A Critical Analysis of the Oxy-Combustion Process: From Mathematical Models to Combustion Product Analysis
Abstract
:1. Introduction
- Post-combustion systems, which involve the separation of carbon dioxide by physical or chemical processes (for example adsorption in organic compounds or solid matrices with a 7–10% efficiency [11]). This process has the advantage of producing a carbon-free H2-based fuel, but it requires additional cost for syngas generation, and it is more expensive compared to other methods [12,13,14].
- Pre-combustion processes, which provide the separation of CO2 from process gases before the combustion. At the end of the process, rich gases in carbon dioxide and water vapor are obtained. The vapor is removed from the recirculating gases and only CO2 is obtained, which is then compressed with an 8–12% efficiency [15]. This process generates minimum changes in the original configuration, but has a high NOx production and low CO2 purity in the flue gas [16,17].
- Oxy-combustion is a technique of burning fuel using pure oxygen, resulting in higher temperatures, lower fuel use, and higher CO2 concentration. It is particularly promising, given the possibility of integrating this technology with other systems mentioned above. In fact, it increases the convective and radiative heat transfer, produces a highly concentrated CO2 stream, and a low NOx production [18] with a 7–11% of efficiency penalty [19].
- Emerging technologies, such as membrane separation, chemical looping combustion, carbonation–calcination cycles, mineralization, etc. The use of these methods reduces energy consumption and consequently the energy penalty of carbon capture from power plants [20,21], but the emerging technologies were not demonstrated at sufficient scales for industrialization.
2. Status of Oxy-Combustion
- the high-pressure operation causes the temperature condensation of water steam in the exhaust gas to be higher, resulting in more efficient latent heat recovery [28];
- the energy required for CCS is reduced because CO2 is delivered at high pressure [29];
- it is possible to decrease the size of the heat exchanger because the exhaust gases have a higher convective heat transfer coefficient [5].
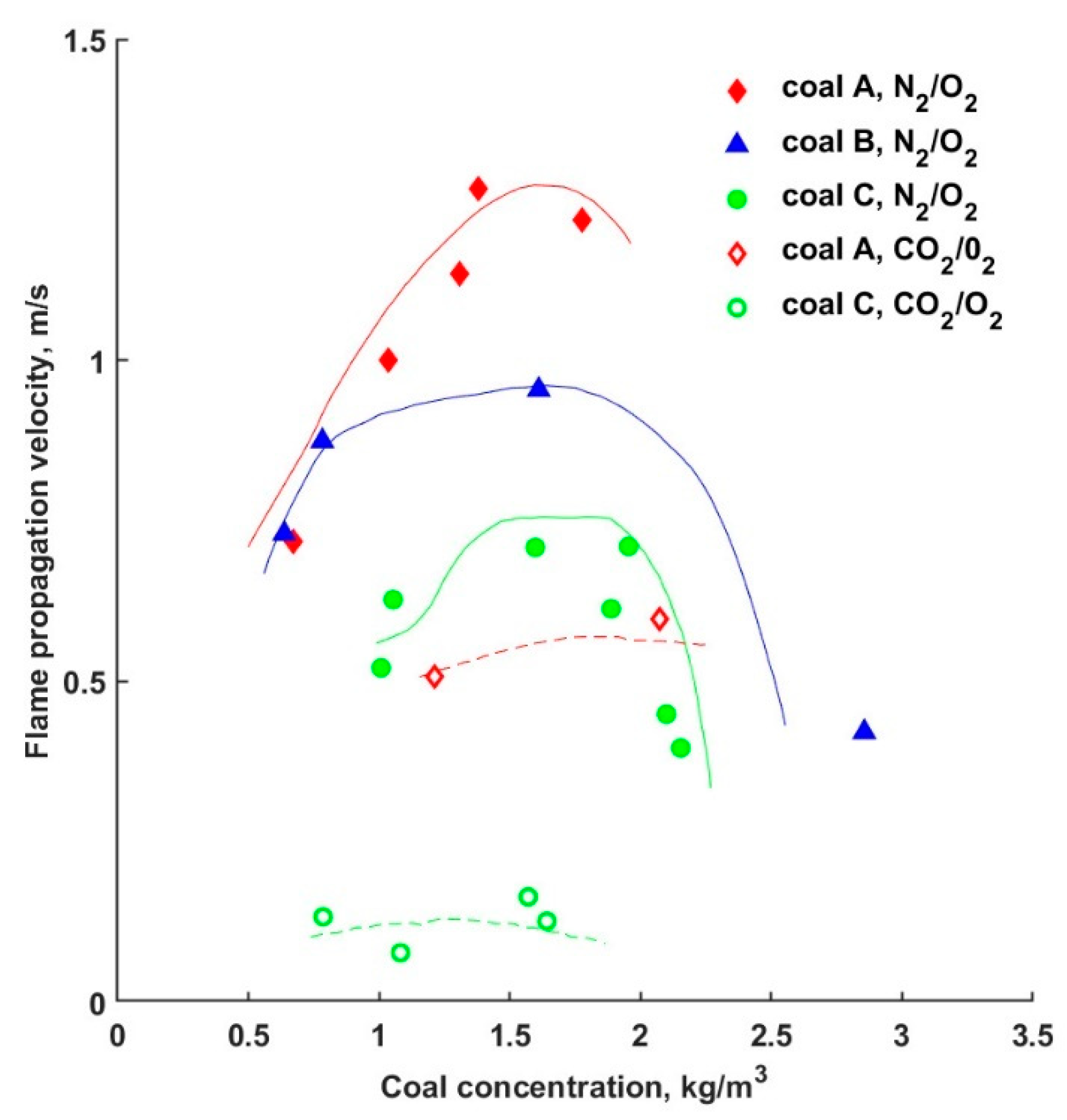
- The concentration of oxygen increases in the process products, while the CO emission decreases. This phenomenon slows down the propagation of the flame in oxyfuel compared to air combustion;
- the O2 purity has no significant effects on the flame temperature;
- wet/dry recirculation;
- burner performance.
3. Mathematical Models
- A single-step scheme that used a single reaction to describe the oxidation of the fuel by the oxidant:
- A two-phase system using carbon dioxide as the medium reaction product. This mechanism is governed by the following two reactions:
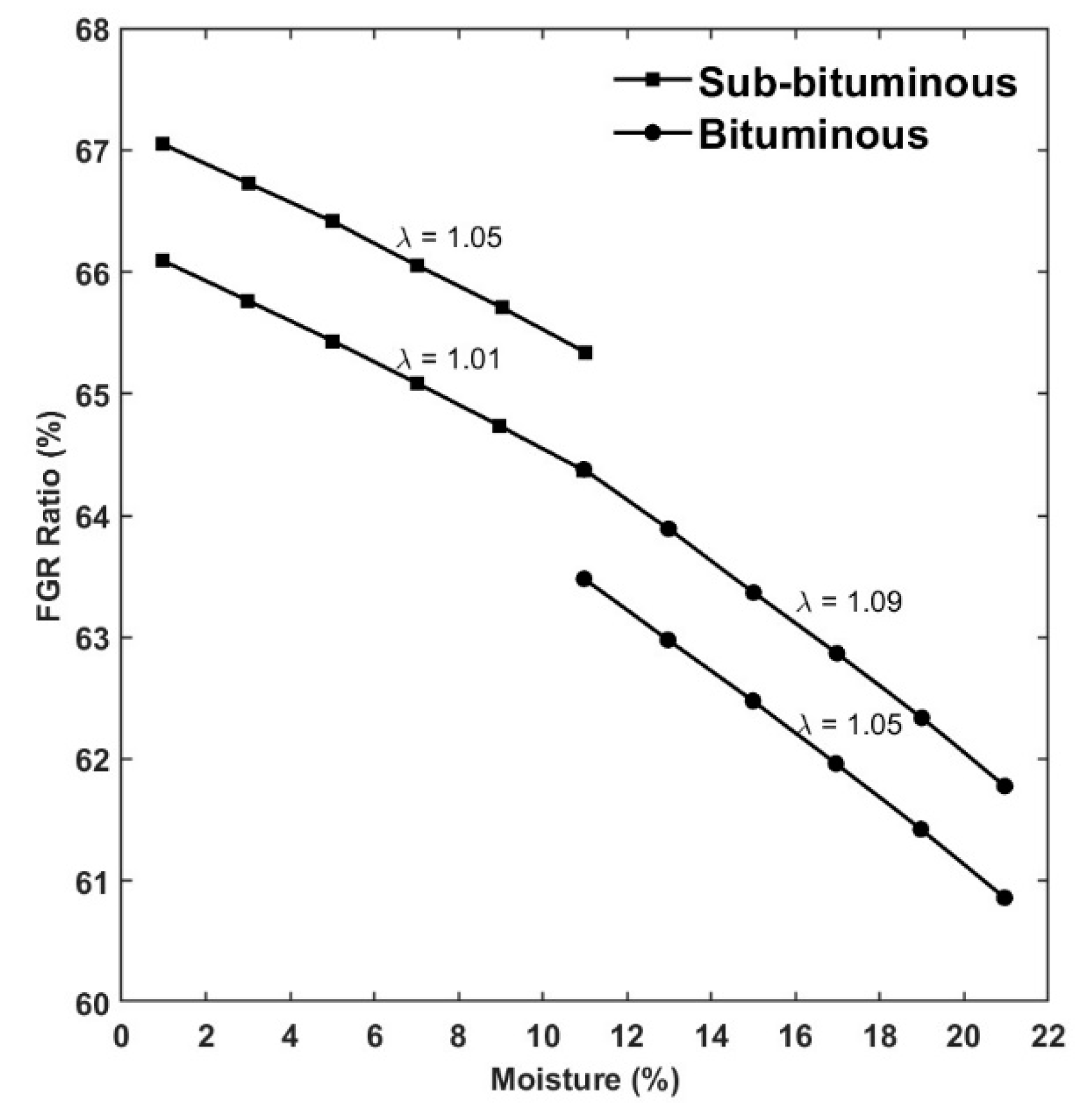
4. Recycles Ratio and Analysis of Flues Gas Recycled (FGR)
- With oxygen addition before coal mills: but only if there is sufficient space in the recirculation duct to ensure proper mixing, otherwise the risk of combustion or explosion increases because primary flux may even cool down due to the low temperature of the incoming oxygen.
- With oxygen addition after mills, but before the burners: it is possible to obtain the same oxygen concentration in the burners, but pure oxygen is likely to come into contact with the recirculated gases at high temperature.
- Oxygen addition directly in burners: there is not an explosion in fuel gas ducts and the oxygen addition, and this would result in a better mixing of fuel and combustion agent in the area close to the near-burner zone.
5. Ignition and Flame Propagation
6. Heat Transfer
- air combustion: 20% oxygen;
- oxyfuel with wet recycle: 23.5–27% oxygen;
- oxyfuel with dry recycle: 25–35%.
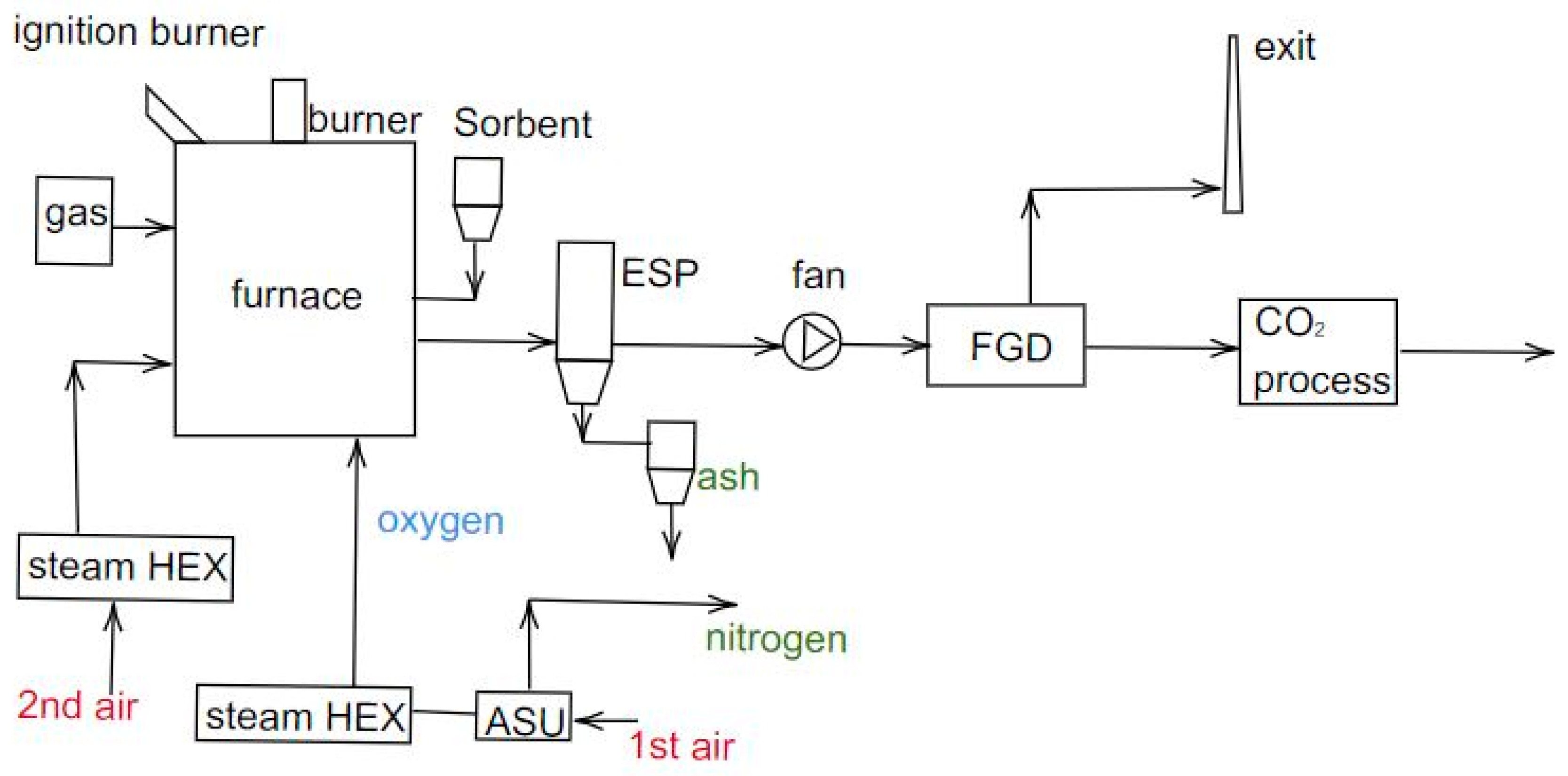
7. Oxygen Separation Methods
- the first method consists in using the energy produced by the power plant as input for the PSA system;
- the second method is to use both electricity and waste heat from the power plants at a high temperature (about 90 °C) for the separation unit;
- the third method involves using electricity and waste heat from the power plant at low temperatures (around 40 °C).
8. Emissions
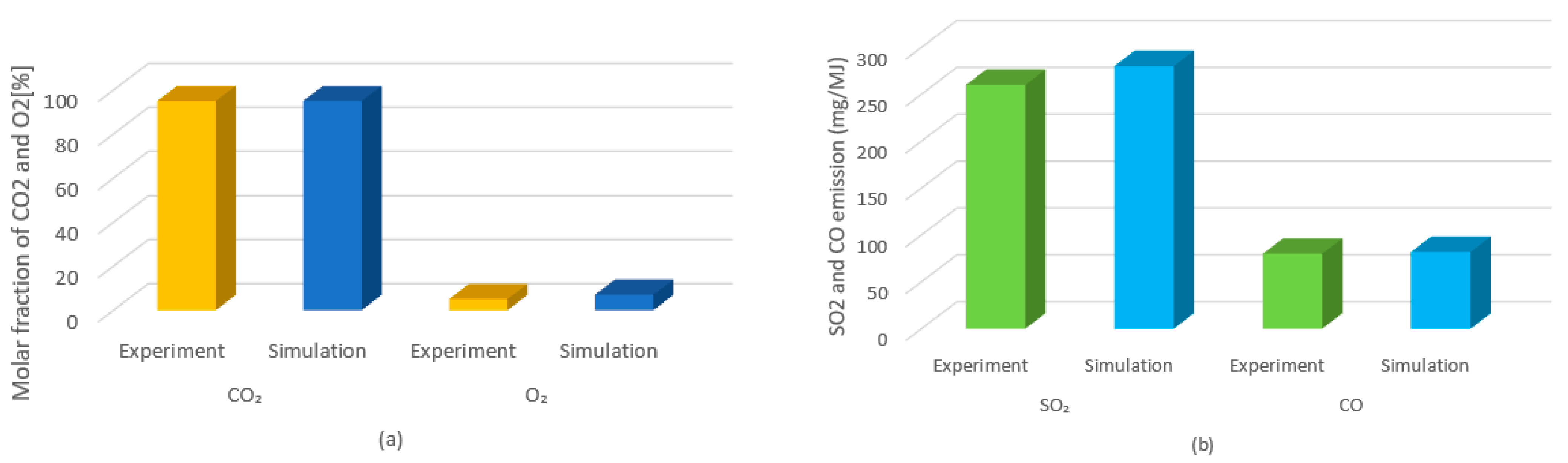
8.1. CO2, NOx and SOx Emissions
- the oxidant has SO2 and increases during combustion;
- the oxidant has oxygen, which decreases the flow in the chamber and increases SO3;
- the replacement of N2 with CO2 increases the SO2/SO3.
8.2. Ash
8.3. Corrosion
8.4. Mercury
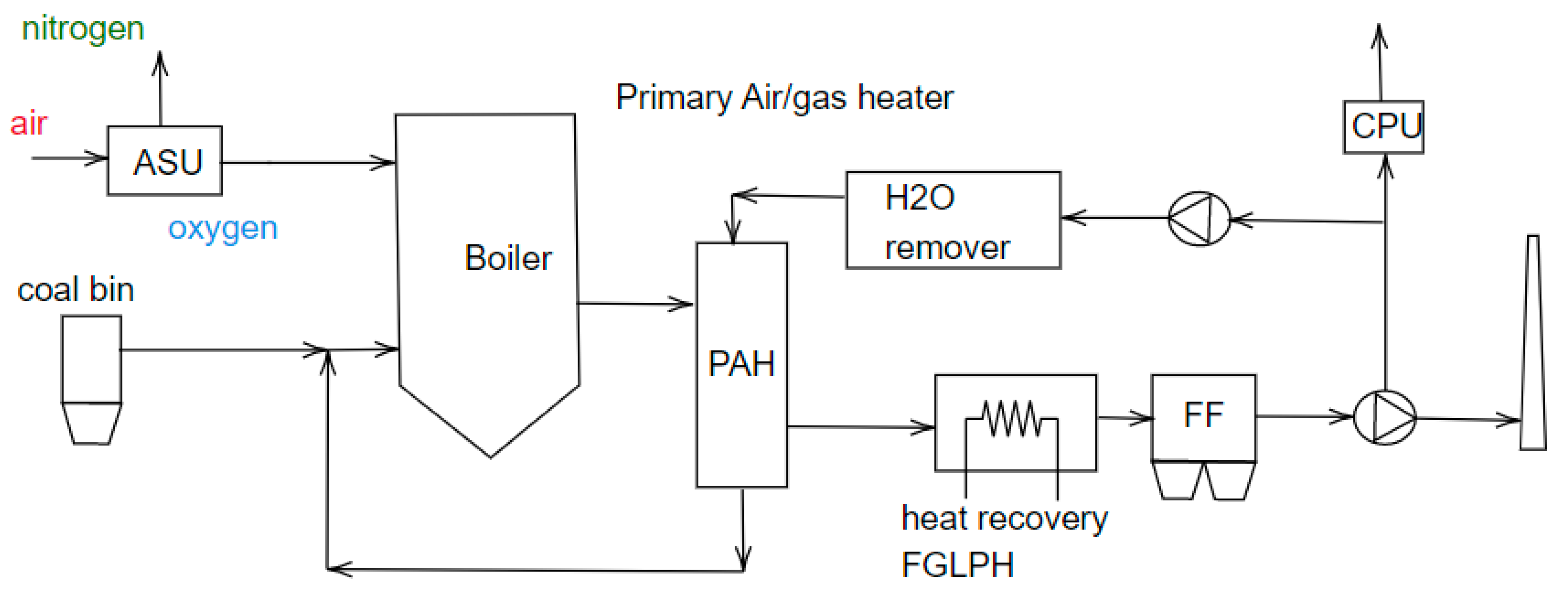
9. Industrial-Scale Pilot Project
10. Conclusions and Future Perspectives
- the possibility to retrofit an existing plant as either oxyfuel with CO2 liquefaction or flue gas liquefaction;
- modify the technology of an existing process;
- oxy-combustion is near-zero-emissions and it has low NOx emissions compared to conventional PF technology.
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Nomenclature
| AFT | adiabatic flame temperature |
| EDC | eddy dissipation concept |
| EES | engineering equation solver |
| CCS | carbon capture sequestration |
| CFD | computational fluid dynamic |
| CLOU | chemical-looping with oxygen uncoupling |
| FGC | flue gas condenser |
| FGD | flue gas desulphurization |
| IFRF | International Flames Research Foundation |
| ITM | ion transport membrane |
| OF25 | oxygen fraction 25% |
| OF27 | oxygen fraction 27% |
| OF29 | oxygen fraction 29% |
| PF | purification technologies |
| PSA | pressure swing adsorption |
| PTSA | pressure temperature swing adsorption |
| RFG | recycled fuel gas |
| VSA | vacuum swing adsorption |
References
- Buhre, B.; Elliott, L.; Sheng, C.; Gupta, R.; Wall, T. Oxy-Fuel combustion technology for coal-Fired power generation. Prog. Energy Combust. Sci. 2005, 31, 283–307. [Google Scholar] [CrossRef]
- Wall, T.F. Combustion processes for carbon capture. Proc. Combust. Inst. 2007, 31, 31–47. [Google Scholar] [CrossRef]
- Available online: www.iea.org (accessed on 2 February 2022).
- Perrone, D. A study of oxy-Coal combustion with wet recycle using CFD modeling. In Energy Procedia, Proceedings of the ATI 2015-70th Conference of the ATI Engineering Association, Naples, Italy, 17–20 May 2015; Elsevier: Amsterdam, The Netherlands, 2015; Volume 82, pp. 900–907. [Google Scholar]
- Zheng, L. Oxy-Fuel Combustion for Power Generation and Carbon Dioxide (CO2) Capture; Elsevier: Sawston, Cambridge, 2011. [Google Scholar]
- Hashmi, R.; Alam, K. Dynamic relationship among environmental regulation, innovation, CO2 emissions, population, and economic growth in OECD countries: A panel investigation. J. Clean. Prod. 2019, 231, 1100–1109. [Google Scholar] [CrossRef]
- Baena-Moreno, F.M.; Rodríguez-Galán, M.; Vega, F.; Alonso-Fariñas, B.; Vilches Arenas, L.F.; Navarrete, B. Carbon capture and utilization technologies: A literature review and recent advances. Energy Sources Part A Recovery Util. Environ. Effects. 2019, 41, 1403–1433. [Google Scholar] [CrossRef]
- Koohestanian, E.; Shahraki, F. Review on principles, recent progress, and future challenges for oxy-Fuel combustion CO2 capture using compression and purification unit. J. Environ. Chem. Eng. 2021, 9, 105777. [Google Scholar] [CrossRef]
- Rolfe, A.; Huang, Y.; Haaf, M.; Pita, A.; Rezvani, S.; Dave, A.; Hewitt, N. Technical and environmental study of calcium carbonate looping versus oxy-Fuel options for low CO2 emission cement plants. Int. J. Greenh. Gas Control 2018, 75, 85–97. [Google Scholar] [CrossRef]
- Madejski, P.; Chmiel, K.; Subramanian, N.; Kuś, T. Methods and techniques for CO2 capture: Review of potential solutions and applications in modern energy technologies. Energies 2022, 15, 887. [Google Scholar] [CrossRef]
- Martelli, E.; Kreutz, T.; Carbo, M.; Consonni, S.; Jansen, D. Shell coal IGCCS with carbon capture: Conventional gas quench vs. innovative configurations. Appl. Energy 2011, 88, 3978–3989. [Google Scholar] [CrossRef]
- Spallina, V.; Gallucci, F.; Romano, M.C.; Annaland, M.V.S. Pre-Combustion packed bed chemical looping (PCCL) technology for efficient H2-Rich gas production processes. Chem. Eng. J. 2016, 294, 478–494. [Google Scholar] [CrossRef]
- Jansen, D.; Gazzani, M.; Manzolini, G.; van Dijk, E.; Carbo, M. Pre-Combustion CO2 capture. Int. J. Greenh. Gas Control. 2015, 40, 167–187. [Google Scholar] [CrossRef] [Green Version]
- Nemitallah, M.A.; Habib, M.A.; Badr, H.M.; Said, S.A.; Jamal, A.; Ben-Mansour, R.; Mokheimer, E.M.A.; Mezghani, K. Oxy-Fuel combustion technology: Current status, applications, and trends. Int. J. Energy Res. 2017, 41, 1670–1708. [Google Scholar] [CrossRef]
- Goto, K.; Yogo, K.; Higashii, T. A review of efficiency penalty in a coal-Fired power plant with post-Combustion CO2 capture. Appl. Energy 2013, 111, 710–720. [Google Scholar] [CrossRef]
- Canepa, R.; Wang, M. Techno-Economic analysis of a CO2 capture plant integrated with a commercial scale combined cycle gas turbine (CCGT) power plant. Appl. Therm. Eng. 2015, 74, 10–19. [Google Scholar] [CrossRef]
- Benhelal, E.; Zahedi, G.; Shamsaei, E.; Bahadori, A. Global strategies and potentials to curb CO2 emissions in cement industry. J. Clean. Prod. 2013, 51, 142–161. [Google Scholar] [CrossRef]
- Yang, X.; Clements, A.; Szuhánszki, J.; Huang, X.; Moguel, O.F.; Li, J.; Gibbins, J.; Liu, Z.; Zheng, C.; Ingham, D.; et al. Prediction of the radiative heat transfer in small and large scale oxy-Coal furnaces. Appl. Energy 2018, 211, 523–537. [Google Scholar] [CrossRef]
- Escudero, A.I.; Espatolero, S.; Romeo, L.M. Oxy-Combustion power plant integration in an oil refinery to reduce CO2 emissions. Int. J. Greenh. Gas Control 2015, 45, 118–129. [Google Scholar] [CrossRef]
- Figueroa, J.D.; Fout, T.; Plasynski, S.; McIlvried, H.; Srivastava, R.D. Advances in CO2 capture technology—The US Department of Energy’s Carbon Sequestration Program. Int. J. Greenh. Gas Control 2008, 2, 9–20. [Google Scholar] [CrossRef]
- Abu-Khader, M.M. Recent progress in CO2 capture/sequestration: A review. Energy Sources Part A 2006, 28, 1261–1279. [Google Scholar] [CrossRef]
- Rubin, E.S.; Mantripragada, H.; Marks, A.; Versteeg, P.; Kitchin, J. The outlook for improved carbon capture technology. Prog. Energy Combust. Sci. 2012, 38, 630–671. [Google Scholar] [CrossRef]
- IEA. International Energy Agency, Medium-Term Coal Market Report 2014; IEA: Paris, France, 2014; ISBN 978-92-64-22188-8. [Google Scholar]
- Horn, F.L.; Steinberg, M. Control of carbon dioxide emissions from a power plant (and use in enhanced oil recovery). Fuel 1982, 61, 415–422. [Google Scholar] [CrossRef]
- Wall, T.; Liu, Y.; Spero, C.; Elliott, L.; Khare, S.; Rathnam, R.; Zeenathal, F.; Moghtaderi, B.; Buhre, B.; Sheng, C.; et al. An overview on oxyfuel coal combustion—State of the art research and technology development. Chem. Eng. Res. Des. 2009, 87, 1003–1016. [Google Scholar] [CrossRef]
- Simmonds, M.; Miracca, I.; Gerdes, K. Oxyfuel technologies for CO2 capture: A techno-Economic overview. In Greenhouse Gas Control Technologies 7; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2005; pp. 1125–1130. [Google Scholar]
- Dobó, Z.; Backman, M.; Whitty, K.J. Experimental study and demonstration of pilot-Scale oxy-Coal combustion at elevated temperatures and pressures. Appl. Energy 2019, 252, 113450. [Google Scholar] [CrossRef]
- Stanger, R.; Wall, T.; Spörl, R.; Paneru, M.; Grathwohl, S.; Weidmann, M.; Scheffknecht, G.; McDonald, D.; Myöhänen, K.; Ritvanen, J.; et al. Oxyfuel combustion for CO2 capture in power plants. Int. J. Greenh. Gas Control 2015, 40, 55–125. [Google Scholar] [CrossRef]
- Yin, C.; Yan, J. Oxy-Fuel combustion of pulverized fuels: Combustion fundamentals and modeling. Appl. Energy 2016, 162, 742–762. [Google Scholar] [CrossRef]
- Normann, F.; Thunman, H.; Johnsson, F. Process analysis of an oxygen lean oxy-Fuel power plant with co-Production of synthesis gas. Energy Convers. Manag. 2009, 50, 279–286. [Google Scholar] [CrossRef]
- Croiset, E.; Thambimuthu, K.V. Coal combustion with flue gas recirculation for CO2 recovery. In Proceedings of the 4th International Conference on Greenhouse Gas Control Technologies, Oxford, UK, 1 August 1999. [Google Scholar]
- Croiset, E.; Thambimuthu, K.; Palmer, A. Coal combustion in O2/CO2 mixtures compared with air. Can. J. Chem. Eng. 2000, 78, 402–407. [Google Scholar] [CrossRef]
- Westbrook, C.K.; Dryer, F.L. Simplified reaction mechanisms for the oxidation of hydrocarbon fuels in flames. Combust. Sci. Technol. 1981, 27, 31–43. [Google Scholar] [CrossRef]
- Westbrook, C.K.; Dryer, F.L. Chemical kinetic modeling of hydrocarbon combustion. Prog. Energy Combust. Sci. 1984, 10, 1–57. [Google Scholar] [CrossRef]
- Jones, W.; Lindstedt, R. Global reaction schemes for hydrocarbon combustion. Combust. Flame 1988, 73, 233–249. [Google Scholar] [CrossRef]
- Andersen, J.; Rasmussen, C.L.; Giselsson, T.; Glarborg, P. Global combustion mechanisms for use in CFD modeling under oxy-Fuel conditions. Energy Fuels 2009, 23, 1379–1389. [Google Scholar] [CrossRef]
- Várhegyi, G.; Szabó, P.; Jakab, E.; Till, F.; Richard, J.R. Mathematical modeling of char reactivity in Ar−O2 and CO2−O2 mixtures. Energy Fuels 1996, 10, 1208–1214. [Google Scholar] [CrossRef]
- Várhegyi, G.; Till, F. Comparison of temperature programmed char combustion in CO2-O2 and Ar-O2 mixtures at elevated pressure. Energy Fuels 1999, 13, 539–540. [Google Scholar] [CrossRef]
- Smart, J.; O’Nions, P.; Riley, G. Radiation and convective heat transfer, and burnout in oxy-Coal combustion. Fuel 2010, 89, 2468–2476. [Google Scholar] [CrossRef]
- Tan, Y.; Croiset, E.; Douglas, M.A.; Thambimuthu, K.V. Combustion characteristics of coal in a mixture of oxygen and recycled flue gas. Fuel 2006, 85, 507–512. [Google Scholar] [CrossRef]
- Toftegaard, M.B.; Brix, J.; Jensen, P.A.; Glarborg, P.; Jensen, A.D. Oxy-Fuel combustion of solid fuels. Prog. Energy Combust. Sci. 2010, 36, 581–625. [Google Scholar] [CrossRef]
- Choi, S.K.; Kim, J.S.; Chung, S.H. Structure of the edge flame in a methane–Oxygen mixing layer. Combust. Theory Model. 2009, 13, 39–56. [Google Scholar] [CrossRef]
- Yin, C.; Rosendahl, L.A.; Kær, S.K. Chemistry and radiation in oxy-Fuel combustion: A computational fluid dynamic modeling study. Fuel 2011, 90, 2519–2529. [Google Scholar] [CrossRef]
- Chen, L.; Yong, S.Z.; Ghoniem, A.F. Oxy-Fuel combustion of pulverized coal: Characterization, fundamentals, stabilization and CFD modeling. Prog. Energy Combust. Sci. 2012, 38, 156–214. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, Z.; Xiang, J.; Zhang, L.; Zhang, S.; Luo, C.; Zhao, Y. Fundamental and technical challenges for a compatible design scheme of oxyfuel combustion technology. Engineering 2015, 1, 139–149. [Google Scholar] [CrossRef]
- Becher, V.; Bohn, J.P.; Dias, P.; Spliethoff, H. Validation of spectral gas radiation models under oxyfuel conditions—Part B: Natural gas flame experiments. Int. J. Greenh. Gas Control 2011, 5, S66–S75. [Google Scholar] [CrossRef]
- Al-Abbas, A.H.; Naser, J. CFD modelling of air-Fired and oxy-Fuel combustion in a 100 kW unit firing propane. In Proceedings of the International Conference on Mechanical Engineering 2011, Dhaka, Bangladesh, 18–20 December 2011. [Google Scholar]
- Andersson, K.; Johnsson, F. Flame and radiation characteristics of gas fired O2/CO2 combustion. Fuel 2007, 86, 656–668. [Google Scholar] [CrossRef]
- Hjärtstam, S.; Andersson, K.; Johnsson, F.; Leckner, B. Combustion characteristics of lignite-Fired oxy-Fuel flames. Fuel 2009, 88, 2216–2224. [Google Scholar] [CrossRef]
- Riaza, J.; Álvarez, L.; Gil, M.V.; Pevida, C.; Pis, J.J.; Rubiera, F. Effect of oxy-Fuel combustion with steam addition on coal ignition and burnout in an entrained flow reactor. Energy 2011, 36, 5314–5319. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, X.; Han, X.; Wang, H. Combustion characteristics of fine-And micro-Pulverized coal in the mixture of O2/CO2. Energy Fuels 2008, 22, 3756–3762. [Google Scholar] [CrossRef]
- Khare, S. Heat Transfer in Pulverised air Furnaces Adapted to Oxyfuel. Master’s Thesis, Universiy of Newcastle, Newcastle, Australia, 2008. [Google Scholar]
- Khare, S.P.; Wall, T.F.; Farida, A.Z.; Liu, Y.; Moghtaderi, B.; Gupta, R.P. Factors influencing the ignition of flames from air-Fired swirl of burners retrofitted to oxy-Fuel. Fuel 2008, 87, 1042–1049. [Google Scholar] [CrossRef]
- Bejarano, P.A.; Levendis, Y.A. Combustion of coal chars in oxygen-Enriched atmospheres. Combust. Sci. Technol. 2007, 179, 1569–1587. [Google Scholar] [CrossRef]
- Levendis, Y.A.; Bejerano, P.A. Reaction times of burning bituminous chars at high O2 partial pressures. In Proceedings of the 31st International Technical Conference on Coal Utilization & Fuel Systems, Clearwater, FL, USA, 21–26 May 2006; Sakkestad, B.A., Ed.; Coal Technology Association: Gaithersburg, MD, USA, 2006; Volume I, pp. 145–157. [Google Scholar]
- Molina, A.; Shaddix, C.R. Effect of O2/CO2-Firing on Coal Particle Ignition; Sandia National Laboratories (SNL): Albuquerque, NM, USA; Livermore, CA, USA, 2005. [Google Scholar]
- Suda, T.; Masuko, K.; Sato, J.I.; Yamamoto, A.; Okazaki, K. Effect of carbon dioxide on flame propagation of pulverized coal clouds in CO2/O2 combustion. Fuel 2007, 86, 2008–2015. [Google Scholar] [CrossRef]
- Tahir, F.; Ali, H.; Baloch, A.A.; Jamil, Y. Performance Analysis of Air and Oxy-Fuel Laminar Combustion in a Porous Plate Reactor. Energies 2019, 12, 1706. [Google Scholar] [CrossRef]
- Kangwanpongpan, T.; Klatt, M.; Krautz, H.J. Challenges of oxyfuel combustion modeling for carbon capture. In EGU General Assembly Conference Abstracts; EGU General Assembly: Vienna, Austria, 2012; p. 9859. [Google Scholar]
- Schiemann, M.; Scherer, V.; Wirtz, S. Optical coal particle temperature measurement under oxy-Fuel conditions: Measurement methodology and initial results. Chem. Eng. Technol. Ind. Chem.-Plant Equip.-Process Eng.-Biotechnol. 2009, 32, 2000–2004. [Google Scholar] [CrossRef]
- Andersson, K.; Johansson, R.; Johnsson, F. Thermal radiation in oxy-Fuel flames. Int. J. Greenh. Gas Control 2011, 5, S58–S65. [Google Scholar] [CrossRef]
- Andersson, K.; Johansson, R.; Johnsson, F.; Leckner, B. Radiation intensity of propane-Fired oxy-Fuel flames: Implications for soot formation. Energy Fuels 2008, 22, 1535–1541. [Google Scholar] [CrossRef]
- Black, S.; Szuhánszki, J.; Pranzitelli, A.; Ma, L.; Stanger, P.J.; Ingham, D.B.; Pourkashanian, M. Effects of firing coal and biomass under oxy-Fuel conditions in a power plant boiler using CFD modelling. Fuel 2013, 113, 780–786. [Google Scholar] [CrossRef]
- Smart, J.P.; Patel, R.; Riley, G.S. Oxy-Fuel combustion of coal and biomass, the effect on radiative and convective heat transfer and burnout. Combust. Flame 2010, 157, 2230–2240. [Google Scholar] [CrossRef]
- Toporov, D.; Bocian, P.; Heil, P.; Kellermann, A.; Stadler, H.; Tschunko, S.; Foster, M.; Kneer, R. Detailed investigation of a pulverized fuel swirl flame in CO2/O2 atmosphere. Combust. Flame 2008, 155, 605–618. [Google Scholar] [CrossRef]
- Banaszkiewicz, T.; Chorowski, M.; Gizicki, W. Comparative analysis of oxygen production for oxy-Combustion application. In Energy Procedia, Proceedings of the 7th Trondheim CCS Conference, TCCS-7, Trondheim, Norway, 5–6 June 2013; Elsevier: Amsterdam, The Netherlands, 2014; Volume 51, pp. 127–134. [Google Scholar]
- Chorowski, M.; Gizicki, W.; Gromada, M. Aerobic Combustion for Dust and Fluidized Bed Combustion Integrated with CO2 Capture, Chapter 10: Oxygen Production for Aerobic Combustion; Nowak, W., Czakiert, T., Eds.; Wydawnictwo Politechniki Czstochowskiej: Czstochowa, Poland, 2012. [Google Scholar]
- Smith, A.R.; Klosek, J. A review of air separation technologies and their integration with energy conversion processes. Fuel Processing Technol. 2001, 70, 115–134. [Google Scholar] [CrossRef]
- Gellings, P.J. Handbook of Solid State Electrochemistry; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Sher, F.; Pans, M.A.; Sun, C.; Snape, C.; Liu, H. Oxy-Fuel combustion study of biomass fuels in a 20 kWth fluidized bed combustor. Fuel 2018, 215, 778–786. [Google Scholar] [CrossRef]
- Tranier, J.-P.; Dubettier, R.; Darde, A.; Perrin, N. Air separation, flue gas compression and purification units for oxy-Coal combustion systems. Energy Procedia 2011, 4, 966–971. [Google Scholar] [CrossRef]
- Castiglioni, R.; Croci, F. Fluid Dynamics Modelling of Natural Gas Oxyfuel Combustion. Master’s Thesis, Polytechnic of Milan, Milan, Italy, 2008. [Google Scholar]
- Nakayama, S.; Noguchi, Y.; Kiga, T.; Miyamae, S.; Maeda, U.; Kawai, M.; Ttanaka, T.; Koyata, K.; Makino, H. Pulverized coal combustion in O2/CO2 mixtures on a power plant for CO2 recovery. Energy Convers. Manag. 1992, 33, 379–386. [Google Scholar] [CrossRef]
- Wu, F.; Argyle, M.D.; Dellenback, P.A.; Fan, M. Progress in O2 separation for oxy-Fuel combustion—A promising way for cost-Effective CO2 capture: A review. Prog. Energy Combust. Sci. 2018, 67, 188–205. [Google Scholar] [CrossRef]
- Zhou, C.; Shah, K.; Song, H.; Zanganeh, J.; Doroodchi, E.; Moghtaderi, B. Integration options and economic analysis of an integrated chemical looping air separation process for oxy-Fuel combustion. Energy Fuels 2016, 30, 1741–1755. [Google Scholar] [CrossRef]
- Bensmann, B.; Hanke-Rauschenbach, R.; Arias, I.P.; Sundmacher, K. Energetic evaluation of high pressure PEM electrolyzer systems for intermediate storage of renewable energies. Electrochim. Acta 2013, 110, 570–580. [Google Scholar] [CrossRef]
- Bhandari, R.; Trudewind, C.A.; Zapp, P. Life cycle assessment of hydrogen production via electrolysis—A review. J. Clean. Prod. 2014, 85, 151–163. [Google Scholar] [CrossRef]
- Smolinka, T.; Günther, M.; Garche, J. Stand und Entwicklungs Potenzial der Wasserelektrolyse zur Herstellung vonWasserstoff aus Regenerativen Energien; Nationale Organisation Wasserstoff-Und Brennstoffzellen Technologie: Berlin, Germany, 2011. [Google Scholar]
- Habib, M.A.; Badr, H.M.; Ahmed, S.F.; Ben-Mansour, R.; Mezghani, K.; Imashuku, S.; la O’, G.J.; Shao-Horn, Y.; Mancini, N.D.; Mitsos, A.; et al. A review of recent developments in carbon capture utilizing oxy-Fuel combustion in conventional and ion transport membrane systems. Int. J. Energy Res. 2011, 35, 741–764. [Google Scholar] [CrossRef]
- Hou, S.S.; Chiang, C.Y.; Lin, T.H. Oxy-Fuel combustion characteristics of pulverized coal under O2/recirculated flue gas atmospheres. Appl. Sci. 2020, 10, 1362. [Google Scholar] [CrossRef]
- Liu, H.; Shao, Y. Predictions of the impurities in the CO2 stream of an oxy-Coal combustion plant. Appl. Energy 2010, 87, 3162–3170. [Google Scholar] [CrossRef]
- Kumar, R.R.; Elliott, L.; Moghtaderi, B.; Gupta, R.; Wall, T. Differences in Coal Reactivity in Air and Oxy-Fuel Conditions and Implications for Coal Burnout. The Clearwater Coal Conference: The 31st International Technical Conference on Coal Utilization and Fuel Systems. Coal Technol. Whats Next 2006, 1, 21–26. [Google Scholar]
- Okazaki, K.; Ando, T. NOx reduction mechanism in coal combustion with recycled CO2. Energy 1997, 22, 207–215. [Google Scholar] [CrossRef]
- Hu, Y.; Naito, S.; Kobayashi, N.; Hasatani, M. CO2, NOx and SO2 emissions from the combustion of coal with high oxygen concentration gases. Fuel 2000, 79, 1925–1932. [Google Scholar] [CrossRef]
- Croiset, E.; Thambimuthu, K.V. NOx and SO2 emissions from O2/CO2 recycle coal combustion. Fuel 2001, 80, 2117–2121. [Google Scholar] [CrossRef]
- Al-Abbas, A.H.; Naser, J.; Dodds, D.; Blicblau, A. Numerical modelling of oxy-Fuel combustion in a full-Scale tangentially-Fired pulverised coal boiler. Procedia Eng. 2013, 56, 375–380. [Google Scholar] [CrossRef]
- Hu, Y.Q.; Kobayashi, N.; Hasatani, M. The reduction of recycled-NOx in coal combustion with O2/recycled flue gas under low recycling ratio. Fuel 2001, 80, 1851–1855. [Google Scholar] [CrossRef]
- Czakiert, T.; Bis, Z.; Muskala, W.; Nowak, W. Fuel conversion from oxy-Fuel combustion in a circulating fluidized bed. Fuel Processing Technol. 2006, 87, 531–538. [Google Scholar] [CrossRef]
- Czakiert, T.; Sztekler, K.; Karski, S.; Markiewicz, D.; Nowak, W. Oxy-Fuel circulating fluidized bed combustion in a small pilot-Scale test rig. Fuel Processing Technol. 2010, 91, 1617–1623. [Google Scholar] [CrossRef]
- Beigzadeh, A.; Shafeen, A.; Abbassi, M.; Salvador, C.; Zanganeh, K.E. Optimized Multi-Pollutant Control in Oxy-Fuel Combustion Systems using CO2 Capture and Compression Process. Energy Procedia 2014, 63, 8134–8143. [Google Scholar] [CrossRef]
- Andersson, K.; Normann, F.; Johnsson, F.; Leckner, B. NO emission during oxy-Fuel combustion of lignite. Ind. Eng. Chem. Res. 2008, 47, 1835–1845. [Google Scholar] [CrossRef]
- Wang, F.; Shen, B.; Yang, J.; Singh, S. Review of mercury formation and capture from CO2-Enriched oxy-Fuel combustion flue gas. Energy Fuels 2017, 31, 1053–1064. [Google Scholar] [CrossRef]
- Santos, S. Fate of Sulphur in Coal during Oxyfuel Combustion with Recycled Flue Gas (Review of the Current State of uNDERSTANDING), IEC Gasification Conference Publication, IEA Greenhouse Gas R&D Programme. 2009. Available online: http://tu-freiberg.de/sites/default/files/media/professur-fuer-energieverfahrenstechnik-und-thermische-rueckstandsbehandlung-16460/publikationen/2009_ssantos-1.pdf (accessed on 24 February 2022).
- Fleig, D.; Normann, F.; Andersson, K.; Johnsson, F.; Leckner, B. The fate of sulphur during oxy-Fuel combustion of lignite. Energy Procedia 2009, 1, 383–390. [Google Scholar] [CrossRef]
- Gu, J.; Shao, Y.; Zhong, W. Study on oxy-Fuel combustion behaviors in a S-CO2 CFB by 3D CFD simulation. Chem. Eng. Sci. 2020, 211, 115262. [Google Scholar] [CrossRef]
- Scheffknecht, G.; Al-Makhadmeh, L.; Schnell, U.; Maier, J. Oxy-Fuel coal combustion—A review of the current state-of-The-Art. Int. J. Greenh. Gas Control 2011, 5, S16–S35. [Google Scholar] [CrossRef]
- Suriyawong, A.; Skeen, S.; Axelbaum, R.L.; Biswas, P. Experimental and modeling study on particle size distribution effects due to oxy-Combustion of coal. Environment and Sustainable Development. In Proceedings of the 23rd Annual International Pittsburgh Coal Conference, PCC-Coal-Energy, Pittsburgh, PA, USA, 25–28 September 2006. [Google Scholar]
- Liu, H.; Okazaki, K. Simultaneous easy CO2 recovery and drastic reduction of SOx and NOx in O2/CO2 coal combustion with heat recirculation. Fuel 2003, 82, 1427–1436. [Google Scholar] [CrossRef]
- Kull, R.; Maier, J.; Mönckert, P.; Scheffknecht, G.; Hjörnhede, A.; Anheden, M. 2008 Summary Report on Fly Ash, Deposit, Slagging and Corrosion under Oxyfuel Conditions in a 500 kW Test Rig. ENCAP Project. Available online: http://www.encapco2.or (accessed on 24 February 2022).
- Schulz, W.; Huenert, D.; Nitschke, H.; Saliwan-Neumann, R.; Kranzmann, A. Comparison of the corrosion behaviour of 9–12% Cr steels in H2O, H2O-CO2 and H2O-CO2-O2. In CORROSION 2009; OnePetro: Atlanta, Georgia, 2009. [Google Scholar]
- Covino, B.S.; Matthes, S.A.; Bullard, S.J. Effect of oxyfuel combustion on superheater corrosion. In Proceedings of the CORROSION 2008, New Orleans, LA, USA, 6–20 March 2008. [Google Scholar]
- Stanger, R.; Wall, T. Sulphur impacts during pulverised coal combustion in oxy-Fuel technology for carbon capture and storage. Prog. Energy Combust. Sci. 2011, 37, 69–88. [Google Scholar] [CrossRef]
- Rathnam, R.K.; Wall, T.; Moghtaderi, B. Reactivity of pulverized coals and their chars in oxyfuel (O2/CO2) and air (O2/N2) conditions. In Proceedings of the 3rd Oxyfuel Combustion Conference, Ponferrada, Spain, 9–13 September 2013. [Google Scholar]
- Edge, P.; Gharebaghi, M.; Irons, R.; Porter, R.; Porter, R.T.J.; Pourkashanian, M.; Smith, D.; Stephenson, P.; Williams, A. Combustion modelling opportunities and challenges for oxy-Coal carbon capture technology. Chem. Eng. Res. Des. 2011, 89, 1470–1493. [Google Scholar] [CrossRef]
- Mcdonald, D.K.; Farzan, H.; Varagani, R.; Docquier, N.; Perrin, N. Evaluation of Oxy-Coal Combustion at a 30 MWth Pilot. In Proceedings of the 1st International Oxyfuel Combustion Conference, Cottbus, Germany, 7–11 September 2009. [Google Scholar]
- Zheng, L.; Furimsky, E. Assessment of coal combustion in O2+ CO2 by equilibrium calculations. Fuel Processing Technol. 2003, 81, 23–34. [Google Scholar] [CrossRef]
- Gharebaghi, M.; Goh, B.; Jones, J.M.; Ma, L.; Pourkashanian, M.; Williams, A. Assessment of fate of mercury in oxy-Coal combustion. In Proceedings of the 1st Oxyfuel Combustion Conference, Cottbus, Germany, 7–11 September 2009; pp. 8–11. [Google Scholar]
- Gharebaghi, M.; Hughes, K.J.; Porter, R.T.J.; Pourkashanian, M.; Williams, A. Mercury speciation in air-Coal and oxy-Coal combustion: A modelling approach. Proc. Combust. Inst. 2011, 33, 1779–1786. [Google Scholar] [CrossRef]
- Fernández-Miranda, N.; Rodríguez, E.; Lopez-Anton, M.A.; García, R.; Martínez-Tarazona, M.R. Un nuovo approccio per trattenere il mercurio nei processi di generazione di energia: Assorbenti carboniosi rigenerabili. Energie 2017, 10, 1311. [Google Scholar] [CrossRef]
- Burchhardt, U.; Giering, R.; Weiß, G. Overview of Burner tests in Vattenfall’s Oxyfuel Pilot Plant. In Proceedings of the 3rd Oxyfuel Combustion Conference, Ponferrada, Spain, 10 September 2013. [Google Scholar]
- Spero, C. Oxyfuel project: Development status. In Proceedings of the 5th Oxyfuel Capacity Building Course, Ponferrada, Spain, 8–9 September 2013; Volume 15. [Google Scholar]
- Spero, C.; Yamada, T.; Nelson, P.; Morrison, T.; Bourhy-Weber, C. Callide oxyfuel project: Combustion and environmental performance. In Proceedings of the 3rd Oxyfuel Combustion Conference, Ponferrada, Spain, 9–13 September 2013; Volume 19. [Google Scholar]
- Spero, C.; Yamada, T.; Rees, G. Callide oxyfuel project: Overview of the commissioning experience. In Proceedings of the 3rd Oxyfuel Combustion Conference, Ponferrada, Spain, 10–12 September 2013. [Google Scholar]
- Uchida, T. Callide oxyfuel project: Boiler retrofit design. In Proceedings of the 2nd IEA Oxyfuel Combustion, Queensland, Australia, 12–16 September 2011. [Google Scholar]
- Sotacarbo. The CO2 technology Centre of Sulcis R&D program on carbon capture, utilization, and storage. In Proceedings of the CCUS Conference, Pittsburgh, PA, USA, 27 April–1 May 2015. [Google Scholar]
- Mills, S.J. Prospects for Coal, CCTs and CCS in the European Union; IEA Clean Coal Centre: London, UK, 2010. [Google Scholar]
- Mathekga, H.I.; Oboirien, B.O.; North, B.C. A review of oxy-Fuel combustion in fluidized bed reactors. Int. J. Energy Res. 2016, 40, 878–902. [Google Scholar] [CrossRef]
- Lupion, M.; Alvarez, I.; Otero, P.; Kuivalainen, R.; Lantto, J.; Hotta, A.; Hack, H. 30 MWth CIUDEN oxy-Cfb boiler-First experiences. Energ. Procedia 2013, 37, 6179–6188. [Google Scholar] [CrossRef]
- Gu, J.; Liu, Q.; Zhong, W.; Yu, A. Study on scale-Up characteristics of oxy-Fuel combustion in circulating fluidized bed boiler by 3D CFD simulation. Adv. Powder Technol. 2020, 31, 2136–2151. [Google Scholar] [CrossRef]
- Li, S.; Xu, M.; Jia, L.; Tan, L.; Lu, Q. Influence of operating parameters on N2O emission in O2/CO2 combustion with high oxygen concentration in circulating fluidized bed. Appl. Energy 2016, 173, 197–209. [Google Scholar] [CrossRef]
- Aamand, L.E.; Leckner, B.; Andersson, S. Formation of nitrous oxide in circulating fluidized-Bed boilers. Energy Fuels 1991, 5, 815–823. [Google Scholar] [CrossRef]
- Kosowska-Golachowska, M.; Luckos, A.; Kijo-Kleczkowska, A. Emissioni inquinanti durante la combustione Oxy-Fuel della biomassa in un combustore CFB su scala da banco. Energie 2022, 15, 706. [Google Scholar] [CrossRef]

| Coal A | Coal B | Coal C | ||||
|---|---|---|---|---|---|---|
| Air | Oxy | Air | Oxy | Air | Oxy | |
| Estimated furnace heat transfer (kW) | 348 | 354 | 368 | 373 | 342 | 347 |
| Estimated heat flux (kW/m2) | 12.6 | 12.8 | 13.3 | 13.5 | 12.3 | 12.5 |
| % Difference, change from air to oxy | +1.6% | +1.5% | +1.6% | / | / | / |
| Water inlet temperature [°C] | 37.8 | 38.1 | 32.8 | 34.2 | -NA | -NA |
| Water exit temperature [°C] | 47.5 | 49.1 | 40.9 | 44.0 | -NA | -NA |
| Estimated furnace heat absorption [kW] | 354 | 392 | 336 | 356 | -NA | -NA |
| Technology | O2 Purity % | Capacity (Tons Per Day) | Quality | Driving Force | Start-Up Time |
|---|---|---|---|---|---|
| Cryogenic Matured | 99+ | Up to 4000 | N, Ar, Kr, Xe; Very good | Electricity | Hours/days |
| Adsorption | 95+ | Up to 300 | N, Bad, ca.11% | Electricity (70–90 °C) | Minutes/hours |
| Membrane (Polymer) | 50 | up to 20 | N | Electricity | Minutes |
| Membrane (ITM) | 99+ | Laboratory scale | N | Electricity (800 °C) | Hours |
| Test ID | O2 (%) | CO2 (%) | CO (ppm) | SO2 (ppm) | NO (ppm) | NO (ng/J) |
|---|---|---|---|---|---|---|
| EB-air | 2.0 | 17 | 51 | 615 | 583 | 211 |
| EB-O2/RFG | 2.1 | 97 | 85 | 1431 | 1332 | 233 |
| SB-air | 2.0 | 17 | 9 | 175 | 707 | 236 |
| SB-O2/RFG | 2.2 | 98 | 75 | 372 | 1183 | 148 |
| LN-air | 3.3 | 17 | 1 | 277 | 771 | 269 |
| LN-O2/RFG | 2.7 | 92 | 14 | 785 | 555 | 68 |
| O2 in Inlet (%) | Emission of CO (mg/MJ) | Mole Fraction of CO2 (%) | Mole Fraction of O2 (%) |
|---|---|---|---|
| 21 | 475 | 95.8 | 3.9 |
| 25 | 250 | 95.1 | 4.6 |
| 30 | 110 | 94.5 | 5.25 |
| 35 | 75 | 93.3 | 6.25 |
| Design Parameter | BFB | CFB |
|---|---|---|
| Combustion temperature [°C] | 700–900 | 800–900 |
| Fluidization velocities [m/s] | 1–3 | 3–10 |
| Fuel particle size [mm] | 0–3 | 0–0.3 |
| Combustion efficiency [%] | 90–96 | 95–99.5 |
| Test | Temperature [°C] | Excess O2 Ratio | NO [mg/MJ] | N2O [mg/MJ] | SO2 [mg/MJ] | CO [mg/MJ] | O2 [%] |
|---|---|---|---|---|---|---|---|
| 1 | 800 | 1.18 | 40.2 | 92.5 | 183.4 | 104.8 | 7.2 |
| 2 | 850 | 1.18 | 42.8 | 76.5 | 194.1 | 58.3 | 7.5 |
| 3 | 900 | 1.18 | 48.2 | 39.6 | 198 | 27.5 | 7.6 |
| 4 | 850 | 1.18 | 37.1 | 125.0 | 259.7 | 84.9 | 5.4 |
| 5 | 850 | 1.18 | 38.2 | 105.1 | 196.2 | 73.8 | 6.2 |
| 6 | 850 | 1.18 | 50.8 | 82.6 | 161.3 | 70.6 | 6.9 |
| 7 | 850 | 1.06 | 27.2 | 42.3 | 244.6 | 483.0 | 3.2 |
| 8 | 850 | 1.11 | 33.9 | 56.6 | 240.1 | 334.3 | 5 |
| 9 | 850 | 1.15 | 39.6 | 76.6 | 241.3 | 189.8 | 6.2 |
| 10 | 850 | 1.18 | 52.7 | 72.2 | 133.6 | 68.8 | 7.6 |
| 11 | 850 | 1.18 | 51.5 | 74.4 | 170.4 | 64.8 | 7.3 |
| 12 | 850 | 1.18 | 37.8 | 81.3 | 207.3 | 75.2 | 7.2 |
| 13 | 850 | 1.18 | 39.0 | 78.7 | 201.5 | 69.1 | 7.5 |
| 14 | 850 | 1.18 | 41.2 | 79.8 | 196.2 | 61.6 | 7.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raho, B.; Colangelo, G.; Milanese, M.; de Risi, A. A Critical Analysis of the Oxy-Combustion Process: From Mathematical Models to Combustion Product Analysis. Energies 2022, 15, 6514. https://doi.org/10.3390/en15186514
Raho B, Colangelo G, Milanese M, de Risi A. A Critical Analysis of the Oxy-Combustion Process: From Mathematical Models to Combustion Product Analysis. Energies. 2022; 15(18):6514. https://doi.org/10.3390/en15186514
Chicago/Turabian StyleRaho, Brenda, Gianpiero Colangelo, Marco Milanese, and Arturo de Risi. 2022. "A Critical Analysis of the Oxy-Combustion Process: From Mathematical Models to Combustion Product Analysis" Energies 15, no. 18: 6514. https://doi.org/10.3390/en15186514







