Abstract
The reduction of CO2 to CO through the reverse water gas shift (RWGS) reaction is an important catalytic step in the overall strategy of CO2 utilization. The product CO can be subsequently used as a feedstock for a variety of useful reactions, including the synthesis of fuels through the Fischer–Tropsch process. Recent works have demonstrated that potassium-promoted molybdenum carbide (K-Mo2C) is a highly selective catalyst for low-temperature RWGS. In this work, we describe the systematic investigation of key parameters in the synthesis of K-Mo2C, and their influence on the overall activity and selectivity for the low-temperature RWGS reaction. Specifically, we demonstrate how catalyst support, precursor calcination, catalyst loading, and long-term ambient storage influence performance of the K-Mo2C catalyst.
1. Introduction
The sustainable conversion of CO2 into fungible liquid fuels represents an attractive alternative (or potential complement) to fossil-based fuel production. In addition to the obvious environmental merits, such a process would enable the production of fuels independent of the logistical burden of fossil fuel refinement and delivery. The orchestration of sustained liquid fuel delivery is of key importance to the U.S. DOD and its allies, as it directly impacts “Freedom of Action” for the Warfighter [1,2]. Accordingly, CO2-to-fuel technologies have been identified as an important focus area, which would enable strategic advantages [3].
To address this, the U.S. Naval Research Laboratory is currently developing a process capable of producing hydrocarbon fuels using CO2 and H2 extracted from seawater [4,5,6,7,8,9,10]. A critical step in this process is the thermocatalytic reduction of CO2 to more reactive intermediates that can be subsequently hydrogenated to useable chemicals and fuels. The reduction of CO2 to CO through the endothermic reverse water gas shift reaction (RWGS) (Equation (1)) is a promising pathway, as the intermediate CO can be further hydrogenated to usable fuels through the well-developed Fischer–Tropsch process [11,12,13,14].
High CO2 conversions and CO selectivities can be readily achieved when the endothermic RWGS reaction is operated at high temperatures (>800 °C), and many catalytic materials including Pt [15], Ni [16], Cu [17], and ZnO [18], have demonstrated high activities for RWGS at elevated temperatures. However, these temperatures can lead to catalyst degradation, safety concerns, and pose limitations to the design and operation of reactors [19,20,21]. For these reasons, RWGS catalysts capable of operating at lower temperatures (250–350 °C) are desirable. Unfortunately, at these temperatures the RWGS reaction is equilibrium limited (16–35% conversions at a 3:1 ratio of H2:CO2) and competes with the thermodynamically favored methanation of CO2 and CO, further decreasing CO yields [11,12]. While the equilibrium limitations of low-temperature RWGS can be overcome by removing the product water and recycling the remaining reactor effluent over the RWGS catalyst [22,23], low-temperature RWGS catalysts must demonstrate high CO selectivity to efficiently utilize CO2 and H2 feedstocks.
A variety of materials have been studied as catalysts for the RWGS reaction across a wide range of operating temperatures, H2:CO2 ratios, and flow rates. The explicit details of these studies have been summarized and tabulated in multiple review articles [11,12,14]. Specifically, for the reverse water gas shift reaction at lower temperatures (from 250–350 °C) precious metals, such as Pt [24,25,26] or Pd [27] supported on metal oxides have demonstrated high activities and selectivities. However, the low terrestrial abundance of these materials makes them non-ideal candidates for industrial-scale CO2 conversions. On the other hand, transition metal carbides (TMCs) have been identified as affordable and effective catalysts for a variety of chemical transformations including CO oxidation, methane reforming, hydrogenation and RWGS [28,29,30,31,32,33]. For the RWGS reaction specifically, TMC catalysts are believed to participate through a redox active pathway, where the active site dissociates CO2 to form CO and an oxy-carbide intermediate, which is subsequently reduced by H2 to form H2O [11,31,34,35]. The addition of chemical promoters to TMCs can further improve catalytic performance by attenuating the material’s electronic, acid-base, and structural properties, enabling improved activities and/or selectivities [33,36,37,38,39,40]. Recent works by our lab and others have demonstrated that the addition of potassium promoters drastically improves the low-temperature RWGS selectivity of TMCs without sacrificing the overall activity of the catalyst [36,41]. Specifically, potassium promoted molybdenum carbide supported on high surface area gamma alumina (K-Mo2C@γ-Al2O3) has demonstrated excellent performance over a range of temperatures, time scales, operating conditions, and reactor scales [23,41,42]. For example, at 300 °C, an H2 to CO2 ratio of 3, and at a gas hourly space velocity of 2.1 mL g−1 s−1, K-Mo2C@γ-Al2O3 demonstrated CO2 conversions of 18.1% and a CO selectivity of 95.9% [41].
Optimization of the K-Mo2C catalyst synthesis is critical to determine how synthetic details can influence important properties, such as catalyst distribution, active surface area, promotional effects, attrition rate, and catalyst-support interactions [43,44]. While previous works have addressed the relationship between potassium content and catalyst performance [41], further systematic investigations focusing on K-Mo2C preparation as it pertains to catalyst activity should be performed. In this work, we investigate the influence of key variables on the performance of the K-Mo2C RWGS catalyst including catalyst loading, calcination temperature, the influence of the γ-Al2O3 support, as well as the effects of long-term catalyst storage under ambient conditions (shelf life).
2. Materials and Methods
Materials: Ammonium molybdate tetrahydrate ((NH4)6Mo7O24·4H2O) 81.0–83.0% MoO3 basis, and potassium carbonate (K2CO3) > 99% were purchased from Sigma Aldrich (St. Louis, MO, USA). γ-Al2O3 powder (>97%) was purchased from STREM Chemicals (Newburyport, MA, USA). All gases used for catalyst synthesis and testing were supplied from Matheson.
Catalyst Preparation: The Mo based catalysts were synthesized by the evaporation deposition method. In a typical synthesis, ammonium molybdate tetrahydrate, and potassium carbonate (when applicable) were dissolved in deionized water in a beaker. γ-Al2O3 powder was then added to the solution, which was then stirred at 60 °C for 48 h to allow for complete evaporation of water. Unless otherwise noted, the materials were then calcined in air using a muffle furnace at 350 °C for 12 h. Finally, following calcination, the materials were carburized in 4 g batches, using a tube furnace flowing a blend of 20% methane and 80% hydrogen gas at 300 mL min−1 for a total of 4 h at 600 °C. Following carburization, the samples were passivated at ambient temperature under flowing a blend of 1% oxygen and 99% nitrogen at 10 mL min−1 overnight. Following passivation, all catalysts were stored in glass vials under ambient atmosphere.
For the preparation of unsupported Mo and K-Mo catalysts, the procedure described above was followed, except γ-Al2O3 was not added during the evaporation deposition method.
X-ray Diffraction (XRD): Measurements were performed on a Rigaku Smartlab X-ray diffractometer using Cu Kα monochromated radiation operated at 40 kV and 44 mA at room temperature over the range of 20–90° 2θ. X-ray diffraction patterns for the catalysts were referenced to reported patterns from multiple databases. Average crystallite size was estimated by performing Scherrer analysis using the (101) reflection of Mo2C at 39.5° 2θ.
BET Surface Area Analysis: Nitrogen adsorption analysis was performed at −196 °C, using a Beckman-Coulter S.A. (Singapore) 3100 Surface Area Analyzer. Prior to the measurement, the catalyst was degassed at 120 °C for 30 min under 4 μmHg vacuum. To account for any temperature induced changes to the catalyst support, the bare γ-Al2O3 was calcined, pelletized and heated under the same carburizing atmosphere as the Mo2C catalysts before BET characterization.
Reactor Studies: Prior to testing, the catalysts were pelletized under a force of 1 ton for 10 min, then ground and sieved to a particle size of 200–350 μm to improve mass transfer. For a typical experiment, 0.5 g of catalyst pellets were loaded into a 0.25 inch diameter stainless-steel reactor. The catalyst bed was reduced under H2 at 300 °C and 0.5 MPa at a flow rate of 50 mL min−1 for a total of 2.5 h. The catalyst bed was then isolated as the rest of the reactor system was pressurized to 2.0 MPa with a blend of N2, CO2, and H2 gases at ratios commensurate with the experimental flow rates. Once pressurized, the reactant gases were introduced to the catalyst bed at a 3:1 H2:CO2 ratio, with flowrates corresponding to the reported gas hourly space velocities (GHSV). N2 was used as an internal standard to quantify CO2 conversion and CO yield, and represented 16% of the reagent gas blend. Reactant flowrates were controlled using programmable mass flow controllers (Brooks SLA5850, Brooks Instrument, Hatfield, PA, USA). Reactions were monitored by gas chromatography (Agilent 7890A, Santa Clara, CA, USA), and all reported data were recorded after steady state had been achieved. Carbon balances between 95–100% were observed for all reported reactor data.
3. Results and Discussion
3.1. Influence of γ-Al2O3 Support
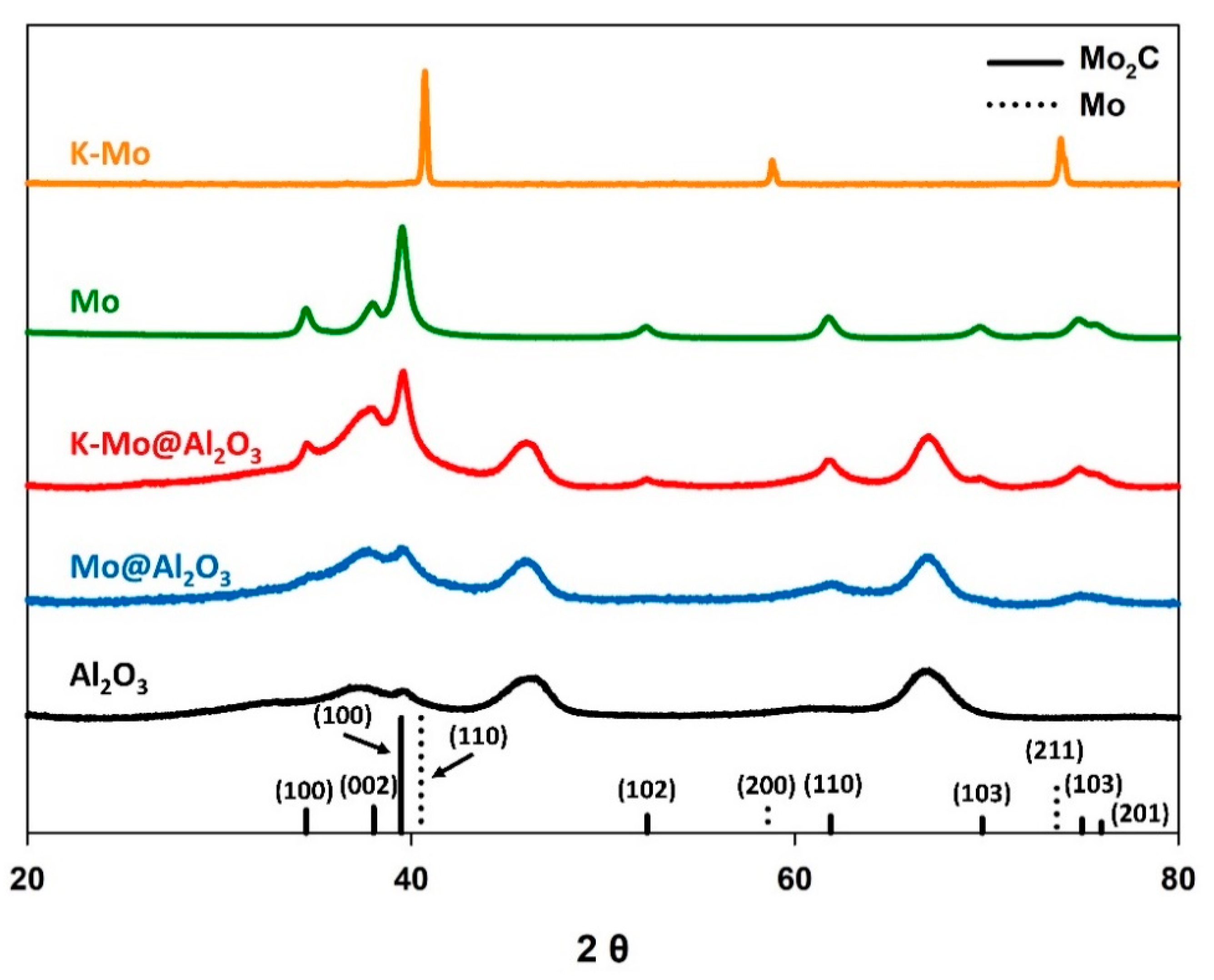
To develop a baseline for Mo2C performance, and to evaluate the influence of the high surface area γ-Al2O3 support on the catalytic activity of K-Mo2C, the potassium promoted molybdenum (K-Mo) precursor was carburized, with and without the γ-Al2O3 support. For comparison, the same procedures were also carried out on identical precursors in absence of the potassium promoter. The four catalysts were prepared under identical conditions, and consisted of molar ratios of 1/4/15 (K/Mo/γ-Al2O3). Figure 1 displays the X-ray diffraction patterns for the resulting materials. The K-Mo precursor supported on the γ-Al2O3 was fully carburized to yield the Mo2C phase. Likewise, when the potassium promoter was excluded, the same Mo2C phase was observed with and without the γ-Al2O3 support, although the signal corresponding to this phase was significantly less prominent for the γ-Al2O3 supported catalyst. Notably, however, when the unsupported K-Mo precursor is treated under identical conditions in the absence of the γ-Al2O3 support, the unsupported material was reduced to metallic molybdenum.
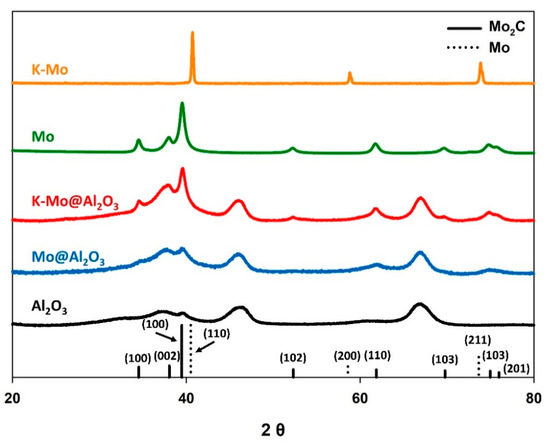
Figure 1.
X-ray diffraction patterns for the various molybdenum-based catalysts, with and/or without the potassium promoter and γ-Al2O3 support. Reference patterns are included for both Mo2C (solid line) and metallic Mo (dotted line), which are labeled with their corresponding Miller indices.
Reduction of the unsupported K-Mo precursor to metallic Mo is likely due to the presence of the alkali dopant, which has been reported to inhibit the carburization of molybdenum species [45,46]. It is therefore interesting to note that, under identical conditions, the presence of the γ-Al2O3 support appears to inhibit complete reduction of the impregnated K and Mo precursors, enabling straightforward carburization to the Mo2C phase. This may be due to the high surface area of Al2O3, which allows for dispersion of the K and Mo precursors across the support, thereby limiting the physical interaction between the impregnated precursors. Alternatively, the direct carburization may be due to interaction between the support and impregnated species, which is known to influence the temperatures of various chemical transformations [47,48,49,50].
The specific surface area of the various catalysts was calculated using N2 physisorption, and are displayed in Table 1, along with the measured activity, selectivity and overall CO yield for the reverse water gas shift reaction at 300 °C. Under the conditions tested, the RWGS reaction is thermodynamically limited to CO yields of 23%.

Table 1.
Performance of Mo-based catalysts with and without γ-Al2O3 and potassium promoter. All catalysts were tested under the following conditions: 3:1- H2:CO2 ratio at 300 °C, 2.0 MPa, and at a GHSV of 3600 L kg−1 h−1.
As expected, the K-Mo2C@γ-Al2O3 displayed the highest selectivity for the product CO, and a clear correlation was observed between surface area and RWGS activity, with the K-Mo2C@γ-Al2O3 displaying the highest overall CO yield. The unsupported Mo2C was measured to have a surface area of 13.7 m2 g−1 and exhibited large CO2 conversions, but poor selectivity for the desired RWGS reaction. The low overall CO selectivity for unpromoted Mo2C observed in this work is inconsistent with recent reports describing high RWGS selectivity for unpromoted molybdenum carbide [51,52]. This disparity is likely due to the increased pressures, lower reaction temperatures, and reduced gas hourly space velocities (GHSV) explored in this work (all of which favor CO2 methanation), and further demonstrates the importance of the potassium promoter’s ability to attenuate catalyst performance. The unsupported K-promoted catalyst exhibited the lowest surface area of 1.0 m2 g−1, and exhibited no observable CO2 conversion under the conditions tested. The lack of activity for the unsupported K-promoted catalyst demonstrates that, under the synthetic conditions explored in this work, efficient K-promoted molybdenum catalysts cannot be accessed without the γ-Al2O3 support.
3.2. Calcination
The temperature programmed carburization of transition metal oxides using gaseous reactants is a convenient and well-known route to yield high surface area carbides [30,53,54,55]. Accordingly, in previous works, K-Mo2C@γ-Al2O3 was prepared though a three-step process: (1) impregnation of metal salts onto the γ-Al2O3 support, (2) calcination of the precursors to the respective metal oxides, and (3) temperature programed carburization of the supported oxides. To understand the influence of calcination on catalyst performance, K-Mo2C@γ-Al2O3 samples were prepared using three different calcination methods prior to carburization: (a) no calcination, (b) calcination at 350 °C, and (c) calcination at 425 °C. All other synthetic procedures were identical.
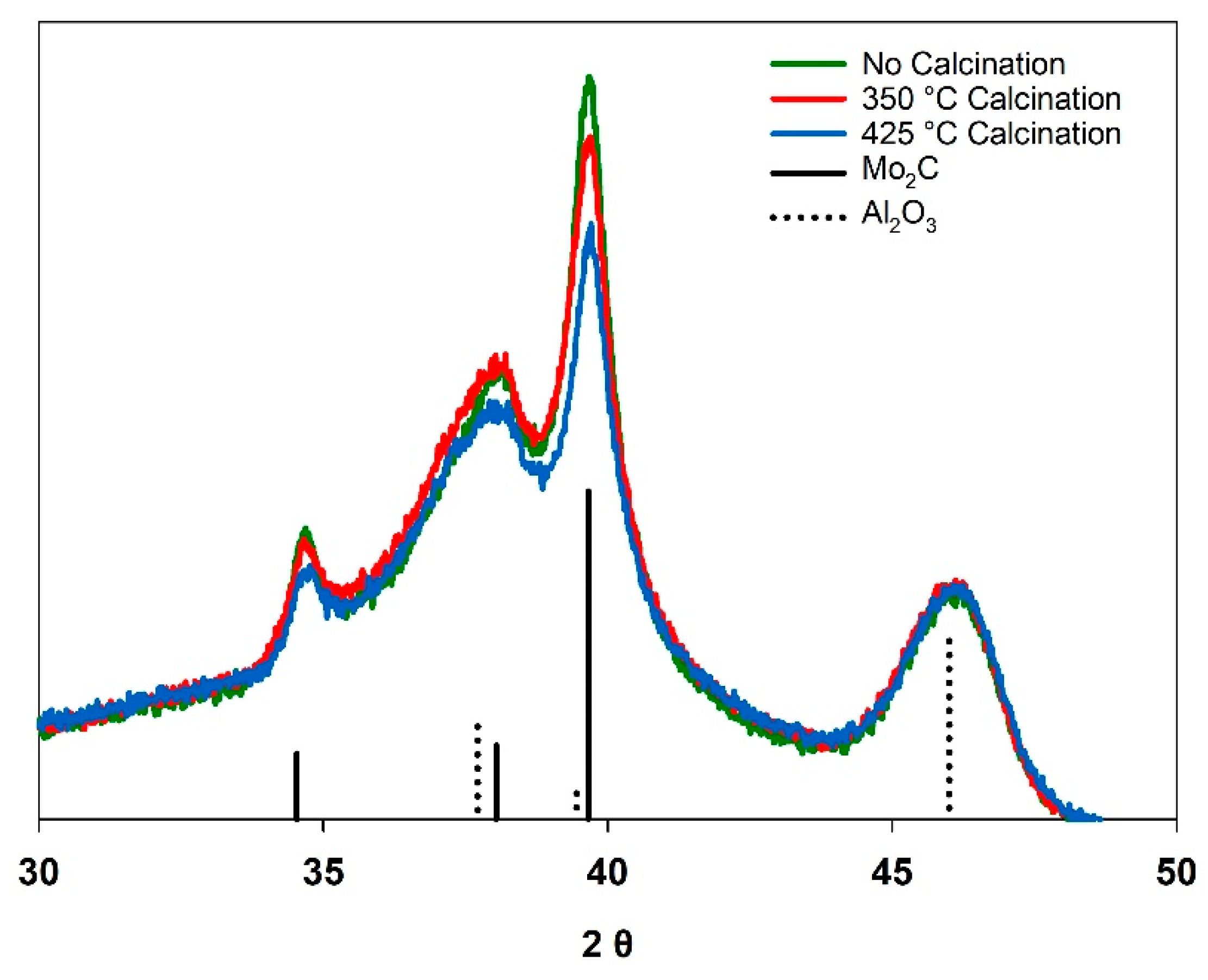
Figure 2 shows overlaid X-ray diffraction patterns (normalized to the γ-Al2O3 support) for the resulting K-Mo2C@γ-Al2O3 samples following carburization, and Table 2 shows the corresponding RWGS activity and selectivity at two different GHSVs. Following carburization, the Mo2C phase was observed for all three samples. A slight decrease in the intensity of the XRD signal corresponding to the Mo2C phase was observed as a function calcination temperature, with the “no calcination” sample exhibiting the most prominent Mo2C pattern. The CO yield for the RWGS reaction also decreased as a function of calcination temperature, with the “no calcination” sample exhibiting the greatest activity and CO yield. The differences in catalyst performance were more pronounced at greater reagent flowrates (GHSV: 18 100 L kg−1 h−1).
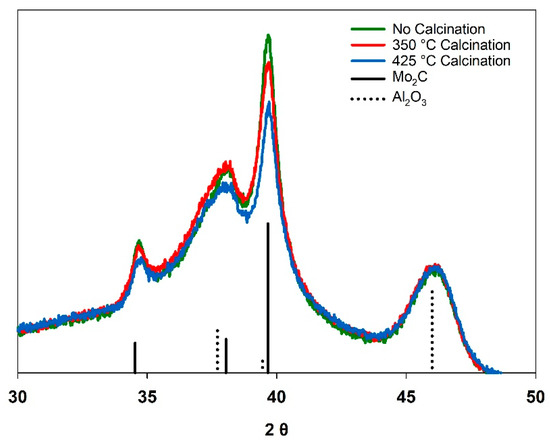
Figure 2.
Normalized X-ray diffraction patterns for K-Mo2@γ-Al2O3 prepared at different calcination temperatures.

Table 2.
RWGS activity for K-Mo2C@γ-Al2O3 prepared using different calcination temperatures. All catalysts were prepared at a molar ratio of 1/4/15 (K/Mo/γ-Al2O3) and were tested under the following conditions: 3:1- H2:CO2 ratio, 2.0MPa, at 300 °C.
To the best of our knowledge, the direct carburization of alumina supported ammonium molybdate ((NH4) 6Mo7O24·4H2O) to Mo2C has yet to be reported in the open literature. Instead, most reports describe calcination of the molybdate precursor to molybdenum trioxide (MoO3), which is then carburized in a subsequent step. This is likely due to the well-known and highly cited work of Boudart [53,56], which originally described the temperature programmed carburization MoO3 using gaseous reactants. However, for many reduced and sulfided transition metal catalysts, calcination of the precursor in air is not ideal and can lead to reduced catalyst activity [50,57]. Additionally, when supported on metal oxides such as alumina, the calcination of ammonium molybdate can lead to interaction between the catalyst precursor and support, which could negatively affect the subsequent carburization step [47,48,49,50].
Although not observable by XRD, calcination likely drives the formation of oxidized species in the precursor material, which cannot be subsequently carburized under the conditions tested in this work, thereby leading to an overall lower quantity of surface-active sites available to catalyze the RWGS reaction. Together, these data demonstrate that calcination of the K-Mo precursor prior to carburization is unnecessary, and in fact detrimental, to the performance of the K-Mo2C RWGS catalysts.
3.3. Catalyst Loading
In an attempt to further optimize the overall performance of the K-Mo2C catalyst for RWGS, the quantity of promoter and catalyst loaded onto the γ-Al2O3 support was varied. All catalysts were directly carburized following impregnation (no calcination step). Table 3 shows the overall CO2 conversion and CO selectivities for the RWGS reaction for four K-Mo2C@γ-Al2O3 catalysts prepared at differing K/Mo/Al2O3 molar ratios: (1/4/30), (1/4/15), (1/4/7.5), and (1/8/15).

Table 3.
Average crystallite size as calculated by Scherrer analysis, and RWGS activity for K-Mo2C@γ-Al2O3 catalysts of various loadings. All catalysts were tested under the following conditions: 3:1- H2:CO2 ratio, 2.0 MPa, at 300 °C and at a GHSV of 18 100 L kg−1 h−1.
(1/4/30) displayed a CO2 conversion of 6.9%, and an overall CO yield of 6.6%, the lowest activity observed of the variously loaded K-Mo2C@γ-Al2O3 catalysts. (1/4/15) displayed a CO2 conversion of 14.1%, and an overall CO yield of 13.7%, demonstrating the greatest activity of the catalysts listed in Table 3. The catalysts with higher loadings, (1/4/7.5) and (1/8/15), displayed CO2 conversions of 11.5% and 13.1%, and overall CO yields of 11.4% and 12.7%, respectively.
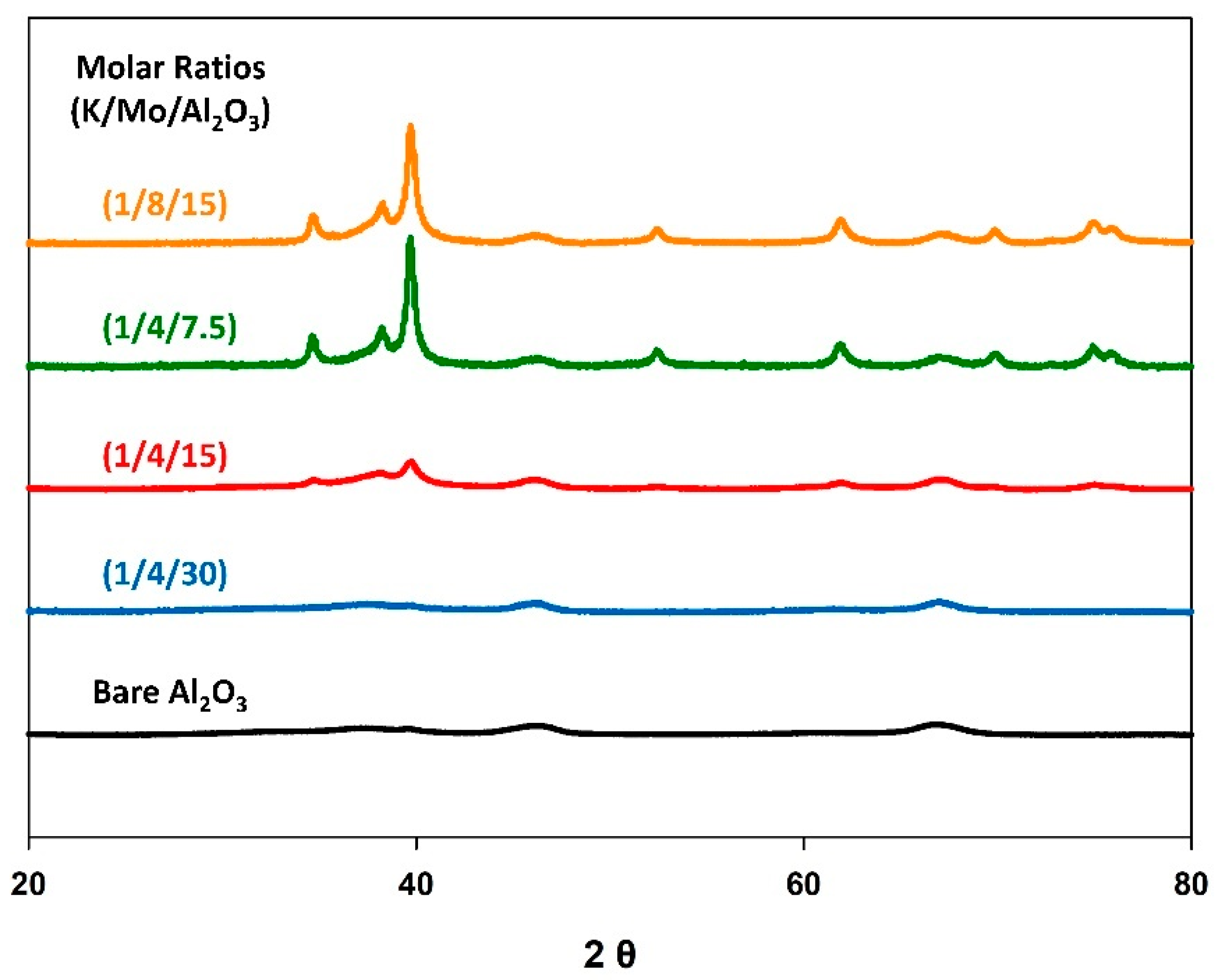
Figure 3 shows the resulting X-ray diffraction patterns for the K-Mo2C@γ-Al2O3 catalysts of differing loadings. Notably, the Mo2C phase was not observed for the (1/4/30) sample, indicating the Mo species were of low surface density, and were finely dispersed across the γ-Al2O3 support [58]. However, reflections corresponding to Mo2C were observed for the other three samples, with the reflection intensities increasing with Mo loading. The average crystallite size of the Mo2C species were calculated through Scherrer analysis, and found to be 9 nm, 17 nm, and 16 nm for (1/4/15), (1/4/7.5), and (1/8/15), respectively.
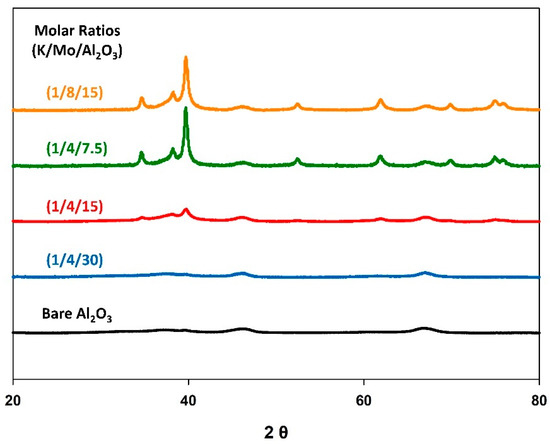
Figure 3.
Normalized X-ray diffraction patterns for the K-Mo2C@γ-Al2O3 of various precursor loadings.
In the current study, the (1/4/30) K-Mo2C@γ-Al2O3 corresponds to the lowest molybdenum loading at roughly 11 wt.%. This is close to the value described in previous literature reports, which estimate that a theoretical monolayer dispersion of Mo across high surface area Al2O3 requires a total Mo loading of about 8.5 wt.% [59,60]. This is in line with the results displayed in Figure 3, which shows no Mo2C signature for the (1/4/30) sample, indicating the Mo2C is of small crystal size (<4 nm), and is well dispersed across the γ-Al2O3 surface. The other catalyst concentrations of (1/4/15), (1/4/7.5), and (1/8/15), correspond to Mo loadings of roughly 20 wt.%, 32 wt.%, and 33 wt.%, respectively. These significantly greater loadings result in a greater concentration of molybdenum species across the γ-Al2O3 surface, ultimately resulting in the formation of larger Mo2C domains visible by XRD. The maximum activity for the Mo loadings tested in this work was achieved using the (1/4/15) sample (~20 wt.% Mo). Again, this is in line with previous reports, which have observed maximum activity for Mo/Al2O3 catalysts in the range of 10–15 wt.% loading [61,62]. These considerations, along with the RWGS activity reported in Table 3, suggest that the maximum catalyst activity for the K-Mo2C@γ-Al2O3 is achieved at loadings just above the quantity required to achieve a theoretical monolayer, and that Mo loadings above a molar ratio of 4/15 (Mo/Al2O3), do not produce a greater quantity of active surface sites due to the corresponding increase Mo2C particle size.
3.4. Shelf Life
In addition to the influence of synthetic conditions, the stability of a catalyst under ambient storage is also important in the practical application of the material. The best performing catalyst in this work, non-calcined with a molar ratio of 1/4/15 (K/Mo/Al2O3), provided a CO yield of 18.7% when tested within two weeks of synthesis (See Table 2). However, after being stored at ambient conditions for 15 months, the same catalyst produced a CO yield of 8.9% under identical conditions. Although measures were taken to passivate the reactive Mo2C surface following carburization, it appears that the Mo2C catalyst can still appreciably degrade over long periods of time. It should also be emphasized, as described in the Experimental Section, that all catalysts were treated with flowing H2 at 300 °C prior to catalytic testing to re-reduce and activate the carbide surface. This degradation in activity is likely due to the gradual oxidation of the Mo2C when stored under ambient conditions. Mehdad et al. have reported similar results, describing the gradual oxidation and adsorption of water for bulk molybdenum and tungsten carbides when stored under air for 11 months [63].
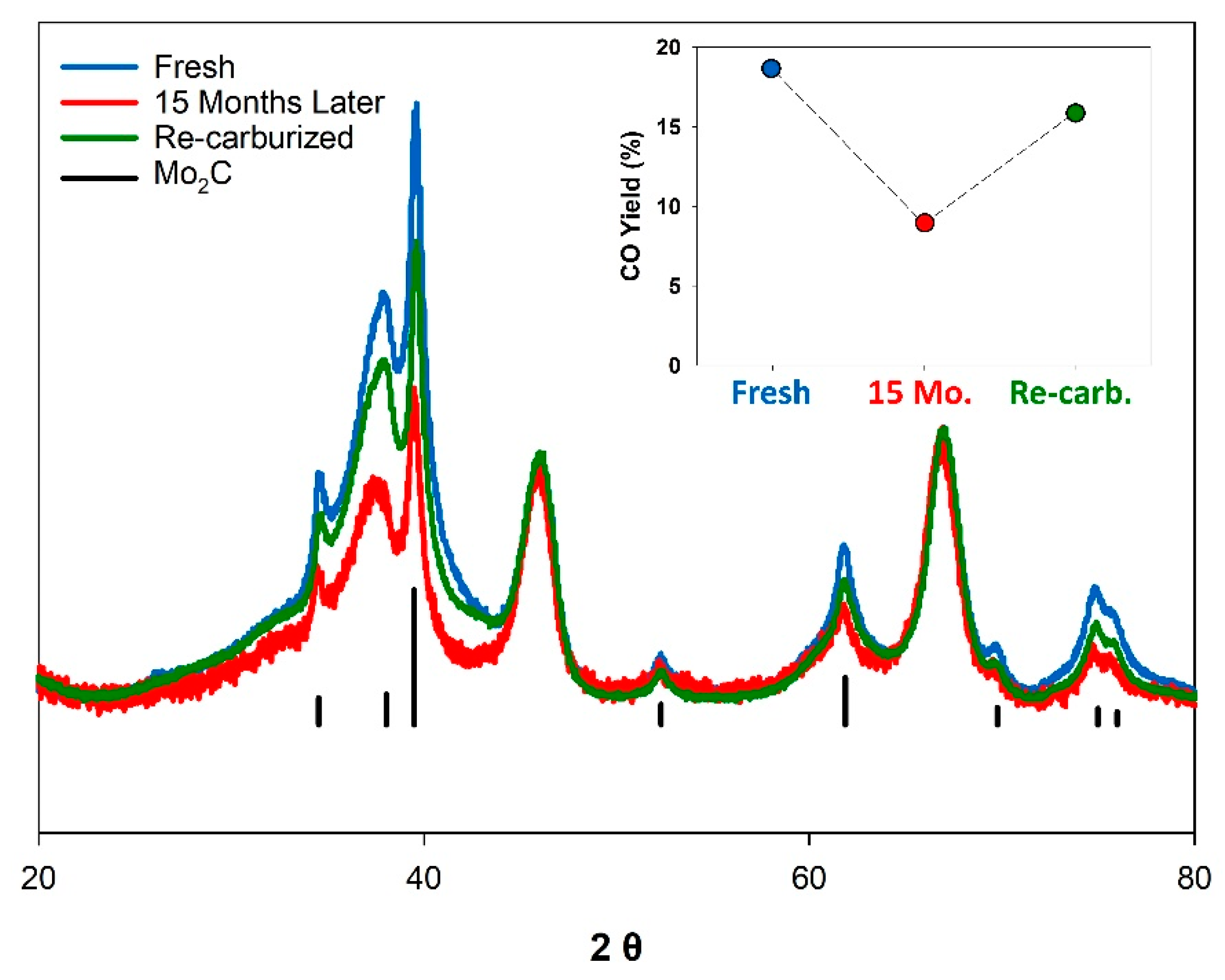
In an attempt to regenerate the catalyst, the 15-month-old K-Mo2C@γ-Al2O3 was re-carburized under conditions identical to the original catalyst synthesis. Figure 4 displays the X-ray diffraction patterns for the fresh, aged, and re-carburized catalyst (normalized to the γ-Al2O3 support). The aged sample clearly displays a loss of crystalline Mo2C, with the reflections for the Mo2C decreasing in intensity, and broadening in width. However, no obvious oxide formation is observed by XRD, indicating that the resulting species following Mo2C degradation are amorphous in nature. The reduced intensity of the Mo2C reflections for the “aged” sample shown in Figure 4 may also be due to the slow incorporation of oxygen into the bulk carbide [63,64]. Upon re-carburization, the sample displayed reflections corresponding to Mo2C which were of greater intensity relative to the “aged” sample. However, the relative intensity of these reflections remained less than the original “Fresh” catalyst, indicating complete re-carburization was not achieved. When tested under identical conditions, the “re-carburized” catalyst demonstrated improved performance, but was still less active than the originally synthesized catalyst, with a CO yield of 15.9%. Previous works have noted that even the passivation of Mo2C with dilute O2 at room temperature can irreversibly alter the freshly synthesized carbide surface [65,66]. It thus appears likely that the long term exposure of the K-Mo2C@γ-Al2O3 to ambient conditions further alters the catalyst composition in a way that cannot be easily reversed.
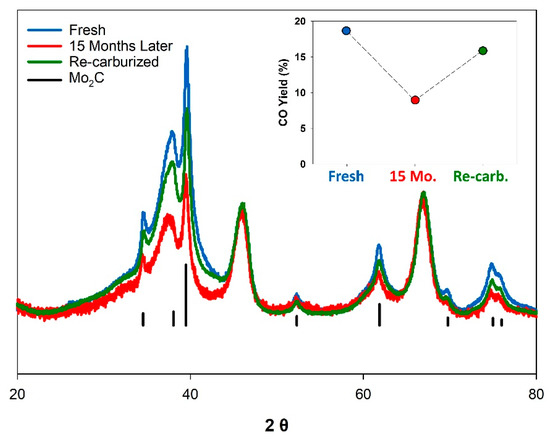
Figure 4.
X-ray diffraction patterns normalized to the γ-Al2O3 signal for “Fresh” (blue), “Aged” (red), and “Re-carburized” (green) K-Mo2C@γ-Al2O3. Inset: CO Yield observed for the three catalysts under identical conditions (tested at 3:1- H2:CO2 ratio, 2.0 MPa, at 300 °C and at a GHSV of 3600 L kg−1 h−1).
This is in line with previous reports [63,67] which have described reduced catalyst performance following re-carburization of transition metal carbides, and suggests that K-Mo2C@Al2O3 should be applied as a catalyst for the RWGS shortly after synthesis, and if necessary, stored under inert conditions before use in order to achieve maximum performance. Additionally, further studies to understand and improve the passivation step of the Mo2C catalyst could be useful for improving the overall performance of Mo2C for the RWGS reaction.
4. Conclusions
Potassium promoted molybdenum carbide is an effective catalyst for the low temperature reverse water gas shift (RWGS) reaction, which can be considered an important step in the overall reduction of CO2 to liquid fuels and other chemicals. To optimize the performance of K-Mo2C@γ-Al2O3 for the RWGS reaction, systematic evaluation of various parameters such as support dependence, catalyst loading, calcination temperature, and catalyst stability under ambient conditions were performed.
Besides providing a high surface area for the dispersion of Mo2C, it was demonstrated that the γ-Al2O3 support has an important influence on the carburization of the K-Mo precursor, enabling a direct route to temperature programmed carburization to Mo2C, which could not be achieved with the unsupported K-Mo precursor under identical conditions. Calcination of the supported K-Mo precursor prior to carburization was found to be slightly detrimental to catalyst performance, with the non-calcined catalyst displaying the greatest degree of carburization and RWGS activity. Optimal loading of the K and Mo precursors on the γ-Al2O3 support was found to consist of molar ratios of 1/4/15 (K/Mo/Al2O3), respectively, which corresponded to the smallest observable reflections for Mo2C as observed by X-ray diffraction. Finally, K-Mo2C@γ-Al2O3 was found to display a “shelf life”, with catalyst performance degrading after being stored under ambient conditions for 15 months. Upon re-carburization, the catalyst performance was improved, providing CO yields close to the freshly synthesized material.
Author Contributions
Conceptualization, J.R.M. and H.D.W.; methodology, J.R.M. and C.F.H.; investigation, J.R.M. and C.F.H.; original draft preparation, J.R.M.; writing—review and editing, J.R.M., H.D.W., C.F.H. and J.W.B.; supervision, J.R.M., J.W.B. and H.D.W.; funding acquisition, H.D.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Office of Naval Research both directly and through the Naval Research Laboratory and OPNAV N45.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Department of Defense. Operational Energy Strategy. 2016. Available online: http://www.acq.osd.mil/eie/Downloads/OE/2016%20DoD%20Operational%20Energy%20Strategy%20WEBc.pdf (accessed on 28 January 2022).
- National, G.; Pillars, H. Summary of the 2018 National Defense Strategy of the United States of America. Available online: https://dod.defense.gov/Portals/1/Documents/pubs/2018-National-Defense-Strategy-Summary.pdf (accessed on 28 January 2022).
- S.1790—116th Congress (2019–2020): National Defense Authorization Act for Fiscal Year 2020. 20 December 2019. Available online: http://www.congress.gov/ (accessed on 28 January 2022).
- Dorner, R.; Hardy, D.; Williams, F. Influence of Gas Feed Composition and Pressure on the Catalytic Conversion of CO2 to Hydrocarbons Using a Traditional Cobalt-Based Fischer- Tropsch Catalyst. Energy Fuels 2009, 23, 4190–4195. [Google Scholar] [CrossRef]
- Willauer, H.D.; Hardy, D.R.; Lewis, M.K.; Ndubizu, E.C.; Williams, F.W. Effects of pressure on the recovery of CO2 by phase transition from a seawater system by means of multilayer gas permeable membranes. J. Phys. Chem. A 2010, 114, 4003–4008. [Google Scholar] [CrossRef] [PubMed]
- Willauer, H.D.; Dimascio, F.; Hardy, D.R.; Williams, F.W. Feasibility of CO2 extraction from seawater and simultaneous hydrogen gas generation using a novel and robust electrolytic cation exchange module based on continuous electrodeionization technology. Ind. Eng. Chem. Res. 2014, 53, 12192–12200. [Google Scholar] [CrossRef]
- Willauer, H.D.; DiMascio, F.; Hardy, D.R.; Lewis, M.K.; Williams, F.W. Development of an Electrochemical Acidification Cell for the Recovery of CO2 and H2 from Seawater. Ind. Eng. Chem. Res. 2011, 50, 9876–9882. [Google Scholar] [CrossRef]
- Willauer, H.D.; Dimascio, F.; Hardy, D.R.; Lewis, M.K.; Williams, F.W. Development of an electrochemical acidification cell for the recovery of CO2 and H2 from seawater II. Evaluation of the cell by natural seawater. Ind. Eng. Chem. Res. 2012, 51, 11254–11260. [Google Scholar] [CrossRef]
- Willauer, H.D.; DiMascio, F.; Hardy, D.R.; Williams, F.W. Development of an Electrolytic Cation Exchange Module for the Simultaneous Extraction of Carbon Dioxide and Hydrogen Gas from Natural Seawater. Energy Fuels 2017, 31, 1723–1730. [Google Scholar] [CrossRef]
- Willauer, H.D.; Hardy, D.R.; Lewis, M.K.; Ndubizu, E.C.; Williams, F.W. Extraction of CO2 from seawater and aqueous bicarbonate systems by ion-exchange resin processes. Energy Fuels 2010, 24, 6682–6688. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Yan, B.; Chen, J.G. Catalytic reduction of CO2 by H2 for synthesis of CO, methanol and hydrocarbons: Challenges and opportunities. Energy Environ. Sci. 2016, 9, 62. [Google Scholar] [CrossRef]
- Daza, Y.; Kuhn, J.N. CO2 conversion by reverse water gas shift catalysis: Comparison of catalysts and mechanisms and their consequences for CO2 conversion to liquid fuels. RSC Adv. 2016, 6, 49675–49691. [Google Scholar] [CrossRef]
- Xie, C.; Chen, C.; Yu, Y.; Su, J.; Li, Y.; Somorjai, G.A.; Yang, P. Tandem Catalysis for CO2 Hydrogenation to C2–C4 Hydrocarbons. Nano Lett. 2017, 17, 3798–3802. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703. [Google Scholar] [CrossRef]
- Kim, S.S.; Lee, H.H.; Hong, S.C. A study on the effect of support’s reducibility on the reverse water-gas shift reaction over Pt catalysts. Appl. Catal. A Gen. 2012, 423–424, 100–107. [Google Scholar] [CrossRef]
- Lu, B.; Kawamoto, K. Preparation of mesoporous CeO2 and monodispersed NiO particles in CeO2, and enhanced selectivity of NiO/CeO2 for reverse water gas shift reaction. Mater. Res. Bull. 2014, 53, 70–78. [Google Scholar] [CrossRef]
- Chen, C.-S.; Cheng, W.-H.; Lin, S. Mechanism of CO Formation in Reverse Water–Gas Shift Reaction over Cu/Al2O3 Catalyst. Catal. Lett. 2000, 68, 45–48. [Google Scholar] [CrossRef]
- Joo, O.S.; Jung, K.D.; Moon, I.; Rozovskii, A.Y.; Lin, G.I.; Han, S.H.; Uhm, S.J. Carbon dioxide hydrogenation to form methanol via a reverse-water-gas-shift reaction (the CAMERE process). Ind. Eng. Chem. Res. 1999, 38, 1808–1812. [Google Scholar] [CrossRef]
- Chen, C.S.; Cheng, W.H.; Lin, S.S. Study of iron-promoted Cu/SiO2 catalyst on high temperature reverse water gas shift reaction. Appl. Catal. A Gen. 2004, 257, 97–106. [Google Scholar] [CrossRef]
- Chen, C.S.; Cheng, W.H.; Lin, S.S. Enhanced activity and stability of a Cu/SiO2 catalyst for the reverse water gas shift reaction by an iron promoter. Chem. Commun. 2001, 1, 1770–1771. [Google Scholar] [CrossRef]
- Su, X.; Yang, X.; Zhao, B.; Huang, Y. Designing of highly selective and high-temperature endurable RWGS heterogeneous catalysts: Recent advances and the future directions. J. Energy Chem. 2017, 26, 854–867. [Google Scholar] [CrossRef]
- Willauer, H.D.; Bradley, M.J.; Baldwin, W.; Hartvigsen, J.J.; Frost, L.; Morse, J.R.; Dimascio, F.; Hardy, D.R.; Hasler, D.J. Evaluation of CO2 Hydrogenation in a Modular Fixed-Bed Reactor Prototype. Catalysts 2020, 10, 970. [Google Scholar] [CrossRef]
- Juneau, M.; Vonglis, M.; Hartvigsen, J.; Frost, L.; Bayerl, D.; Dixit, M.; Mpourmpakis, G.; Morse, J.R.; Baldwin, J.W.; Willauer, H.D.; et al. Assessing the viability of K-Mo2C for reverse water-gas shift scale-up: Molecular to laboratory to pilot scale. Energy Environ. Sci. 2020, 13, 2524–2539. [Google Scholar] [CrossRef]
- Kattel, S.; Yu, W.; Yang, X.; Yan, B.; Huang, Y.; Wan, W.; Liu, P.; Chen, J.G. CO2 Hydrogenation over Oxide-Supported PtCo Catalysts: The Role of the Oxide Support in Determining the Product Selectivity. Angew. Chem. Int. Ed. 2016, 55, 7968–7973. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Su, X.; Duan, H.; Liang, B.; Huang, Y.; Zhang, T. Catalytic performance of the Pt/TiO2 catalysts in reverse water gas shift reaction: Controlled product selectivity and a mechanism study. Catal. Today 2017, 281, 312–318. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Chen, J.G. Trends in the catalytic reduction of CO2 by hydrogen over supported monometallic and bimetallic catalysts. J. Catal. 2013, 301, 30–37. [Google Scholar] [CrossRef]
- Kwak, J.H.; Kovarik, L.; Szanyi, J. Heterogeneous catalysis on atomically dispersed supported metals: CO2 reduction on multifunctional Pd catalysts. ACS Catal. 2013, 3, 2094–2100. [Google Scholar] [CrossRef]
- Patterson, P.M.; Das, T.K.; Davis, B.H. Carbon monoxide hydrogenation over molybdenum and tungsten carbides. Appl. Catal. A Gen. 2003, 251, 449–455. [Google Scholar] [CrossRef]
- Oshikawa, K.; Nagai, M.; Omi, S. Characterization of molybdenum carbides for methane reforming by TPR, XRD, and XPS. J. Phys. Chem. B 2001, 105, 9124–9131. [Google Scholar] [CrossRef]
- Claridge, J.B.; York, A.P.E.; Brungs, A.J.; Marquez-alvarez, C.; Sloan, J.; Tsang, S.C.; Green, M.L.H. New Catalysts for the Conversion of Methane to Synthesis Gas: Molybdenum and Tungsten Carbide. J. Catal. 1998, 100, 85–100. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Yang, X.; Boscoboinik, J.A.; Chen, J.G. Molybdenum Carbide as Alternative Catalysts to Precious Metals for Highly Selective Reduction of CO2 to CO. Angew. Chemie 2014, 126, 6823–6827. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, Z.; Jiang, Q.; Wu, K.H.; Gong, H.; Liu, Y. Molybdenum carbide clusters for thermal conversion of CO2 to CO via reverse water-gas shift reaction. J. Energy Chem. 2020, 50, 37–43. [Google Scholar] [CrossRef]
- Juneau, M.; Pope, C.; Liu, R.; Porosoff, M.D. Support acidity as a descriptor for reverse water-gas shift over Mo2C-based catalysts. Appl. Catal. A Gen. 2021, 620, 118034. [Google Scholar] [CrossRef]
- Roy, S.; Cherevotan, A.; Peter, S.C. Thermochemical CO2 Hydrogenation to Single Carbon Products: Scientific and Technological Challenges. ACS Energy Lett. 2018, 3, 1938–1966. [Google Scholar] [CrossRef]
- Dietz, L.; Piccinin, S.; Maestri, M. Mechanistic insights into CO2 activation via reverse water—Gas shift on metal surfaces. J. Phys. Chem. C 2015, 119, 4959–4966. [Google Scholar] [CrossRef]
- Morse, J.R.; Juneau, M.; Baldwin, J.W.; Porosoff, M.D.; Willauer, H.D. Alkali promoted tungsten carbide as a selective catalyst for the reverse water gas shift reaction. J. CO2 Util. 2020, 35, 38–46. [Google Scholar] [CrossRef]
- Correlation, T.; Oosthuizen, G.J. The Correlation Between Catalyst Surface Basicity and Hydrocarbon Selectivity in the Fischer Tropsch Synthesis. J. Catal. 1968, 11, 18–24. [Google Scholar]
- Saeidi, S.; Amin, N.A.S.; Rahimpour, M.R. Hydrogenation of CO2 to value-added products—A review and potential future developments. J. CO2 Util. 2014, 5, 66–81. [Google Scholar] [CrossRef]
- Gnanamani, M.K.; Hamdeh, H.H.; Shafer, W.D.; Hopps, S.D.; Davis, B.H. Hydrogenation of carbon dioxide over iron carbide prepared from alkali metal promoted iron oxalate. Appl. Catal. A Gen. 2018, 564, 243–249. [Google Scholar] [CrossRef]
- Woo, H.C.; Park, K.Y.; Kim, Y.G.; Namau]Jong ShikChung, I.S.; Lee, J.S. Mixed alcohol synthesis from carbon monoxide and dihydrogen over potassium-promoted molybdenum carbide catalysts. Appl. Catal. 1991, 75, 267–280. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Baldwin, J.W.; Peng, X.; Mpourmpakis, G.; Willauer, H.D. Potassium-Promoted Molybdenum Carbide as a Highly Active and Selective Catalyst for CO2 Conversion to CO. ChemSusChem 2017, 10, 2408–2415. [Google Scholar] [CrossRef]
- Porosoff, M.; Willauer, H.D. Alkali metal doped molybdenum carbide supported on gamma-alumina for selective CO2 hydrogenation into CO 2019. U.S. Patent 11,266,980, 11 July 2019. [Google Scholar]
- Ro, I.; Resasco, J.; Christopher, P. Approaches for Understanding and Controlling Interfacial Effects in Oxide-Supported Metal Catalysts. ACS Catal. 2018, 8, 7368–7387. [Google Scholar] [CrossRef]
- Munnik, P.; De Jongh, P.E.; De Jong, K.P. Recent Developments in the Synthesis of Supported Catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef]
- Christensen, J.M.; Duchstein, L.D.L.; Wagner, J.B.; Jensen, P.A.; Temel, B.; Jensen, A.D. Catalytic conversion of syngas into higher alcohols over carbide catalysts. Ind. Eng. Chem. Res. 2012, 51, 4161–4172. [Google Scholar] [CrossRef]
- Kojima, R.; Aika, K.I. Molybdenum nitride and carbide catalysts for ammonia synthesis. Appl. Catal. A Gen. 2001, 219, 141–147. [Google Scholar] [CrossRef]
- Said, A.A. Mutual influences between ammonium heptamolybdate and γ-alumina during their thermal treatments. Thermochim. Acta 1994, 236, 93–104. [Google Scholar] [CrossRef]
- Vo, D.V.N.; Adesina, A.A. Kinetics of the carbothermal synthesis of Mo carbide catalyst supported on various semiconductor oxides. Fuel Process. Technol. 2011, 92, 1249–1260. [Google Scholar] [CrossRef]
- Hiraishi, J.; Nishijima, A. Support Effect on the Catalytic Activity and Properties Molybdenum Catalysts. J. Catal. 1988, 110, 275–284. [Google Scholar]
- Thomazeau, C.; Martin, V.; Afanasiev, P. Effect of support on the thermal decomposition of (NH4)6Mo7O24·4H2O in the inert gas atmosphere. Appl. Catal. A Gen. 2000, 199, 61–72. [Google Scholar] [CrossRef]
- Liu, X.; Kunkel, C.; Ramírez De La Piscina, P.; Homs, N.; Viñes, F.; Illas, F. Effective and Highly Selective CO Generation from CO2 Using a Polycrystalline α-Mo2C Catalyst. ACS Catal. 2017, 7, 4323–4335. [Google Scholar] [CrossRef]
- Marquart, W.; Raseale, S.; Prieto, G.; Zimina, A.; Sarma, B.B.; Grunwaldt, J.D.; Claeys, M.; Fischer, N. CO2 Reduction over Mo2C-Based Catalysts. ACS Catal. 2021, 11, 1624–1639. [Google Scholar] [CrossRef]
- Lee, J.S.; Oyama, S.T.; Boudart, M. Molybdenum Carbide Catalysts 1. Synthesis of Unsupported Powders. J. Catal. 1987, 106, 125–133. [Google Scholar] [CrossRef]
- Ribeiro, F.H.; Betta, R.A.D.; Guskey, G.J.; Boudart, M. Preparation and Surface Composition of Tungsten Carbide Powders with High Specific Surface Area. Chem. Mater. 1991, 3, 805–812. [Google Scholar] [CrossRef]
- Li, S.; Kim, W.B.; Lee, J.S. Effect of the Reactive Gas on the Solid-State Transformation of Molybdenum Trioxide to Carbides and Nitrides. Chem. Mater. 1998, 10, 1853–1862. [Google Scholar] [CrossRef]
- Volpe, L.; Boudart, M. Compounds of molybdenum and tungsten with high specific surface area. II. Carbides. J. Solid State Chem. 1985, 59, 348–356. [Google Scholar] [CrossRef]
- Thiollier, A.; Afanasiev, P.; Cattenot, M.; Vrinat, M. Preparation and properties of chromium-containing hydrotreating catalysts (Ni-Mo)/ZrO2-Cr2O3. Catal. Lett. 1998, 55, 39–45. [Google Scholar] [CrossRef]
- Fountzoula, C.; Spanos, N.; Matralis, H.K.; Kordulis, C. Molybdenum-titanium oxide catalysts: The influence of the preparation conditions on their activity for the selective catalytic reduction of NO by NH3. Appl. Catal. B Environ. 2002, 35, 295–304. [Google Scholar] [CrossRef]
- Massoth, F.E. Characterization of Molybdena Catalysts. Adv. Catal. 1979, 27, 265–310. [Google Scholar]
- Okamoto, Y.; Imanaka, T. Interaction chemistry between molybdena and alumina: Infrared studies of surface hydroxyl groups and adsorbed carbon dioxide on aluminas modified with molybdate, sulfate, or fluorine anions. J. Phys. Chem. 1988, 92, 7102–7112. [Google Scholar] [CrossRef]
- Okamoto, Y.; Ishihara, S.Y.; Kawano, M.; Satoh, M.; Kubota, T. Preparation of Co-Mo/Al2O3 model sulfide catalysts for hydrodesulfurization and their application to the study of the effects of catalyst preparation. J. Catal. 2003, 217, 12–22. [Google Scholar] [CrossRef]
- Okamoto, Y.; Maezawa, A.; Imanaka, T. Active sites of molybdenum sulfide catalysts supported on Al2O3 and TiO2 for hydrodesulfurization and hydrogenation. J. Catal. 1989, 120, 29–45. [Google Scholar] [CrossRef]
- Mehdad, A.; Jentoft, R.E.; Jentoft, F.C. Passivation agents and conditions for Mo2C and W2C: Effect on catalytic activity for toluene hydrogenation. J. Catal. 2017, 347, 89–101. [Google Scholar] [CrossRef]
- Leary, K.J.; Michaels, J.N.; Stacy, A.M. Carbon and Oxygen Atom Mobility during Activation of Mo2C Catalysts. J. Catal. 1986, 101, 301–313. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Liang, C.; Chen, X.; Ying, P.; Li, C. In situ FT-IR spectroscopic studies of CO adsorption on fresh Mo2C/Al2O3 catalyst. J. Phys. Chem. B 2003, 107, 7088–7094. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wu, Z.; Liang, C.; Ying, P.; Feng, Z.; Li, C.; Academy, C. An IR study on the surface passivation of Mo2C/Al2O3 catalyst with O2, H2O and CO2. Phys. Chem. Chem. Phys. 2004, 6, 5603–5608. [Google Scholar] [CrossRef]
- Shou, H.; Ferrari, D.; Barton, D.G.; Jones, C.W.; Davis, R.J. Influence of passivation on the reactivity of unpromoted and Rb-promoted Mo2C nanoparticles for CO hydrogenation. ACS Catal. 2012, 2, 1408–1416. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).