Abstract
Increasing environmental pollution is driving an increase in the production and use of biofuels. The cost price of biodiesel could be reduced by using low-quality oilseeds unfit for human consumption and by applying the simultaneous oil extraction and transesterification process, avoiding the oil pressure stage. The purpose of this study was to investigate the enzymatic biofuel production process (in situ) by using rapeseed with high oil acidity for simultaneous oil extraction and transesterification with a mixture of butanol and mineral diesel fuel. The investigation of the in situ process was performed using a mixture of butanol and mineral diesel and the most effective biocatalyst Lipozyme TL IM was selected. The novelty of this paper consists of the fact that mineral diesel was used as the oil extractant, and the amount chosen was such that, at the end, a mixture of fuel with a ratio 9:1 of mineral diesel to biodiesel was be produced. The experiments were carried out using ground rapeseeds under laboratory conditions. The efficiency of oil extraction was investigated by the FTIR spectrometry method, and the efficiency of transesterification was determined by the gas chromatography method. It was found that the optimal reaction duration was 7 h, reaction temperature was 40 °C, and lipase content was 6% (from the oil content in rapeseed). An oil extraction efficiency of 99.92 ± 0.04 (w/w) was observed at these conditions. A transesterification degree of 99.08 ± 0.08% (w/w) met with the requirements of the standards for biodiesel fuel. The physical and chemical properties of the produced fuel mixture met the requirements of the standards for mineral diesel and biodiesel; therefore, it can be used in diesel engines.
1. Introduction
Currently, diesel fuel is still one of the most widely used forms of fuel, without which the efficient operation of transport systems is unimaginable. Diesel is also a critical fuel used in the field of energy [1]. However, in recent decades, growing concern about environmental and pollution problems has led to the search for more eco-friendly sources of energy and fuel. Mineral diesel is one of the main sources of energy that promotes environmental pollution, along with other petroleum products such as gasoline [2]. With the growing concern about environmental pollution and the increasing concentrations of greenhouse gases entering the atmosphere, the aim is to find more efficient ways to provide the necessary energy with the least possible negative impact on the environment [3].
Often, biodiesel is considered the solution. The industrial development and use of biodiesel production in the transport sector of the European Union has been going on for almost two decades and is gaining increasing attention. Biodiesel has many advantages over mineral diesel fuel. It is produced from natural raw materials—vegetable oil. Biodiesel is characterized by such properties as causing less environmental pollution by the harmful components in exhaust gases and having a rapid biological degradability. The development of biodiesel production could allow the gradual reduction in the consumption of fuel produced from oil. However, compared to the synthesis of mineral diesel, the process of production of biodiesel is still quite complex and expensive, so it is necessary to look for new raw materials and advanced technologies to expand the industrial production of biodiesel [4].
One of the latest technologies for the production of biodiesel, using which the cost of production could be reduced, is the possibility of using the simultaneous process of oil extraction from raw materials and transesterification into biodiesel (in situ). During this process, the stage of extracting oil from oilseeds is avoided [5]. The process of extracting and purifying the oil requires a lot of energy and accounts for about 70% of the total energy consumption of the biodiesel production process [6]. For this reason, the production price of biodiesel could be significantly reduced through the in situ process [7]. Table 1 provides a comparison of the stages of traditional and in situ transesterification processes. This process does not require the commonly used oil solvent hexane, since the alcohol used for transesterification acts as an oil extraction agent, and the formed fatty acid esters also have good oil solvent properties; as a result, the efficiency of oil extraction increases.

Table 1.
Comparison of the stages of traditional and in situ biodiesel production processes.
Various types of fatty raw materials and wastes could be used for the production of biodiesel [8,9,10]. The biggest amount of biodiesel (around 84%) in the EU is produced from food-grade rapeseed oil. Sunflower, corn, and palm oil are also used for biodiesel synthesis. Recently, there has been interest in the use of microalgae oil [11]. However, the production of biodiesel from vegetable oil competes strongly with the production of oil for the food industry. Therefore, for the production of biodiesel, it is useful to look for oily waste and raw materials that are not suitable for human consumption. In order to avoid competition with the food sector, it is appropriate to use rapeseed oil of poor quality that unsuitable for human consumption in biodiesel production. The content of such oil reaches about 10% of the total amount of rapeseed oil. The use of non-food oil would address social problems related to the use of food raw materials for fuel synthesis and rising prices of food-grade oil. Scientists have conducted research using low-quality rapeseed oil (high acidity oil or waste oil resulting from cooking), applying chemical [12] and biotechnological [13] methods. Studies have also already begun on the simultaneous oil extraction and transesterification process in situ. Their results have shown that the speed of the in situ process can be increased with the help of additional solvents in the reaction medium. Most of the experiments were conducted using hexane [14,15,16]. However, the use of solvents causes certain technological and environmental problems. They need to be removed from the reaction product; solvent loss is possible during disposal. Given that mixtures of mineral diesel and biodiesel are currently used in the transport sector, our idea was to use mineral diesel as an extraction agent in the in situ process, obtaining fuel mixtures suitable for use in the transport sector. Sendzikiene et al. studied the process of simultaneous oil extraction and transesterification using food-grade rapeseed. A 97.6 wt% yield of esters was obtained using Lipozyme RM IM as a catalyst and mixture of butanol and mineral diesel as an oil extraction and transesterification agent [17]. Kojima et al. applied the biotechnological (catalyst Candida cylindracea) in situ process for waste-activated bleaching earth transesterification using a methanol and mineral diesel mixture, obtaining a fatty acid methyl ester (FAME) content in the sample of 10% (w/w) and achieving a yield of 100 wt% esters [18]. The in situ process of biodiesel synthesis using low-quality (with higher acidity) rapeseed and mineral diesel as an extractant in biotechnological biodiesel synthesis has been studied using methanol and ethanol for transesterification and obtaining mixtures containing 10% FAME and 90% mineral diesel [19,20]. Butanol can be obtained from renewable sources, so its use for biodiesel synthesis is attractive and increases the share of renewables. However, studies on the in situ process for the production of fuel mixtures using low-quality rapeseed seeds, biocatalysts, butanol, and mineral diesel have not been carried out.
The purpose of this study was to Investigate the enzymatic biofuel production process by using rapeseed with high oil acidity for simultaneous oil extraction and transesterification with a mixture of butanol and mineral diesel fuel and to assess the characteristics and suitability of the product obtained for use as diesel fuel.
2. Materials and Method
2.1. Materials
Rapeseed was cultivated at the Experimental Station of Agriculture Academy at Vytautas Magnus University (Lithuania). Arctic mineral diesel (Class 1) was acquired from a local market (Lithuania); butanol (99.8%) was purchased from Sigma-Aldrich.
The following fermented preparations produced by A/S Novozymes (Copenhagen, Denmark) were used as catalysts for transesterification experiments:
- lipases from Candida Antarctica: Novozyme 435 (600 U/g); Lipozyme 435 (600 U/g); Lipozyme CALB (5 kU/g);
- lipases from Thermomyces lanuginosus: Lipozyme TL IM (≥3 kU/g); Lipolase 100L (122 kU/g); Lecitase Ultra (150 U/g); Lipozyme TL 100L (100 kU/g); Lipex 100L (10 kU/g);
- lipases from Rhizomucor miehei: Lipozyme RM IM (>30 U/g); Palatase 20000L (≥20 kU/g);
- lipase from Aspergillus oryzae—Resinase A 2X (119.6 kU/g).
2.2. Quality Assessment of Raw Material and Its Oil
The following methodologies were used to determine the properties of low-quality rapeseed, and its oil: oil content in raw materials (standard—LST EN ISO 659), fatty acid composition of oil (standards—EN ISO 5509 and EN ISO 5508), acidity of oil (standard—LST EN ISO 660), and moisture (standard—LST EN ISO 665).
2.3. Selection of Lipase for In Situ Transesterification Process
Rapeseed (with a known amount of oil) was crushed (to an average particle size of 0.315 mm) and weighed. The crushed seeds were mixed with mineral diesel, choosing a ratio of oil to mineral diesel of 1:9 (w/w), and placed in laboratory reactor containing heaters, mixers, and reflux condensers. These samples were mixed at a constant (250 min−1) rotation speed whilst increasing the temperature to 40 °C. When the required temperature was reached, a weighed amount of alcohol and biocatalyst was added to the laboratory reactor and reaction was performed for 5 h. The molar ratio of butanol to oil was 5:1. Lipase content (from the oil content in the rapeseed sample) was 7%. After the reaction was completed, the resulting fuel blends were filtered from the catalyst and rapeseed residues. The glycerol fraction was separated in a separatory funnel and the resulting fuel blends were washed twice with distilled water. A rotary evaporator was used to remove residual water and unreacted alcohol. Silica gel was used for the complete removal of moisture residues. The content of rapeseed oil and rapeseed oil butyl esters in the reaction product was determined by applying the Fourier transform infrared spectroscopy method and using an FTIR Spectrum RX-I spectrophotometer (Perkin Elmer). For sample preparation, the method described in EN 14078 was applied. Spectral measurements were performed at a wavelength of 1600 to 1900 cm−1 as described in our previous publications [19,20].
The content of rapeseed oil butyl esters (RBE) was evaluated by applying thin-layer chromatography (TLC) according to the method described and modified previously [17]. Samples were selected by TLC using the most efficient lipases also analyzed by gas chromatography.
2.4. Optimization of the Lipase-Catalysed In Situ Transesterification
Experiments were performed (according to section of lipase for the in situ transesterification process) using the selected lipase, Lipozyme TL IM. The alcohol-to-oil molar ratio was 5:1, the temperature varied from 25 to 40 °C, the process time was from 1 to 9 h and the enzyme concentration range from 3 to 6%. All samples prepared for the in situ reactions were analyzed by gas chromatography method.
2.5. Determination of the Rapeseed Oil Extraction Effectiveness and Ester Yield in the Reaction Product
The content of rapeseed oil and rapeseed oil butyl esters in the reaction product was determined by applying the Fourier transform infrared spectroscopy method and using an FTIR Spectrum RX-I spectrophotometer (Perkin Elmer). For sample preparation, the method described in EN 14078 was applied. Spectral measurements were performed at a wavelength of 1600 to 1900 cm−1 as described in our previous publications [19,20].
2.6. Determination of Transesterification Degree
A gas chromatographic method was used for quantitative analysis in order to check whether the produced fuel blends met the requirements of the standard EN 14214 for the content of glycerides in the produced biodiesel. Analyses were performed using a “Perkin Elmer Clarus 500” gas chromatograph (flame ionization detector; column–Restek MXT-Biodiesel TG (15 m–0.32 mm–0.10 μm)) according to the requirements of the EN 14105 standard. The degree of transesterification was calculated by applying the method described in previous publications [13].
After evaluating the results of the gas chromatography, the most efficient lipase was selected for further research of the enzymatic in situ transesterification process. The same gas chromatography method used to study the optimization of the process conditions. Each sample was analyzed three times. The average of the three measurements taken as a result.
2.7. Determination of Physical and Chemical Properties of Fuel Blend
The physical and chemical properties of the produced biodiesel were analyzed according to the requirements of the standards EN ISO 14214 and EN ISO 590.
All tests were performed in three replicates, and the arithmetic mean was taken as the result.
3. Results and Discussions
3.1. Quality of Rapeseed and Their Oil
The characteristics of the rapeseeds and their oil used for experiments were established; seed moisture, oil content, and acidity were determined. It was found that the oil content was 45.0 ± 2.0%. As a rule, oil content in rapeseed can amount to up to 50% of the dry weight [21]. The moisture content of the analyzed rapeseed was 6.3 ± 1.5% (by weight). The acid content of the rapeseed oil was 2.63 ± 0.25 mg KOH g−1. Other researchers have reported the acid content of rapeseed oil to be in a range from 0.89 to 12.25 mg KOH g−1 and have determined that the acid content of oil is influenced by the storage and transportation of seeds [12].
3.2. Efficacy of Lipases in In Situ Synthesis
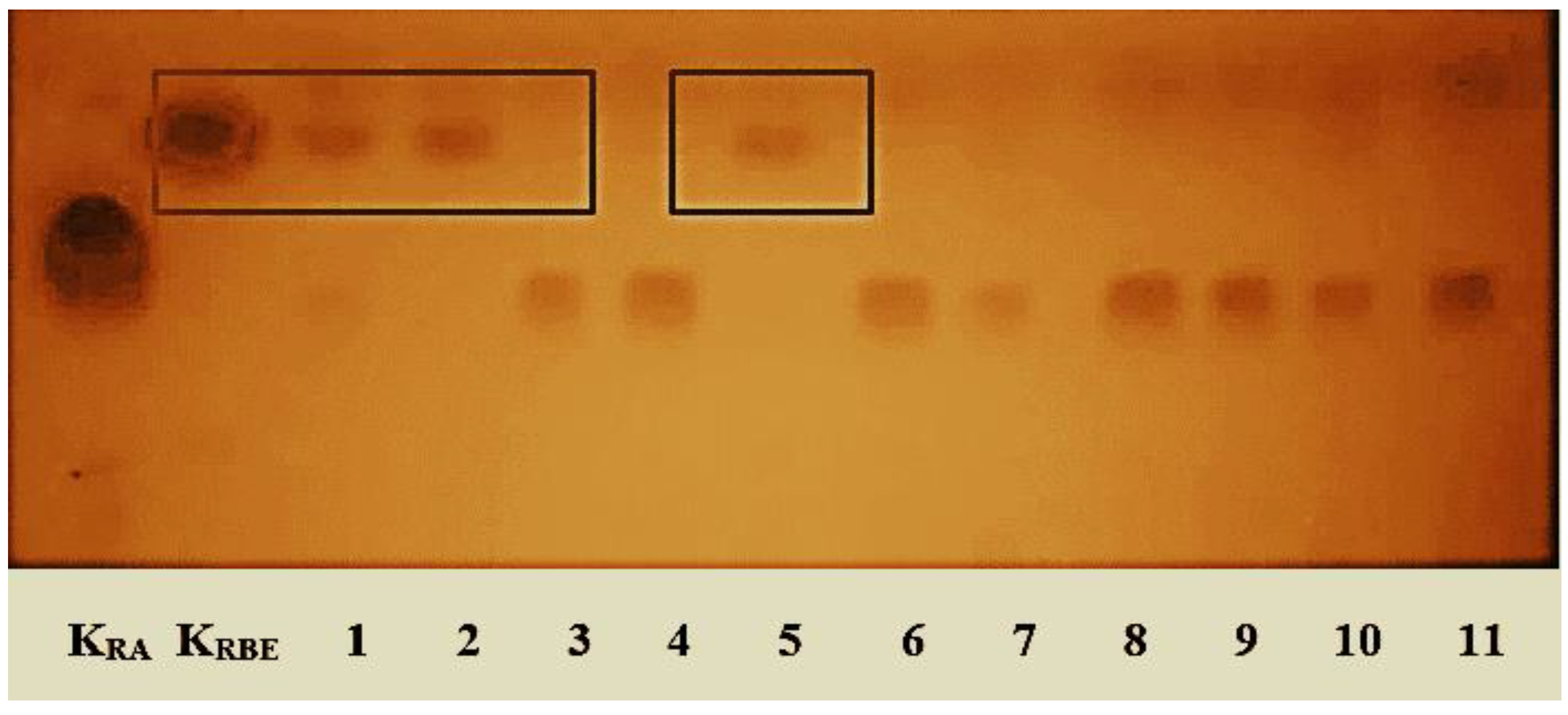
In the first stage, a study of the effectiveness of lipases was carried out and a more effective biodiesel synthesis lipase was selected, which was used to optimize the process. Eleven different lipases were studied. The in situ process was carried out for 5 h at 40 °C. The resulting fuel mixtures were examined by thin-layer chromatography (Figure 1).
Figure 1.
Efficacy of lipases in the in situ process catalyzed by rapeseed oil lipase using butanol: KRO—rapeseed oil, KRBE—rapeseed oil fatty acid butyl esters and lipases: (1—Lipozyme RM IM, 2—Lipozyme TL IM, 3—Novozyme 435, 4—Lipozyme 435, 5—Lipolase 100L, 6—Lecitase Ultra, 7—Resinase A 2X, 8—Palatase 20000L, 9—Lipozyme CALB, 10—Lipozyme TL 100L, 11—Lipex 100L).
The following three lipases were found to have the highest efficiency: Lipozyme RM IM, Lipozyme TL IM, and Lipolase 100L. With the other eight lipases, RBE were almost non-formed or formed, but a lot of unreacted oil remained. The resulting fuel mixtures obtained using the efficient lipases by were tested by gas chromatography to assess the degree of transesterification and the glyceride content in the mixtures and to select one of the most effective lipases suitable for the in situ process using butanol (Table 2).

Table 2.
The most efficient lipases using the in situ process.
A comparison of the effectiveness of all the lipases studied showed that the most effective lipase in the in situ process was immobilized lipase, Lipozyme TL IM, since not only was the highest degree of transesterification obtained with this lipase, but also the lowest levels of mono-, di- and triglycerides were obtained compared to the other lipases used. Other scientists have also found that Lipozyme TL IM is suitable for the enzymatic transesterification process in the production of biodiesel from mango oil by transesterification with methanol [22]. In addition, the enzymatic synthesis of biodiesel using different alcohols has been studied using sunflower, cucumber, olive, and soybean oil and has been found to be more effective with methanol than with ethanol, and it was determined that the Lipozyme TL IM was more effective as a biocatalyst than the lipase Novozyme 435 [23]. Our previous studies using the in situ process in the production of fuel mixtures from low-quality seeds and using the different alcohols methanol [20] and ethanol [19] also confirmed that the Lipozyme TL IM had a higher efficiency. It can be said that the results of the studies confirmed the results obtained by other researchers. Therefore, the lipase Lipozyme TL IM was chosen as the most suitable biocatalyst for further research.
3.3. Optimal Conditions of Enzymatic In Situ Process
3.3.1. Effectiveness of Simultaneous Oil Extraction and Transesterification
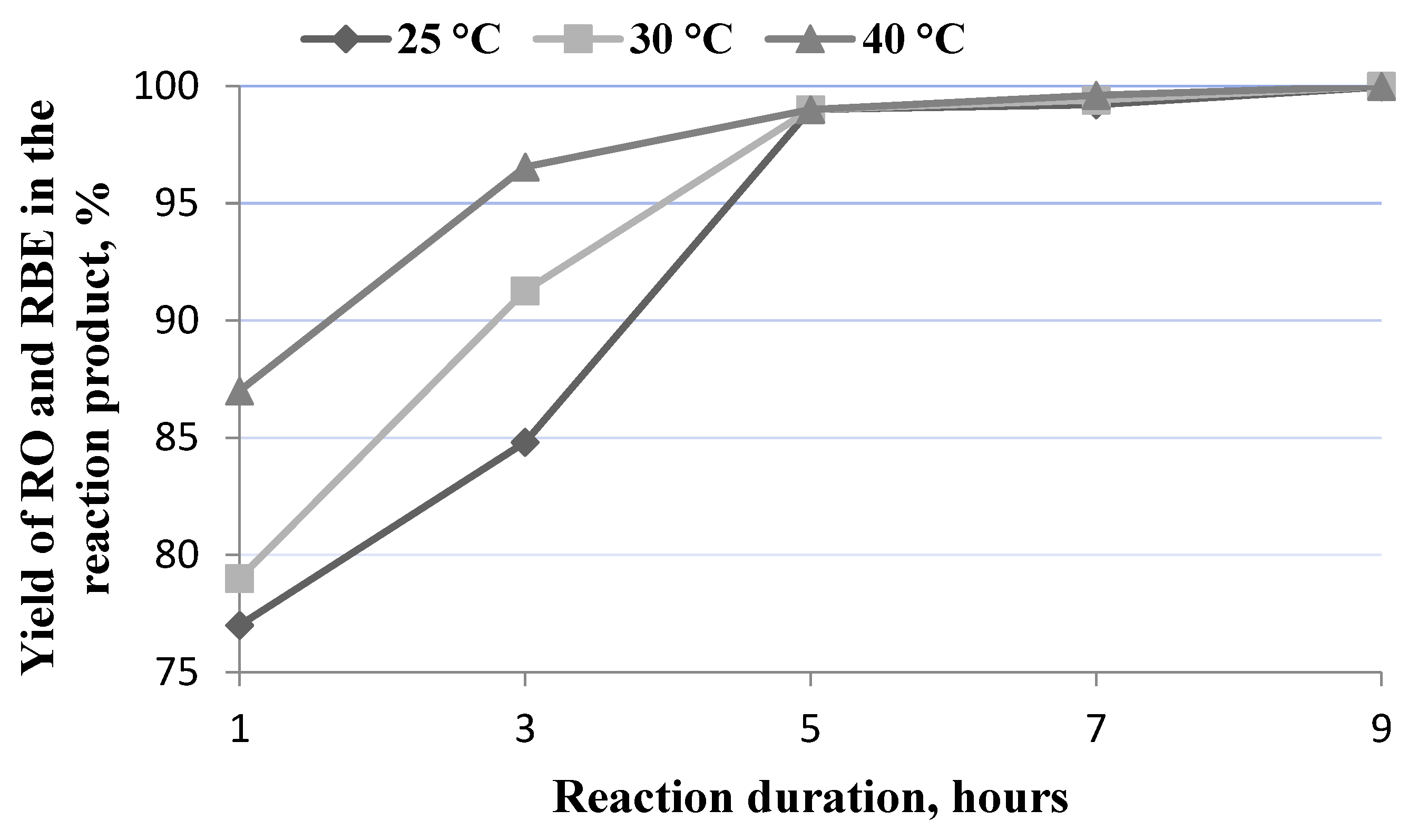
In the in situ process, it is important to extract the maximum amount of oil from oil seeds, the more it is extracted, the higher the yield of biofuels that can be obtained. Due to the fact that, during the in situ process, both the process of oil extraction and transesterification takes place simultaneously, assessing their rate and optimal duration separately is difficult so, at the initial stage, the infrared spectral analysis method was used to evaluate the total amount of oil and rapeseed butyl esters in reaction product. The processes of oil extraction and transesterification of oil depends on the temperature and duration; therefore, it was necessary to determine under what conditions the maximum content of oil is extracted from the seed and under what conditions the maximum yield of RBE is obtained. In the case where the extraction of the oil from rapeseed required a longer time than the transesterification of the extracted oil, the duration of the in situ process was determined by the optimum oil extraction time and the temperature, or in reverse, in the case of a lower transesterification rate compared with the extraction rate, the maximum yield of biodiesel was determined by the optimal duration and temperature of transesterification. The tests were carried out at temperatures of 25 °C, 30 °C, and 40 °C. Higher temperatures were not chosen because higher temperatures not only increase the energy costs of the process, but also the denaturation of enzymes may occur. The total content of rapeseed oil (RO) and rapeseed oil fatty butyl esters (RBE) was determined in the reaction product (Figure 2).
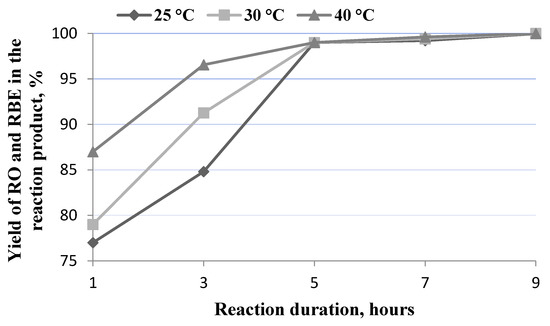
Figure 2.
Dependence of RO and RBE yield on reaction time and temperature.
The data submitted show that both the temperature and duration of the process had a direct impact on the efficiency of the extraction and the transesterification processes. A high yield of oil and rapeseed oil butyl esters was obtained at lower temperatures, but for a longer duration of the process, or vice versa. When process duration was lower than 5 h, temperature had a great impact on the product yield. At a reaction time of 1 h and a temperature of 25 °C, 30 °C, or 40 °C, the RO and RBE yield was 77.12 ± 0.04%, 79.15 ± 0.03%, and 87.23 ± 0.05%, respectively. When the reaction time was extended to 3 h at a reaction temperature of 25 °C or 30 °C, the RO and RBE yield increased to 84.80 ± 0.03% and 91.27 ± 0.03%, respectively. At 40 °C, a yield of 96.55 ± 0.02% was determined. At 5, 7, or 9 h reaction time, the RO and RBE yield was very similar to all the selected reaction temperatures and reached a value of more than 99%. At the lowest temperature of 25 °C, the maximum RO and RBE yield of 99.96 ± 0.02% was obtained with a significantly longer reaction time of 9 h.
The results obtained show that a mixture of mineral diesel and butanol was a suitable extraction agent in the in situ process. Other scientists who evaluated the effectiveness of the enzymatic in situ process using high-quality rapeseed and a mixture of butanol and mineral diesel for oil extraction and transesterification found that the optimum process temperature was 40 °C [17]. The research data has shown that the optimal temperature for using a biocatalyst in the synthesis of biodiesel in hexane is 30–55 °C when transesterification is performed with methanol, ethanol, butanol, and propanol [24,25].
The results of our studies show that, in order to obtain a high yield of RO and RBE, the reaction time should be at least 5 h. The results of studies carried out by other scientists have shown that the optimal duration of the enzymatic in situ process is quite diverse, i.e., from 2 to 67 h [7,16,18]. Kojima et al. applied in situ transesterification experiments using waste-activated bleaching earth as a raw material and methanol and a mineral diesel mixture as an oil extraction medium and transesterification agent, and they achieved a 100% FAME yield after 2–3 h [18] while Tran et al. achieved a FAME yield of 97.3% using Chlorella vulgaris (ESP-31) only after 48 h [16]. In addition, it has been found that the minimum optimal reaction time and temperature are found when the shortest chain of alcohol (methanol) is used for transesterification. Meanwhile, when a longer-chain alcohol is used for the in situ process, a higher temperature and a longer reaction time are required [19,20].
3.3.2. Optimal Transesterification Temperature and Duration
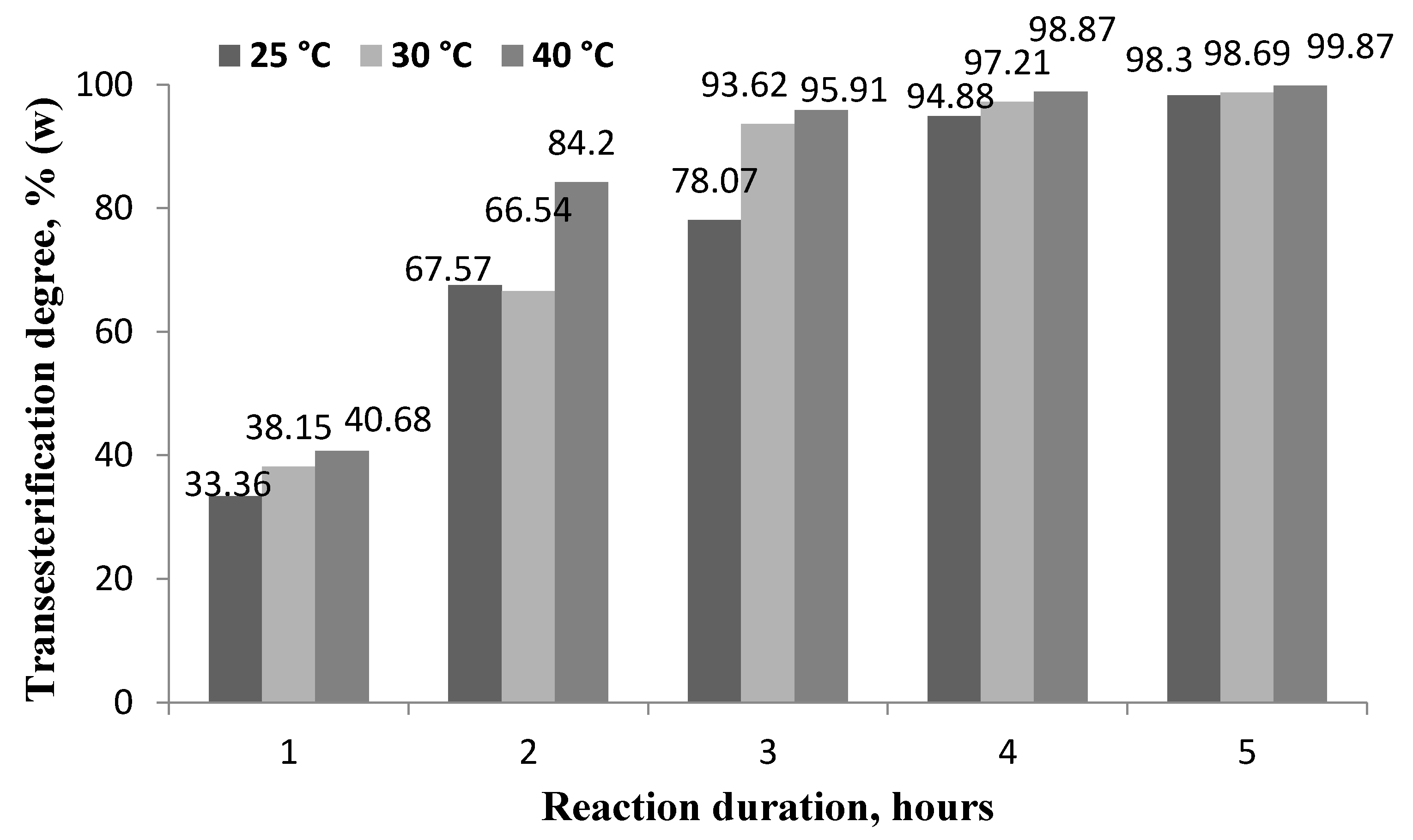
With the simultaneous process of extraction and transesterification of triglycerides, it is important not only to extract all the oil contained in the oily seeds, but also to convert as much of it as possible into fatty acid esters, i.e., biodiesel. In accordance with the requirements of the standard EN 14214, biodiesel must contain at least 96.5% esters and residues may consist of mono-, di-, and triglycerides. This means that the transesterification efficiency must be sufficiently high, and the residual amount of non-transesterified triglycerides must be very low. The dependence of the transesterification degree on the reaction temperature and duration and the glyceride content in biodiesel are shown in Figure 3 and Table 3.
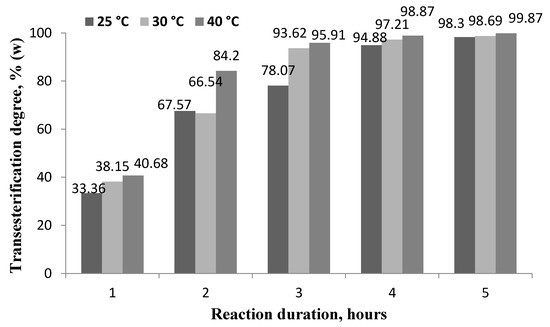
Figure 3.
Dependence of transesterification degree on the reaction temperature and duration at a molar ratio of RO to butanol of 1:5 and a lipase concentration of 7%.

Table 3.
Dependence of glyceride content in reaction product on reaction temperature and duration.
The minimum transesterification degree (33.36 ± 0.08%) was obtained after 1 h at 25 °C. The maximum transesterification degree (99.93 ± 0.02%) was achieved after 9 h at 40 °C, but not all samples met the biodiesel standard requirements for glyceride content (Table 3). According to the requirements of the standard EN 14214, biodiesel must contain no more than 0.7% (w/w) monoglycerides, no more than 0.2% (w/w) diglycerides, and no more than 0.2% (w/w) triglycerides.
At a reaction time of 1, 3, and 5 h, even with an increase in the reaction temperature from 25 °C to 40 °C, the glyceride content in biodiesel was higher than required by the standard. With a reaction time of 7 h and a reaction temperature of 25 °C, neither the transesterification degree (94.88% w/w) nor the glyceride content met the requirements of the standard (mono glyceride content of 1.28 ± 0.02%, diglyceride content of 1.02 ± 0.04%, and triglyceride content of 0.57 ± 0.03%); similar results were obtained at 30 °C and 7 h. This can be explained by the fact that the process of transesterification occurs in three stages, the first with the formation of diglycerides and one molecule of fatty acid butyl esters, and the second with the formation of monoglycerides. It is only after the third stage, when a third fatty acid butyl ester molecule is formed from the monoglyceride, that the highest content of esters and the level of glycerides corresponding to the standard are obtained A certain amount of time is required for a full reaction of triglycerides, while it is shorter at elevated temperatures.
At the same time and increased temperature of 40 °C obtained a biodiesel content that met the requirements of the standard EN 14214. After 9 h at a temperature of 30 °C and 40 °C, the biodiesel produced met the requirements of the standard for both ester and glyceride content.
Summarizing the results of the studies, it can be stated that the optimal reaction time of the in situ process using butanol was 7 h at a reaction temperature of 40 °C. Sendzikiene et al. [17] conducted similar studies using high-quality rapeseed for simultaneous oil extraction and transesterification. In addition, mineral diesel was used as a solvent in order to obtain fuel mixtures containing mineral diesel and rapeseed butyl esters. The optimal determined conditions for the in situ process were as follows: mineral diesel-to-oil ratio 9:1 (w/w), butanol-to-oil molar ratio 31:1, Lipozyme RM IM content 5.2% (in terms of oil content), reaction time 19.6 h, and reaction temperature 40 °C.
The results of previous studies on the in situ process for biodiesel synthesis using low-quality rapeseeds with various alcohols as acyl receptors and a catalyst Lipozyme TL IM have indicated that the rate of the in situ process is higher using methanol (5 h at 25 °C) [20]. When using longer chain alcohols, the process takes longer and requires a higher temperature [19].
The results of research performed by other authors have shown that the optimal process duration at a temperature of 30 °C is 7 h when using ethanol. In our case, butanol was used for the simultaneous oil extraction and transesterification, and the same optimal duration of the process was determined. However, a higher optimal temperature of 40 °C was required to obtain a high yield of rapeseed butyl esters.
3.3.3. Optimal Amount of Lipase
After determining the optimal reaction time and temperature, the influence of the lipase (Lipozyme TL IM) content on the yield of rapeseed butyl esters was investigated. The amount of lipase selected in the study was 3%, 4%, 5%, and 6% by weight of the oil contained in the rapeseed. The molar ratio of butanol to rapeseed oil was 5:1.
The reaction product was tested to determine the rapeseed oil and rapeseed oil butyl ester yields. The transesterification degree and the levels of glycerides contained in the biodiesel were also analyzed. The results of the experiments are presented in Table 4.

Table 4.
Dependence of the yield of rapeseed oil, rapeseed oil butyl esters, the transesterification degree, and the glyceride content in reaction product on the lipase amount.
The data show that the yield of rapeseed oil and rapeseed oil butyl esters in the reaction product was greater than 98% (w/w). This indicates that, almost all the oil was extracted from the rapeseed.
The high yield was obtained due to the use of a mixture of mineral diesel and alcohol in order to maximize the effectiveness of the simultaneous rapeseed oil extraction and transesterification. The amount of lipase in the reaction mixture had a great influence on the transesterification degree; by increasing the amount of lipase in the reaction medium, a higher transesterification degree was obtained. The highest transesterification degree of 99.08 ± 0.08% (w/w) was achieved using 6% of the lipase Lipozyme TL IM. The levels of glycerides in the biodiesel in this case also met the requirements of the standard EN 14214. The reaction products obtained with a lower lipase content had excess levels of unreacted mono-, di-, and triglycerides.
According to data from other authors, the optimal Lipozyme RM IM amount was 5.2% for simultaneous rapeseed oil extraction and transesterification with a butanol and mineral diesel mixture [17]. According to Antczak et al., the optimum concentration of the lipase Lipozyme TL IM is 5% for the simultaneous extraction and transesterification with ethanol for biodiesel synthesis [25,26].
In the enzymatic in situ process, biomass of microalgae Ankistrodesmus sp. was also used as a raw material for oil extraction and transesterification with ethanol in diesel fuel media. A high yield of esters was obtained at an optimal Lipozyme TL IM concentration of 9.6% (of oil content) [27]. The results of some studies have shown that in the production of mixtures of biodiesel 10% and mineral diesel 90% by applying the in situ process and using rapeseeds containing oil of a higher acidity, in the case of the application of methanol or ethanol as an acyl receptor, the optimal amount of lipase Lipozyme TL mixture should be at least 5% [19,20]. However, the results of our studies show that the application of butanol required a higher content of the biocatalyst, which must be at least 6% by weight of the oil contained in the rapeseed.
3.4. Quality Indicators of Fuel Blends
Biodiesel, which is used in diesel engines, must comply with the requirements of the biodiesel standard EN 14214, but the resulting fuel mixtures in this study contained 90% mineral diesel, so it is also important to take into account the requirements of the EN 590 standard for mineral diesel. The quality indicators of the fuel mixtures produced, in which biodiesel, i.e., rapeseed oil butyl esters, accounted for 10% by weight, were determined and the results obtained were compared to the requirements of standards and the published characteristics of other fuel mixtures containing mineral diesel and 10% rapeseed oil methyl or ethyl esters.
Based on the results of the tests presented in Table 5, the mixtures of mineral diesel and rapeseed butyl esters complied with the requirements for ester content and glyceride content specified in the standard EN 14214, and these indicators were also met by the fuel mixtures containing mineral diesel and rapeseed methyl and ethyl esters [19,20].

Table 5.
Quality indicators of fuel mixtures.
Based on the results of the tests presented in Table 5, the mixtures of mineral diesel and rapeseed butyl esters complied with the requirements for ester content and glyceride content specified in the standard EN 14214, and these indicators were also met by fuel mixtures containing mineral diesel and rapeseed methyl and ethyl esters [19,20].
According to the requirements of the mineral diesel standard EN 590, the fuel density should be between 820 and 845 kg m−3, and according to the requirements of biodiesel standard EN 14214, the density value should be within the range of 860–890 kg m−3. The density of the resulting fuel mixtures containing rapeseed butyl esters reached 831 kg m−3. Only the fuel mixtures containing rapeseed oil ethyl esters (density—819 kg m−3) did not meet this requirement, but the resulting density was very close to the required norm. In accordance with requirements of EN 590, the viscosity should be at least 2 mm2s−1 and not more than 4.5 mm2s−1. This requirement was met by the produced fuel mixture and, therefore, such fuels could lubricate parts of the fuel system sufficiently well. All the fuel mixtures produced also complied with the flash point requirements, which should be at least 55 °C in accordance with the requirements of the standard EN 590. Sulphur in the fuel mixtures was not present and this is a positive feature from an environmental point of view. Because high-sulphur fuels have a negative impact on the environment, as emissions of sulphur oxides increase, humans are affected. Oxidation resistance is a measure of fuel stability, and this is especially important for biofuels, which, when stored for a longer period of time, begin oxidation processes, during which compounds are formed that impair the properties of the fuel. The oxidation stability of the fuel mixtures containing RBE reached 12.84 h and was higher than the oxidation stability of mixtures containing rapeseed ethyl esters, which reached 7.49 h but was lower than the oxidation stability of the fuel mixtures containing 10% of rapeseed methyl ethers (17.87 h).
The degree of corrosion of all the fuel mixtures was one, so they met the requirements of both the mineral diesel and biodiesel standards.
It is important that fuel can be used in cold periods. The low-temperature characteristics of fuel can be assessed by examining the cold filter plugging point (CFPP). According to the requirements of EN 590, the CFPP should be 5 °C for fuel used in summer, minus 15 °C for fuel used during the transition period and minus 32 °C in winter. The RBE containing the fuel mixture complied with the requirements of CFPP for winter fuel. It was determined that CFPP value was minus 33 °C, while the CFPP value for the mixtures containing rapeseed methyl esters found by other authors was even lower and reached minus 36 °C, and the CFPP value for the mixtures containing rapeseed ethyl esters was equal to minus 37 °C.
Similar physical and chemical properties of fuel mixtures (diesel 90%–biodiesel 10%) were determined by other scientists, where biodiesel was made from edible waste oil, chichen fat, palm kernel oil, and goat fat [28].
Summarizing the results of studies on the physical and chemical properties of fuel mixtures, it can be stated that the product obtained by applying the in situ process, containing 10% rapeseed butyl esters in a mixture with mineral diesel, met the requirements of the standards and can be used in diesel engines even in the winter period.
4. Conclusions
In order to reduce the cost of biofuel production, it is appropriate to apply the process of simultaneous extraction of oil directly from ground rapeseed and the transesterification of the extracted oil. It is possible to reduce the cost of this process by using poor-quality rapeseed with a higher acidity of the oil. The use of a mixture of mineral diesel and butanol for simultaneous oil extraction and transesterification (in situ) makes it possible to directly obtain a fuel mixture in which biodiesel accounts for 10%. From an environmental point of view, it is appropriate to apply enzymatic processes in biodiesel synthesis. Therefore, this paper studied the effects of using lipases as a catalyst. The results of the evaluation of the effectiveness of the lipases showed that a higher catalytic activity in the in situ process was characterized by the biocatalyst Lipozyme TL IM. The results obtained showed a tendency that the efficiency of oil extraction was more influenced by the reaction time than the temperature. The optimal conditions for the simultaneous oil extraction and transesterification process were determined as follows: reaction duration of 7 h, a reaction temperature of 40 °C and a lipase content of 6% (from the oil content in rapeseed). An oil extraction efficiency of 99.92 ± 0.04% (w/w) was observed at these conditions. The transesterification degree of 99.08 ± 0.08% (w/w) met with the requirements of the standards for biodiesel fuel. The physical and chemical properties of the produced fuel blends met the requirements of the standards EN 14214 and EN 590, and therefore they can be used in diesel engines.
The process of simultaneous extraction and transesterification of oil is attractive from an environmental point of view due to the following aspects: the stage of extraction of oil from oily seeds and the associated energy and material costs are avoided, raw materials unsuitable for food and feed can be used, butanol used for transesterification can be obtained from raw materials of plant origins, and chemical catalysts are replaced by biocatalysts. In this process, a fuel mixture containing 10% biodiesel can be obtained directly. Such a mixture is currently produced by mixing mineral diesel and biodiesel and is used in the transport sector.
Author Contributions
Conceptualization, E.S.; methodology, E.S. and V.M.; investigation M.S., E.S. and V.M.; data curation and methodology M.S. and E.S.; resources E.S.; writing—original draft preparation M.S., E.S. and V.M.; writing—review and editing, E.S. and V.M.; supervision, E.S. and V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
Authors are thankful for biocatalyst (Lipozyme TL IM, Novozyme 435, Lipozyme 435, Lipolase 100L, Lecitase Ultra, Resinase A 2X, Palatase 20000L, Lipozyme CALB, Lipozyme TL 100L, Lipex 100L) JSC “Biopolis” (Lithuania).
Conflicts of Interest
The authors declare no conflict of interest.
References
- U.S. Department of Energy. Biodiesel Handling and Use Guide, 8th ed.; U.S. Department of Energy: Washington, DC, USA, 2020.
- Alexander, D.; Schwandt, H. The Impact of Car Pollution on Infant and Child Health: Evidence from Emissions Cheating; CEPR Discussion Paper No. DP13805; IZA Institute of Labor Economics: Bonn, Germany, 2019. [Google Scholar]
- Karmakar, B.; Halder, G. Progress and future of biodiesel synthesis: Advancements in oil extraction and conversion technologies. Energy Convers. Manag. 2019, 182, 307–339. [Google Scholar] [CrossRef]
- Sarno, M.; Iuliano, M. Biodiesel production from waste cooking oil. Green Process. Synth. 2019, 8, 828–836. [Google Scholar] [CrossRef]
- Lei, H.; Ding, X.; Zhang, H.; Chen, X.; Li, Y.; Zhang, H.; Wang, Z. In situ production of fatty acid methyl ester from low quality rice bran: An economical route for biodiesel production. Fuel 2010, 89, 1475–1479. [Google Scholar] [CrossRef]
- Zeng, J.; Wang, X.; Zhao, B.; Sun, J.; Wang, Y. Rapid In Situ Transesterification of Sunflower Oil. Ind. Eng. Chem. Res. 2009, 48, 850–856. [Google Scholar] [CrossRef]
- Makareviciene, V.; Sendzikiene, E.; Gumbyte, M. Application of Simultaneous Oil Extraction and Transesterification in Biodiesel Fuel Synthesis: A Review. Energies 2020, 13, 2204. [Google Scholar] [CrossRef]
- Ambat, I.; Srivastava, V.; Sillanpää, M. Recent advancement in biodiesel production methodologies using various feedstock: A review. Renew. Sustain. Energy Rev. 2018, 90, 356–369. [Google Scholar] [CrossRef]
- Makareviciene, V.; Sinkuniene, D.; Sendzikiene, E. Optimisation of enzymatic transesterification of linseed oil and pork lard mixture with ethanol using response surface methodology. J. Renew. Sustain. Energy 2015, 7, 053119. [Google Scholar] [CrossRef]
- Makareviciene, V.; Gumbyte, M.; Yunik, A.; Kalenska, S.; Kalenskii, V.; Rachmetov, D.; Sendzikiene, E. Opportunities for the use of chufa sedge in biodiesel production. Ind. Crops Prod. 2013, 50, 633–637. [Google Scholar] [CrossRef]
- Kostić, M.D.; Bazargan, A.; Stamenković, O.S.; Veljković, V.B.; McKay, G. Optimization and kinetics of sunflower oil methanolysis catalyzed by calcium oxide-based catalyst derived from palm kernel shell biochar. Fuel 2016, 163, 304–313. [Google Scholar] [CrossRef]
- Kwiecien, J.; Hájek, M.; Skopal, F. The effect of the acidity of rapeseed oil on its transesterification. Bioresour. Technol. 2009, 100, 5555–5559. [Google Scholar] [CrossRef][Green Version]
- Sendzikiene, E.; Sinkuniene, D.; Kazanceva, I.; Kazancev, K. Optimization of low quality rapeseed oil transesterification with butanol by applying the response surface methodology. Renew. Energy 2016, 87, 266–272. [Google Scholar] [CrossRef]
- Shuit, S.H.; Lee, K.T.; Kamaruddin, A.H.; Yusup, S. Reactive extraction and in situ esterification of Jatropha curcas L. seeds for the production of biodiesel. Fuel 2010, 89, 527–530. [Google Scholar] [CrossRef]
- Shuit, S.H.; Lee, K.T.; Kamaruddin, A.H.; Yusup, S. Reactive extraction of Jatropha curcas L. seed for production of biodiesel: Process optimization study. Environ. Sci. Technol. 2010, 44, 4361–4367. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.T.; Yeh, K.L.; Chen, C.L.; Chang, J.S. Enzymatic transesterification ofmicroalgal oil from Chlorella vulgaris ESP-31 for biodiesel synthesis using immobilized Burkholderia lipase. Bioresour. Technol. 2012, 108, 119–127. [Google Scholar] [CrossRef]
- Sendzikiene, E.; Makareviciene, V.; Gumbyte, M. Reactive extraction and fermental transesterification of rapeseed oil with butanol in diesel fuel media. Fuel Process. Technol. 2015, 138, 758–764. [Google Scholar] [CrossRef]
- Kojima, S.; Du, D.; Sato, M.; Park, E.Y. Efficient production of fatty acid methyl ester from waste activated bleaching earth using diesel oil as organic solvent. J. Biosci. Bioeng. 2004, 98, 420–424. [Google Scholar] [CrossRef]
- Santaraite, M.; Sendzikiene, E.; Makareviciene, V.; Kazancev, K. Biodiesel Production by Lipase-Catalyzed in Situ Transesterification of Rapeseed Oil Containing a High Free Fatty Acid Content with Ethanol in Diesel Fuel Media. Energies 2020, 13, 2588. [Google Scholar] [CrossRef]
- Sendzikiene, E.; Santaraite, M.; Makareviciene, V. Lipase-Catalysed In Situ Transesterification of Waste Rapeseed Oil to Produce Diesel-Biodiesel Blends. Processes 2020, 8, 1118. [Google Scholar] [CrossRef]
- Abbadi, A.; Leckband, G. Rapeseed breeding for oil content, quality, and sustainability. Eur. J. Lipid Sci. Technol. 2011, 113, 1198–1206. [Google Scholar] [CrossRef]
- Thliveros, P.; Uçkun Kiran, E.; Webb, C. Microbial biodiesel production by direct methanolysis of oleaginous biomass. Bioresour. Technol. 2014, 157, 181–187. [Google Scholar] [CrossRef]
- Hernández-Martín, E.; Otero, C. Different enzyme requirements for the synthesis of biodiesel: Novozym® 435 and Lipozyme® TL IM. Bioresour. Technol. 2008, 99, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Shamel, M.M.; Hasan, M.; Ramachandran, B. Operational stability of lipase enzyme: Effect of temperature and shear. Asia Pac. J. Chem. Eng. 2005, 13, 599–604. [Google Scholar] [CrossRef]
- Haas, J.; Piazza, G.J.; Foglia, T.A. Enzymatic approaches to the production of biodiesel fuels. Lipid Biotechnol. 2002, 29, 587–598. [Google Scholar]
- Antczak, S.M.; Kubiak, A.; Antczak, T.; Bielecki, S. Enzymatic biodiesel synthesis—Key factors affecting efficiency of the process. Renew. Energy 2009, 34, 1185–1194. [Google Scholar] [CrossRef]
- Makareviciene, V.; Gumbyte, M.; Sendzikiene, E. Simultaneous extraction of microalgae Ankistrodesmus sp. oil and enzymatic transesterification with ethanol in the mineral diesel medium. Food Bioprod. Process. 2019, 116, 89–97. [Google Scholar] [CrossRef]
- Foroun, R.; Esmaeili, H.; Mousavi, S.M.L.; Hashemi, S.A.; Yeganeh, G. The Physical Properties of Biodiesel-Diesel Fuel Produced via Transesterification Process from Different Oil Sources. Phys. Chem. Res. 2019, 7, 415–424. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).