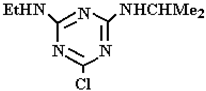
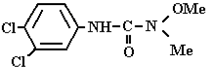
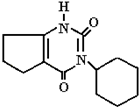
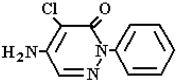
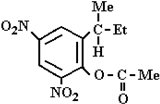
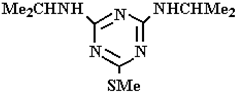
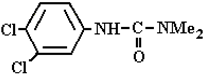
Abstract
The decomposition of seven herbicides (atrazine, linuron, lenacil, chloridazon, dinoseb acetate, prometryn, and diuron) was carried out by detonative combustion. The investigated blasting material was produced on the basis of porous ammonium nitrate, which served as an oxidizer, while the pesticides played the role of the fuel. Detonative decomposition of the mixtures was carried out in blast-holes in soil. The efficiency of the decomposition process was assessed using the techniques of gas chromatography, high-efficiency liquid chromatography, and additionally by biological tests according to the grading of the European Weed Research Council. The results demonstrate an efficient decomposition of the tested herbicides. In the tested soil samples taken after the detonation decomposition of the herbicide, no symptoms of phytotoxic effects on the plants were found. This was confirmed by the lack (or at most negligible amounts) of residual herbicides in the soil samples. Only for the samples of chloradizine and diuron were large amounts of residual biologically active substance found.
1. Introduction
The rapid increase in the emergence of resistant super weeds seen in agriculture requires not only the increased use of herbicides, but also leads to the necessity of creating new forms of herbicides. Genetic modifications of plants also require the application of new selective pesticides. The storage of unwanted expired pesticides can also lead to many problems. The Food and Agriculture Organization reports that the total amount of out-of-date pesticides, of all types, is 400,000–500,000 tons [1]. Over 20% of them are organic, halogen derivative substances. In Africa and the Middle East, about 47,000 tons of pesticide are estimated to be stored. In Poland, within just the past 20 years, 60,000 tons of pesticides were stored that had never been used, and there is now a need to neutralize them [2].
Many chemical, biological [3,4,5,6,7,8], and thermal [9,10,11,12,13] methods of pesticide neutralization have been devised. Numerous studies have shown that not all technologies are universally effective, and many of them are designed to only work for a specified group of substances (for example, organochlorines, organophosphates, carbamates, etc.).
Here, an investigation was carried out to determine the applicability of detonative combustion to decompose distinct herbicides with different functional groups, in the same way as it has been previously applied to the decomposition of 4,6-dinitro-o-cresole (DNOC) [14,15].
Currently, two methods are predominantly used for the determination of the present pesticide content in environmental samples, namely high-pressure liquid chromatography (HPLC) and gas chromatography (GC), which use specific detectors for certain groups of chemical compounds. Regarding the analytical methods for the determination of herbicide content in various materials (water, soil, plants, animal tissue etc.) [16,17,18,19], the investigation procedure can be reduced to the isolation of the herbicide by means of solvent extraction. This is followed by purification of the obtained solution and its standardization and final determination. The most frequently applied method of determination is gas chromatography, but for substances that are likely to decompose when experiencing the temperature conditions necessary during GC analysis, liquid chromatography can serve as an alternative method. As an auxiliary method, the thin-layer chromatography method has also been applied.
We carried out an assessment of the efficiency of the decomposition (by detonative combustion) of several herbicides by means of liquid chromatography. The results confirmed the decomposition of the herbicides. These findings were then correlated with results obtained from biological tests.
2. Materials and Methods
2.1. Preparation of Herbicide Samples for Detonative Combustion
We decided that the herbicide would be a component of the heterogenetic explosive substance of the type ANFO (ammonium nitrate/fuel oil), in which the herbicide replaces fuel oil. The compositions of the porous ammonium nitrate and herbicide were designed in such a way that the density of the obtained explosive blend was d = 0.900 kg/dm3, and the oxygen balance was B = 0%. For the designed blends, the performance parameters were calculated in order to establish an explosion temperature that should be high enough to decompose the herbicide. The results of the calculation obtained for various exemplary blends are shown in Table 1. The parameters of the explosive material ANFO, obtained from ammonium nitrate and liquid paraffin, are given for comparison.

Table 1.
Thermodynamic parameters of the explosive blends based on ammonium nitrate and a herbicide component.
By using a specially prepared composition of the materials, we are able to obtain rapid reactions with detonation speeds reaching 4000 m/s. In this combustion reaction, a herbicide should be converted to simple compounds, such as H2O, CO2, N2, etc. The mechanism of the herbicide’s decomposition should ideally be undertaken by quick heating to high temperatures (temperature of the detonation products before their expansion is up to 2400 °C, and the temperature of the detonative wave front is approximately one hundred times higher). Under such conditions, the high energy of the bond vibrations causes their cleavage and the degradation of the compound. Recombination of the generated moieties is prevented by oxidation and quick cooling. The presence of traces of aggregate in the environment near the explosion is caused by the movement of fine crushed rocks to the analyzed sample.
The production of an ammonium nitrate/fuel oil (ANFO) charge (here, porous ammonium nitrate with herbicide as a fuel), requires suitable conditions allowing the maintenance of the run of combustion reactions in a detonative way. To obtain these conditions, the following tasks were realized:
- ⮚
- Calculation of useable parameters for the considered mixtures. Calculations were carried out under the assumption of a stoichiometric course of detonative combustion reaction, i.e., with zero oxygen balance.
- ⮚
- The determination of higher calorific value, composition of elements, and global formula of hypothetic component, in the case when the method is applied to expired herbicides.
It was assumed that during detonation of ANFO, the airborne soil particles fall back into the crater. The amount of charge was taken such that the crater after detonation had a radius r = 1.25–1.50 m and a depth of 0.90–1.20 m. The soil samples were retrieved from a depth of 1.4 m. The prepared ANFO charge with herbicide, of a total mass of 2 kg, was placed in a pentrite cartridge (PET) container with a detonator and sand. After the arming of a percussive charge with a non-electric initiation system, the Non-Electric Detonator (NONEL), the container was placed in blast-hole at the depth of 1.20 m, and then tamping was performed to protect the hole.
2.2. Preparation of the Soil Substrate for the Biological Test
After detonation of the charge, soil samples were subjected to biological tests [20]. In a funnel, five holes were drilled by means of a manual earth drill. The depth of the holes was 140 cm (relative to the level before detonation). Sample material from each hole was tipped on foil, and precisely mixed for further analysis. Until further processing occurred, it was maintained at a low temperature [21].
2.3. Preparation of Soil Extracts for Chromatographic Analysis
Soil samples of 50 g were subsequently mixed with two portions of methylene chloride, 50 cm3 each, and then extraction was carried out by an ultrasonic treatment [22]. Extracts were filtered, and in a vacuum evaporator, finally dissolved in 10 cm3 of acetone. Prepared samples were analyzed to investigate the content of biologically active substances.
2.4. Preparation of Soil Extracts to Biological Tests
Samples of 5000 g were subsequently mixed with two portions of methylene chloride, 5000 cm3 each, and extraction was carried out for 30 min in a Rotavapor R-250 with a flask of capacity 20 dm3. Following this, the extracts were filtered and evaporated to a dry mass. The dry remnants were washed out from the flask walls with 1000 cm3 of acetone, and the obtained solution was subsequently concentrated to a total volume of 10 cm3. Thereafter, the sample was standardized by diluting the concentrated solution with distilled water to a volume of 100 cm3. Finally, the sample of the extract was used for the biological tests.
2.5. Determination of Herbicide Residue in Extracts by Gas Chromatography
To determine the herbicide residue, a Varian 3400 gas chromatograph was used. The chromatograph was equipped with an electron capture detector (ECD) and an IBDH integrator assuring automatic data analysis. Chromatographic separation was carried out by means of a dimethyl silicone capillary column.
The inner diameter of the column was 0.25 mm, the thickness of the phase film was 0.25 µm, and the length was 30 m. The thermal conditions for decomposition were a batcher temperature of 280 °C and a detector temperature of 300 °C; the temperature of the column was 80 °C for 5 min, and then it was increased to 290 °C at a rate of 10 °C/min. The carrier gas was helium, and the flow rate was 1 mL/min.
Soil extracts, besides the possible contents of herbicides, also contain organic compounds, which are volatile under thermal conditions typical for gas chromatography. Both genuine soil compounds as well as herbicides are likely to be detected. About half of the determined chemical compounds eluted beyond the elution range of soil components. The remainder of them elute in the same, or similar, retention times.
2.6. Determination of Herbicide Residue in Extracts by Means of Liquid Chromatography
Diuron, a herbicide that decomposes when experiencing the thermal conditions required for gas chromatography, was analyzed by means of a high-efficiency liquid chromatography method. The liquid chromatograph Varian 4000 was equipped with a detector for measuring UV absorption (the wavelength during measurements was 248 nm). Analyses were made for reversed phase systems. A column with a length of 250 mm and an inner diameter of 4.6 mm was filled with Nucleosilem C 18 (silica gel with chemically bonded octodecylene). The polar moving phase was a mixture of acetonitrile and water in the proportion 1:1. The flow rate of the moving phase was 1.2 mL/min, and its pressure was 13.1 MPa. The identification of the herbicide was based on a comparison to the retention times of standard substances. Model curves were drawn from measurements of the absorbance of herbicides in solutions of definite concentrations, which were injected into definite volumes. In the case of the detection of the herbicide residue in samples, a definite volume of standard extract was injected to the chromatograph, and its absorbance was determined. Results were taken from a calibration curve.
2.7. Biological Tests
- Biological tests were carried out in three series:
- ⮚
- 1st series:
On the drawn soil samples, after detonative decomposition of the herbicides, seeds of oat and charlock were sown. The same control tests were carried out on floral (pure) soil.
- ⮚
- 2nd series:
On the floral (pure) soil, seeds of oat and charlock were sown, and in the third leaf phase, they were treated with the soil extracts from the detonative decomposition of herbicides.
- ⮚
- 3rd series:
On the floral (pure) soil, seeds of oat and charlock were sown, and in the third leaf phase, they were sprayed or their roots were treated with a standard solution of herbicide in the dose recommended by the producer (reference test).
All the tests were carried out at the same time, under the same conditions, and the plants were kept in an environment assuring the proper conditions for vegetation. The seeds that were used for tests had been pre-selected (only robust and shapely seeds had been taken) to assure the best efficiency of sprouting.
Thirty seeds of charlock were sown on soil and placed in plastic plates of 14 cm diameter and 3 cm depth. In each series, seven plates were used (two of them for control purposes). After sprouting (in the second cotyledon phase), weak plants were removed, and 25 of the shapeliest plants remained. The same number of seeds as in the first series were then sown.
Eighteen seeds of oat were sown in a similar way as the charlock ones. After sprouting, weak plants were removed, and 15 of the shapeliest plants were left for further research. The same number of seeds as in the first series were then sown.
The plants were under observation at specified times every day, and the assessment was performed in five stages for the first series (the first assessment on the 4th day after the start of sprouting, the second assessment on the 7th day, the third assessment on the 11th day after sprouting, and the fourth and fifth assessments after 19 and 21 days after sprouting, respectively). Assessments were performed in four stages for the 2nd and 3rd series (the first assessment after three days of spraying, the second assessment 7 days after the first stage, the third one after 14 days, and the fourth one after 21 days).
Applied herbicides, species of plants, and application methods are shown in Table 2.

Table 2.
Species of plants and methods of herbicide application.
3. Results and Discussion
The results of the chromatography determination of herbicide residue in soil after detonative decomposition are listed in Table 3 and presented on chromatograms in Figure 1, Figure 2, Figure 3, Figure 4 and Figure 5. These contain the determinations of residue in samples from the neutral extraction of soil. An acid extraction of soil was also carried out, but in this case, there were no differences seen in chromatograms; thus, this part is not included here.

Table 3.
Results of the determination of herbicide residue in soil.
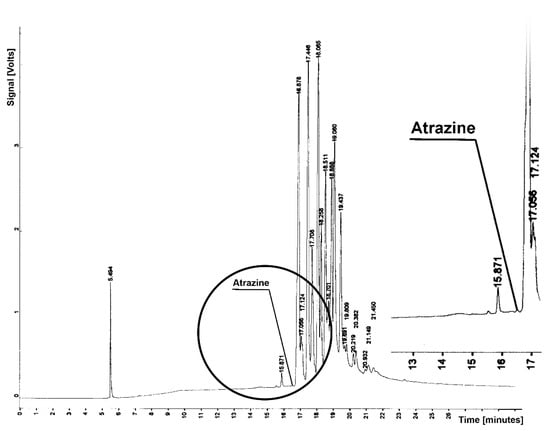
Figure 1.
Chromatogram from determination of atrazine residue in sample from soil extraction; tR = 16.576—atrazine.
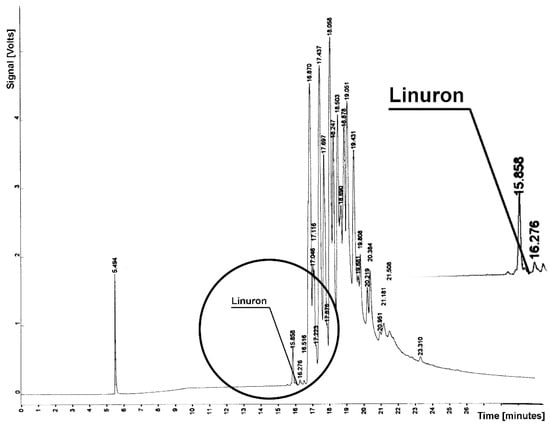
Figure 2.
Chromatogram from determination of linuron residue in sample from soil extraction; tR = 16.072—linuron.
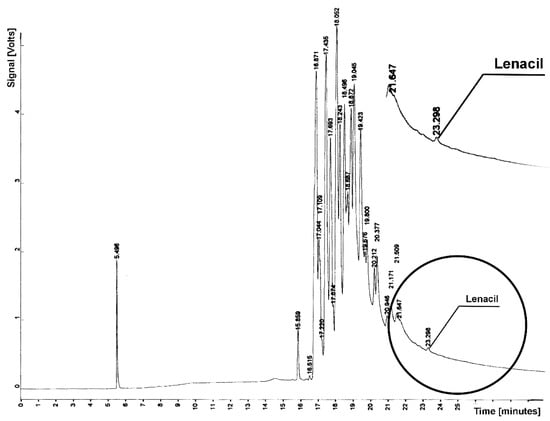
Figure 3.
Chromatogram from determination of lenacil residue in sample from soil extraction; tR = 23.298—lenacil.
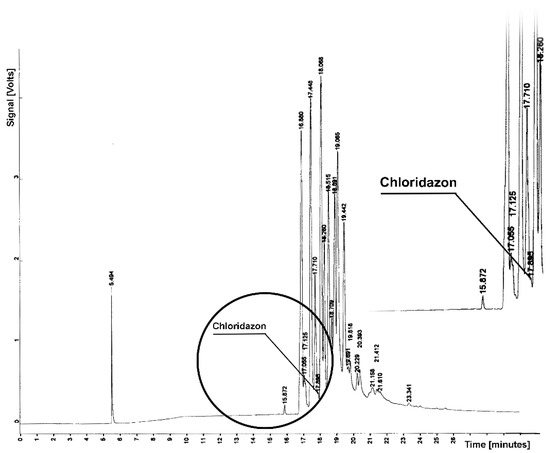
Figure 4.
Chromatogram from determination of chloridazon residue in sample from soil extraction; tR = 17.895—chloridazon.
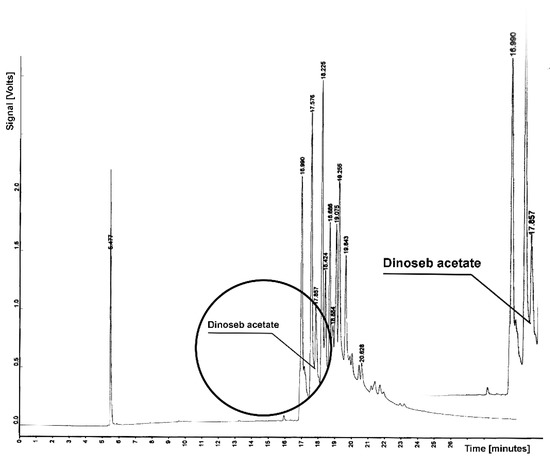
Figure 5.
Chromatogram from determination of dinoseb acetate residue in sample from soil extraction; tR = 17.651—dinoseb acetate.
In the case of herbicides for which the bdl result was obtained, a small amount of the compound in the sample was detected (Table 3). The method used does not detect such low concentrations. For two herbicides (chloridazone and diuron), the highest amounts of the substance in the sample were detected, 9.28 mg/kg and 22.2 mL/kg, respectively.
Figure 1 and Figure 2 present chromatograms from the determination of in the sample atrazine and linuron. These substances elute from the column before soil components, for Tr = 16.576 (atrazine) and tR = 16.072 (linuron). Chromatograms do not contain a peak or a suggestion of the detector signal that would demonstrate the herbicide residue. At the retention times mentioned, an almost horizontal line appears in the chromatograms, proving the absence of these herbicides.
Lenacil elutes from the column after natural soil compounds for tR = 23.298 (Figure 3). For the determined retention time of lenacil, a flat line also appears on the chromatogram, which proves the absence of this herbicide.
Chloridazon elutes from the column of soil components for retention times tR = 17.895 (Figure 4). The chromatogram shows a clear peak with the retention time determined, which proves the presence of this herbicide in the soil.
Figure 5 presents chromatograms with marks of the retention parameter dinoseb acetate, which elutes from the column of soil components for tR = 17.651. For the determined retention time, no presence of this compound (dinoseb) was found, and the peak in the chromatogram is flat.
Prometrine elutes at tR = 17.703, together with the soil sample at tR = 17.708, from a proportion of peak value for reference signal from tR = 18.064 to tR = 17.708 with the allowance of blank test chromatograms and analyzed tests. Extracts did not contain any residue of prometrine.
Diuron decomposes in thermal conditions of gas chromatography, and it was determined by means of a high-efficiency liquid chromatography method instead. An absorbance of analyzed extracts was measured for light with a wavelength of 248 nm, and the content of diuron was read from the calibration curve.
An assessment of the effect of herbicides on charlock and oat was carried out according to the nine-grade EWRC scale (Table 4) [23].

Table 4.
Quality valuation of herbicide influence on plants according to the 9-grade EWRC scale.

Table 5.
Results of the 1st series of biological tests.

Table 6.
Results of the 2nd series of biological tests.

Table 7.
Results of the 3rd series of biological tests (reference series).
In none of the biological tests was a phytotoxic influence of applied extract on the plants observed. Incomplete sprouting (shown in Table 1) is a result of the lack of full (100%) sprouting efficiency, in spite of preliminary selection of the seeds. The outer appearance of the seeds was the criterion for selection, which proved to be an insufficient measure.
The results of the tests confirm the absence (or at most, trace amounts) of herbicides in the soil that had been sampled from the locations of detonative combustion.
Symptoms of the phytotoxic influence of herbicides were first observed in the charlock samples sprayed with dinoseb acetate and bentazone. Due to the complete destruction of the plants, the observations were stopped on the 12th day after the application of dinoseb acetate or bentazone. For the same reasons, the observations of the oat plants were stopped 18 days after spraying.
The oat plants that had been sprayed with atrazine demonstrated the minimum number of symptoms of phytotoxic effect during the overall course of the test. The other plants were completely withered 24 days after being sprayed with the herbicides.
4. Conclusions
In the examined soil samples that were retrieved after a detonative decomposition of herbicide, symptoms of a phytotoxic influence on the tested plants was not observed. This confirms the lack, or at most only negligible trace amounts, of residual herbicides found in the soil samples.
In the case of chloradizine and diuron samples retrieved after detonation, they contained high amounts of a residual biologically active substance, as can be seen in the results of the gas and liquid chromatography analysis. This was probably caused by an incomplete detonation of the sample. Nevertheless, it did not show any influence in the biological tests.
Author Contributions
Conceptualization, J.B.; Methodology, J.B.; Writing—original draft, K.B. and J.B.; Writing—original draft preparation, K.B.; Writing—review and editing, K.B. All authors have read and agreed to the published version of the manuscript.
Funding
The publication was implemented as part of the statutory work 11.11.100 597.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author (K.B.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. Prevention and Disposal of Obsolete Pesticides. Available online: www.fao.org/agriculture/crops/obsolete-pesticides/where-stocks/en/ (accessed on 3 August 2022).
- Gałuszka, A.; Migaszewski, M.; Manecki, P. Pesticide Burial Grounds in Poland: A Review. Environ. Int. 2011, 37, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, T.; Russel, R.; Selleck, M. Using Enzymes to Clean Pesticide Residues. Pestic. Outlook 2002, 13, 149–151. [Google Scholar] [CrossRef]
- Barriuso, E.; Benoit, P.; Dubus, I.G. Formation of Pesticide Nonextractable (Bound) Residues in Soil: Magnitude, Controlling Factors and Reversibility. Environ. Sci. Technol. 2008, 42, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Siddique, T.; Arshad, M.; Saleem, M. Bioremediation and Phytoremediation of Pesticides: Recent Advances. Crit. Rev. Environ. Sci. Technol. 2009, 39, 843–907. [Google Scholar] [CrossRef]
- Rao, M.A.; Scelza, R.; Scotti, R.; Gianfreda, L. Role of Enzymes in the Remediation of Polluted Environments. J. Soil Sci. Plant Nutr. 2010, 10, 333–353. Available online: https://www.researchgate.net/publication/228819276_Role_of_enzymes_in_the_remediation_of_polluted_environments/link/09e4150d0243fdf3fb000000/download (accessed on 8 August 2022). [CrossRef]
- Chaplain, V.; Mamy, L.; Vieublé-Gonod, L.; Mougin Ch Benoit, P.; Barriuso, E.; Nélieu, S. Fate of Pesticides in Soils: Toward an Integrated Approach of Influential Factors. In Pesticides in the Modern World-Risks and Benefits; Stoytcheva, M., Ed.; Intech OpenAcess: London, UK, 2011; Chapter 29; pp. 535–550. [Google Scholar]
- Uqab, B.; Mudasir, S.; Nazir, R. Review on Bioremediation of Pesticides. J. Bioremediat. Biodegrad. 2016, 7, 343. Available online: https://www.omicsonline.org/open-access-pdfs/review-on-bioremediation-of-pesticides-2155-6199-1000343.pdf (accessed on 9 August 2022).
- Schneider, D.; Beckstrom, B.D. Cleanup of Contaminated Soils by Pyrolysis in an Indirectly Heated Rotary Kiln. Environ. Progress. 1990, 9, 165–168. [Google Scholar] [CrossRef]
- Stobiecki, S.; Cieszkowski, J.; Siłowiecki, A.; Stobiecki, T. Disposal of Pesticides as an Alternative Fuel in Cement Kiln. In Proceedings of the Project Outline 6th International HCH and Pesticides Forum, Poznań, Poland, 20–22 March 2001; pp. 285–289. Available online: http://www.hchforum.com/6th/forum_book/A.5.8.pdf (accessed on 11 August 2022).
- Senneca, O.; Scherillo, F.; Nunziata, A. Thermal Degradation of Pesticides under Oxidative Conditions. J. Anal. Appl. Pyrolysis 2007, 80, 61–76. [Google Scholar] [CrossRef]
- Karstensen, K.H. Guidelines for Treatment of Hazardous Wastes and Co-Processing of AFRs in Cement Kilns; Report 66011/E1101/KHK; Department for Environmental Affairs and Tourism: Pretoria, South Africa, 2008. [Google Scholar]
- Cement Kilns. Center for Health, Environment & Justice. Available online: https://chej.org/wp-content/uploads/Cement-Kilns-PUB-0401.pdf (accessed on 11 August 2022).
- Biegańska, J. Neutralization of 4,6-Dinitro-o-cresol Waste Pesticide by Means of Detonative Combustion. Environ. Sci. Technol. 2005, 39, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Biegańska, J.; Harat, A.; Zyzak, W. Neutralizing of Waste Pesticides from Dumping Grounds by Means of Explosive Burning. Inżynieria Ekol. 2013, 33, 13–20. [Google Scholar] [CrossRef]
- Amistadi, M.K.; Hall, J.K.; Bogus, E.R.; Mumm, R.O. Comparison of Gas Chromatography and Immunoassay Methods for the Detection of Atrazine in Water and Soil. J. Environ Sci. Health B 1997, 32, 845–860. [Google Scholar] [CrossRef] [PubMed]
- Polese, L.; Dores, E.F.G.C.; Jardim, E.F.G.; Navickiene, S.; Ribeiro, M.L. Determination of Herbicides Residues in Soil by Small Scale Extraction. Eclet. Quím. 2002, 27. Available online: https://www.researchgate.net/publication/26353260_Determination_of_herbicides_residues_in_soil_by_small_scale_extraction/link/09e415020da55affae000000/download (accessed on 11 August 2022). [CrossRef]
- Takatori, S.; Okihashi, M.; Okamoto, Y.; Kitagawa, Y.; Kakimoto, S.; Murata, H.; Sumimoto, T.; Tanaka, Y. A Rapid and Easy Multiresidue Method for the Determination of Pesticide Residues in Vegetables, Fruits, and Cereals Using Liquid Chromatography/Tandem Mass Spectrometry. J. AOAC Int. 2008, 91, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Delwichem, K.B.; Lehmann, J.; Walter, M.T. Atrazine Leaching from Biochar-amended Soils. Chemosphere 2014, 95, 346–352. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Crawford, J.W.; Álvarez, M.C.D.; Moreno, R.G. Soil Management for Sustainable Agriculture. Appl. Environ. Soil Sci. 2012, 2012, 850739. Available online: https://downloads.hindawi.com/journals/aess/2012/850739.pdf (accessed on 11 August 2022). [CrossRef]
- Ozcan, S.; Tor, A.; Aydin, M.E. Analytical Methods for Viable and Rapid Determination of Organochlorine Pesticides in Water and Soil Samples. In Pesticides—Strategies for Pesticides Analysis; Stoytcheva, M., Ed.; InTech: UK, London, 2011; Chapter 3; pp. 59–82. Available online: https://www.intechopen.com/chapters/12949 (accessed on 7 August 2022).
- Ashley, K.; Wise, T.J.; Mercado, W.; Parry, D.B. Ultrasonic Extraction and Field Portable Anodic Stripping Voltarnmetry Measurement of Lead in Dust Wipe Samples. J. Hazard. Mater. 2001, 83, 41–50. [Google Scholar] [CrossRef]
- European Weed Research Council (EWRC). Report of 3rd and 4th Meetings of EWRC; Committee of Methods in Weed Research, Weed Research: Oxford, UK, 1964; Volume 4. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).