1. Introduction
The terms “global warming” and “climate change” refer to the large-scale impacts of human actions such as the burning of fossil fuels and extensive deforestation that contribute to a rise in the level of greenhouse gases in the atmosphere [1]. The Intergovernmental Panel on Climate Change (IPCC) asserts that the problem of greatest concern for humankind in this century is climate change, stimulated by an increase in the global average surface temperature of 1.1 °C since 1900 [2,3]. The global water cycle is being disrupted by climate change, resulting in droughts in some regions and extreme rainfall and flooding in others [4]. Continued warming will accelerate permafrost melting, which can cause a loss of seasonal snow cover, melting glaciers, and a reduction in the summer Arctic sea ice. Coastal communities across the world will be impacted, and lowland areas will see more frequent floods and severe shoreline erosion as sea levels rise [5]. More frequent ocean heat waves, ocean acidification, and reduced oxygen levels in the sea are all consequences of climate change that can have an impact on marine ecosystems and the livelihoods of people who depend on them. Cities, in particular, suffer significant effects of climate change. Urbanization increases the severity and frequency of heat waves as well as extreme precipitation [6]. The “Paris Agreement”, which seeks to restrict the rise in the average global temperature above its preindustrial level to 1.5 to 2 °C, was signed by 175 nations with the goal of minimizing the harm that is caused by climate change [7]. Removing greenhouse gases (GHGs) from the atmosphere is one important way to help achieve this target.
2. Greenhouse Gases in the Atmosphere
Carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), hydrofluorocarbons (HFCs), perfluorocarbons (PFCs), and sulfur hexafluoride (SF6) are the six GHGs that must be restricted in the atmosphere, according to the Kyoto Protocol [8]. The three most significant GHGs, with their respective average global concentration and their contribution to global warming, are: CO2, 410.5 ppm, 66%; CH4, 1877 ppb, 16%; N2O, 332.0 ppb, 7%. The leading contributor to global warming is CO2. The increase in average global temperature will reach 3.8 °C if the concentration of CO2 in the atmosphere doubles, and it will drop by 3.6 °C if it is cut in half [9]. CH4 presently contributes less to global warming, but its effect cannot be ignored. One ton of CH4 causes as much global warming as 86 tons of CO2 does [10]. Radiative forcing could be decreased by 16% within 10–20 years by returning the atmospheric CH4 concentrations to the pre-industrial levels of 760 ppb [11]. It is therefore desirable—even imperative—to hold the amounts of CO2 and CH4 in the atmosphere to specific levels.
2.1. Capture and Removal of CO2
Strategies such as afforestation and vegetation restoration are natural and eco-friendly approaches to reducing the level of CO2 in the atmosphere. The potential of nature to absorb CO2, however, will take more than tens of thousands of years to achieve the goal of carbon neutrality if we are reliant just on the ecosystem. It is not practical to address climate change only by planting a great many trees quickly. Other approaches are also needed. Carbon capture and storage (CCS) is a way to reduce carbon emissions by capturing and storing CO2 at its source [12].
CO2 can be captured in a variety of ways, including electrochemically [13], through liquid-absorbency [14], and physically [15,16]. The cost of capturing one ton of CO2 with CCS technology is $36 to $53 [17], which is cost-effective. The active deployment of CCS technology has not been widely adopted because there are still some technical challenges to be solved [18,19]. Although CCS can dramatically lower emissions from the electric-generation sector (coal-fired power plants), it is unable to capture emissions from the construction and transportation sectors, making it challenging for CCS technology alone to reach the goal of the Paris Agreements. Active emission reduction strategies can more effectively address dispersed carbon emission sources and accomplish decarbonization. The most feasible alternate technique, known as bio-energy with carbon capture and storage (BECCS), involves absorbing CO2 from the atmosphere by having plants or crops grow and with the use of CCS technology [20]. The trees may be burnt for energy while the CO2 that is emitted during the combustion is captured using the CCS technology. The trapped CO2 is kept underground, where it is preserved from escaping back into the atmosphere, and the process is repeated. The technique has the potential to eventually take all of the excess CO2 out of the atmosphere if it is used on a large enough scale [21]. However, BECCS requires a large forested area, which can result in a shortage of farm land and fresh water, and have other negative consequences [22]. Direct air capture (DAC) is the strategy of chemically directly converting CO2 from the atmosphere into compounds like carbonates [23]. Figure 1 shows that relative to BECCS, DAC can lessen the use of land and water, but its high cost is still a major barrier to its commercialization. The standard DAC system costs around $600 per ton of CO2 that is captured [24]. According to a recent report [25], a pilot plant can decrease the cost to $113–232 per ton of CO2 that is captured by implementing appropriate energy-saving measures, but this is still significantly more expensive than CCS.
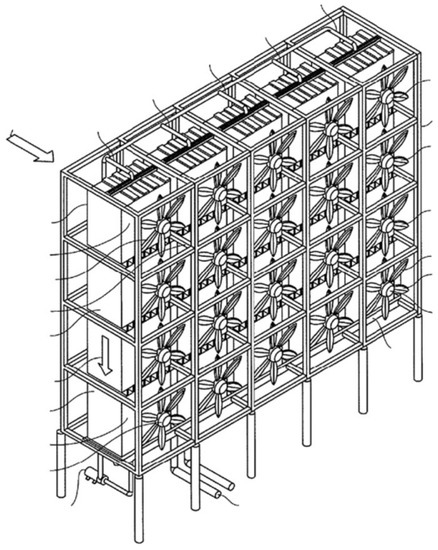
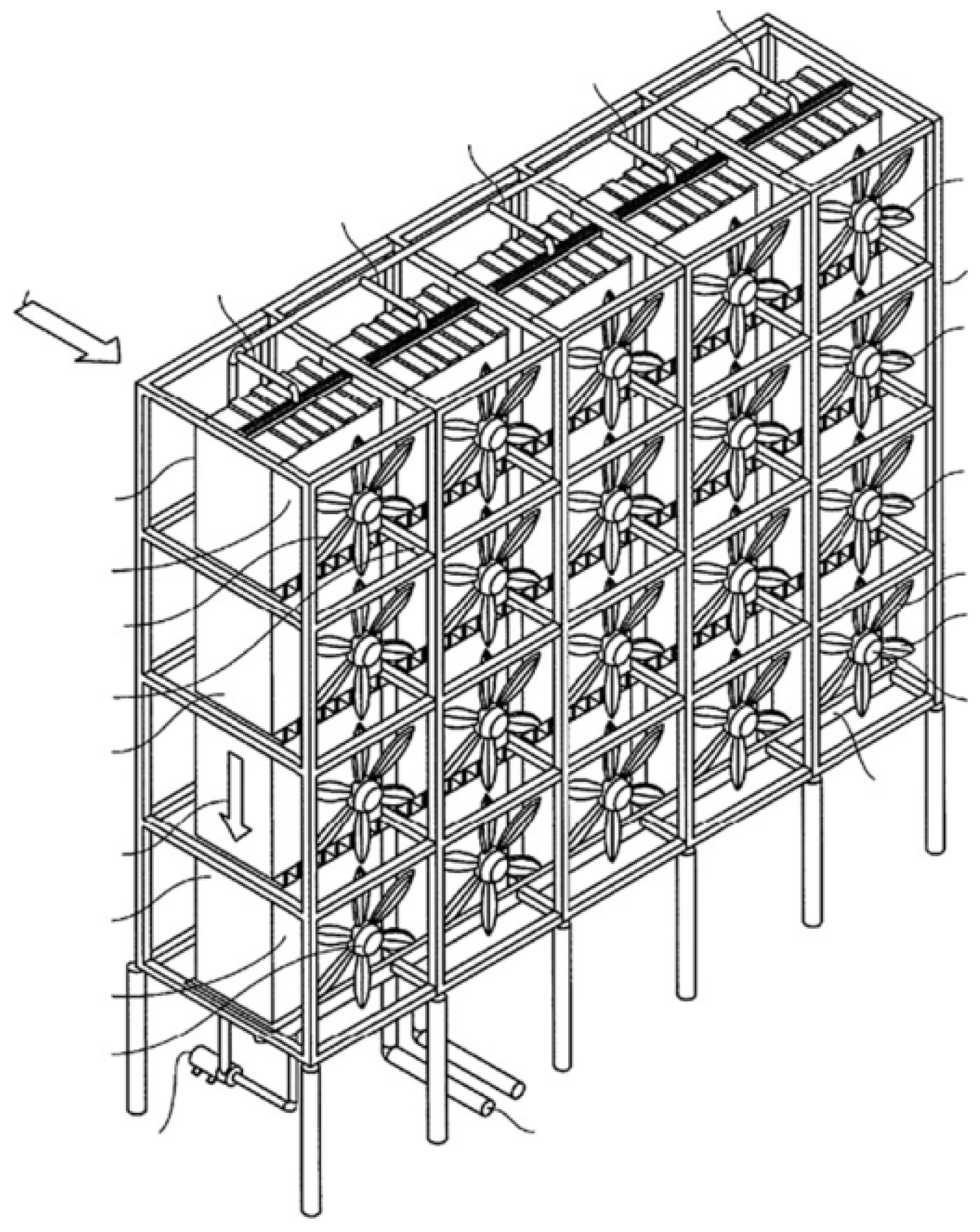
Figure 1.
Schematic of the DAC device [26].
2.2. Capture and Removal of CH4
In fact, planning only for the removal of CO2 from the atmosphere is not enough. Even if no additional CO2 is produced, the existing carbon store (515 Gt) can continue to contribute to global warming for decades to come [27]. The oceans also store a substantial amount of CO2. It is released into the atmosphere when the level of CO2 in the atmosphere drops due to the breakdown of carbonic acid in the seas [28].
Reducing atmospheric CH4 levels, according to the United Nations Environment Programme (UNEP), is another efficient strategy to combat climate change [29]. It can reduce warming by 0.4 to 0.5 °C by 2050. Rising ocean temperatures will cause CH4 (1146 Gt) in the form of flammable ice to become unstable and release itself into the atmosphere, thereby exacerbating global warming [30]. There are few experiments currently being done on atmospheric CH4 removal. Thermal catalysis is regarded by certain researchers as a potent technique for removing atmosphere CH4. The disadvantages of thermal catalysis, which frequently involves high temperatures and pressures, include considerable energy consumption, difficult reaction conditions, and some safety risks. CH4 has a high C-H bond energy (413 kJ/mol) and a relatively stable structure. It is presently not economical to employ thermal catalytic technology on a wide scale to degrade atmospheric CH4 because it demands temperatures of more than 700 °C. The limit of the reaction conditions can be lowered by catalyst improvement. Brenneis et al. [31] added copper-treated zeolite particles to a reaction tube through which air passed. The zeolite was capable of capturing and converting all of the CH4 in the air when heated it was to 310 °C. The only place it can actually be utilized is in a lab, and even there, the reaction temperature is too high for the process to be practical.
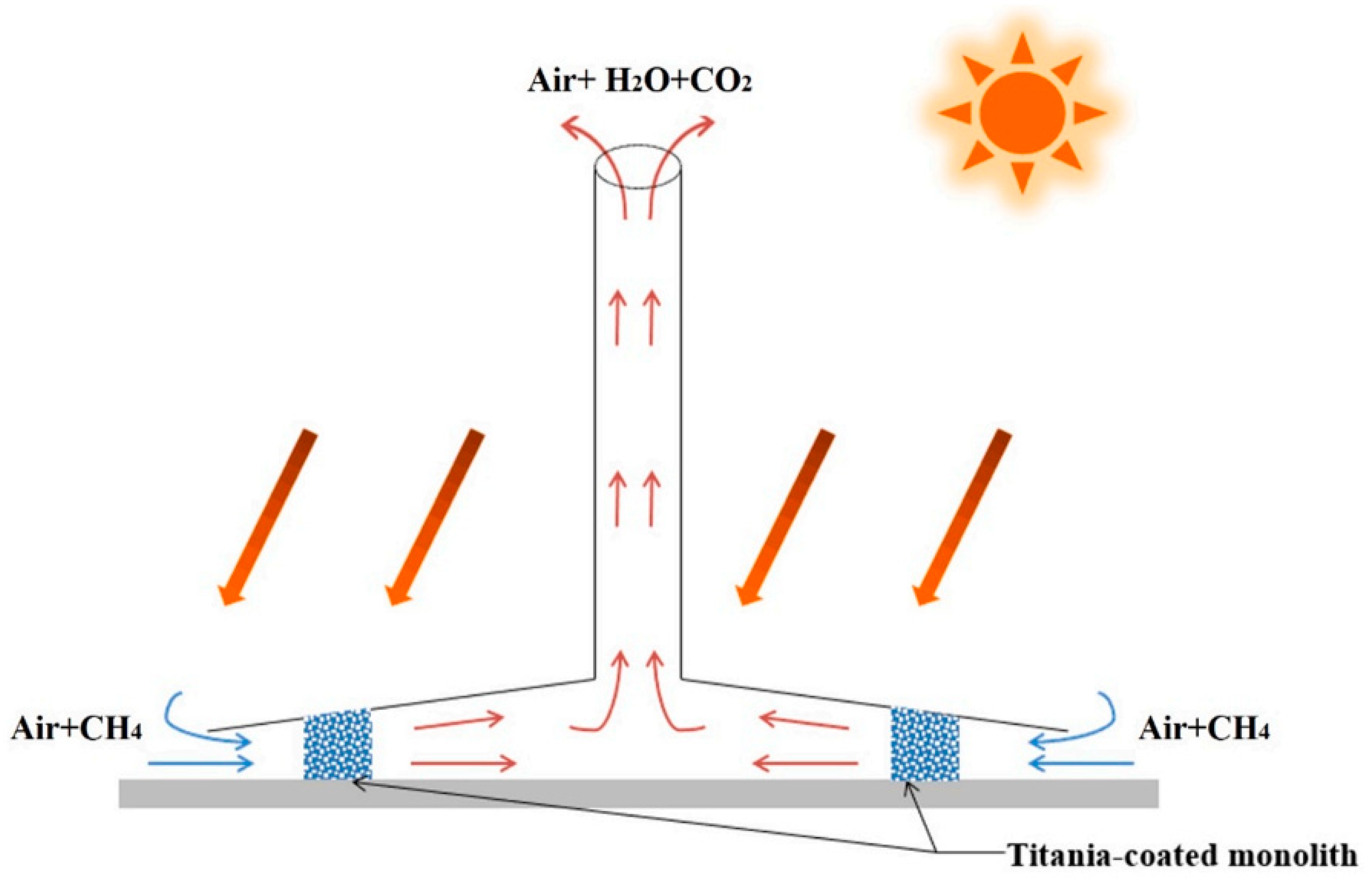
Photocatalytic semiconductor technology can be used to remove pollutants at room temperature and pressure, and the most common pollutants, such as indoor formaldehyde and volatile organic compounds (VOCs), have achieved good degradation rates. De Richter [32] presented a solar chimney power plant that was integrated with photocatalytic reactors (SCPP-PCRs) to address the problem of climate change. The system utilizes a natural convection mechanism to provide a powerful airflow and a driving force without the need for fuel. The canopy is coupled with a photocatalytic reactor. Atmospheric CH4 that comes into contact with the photocatalyst is converted to CO2 and H2O under solar irradiation. Ming et al. [33] confirmed the feasibility of SCPP-PCRs in combating global climate change by using a numerical simulation method, as shown in Figure 2. A good photocatalyst is required for the system to achieve the best performance. Chen et al. [34] carried out experiments on the oxidation of CH4 by a new synthetic photocatalyst (Ag/ZnO), and they found that nano-scale zinc oxide could effectively oxidize 100% of CH4 under sunlight irradiation, and the activity of the silver-plated ZnO semiconductor remained stable after multiple uses. The reaction rate depended on the concentration of CH4; it was faster at lower concentrations and less susceptible to temperature changes after the light conditions were set up. The experimental findings demonstrate that the photocatalytic technique that is utilizing Ag-ZnO has special benefits for low levels of atmospheric CH4 that are challenging to handle by thermal catalysis. Other photocatalysts with great potential include g-C3N4@Cs0.33WO3, ZnO/CuO, and Ga2O3/AC. The DAC system can still be used to capture and degrade atmospheric CH4 by creating a continuous stream of air with a fan, then absorbing the CH4 with organic solutions or removing it using a honeycomb photocatalytic reactor that is positioned behind the fan [35]. However, if the chemical absorption is considerable, then the employment of the DAC results in excessive energy consumption and subsequent maintenance expenses.
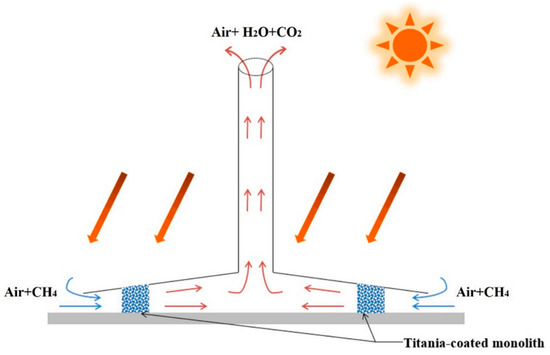
Figure 2.
Schematic of the SCPP-PCRs [33].
3. Conclusions
It is essential to continue studying methods for removing greenhouse gases from the atmosphere. The following are a few major conclusions:
- The disadvantages of the current methods for quick, large-scale CO2 removal from the atmosphere can include significant energy consumption, high investment, and high maintenance costs. Transformative technologies are needed in this field.
- The removal of atmospheric CH4 is a worthwhile goal, but because this gas is so rarefied, and removing it remains a challenge.
- Although the study of SCPP-PCRs is still in its early stages, it appears to be a promising technology that can not only decrease CO2 emissions from thermal power plants but also the diminish greenhouse gases that are in the air.
Funding
This research was funded by [the National Key Research and Development Plan] grant number [No. 2019YFE0197500], and [the European Commission H2020 Marie Curie Research and Innovation Staff Exchange (RISE) award] grant number [No. 871998].
Conflicts of Interest
The authors declare no conflict of interest.
References
- Houghton, J. Global warming. Rep. Prog. Phys. 2005, 68, 1343–1403. [Google Scholar] [CrossRef]
- Koç, İ. Changes that may occur in temperature, rain, and climate types due to global climate change: The example of Düzce. Turk. J. Agric. -Food Sci. Technol. 2021, 9, 1545–1554. [Google Scholar] [CrossRef]
- Allan, R.P.; Hawkins, E.; Bellouin, N.; Collins, B. IPCC, 2021: Summary for Policymakers; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Shiklomanov, I.A.; Rodda, J.C. World Water Resources at the Beginning of the Twenty-First Century; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Tebaldi, C.; Ranasinghe, R.; Vousdoukas, M.; Rasmussen, D.J.; Vega-Westhoff, B.; Kirezci, E.; Kopp, R.E.; Sriver, R.; Mentaschi, L. Extreme sea levels at different global warming levels. Nat. Clim. Chang. 2021, 11, 746–751. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global Warming, Climate Change, and Environmental Pollution: Recipe for a Multifactorial Stress Combination Disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef]
- Vigueras-Zúñiga, M.O.; Ramírez-Ruíz, C.A.; Herrera-May, A.L.; Tejeda-del-Cueto, M.E. Numerical and Experimental Analysis of the Effect of a Swirler with a High Swirl Number in a Biogas Combustor. Energies 2021, 14, 2768. [Google Scholar] [CrossRef]
- Bohringer, C. The Kyoto Protocol: A Review and Perspectives. Oxf. Rev. Econ. Policy 2003, 19, 451–466. [Google Scholar] [CrossRef]
- Al-Ghussain, L. Global warming: Review on driving forces and mitigation. Environ. Prog. Sustain. Energy 2019, 38, 13–21. [Google Scholar] [CrossRef]
- Mousavi Motlagh, S.F.; Sohani, A.; Djavad Saghafi, M.; Sayyaadi, H.; Nastasi, B. Acquiring the Foremost Window Allocation Strategy to Achieve the Best Trade-Off among Energy, Environmental, and Comfort Criteria in a Building. Energies 2021, 14, 3962. [Google Scholar] [CrossRef]
- Kuylenstierna, J.C.; Michalopoulou, E.; Malley, C. Global Methane Assessment: Benefits and Costs of Mitigating Methane Emissions. Stockholm Environment Institute. 2021. Available online: https://policycommons.net/artifacts/1528411/global-methane-assessment/2218096/ (accessed on 1 July 2022).
- Romanak, K.; Fridahl, M.; Dixon, T. Attitudes on Carbon Capture and Storage (CCS) as a Mitigation Technology within the UNFCCC. Energies 2021, 14, 629. [Google Scholar] [CrossRef]
- Sharifian, R.; Wagterveld, R.; Digdaya, I.; Xiang, C.; Vermaas, D. Electrochemical carbon dioxide capture to close the carbon cycle. Energy Environ. Sci. 2021, 14, 781–814. [Google Scholar] [CrossRef]
- Ochedi, F.O.; Yu, J.; Yu, H.; Liu, Y.; Hussain, A. Carbon dioxide capture using liquid absorption methods: A review. Environ. Chem. Lett. 2021, 19, 77–109. [Google Scholar] [CrossRef]
- Lin, J.-B.; Nguyen, T.T.; Vaidhyanathan, R.; Burner, J.; Taylor, J.M.; Durekova, H.; Akhtar, F.; Mah, R.K.; Ghaffari-Nik, O.; Marx, S. A scalable metal-organic framework as a durable physisorbent for carbon dioxide capture. Science 2021, 374, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Abd, A.A.; Othman, M.R.; Kim, J. A review on application of activated carbons for carbon dioxide capture: Present performance, preparation, and surface modification for further improvement. Environ. Sci. Pollut. Res. Int. 2021, 28, 43329–43364. [Google Scholar] [CrossRef] [PubMed]
- Rubin, E.S.; Davison, J.E.; Herzog, H.J. The cost of CO2 capture and storage. Int. J. Greenh. Gas Control 2015, 40, 378–400. [Google Scholar] [CrossRef]
- Mantripragada, H.C.; Zhai, H.; Rubin, E.S. Boundary Dam or Petra Nova–Which is a better model for CCS energy supply? Int. J. Greenh. Gas Control 2019, 82, 59–68. [Google Scholar] [CrossRef]
- Reiner, D.M. Learning through a portfolio of carbon capture and storage demonstration projects. Nat. Energy 2016, 1, 15011. [Google Scholar] [CrossRef]
- Muratori, M.; Bauer, N.; Rose, S.K.; Wise, M.; Daioglou, V.; Cui, Y.; Kato, E.; Gidden, M.; Strefler, J.; Fujimori, S. EMF-33 insights on bioenergy with carbon capture and storage (BECCS). Clim. Chang. 2020, 163, 1621–1637. [Google Scholar] [CrossRef]
- Muri, H. The role of large—Scale BECCS in the pursuit of the 1.5 C target: An Earth system model perspective. Environ. Res. Lett. 2018, 13, 044010. [Google Scholar] [CrossRef]
- Heck, V.; Gerten, D.; Lucht, W.; Popp, A. Author Correction: Biomass-based negative emissions difficult to reconcile with planetary boundaries. Nat. Clim. Chang. 2018, 8, 345. [Google Scholar] [CrossRef]
- Santori, G.; Charalambous, C.; Ferrari, M.-C.; Brandani, S. Adsorption artificial tree for atmospheric carbon dioxide capture, purification and compression. Energy 2018, 162, 1158–1168. [Google Scholar] [CrossRef] [Green Version]
- House, K.Z.; Baclig, A.C.; Ranjan, M.; van Nierop, E.A.; Wilcox, J.; Herzog, H.J. Economic and energetic analysis of capturing CO2 from ambient air. Proc. Natl. Acad. Sci. USA 2011, 108, 20428–20433. [Google Scholar] [CrossRef] [PubMed]
- Keith, D.W.; Holmes, G.; St. Angelo, D.; Heidel, K. A Process for Capturing CO2 from the Atmosphere. Joule 2018, 2, 1573–1594. [Google Scholar] [CrossRef]
- de_Richter, R.K.; Ming, T.; Caillol, S. Fighting global warming by photocatalytic reduction of CO2 using giant photocatalytic reactors. Renew. Sustain. Energy Rev. 2013, 19, 82–106. [Google Scholar] [CrossRef]
- Matthews, H.D.; Caldeira, K. Stabilizing climate requires near-zero emissions. Geophys. Res. Lett. 2008, 35, 1–5. [Google Scholar] [CrossRef]
- Vichi, M.; Navarra, A.; Fogli, P. Adjustment of the natural ocean carbon cycle to negative emission rates. Clim. Chang. 2013, 118, 105–118. [Google Scholar] [CrossRef]
- Blok, K.; Hare, W.; Hohne, N.; Kainuma, M.; Kejun, J.; Lee, D.S.; Rogelj, J.; Shukla, A.; Arent, D.; Bogner, J. Bridging the Emissions Gap: A UNEP Synthesis Report. The United Nations Environment Programme (UNEP). 2011. Available online: https://wedocs.unep.org/handle/20.500.11822/7996 (accessed on 1 July 2022).
- Kretschmer, K.; Biastoch, A.; Rüpke, L.; Burwicz, E. Modeling the fate of methane hydrates under global warming. Glob. Biogeochem. Cycles 2015, 29, 610–625. [Google Scholar] [CrossRef]
- Brenneis, R.J.; Johnson, E.P.; Shi, W.; Plata, D.L. Atmospheric-and Low-Level Methane Abatement via an Earth-Abundant Catalyst. ACS Environ. Au 2021, 2, 223–231. [Google Scholar] [CrossRef]
- de Richter, R.; Ming, T.Z.; Davies, P.; Liu, W.; Caillol, S. Removal of non-CO2 greenhouse gases by large-scale atmospheric solar photocatalysis. Prog. Energy Combust. 2017, 60, 68–96. [Google Scholar] [CrossRef]
- Ming, T.; Gui, H.; Shi, T.; Xiong, H.; Wu, Y.; Shao, Y.; Li, W.; Lu, X.; de Richter, R. Solar chimney power plant integrated with a photocatalytic reactor to remove atmospheric methane: A numerical analysis. Sol. Energy 2021, 226, 101–111. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Pan, X.; Cortie, D.; Huang, X.; Yi, Z. Photocatalytic oxidation of methane over silver decorated zinc oxide nanocatalysts. Nat. Commun. 2016, 7, 12273. [Google Scholar] [CrossRef] [Green Version]
- Ming, T.; Li, W.; Yuan, Q.; Davies, P.; De Richter, R.; Peng, C.; Deng, Q.; Yuan, Y.; Caillol, S.; Zhou, N. Perspectives on removal of atmospheric methane. Adv. Appl. Energy 2022, 5, 100085. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).