Abstract
Beside steam reforming, methane pyrolysis is an alternative method for hydrogen production. ‘Turquoise’ hydrogen with solid carbon is formed in the pyrolysis process, contrary to ‘grey’ or ‘blue’ hydrogen via steam methane reforming, where waste carbon dioxide is produced. Thermal pyrolysis is conducted at higher temperatures, but catalytic decomposition of methane (CDM) is a promising route for sustainable hydrogen production. CDM is generally carried out over four types of catalyst: nickel, carbon, noble metal and iron. The applied reactors can be fixed bed, fluidized bed, plasma bed or molten-metal reactors. Two main advantages of CDM are that (i) carbon-oxide free hydrogen, ideal for fuel cell applications, is formed and (ii) the by-product can be tailored into carbon with advanced morphology (e.g., nanofibers, nanotubes). The aim of this review is to reveal the very recent research advances of the last two years achieved in the field of this promising prospective technology.
1. Introduction
One of the long-term effects of the chemical industry on the environment is the accumulation of greenhouse gases, leading to climate change. Reducing carbon dioxide (CO2) emissions by prioritizing the diversification of energy resources is a great challenge for the world economy. Production of hydrogen from natural gas by a smaller environmental impact method and its application as a clean source of energy is a possible solution for these problems [1].
Recently, steam methane (CH4) reforming (SMR: CH4 + H2O ↔ CO + 3H2) followed by the water–gas shift reaction (WGS: CO + H2O ↔ CO2 + H2) is the most common, dominant process for large-scale hydrogen production, with a simultaneous long life of the used catalysts. Despite its long-time commercial optimization, the endothermic SMR process is expensive due to its high capital costs and energy consumption, and it produces CO2 in a significant amount. In addition, the burning of methane that ensures the heat necessary for the process produces even more carbon dioxide [2]. The production of every cubic meter of hydrogen is accompanied by the emission of a half m3 of carbon dioxide. Pure H2 must be separated from CO2, CH4 and other impurities in effluent gas streams using energy-intensive (adsorption-, cryogenic-, or membrane-) separation processes. The development of new processes to produce hydrogen without concomitant formation of CO2 is necessary to overcome the drawbacks of steam reforming [3]. The term ‘grey hydrogen’ refers to carbon dioxide emissions being released to the atmosphere, and ‘blue hydrogen’ is used when emissions are captured through carbon capture and storage (CCS). Hydrogen produced from renewable energy sources is often referred to as ‘green hydrogen’.
Thermal dissociation or pyrolysis of methane is a technologically simpler, one-step process (CH4 → C + 2H2) compared with steam reforming, and it does not produce carbon oxides. Per mole of methane, only half as much hydrogen is produced compared to SMR+WGS, but less energy input is required (74.5 kJ for methane pyrolysis, 164.6 kJ for SMR+WGS), and solid carbon is coproduced (rather than CO2). Carbon oxides produced during the SMR process would poison the catalysts for ammonia synthesis and must be completely removed [2]. Following pyrolysis, carbon oxides are not present as pollutants, and small amounts of unconverted CH4 can be tolerated in the hydrogen used, e.g., in a fuel cell or in ammonia production. In addition, pure carbon is a valuable by-product; it can be used as electrodes or additives (in, e.g., concrete, asphalt, tires) or in microelectronics (carbon nanotubes or graphitic nano-fibres) [3]. Hydrogen produced using methane pyrolysis is often referred to as ‘turquoise hydrogen’.
Methane is one of the most stable organic molecules; therefore, its thermal dissociation occurs at very high, 1000–1100 °C, temperatures, which can be decreased significantly (500–1000 °C) using catalytic pyrolysis or in other words catalytic decomposition of methane (CDM). The order of methane decomposition activity by transition metals is as follows: Co, Ru, Ni, Rh > Pt, Re, Ir > Pd, Cu, W, Fe, Mo [4]. Reaction equilibrium favours high temperature and low pressure to achieve high CH4 conversion, but catalysts can be sintered and therefore deactivated at high temperatures. However, the main challenge for the practical application of these catalysts is their rapid deactivation due to carbon accumulation on their surfaces. Cyclic regeneration of the catalyst by gasification or combustion in a fluidized bed reactor can extend its lifetime of operation [4]. If it occurs with steam or oxygen, it results in significant CO2 emissions, which contradicts the idea of producing turquoise hydrogen. Therefore, instead of burning the deposited carbon by-product, it must be removed and valourized [5].
The aim of this review is to summarize the progress in catalyst development and reactor types in methane pyrolysis and to reveal the recent advances of this promising prospective technology. Many reviews [3,4,5,6,7,8,9,10,11] have examined the CDM literature, and here we try to refresh them with the latest (2021–2022) results.
The survey of the last two year’s publications in the field of methane pyrolysis research led us to note a frequent problem concerning the evaluating points of catalytic performance. CH4 conversion, H2 yield/selectivity and carbon productivity (gcarbon/gcatalyst, called frequently as carbon yield) are used generally to describe CDM performance. Indeed, CH4 conversion and H2 yield can be followed during the reaction via analysing the gas phase products, while the carbon yield can be directly determined only after the reaction by measuring all carbon accumulated during the time-on-stream. Space-time yield (STY) of hydrogen is a good measure of the reaction. In most cases, all of these measurable characteristics are not given, only some of them. Moreover, the way of their determination/calculation is sometimes not clearly described. If significantly lower H2 yield is listed along with a relatively high CH4 conversion, it indicates that H2 selectivity is much lower than 1. In this case, the appearance of a product other than hydrogen and carbon should be mentioned. Sometimes, H2 selectivity equal to 1 is supposed for calculations. Occasionally, only the H2 vol% is given instead of the H2 yield. In general, the examination of hydrogen and also carbon balance is recommended, and the missing part (if it is significant, such as H2 selectivity ~0.5) should be traced in both the gas phase and in solid products to gain reliable catalytic results and to better understand the process.
2. Conventional Catalyst Development
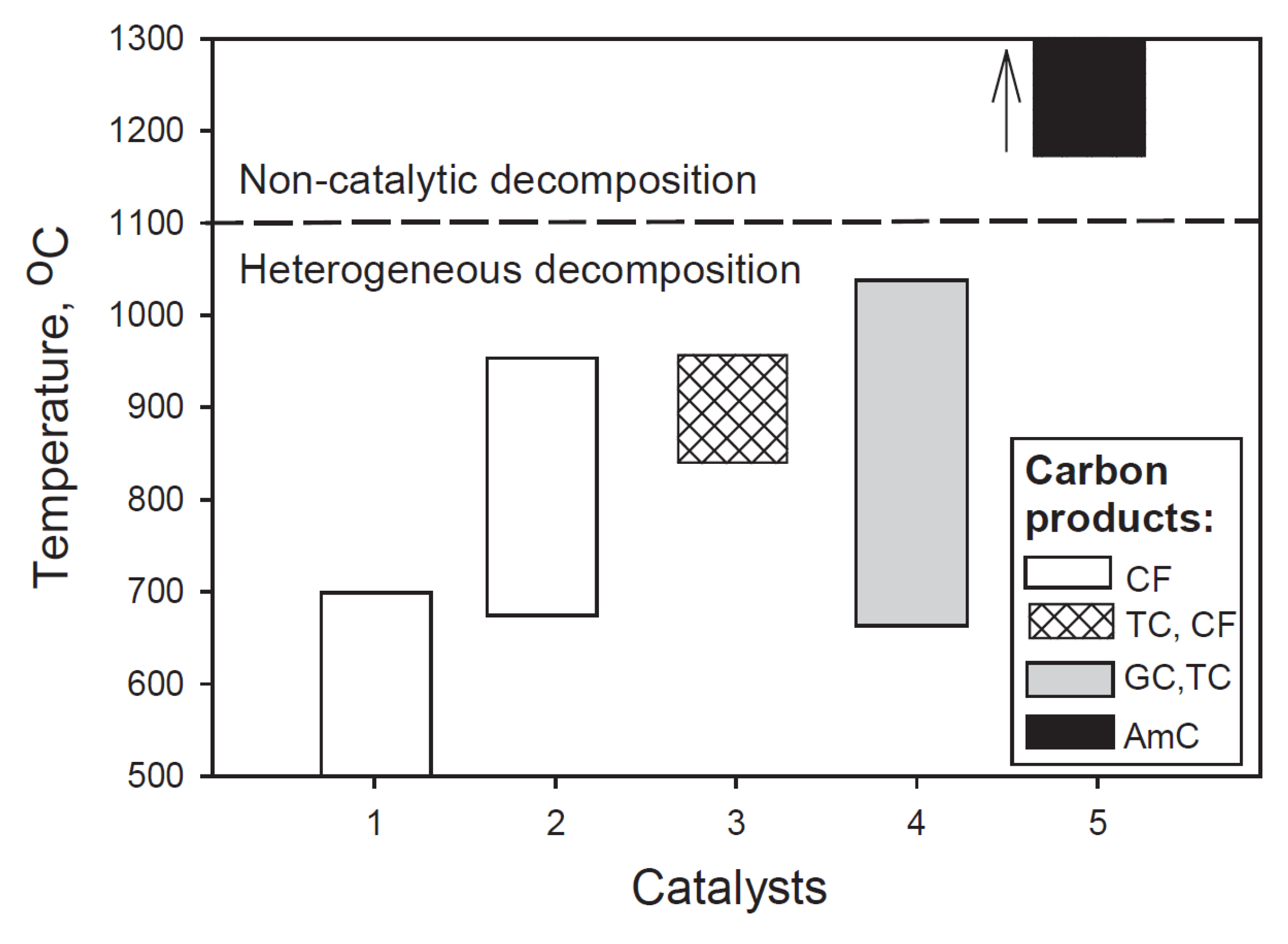
Generally, four types of material have been applied as conventional catalysts in the CDM process, related to their increasing preferred temperature ranges: nickel-, iron-, doped noble metal- and carbon-based catalysts [9]. Mostly, carbon filaments are formed on nickel and iron catalysts, graphitic- and turbostratic carbon on noble metals and turbostratic carbon and carbon filaments on carbon-based catalysts (Figure 1) [12].
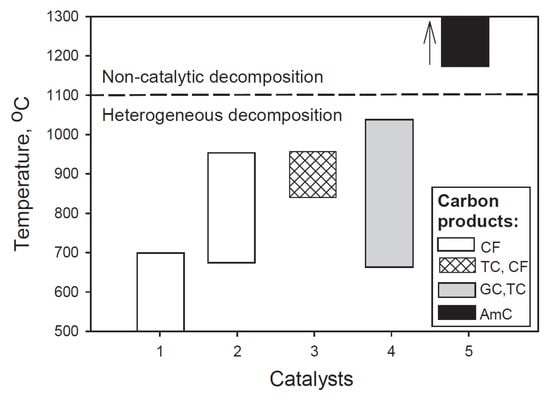
Figure 1.
Graphical representation of the bulk of literature data on catalysts, preferred temperature range and carbon products related to catalytic methane decomposition reaction. Catalysts: 1-Ni-based, 2-Fe-based, 3-carbon-based, 4-summary of data related to Co, Ni, Fe, Pd, Pt, Cr, Ru, Mo, W catalysts, 5-non-catalytic decomposition. Carbon products: CF-carbon filaments, TC-turbostratic carbon, GC-graphitic carbon, AmC-amorphous carbon. (Copied from Ref. [12] with Elsevier permission).
2.1. Nickel-Containing Catalysts
Nickel-containing catalysts can be classified as oxide (Al2O3, SiO2, MgO and their mixtures) and carbon supported, one- and two-metallic (Ni, NiFe, NiCo, NiCu, etc.) catalysts. The main advantage of these catalysts is that they are working at the lowest (500–750 °C) reaction temperatures, with 80–85% methane conversion, but they become deactivated fast at higher temperatures [9]. Dipu [4] overviewed the advancements (from 2015 to 2020) in CDM for Ni-based catalysts. The influence of promoter, metal composition, support, admixture, synthesis method and operating parameters were discussed.
Undoped oxide-supported Ni catalysts were efficient in the 500–750 °C temperature range with high (40–90 wt%) metal loading over SiO2, Al2O3, MgO, MCM-22 and CeO2 supports [9,10]. Ni/MgO catalysts with mesoporous structures synthesized by hydrothermal method presented increasing methane conversion (28 → 49%) and hydrogen yield (33 → 53%) with increasing nickel content (10 → 40%) at 600 °C reaction temperature (Table 1). Only the NiO-MgO phase as a solid solution was formed in the calcined catalysts [13]. Xu et al. [14] prepared a series of 10 wt% nickel-containing Ni/Al2O3 and Ni/MgAl2O4 catalysts with varying synthesis and pre-treatment methods and examined their initial methane turnover and carbon co-product selectivity in the CDM reaction at 650 °C (Table 1). They found that methane turnover increases with Ni particle size and small (<10 nm) Ni particles are selective toward the formation of graphitic carbon layers, while large (>20 nm) Ni particles are selective toward the formation of carbon nanotubes (CNTs). Catalyst deactivation is due to the fragmentation of Ni particles into smaller Ni particles, followed by their encapsulation with graphitic carbon layers (but eventually all the samples were deactivated within an hour of reaction). Chen and Lua [15] prepared Ni/SBA-15 catalysts by electroless nickel plating. The 32 wt% Ni/SBA-15 catalyst exhibited the best catalytic performance and the highest resistance to deactivation, with a stable 44% methane conversion at 575 °C (Table 1).
Modified (doped) bi- and trimetallic oxide-supported nickel catalysts were also tested to increase the stability and catalytic activity at higher temperatures. The second metal is expected to change the nickel properties if sufficient interaction is provided between the two metals (for example, via alloy formation), which can influence the methane cracking rate, carbon migration rate or simply the dispersion of the catalyst or interaction of metal components with the support. Ahmed et al. [16] investigated pre-reduced 40% Ni-10% Mo/CeO2 and 40% Ni-10% Mo/CeO2-SiO2 catalysts in pure CH4 stream at 700 °C and revealed that the mixed support results in poorer catalytic properties in terms of hydrogen and carbon yield due to larger particle size (Table 1). This behaviour was explained by the greater sintering of metal particles on the SiO2-containing support caused by the weak metal-support interaction. The XRD, TEM, TGA and Raman findings of spent catalysts demonstrated the production of MWCNTs with a higher yield, quality, graphitization degree and thermal stability over Ni–Mo/CeO2 catalysts.
Ni–Cu/Al2O3 alloy catalysts were prepared from Ni–Cu–Al hydrotalcite-like compounds and tested for methane decomposition at 650 °C (Table 1). Alloying Ni with Cu caused a decrease in methane conversion, but significantly enhanced the catalytic life and carbon yield. The highest carbon yield was obtained at an atomic ratio of Ni:Cu = 7:3, which is approximately 78 times that of the Ni/Al2O3 catalyst. Carbon morphology was changed from thin carbon nanotubes (CNTs) to thick fishbone-carbon nanofibers (CNF)s and platelet-CNFs, depending on the copper content [17].
Bimetallic Ni-Co/γ-Al2O3 catalysts were more active than monometallic Ni/γ-Al2O3, presenting 86 and 15% methane conversions and 51 and 12% hydrogen yields at 600 °C temperature, respectively (Table 1). Due to the formation of surface bimetallic Ni-Co alloys, the reducibility of the catalyst was facilitated, while the dispersion of particles increased [18]. Cu–Zn-promoted Ni–Co/Al2O3 catalysts with fixed 50 wt% Ni loading gave the highest methane conversion of 85% at 700 °C (Table 1). Zn addition improved the stability of the catalyst by retaining the active metal size during the CDM reaction. Zn promoted the growth of reasonably long and thin carbon nanotubes [19].
A multi-metallic nickel-based catalyst with an optimal composition (60%Ni-5%Cu-5%Zn/Al2O3) was used for CDM in a fluidized bed reactor under bubbling conditions. Higher than 90% methane conversion was achieved with high-quality CNTs (Table 1). The carbon product separation was effectively performed via ultrasonication of the spent catalyst and by using a strong magnet to collect the ferromagnetic nickel-loaded catalyst material in ethanol suspension. The complete carbon removal was carried out by a further recalcination step, and the regenerated catalyst regained its full activity [20].
The effects of Ni loading and gas hourly space velocity (GHSV) over Ni supported on palm oil fuel ash (Ni-POFA with high SiO2 content) catalysts were investigated in CDM at 550 °C for 6 h (Table 1). Over optimum 15 wt% Ni loading and 7000 mL/g h GHSV, the Ni-POFA catalyst performed at 87% initial CH4 conversion and 27% initial H2 yield [21].
Graphene-encapsulated nickel nanoparticles (Ni@G) prepared from nickel nitrate and kraft lignin showed high catalytic activity for methane decomposition at temperatures of 800 to 900 °C and exhibited long-term stability of 600 min time-on-stream (TOS) without apparent deactivation. The methane conversion was 88% and the hydrogen production was 95 vol% at 900 °C over a 25 wt% Ni@G catalyst (Table 1). During the CDM process, graphene shells over Ni@G nanoparticles were cracked and peeled off the nickel cores. Both the exposed nickel nanoparticles and the cracked graphene shells may participate in the CDM reaction, explaining the high activity of the dual (metallic nickel and graphenic carbon) catalytic system. Graphene nanoplatelets, fluffy graphene, 3D fluffy graphene, and 3D graphitic nanochips were formed as the main solid products, depending on the reaction time [22].
Ni and Ni–Pd alloy supported on CNTs with various atomic ratios were synthesized and tested for CDM. The addition of Pd to Ni stabilized the metal particles and terminated the CNT growth due to coking. Increasing the CH4 molar fraction from 30 to 100% over the NiPd/CNT catalyst with optimum 10:1 Ni:Pd atomic ratio, the conversion decreased from 55 to 35%, but the hydrogen production rate increased and remained stable at 600 °C for 6 h (Table 1). A self-sustained cyclic reaction–regeneration process was demonstrated by leaching out the metal from the produced CNTs/CNFs catalyst mixture and re-synthesizing the 10Ni–1Pd/CNT catalyst [23].
2.2. Iron Containing Catalysts
Iron-based catalysts are cheaper, but they generally work at higher temperatures than nickel-containing ones (Figure 1). Natural iron ores (Tierga and Ilmenite) were used as CDM catalysts. Tierga (iron oxide) exhibited high stability and activity (70 vol% H2) at 850 °C when methane also acted as a reducing agent (Table 1). The activation with CH4 led to the initial fragmentation of the α-Fe phase and inhibition of large amounts of Fe3C formation. Hybrid nanomaterials composed of graphite sheets and carbon nanotubes with a high degree of graphitization were obtained [24].
Carbonaceous nanomaterials (CNMs) were obtained over stainless-steel foams via CDM. The maximum productivity attained was 0.116 g C/g foam h operating at 950 °C with a feed ratio of CH4/H2 = 3 (Table 1). The formation of graphene-related materials (GRMs, mainly few-layer graphene and graphene) was favoured above 900 °C; at lower temperatures carbon nanotubes were produced [25].
Carbon-encapsulated iron nanoparticles (CEINPs) with varying iron concentrations (20, 30 and 40 wt%) were investigated for methane decomposition between 700 and 800 °C in a semicontinuous flow fixed-bed reactor. The graphitic shell prevented atmospheric oxidation and sintering at high temperature, improving the thermal stability of the catalysts. The highest initial methane conversion (96%) was reached over the 30 wt% Fe containing catalyst at 800 °C and dropped to 37% after 180 min of reaction (Table 1). Four different active sites were suggested in the catalyst: graphite, graphite encapsulated iron nanoparticles, uncovered iron nanoparticles and activated carbon. Catalyst deactivation was due to carbon deposition from CH4 in the form of coke and graphite on non-encapsulated Fe nanoparticles [26].
2.3. Noble Metal Catalysts
The addition of noble metals into supported-metal catalysts provided better activity and stability in comparison with the single metal in CDM [9]. Only the Ni-Pd/CNT catalyst [23] discussed above among Ni-based catalysts appeared during the period 2021–2022.
2.4. Carbon Based Catalysts
Due to their availability, durability, low cost, abundant porosity, molecular activation and tolerance to high temperatures, carbon catalysts are frequently used in CDM [9]. Metal-free carbon catalysts (activated carbon (AC), mesoporous carbon (MC) and carbon black (CB)) have been investigated for CDM. CB showed greater stability over the course of the reaction than AC and MC. The lifetime of CB catalysts and the possibility of their regeneration are fundamental for their potential applicability in industry. A strong relationship between the number and type of oxygen-containing functional groups and methane conversion was found. The action of epoxy and quinone functional groups for methane activation was confirmed: epoxy groups gently activate methane, quinone groups tend to accelerate coke formation [27].
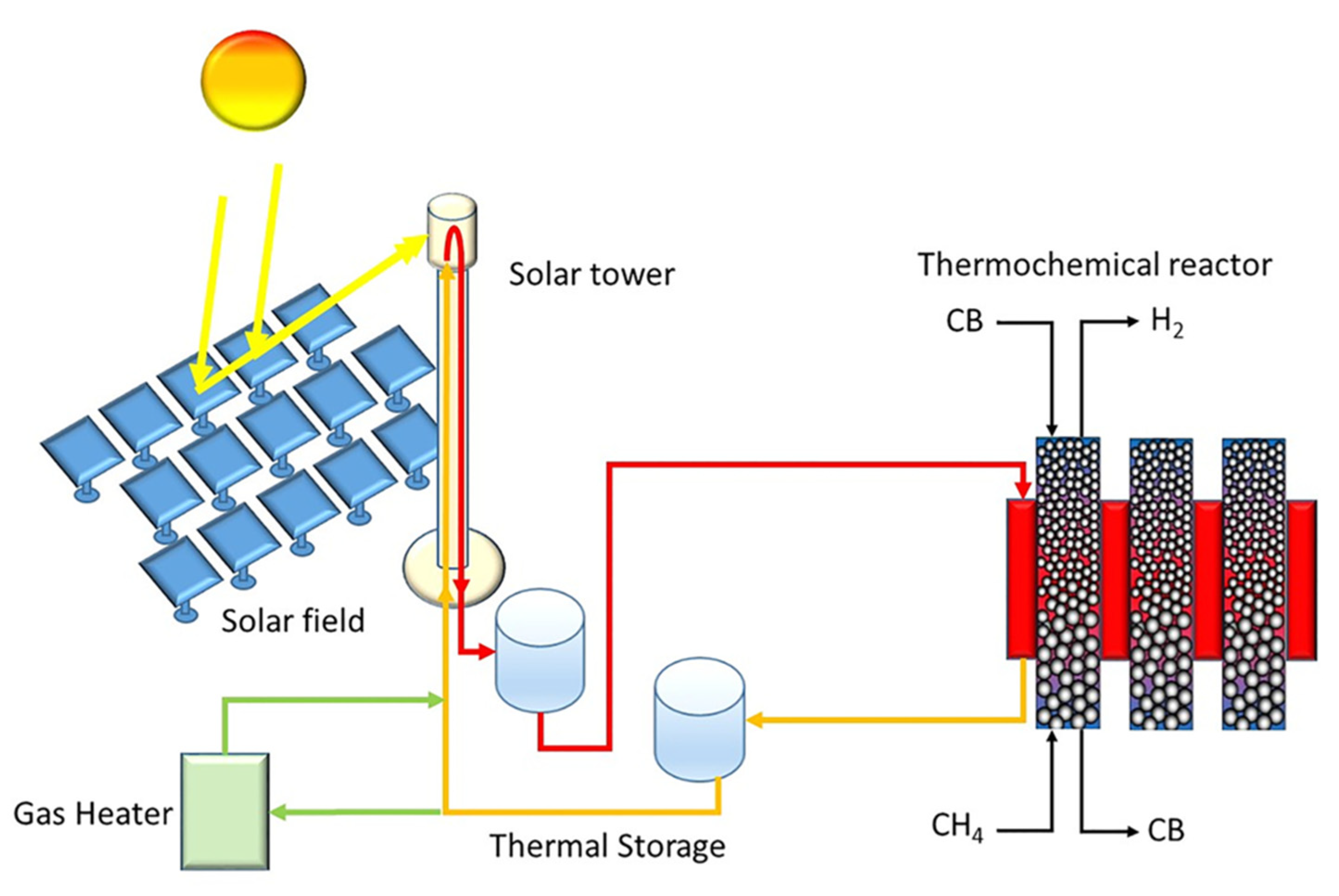
Boretti published two papers on the perspectives of solar-driven CDM producing “aquamarine” hydrogen over carbon black [28] and other [29] catalysts. The basic idea of the thermochemical reactor setup was developed by a group led by BASF: the reaction occurs at about 1000 °C in a moving carbon-bed reactor. According to the solar-driven approach described by Boretti (Figure 2) [28], the thermal energy is provided by molten salt, MgCl2-KCl, flowing from a hot thermal energy storage tank to the cold thermal energy storage tank through the reactor, and from the cold tank to the hot tank through a higher-concentration solar receiver. The perspective could be extended to supported metal-based catalysts such as Fe, Ni, Co and Cu on metal oxide supports such as SiO2, Al2O3 and TiO2, and carbon-based catalysts such as carbon blacks, carbon nanotubes and activated carbons [29].
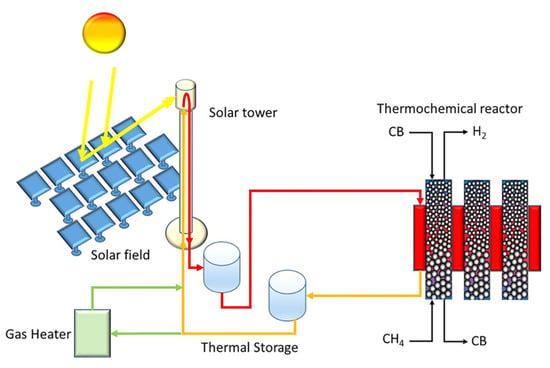
Figure 2.
Sketch of a novel concentrated solar energy-driven version of a theoretical plant for catalytic thermal pyrolysis of CH4 with CB as a catalyst, with the addition of a gas heater. (Adapted from Ref. [28] with Wiley permission).

Table 1.
Results on best-performing solid catalysts in methane pyrolysis in 2021–2022 publications.
Table 1.
Results on best-performing solid catalysts in methane pyrolysis in 2021–2022 publications.
| Catalyst | Preparation | Pretreatment | Feed Rate * (mL/min) | Temp. (°C) | React. Time | Max. CH4 Conversion | H2 Productivity | Carbon Productivity | Carbon Morphology | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 200 mg 40% Ni/MgO | hydrothermal | 850 °C 1 h red. | 200 (33%/N2) | 600 | 4.5 h | 49% | 53% yield | [13] | ||
| 300 mg 10% Ni/Al2O3 | sol-gel | 650 °C 3 h red. | 70 (30%/N2) | 650 | 1 h | 46% | [14] | |||
| 140 mg 32% Ni/SBA-15 | electroless plating | 450 °C 4 min red. | 15 (50%/N2) | 575 | 750 min | 44% | [15] | |||
| 500 mg 40% Ni-10% Mo/CeO2 | coimpregnation | 700 °C 1 h red. | 50 (100% CH4) | 700 | 3 h | 73% yield | 537 wt% | MWCNT | [16] | |
| 10 mg Ni-Cu (7:3)/Al2O3 | coprecipitation | 800 °C 30 m red. | 25 (20%/N2) | 650 | 19 h | 68% | 133 wt% | CNT, CNF | [17] | |
| 2 mL 1% Ni-2% Co/Al2O3 | impregnation | 600 °C 6 h red. | 160 (6%/N2) | 600 | 5 h | 86% | 51% yield | 11 wt% | [18] | |
| 100 mg 50% Ni-CoCuZn/Al2O3 | coprecipitation | 750 °C 3 h red. | 42 L/g/h (50%/N2) | 700 | 80 h | 85% | 265 wt% | CNT | [19] | |
| 5g 60% Ni-5% Cu-5% Zn/Al2O3 | impregnation | 550 °C 5 h red. | 180 (25%/N2) | 750 | 180 min | >90% | CNT | [20] | ||
| 80 mg 15% Ni/POFA (SiO2) | combustion | no pretreatment | 60 (20%/N2) | 550 | 6 h | 87% | 27% yield | filament | [21] | |
| 10 g 25% Ni@G | coprecipitation | 300 °C 0.5 h in N2 | 150 (67%/Ar) | 900 | 10 h | 88% | 95 vol% H2 | 4.46 g/g Ni | G, graphite | [22] |
| 200 mg 10% Ni-1% Pd/CNT | solvothermal | 400 °C 4 h red. | 30 (30%/N2) | 600 | 6 h | 55% | 90% select. | CNT, CNF | [23] | |
| 600 mg iron ores | natural | 900 °C 1 h in CH4 | 20 (100% CH4) | 850 | 3 h | 56% | 70 vol% H2 | 1.63 g/g cat. | CNT, graphite | [24] |
| 200 mg stainless steel foam | commercial | 900 °C ox. + red. | 700 (43%/14% H2/N2) | 950 | <5% | 116 g/(g foam * h) | GRM, CNT | [25] | ||
| 500 mg 30% Fe@C | impregnation | 1000 °C in N2 | 100 (5%/N2) | 800 | 3 h | 96% | 0.39 g/g cat. | coke, graphite | [26] | |
| 1.1 mL Active Carbon (AC) | commercial | no pretreatment | 60 (50%/N2) | 1000 | 45 min | 37% | [27] | |||
| 200 mg 10% Co/BFA | impregnation | 700 °C 5 h calc. | 40 (100% CH4) | 700 | 330 min | 63% | 30% yield | whisker | [30] | |
| 500 mg 5% Co/CeO2-BFA | impregnation | 700 °C 3 h calc. | 20 (100% CH4) | 850 | 34 h | 71% | 45% yield | [31] | ||
| 25 mg 24% Co-6% Cu/C | coimpregnation | 850 °C 75 m red. | 700 (29%/14% H2/N2) | 750 | 11 h | 0.29 g/g cat. | CNT | [32] | ||
| 500 mg 40% Mo/MgO | impregnation | 700 °C 1 h red. | 60 (100% CH4) | 800 | 2 h | 68% | 68% yield | 180 wt% | MWCNT | [33] |
* Methane content (vol%)/inert gas in brackets. + Fluid-bed reactor; all other references used fixed-bed reactors.
2.5. Other Catalysts
Other new catalysts also appeared in the literature of CDM beside the above mentioned four groups of catalysts. Cobalt-containing catalysts are discussed in three papers. Co-loaded biomass fly ash (10% Co/BFA) was employed for methane decomposition at 700 °C in a fixed-bed reactor with a stable 63% CH4 conversion and greater than 30% hydrogen yield for 330 min time-on-stream (Table 1) [30]. The same group of authors reported 71% methane conversion with 45% hydrogen yield at 850 °C for 34 h on stream activity using a 5% Co/CeO2-BFA catalyst (Table 1) [31]. Henao et al. [32] studied the influence of the reaction temperature (650–950 °C) and feed composition (7%–43% of CH4 and H2) on the yield and CNTs quality of CDM using a Co-Cu/cellulose-derived carbon catalyst. Below 800 °C, the reaction was selective towards the formation of CNTs, while above 800 °C, the obtained nanomaterial exhibited a graphite-like morphology similar to Ref. [25]. The best operating parameters for growing CNTs with high productivity (0.29 gC/gcat∙h) and quality were found at 750 °C under 29% CH4: 14% H2: 57%N2 (Table 1).
Awadallah et al. [33] prepared 20–50% Mo/MgO catalysts by impregnation and declared that mainly Mo, reduced from the MgMoO4 phase, was the catalytically active site in methane decomposition. The presence of non-interacting MoO3 was not beneficial concerning activity and longevity at 50% Mo-loading. Multi-walled carbon nanotube bundles were deposited at 800 °C with very uniform and narrow diameters. The maximum carbon yield was 180% for the 40% Mo/MgO catalyst with 68% final methane conversion after 120 min reaction time (Table 1). The highest initial methane conversion was 75% for the 50% Mo/MgO catalyst, but it decreased to 59% final conversion, resulting in a 140% carbon yield due to the presence of MoO3 species.
Kreuger et al. [34] studied methane pyrolysis over nonporous α-Al2O3 surfaces in the range of 900–1300 °C in single-particle and fixed-bed reactors. The selectivity towards carbon (and hydrogen) was initially low (38% at 1000 °C) with 20% methane conversion over the fresh catalyst, indicating an activation process for the formation of carbon and hydrogen from the intermediate products (e.g., benzene), but later a temperature-dependent maximum in carbon loading was observed. They were able to predict carbon growth and CH4 conversion as a function of temperature, specific bed area, carbon loading and gas composition based on the parametrization of kinetic models.
The great challenge in methane pyrolysis is to obtain very pure hydrogen with high yield and gain separated, precious, possibly nanostructured carbon such as carbon nanotubes at the same time. If the process heat of the endothermic reaction is provided from the combustion of H2 product, the efficiency of hydrogen production is lowered. Metal-containing catalysts can provide graphitic, structured carbon, but the product separation and catalyst regeneration is demanding. Moreover, the exact conditions of its formation and tailoring of a desired carbon structure with the use of the appropriate catalyst is again a future task that should be solved. In the case of purely carbon-based catalysts, separation of pyrolytic carbon from the carbon catalyst is of less importance, but we should keep in mind that the structure of carbon products is usually less ordered. Environmental issues may suggest a preference for the application of Fe-based and carbon-supported systems, with special attention on the future utilization of the carbon product. We must note that catalyst regeneration without CO2 formation remains the next rather challenging issue of the methane pyrolysis process. Because all the above-mentioned case studies are still at research level, expertise is needed from a technological point of view to plan the scale-up steps and study the influence of process parameters if a prospective catalyst family is found. However, the responsibility and role of basic research is inevitable at the present stage of catalytic methane pyrolysis to clarify the interaction of catalyst structure and performance influencing the purity of H2 and carbon products.
3. Reactors Used in Methane Pyrolysis
Four different reactor configurations can be generally applied for CDM: fixed-bed, fluidized-bed, plasma-bed and molten-metal reactors [9]. Fixed-bed reactors are the most commonly used ones, but are usually preferred on a laboratory scale only. Their main drawback is the filling of the reactor with the carbon product during long-term experiments. A better prospective reactor for large-scale operation is the fluidized-bed reactor because it is suitable for the continuous addition of catalyst particles and withdrawal of solid carbon coproducts. A pressure drop does not increase significantly, and the operation for longer times is possible. The vigorous movement of the particles allows efficient heat and mass transfer between the gas and the solid catalyst. Two parallel reactors can operate in a cyclic mode by switching the natural gas feed and the regeneration agent stream (air, steam) between the two reactors. The plasma bed reactor is mainly operated by arc plasma, where a glow-like jet region is used. Its drawback is its low energy efficiency. The molten metal or liquid–bubble-column reactor operates with molten media, such as molten metals (Ti, Pb, Sn, Ga), molten-metal alloys (Ni−Bi, Cu−Bi) or molten salts (KBr, NaBr, NaCl, NaF, MnCl2, KCl). The main advantage of liquid–bubble-column reactors is the easy separation of the carbon by-product from the liquid medium due to density differences. Their drawbacks are the limited stability of the molten media at the required high operating temperatures (>900–1000 °C) and the corrosion at such high temperatures [35]. The schematic diagrams of these reactor types are given in Ref. [9].
4. Molten Media Pyrolysis of Methane
Methane pyrolysis in molten metals/salts to prevent both reactor coking and rapid catalyst deactivation is the most promising alternative to conventional pyrolysis. This strategy can fundamentally solve the problem of carbon deactivation because, at the liquid–solid interface between the molten catalyst and the carbon product, the generated carbon may be separated from the active site and removed continuously from the reactor [36]. As a recent review [11] discusses this process in detail, we present here only the very latest results in this area.
Methane pyrolysis was performed in a quartz bubble column using molten gallium as a heat transfer agent and catalyst. A maximum conversion of 91% was achieved at 1119 °C and ambient pressure, with a residence time of the bubbles in the liquid of 0.5 s (Table 2) [37].

Table 2.
Results of methane pyrolysis 2021–2022 publications in molten media.
Methane bubbled through molten tin at 1000 °C and ambient pressure with an increasing flow rate from 25 to 250 mL/min (Table 2) decreased the mole fraction of hydrogen from 12 to 4.4% in the resulting gas mixture due to the shortening contact between the gas bubbles and the tin melt. A unique float-type structure placed inside the reactor solved the problem of continuous removal of the carbon deposits generated during methane pyrolysis and controlled the metal melt level [38].
Nitrogen-diluted methane was bubbled through molten tin, tin-nickel, and tin-copper alloys in an alumina tube reactor at 950–1050 °C. A maximum 19% conversion was achieved over the 5% Ni containing alloy at 1050 °C in a 10 cm molten-metal column (Table 2) [39].
Methane conversion was measured using a bubble column reactor at 700–1000 °C temperatures by injecting 1:1 Ar:CH4 reactant gas mixture into molten KCl (Table 2). The melt acted as a carbon-separating agent and as a pyrolytic catalyst and enabled 40 h of continuous running without catalytic deactivation, with an apparent activation energy of 277 kJ/mole. Heating the solid product at 1200 °C produced the highest purity carbon (97.2 at%), with some salt residues [40].
Alkali halides (NaBr, KBr, KCl, NaCl) and an eutectic mixture of NaBr:KBr (49:51 mol%) as the liquid media were tested for CH4 pyrolysis and characterized the properties of the generated carbons. Significantly lower activation energies (224–278 kJ/mol) were found than those measured during non-catalytic gas-phase methane pyrolysis (~422 kJ/mol). The purity of the washed carbon samples was in the range of 92–97% [41]. The same group of authors [42] tested alumina-supported La, Ni, Co and Mn catalysts as particle suspensions in molten NaBr-KBr at 850–1000 °C for CDM. The increase in the molar Co:Mn ratio from zero to two increased the CH4 conversion at 1000 °C from 5 to 10%, and they observed a stable performance over ca. 24 h of methane pyrolysis and product selectivities for hydrogen near unity. An enhanced conversion was measured using the smaller catalyst particle size ranges (Table 2).
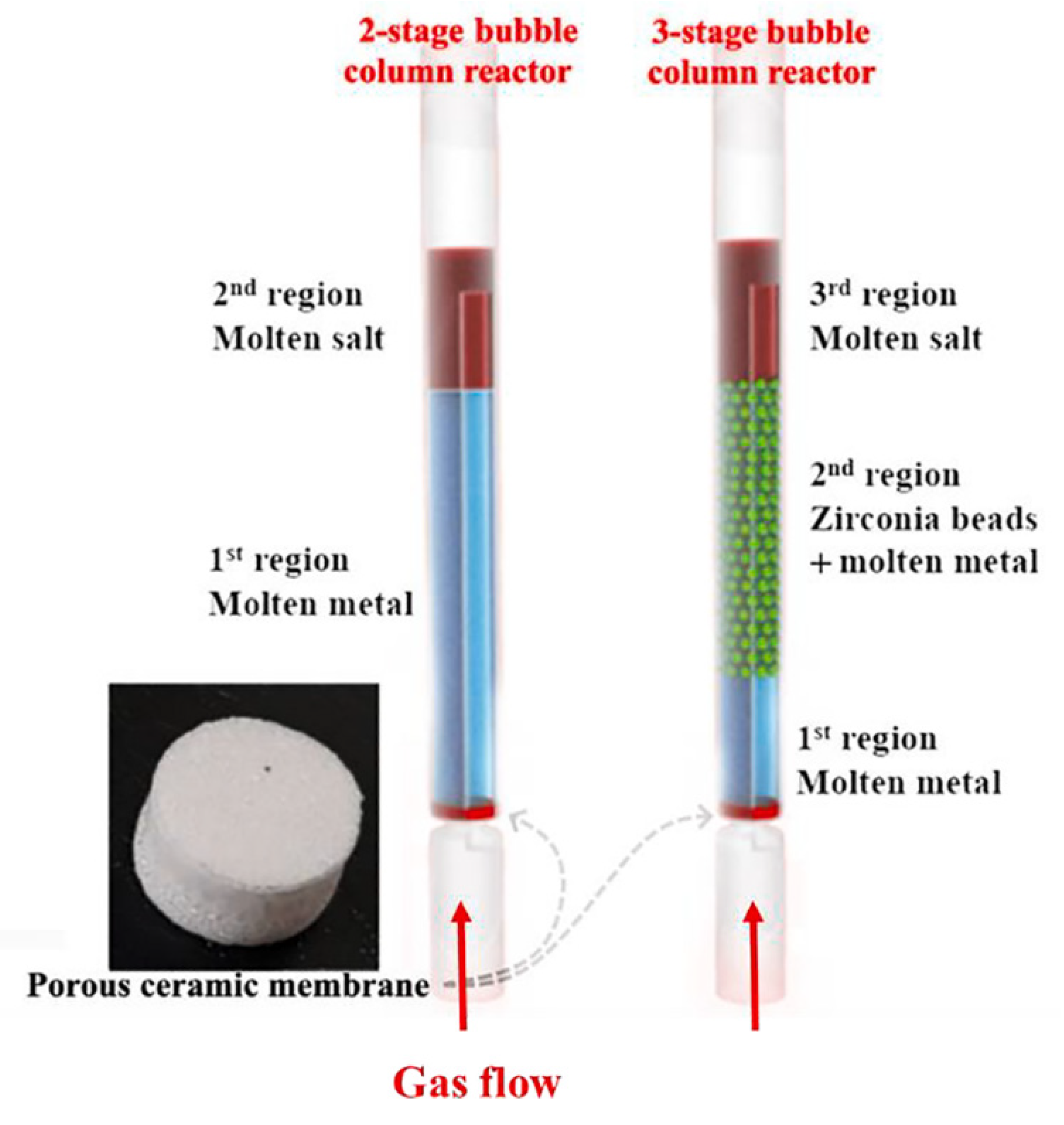
Noh et al. [43] applied bubble-column reactors containing molten Ni-Bi alloy (bottom), molten NaBr (top) in two-stage, and zirconia (between them) in three-stage reactors (Figure 3) for CDM with 32 and 38% stable methane conversion rates, respectively, at 985 °C up to 50 h operation (Table 2). The enhanced methane pyrolysis rates with the use of zirconia beads indicate that they can be employed to control the bubble behaviour and to enhance the contact area between bubbles and the liquid catalyst surface for an efficient bubble column reactor with liquid metal alloy catalysts.
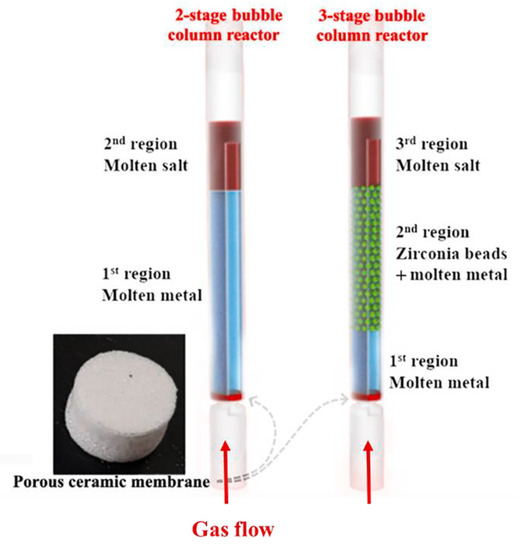
Figure 3.
The structures of the two-stage and three-stage bubble-column reactors implemented for methane decomposition reaction. (Copied from Ref. [43] with Elsevier permission).
The two main problems in this field are high process temperatures, which require high energy usage, and corrosion of reactor material due to high temperatures. Nevertheless, the advantages of this process, such as prevention of reactor coking and rapid catalyst deactivation, separation of carbon by-product from the catalyst, improvement of heat transfer (and thermal inertia) owing to the high heat capacity of molten media and the enhancement of the gas residence time due to the liquid viscosity, compensate for the mentioned problems. Molten salts are weaker catalysts than molten metals, but their application reduces the investment cost of CDM. Salts melt at a relatively lower temperature compared with metals, they have low vapor pressure diminishing salt evaporation and they are less expensive and less dense than metals, and carbon contaminated with salt is much easier to purify because the salt is flushable by dissolution in water [11].
5. Reaction Mechanism and Aspects of Industrialization
The reaction mechanisms and industrialization possibilities of methane pyrolysis are summarized in Ref. [35]. It is generally accepted that the rate-limiting step is the splitting of methane into a methyl radical and a hydrogen atom in noncatalytic methane pyrolysis. Some works have proposed a molecular adsorption mechanism, whereas a dissociative adsorption model has been described in other studies for catalytic methane pyrolysis. Methane is first adsorbed on the catalyst surface and then dissociates following a series of stepwise surface dehydrogenation reactions according to the molecular adsorption mechanism. Methane dissociates upon adsorption on the catalytic active sites, generating chemisorbed CH3 and H fragments according to the dissociative adsorption model, which is followed by the same surface dissociation reactions described by the molecular adsorption mechanism. The vapor−liquid−solid (VLS) model was applied for the growth of filamentous carbon, which includes carbon nanotubes and nanofibers. It supposes carbon diffusion through the metal (assumed to have the properties of a liquid) particles as the rate-limiting stage. Nevertheless, there is no general agreement, and the reaction mechanism involved in methane pyrolysis, as well as the overall rate-limiting step, is still unclear and must depend on the type of catalyst material, reactor design, temperature and other process parameters [35].
The quality and sale of the carbon coproduct may improve the economic efficiency of the industrial pyrolysis of methane. The characteristics of the carbon depend on the catalyst used and the reaction conditions. The formation of carbon nanotubes and nano-fibres usually occurs over metal catalysts. At high operation temperatures, as the diameter and length of the carbon nanofilaments decrease, their crystallinity and graphitization degree increase. Carbon blacks are formed over activated carbons, and they produce amorphous turbostratic structures. The use of carbon nanotubes as a catalyst favours the growth of their walls, leading to the formation of multiwalled carbon nanotubes. A suitable experimental setup must still be found to industrialize the process, and the possible commercialization or storage of the purified carbon coproduct still remains a challenge [35].
The industrialization of methane pyrolysis is limited, and the presented works are mainly at the research stage. High processing temperatures, deactivation of catalytic systems (e.g., by sintering of active sites and/or by carbon deposition) and difficulties with removing the formed carbon hinder the application possibilities. Catalyst structure and process parameters control the morphology of carbon co-products. The aim of regeneration is to prolong the lifetime of the catalyst and to remove the poisoning carbon by gasification without destroying the useful carbon products. Gasification can be catalysed by the CDM catalyst itself into carbon oxides (CO2 or CO) using oxygen, water or CO2 as a reactant. Generally, the most deactivating carbon forms, which are in close contact with the catalytic active sites, are of the most reactive ones. The selective removal of such carbons can be enhanced by optimizing the temperature and the oxygen/water/carbon-dioxide concentration. However, all these types of gasification produce carbon oxides, which corrupt the eco-friendly nature of CDM and may also cause catalyst oxidation. The hydrogenation of deactivating carbon to CH4 is also a promising, but less studied type of gasification [10]. With this method, carbon oxide formation and also the oxidation of the active metal(s) can be fully avoided; furthermore, this method is technologically more facile compared to the oxidative regeneration methods, because no change between reductive and oxidative steps is needed.
6. Summary, Conclusions and Perspectives
As can be seen in the number of reviewed publications, nickel-containing catalysts and molten media pyrolysis attracted the highest attention regarding CDM during the period 2021–2022. The main advantage of nickel-containing catalysts in conventional methane pyrolysis is their relatively low working temperature, but their drawback is their generally fast deactivation, especially at higher temperatures. A possible solution could be the application of graphene-encapsulated nickel nanoparticles as catalysts, which, according to Ref. [22], show long-term stability without apparent deactivation at 900 °C with high methane conversion and hydrogen production. The outer graphene shell breaks up and the naked nickel core will be a new continuously regenerated active site during the reaction, and the graphene products also serve as carbon-based catalysts and valuable solid products in the CDM reaction. A similar but iron-based catalyst (carbon-encapsulated iron nanoparticles [26]) also presented high initial methane conversions, but this catalyst deactivated quickly.
In agreement with one of the latest reviews [11], solar energy utilization can be a promising option for CDM. The two papers of Boretti [28,29] outline such a perspective: the usage of solar-derived thermal energy provided by a molten-salt flow for methane pyrolysis is a very promising project for the future. This solar–thermal driven catalytic decomposition of methane produces ‘aquamarine’ hydrogen and carbon particles of commercial interest at a reduced cost. The design and development of a high-efficiency reactor for the proposed temperature range capable of continuous operation is the most critical aspect of the project.
Due to its many advantages over conventional pyrolysis and technological novelty, CDM in molten media reached the highest publication activity in the present and last year. Papers on pure molten metals through to metal alloys and oxide-supported metal catalysts suspended in molten salt mixtures were published, showing the development of this research area. In the latest review [11], molten salts and Ni-Bi alloys were declared as the most promising and most efficient for methane pyrolysis in molten media. Recently, a molten NaBr-KBr mixture [41,42] and Ni-Bi alloys [43] as CDM catalysts were developed even further: alumina-supported Co-Mn oxide catalysts increased and stabilized the methane conversion in the salt mixture [42], zirconia beads enhanced methane pyrolysis rates in a three-stage bubble-column reactor containing molten Ni-Bi alloy, zirconia and molten NaBr [43].
Optimizing the CDM process to produce both the desired grade of carbon and fuel cell-grade hydrogen, particularly if a carbon with very tight product specification is being produced, could be a major challenge. The high reaction temperatures required for CH4 conversion limit the choice of materials of construction, adversely impact catalyst life, and exacerbate heat losses. Most catalysts produce both amorphous and structured carbons. Amorphous carbons such as activated carbon and carbon black are more active for CDM than structured carbons such as graphite and diamond. Graphite used in lithium-ion batteries and nanotube- and nanofiber carbons are high-value products. The yield of high-value forms of carbon is critical for successful commercial implementation. Separation of the catalyst and the carbon by-product remains a challenge. Catalysts should be recovered and fully regenerated without burning the highly valued carbon products. The major benefit of molten-metal technology is the easy separation of carbon from molten metal due to density differences; however, high temperature for conversion is still required [44].
Conclusively definite progress has been reached in this research area, but even the BASF plant developed for industrial application of methane pyrolysis is still in its infancy. According to a BASF report in 2021 [45], methane pyrolysis for large-scale production should be available by 2030 at the latest. The existing large conventional and unconventional methane deposits (methane clathrates, natural gas hydrates occurring in deep seas and permafrost) are a precious source for hydrogen energy. Their exploitation is highly required by optimized methane pyrolysis processes in an environmentally friendly manner in the near future.
Author Contributions
Writing—original draft preparation, T.I.K.; writing—review and editing, T.I.K., A.B. and A.H.; visualization, M.N.; supervision, T.I.K. and A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Support by Hungarian-Egyptian bilateral project # NKM 2019-12 is acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bakenne, A.; Nuttall, W.; Kazantzis, N. Sankey-Diagram-based insights into the hydrogen economy of today. Int. J. Hydrogen Energy 2016, 41, 7744–7753. [Google Scholar] [CrossRef]
- Upham, D.C.; Agarwal, V.; Khechfe, A.; Snodgrass, Z.R.; Gordon, M.J.; Metiu, H.; McFarland, E.W. Catalytic molten metals for the direct conversion of methane to hydrogen and separable carbon. Science 2017, 358, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Hoekman, S.K. Production of CO2-Free Hydrogen From Methane Dissociation: A Review. Environ. Prog. Sustain. Energy 2014, 33, 213–219. [Google Scholar] [CrossRef]
- Dipu, A.L. Methane decomposition into COx-free hydrogen over a Ni based catalyst: An overview. Int. J. Energy Res. 2021, 45, 9858–9877. [Google Scholar] [CrossRef]
- Abbas, H.F.; Daud, W.M.A.W. Hydrogen production by methane decomposition: A review. Int. J. Hydrogen Energy 2010, 35, 1160–1190. [Google Scholar] [CrossRef]
- Fidalgo, B.; Menéndez, J.Á. Carbon Materials as Catalysts for Decomposition and CO2 Reforming of Methane: A Review. Chin. J. Catal. 2011, 32, 207–216. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Wang, G. Methane decomposition to COx-free hydrogen and nano-carbon material on group 8–10 base metal catalysts: A review. Catal. Today 2011, 162, 1–48. [Google Scholar] [CrossRef]
- Ashik, U.P.M.; Daud, W.M.A.W.; Abbas, -H.F. Production of greenhouse gas free hydrogen by thermocatalytic decomposition of methane—A review. Renew. Sustain. Energy Rev. 2015, 44, 221–256. [Google Scholar] [CrossRef]
- Qian, J.X.; Chen, T.W.; Enakonda, L.R.; Liu, D.B.; Mignani, G.; Basset, J.-M.; Zhou, L. Methane decomposition to produce COx-free hydrogen and nano-carbon over metal catalysts: A review. Int. J. Hydrogen Energy 2020, 45, 7981–8001. [Google Scholar] [CrossRef]
- Alves, L.; Pereira, V.; Lagarteira, T.; Mendes, A. Catalytic methane decomposition to boost the energy transition: Scientific and technological advancements. Renew. Sustain. Energy Rev. 2021, 137, 110465. [Google Scholar] [CrossRef]
- Msheik, M.; Rodat, S.; Abanades, S. Methane Cracking for Hydrogen Production: A Review of Catalytic and Molten Media Pyrolysis. Energies 2021, 14, 3107. [Google Scholar] [CrossRef]
- Muradov, N.Z.; Veziroglu, T.N. From hydrocarbon to hydrogen–carbon to hydrogen economy. Int. J. Hydrogen Energy 2005, 30, 225–237. [Google Scholar] [CrossRef]
- Dalenjan, M.B.; Rashidi, A.; Khorasheh, F.; Ardjmand, M. Effect of Ni ratio on mesoporous Ni/MgO nanocatalyst synthesized by one-step hydrothermal method for thermal catalytic decomposition of CH4 to H2. Int. J. Hydrogen Energy 2022, 47, 11539–11551. [Google Scholar] [CrossRef]
- Xu, M.; Lopez-Ruiz, J.A.; Kovarik, L.; Bowden, M.E.; Davidson, S.D.; Weber, R.S.; Wang, I.W.; Hu, J.; Dagle, R.A. Structure sensitivity and its effect on methane turnover and carbon co-product selectivity in thermocatalytic decomposition of methane over supported Ni catalysts. Appl. Catal. A Gen. 2021, 611, 117967. [Google Scholar] [CrossRef]
- Chen, Q.; Lua, A.C. Synthesis of electroless Ni catalyst supported on SBA-15 for hydrogen and carbon production by catalytic decomposition of methane. Int. J. Energy Res. 2021, 45, 2810–2823. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Awadallah, A.E.; Aboul-Enein, A.A.; Solyman, S.M.; Aboul-Gheit, N.A.K. Non-oxidative Decomposition of CH4 Over CeO2 and CeO2–SiO2 Supported Bimetallic Ni–Mo Catalysts. Catal. Lett. 2022, 152, 2789–2800. [Google Scholar] [CrossRef]
- Lin, X.; Zhu, H.; Huang, M.; Wan, C.; Li, D.; Jiang, L. Controlled preparation of Ni–Cu alloy catalyst via hydrotalcite-like precursor and its enhanced catalytic performance for methane decomposition. Fuel Process. Technol. 2022, 233, 107271. [Google Scholar] [CrossRef]
- Yergaziyeva, G.; Makayeva, N.; Shaimerden, Z.; Soloviev, S.; Telbayeva, M.; Akkazin, E.; Ahmetova, F. Catalytic Decomposition of Methane to Hydrogen over Al2O3 Supported Mono– and Bimetallic Catalysts. Bull. Chem. React. Eng. Catal. 2022, 17, 1–12. [Google Scholar] [CrossRef]
- Alwan, B.A.A.; Shah, M.; Danish, M.; Mesfer, M.K.A.; Khan, M.I.; Natarajan, V. Enhanced methane decomposition over transition metal-based tri-metallic catalysts for the production of COx free hydrogen. J. Indian Chem. Soc. 2022, 99, 100393. [Google Scholar] [CrossRef]
- Parmar, K.R.; Pant, K.K.; Roy, S. Blue hydrogen and carbon nanotube production via direct catalytic decomposition of methane in fluidized bed reactor: Capture and extraction of carbon in the form of CNTs. Energy Convers. Manag. 2021, 232, 113893. [Google Scholar] [CrossRef]
- Hanifa, N.H.E.; Ismail, M.; Ideris, A. Methane decomposition over Ni supported on palm oil fuel ash (Ni-POFA) catalyst. Chem. Eng. Res. Des. 2022, 178, 224–231. [Google Scholar] [CrossRef]
- Yan, Q.; Ketelboeter, T.; Cai, Z. Production of COx-Free Hydrogen and Few-Layer Graphene Nanoplatelets by Catalytic Decomposition of Methane over Ni-Lignin-Derived Nanoparticles. Molecules 2022, 27, 503. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.W.; Dagle, R.A.; Khan, T.S.; Lopez-Ruiz, J.A.; Kovarik, L.; Jiang, Y.; Xu, M.; Wang, Y.; Jiang, C.; Davidson, S.D.; et al. Catalytic decomposition of methane into hydrogen and high-value carbons: Combined experimental and DFT computational study. Catal. Sci. Technol. 2021, 11, 4911–4921. [Google Scholar] [CrossRef]
- Silva, J.A.; Santos, J.B.O.; Torres, D.; Pinilla, J.L.; Suelves, I. Natural Fe-based catalysts for the production of hydrogen and carbon nanomaterials via methane decomposition. Int. J. Hydrogen Energy 2021, 46, 35137–35148. [Google Scholar] [CrossRef]
- Cazana, F.; Latorre, N.; Tarifa, P.; Royo, C.J.; Sebastian, V.; Romeo, E.; Centeno, M.A.; Monzon, A. Performance of AISI 316L-stainless steel foams towards the formation of graphene related nanomaterials by catalytic decomposition of methane at high temperature. Catal. Today 2022, 383, 236–246. [Google Scholar] [CrossRef]
- Kundu, R.; Ramasubramanian, V.; Neeli, S.T.; Ramsurn, H. Catalytic Pyrolysis of Methane to Hydrogen over Carbon (from cellulose biochar) Encapsulated Iron Nanoparticles. Energy Fuels 2021, 35, 13523–13533. [Google Scholar] [CrossRef]
- Kim, S.E.; Jeong, S.K.; Park, K.T.; Lee, K.Y.; Kim, H.J. Effect of oxygen-containing functional groups in metal-free carbon catalysts on the decomposition of methane. Catal. Commun. 2021, 148, 106167. [Google Scholar] [CrossRef]
- Boretti, A. Concentrated solar energy-driven carbon black catalytic thermal decomposition of methane. Int. J. Energy Res. 2021, 45, 21497–21508. [Google Scholar] [CrossRef]
- Boretti, A. A perspective on the production of hydrogen from solar-driven thermal decomposition of methane. Int. J. Hydrogen Energy 2021, 46, 34509–34514. [Google Scholar] [CrossRef]
- Munawar, M.A.; Khoja, A.H.; Hassan, M.; Liaquat, R.; Naqvi, S.R.; Mehran, M.T.; Abdullah, A.; Saleem, F. Biomass ash characterization, fusion analysis and its application in catalytic decomposition of methane. Fuel 2021, 285, 119107. [Google Scholar] [CrossRef]
- Raza, J.; Khoja, A.H.; Naqvi, S.R.; Mehran, M.T.; Shakir, S.; Liaquat, R.; Tahir, M.; Ali, G. Methane decomposition for hydrogen production over biomass fly ash-based CeO2 nanowires promoted cobalt catalyst. J. Environ. Chem. Eng. 2021, 9, 105816. [Google Scholar] [CrossRef]
- Henao, W.; Cazaña, F.; Tarifa, P.; Romeo, E.; Latorre, N.; Sebastian, V.; Delgado, J.J.; Monzón, A. Selective synthesis of carbon nanotubes by catalytic decomposition of methane using Co-Cu/cellulose derived carbon catalysts: A comprehensive kinetic study. Chem. Eng. J. 2021, 404, 126103. [Google Scholar] [CrossRef]
- Awadallah, A.E.; Deyab, M.A.; Ahmed, H.A. Mo/MgO as an efficient catalyst for methane decomposition into COx-free hydrogen and multi-walled carbon nanotubes. J. Environ. Chem. Eng. 2021, 9, 106023. [Google Scholar] [CrossRef]
- Kreuger, T.; van Swaaij, W.P.M.; Bos, A.N.R.; Kersten, S.R.A. Methane decomposition kinetics on unfunctionalized alumina surfaces. Chem. Eng. J. 2022, 427, 130412. [Google Scholar] [CrossRef]
- Sánchez-Bastardo, N.; Schlögl, R.; Ruland, H. Methane Pyrolysis for Zero-Emission Hydrogen Production: A Potential Bridge Technology from Fossil Fuels to a Renewable and Sustainable Hydrogen Economy. Ind. Eng. Chem. Res. 2021, 60, 11855–11881. [Google Scholar] [CrossRef]
- Fan, Z.; Weng, W.; Zhou, J.; Gu, D.; Xiao, W. Catalytic decomposition of methane to produce hydrogen: A review. J. Energy Chem. 2021, 58, 415–430. [Google Scholar] [CrossRef]
- Pérez, B.J.L.; Jiménez, J.A.M.; Bhardwaj, R.; Goetheer, E.; Annaland, M.V.S.; Gallucci, F. Methane pyrolysis in a molten gallium bubble column reactor for sustainable hydrogen production: Proof of concept & techno-economic assessment. Int. J. Hydrogen Energy 2021, 46, 4917–4935. [Google Scholar] [CrossRef]
- Kudinov, I.V.; Pimenov, A.A.; Kryukov, Y.A.; Mikheeva, G.V. A theoretical and experimental study on hydrodynamics, heat exchange and diffusion during methane pyrolysis in a layer of molten tin. Int. J. Hydrogen Energy 2021, 46, 10183–10190. [Google Scholar] [CrossRef]
- Zaghloul, N.; Kodama, S.; Sekiguchi, H. Hydrogen Production by Methane Pyrolysis in a Molten-Metal Bubble Column. Chem. Eng. Technol. 2021, 44, 1986–1993. [Google Scholar] [CrossRef]
- Boo, J.; Ko, E.H.; Park, N.-K.; Ryu, C.; Kim, Y.-H.; Park, J.; Kang, D. Methane Pyrolysis in Molten Potassium Chloride: An Experimental and Economic Analysis. Energies 2021, 14, 8182. [Google Scholar] [CrossRef]
- Parkinson, B.; Patzschke, C.F.; Nikolis, D.; Raman, S.; Dankworth, D.C.; Hellgardt, K. Methane pyrolysis in monovalent alkali halide salts: Kinetics and pyrolytic carbon properties. Int. J. Hydrogen Energy 2021, 46, 6225–6238. [Google Scholar] [CrossRef]
- Patzschke, C.F.; Parkinson, B.; Willis, J.J.; Nandi, P.; Love, A.M.; Raman, S.; Hellgardt, K. Co-Mn catalysts for H2 production via methane pyrolysis in molten salts. Chem. Eng. J. 2021, 414, 128730. [Google Scholar] [CrossRef]
- Noh, Y.G.; Lee, Y.J.; Kim, J.; Kim, Y.K.; Ha, J.S.; Kalanur, S.S.; Seo, H. Enhanced efficiency in CO2-free hydrogen production from methane in a molten liquid alloy bubble column reactor with zirconia beads. Chem. Eng. J. 2022, 428, 131095. [Google Scholar] [CrossRef]
- Dagle, R.A.; Dagle, V.; Bearden, M.; Holladay, J.; Krause, T.; Ahmed, S. An Overview of Natural Gas Conversion Technologies for Co-Production of Hydrogen and Value-Added Solid Carbon Products; Argonne National Laboratory: Lemont, IL, USA, 2017. [Google Scholar] [CrossRef]
- BASF Report. Innovative Processes for Climate-Smart Chemistry. 2021. Available online: https://report.basf.com/2021/en/shareholders/basf-on-the-capital-market/methane-pyrolysis.html (accessed on 27 June 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).