. [Google Scholar] [CrossRef]
- Bu, L.; Zhang, N.; Guo, S.; Zhang, X.; Li, J.; Yao, J.; Wu, T.; Lu, G.; Ma, J.Y.; Su, D.; et al. Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Science 2016, 354, 1410–1414. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, J.; Huang, Q.; Zhou, Y.; Zou, Z.; Yang, H. Interconnected nanoparticle-stacked platinum-based nanosheets as active cathode electrocatalysts for passive direct methanol fuel cells. J. Electroanal. Chem. 2018, 828, 50–58. [Google Scholar] [CrossRef]
- Choi, B.; Nam, W.H.; Chung, D.Y.; Park, I.S.; Yoo, S.J.; Song, J.C.; Sung, Y.E. Enhanced methanol tolerance of highly Pd rich Pd-Pt cathode electrocatalysts in direct methanol fuel cells. Electrochim. Acta 2015, 164, 235–242. [Google Scholar] [CrossRef]
- Yan, X.H.; Zhao, T.S.; An, L.; Zhao, G.; Zeng, L. A novel cathode architecture with a thin reaction layer alleviates mixed potentials and catalyst poisoning in direct methanol fuel cells. Int. J. Hydrogen Energy 2015, 40, 16540–16546. [Google Scholar] [CrossRef]
- Shao, Y.; Dodelet, J.; Wu, G.; Zelenay, P. PGM-Free Cathode Catalysts for PEM Fuel Cells: A Mini-Review on Stability Challenges. Adv. Mater. 2019, 31, 1807615. [Google Scholar] [CrossRef]
- Goswami, C.; Hazarika, K.K.; Bharali, P. Transition metal oxide nanocatalysts for oxygen reduction reaction. Mater. Sci. Energy Technol. 2018, 1, 117–128. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.-C.; An, B.; Huang, R.; Wang, C.; Zhou, Z.; Lin, W. Networking Pyrolyzed Zeolitic Imidazolate Frameworks by Carbon Nanotubes Improves Conductivity and Enhances Oxygen-Reduction Performance in Polymer-Electrolyte-Membrane Fuel Cells. Adv. Mater. 2017, 29, 1604556. [Google Scholar] [CrossRef]
- Nie, Y.; Li, L.; Wei, Z. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction. Chem. Soc. Rev. 2015, 44, 2168–2201. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Takeyasu, K.; Nakamura, J. Active Sites and Mechanism of Oxygen Reduction Reaction Electrocatalysis on Nitrogen-Doped Carbon Materials. Adv. Mater. 2019, 31, 1804297. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Song, M.; Wang, J.; Liu, X. Recent Progress in Nitrogen-Doped Metal-Free Electrocatalysts for Oxygen Reduction Reaction. Catalysts 2018, 8, 196. [Google Scholar] [CrossRef]
- Sa, Y.J.; Kim, J.H.; Joo, S.H. Recent progress in the identification of active sites in pyrolyzed Fe–N/C catalysts and insights into their role in oxygen reduction reaction. J. Electrochem. Sci. Technol. 2017, 8, 169–182. [Google Scholar] [CrossRef]
- Wu, G.; Santandreu, A.; Kellogg, W.; Gupta, S.; Ogoke, O.; Zhang, H.; Wang, H.L.; Dai, L. Carbon nanocomposite catalysts for oxygen reduction and evolution reactions: From nitrogen doping to transition-metal addition. Nano Energy 2016, 29, 83–110. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, Z.; Dai, L. Carbon-based electrocatalysts for advanced energy conversion and storage. Sci. Adv. 2015, 1, e1500564. [Google Scholar] [CrossRef]
- Jaouen, F.; Proietti, E.; Lefèvre, M.; Chenitz, R.; Dodelet, J.P.; Wu, G.; Chung, H.T.; Johnston, C.M.; Zelenay, P. Recent advances in non-precious metal catalysis for oxygen-reduction reaction in polymer electrolyte fuel cells. Energy Environ. Sci. 2011, 4, 114–130. [Google Scholar] [CrossRef]
- Martinez, U.; Babu, S.K.; Holby, E.F.; Zelenay, P. Durability challenges and perspective in the development of PGM-free electrocatalysts for the oxygen reduction reaction. Curr. Opin. Electrochem. 2018, 9, 224–232. [Google Scholar] [CrossRef]
- Banham, D.; Kishimoto, T.; Sato, T.; Kobayashi, Y.; Narizuka, K.; Ozaki, J.-i.; Zhou, J.Y.; Marquez, E.; Bai, K.; Ye, S. New insights into non-precious metal catalyst layer designs for proton exchange membrane fuel cells: Improving performance and stability. J. Power Sources 2017, 344, 39–45. [Google Scholar] [CrossRef]
- Banham, D.; Ye, S.; Pei, K.; Ozaki, J.I.; Kishimoto, T.; Imashiro, Y. A review of the stability and durability of non-precious metal catalysts for the oxygen reduction reaction in proton exchange membrane fuel cells. J. Power Sources 2015, 285, 334–348. [Google Scholar] [CrossRef]
- Zagal, J.H.; Recio, F.J.; Gutierrez, C.A.; Zuñiga, C.; Páez, M.A.; Caro, C.A. Towards a unified way of comparing the electrocatalytic activity MN4 macrocyclic metal catalysts for O2 reduction on the basis of the reversible potential of the reaction. Electrochem. Commun. 2014, 41, 24–26. [Google Scholar] [CrossRef]
- Chen, Z.; Higgins, D.; Yu, A.; Zhang, L.; Zhang, J. A review on non-precious metal electrocatalysts for PEM fuel cells. Energy Environ. Sci. 2011, 4, 3167–3192. [Google Scholar] [CrossRef]
- Lefèvre, M.; Proietti, E.; Jaouen, F.; Dodelet, J.P. Iron-Based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells. Science 2009, 324, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Piela, B.; Olson, T.S.; Atanassov, P.; Zelenay, P. Highly methanol-tolerant non-precious metal cathode catalysts for direct methanol fuel cell. Electrochim. Acta 2010, 55, 7615–7621. [Google Scholar] [CrossRef]
- Serov, A.; Shum, A.D.; Xiao, X.; De Andrade, V.; Artyushkova, K.; Zenyuk, I.V.; Atanassov, P. Nano-structured platinum group metal-free catalysts and their integration in fuel cell electrode architectures. Appl. Catal. B Environ. 2018, 237, 1139–1147. [Google Scholar] [CrossRef]
- Normile, S.J.; Sabarirajan, D.C.; Calzada, O.; De Andrade, V.; Xiao, X.; Mandal, P.; Parkinson, D.Y.; Serov, A.; Atanassov, P.; Zenyuk, I.V. Direct observations of liquid water formation at nano- and micro-scale in platinum group metal-free electrodes by operando X-ray computed tomography. Mater. Today Energy 2018, 9, 187–197. [Google Scholar] [CrossRef]
- Gómez, J.C.; Moliner, R.; Lázaro, M. Palladium-Based Catalysts as Electrodes for Direct Methanol Fuel Cells: A Last Ten Years Review. Catalysts 2016, 6, 130. [Google Scholar] [CrossRef]
- Shao, M. Palladium-based electrocatalysts for hydrogen oxidation and oxygen reduction reactions. J. Power Sources 2011, 196, 2433–2444. [Google Scholar] [CrossRef]
- Wen, C.; Wei, Y.; Tang, D.; Sa, B.; Zhang, T.; Chen, C. Improving the electrocatalytic properties of Pd-based catalyst for direct alcohol fuel cells: Effect of solid solution. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Zhang, L.; Chang, Q.; Chen, H.; Shao, M. Recent advances in palladium-based electrocatalysts for fuel cell reactions and hydrogen evolution reaction. Nano Energy 2016, 29, 198–219. [Google Scholar] [CrossRef]
- Baglio, V.; Aricò, A.S.; Stassi, A.; D’Urso, C.; Di Blasi, A.; Luna, A.M.C.; Antonucci, V. Investigation of Pt–Fe catalysts for oxygen reduction in low temperature direct methanol fuel cells. J. Power Sources 2006, 159, 900–904. [Google Scholar] [CrossRef]
- Salgado, J.R.C.; Antolini, E.; Gonzalez, E.R. Carbon supported Pt–Co alloys as methanol-resistant oxygen-reduction electrocatalysts for direct methanol fuel cells. Appl. Catal. B Environ. 2005, 57, 283–290. [Google Scholar] [CrossRef]
- Gavidia, L.M.R.; García, G.; Anaya, D.; Querejeta, A.; Alcaide, F.; Pastor, E. Carbon-supported Pt-free catalysts with high specificity and activity toward the oxygen reduction reaction in acidic medium. Appl. Catal. B Environ. 2016, 184, 12–19. [Google Scholar] [CrossRef]
- Gavidia, L.R.; Sebastián, D.; Pastor, E.; Aricò, A.; Baglio, V. Carbon-Supported Pd and PdFe Alloy Catalysts for Direct Methanol Fuel Cell Cathodes. Materials 2017, 10, 580. [Google Scholar] [CrossRef]
- Lo Vecchio, C.; Sebastián, D.; Alegre, C.; Aricò, A.S.; Baglio, V. Carbon-supported Pd and Pd-Co cathode catalysts for direct methanol fuel cells (DMFCs) operating with high methanol concentration. J. Electroanal. Chem. 2018, 808, 464–473. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, J.; Lv, Q.; Liu, C.; Li, Q.; Xing, W. Promotional effect of phosphorus doping on the activity of the Fe-N/C catalyst for the oxygen reduction reaction. Electrochim. Acta 2015, 155, 335–340. [Google Scholar] [CrossRef]
- Karim, N.A.; Kamarudin, S.K.; Loh, K.S. Performance of a novel non-platinum cathode catalyst for direct methanol fuel cells. Energy Convers. Manag. 2017, 145, 293–307. [Google Scholar] [CrossRef]
- Karim, N.A.; Kamarudin, S.K. Novel heat-treated cobalt phthalocyanine/carbon-tungsten oxide nanowires (CoPc/C-W18O49) cathode catalyst for direct methanol fuel cell. J. Electroanal. Chem. 2017, 803, 19–29. [Google Scholar] [CrossRef]
- Karim, N.A.; Kamarudin, S.K. An overview on non-platinum cathode catalysts for direct methanol fuel cell. Appl. Energy 2013, 103, 212–220. [Google Scholar] [CrossRef]
- Karim, N.A.; Kamarudin, S.K.; Shyuan, L.K.; Yaakob, Z.; Daud, W.R.W.; Khadum, A.A.H. Novel cathode catalyst for DMFC: Study of the density of states of oxygen adsorption using density functional theory. Int. J. Hydrogen Energy 2014, 39, 17295–17305. [Google Scholar] [CrossRef]
- Baranton, S.; Coutanceau, C.; Léger, J.M.; Roux, C.; Capron, P. Alternative cathodes based on iron phthalocyanine catalysts for mini- or micro-DMFC working at room temperature. Electrochim. Acta 2005, 51, 517–525. [Google Scholar] [CrossRef]
- Negro, E.; Videla, A.H.A.M.; Baglio, V.; Aricò, A.S.; Specchia, S.; Koper, G.J.M. Fe-N supported on graphitic carbon nano-networks grown from cobalt as oxygen reduction catalysts for low-temperature fuel cells. Appl. Catal. B Environ. 2015, 166–167, 75–83. [Google Scholar] [CrossRef]
- Li, Q.; Wang, T.; Havas, D.; Zhang, H.; Xu, P.; Han, J.; Cho, J.; Wu, G. High-Performance Direct Methanol Fuel Cells with Precious-Metal-Free Cathode. Adv. Sci. 2016, 3, 1600140. [Google Scholar] [CrossRef]
- Park, J.C.; Choi, C.H. Graphene-derived Fe/Co-N-C catalyst in direct methanol fuel cells: Effects of the methanol concentration and ionomer content on cell performance. J. Power Sources 2017, 358, 76–84. [Google Scholar] [CrossRef]
- Serov, A.; Artyushkova, K.; Niangar, E.; Wang, C.; Dale, N.; Jaouen, F.; Sougrati, M.T.; Jia, Q.; Mukerjee, S.; Atanassov, P. Nano-structured non-platinum catalysts for automotive fuel cell application. Nano Energy 2015, 16, 293–300. [Google Scholar] [CrossRef]
- Serov, A.; Artyushkova, K.; Atanassov, P. Fe-N-C Oxygen Reduction Fuel Cell Catalyst Derived from Carbendazim: Synthesis, Structure, and Reactivity. Adv. Energy Mater. 2014, 4, 1301735. [Google Scholar] [CrossRef]
- Videla, A.H.A.M.; Sebastián, D.; Vasile, N.S.; Osmieri, L.; Aricò, A.S.; Baglio, V.; Specchia, S. Performance analysis of Fe–N–C catalyst for DMFC cathodes: Effect of water saturation in the cathodic catalyst layer. Int. J. Hydrogen Energy 2016, 41, 22605–22618. [Google Scholar] [CrossRef]
- Videla, A.H.A.M.; Osmieri, L.; Armandi, M.; Specchia, S. Varying the morphology of Fe-N-C electrocatalysts by templating Iron Phthalocyanine precursor with different porous SiO2 to promote the Oxygen Reduction Reaction. Electrochim. Acta 2015, 177, 43–50. [Google Scholar] [CrossRef]
- Wang, Y.C.; Huang, L.; Zhang, P.; Qiu, Y.T.; Sheng, T.; Zhou, Z.Y.; Wang, G.; Liu, J.G.; Rauf, M.; Gu, Z.Q.; et al. Constructing a triple-phase interface in micropores to boost performance of Fe/N/C catalysts for direct methanol fuel cells. ACS Energy Lett. 2017, 2, 645–650. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, Z.Y.; Lai, Y.J.; You, Y.; Liu, J.G.; Wu, X.L.; Terefe, E.; Chen, C.; Song, L.; Rauf, M.; et al. Phenylenediamine-based FeNx/C catalyst with high activity for oxygen reduction in acid medium and its active-site probing. J. Am. Chem. Soc. 2014, 136, 10882–10885. [Google Scholar] [CrossRef]
- Osmieri, L.; Escudero-Cid, R.; Videla, A.H.A.M.; Ocón, P.; Specchia, S. Performance of a Fe-N-C catalyst for the oxygen reduction reaction in direct methanol fuel cell: Cathode formulation optimization and short-term durability. Appl. Catal. B Environ. 2017, 201, 253–265. [Google Scholar] [CrossRef]
- Osmieri, L.; Videla, A.H.A.M.; Armandi, M.; Specchia, S. Influence of different transition metals on the properties of Me–N–C (Me = Fe, Co, Cu, Zn) catalysts synthesized using SBA-15 as tubular nano-silica reactor for oxygen reduction reaction. Int. J. Hydrogen Energy 2016, 41, 22570–22588. [Google Scholar] [CrossRef]
- Sebastián, D.; Baglio, V.; Aricò, A.S.; Serov, A.; Atanassov, P. Performance analysis of a non-platinum group metal catalyst based on iron-aminoantipyrine for direct methanol fuel cells. Appl. Catal. B Environ. 2016, 182, 297–305. [Google Scholar] [CrossRef]
- Sebastián, D.; Serov, A.; Artyushkova, K.; Atanassov, P.; Aricò, A.S.; Baglio, V. Performance, methanol tolerance and stability of Fe-aminobenzimidazole derived catalyst for direct methanol fuel cells. J. Power Sources 2016, 319, 235–246. [Google Scholar] [CrossRef]
- Osmieri, L.; Escudero-Cid, R.; Armandi, M.; Videla, A.H.A.M.; Fierro, J.L.G.; Ocón, P.; Specchia, S. Fe-N/C catalysts for oxygen reduction reaction supported on different carbonaceous materials. Performance in acidic and alkaline direct alcohol fuel cells. Appl. Catal. B Environ. 2017, 205, 637–653. [Google Scholar] [CrossRef]
- Liu, J.; Li, E.; Ruan, M.; Song, P.; Xu, W. Recent progress on Fe/N/C electrocatalysts for the oxygen reduction reaction in fuel cells. Catalysts 2015, 5, 1167–1192. [Google Scholar] [CrossRef]
- Sebastián, D.; Serov, A.; Matanovic, I.; Artyushkova, K.; Atanassov, P.; Aricò, A.S.; Baglio, V. Insights on the extraordinary tolerance to alcohols of Fe-N-C cathode catalysts in highly performing direct alcohol fuel cells. Nano Energy 2017, 34, 195–204. [Google Scholar] [CrossRef]
- Lo Vecchio, C.; Aricò, A.S.; Baglio, V. Application of low-cost Me-N-C (Me = Fe or Co) electrocatalysts derived from edta in direct methanol fuel cells (DMFCs). Materials 2018, 11, 1193. [Google Scholar] [CrossRef]
- Lo Vecchio, C.; Serov, A.; Romero, H.; Lubers, A.; Zulevi, B.; Aricò, A.S.; Baglio, V. Commercial platinum group metal-free cathodic electrocatalysts for highly performed direct methanol fuel cell applications. J. Power Sources 2019, 437, 226948. [Google Scholar] [CrossRef]
- Xu, X.; Xia, Z.; Zhang, X.; Sun, R.; Sun, X.; Li, H.; Wu, C.; Wang, J.; Wang, S.; Sun, G. Atomically dispersed Fe-N-C derived from dual metal-organic frameworks as efficient oxygen reduction electrocatalysts in direct methanol fuel cells. Appl. Catal. B Environ. 2019, 259, 118042. [Google Scholar] [CrossRef]
- Pu, L.; Zhang, H.; Yuan, T.; Zou, Z.; Zou, L.; Li, X.M.; Yang, H. High performance platinum nanorod assemblies based double-layered cathode for passive direct methanol fuel cells. J. Power Sources 2015, 276, 95–101. [Google Scholar] [CrossRef]
- Wang, G.; Lei, L.; Jiang, J.; Zhou, Y.; Huang, Q.; Zou, Z.; Jiang, S.P.; Yang, H. An ordered structured cathode based on vertically aligned Pt nanotubes for ultra-low Pt loading passive direct methanol fuel cells. Electrochim. Acta 2017, 252, 541–548. [Google Scholar] [CrossRef]
- Viva, F.A.; Olah, G.A.; Prakash, G.K.S. Characterization of Pt supported on commercial fluorinated carbon as cathode catalysts for Polymer Electrolyte Membrane Fuel Cell. Int. J. Hydrogen Energy 2017, 42, 15054–15063. [Google Scholar] [CrossRef]
- Pu, L.; Zou, L.; Zhou, Y.; Zou, Z.; Yang, H. High performance MWCNT-Pt nanocomposite-based cathode for passive direct methanol fuel cells. RSC Adv. 2017, 7, 12329–12335. [Google Scholar] [CrossRef]
- Fan, C.; Wang, G.; Zou, L.; Fang, J.; Zou, Z.; Yang, H. Composition- and shape-controlled synthesis of the PtNi alloy nanotubes with enhanced activity and durability toward oxygen reduction reaction. J. Power Sources 2019, 429, 1–8. [Google Scholar] [CrossRef]
- Martinaiou, I.; Videla, A.H.A.M.; Weidler, N.; Kübler, M.; Wallace, W.D.Z.; Paul, S.; Wagner, S.; Shahraei, A.; Stark, R.W.; Specchia, S.; et al. Activity and degradation study of an Fe-N-C catalyst for ORR in Direct Methanol Fuel Cell (DMFC). Appl. Catal. B Environ. 2020, 262, 118217. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef]
- Ismail, M.S.; Ingham, D.B.; Ma, L.; Hughes, K.J.; Pourkashanian, M. Effects of catalyst agglomerate shape in polymer electrolyte fuel cells investigated by a multi-scale modelling framework. Energy 2017, 122, 420–430. [Google Scholar] [CrossRef]
- Zawodzinski, T.; Wieckowski, A.; Mukerjee, S.; Neurock, M. Integrated Theoretical and Experimental Studies of Fuel Cell Electrocatalysts. Electrochem. Soc. Interface 2007, 16, 37–41. [Google Scholar] [CrossRef]
- Karim, N.A.; Yahya, N.; Kejuruter, J. A Short Overview Current Research of Catalyst for Methanol Oxidation Reaction in Direct Methanol Fuel Cell (DMFC) from Experimental and Theoretical Aspect. J. Kejuruter. 2018, 1, 9–17. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Han, D.; Gwak, G.; Ju, H. Numerical modeling and simulations of active direct methanol fuel cell (DMFC) systems under various ambient temperatures and operating conditions. Int. J. Hydrogen Energy 2017, 42, 1736–1750. [Google Scholar] [CrossRef]
- Falcão, D.S.; Oliveira, V.B.; Rangel, C.M.; Pinto, A.M.F.R. Experimental and modeling studies of a micro direct methanol fuel cell. Renew. Energy 2015, 74, 464–470. [Google Scholar] [CrossRef]
- Oliveira, V.B.; Rangel, C.M.; Pinto, A.M.F.R. One-dimensional and non-isothermal model for a passive DMFC. J. Power Sources 2011, 196, 8973–8982. [Google Scholar] [CrossRef]
- Oliveira, V.B.; Falcão, D.S.; Rangel, C.M.; Pinto, A.M.F.R. Heat and mass transfer effects in a direct methanol fuel cell: A 1D model. Int. J. Hydrogen Energy 2008, 33, 3818–3828. [Google Scholar] [CrossRef]
- Liu, C.Y.; Chang, C.C.; Ho, J.J.; Li, E.Y. First-Principles Study on CO Removing Mechanism on Pt-Decorated Oxygen-Rich Anode Surfaces (Pt2/o-MO2(110), M = Ru and Ir) in DMFC. J. Phys. Chem. C 2017, 121, 9825–9832. [Google Scholar] [CrossRef]
- Park, S.; Shao, Y.; Liu, J.; Wang, Y. Oxygen electrocatalysts for water electrolyzers and reversible fuel cells: Status and perspective. Energy Environ. Sci. 2012, 5, 9331–9344. [Google Scholar] [CrossRef]
- Basri, S.; Kamarudin, S.K.; Daud, W.R.W.; Yaakob, Z.H.; Khadum, A.A. Study on kinetic energy of a novel metal composite for anode catalyst in direct methanol fuel cell. Int. J. Energy Res. 2015, 39, 181–190. [Google Scholar] [CrossRef]
- Ghosh, A.; Ramaprabhu, S. An efficient and durable novel catalyst support with superior electron-donating properties and fuel diffusivity for a direct methanol fuel cell. Catal. Sci. Technol. 2017, 7, 5079–5091. [Google Scholar] [CrossRef]
- Liu, L.; Zeng, G.; Chen, J.; Bi, L.; Dai, L.; Wen, Z. N-doped porous carbon nanosheets as pH-universal ORR electrocatalyst in various fuel cell devices. Nano Energy 2018, 49, 393–402. [Google Scholar] [CrossRef]

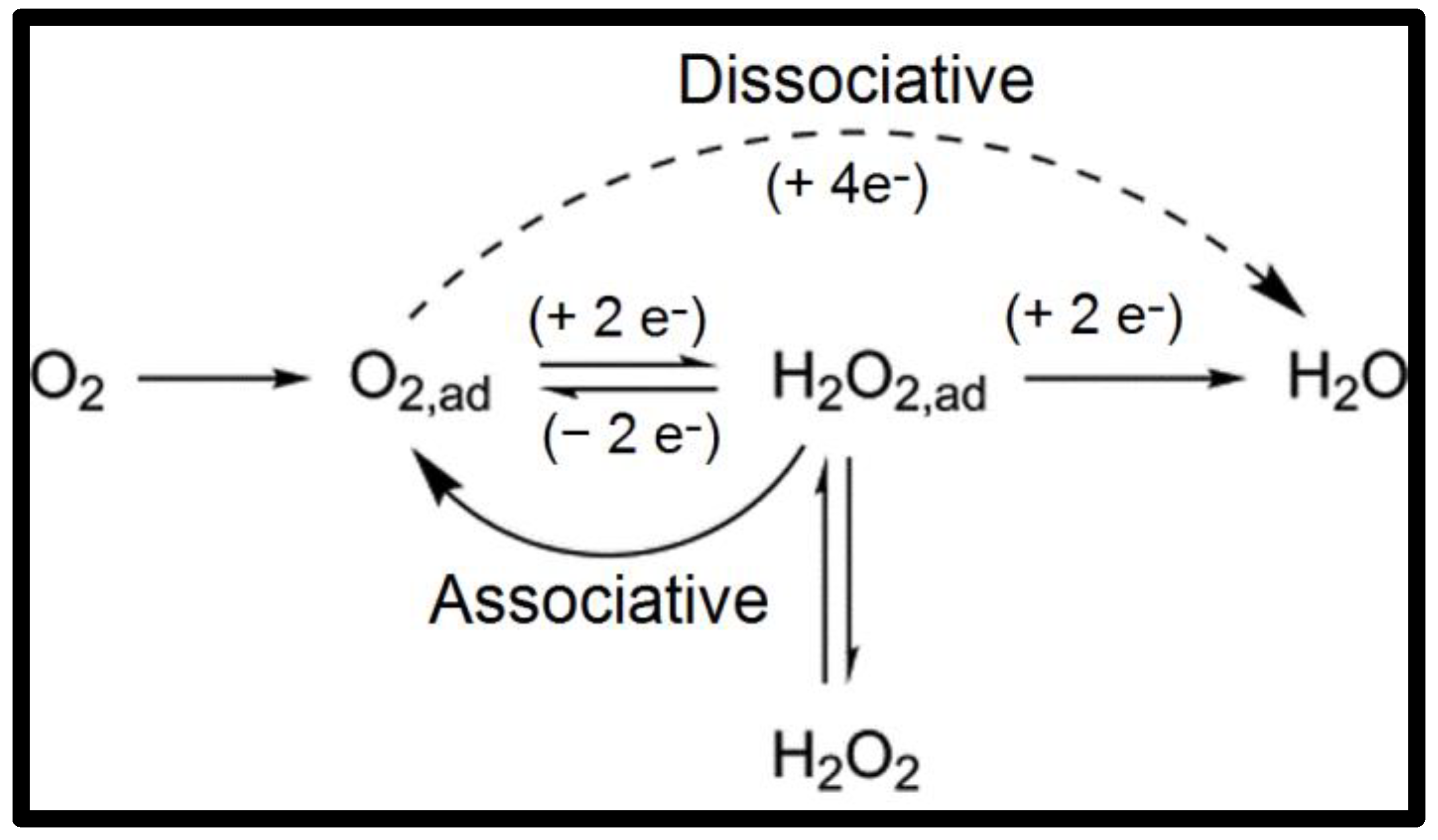
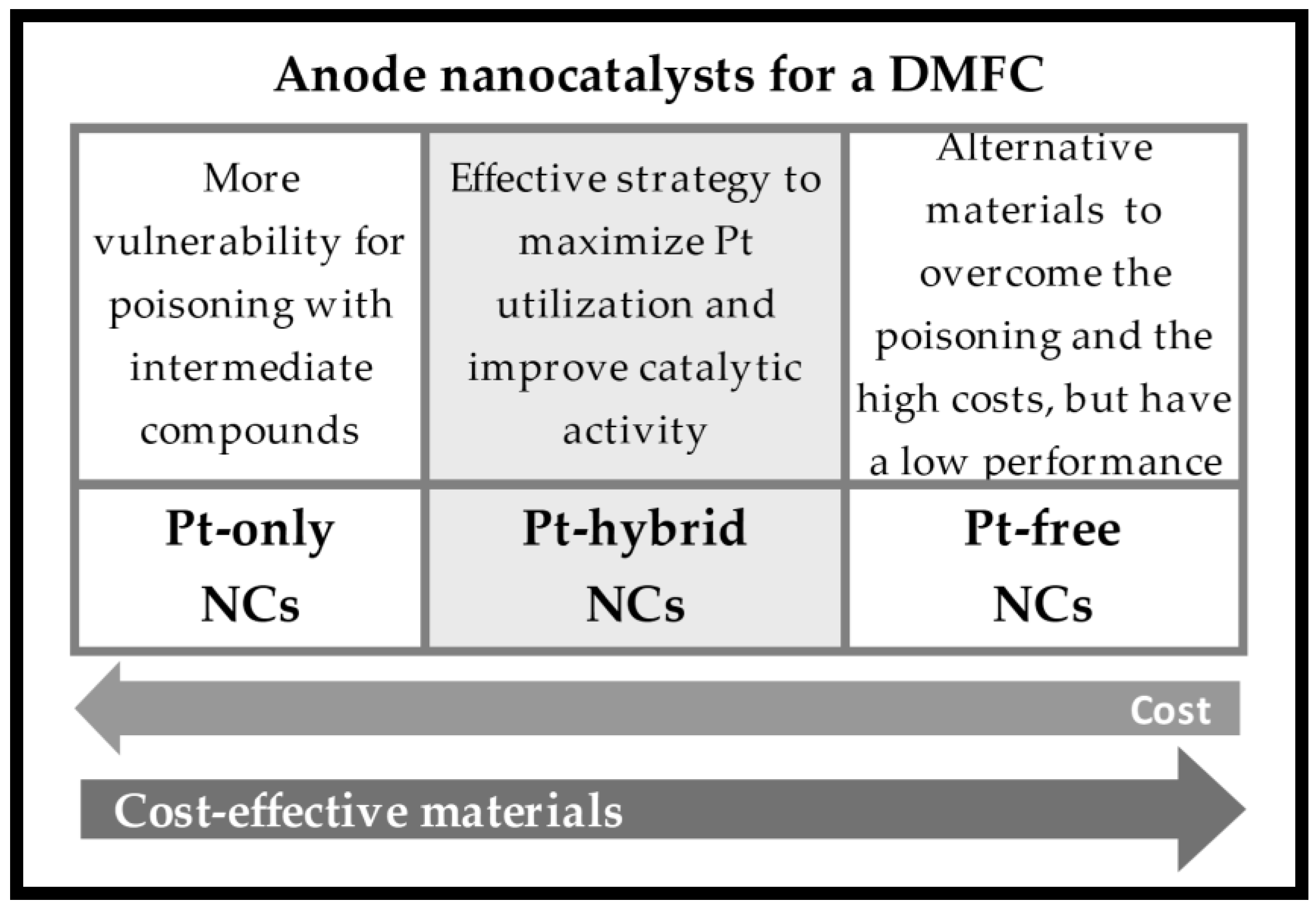
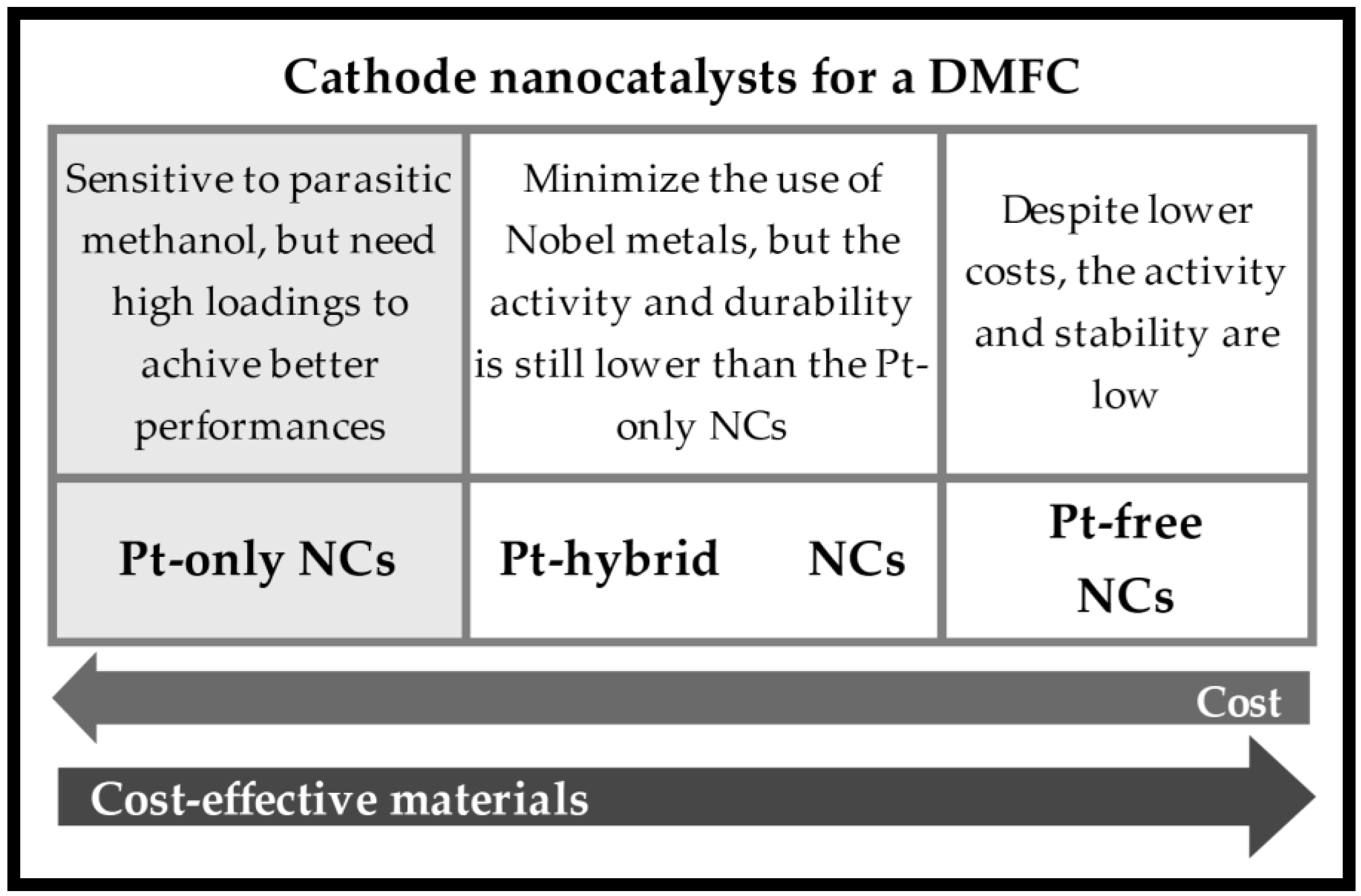
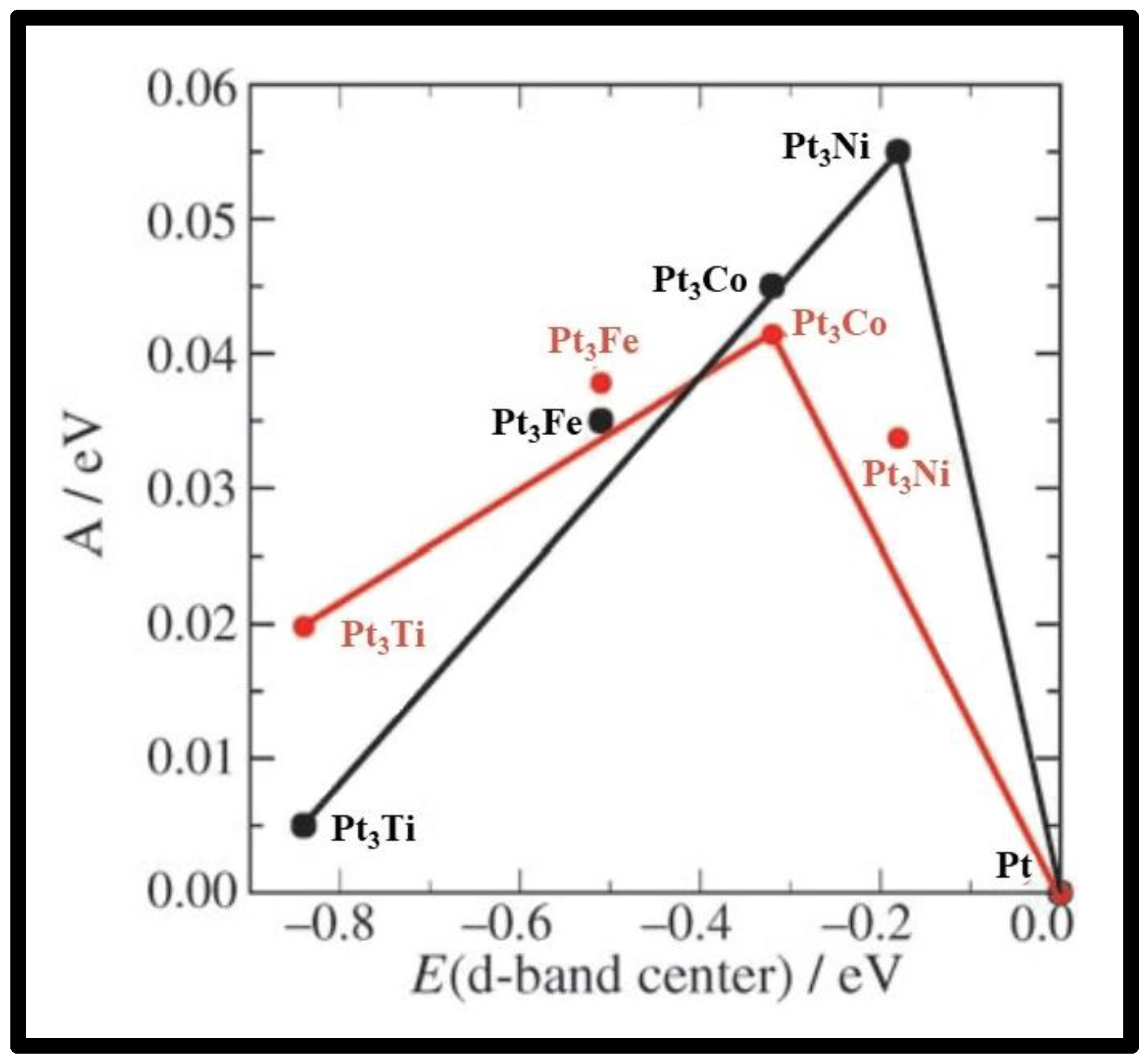
| NCs | Pt Load (mg cm−2) | [CH3OH] (M) | T (°C) | DMFC Mode | Max. Power Density (mW cm−2) | Ref. |
|---|---|---|---|---|---|---|
| Pt@RFC | 0.75 | 1 | 40 | Active | 33.8 | [193] |
| Pt–Ru/C-commercial | 2.0 | 27.7 | ||||
| Pt–Ru/CNF-BM | 2.0 | 2 | 40 | Active | (7.0) | [125] |
| Pt–Ru/CNF-SFM | (7.7) | |||||
| Pt–Ru/CNF-SFMTT | (7.2) i | |||||
| Pt–Ru/CNF-MeOH | (6.8) | |||||
| Pt–Ru/C-commercial | (5.4) | |||||
| Pt–Sn/C-PANI | 4 | 2 | (RT) | Passive | 4 | [151] |
| Pt–Ru/C-commercial | 3.5 i | |||||
| Pt–MnO2/rGO-L | 2 | 2 | 60 | Passive | 26.31 | [194] |
| Pt–MnO2/rGO | 21.11 | |||||
| Pt/rGO | 16.13 | |||||
| Pt–Ru-Fe/NG | 4 § | 2 | 40 | Active | 57 | [195] |
| Pt–Ru-Fe-Co/NG | 4 ¢ | 2 | 40 | Active | 68 | [196] |
| Pt–Ru/NGA | 2.5 ¥ | 2 | 30 | Active | 32 i | [197] |
| Pt–Ru/GA | 25 i | |||||
| Pt–Ru/C-commercial | 23 i | |||||
| Pt/C-commercial | 2.5 | 13 i | ||||
| Pt–NiTiO3/C | 0.5 | 0.5 | 45 | Active | 9.6 | [198] |
| Pt–Ru/N-CNT | 4 | 3 | (RT) | Passive | 26.10 | [199] |
| Pt–Ru/CNT | 22.12 | |||||
| Pt–Ru/C | 16.13 | |||||
| Pt–Ru/C-commercial | 2–17 |
| NCs | Pt Load (mg cm−2) | [CH3OH] (M) | T (°C) | DMFC Mode | Max. Power Density (mW cm−2) | Ref. |
|---|---|---|---|---|---|---|
| Pt/C-commercial | 2 | 2 | 25 | Passive | 30.2 | [293] |
| Pt/C (DLC1) | 1 | 29.1 | ||||
| Pt/C (DLC2) | 2 | 39.4 | ||||
| Pt/C-commercial | 0.83 | 2 | 25 | Passive | 15.5 | [294] |
| Pt/C-commercial | 1 | 17.4 | ||||
| Pt/C-commercial | 2 | 25.4 | ||||
| Pt/C (S-Pt) | 0.12 | 15.1 | ||||
| Pt/C (D-Pt) | 0.22 | 20.8 | ||||
| Pt/CFX (O2 LF) | 4 | 1 | 30 | Active | 23 | [295] |
| Pt/C (O2 LF) | 14 i | |||||
| Pt/C | 1 | 2 | 25 | Passive | 16.2 | [296] |
| Pt/MWNTs (3:1) | 14.7 | |||||
| Pt/MWNTs (1:1) | 18.1 | |||||
| Pt/MWNTs (1:2) | 28.5 | |||||
| Pt/MWNTs (1:3) | 24.2 | |||||
| Pt/MWNTs (1:2) | 0.5 | 19.2 | ||||
| Pd19Pt1/C | 2.8 * | 2 | 30 | Active | 45 i | [238] |
| Pd19Pt1/C | 4 | 40 i | ||||
| Pd19Pt1/C | 6 | 35 i | ||||
| Pt/C | 2 | 30 i | ||||
| Pt/C | 4 | 23 i | ||||
| Pt/C | 6 | 15 i | ||||
| Pt NSs/C | 1.0 | 2 | 25 | Passive | 30.6 | [237] |
| Pt–Ni NSs/C | 0.8 | 32.2 | ||||
| Pt/C | 1.0 | 17.4 | ||||
| Pt/C | 2.0 | 29.9 | ||||
| Pt–Ni NTs | 1.0 | 4 | RT | Passive | 25.1 | [297] |
| Pt/C | 18.6 | |||||
| FeAAPyr 7.4 | 7.4 *** | 10 | 30 | Active | (9) i | [285] |
| FeAAPyr 2.7 | 2.7 *** | (7) i | ||||
| Pt/C | 1 | (5) i | ||||
| FeABZIM | 3.0 *** | 10 | 30 | Active | 6.7 | [286] |
| Fe–N–C (S) | 2.97 | 1 | 25 | Active | 1.5 i | [298] |
| 1.74 | 2 i | |||||
| Fe–N–C (S) | 2.97 | 40 | 4 i | |||
| 1.74 | 4 i |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sá, M.H.; Moreira, C.S.; Pinto, A.M.F.R.; Oliveira, V.B. Recent Advances in the Development of Nanocatalysts for Direct Methanol Fuel Cells. Energies 2022, 15, 6335. https://doi.org/10.3390/en15176335
de Sá MH, Moreira CS, Pinto AMFR, Oliveira VB. Recent Advances in the Development of Nanocatalysts for Direct Methanol Fuel Cells. Energies. 2022; 15(17):6335. https://doi.org/10.3390/en15176335
Chicago/Turabian Stylede Sá, Maria H., Catarina S. Moreira, Alexandra M. F. R. Pinto, and Vânia B. Oliveira. 2022. "Recent Advances in the Development of Nanocatalysts for Direct Methanol Fuel Cells" Energies 15, no. 17: 6335. https://doi.org/10.3390/en15176335
APA Stylede Sá, M. H., Moreira, C. S., Pinto, A. M. F. R., & Oliveira, V. B. (2022). Recent Advances in the Development of Nanocatalysts for Direct Methanol Fuel Cells. Energies, 15(17), 6335. https://doi.org/10.3390/en15176335








