Experimental Study on the Catalyst-Coated Membrane of a Proton Exchange Membrane Electrolyzer
Abstract
1. Introduction
2. Experimental
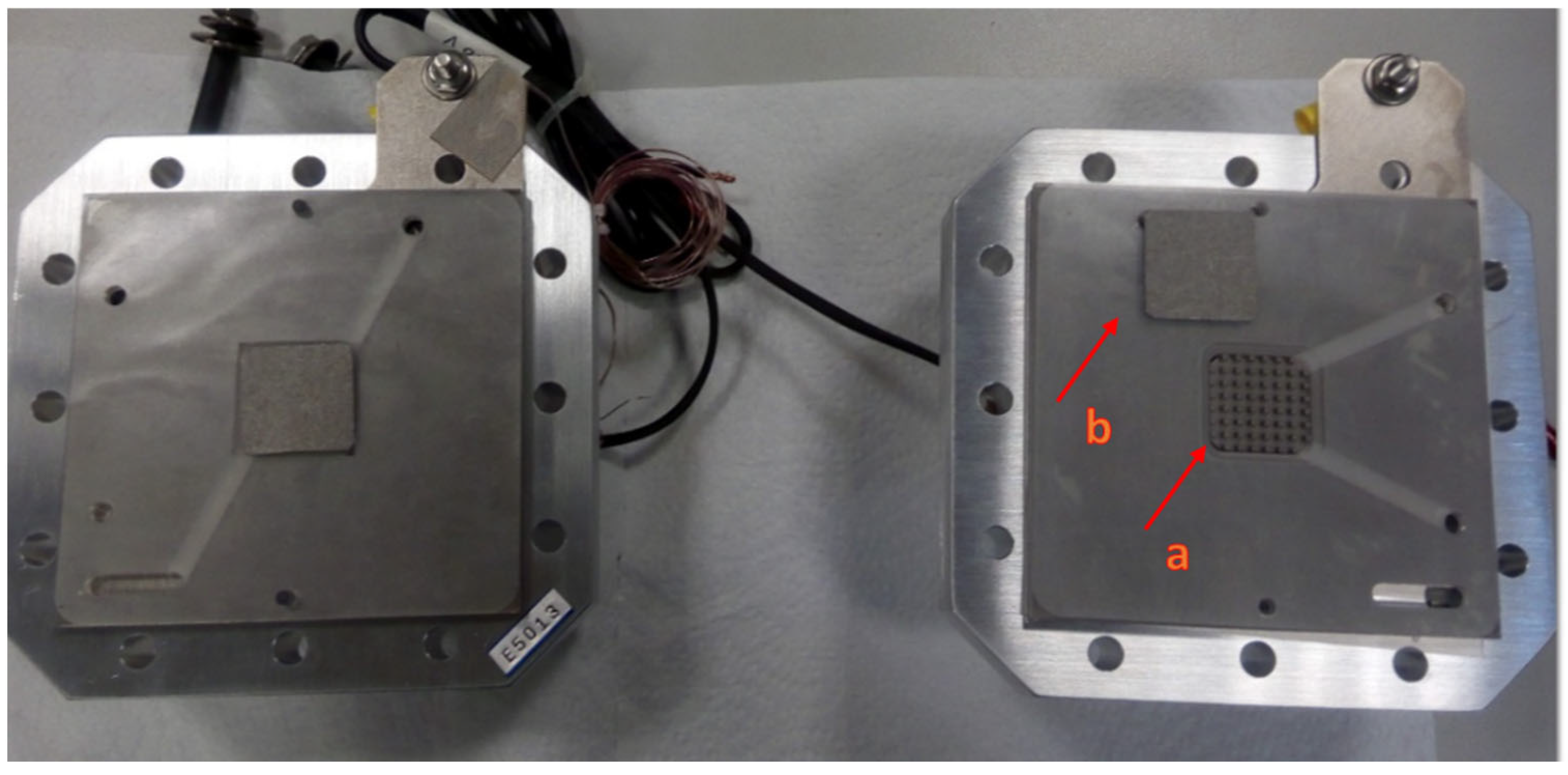
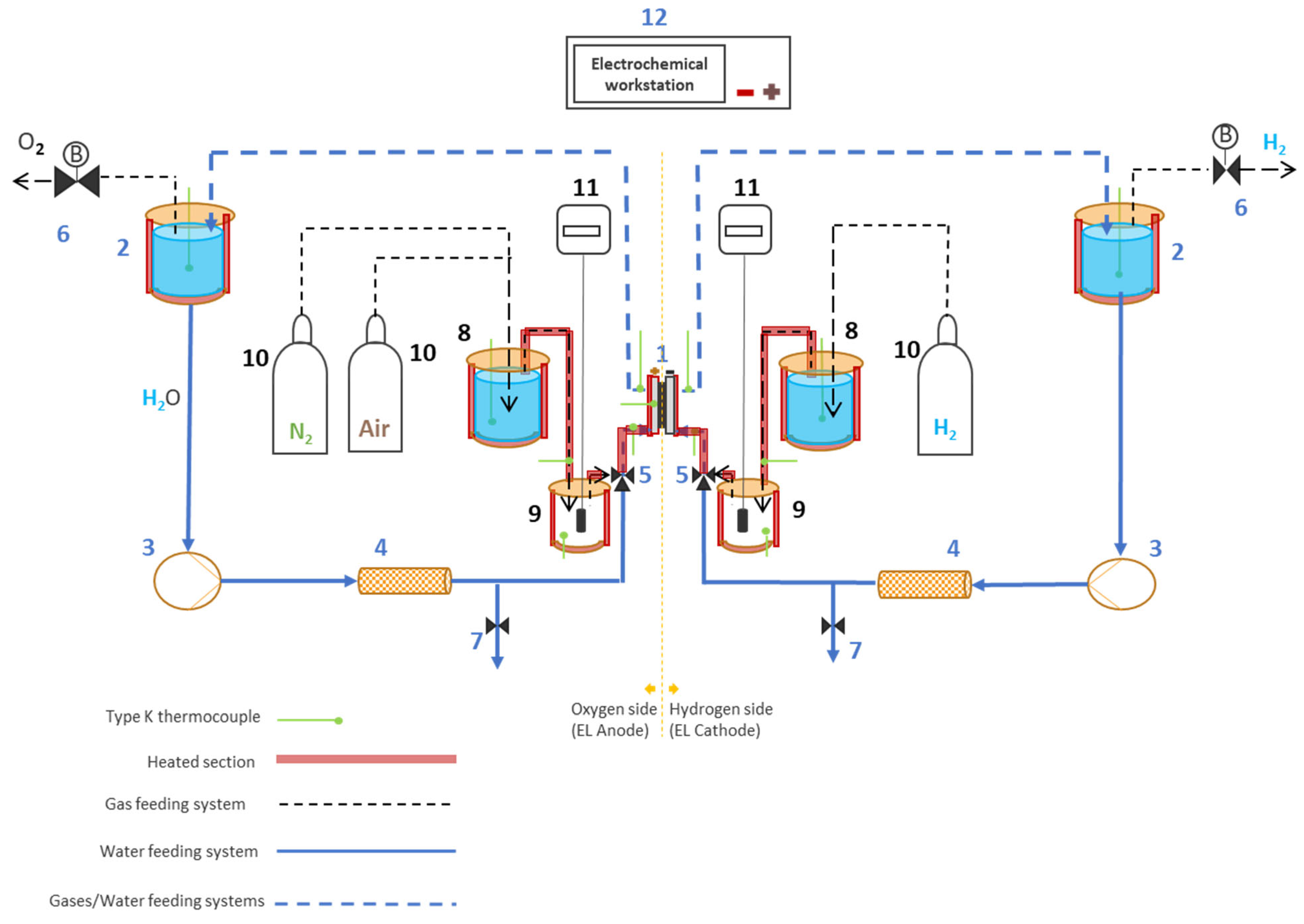
2.1. Electrolyzer
2.2. CCMs
2.3. CCM In-House (8) Preparation Procedure
2.4. Test Setup
2.5. Conditioning
2.6. Electrochemical Characterization
3. Discussion and Results
3.1. Suppliers Comparison
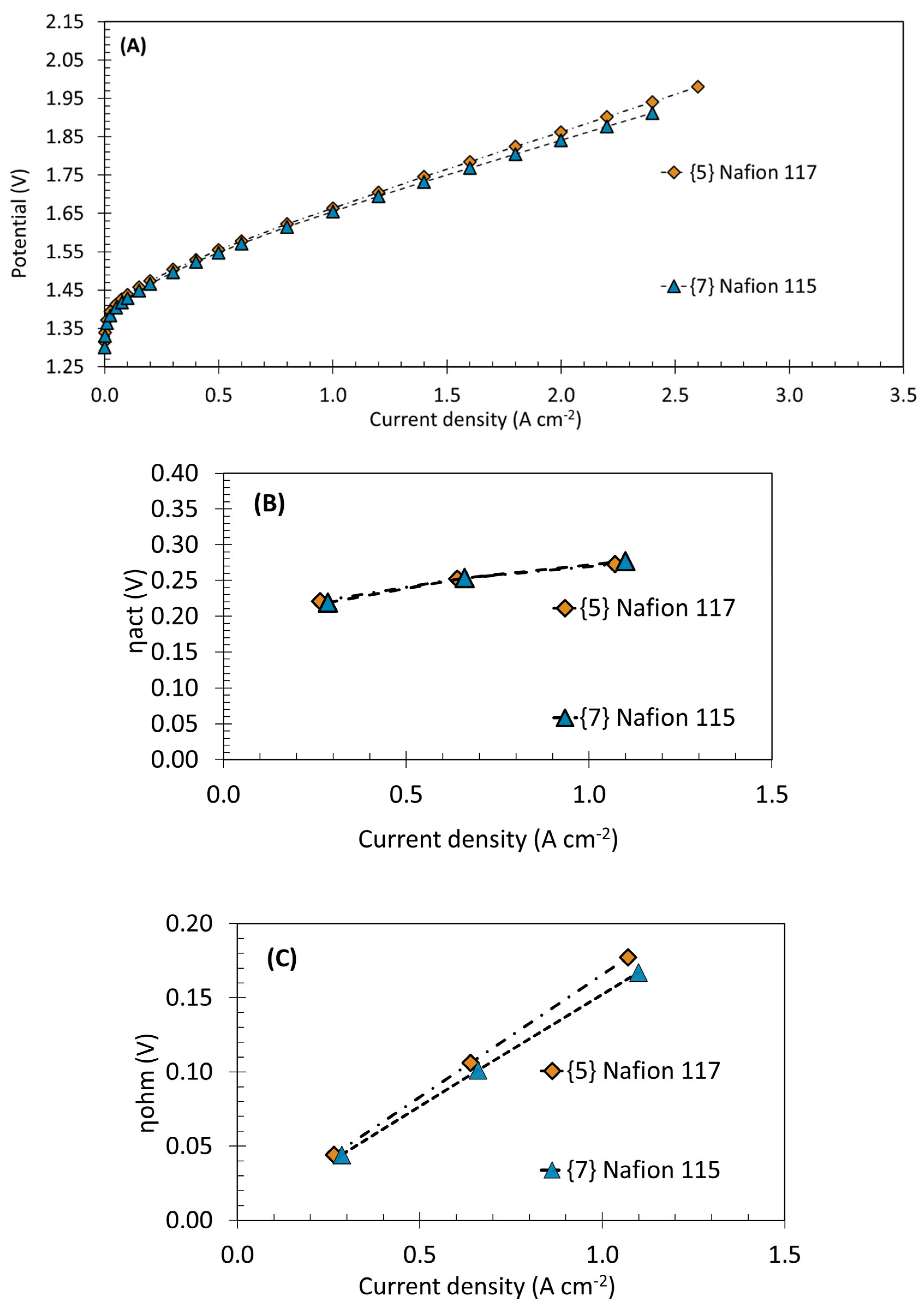
3.2. Membrane Thickness Effect
3.3. Catalyst Type Effect
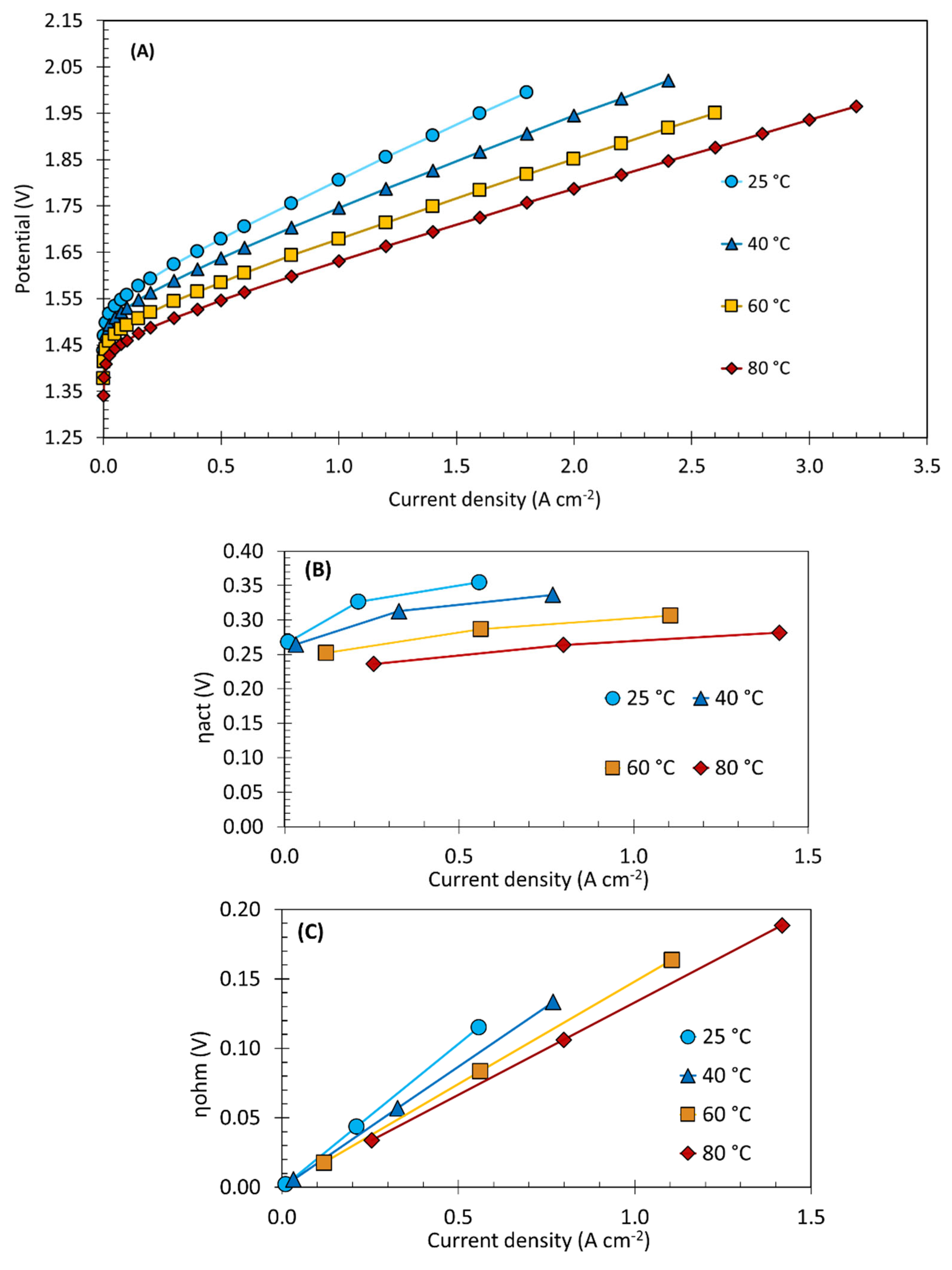
3.4. Temperature and Operating Voltage Analysis
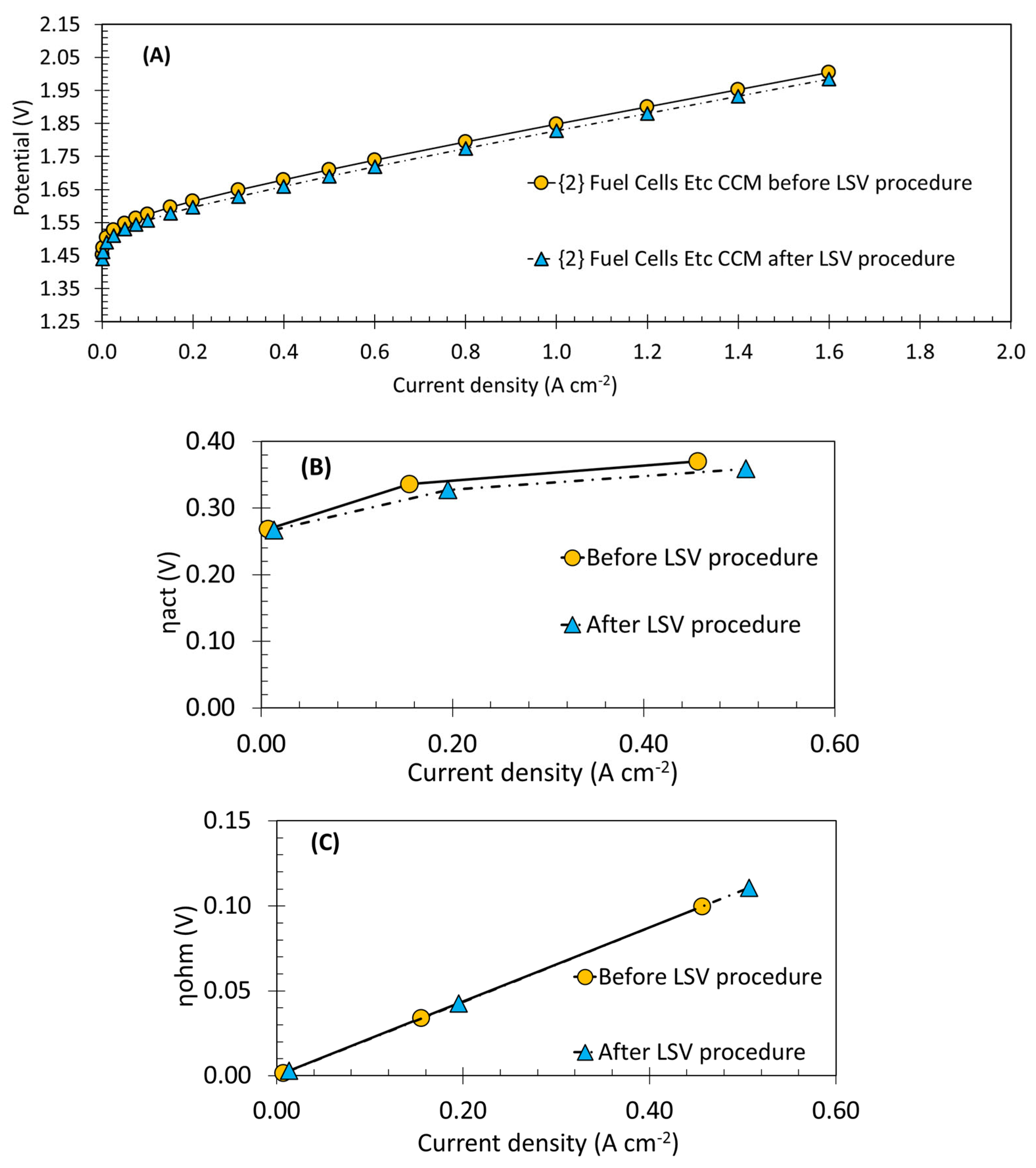
3.5. Hydrogen Purge
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- IEA. Net Renewable Capacity Additions by Technology, 2017–2023. Available online: https://www.iea.org/data-and-statistics/charts/net-renewable-capacity-additions-by-technology-2017-2023 (accessed on 20 September 2022).
- World Energy: Issues Monitor 2019. In World Energy: Issues Monitor; World Energy Council: London, UK, 2019.
- IRENA (Ed.) Hydrogen from Renewable Power: Technology Outlook for the Energy Transition; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2018. [Google Scholar]
- Akinyele, D.O.; Rayudu, R.K. Review of Energy Storage Technologies for Sustainable Power Networks. Sustain. Energy Technol. Assess. 2014, 8, 74–91. [Google Scholar] [CrossRef]
- Zohuri, B.; McDaniel, P. Chapter 8-Energy storage technologies and their role in Renewable Integration. In Introduction to Energy Essentials; Zohuri, B., McDaniel, P., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 277–320. [Google Scholar]
- Price, A. Chapter 1—The Exploitation of Renewable Sources of Energy for Power Generation. In Electrochemical Energy Storage for Renewable Sources and Grid Balancing; Garche, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–12. [Google Scholar]
- Buttler, A.; Spliethoff, H. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: A review. Renew. Sustain. Energy Rev. 2018, 82, 2440–2454. [Google Scholar] [CrossRef]
- LeRoy, R.L. Industrial water electrolysis: Present and future. Int. J. Hydrogen Energy 1983, 8, 401–417. [Google Scholar] [CrossRef]
- Briguglio, N.; Antonucci, V. Chapter 1—Overview of PEM Electrolysis for Hydrogen Production. In PEM Electrolysis for Hydrogen Production; CRC Press: Boca Raton, FL, USA, 2016; pp. 1–9. [Google Scholar]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Ayers, K.E.; Anderson, E.B.; Capuano, C.; Carter, B.; Dalton, L.; Hanlon, G.; Manco, J.; Niedzwiecki, M. Research Advances towards Low Cost, High Efficiency PEM Electrolysis. ECS Trans. 2010, 33, 3–15. [Google Scholar] [CrossRef]
- Lettenmeier, P.; Wang, R.; Abouatallah, R.; Saruhan, B.; Freitag, O.; Gazdzicki, P.; Morawietz, T.; Hiesgen, R.; Gago, A.S.; Friedrich, K.A. Low-Cost and Durable Bipolar Plates for Proton Exchange Membrane Electrolyzers. Sci. Rep. 2017, 7, 44035. [Google Scholar] [CrossRef]
- Russell, J.H.; Nuttall, L.J.; Fickett, A.P. Hydrogen Generation by Solid Polymer Electrolyte Water Electrolysis. Am. Chem. Soc. Div. Fuel Chem. Prepr. 1973, 18, 24–33. [Google Scholar]
- Barbir, F. Chapter 4—Main Cell Components, Materials Properties and Processes. In PEM Fuel Cells: Theory and Practice; Academic Press: Burlington, MA, USA, 2005; pp. 73–113. [Google Scholar]
- Ito, H. Chapter 6—Membranes. In PEM Electrolysis for Hydrogen Production; CRC Press: Boca Raton, FL, USA, 2016; pp. 119–134. [Google Scholar]
- Ito, H.; Maeda, T.; Nakano, A.; Takenaka, H. Properties of Nafion membranes under PEM water electrolysis conditions. Int. J. Hydrogen Energy 2011, 36, 10527–10540. [Google Scholar] [CrossRef]
- Smolinka, T.; Ojong, E.T.; Lickert, T. Chapter 2—Fundamentals of PEM Water Electrolysis. In PEM Electrolysis for Hydrogen Production; CRC Press: Boca Raton, FL, USA, 2016; pp. 11–33. [Google Scholar]
- Miles, M.H.; Thomason, M.A. Periodic Variations of Overvoltages for Water Electrolysis in Acid Solutions from Cyclic Voltammetric Studies. J. Electrochem. Soc. 1976, 123, 1459–1461. [Google Scholar] [CrossRef]
- Miles, M.H.; Klaus, E.A.; Gunn, B.P.; Locker, J.R.; Serafin, W.E.; Srinivasan, S. The oxygen evolution reaction on platinum, iridium, ruthenium and their alloys at 80 °C in acid solutions. Electrochim. Acta 1978, 23, 521–526. [Google Scholar] [CrossRef]
- Galizzioli, D.; Tantardini, F.; Trasatti, S. Ruthenium dioxide: A new electrode material. I. Behaviour in acid solutions of inert electrolytes. J. Appl. Electrochem. 1974, 4, 57–67. [Google Scholar] [CrossRef]
- Bender, G.; Carmo, M.; Smolinka, T.; Gago, A.; Danilovic, N.; Mueller, M.; Ganci, F.; Fallisch, A.; Lettenmeier, P.; Friedrich, K.; et al. Initial approaches in benchmarking and round robin testing for proton exchange membrane water electrolyzers. Int. J. Hydrogen Energy 2019, 44, 9174–9187. [Google Scholar] [CrossRef]
- Sunde, M.T.S. Chapter 3—Electrocatalysts for Oxygen Evolution Reaction (OER). In PEM Electrolysis for Hydrogen Production; CRC Press: Boca Raton, FL, USA, 2016; pp. 36–58. [Google Scholar]
- Carmo, M.; Lüke, W.; Stolten, D. Chapter 4—Electrocatalysts for the Hydrogen Evolution Reaction. In PEM Electrolysis for Hydrogen Production; CRC Press: Boca Raton, FL, USA, 2016; pp. 65–85. [Google Scholar]
- Immerz, C.; Paidar, M.; Papakonstantinou, G.; Bensmann, B.; Bystron, T.; Vidakovic-Koch, T.; Bouzek, K.; Sundmacher, K.; Hanke-Rauschenbach, R. Effect of the MEA design on the performance of PEMWE single cells with different sizes. J. Appl. Electrochem. 2018, 48, 701–711. [Google Scholar] [CrossRef]
- Ma, L.; Sui, S.; Zhai, Y. Investigations on high performance proton exchange membrane water electrolyzer. Int. J. Hydrogen Energy 2009, 34, 678–684. [Google Scholar] [CrossRef]
- Xu, W.; Scott, K. The effects of ionomer content on PEM water electrolyser membrane electrode assembly performance. Int. J. Hydrogen Energy 2010, 35, 12029–12037. [Google Scholar] [CrossRef]
- Rasten, E.; Hagen, G.; Tunold, R. Electrocatalysis in water electrolysis with solid polymer electrolyte. Electrochim. Acta 2003, 48, 3945–3952. [Google Scholar] [CrossRef]
- Slavcheva, E.; Radev, I.; Bliznakov, S.; Topalov, G.; Andreev, P.; Budevski, E. Sputtered iridium oxide films as electrocatalysts for water splitting via PEM electrolysis. Electrochim. Acta 2007, 52, 3889–3894. [Google Scholar] [CrossRef]
- Tsotidris, G.; Pilenga, A. EU Harmonized Protocols for Testing of Low Temperature Water Electrolysis; Publications Office of the European Union: Luxembourg, 2021. [Google Scholar]
- Rheinländer, P.J.; Durst, J. Transformation of the OER-Active IrOx Species under Transient Operation Conditions in PEM Water Electrolysis. J. Electrochem. Soc. 2021, 168, 024511. [Google Scholar] [CrossRef]
- Siracusano, S.; Baglio, V.; Grigoriev, S.; Merlo, L.; Fateev, V.; Aricò, A.S. The influence of iridium chemical oxidation state on the performance and durability of oxygen evolution catalysts in PEM electrolysis. J. Power Sources 2017, 366, 105–114. [Google Scholar] [CrossRef]
- Millet, P. Chapter 10—Characterization tools for Polymer Electrolyte Membrane (PEM) Water electrolyzers. In PEM Electrolysis for Hydrogen Production: Principles and Applications; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Fouda-Onana, F.; Chandesris, M.; Médeau, V.; Chelghoum, S.; Thoby, D.; Guillet, N. Investigation on the degradation of MEAs for PEM water electrolysers part I: Effects of testing conditions on MEA performances and membrane properties. Int. J. Hydrogen Energy 2016, 41, 16627–16636. [Google Scholar] [CrossRef]
- Lettenmeier, P.; Wang, R.; Abouatallah, R.; Helmly, S.; Morawietz, T.; Hiesgen, R.; Kolb, S.; Burggraf, F.; Kallo, J.; Gago, A.; et al. Durable Membrane Electrode Assemblies for Proton Exchange Membrane Electrolyzer Systems Operating at High Current Densities. Electrochim. Acta 2016, 210, 502–511. [Google Scholar] [CrossRef]
- Gago, A.; Ansar, S.; Saruhan, B.; Schulz, U.; Lettenmeier, P.; Cañas, N.; Gazdzicki, P.; Morawietz, T.; Hiesgen, R.; Arnold, J.; et al. Protective coatings on stainless steel bipolar plates for proton exchange membrane (PEM) electrolysers. J. Power Sources 2016, 307, 815–825. [Google Scholar] [CrossRef]
- Ferreira, R.; Falcão, D.; Oliveira, V.; Pinto, A. Experimental study on the membrane electrode assembly of a proton exchange membrane fuel cell: Effects of microporous layer, membrane thickness and gas diffusion layer hydrophobic treatment. Electrochim. Acta 2017, 224, 337–345. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Zhang, S.; Wang, H.; Wu, J.; Sun, J.C.; Hiesgen, R.; Friedrich, K.A.; Schulze, M.; Haug, A. Degradation of a polymer exchange membrane fuel cell stack with Nafion® membranes of different thicknesses: Part I. In situ diagnosis. J. Power Sources 2010, 195, 7594–7599. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.; Leung, D.Y. Energy and exergy analysis of hydrogen production by a proton exchange membrane (PEM) electrolyzer plant. Energy Convers. Manag. 2008, 49, 2748–2756. [Google Scholar] [CrossRef]
- Saeba, D.; Patcharavorachot, Y.; Hacker, V.; Assabumrungrat, S.; Arpornwichanop, A.; Authayanun, S. Analysis of Unbalanced Pressure PEM Electrolyzer for High Pressure Hydrogen Production. Chem. Eng. Trans. 2017, 57, 1615–1620. [Google Scholar]
- Sapountzi, F.M.; Gracia, J.M.; Weststrate, C.-J.; Fredriksson, H.O.; Niemantsverdriet, J.W. Electrocatalysts for the generation of hydrogen, oxygen and synthesis gas. Prog. Energy Combust. Sci. 2017, 58, 1–35. [Google Scholar] [CrossRef]
- Siracusano, S.; Van Dijk, N.; Payne-Johnson, E.; Baglio, V.; Aricò, A. Nanosized IrOx and IrRuOx electrocatalysts for the O2 evolution reaction in PEM water electrolysers. Appl. Catal. B Environ. 2015, 164, 488–495. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, H.; Chen, G.; Zhang, Y. Study of IrxRu1−xO2 oxides as anodic electrocatalysts for solid polymer electrolyte water electrolysis. Electrochimica Acta 2009, 54, 6250–6256. [Google Scholar] [CrossRef]
- Hegge, F.; Lombeck, F.; Ortiz, E.C.; Bohn, L.; Von Holst, M.; Kroschel, M.; Hübner, J.; Breitwieser, M.; Strasser, P.; Vierrath, S. Efficient and Stable Low Iridium Loaded Anodes for PEM Water Electrolysis Made Possible by Nanofiber Interlayers. ACS Appl. Energy Mater. 2020, 3, 8276–8284. [Google Scholar] [CrossRef]
- Trasatti, S. Electrocatalysis in the anodic evolution of oxygen and chlorine. Electrochim. Acta 1984, 29, 1503–1512. [Google Scholar] [CrossRef]
- Frensch, S.H.; Fouda-Onana, F.; Serre, G.; Thoby, D.; Araya, S.S.; Kær, S.K. Influence of the operation mode on PEM water electrolysis degradation. Int. J. Hydrogen Energy 2019, 44, 29889–29898. [Google Scholar] [CrossRef]
- Rakousky, C.; Reimer, U.; Wippermann, K.; Kuhri, S.; Carmo, M.; Lueke, W.; Stolten, D. Polymer electrolyte membrane water electrolysis: Restraining degradation in the presence of fluctuating power. J. Power Sources 2017, 342, 38–47. [Google Scholar] [CrossRef]


| Number | CCM | Supplier | ||
|---|---|---|---|---|
| Nafion Membrane | Electrocatalysts | |||
| Anode Loading (mg cm−2) | Cathode Loading (mg cm−2) | |||
| 1 | 115 | Ir black (2.0) | Pt on advanced carbon (1.0) | QuinTech |
| 2 | 115 | Ir black (2.0) | Pt/C 60% (1.0) | Fuel Cells Etc |
| 3 | 115 | Ir black (3.0) | Pt black (3.0) | Fuel Cells Etc |
| 4 | 115 | IrOx (3.0) | Pt black (3.0) | Fuel Cells Etc |
| 5 | 117 | IrRuOx (3.0) | Pt black (3.0) | Fuel Cells Etc |
| 6 | 115 | IrRuOx (1.5)Pt black (1.5) | Pt black (3.0) | Fuel Cells Etc |
| 7 | 115 | IrRuOx (3.0) | Pt black (3.0) | Fuel Cells Etc |
| 8 | 115 | Ir black (2.0) | Pt/C 20% (1.0) | In-house |
| (1) QuinTech Ir Black (2.0 mg cm−2) | (2) Fuel Cells Etc Ir Black (2.0 mg cm−2) | (3) Ir Black | (4) IrOx | (5) IrRuOx Nafion 117 | (6) IrRuOx/Pt Black | (7) IrRuOx Nafion 115 | |
|---|---|---|---|---|---|---|---|
| Average voltage improvement (%) | 0.7 | 1.1 | 1.0 | 2.0 | 0.6 | 0.7 | 0.5 |
| Charge density increase (%) (from CV scans at 20 mV s−1) | 25 | 30 | 24 | 53 | 6 | 28 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, A.G.; Ferreira, R.; Falcão, D.; Pinto, A.M.F.R. Experimental Study on the Catalyst-Coated Membrane of a Proton Exchange Membrane Electrolyzer. Energies 2022, 15, 7937. https://doi.org/10.3390/en15217937
Rocha AG, Ferreira R, Falcão D, Pinto AMFR. Experimental Study on the Catalyst-Coated Membrane of a Proton Exchange Membrane Electrolyzer. Energies. 2022; 15(21):7937. https://doi.org/10.3390/en15217937
Chicago/Turabian StyleRocha, Amadeu Gomes, Rui Ferreira, Daniela Falcão, and Alexandra M. F. R. Pinto. 2022. "Experimental Study on the Catalyst-Coated Membrane of a Proton Exchange Membrane Electrolyzer" Energies 15, no. 21: 7937. https://doi.org/10.3390/en15217937
APA StyleRocha, A. G., Ferreira, R., Falcão, D., & Pinto, A. M. F. R. (2022). Experimental Study on the Catalyst-Coated Membrane of a Proton Exchange Membrane Electrolyzer. Energies, 15(21), 7937. https://doi.org/10.3390/en15217937









