Life Cycle Analysis of Food Waste Valorization in Laboratory-Scale
Abstract
:1. Introduction
2. Materials and Methods
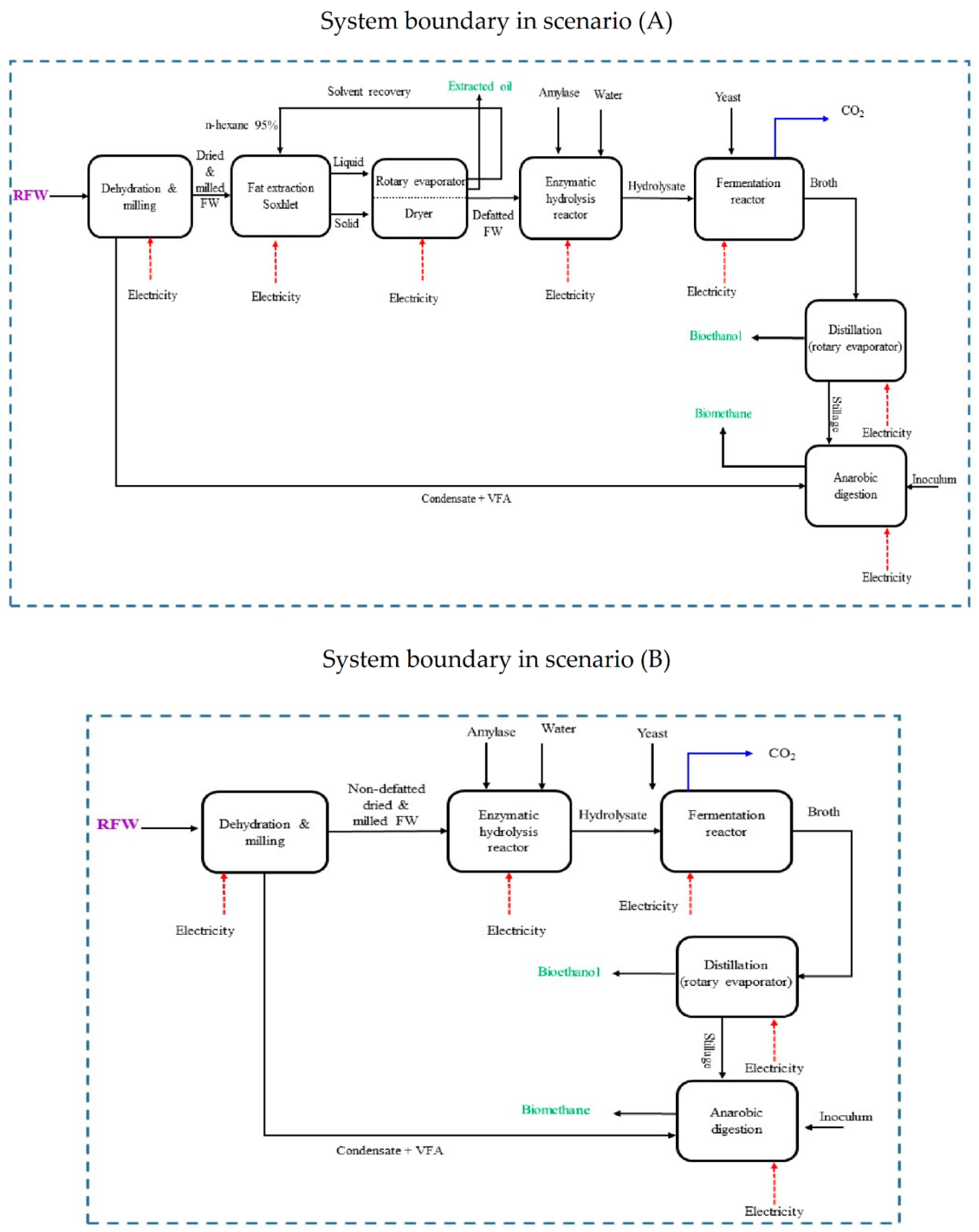
2.1. Descriptions of Food Waste Conversion at Laboratory Scale
2.2. Descriptions of LCA Scenarios
2.2.1. Goals and Scope Definition
2.2.2. Inventory Analysis
2.2.3. Impact Assessment
3. Results and Discussions
3.1. Characterization Results
Avoided Burdens
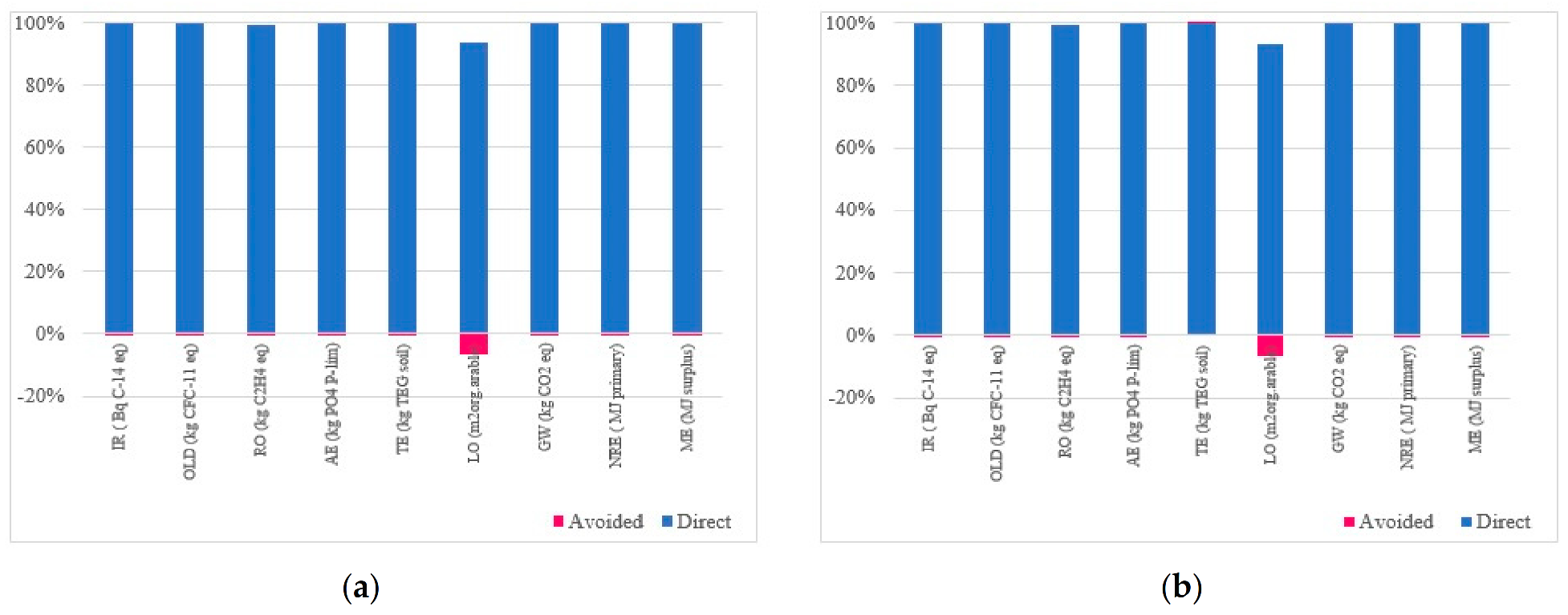
3.2. Normalization Results
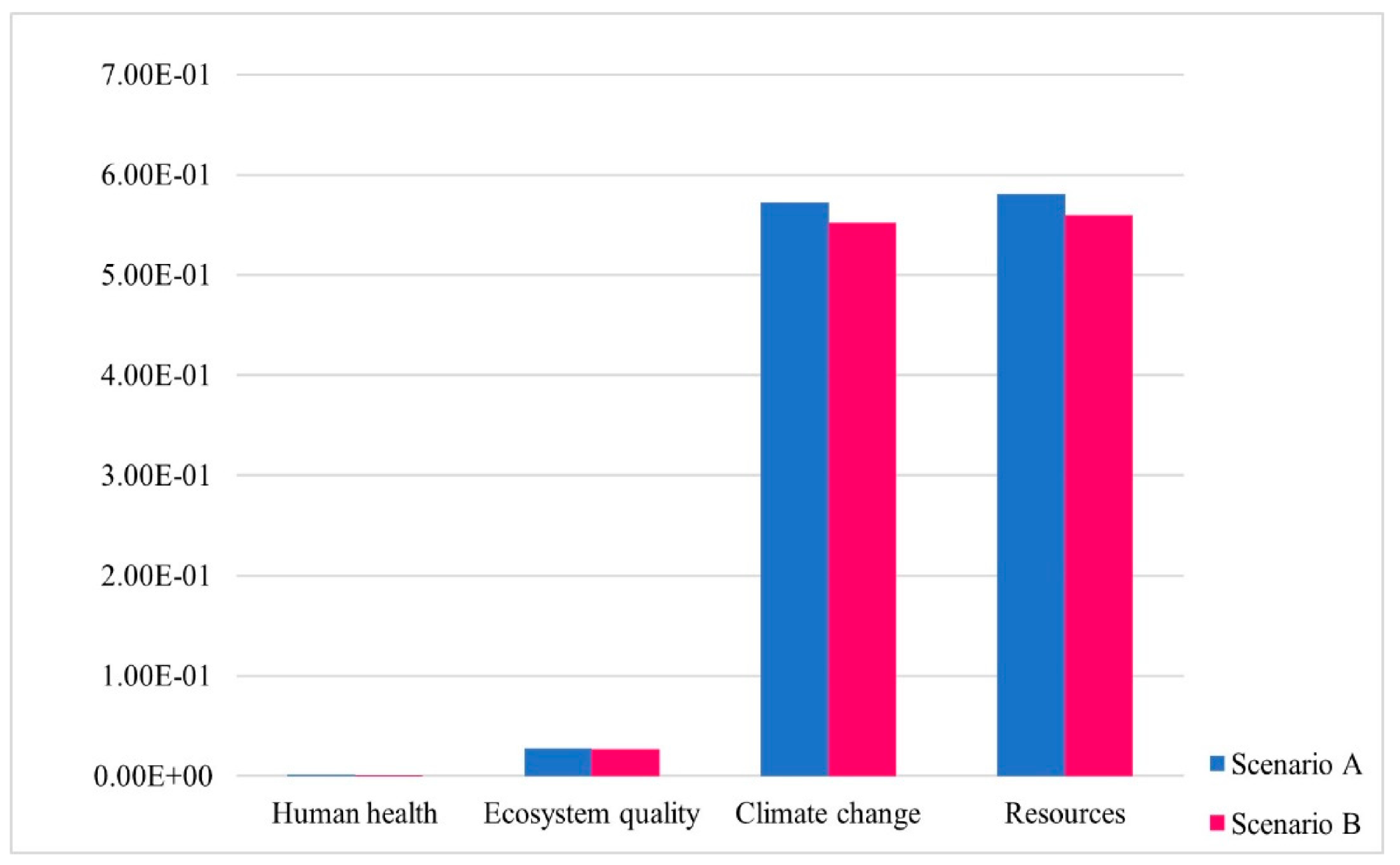
3.3. Single Score LCIA Results
3.4. Sensitivity Analysis
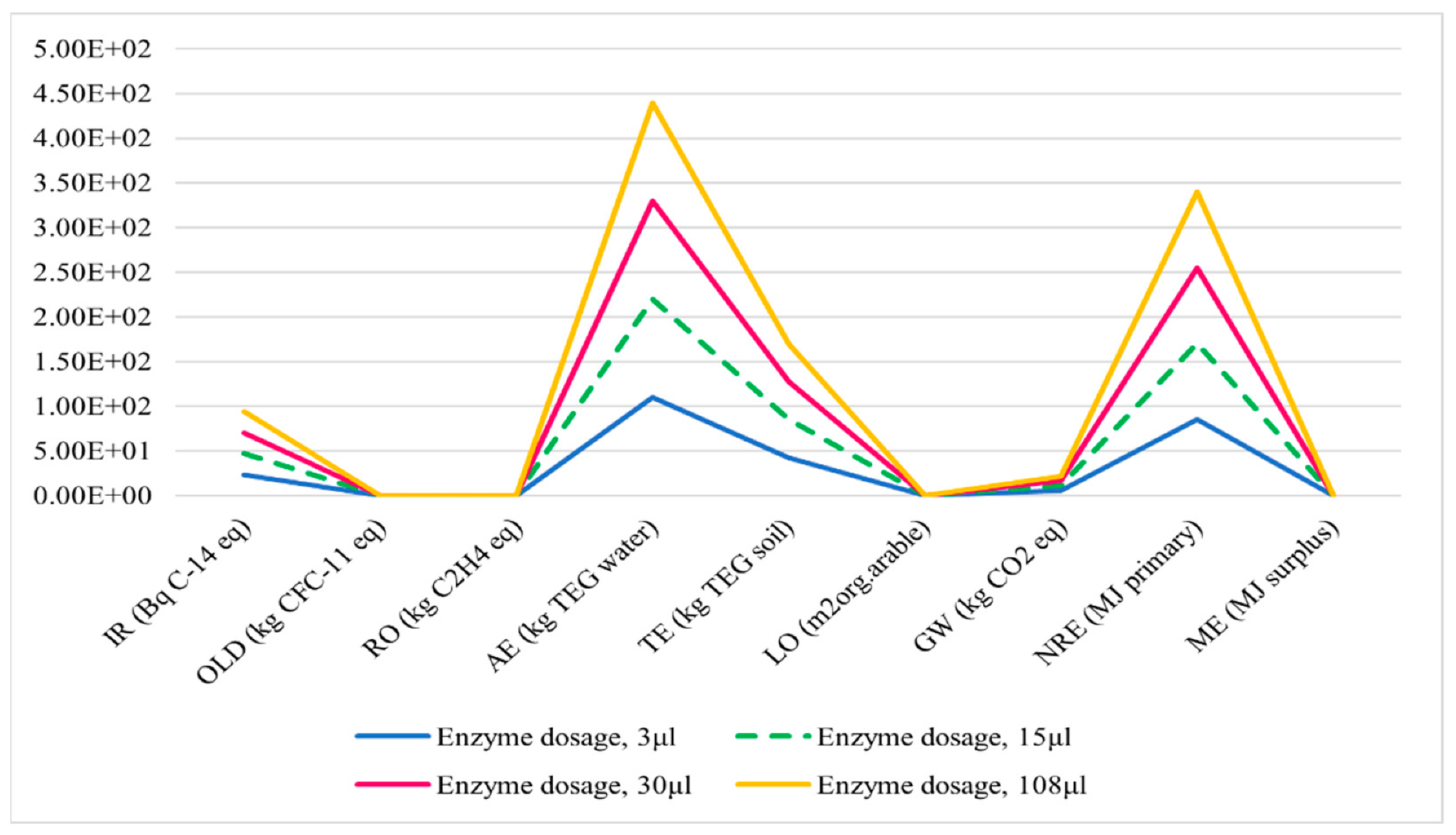
3.4.1. Enzyme Loading
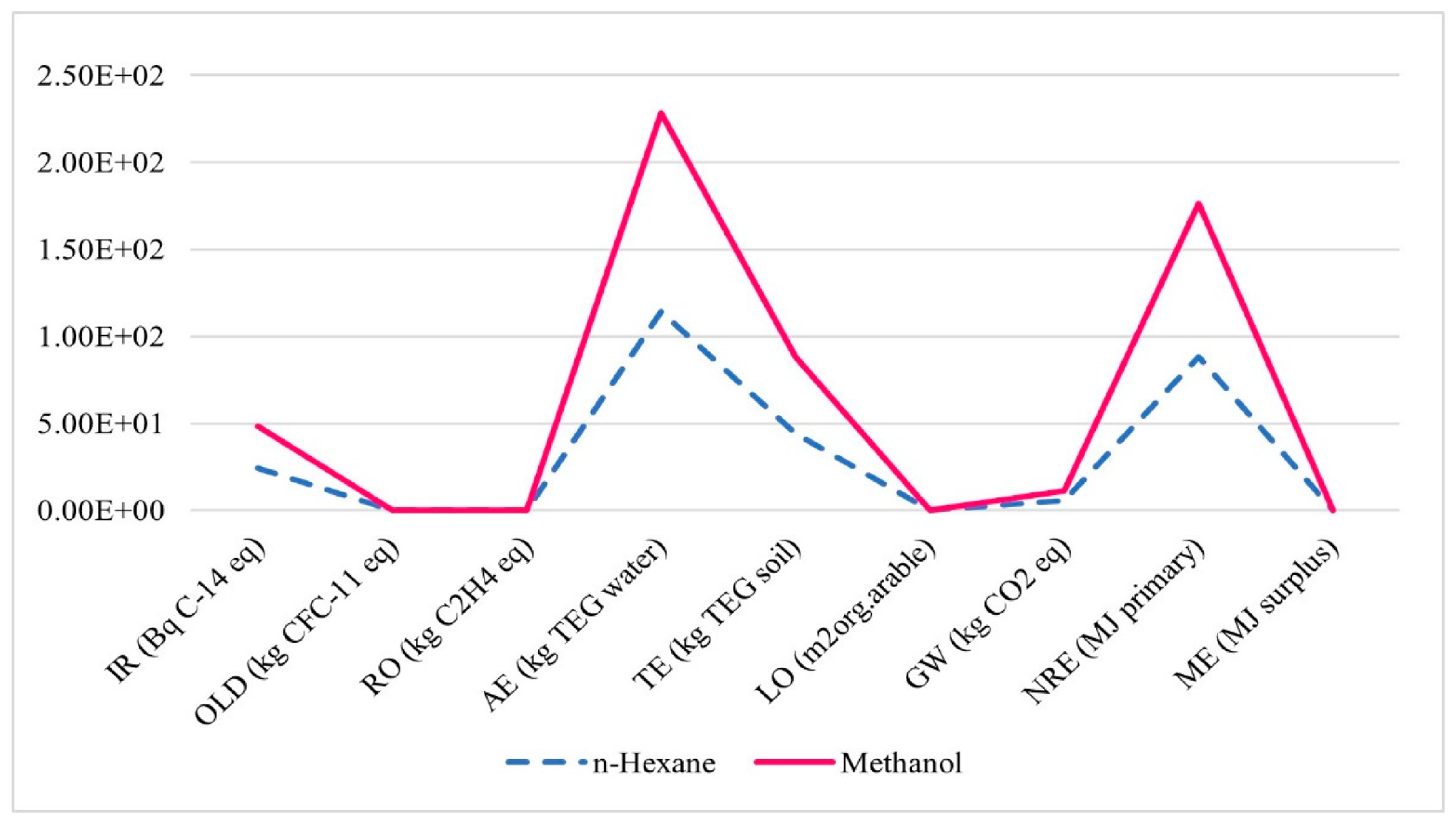
3.4.2. Solvent Type in the Fat Extraction Process
3.5. Limitations of the Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| List of Acronyms | |
| LCA | Life Cycle Assessment |
| FU | Functional Unit |
| EU | European Union |
| EEA | European Environment Agency |
| 1G | First-generation |
| 2G | Second-generation |
| RFW | Raw Food Waste |
| LUC | Land Use Change |
| FW | Food Waste |
| DFW | Defatted Food Waste |
| NDFW | Non-defatted Food Waste |
| ISO | International Standards Organization |
| VFA | Volatile Fatty Acids |
| GWP | Global Warming Potential |
| CC | Climate Change |
| IPCC | Intergovernmental Panel on Climate Change |
| BMP | Biomethane Potential |
| UEST | Unit of Environmental Science and Technology |
| NTUA | National Technical University of Athens |
| VFA | Volatile Fatty Acid |
| LCIA | Life cycle impact assessment |
| VS | Volatile solids |
| TS | Total solids |
| TP | Total phosphorus |
| TN | Total nitrogen |
References
- Bio-Waste in Europe: Turning Challenges into Opportunities; European Environmental Agency: Copenhagen, Denmark, 2020; Available online: https://www.eea.europa.eu/publications/bio-waste-in-europe (accessed on 1 August 2022).
- Vohra, M.; Manwar, J.; Manmode, R.; Padgilwar, S.; Patil, S. Bioethanol production: Feedstock and current technologies. J. Environ. Chem. Eng. 2014, 2, 573–584. [Google Scholar] [CrossRef]
- Soleymani Angili, T.; Grzesik, K.; Rödl, A.; Kaltschmitt, M. Life Cycle Assessment of Bioethanol Production: A Review of feedstock, technology and methodology. Energies 2021, 14, 2939. [Google Scholar] [CrossRef]
- Elginoz, N.; Khatami, K.; Owusu-Agyeman, I.; Cetecioglu, Z. Life Cycle Assessment of an Innovative Food Waste Management System. Front. Sustain. Food Syst. 2020, 4, 23. [Google Scholar] [CrossRef]
- Dahiya, S.; Kumar, A.N.; Sravan, J.S.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Food waste biorefinery: Sustainable strategy for circular bioeconomy. Bioresour. Technol. 2018, 248, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Khoshnevisan, B.; Tabatabaei, M.; Tsapekos, P.; Rafiee, S.; Aghbashlo, M.; Lindeneg, S.; Angelidaki, I. Environmental life cycle assessment of different biorefinery platforms valorizing municipal solid waste to bioenergy, microbial protein, lactic and succinic acid. Renew. Sustain. Energy Rev. 2020, 117, 109493. [Google Scholar] [CrossRef]
- Fernandez-Mena, H.; MacDonald, G.K.; Pellerin, S.; Nesme, T. Co-benefits and Trade-Offs From Agro-Food System Redesign for Circularity: A Case Study With the FAN Agent-Based Model. Front. Sustain. Food Syst. 2020, 4, 41. [Google Scholar] [CrossRef]
- Liu, Y.; Lyu, Y.; Tian, J.; Zhao, J.; Ye, N.; Zhang, Y.; Chen, L. Review of waste biorefinery development towards a circular economy: From the perspective of a life cycle assessment. Renew. Sustain. Energy Rev. 2021, 139, 110716. [Google Scholar] [CrossRef]
- IEA Bioenergy—Task42 Biorefining; Report; IEA Bioenergy: Wageningen, The Netherlands, 2014.
- Ahlgren, S.; Björklund, A.; Ekman, A.; Karlsson, H.; Berlin, J.; Börjesson, P.; Ekvall, T.; Finnveden, G.; Janssen, M.; Strid, I. Review of methodological choices in LCA of biorefi nery systems—Key issues and recommendations. Biofuels Bioprod. Biorefining 2015, 9, 606–619. [Google Scholar] [CrossRef]
- Lam, C.-M.; Yu, I.K.M.; Hsu, S.-C.; Tsang, D.C.W. Life-cycle assessment on food waste valorisation to value-added products. J. Clean. Prod. 2018, 199, 840–848. [Google Scholar] [CrossRef]
- Karka, P.; Papadokonstantakis, S.; Kokossis, A. Cradle-to-gate assessment of environmental impacts for a broad set of biomass-to-product process chains. Int. J. Life Cycle Assess. 2017, 22, 1418–1440. [Google Scholar] [CrossRef]
- Opatokun, S.A.; Lopez-Sabiron, A.M.; Ferreira, G.; Strezov, V. Life cycle analysis of energy production from food waste through anaerobic digestion, pyrolysis and integrated energy system. Sustainability 2017, 9, 1804. [Google Scholar] [CrossRef]
- Xu, C.; Shi, W.; Hong, J.; Zhang, F.; Chen, W. Life cycle assessment of food waste-based biogas generation. Renew. Sustain. Energy Rev. 2015, 49, 169–177. [Google Scholar] [CrossRef]
- Jeswiet, J.; Hauschild, M. EcoDesign and future environmental impacts. Mater. Des. 2005, 26, 629–634. [Google Scholar] [CrossRef]
- Galli, F.; Pirola, C.; Previtali, D.; Manenti, F.; Bianchi, C.L. Eco design LCA of an innovative lab scale plant for the production of oxygen-enriched air. Comparison between economic and environmental assessment. J. Clean. Prod. 2018, 171, 147–152. [Google Scholar] [CrossRef]
- Salimi, E.; Taheri, M.E.; Passadis, K.; Novacovic, J.; Barampouti, E.M.; Mai, S.; Moustakas, K.; Malamis, D.; Loizidou, M. Valorisation of restaurant food waste under the concept of a biorefinery. Biomass Convers. Biorefinery 2021, 11, 661–671. [Google Scholar] [CrossRef]
- Salimi, E.; Saragas, K.; Taheri, M.E.; Novakovic, J.; Barampouti, E.M.; Mai, S.; Moustakas, K.; Malamis, D.; Loizidou, M. The Role of Enzyme Loading on Starch and Cellulose Hydrolysis of Food Waste. Waste Biomass Valorization 2019, 10, 3753–3762. [Google Scholar] [CrossRef]
- Taheri, M.E.; Salimi, E.; Saragas, K.; Novakovic, J.; Barampouti, E.M.; Mai, S.; Malamis, D.; Moustakas, K.; Loizidou, M. Effect of pretreatment techniques on enzymatic hydrolysis of food waste. Biomass Convers. Biorefinery 2021, 11, 219–226. [Google Scholar] [CrossRef]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.; Guwy, A.; Kalyuzhnyi, S.; Jenicek, P.; Van Lier, J. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef]
- Drosg, B.; Wirthensohn, T.; Konrad, G.; Hornbachner, D.; Resch, C.; Wäger, F.; Loderer, C.; Waltenberger, R.; Kirchmayr, R.; Braun, R. Comparing centralised and decentralised anaerobic digestion of stillage from a large-scale bioethanol plant to animal feed production. Water Sci. Technol. 2008, 58, 1483–1490. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Koike, Y.; Liu, K.; An, M.Z.; Morimura, S.; Wu, X.L.; Kida, K. Ethanol production from kitchen waste using the flocculating yeast Saccharomyces cerevisiae strain KF-7. Biomass Bioenergy 2008, 32, 1037–1045. [Google Scholar] [CrossRef]
- Koike, Y.; An, M.; Tang, Y.; Syo, T.; Osaka, N.; Morimura, S.; Kida, K. Production of fuel ethanol and methane from garbage by high-efficiency two-stage fermentation process. J. Biosci. Bioeng. 2009, 108, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Nishimura, H.; Wang, Y.; Sun, Z.; Tang, Y.; Kida, K.; Morimura, S. Effect of organic loading rate on thermophilic methane fermentation of stillage eluted from ethanol fermentation of waste paper and kitchen waste. J. Biosci. Bioeng. 2019, 127, 582–588. [Google Scholar] [CrossRef]
- Guinée, J.B.; Gorrée, M.; Heijungs, R.; Huppes, G.; Kleijn, R.; de Koning, A.; van Oers, L.; Wegener Sleeswijk, A.; Suh, S.; Udo de Haes, H.A.; et al. (Eds.) Handbook on Life Cycle Assessment. An Operational Guide to the ISO Standards; Institute for Environmental Sciences, Leiden University: Leiden, The Netherland, 2002. [Google Scholar]
- Standard ISO14040. ISO (International Organization for Standardization). Environmental Management—Life Cycle Assessment—Principles and Framework; ISO: Geneva, Switzerland, 2006.
- Standard ISO 14044. ISO (International Organization for Standardization). Environmental Management—Life Cycle Assessment—Requirements and Guidelines; ISO: Geneva, Switzerland, 2006.
- Kralisch, D.; Ott, D.; Gericke, D. Rules and benefits of Life Cycle Assessment in green chemical process and synthesis design: A tutorial review. Green Chem. 2015, 17, 123–145. [Google Scholar] [CrossRef]
- Wernet, G.; Bauer, C.; Steubing, B.; Reinhard, J.; Moreno-ruiz, E.; Weidema, B. The ecoinvent database version 3 (part I): Overview and methodology. Int. J. Life Cycle Assess. 2016, 3, 1218–1230. [Google Scholar] [CrossRef]
- Papadaskalopoulou, C.; Sotiropoulos, A.; Novacovic, J.; Barabouti, E.; Mai, S.; Malamis, D.; Kekos, D.; Loizidou, M. Comparative life cycle assessment of a waste to ethanol biorefinery system versus conventional waste management methods. Resour. Conserv. Recycl. 2019, 149, 130–139. [Google Scholar] [CrossRef]
- Dunn, J.B.; Mueller, S.; Wang, M.; Han, J. Energy consumption and greenhouse gas emissions from enzyme and yeast manufacture for corn and cellulosic ethanol production. Biotechnol. Lett. 2012, 34, 2259–2263. [Google Scholar] [CrossRef] [PubMed]
- Hertwich, E.G.; Hammitt, J.K. A decision-analytic framework for impact assessment part I: LCA and decision analysis. Int. J. Life Cycle Assess. 2001, 6, 5–12. [Google Scholar] [CrossRef]
- Pennington, D.W.; Potting, J.; Finnveden, G.; Lindeijer, E.; Jolliet, O.; Rydberg, T.; Rebitzer, G. Life cycle assessment Part 2: Current impact assessment practice. Environ. Int. 2004, 30, 721–739. [Google Scholar] [CrossRef]
- Humbert, S.; Schryver, A.; Bengoa, X.; Margni, M.; Jolliet, O. A User Guide for the Life Cycle Impact Assessment Methodolog IMPACT 2002+, version Q2.21, Quantis Sustainability Counts. 2012. Available online: https://www.quantis-intl.com/pdf/IMPACT2002+_UserGuide_for_vQ2.21_30April2014a.pdf (accessed on 1 August 2022).
- Pallas, G.; Vijver, M.G.; Peijnenburg, W.J.; Guinée, J. Life cycle assessment of emerging technologies at the lab scale The case of nanowire-based solar cells. J. Ind. Ecol. 2020, 24, 193–204. [Google Scholar] [CrossRef]
- Ang, P.; Mothe, S.R.; Chennamaneni, L.R.; Aidil, F.; Khoo, H.H.; Thoniyot, P. Laboratory-Scale Life-Cycle Assessment: A Comparison of Existing and Emerging Methods of Poly(ε-caprolactone) Synthesis. Sustain. Chem. Eng. 2021, 9, 669–683. [Google Scholar] [CrossRef]
- Colangelo, F.; Navarro, T.G.; Farina, I.; Petrillo, A. Comparative LCA of concrete with recycled aggregates: A circular economy mindset in Europe. Int. J. Life Cycle Assess. 2020, 25, 1790–1804. [Google Scholar] [CrossRef]



| Characteristics | Value | Unit |
|---|---|---|
| Initial moisture | 50.9 ± 0.01 | % w/w w.b. |
| pH | 5.65 ± 0.13 | - |
| Conductivity | 2.72 ± 0.10 | mS/cm w.b. |
| BOD5 | 0.77 ± 0.17 | g/gRFW w.b. |
| COD | 0.85 ± 0.09 | g/gRFW w.b. |
| VS/TS | 1.05 ± 0.05 | % w/w w.b. |
| Carbon | ||
| -TC | 50.92 ± 2.12 | % w/w d.b. |
| -IC | ND | % w/w d.b. |
| -TOC | 50.92 ± 1.96 | % w/w d.b. |
| Nitrogen | ||
| -TN | 1.67 ± 0.02 | % w/w d.b. |
| -TKN | 1.67 ± 0.02 | % w/w d.b. |
| Phosphorous | ||
| -TP | 0.23 ± 0.03 | % w/w d.b. |
| Metallic elements | ||
| -Na | 6.645 ± 0.125 | mg/g d.b. |
| -Mg | 0.767 ± 0.101 | mg/g d.b. |
| -K | 4.479 ± 0.053 | mg/g d.b. |
| -Ca | 4.597 ± 0.061 | mg/g d.b. |
| -Cr | ND | mg/g d.b. |
| -Mn | 0.012 ± 0.002 | mg/g d.b. |
| -Fe | ND | mg/g d.b. |
| -Ni | ND | mg/g d.b. |
| -Cu | ND | mg/g d.b. |
| -Zn | 0.024 ± 0.003 | mg/g d.b. |
| -Cd | ND | mg/g d.b. |
| -Pb | ND | mg/g d.b. |
| Nutritional value | ||
| -Fats | 10.55 ± 0.35 | % w/w d.b. |
| -Proteins | 9.43 ± 0.55 | % w/w d.b. |
| -Carbohydrates | ||
| Free glucose | 1.24 ± 0.14 | % w/w d.b. |
| Total reducing sugars | 7.07 ± 0.20 | % w/w d.b. |
| Starch | 57.82 ± 0.77 | % w/w d.b. |
| Cellulose | 5.79 ± 0.19 | % w/w d.b. |
| Hemicellulose | 6.19 ± 0.48 | % w/w d.b. |
| Impact Category | Unit | Abbreviation |
|---|---|---|
| Ozone layer depletion | kg CFC-11 into air-eq | OD |
| Respiratory (organics) | kg ethylene into air-eq | RO |
| Ionizing radiation | Bq Carbon-14 into air-eq | IR |
| Land occupation | m2 organic arable land-eq.y | LO |
| Global warming | kg CO2 into air-eq | GW |
| Non-renewable energy | MJ crude oil-eq | NRE |
| Mineral extraction | in MJ Iron-eq | ME |
| Terrestrial ecotoxicity | kg Triethylene glycol into soil-eq | TE |
| Aquatic ecotoxicity | kg Triethylene glycol into water-eq | AE |
| Damage Category | Unit | Scenario A | Scenario B |
|---|---|---|---|
| Human health | DALY | 8.75 × 10−7 | 8.43 × 10−7 |
| Ecosystem quality | PDF.m2.y | 2.73 × 10−5 | 2.63 × 10−5 |
| Climate change | Kg CO2 eq | 5.73 × 10−4 | 5.52 × 10−4 |
| Resources | MJ primary | 5.81 × 10−4 | 5.60 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soleymani Angili, T.; Grzesik, K.; Salimi, E.; Loizidou, M. Life Cycle Analysis of Food Waste Valorization in Laboratory-Scale. Energies 2022, 15, 7000. https://doi.org/10.3390/en15197000
Soleymani Angili T, Grzesik K, Salimi E, Loizidou M. Life Cycle Analysis of Food Waste Valorization in Laboratory-Scale. Energies. 2022; 15(19):7000. https://doi.org/10.3390/en15197000
Chicago/Turabian StyleSoleymani Angili, Tahereh, Katarzyna Grzesik, Erfaneh Salimi, and Maria Loizidou. 2022. "Life Cycle Analysis of Food Waste Valorization in Laboratory-Scale" Energies 15, no. 19: 7000. https://doi.org/10.3390/en15197000
APA StyleSoleymani Angili, T., Grzesik, K., Salimi, E., & Loizidou, M. (2022). Life Cycle Analysis of Food Waste Valorization in Laboratory-Scale. Energies, 15(19), 7000. https://doi.org/10.3390/en15197000






