Tri-Reforming of Methane: Thermodynamics, Operating Conditions, Reactor Technology and Efficiency Evaluation—A Review
Abstract
:1. Introduction
2. Scope of the Current Review
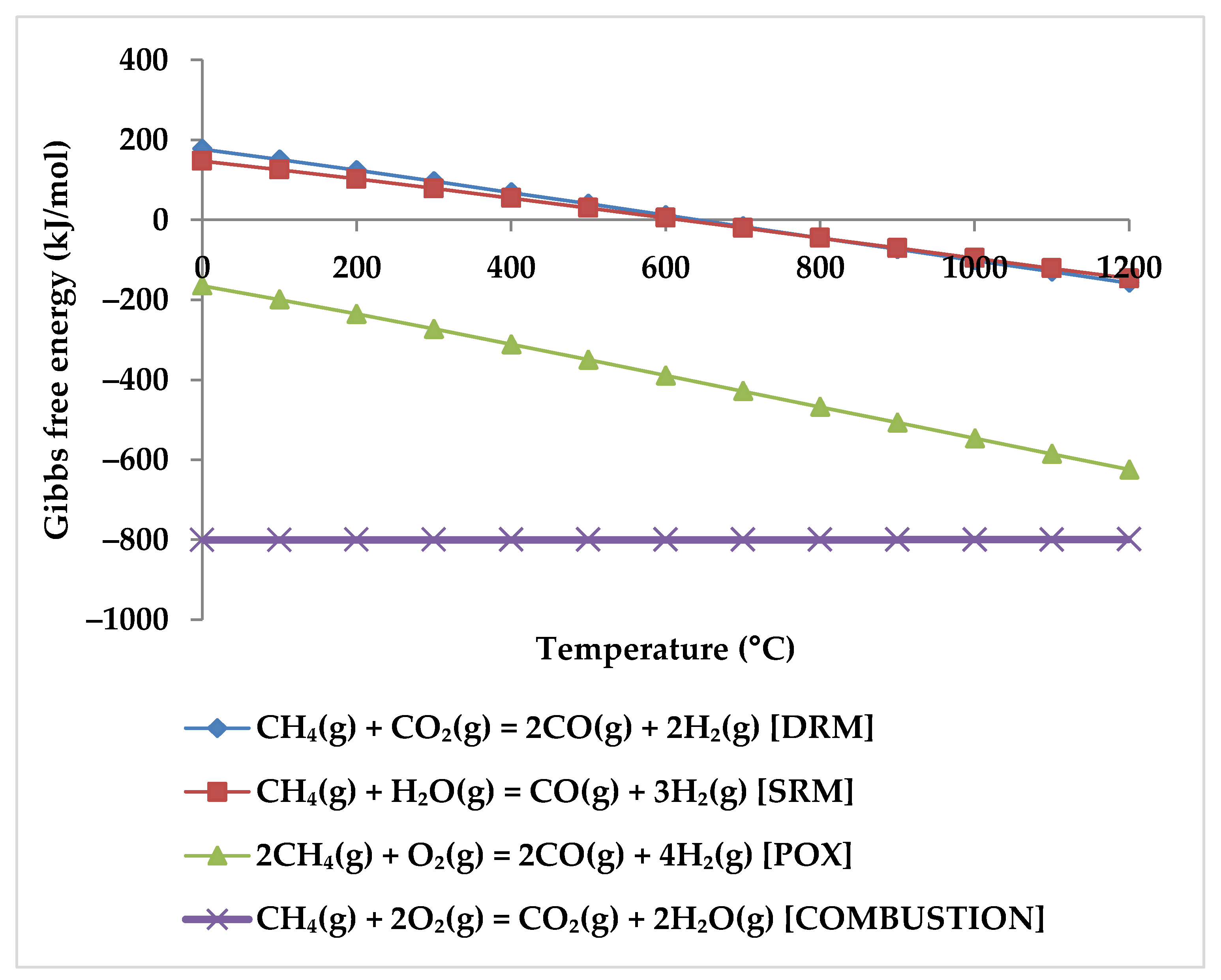
3. Feasibility Studies
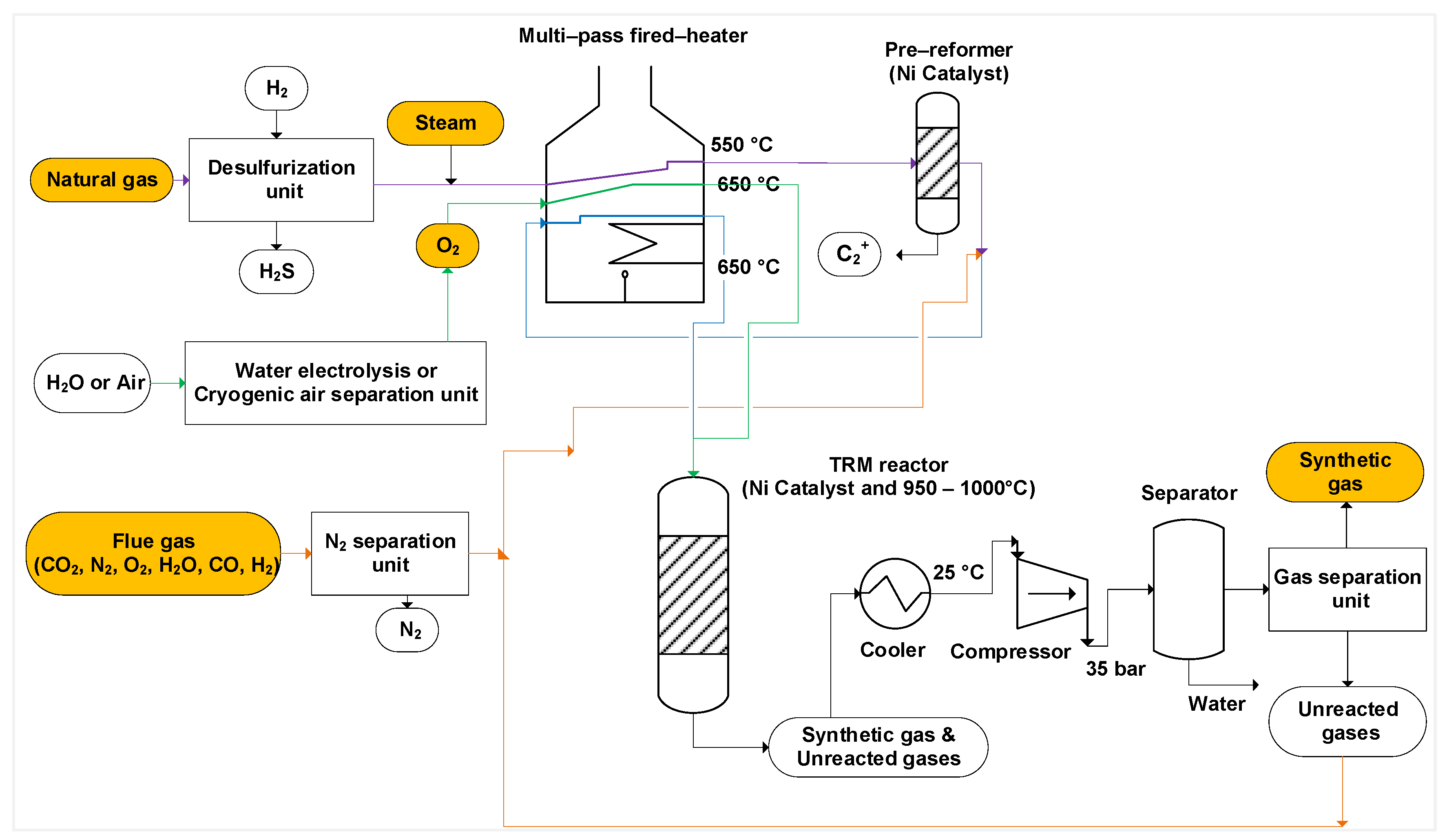
4. TRM Process
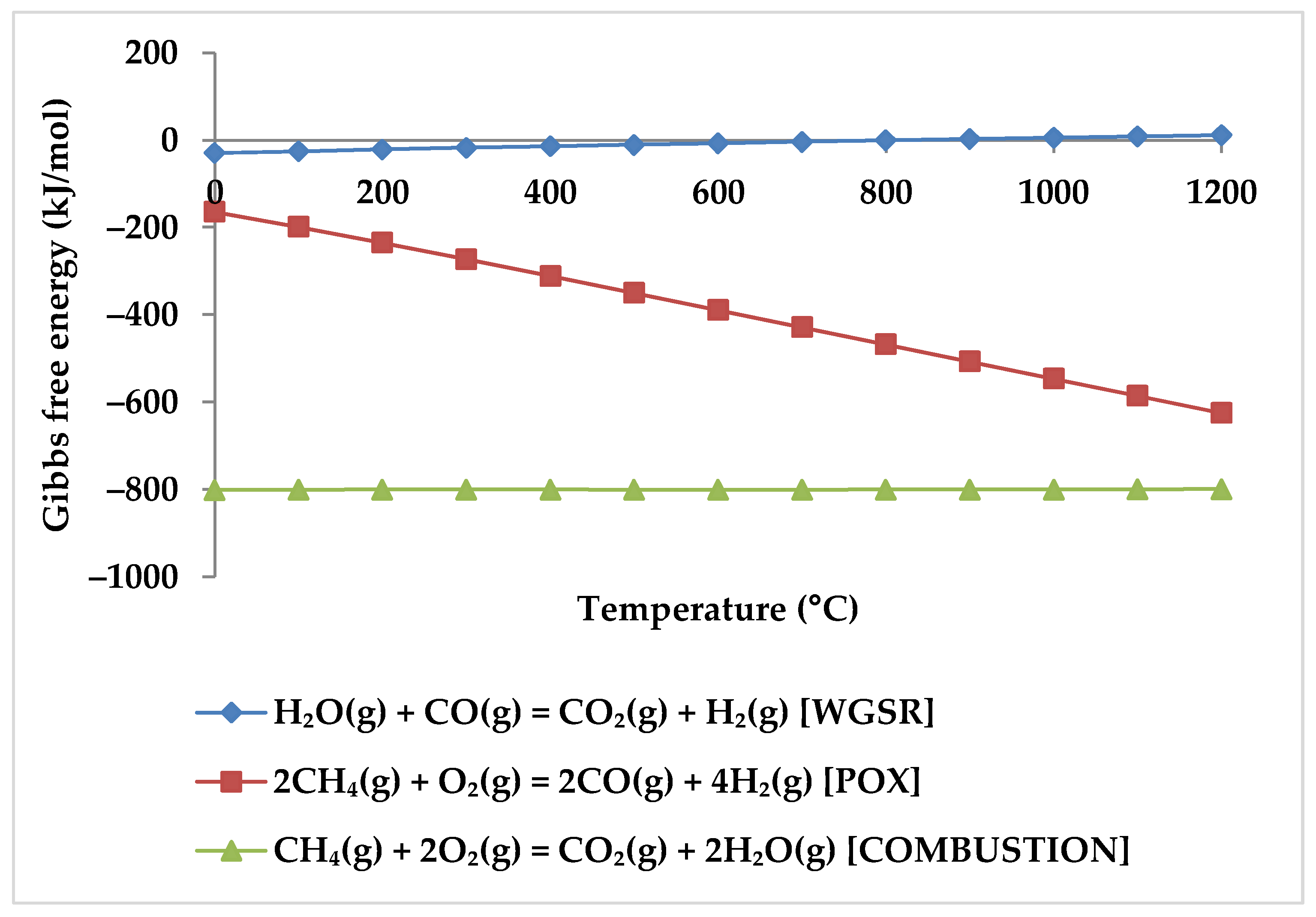
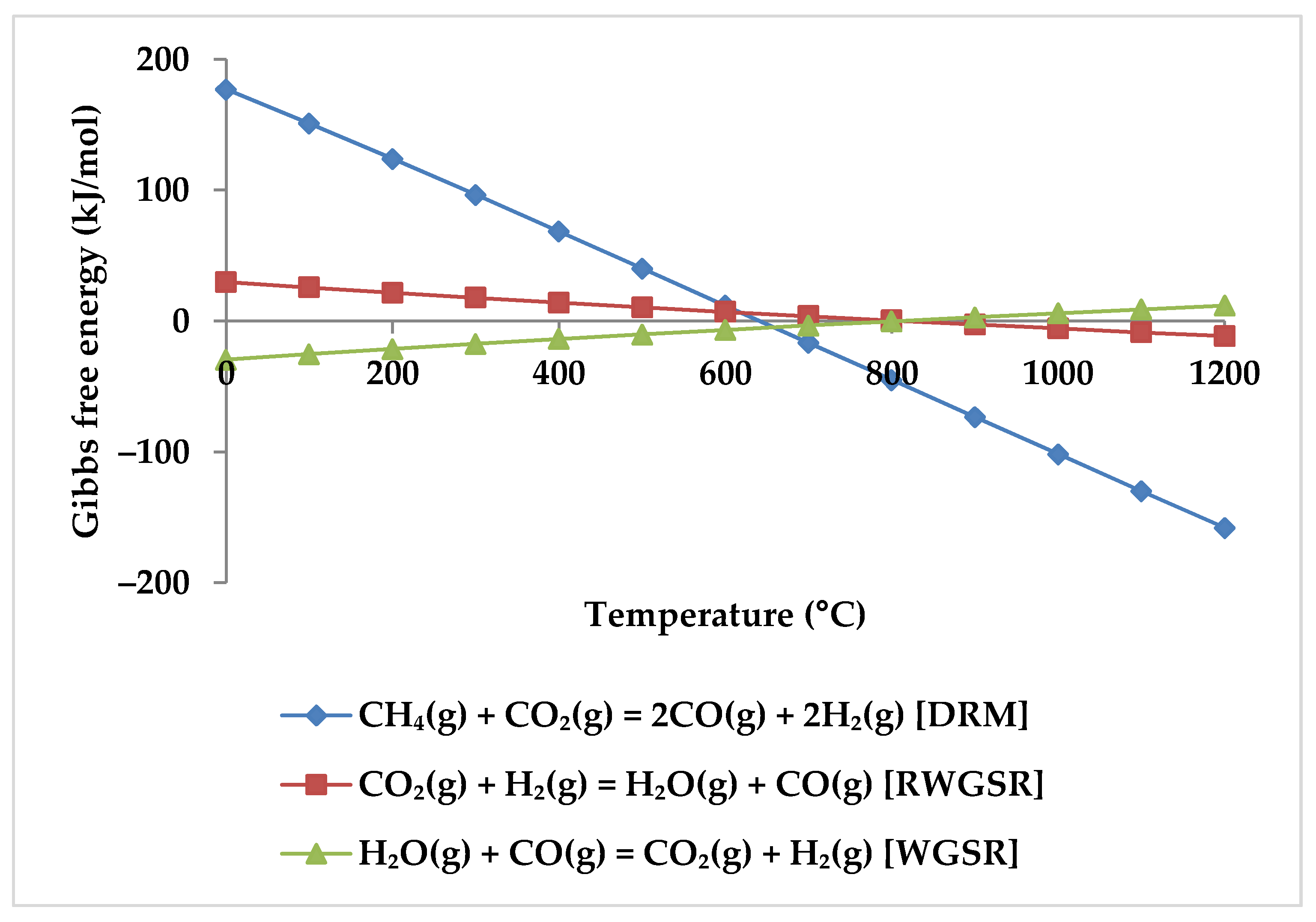
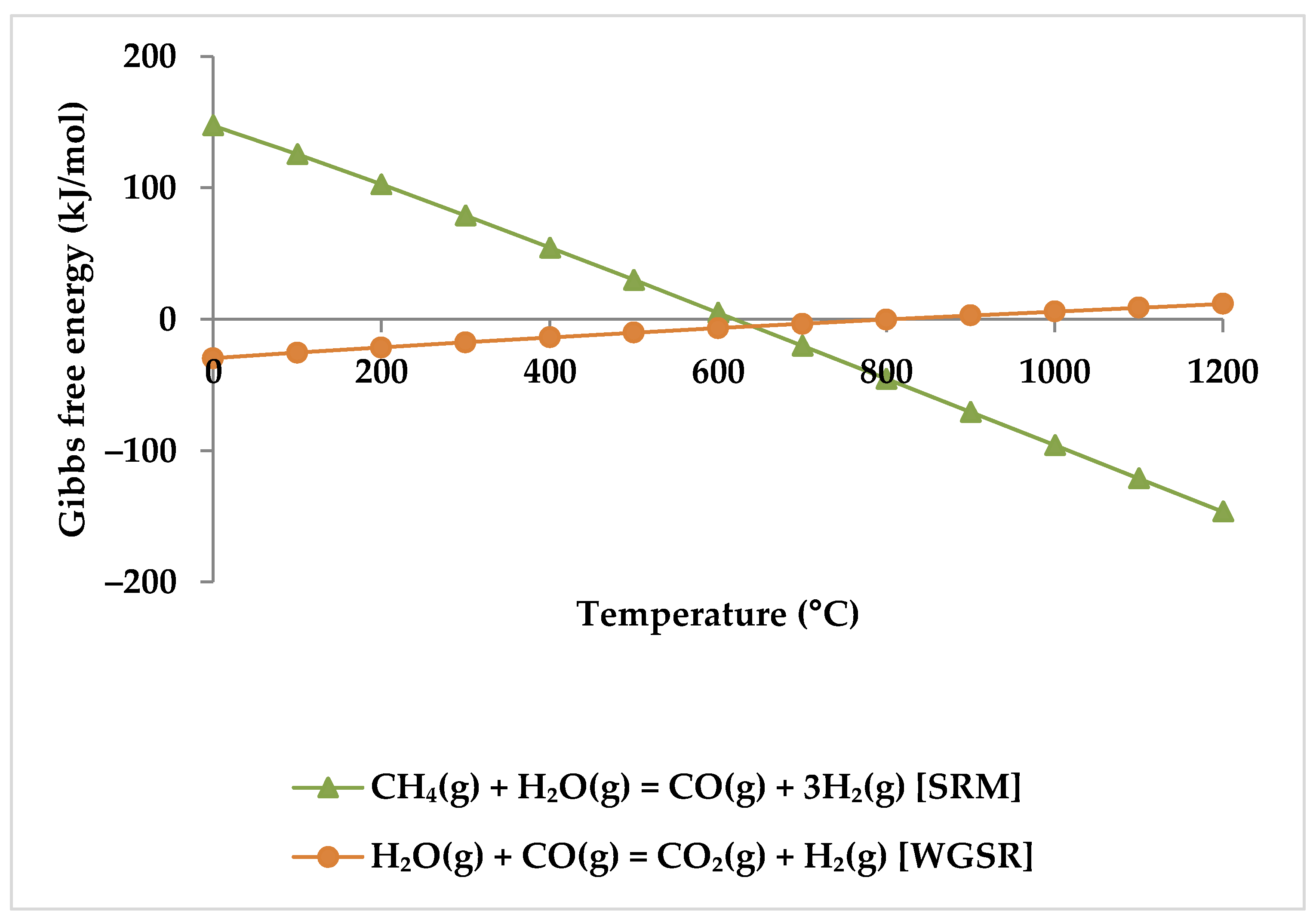
5. Thermodynamic Analysis
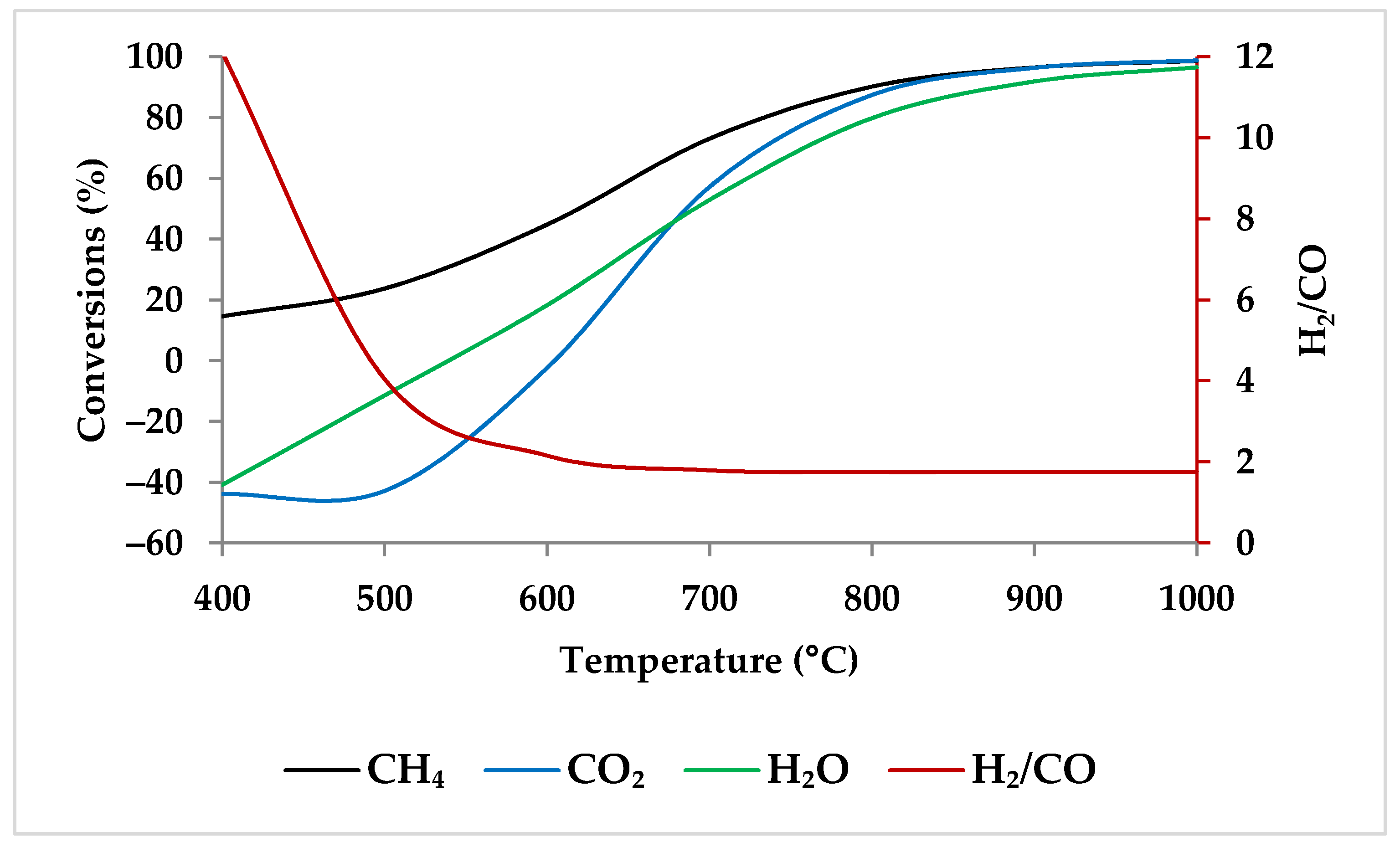
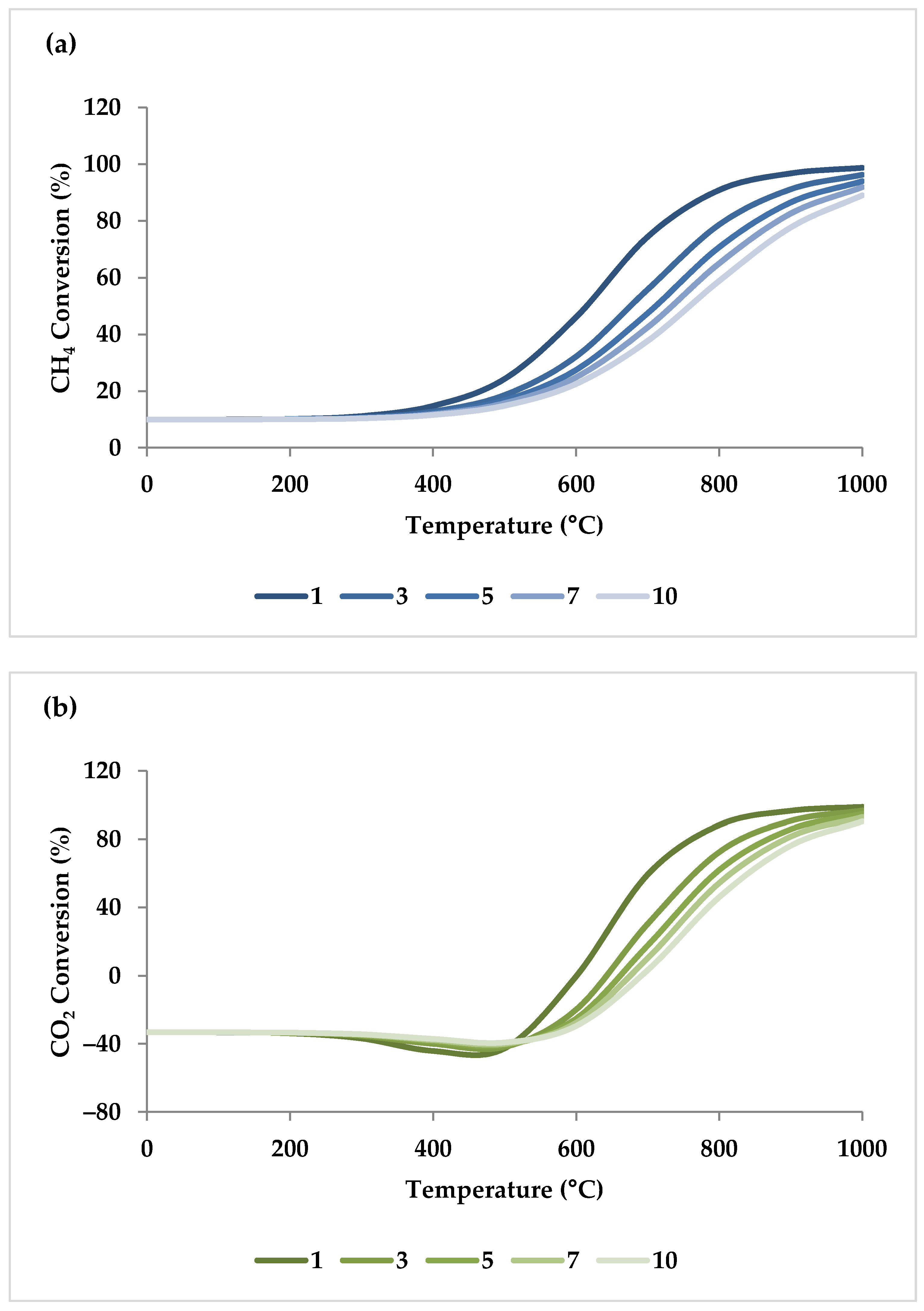
5.1. Effect of Temperature
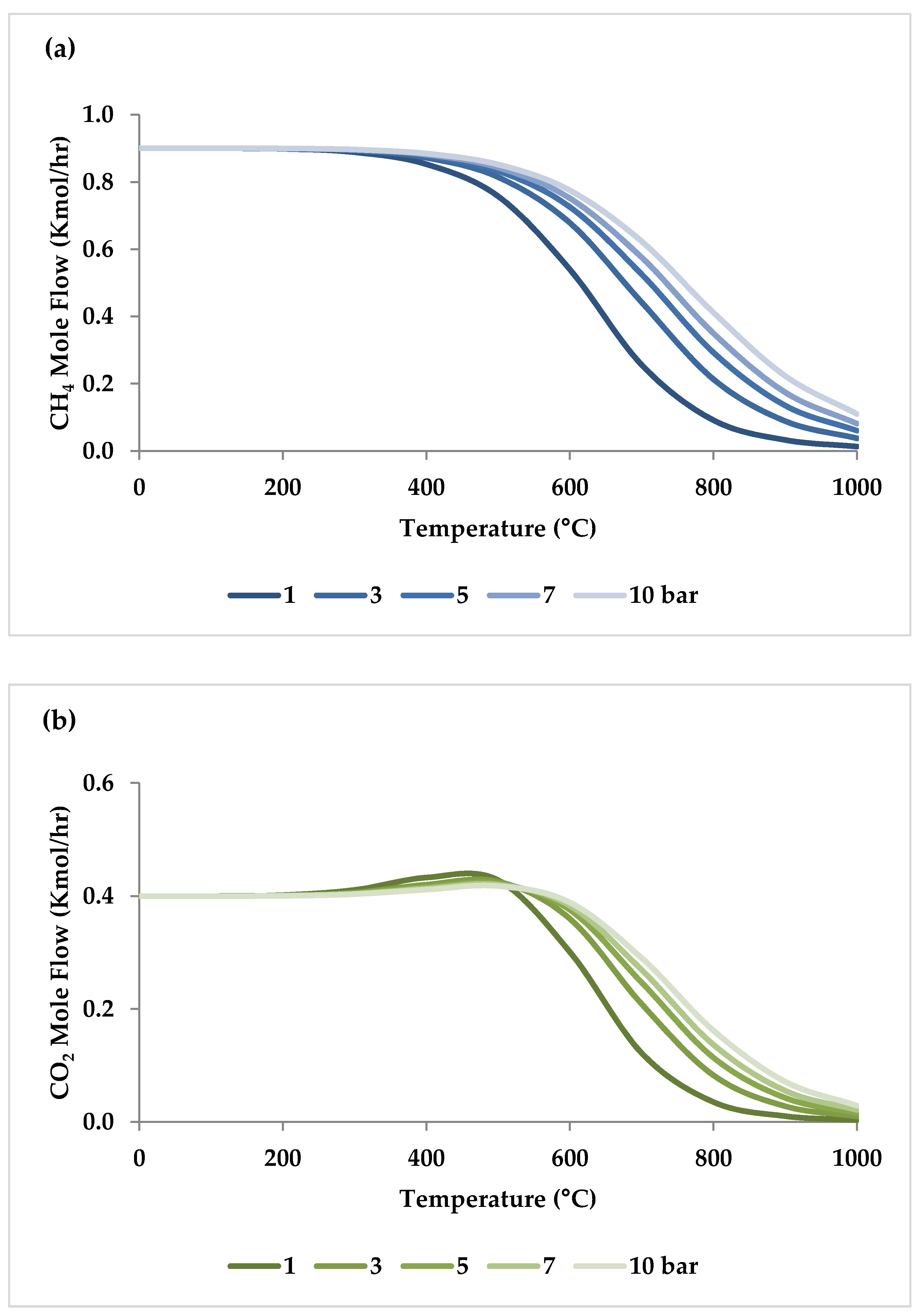
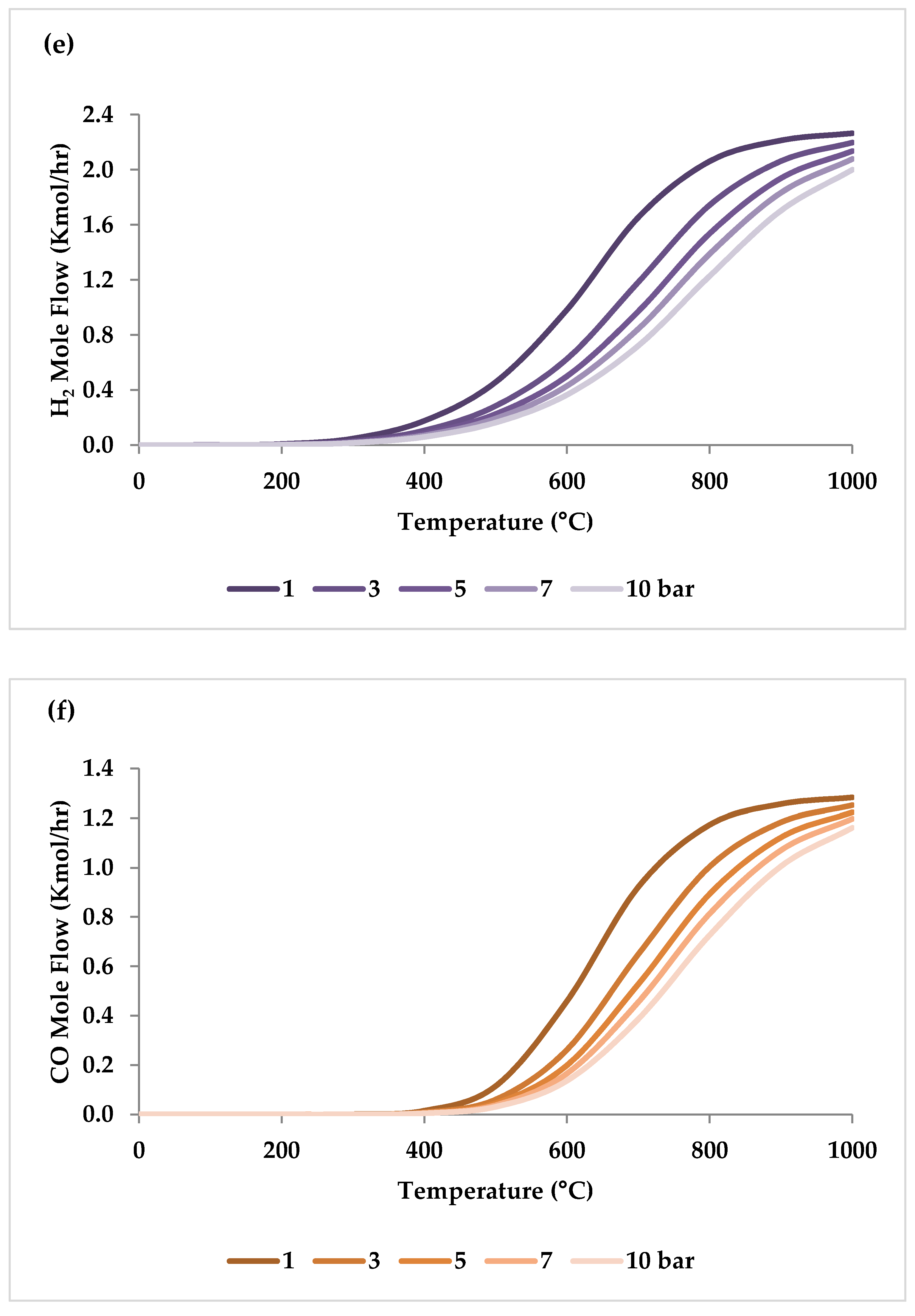
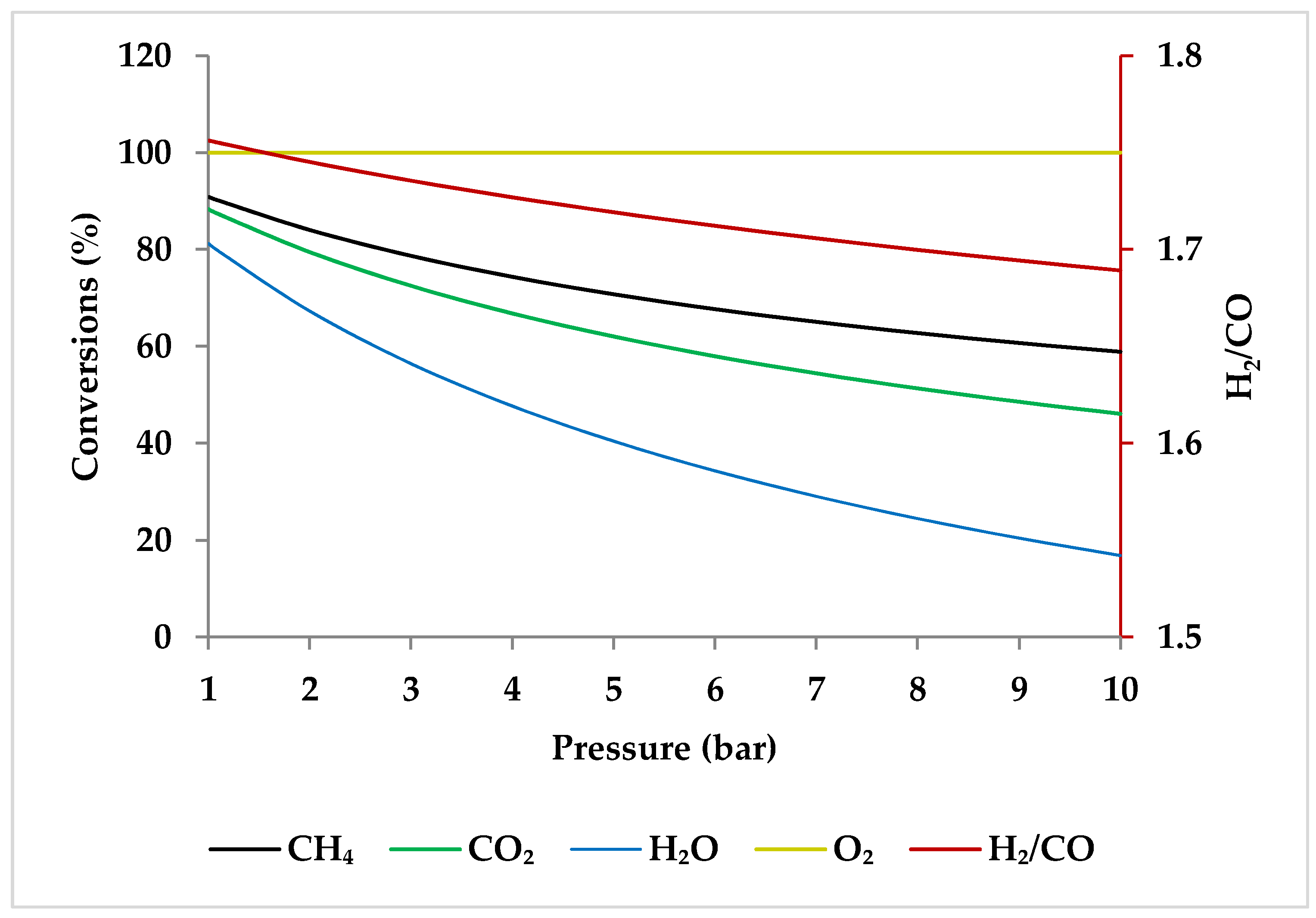
5.2. Effect of Pressure
5.3. Effect of CH4/Flue Gas Ratio
5.4. Effect of O2/CH4 Ratio
5.5. Effect of H2O/CH4 Ratio
5.6. Effect of CO2/CH4 Ratio
5.7. Effect of O2/CO2 Ratio
5.8. Effect of H2O/CO2 Ratio
6. Optimal Operating Conditions and Feed Compositions
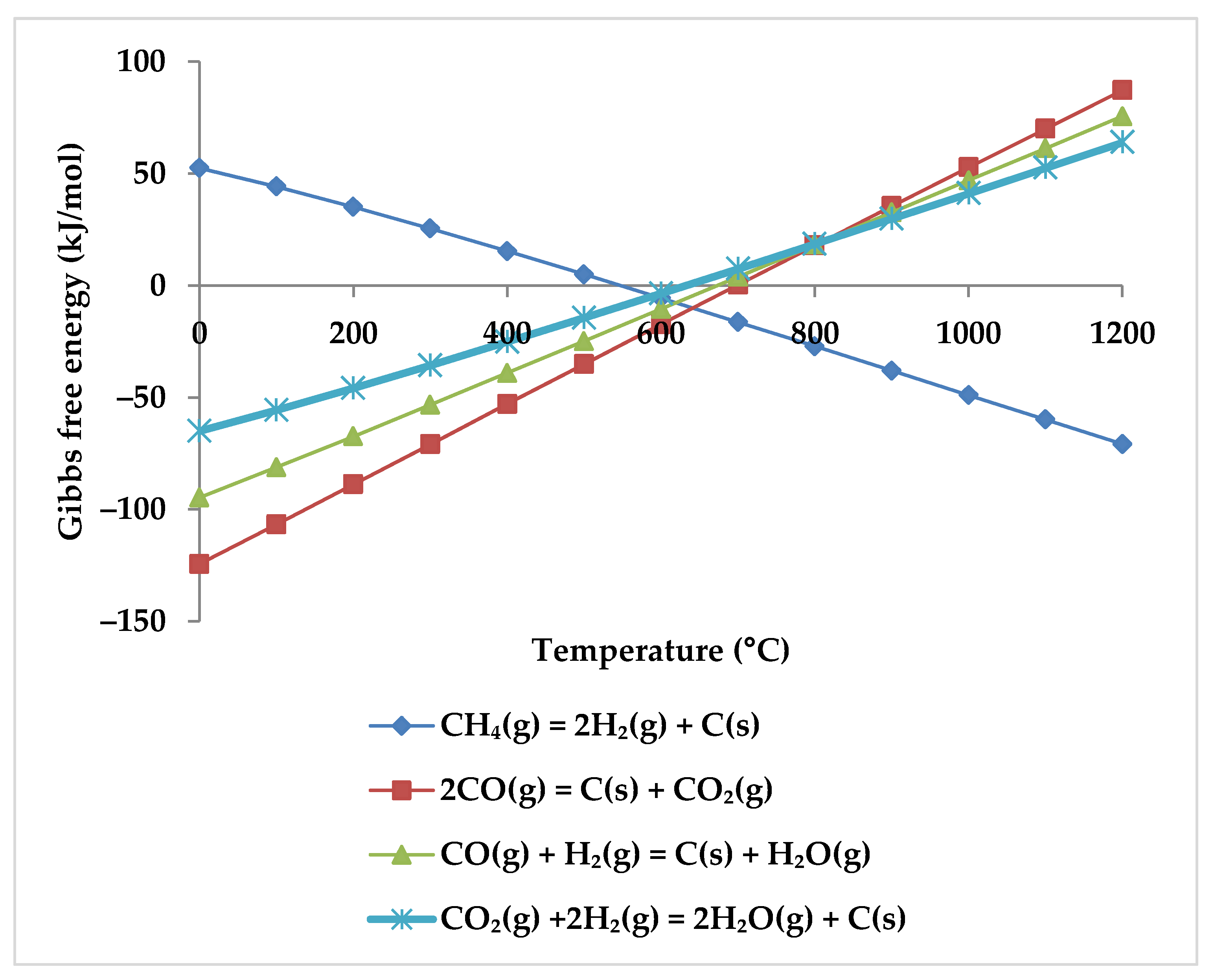
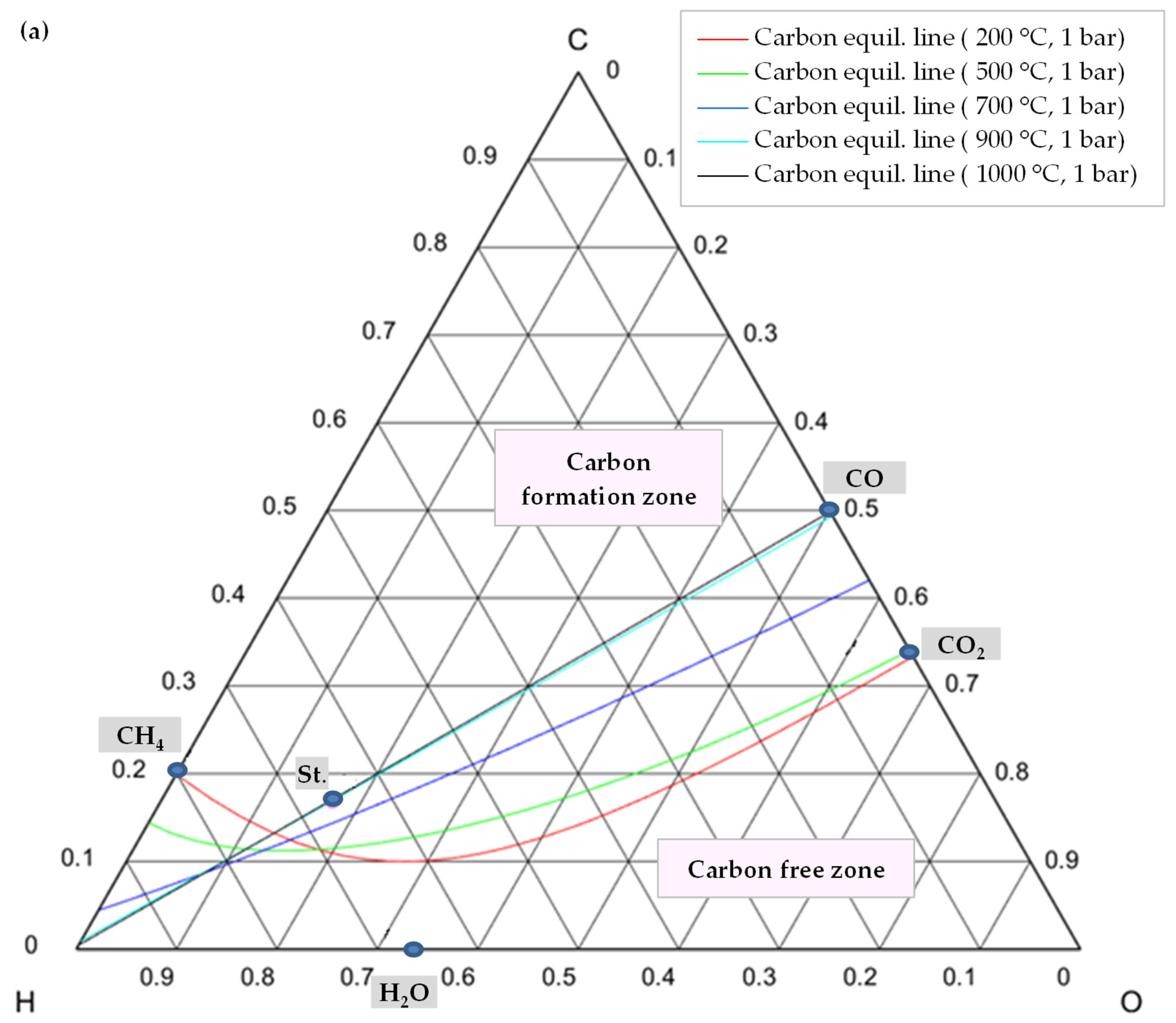
7. Coke Formation Assessment
8. Reactor Technology Evaluation
9. Energetic and Exergetic Considerations
| Process | Compared to | Saving Type: Amount % | Efficiency Type: Increased Amount % | Ref. |
|---|---|---|---|---|
| CO2 separation unit | Chemical absorption with alkanolamines | Energy of a coal-fired power plant: 20% | [90,91] | |
| TRM reaction | BRM reactions | Energy: 29% | [67] | |
| TRM of the flue gases released from coal- and gas-fired burners of power stations containing N2 | Conventional methanol production with SRM | Fuel: 31% | Exergy: 72% | [93] |
| TRM of the flue gases released from coal- and gas-fired burners of power stations containing N2 | Conventional H2 production with SRM | Fuel: 75% | Exergy: 74% | [93] |
| Combined TRM with COG and WGS unit | Conventional CTM | Carbon utilization: 4.3% | [95] | |
| Energy: 11.4% | ||||
| Combined TRM with COG | Conventional CTM | Carbon utilization: 14.5% | [95] | |
| Energy: 16.8% | ||||
| Combined TRM with CTEG and COGS | CTEG | Exergy: 30.5% | [63] | |
| Combined CTEG and SRM | Exergy: 10.3% | |||
| Combined CTEG and DRM | Exergy: 5.9% | |||
| Combined CTEG and BRM | Exergy: 2.0% | |||
| Combined TRM with oxy-fuel combustion equipped with water electrolysis unit | Direct CO2 hydrogenation method for methanol production | Net energy: 10% | [41] | |
| Combined TRM with methanol production WITH heat integration | Combined TRM with methanol production WITHOUT heat integration | Gross energy: 34.3% (minimizing utility cost) | [53] | |
| 32.2% (minimizing utility cost and capital cost) | ||||
| Combined TRM with methanol production with heat integration | Combined BRM with methanol production with heat integration | Specific energy: 4.0 kWh/kgCO2 | [53] | |
| Combined TRM with DME production WITH optimal heat exchanger network | Combined TRM with DME production WITHOUT optimal heat exchanger network | Gross energy: 37% (whole process) | [97] | |
| 33.3% (DME process) | ||||
| Combined TRM with DME production with optimal heat exchanger network | Combined BRM with DME production with optimal heat exchanger network | Energy: 0.97 kWh/kgCO2 | [97] |
| Design Factor | Effect on Energy Efficiency | Effect on Exergy Efficiency |
|---|---|---|
| Reactor inlet temperature | D 1 | N 4 |
| H2O concentration | D | I |
| Reactor pressure | I 2 | N |
| CO2 concentration | I | I |
| Air concentration | O 3 | O |
| Reactor length | O | I |
10. Economic Assessment
11. Environmental Assessment
12. Safety Assessment
13. Conclusions and Outlook
- Optimization studies have been independently undertaken focusing only on one or two specific aspects of TRM technology; thus, an integrated optimization of a TRM process should be performed to find the optimal design while taking safety, environmental, and economic aspects into account.
- Determination of the ideal operating conditions from economic and efficiency points of view.
- A comprehensive sensitivity analysis on operational parameters of the process.
- Determination of the optimal O2 concentration considering both in situ coke removal, energy balance and safety of the process.
- Pilot studies on different reactor configurations to find the best design, since most studies on reactors are of a theoretical nature.
- Investigation on the economic feasibility and commercial viability of the proposed reactor and process configurations.
- Commercialization feasibility study of using membrane technology to separate nitrogen from flue gases for industrial levels.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASU | Air Separation Unit |
| BRM | Bi-Reforming of Methane |
| CAPEX | Capital Cost |
| COG | Coke Oven Gas |
| COGS | Coke Oven Gas Separation |
| CTEG | Coal To Ethylene Glycol |
| CTM | Coal To Methanol |
| DCF | Discounted Cash Flow |
| DME | Dimethyl Ether |
| DRM | Dry Reforming of Methane |
| GAMS | General Algebraic Modeling System |
| GHG | Greenhouse Gas |
| GHSV | Gas Hourly Space Velocity |
| HHV | Higher Heating Value (kJ/mole) |
| NPT | Net Payout Time |
| NPV | Net Present Value (USD) |
| OPEX | Operating Cost |
| POX | Partial Oxidation of Methane |
| PSA | Pressure Swing Adsorption |
| RWGSR | Reverse Water Gas Shift Reaction |
| SRM | Steam Reforming of Methane |
| TRM | Tri-Reforming of Methane |
| WGS | Water Gas Shift |
| WGSR | Water Gas Shift Reaction |
| Nomenclature | |
| n | Molar flow rate (mole/s) |
| W | Power (kW) |
| HHV | Higher heating value per molar rate (kJ/mole) |
| η | Energy efficiency |
| Superscripts | |
| COMP | Compressor |
References
- Abas, N.; Kalair, A.; Khan, N. Review of fossil fuels and future energy technologies. Futures 2015, 69, 31–49. [Google Scholar] [CrossRef]
- Barelli, L.; Bidini, G.; Gallorini, F.; Servili, S. Hydrogen production through sorption-enhanced steam methane reforming and membrane technology: A review. Energy 2008, 33, 554–570. [Google Scholar] [CrossRef]
- Goeppert, A.; Czaun, M.; Jones, J.-P.; Surya Prakash, G.K.; Olah, G.A. Recycling of carbon dioxide to methanol and derived products–closing the loop. Chem. Soc. Rev. 2014, 43, 7995–8048. [Google Scholar] [CrossRef]
- Song, C.; Pan, W. Tri-reforming of methane: A novel concept for catalytic production of industrially useful synthesis gas with desired H2/CO ratios. Catal. Today 2004, 98, 463–484. [Google Scholar] [CrossRef]
- Song, C. Tri-reforming: A new process for reducing CO2 emissions. Chem. Innov. 2001, 31, 21–26. [Google Scholar]
- Song, C. Tri-reforming: A new process concept for effective conversion and utilization of CO2 in flue gas from electric power plants. Am. Chem. Soc. Div. Fuel Chem. Prepr. 2000, 45, 772–776. [Google Scholar]
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Nguyen, T.B.H.; Zondervan, E. Methanol production from captured CO2 using hydrogenation and reforming technologies_ environmental and economic evaluation. J. CO2 Util. 2019, 34, 1–11. [Google Scholar] [CrossRef]
- Minutillo, M.; Perna, A. A novel approach for treatment of CO2 from fossil fired power plants, Part A: The integrated systems ITRPP. Int. J. Hydrogen Energy 2009, 34, 4014–4020. [Google Scholar] [CrossRef]
- Cho, W.; Song, T.; Mitsos, A.; McKinnon, J.T.; Ko, G.H.; Tolsma, J.E.; Denholm, D.; Park, T. Optimal design and operation of a natural gas tri-reforming reactor for DME synthesis. Catal. Today 2009, 139, 261–267. [Google Scholar] [CrossRef]
- Shu, J.; Grandjean, B.P.A.; Kaliaguine, S. Methane steam reforming in asymmetric Pd- and Pd-Ag/porous SS membrane reactors. Appl. Catal. A Gen. 1994, 119, 305–325. [Google Scholar] [CrossRef]
- Liu, C.-j.; Ye, J.; Jiang, J.; Pan, Y. Progresses in the Preparation of Coke Resistant Ni-based Catalyst for Steam and CO2 Reforming of Methane. ChemCatChem 2011, 3, 529–541. [Google Scholar] [CrossRef]
- Souza, M.M.V.M.; Schmal, M. Autothermal reforming of methane over Pt/ZrO2/Al2O3 catalysts. Appl. Catal. A Gen. 2005, 281, 19–24. [Google Scholar] [CrossRef]
- Song, C.; Pan, W. Tri-reforming of methane: A novel concept for synthesis of industrially useful synthesis gas with desired H2/CO ratios using CO2 in flue gas of power plants without CO2 separation. Am. Chem. Soc. Div. Fuel Chem. Prepr. 2004, 49, 62986. [Google Scholar]
- Rostrup-Nielsen, J. Mechanisms of carbon formation on nickel-containing catalysts. J. Catal. 1977, 48, 155–165. [Google Scholar] [CrossRef]
- Lee, S.-H.; Cho, W.; Ju, W.-S.; Cho, B.-H.; Lee, Y.-C.; Baek, Y.-S. Tri-reforming of CH4 using CO2 for production of synthesis gas to dimethyl ether. Catal. Today 2003, 87, 133–137. [Google Scholar] [CrossRef]
- Moon, D.J. Development of tri-reforming catalyst for utilization of CO2 in SOFC and MCFC fuel reforming applications. In Proceedings of the 8th International Conference on Carbon Dioxide Utilization, Oslo, Norway, 20–23 June 2005. [Google Scholar]
- Mignard, D. Methanol synthesis from flue-gas CO2 and renewable electricity: A feasibility study. Int. J. Hydrogen Energy 2003, 28, 455–464. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Gossage, J.L.; Lou, H.H.; Benson, T.J. Thermodynamic Analyses of Tri-reforming Reactions to Produce Syngas. Energy Fuels 2014, 28, 2717–2726. [Google Scholar] [CrossRef]
- Dwivedi, A.; Gudi, R.; Biswas, P. An improved tri-reforming based methanol production process for enhanced CO2 valorization. Int. J. Hydrogen Energy 2017, 42, 23227–23241. [Google Scholar] [CrossRef]
- Liu, J. Kinetics, Catalysis and Mechanism of Methane Steam Reforming. Master’s Thesis, Worcester Polytechnic Institute, Worcester, MA, USA, 2007. [Google Scholar]
- Maciel, L.J.L.; de Souza, A.E.Á.M.; Cavalcanti-Filho, V.O.; Knoechelmann, A.; de Abreu, C.A.M. Kinetic evaluation of the tri-reforming process of methane for syngas production. React. Kinet. Mech. Catal. 2010, 101, 407–416. [Google Scholar] [CrossRef]
- Pham Minh, D.; Pham, X.-H.; Siang, T.J.; Vo, D.-V.N. Review on the catalytic tri-reforming of methane—Part I: Impact of operating conditions, catalyst deactivation and regeneration. Appl. Catal. A Gen. 2021, 621, 118202. [Google Scholar] [CrossRef]
- Pham, X.-H.; Ashik, U.P.M.; Hayashi, J.-I.; Pérez Alonso, A.; Pla, D.; Gómez, M.; Pham Minh, D. Review on the catalytic tri-reforming of methane—Part II: Catalyst development. Appl. Catal. A Gen. 2021, 623, 118286. [Google Scholar] [CrossRef]
- Solov’ev, S.A.; Gubareni, Y.V.; Kurilets, Y.P.; Orlik, S.N. Tri-reforming of methane on structured Ni-containing catalysts. Theor. Exp. Chem. 2012, 48, 199–205. [Google Scholar] [CrossRef]
- Arab Aboosadi, Z.; Farhadi Yadecoury, M. Thermally Intensification of Steam Reforming Process by Use of Methane Tri-Reforming: A Review. Int. J. Chem. React. Eng. 2019, 17. [Google Scholar] [CrossRef]
- Soloviev, S.O.; Gubareni, I.V.; Orlyk, S.M. Oxidative Reforming of Methane on Structured Nickel–Alumina Catalysts: A Review. Theor. Exp. Chem. 2018, 54, 293–315. [Google Scholar] [CrossRef]
- Alves, H.J.; Bley Junior, C.; Niklevicz, R.R.; Frigo, E.P.; Frigo, M.S.; Coimbra-Araújo, C.H. Overview of hydrogen production technologies from biogas and the applications in fuel cells. Int. J. Hydrogen Energy 2013, 38, 5215–5225. [Google Scholar] [CrossRef]
- Web of Science. Available online: https://www.webofscience.com/wos/woscc/summary/33536868-4d73-4730-868c-64c60421ef64-4e95dd9f/relevance/1 (accessed on 31 December 2021).
- Tjatjopoulos, G.J.; Vasalos, I.A. Feasibility Analysis of Ternary Feed Mixtures of Methane with Oxygen, Steam, and Carbon Dioxide for the Production of Methanol Synthesis Gas. Ind. Eng. Chem. Res. 1998, 37, 1410–1421. [Google Scholar] [CrossRef]
- Pan, W.; Zheng, J.; Song, C. Catalytic tri-reforming of methane using flue gas from fossil fuel-based power plants. Fuel Chem. Div. Prep. 2002, 47, 262–264. [Google Scholar]
- Choudhary, V.R.; Uphade, B.S.; Mamman, A.S. Simultaneous steam and CO2 reforming of methane to syngas over NiO/MgO/SA-5205 in presence and absence of oxygen. Appl. Catal. A Gen. 1998, 168, 33–46. [Google Scholar] [CrossRef]
- Won-Jun, C.; Yong-Gi, M.; Taek-Yong, S.; Hyen-Chan, L.; Young-Soon, B.; Douglas, D.; Glen, K.; Chang-Woo, C. Production of DME from CBM by KOGAS DME process. Trans. Korean Hydrogen New Energy Soc. 2011, 22, 925–933. [Google Scholar]
- Song, C.; Pan, W.; Srimat, S.T. Tri-reforming of Natural Gas Using CO2 in Flue Gas of Power Plants without CO2 Pre-separation for Production of Synthesis Gas with Desired H2/CO Ratios. In Environmental Challenges and Greenhouse Gas Control for Fossil Fuel Utilization in the 21st Century; Maroto-Valer, M.M., Song, C., Soong, Y., Eds.; Springer: Boston, MA, USA, 2002; pp. 247–267. [Google Scholar]
- Schmal, M.; Toniolo, F.S.; Kozonoe, C.E. Perspective of catalysts for (Tri) reforming of natural gas and flue gas rich in CO2. Appl. Catal. A Gen. 2018, 568, 23–42. [Google Scholar] [CrossRef]
- Jarungthammachote, S. Optimum feed ratio analysis for tri-reforming of methane using thermodynamic equilibrium method. Sci. Technol. Asia 2015, 20, 68–79. [Google Scholar]
- Özkara-Aydınoğlu, Ş. Thermodynamic equilibrium analysis of combined carbon dioxide reforming with steam reforming of methane to synthesis gas. Int. J. Hydrogen Energy 2010, 35, 12821–12828. [Google Scholar] [CrossRef]
- Yuan, M.; Narakornpijit, K.; Haghpanah, R.; Wilcox, J. Consideration of a nitrogen-selective membrane for postcombustion carbon capture through process modeling and optimization. J. Membr. Sci. 2014, 465, 177–184. [Google Scholar] [CrossRef]
- Dwivedi, A.; Gudi, R.; Biswas, P. Oxy-fuel combustion based enhancement of the tri-reforming coupled methanol production process for CO2 valorization. J. CO2 Util. 2018, 24, 376–385. [Google Scholar] [CrossRef]
- Fekri Lari, M.; Farsi, M.; Rahimpour, M.R. Modification of a tri-reforming reactor based on the feeding policy to couple with methanol and GTL units. Chem. Eng. Res. Des. 2019, 144, 107–114. [Google Scholar] [CrossRef]
- Shi, C.; Labbaf, B.; Mostafavi, E.; Mahinpey, N. Methanol production from water electrolysis and tri-reforming: Process design and technical-economic analysis. J. CO2 Util. 2020, 38, 241–251. [Google Scholar] [CrossRef]
- Gas, U. Chemical Composition of Natural Gas—Union Gas. 2017. Available online: https://www.uniongas.com/about-us/about-natural-gas/Chemical-Composition-of-Natural-Gas (accessed on 26 September 2017).
- Zhang, Y. A Catalytic and Process Design for the Utilization of Waste CO2 Using Tri-Reforming; Lamar University-Beaumont: Beaumont, TX, USA, 2014. [Google Scholar]
- Dwivedi, A.; Gudi, R.; Biswas, P. An oxy-fuel combustion based tri-reforming coupled methanol production process with improved hydrogen utilization. Int. J. Greenh. Gas Control. 2020, 93, 102905. [Google Scholar] [CrossRef]
- Dwivedi, A.; Gudi, R.; Biswas, P. An improved water electrolysis and oxy-fuel combustion coupled tri-reforming process for methanol production and CO2 valorization. J. Environ. Chem. Eng. 2021, 9, 105041. [Google Scholar] [CrossRef]
- Borreguero, A.M.; Dorado, F.; Capuchino-Biezma, M.; Sánchez-Silva, L.; García-Vargas, J.M. Process simulation and economic feasibility assessment of the methanol production via tri-reforming using experimental kinetic equations. Int. J. Hydrogen Energy 2020, 45, 26623–26636. [Google Scholar] [CrossRef]
- Ren, H.-P.; Song, Y.-H.; Liu, Z.-T.; Liu, Z.-W. Key Factors on the Pressurized Tri-Reforming of Methane over Ni-SiO2. In Chemical Reaction Engineering—Boston; Wei, J., Georgakis, C., Eds.; American Chemical Society: Washington, DC, USA, 1982; pp. 155–169. [Google Scholar]
- Zhou, C.; Zhang, L.; Swiderski, A.; Yang, W.; Blasiak, W. Study and development of a high temperature process of multi-reformation of CH4 with CO2 for remediation of greenhouse gas. Energy 2011, 36, 5450–5459. [Google Scholar] [CrossRef]
- Majewski, A.J.; Wood, J. Tri-reforming of methane over Ni@SiO2 catalyst. Int. J. Hydrogen Energy 2014, 39, 12578–12585. [Google Scholar] [CrossRef]
- Kozonoe, C.E.; Brito Alves, R.M.; Schmal, M. Influence of feed rate and testing variables for low-temperature tri-reforming of methane on the Ni@MWCNT/Ce catalyst. Fuel 2020, 281, 118749. [Google Scholar] [CrossRef]
- Challiwala, M.S.; Ghouri, M.M.; Linke, P.; El-Halwagi, M.M.; Elbashir, N.O. A combined thermo-kinetic analysis of various methane reforming technologies: Comparison with dry reforming. J. CO2 Util. 2017, 17, 99–111. [Google Scholar] [CrossRef]
- Lino, A.V.P.; Assaf, E.M.; Assaf, J.M. Adjusting Process Variables in Methane Tri-reforming to Achieve Suitable Syngas Quality and Low Coke Deposition. Energy Fuels 2020, 34, 16522–16531. [Google Scholar] [CrossRef]
- Zhang, Y.; Cruz, J.; Zhang, S.; Lou, H.H.; Benson, T.J. Process simulation and optimization of methanol production coupled to tri-reforming process. Int. J. Hydrogen Energy 2013, 38, 13617–13630. [Google Scholar] [CrossRef]
- Singha, R.K.; Das, S.; Pandey, M.; Kumar, S.; Bal, R.; Bordoloi, A. Ni nanocluster on modified CeO2–ZrO2 nanoporous composite for tri-reforming of methane. Catal. Sci. Technol. 2016, 6, 7122–7136. [Google Scholar] [CrossRef]
- Chein, R.-Y.; Wang, C.-Y.; Yu, C.-T. Parametric study on catalytic tri-reforming of methane for syngas production. Energy 2017, 118, 1–17. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Zhang, X.; Mi, Z. Thermodynamic analysis of autothermal steam and CO2 reforming of methane. Int. J. Hydrogen Energy 2008, 33, 2507–2514. [Google Scholar] [CrossRef]
- Arab Aboosadi, Z.; Jahanmiri, A.H.; Rahimpour, M.R. Optimization of tri-reformer reactor to produce synthesis gas for methanol production using differential evolution (DE) method. Appl. Energy 2011, 88, 2691–2701. [Google Scholar] [CrossRef]
- De Roseno, K.T.C.; Antunes, R.A.; Alves, R.M.B.; Schmal, M. Tri-Reforming of Methane over NdM0.25Ni0.75O3 (M = Cr, Fe) Catalysts and the Effect of CO2 Composition. Catal. Lett. 2021, 151, 3639–3655. [Google Scholar] [CrossRef]
- Singha, R.K.; Shukla, A.; Yadav, A.; Adak, S.; Iqbal, Z.; Siddiqui, N.; Bal, R. Energy efficient methane tri-reforming for synthesis gas production over highly coke resistant nanocrystalline Ni–ZrO2 catalyst. Appl. Energy 2016, 178, 110–125. [Google Scholar] [CrossRef]
- Walker, D.M.; Pettit, S.L.; Wolan, J.T.; Kuhn, J.N. Synthesis gas production to desired hydrogen to carbon monoxide ratios by tri-reforming of methane using Ni–MgO–(Ce,Zr)O2 catalysts. Appl. Catal. A Gen. 2012, 445–446, 61–68. [Google Scholar] [CrossRef]
- Djaidja, A.; Libs, S.; Kiennemann, A.; Barama, A. Characterization and activity in dry reforming of methane on NiMg/Al and Ni/MgO catalysts. Catal. Today 2006, 113, 194–200. [Google Scholar] [CrossRef]
- Wu, K.T.; Yu, C.T.; Chein, R.Y. Numerical Modeling on Catalytic Tri-reforming Reaction of Methane for Syngas Production. Energy Procedia 2017, 105, 4198–4203. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, J.; Chu, G.; Zhou, H.; Zhang, D. Optimal design, thermodynamic and economic analysis of coal to ethylene glycol processes integrated with various methane reforming technologies for CO2 reduction. Energy Convers. Manag. 2021, 244, 114538. [Google Scholar] [CrossRef]
- Jardim, S.S.Q.; Graciano, J.E.A.; Alves, R.M.B. Analysis of the Tri-Reforming of Methane in a Membrane Reactor. In 29th European Symposium on Computer Aided Process Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 517–522. [Google Scholar]
- García-Vargas, J.M.; Valverde, J.L.; de Lucas-Consuegra, A.; Gómez-Monedero, B.; Dorado, F.; Sánchez, P. Methane tri-reforming over a Ni/β-SiC-based catalyst: Optimizing the feedstock composition. Int. J. Hydrogen Energy 2013, 38, 4524–4532. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, S.; Zhang, J.; Liu, C.; Zhang, D.; Zhou, H.; Mei, S.; Gao, M.; Liu, H. Thermodynamic and techno-economic analyses of a novel integrated process of coal gasification and methane tri-reforming to ethylene glycol with low carbon emission and high efficiency. Energy 2021, 229, 120713. [Google Scholar] [CrossRef]
- Gaber, C.; Demuth, M.; Prieler, R.; Schluckner, C.; Schroettner, H.; Fitzek, H.; Hochenauer, C. Experimental investigation of thermochemical regeneration using oxy-fuel exhaust gases. Appl. Energy 2019, 236, 1115–1124. [Google Scholar] [CrossRef]
- Pino, L.; Vita, A.; Cipitì, F.; Laganà, M.; Recupero, V. Hydrogen production by methane tri-reforming process over Ni–ceria catalysts: Effect of La-doping. Appl. Catal. B Environ. 2011, 104, 64–73. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, K.; Fang, L.; Li, Y. Thermodynamic Analysis of Hydrogen Production from Oxidative Steam Reforming of Ethanol. Energy Fuels 2008, 22, 1365–1370. [Google Scholar] [CrossRef]
- Roy, P.S.; Raju, A.S.K.; Kim, K. Influence of S/C ratio and temperature on steam reforming of model biogas over a metal-foam-coated Pd–Rh/(CeZrO2–Al2O3) catalyst. Fuel 2015, 139, 314–320. [Google Scholar] [CrossRef]
- Fiaschi, D.; Baldini, A. Joining semi-closed gas turbine cycle and tri-reforming: SCGT-TRIREF as a proposal for low CO2 emissions powerplants. Energy Convers. Manag. 2009, 50, 2083–2097. [Google Scholar] [CrossRef]
- Okonkwo, O.; Yablonsky, G.; Biswas, P. Thermodynamic analysis of tri-reforming of oxy-fuel combustion exhaust gas. J. CO2 Util. 2020, 39, 101156. [Google Scholar] [CrossRef]
- Nikoo, M.K.; Amin, N.A.S. Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process. Technol. 2011, 92, 678–691. [Google Scholar] [CrossRef]
- Chein, R.-Y.; Hsu, W.-H. Thermodynamic analysis of syngas production via tri-reforming of methane and carbon gasification using flue gas from coal-fired power plants. J. Clean. Prod. 2018, 200, 242–258. [Google Scholar] [CrossRef]
- Rezaei, E.; Catalan, L.J.J. Evaluation of CO2 utilization for methanol production via tri-reforming of methane. J. CO2 Util. 2020, 42, 101272. [Google Scholar] [CrossRef]
- Adesina, A.A. The role of CO2 in hydrocarbon reforming catalysis: Friend or foe? Curr. Opin. Chem. Eng. 2012, 1, 272–280. [Google Scholar] [CrossRef]
- Gaber, C.; Demuth, M.; Prieler, R.; Schluckner, C.; Hochenauer, C. An experimental study of a thermochemical regeneration waste heat recovery process using a reformer unit. Energy 2018, 155, 381–391. [Google Scholar] [CrossRef]
- Jaworski, Z.; Pianko-Oprych, P. A Comparative Thermodynamic Study of Equilibrium Conditions for Carbon Deposition from Catalytic C–H–O Reformates. Energies 2018, 11, 1177. [Google Scholar] [CrossRef]
- Gates, B.C.; Katzer, J.R.; Schuit, G.C.A. Chemistry of Catalytic Processes; McGraw-Hill: New York, NY, USA, 1979. [Google Scholar]
- Trimm, D.L. The regeneration or disposal of deactivated heterogeneous catalysts. Appl. Catal. A Gen. 2001, 212, 153–160. [Google Scholar] [CrossRef]
- Jardim, S.S.Q. Tri-Reforming of CO2-Rich Natural Gas: Equilibrium Analysis and Reactor Technology Evaluation. Ph.D. Thesis, University of São Paulo, São Paulo, SP, Brazil, 2019. [Google Scholar]
- Khajeh, S.; Arab Aboosadi, Z.; Honarvar, B. A comparative study between operability of fluidized-bed and fixed-bed reactors to produce synthesis gas through tri-reforming. J. Nat. Gas Sci. Eng. 2014, 19, 152–160. [Google Scholar] [CrossRef]
- Andorf, R.; Mleczko, L.; Schweer, D.; Baerns, M. Oxidative coupling of methane in a bubbling fluidized bed reactor. Can. J. Chem. Eng. 1991, 69, 891–897. [Google Scholar] [CrossRef]
- De Lasa, H.; Dogammau, G.; Ravella, A. Chemical Reactor Technology for Environmentally Safe Reactors and Products; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Tomishige, K. Syngas production from methane reforming with CO2/H2O and O2 over NiO–MgO solid solution catalyst in fluidized bed reactors. Catal. Today 2004, 89, 405–418. [Google Scholar] [CrossRef]
- Farsi, M.; Fekri Lari, M.; Rahimpour, M.R. Development of a green process for DME production based on the methane tri-reforming. J. Taiwan Inst. Chem. Eng. 2020, 106, 9–19. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Arab Aboosadi, Z.; Jahanmiri, A.H. Synthesis gas production in a novel hydrogen and oxygen perm-selective membranes tri-reformer for methanol production. J. Nat. Gas Sci. Eng. 2012, 9, 149–159. [Google Scholar] [CrossRef]
- Khademi, M.H.; Alipour-Dehkordi, A.; Tabesh, M. Optimal design of methane tri-reforming reactor to produce proper syngas for Fischer-Tropsch and methanol synthesis processes: A comparative analysis between different side-feeding strategies. Int. J. Hydrogen Energy 2021, 46, 14441–14454. [Google Scholar] [CrossRef]
- Ahmed, M.Z.; Padhiyar, N. Comparative study of the optimal operation of methane reforming process in cylindrical and spherical reactors using multi-objective optimization. Int. J. Hydrogen Energy 2021, 46, 7060–7072. [Google Scholar] [CrossRef]
- DOE/FE. Carbon Sequestration: State of the Science; Office of Science and Office of Fossil Energy, US Department of Energy: Washington, DC, USA, February 1999.
- DOE/FE. Capturing Carbon Dioxide; Office of Fossil Energy, U.S. Department of Energy: Washington, DC, USA, 1999.
- Cañete, B.; Gigola, C.E.; Brignole, N.B. Synthesis Gas Processes for Methanol Production via CH4 Reforming with CO2, H2O, and O2. Ind. Eng. Chem. Res. 2014, 53, 7103–7112. [Google Scholar] [CrossRef]
- Halmann, M.; Steinfeld, A. Thermoneutral tri-reforming of flue gases from coal- and gas-fired power stations. Catal. Today 2006, 115, 170–178. [Google Scholar] [CrossRef]
- Minutillo, M.; Perna, A. A novel approach for treatment of CO2 from fossil fired power plants. Part B: The energy suitability of integrated tri-reforming power plants (ITRPPs) for methanol production. Int. J. Hydrogen Energy 2010, 35, 7012–7020. [Google Scholar] [CrossRef]
- Qian, Y.; Man, Y.; Peng, L.; Zhou, H. Integrated Process of Coke-Oven Gas Tri-Reforming and Coal Gasification to Methanol with High Carbon Utilization and Energy Efficiency. Ind. Eng. Chem. Res. 2015, 54, 2519–2525. [Google Scholar] [CrossRef]
- Díez-Ramírez, J.; Dorado, F.; Martínez-Valiente, A.; García-Vargas, J.M.; Sánchez, P. Kinetic, energetic and exergetic approach to the methane tri-reforming process. Int. J. Hydrogen Energy 2016, 41, 19339–19348. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Benson, T. A conceptual design by integrating dimethyl ether (DME) production with tri-reforming process for CO2 emission reduction. Fuel Process. Technol. 2015, 131, 7–13. [Google Scholar] [CrossRef]
- Sadeghi, M.; Jafari, M.; Yari, M.; Mahmoudi, S.M.S. Exergoeconomic assessment and optimization of a syngas production system with a desired H2/CO ratio based on methane tri-reforming process. J. CO2 Util. 2018, 25, 283–301. [Google Scholar] [CrossRef]
- Nguyen, T.B.H.; Zondervan, E. Modeling and simulation of novel bi-And tri-reforming processes for the production of renewable methanol. Chem. Eng. Trans. 2019, 74, 655–660. [Google Scholar]
- Dwivedi, A.; Gudi, R.; Biswas, P. Sensitivity based optimization of the Tri-reforming based CO2 valorization process. IFAC-PapersOnLine 2016, 49, 359–364. [Google Scholar] [CrossRef]
- Korobitsyn, M.A.; van Berkel, F.P.F.; Christie, G.M.; Huijsmans, J.P.P.; van der Klein, C.A.M. SOFC as a Gas Separator; Final Report NOVEM Contract; Netherlands Agency for Energy and Environment NOVEM B.V.: The Hague, The Netherlands, 2000. [Google Scholar]
- Lau, F.; Doong, S.-J. Hydrogen Production Process from Carbonaceous Materials Using Membrane Gasifier. U.S. Patent 2005/0039400A1, 24 February 2005. [Google Scholar]
- Abashar, M. Coupling of steam and dry reforming of methane in catalytic fluidized bed membrane reactors. Int. J. Hydrogen Energy 2004, 29, 799–808. [Google Scholar] [CrossRef]
- Solomon, S.; Qin, D.; Manning, M.; CHEN, Z.; Marquis, M.; Averyt, K.B.; Tignor, M.; Miller, H.L. Climate change 2007: Synthesis Report. In Contribution of Working Group I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Summary for Policymakers; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Werder, M. Life cycle assessment of the conventional and solar thermal production of zinc and synthesis gas. Energy 2000, 25, 395–409. [Google Scholar] [CrossRef]
- Galindo Cifre, P.; Badr, O. Renewable hydrogen utilisation for the production of methanol. Energy Convers. Manag. 2007, 48, 519–527. [Google Scholar] [CrossRef]
- Luu, M.T.; Milani, D.; Bahadori, A.; Abbas, A. A comparative study of CO2 utilization in methanol synthesis with various syngas production technologies. J. CO2 Util. 2015, 12, 62–76. [Google Scholar] [CrossRef]
- Taghdisian, H.; Farhadi, F.; Pishvaie, M.R. An optimization-oriented green design for methanol plants. J. Chem. Technol. Biotechnol. 2012, 87, 1111–1120. [Google Scholar] [CrossRef]
- Bazzanella, A.; Ausfelder, F. Low Carbon Energy and Feedstock for the European Chemical Industry: Technology Study; DECHEMA, Gesellschaft für Chemische Technik und Biotechnologie eV: Frankfurt, Germany, 2017. [Google Scholar]
- Specchia, S. Fuel processing activities at European level: A panoramic overview. Int. J. Hydrogen Energy 2014, 39, 17953–17968. [Google Scholar] [CrossRef]
- Alipour-Dehkordi, A.; Khademi, M.H. O2, H2O or CO2 side-feeding policy in methane tri-reforming reactor: The role of influencing parameters. Int. J. Hydrogen Energy 2020, 45, 15239–15253. [Google Scholar] [CrossRef]
- Coronas, J.; Menéndez, M.; Santamaría, J. The porous-wall ceramic membrane reactor: An inherently safer contacting device for gas-phase oxidation of hydrocarbons. J. Loss Prev. Process. Ind. 1995, 8, 97–101. [Google Scholar] [CrossRef]
- Maroto-Valer, M.M.; Song, C.; Soong, Y. (Eds.) Environmental Challenges and Greenhouse Gas Control for Fossil Fuel Utilization in the 21st Century; Springer: Boston, MA, USA, 2002. [Google Scholar]
- Bhavsar, S.; Veser, G. Chemical looping beyond combustion: Production of synthesis gas via chemical looping partial oxidation of methane. RSC Adv. 2014, 4, 47254–47267. [Google Scholar] [CrossRef]
- Deshmukh, S.A.R.K.; Heinrich, S.; Mörl, L.; van Sint Annaland, M.; Kuipers, J.A.M. Membrane assisted fluidized bed reactors: Potentials and hurdles. Chem. Eng. Sci. 2007, 62, 416–436. [Google Scholar] [CrossRef]
- Khajeh, S.; Arab Aboosadi, Z.; Honarvar, B. Optimizing the fluidized-bed reactor for synthesis gas production by tri-reforming. Chem. Eng. Res. Des. 2015, 94, 407–416. [Google Scholar] [CrossRef]
- Kummer, A.; Varga, T. What do we know already about reactor runaway?—A review. Process. Saf. Environ. Prot. 2021, 147, 460–476. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, C.; Cai, D.; Blair, E.; Qian, W.; Wei, F. The analysis of hot spots in large scale fluidized bed reactors. RSC Adv. 2017, 7, 20186–20191. [Google Scholar] [CrossRef]
- Al-Sherehy, F.A.; Adris, A.M.; Soliman, M.A.; Hughes, R. Avoidance of flammability and temperature runaway during oxidative dehydrogenation using a distributed feed. Chem. Eng. Sci. 1998, 53, 3965–3976. [Google Scholar] [CrossRef]
- Alipour-Dehkordi, A.; Khademi, M.H. Use of a micro-porous membrane multi-tubular fixed-bed reactor for tri-reforming of methane to syngas: CO2, H2O or O2 side-feeding. Int. J. Hydrogen Energy 2019, 44, 32066–32079. [Google Scholar] [CrossRef]
- Elbadawi, A.H.; Ge, L.; Li, Z.; Liu, S.; Wang, S.; Zhu, Z. Catalytic partial oxidation of methane to syngas: Review of perovskite catalysts and membrane reactors. Catal. Rev. 2021, 63, 1–67. [Google Scholar] [CrossRef]
- Dedov, A.G.; Loktev, A.S.; Komissarenko, D.A.; Mazo, G.N.; Shlyakhtin, O.A.; Parkhomenko, K.V.; Kiennemann, A.A.; Roger, A.-C.; Ishmurzin, A.V.; Moiseev, I.I. Partial oxidation of methane to produce syngas over a neodymium–calcium cobaltate-based catalyst. Appl. Catal. A Gen. 2015, 489, 140–146. [Google Scholar] [CrossRef]
- Rabe, S.; Truong, T.-B.; Vogel, F. Catalytic autothermal reforming of methane: Performance of a kW scale reformer using pure oxygen as oxidant. Appl. Catal. A Gen. 2007, 318, 54–62. [Google Scholar] [CrossRef]
- Tang, M.; Xu, L.; Fan, M. Progress in oxygen carrier development of methane-based chemical-looping reforming: A review. Appl. Energy 2015, 151, 143–156. [Google Scholar] [CrossRef]
- Jiang, H.; Li, H.; Xu, H.; Zhang, Y. Preparation of Ni/MgxTi1−xO catalysts and investigation on their stability in tri-reforming of methane. Fuel Process. Technol. 2007, 88, 988–995. [Google Scholar] [CrossRef]
- García-Vargas, J.M.; Valverde, J.L.; Díez, J.; Dorado, F.; Sánchez, P. Catalytic and kinetic analysis of the methane tri-reforming over a Ni–Mg/β-SiC catalyst. Int. J. Hydrogen Energy 2015, 40, 8677–8687. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, K.; Choudary, N.V.; Pant, K.K. Effect of support materials on the performance of Ni-based catalysts in tri-reforming of methane. Fuel Process. Technol. 2019, 186, 40–52. [Google Scholar] [CrossRef]
- Kang, J.S.; Kim, D.H.; Lee, S.D.; Hong, S.; Moon, D.J. Nickel-based tri-reforming catalyst for the production of synthesis gas. Appl. Catal. A Gen. 2007, 332, 153–158. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Rajput, A.M.; Prabhakar, B. NiO/CaO-Catalyzed Formation of Syngas by Coupled Exothermic Oxidative Conversion and Endothermic CO2 and Steam Reforming of Methane. Angew. Chem. Int. Ed. Engl. 1994, 33, 2104–2106. [Google Scholar] [CrossRef]





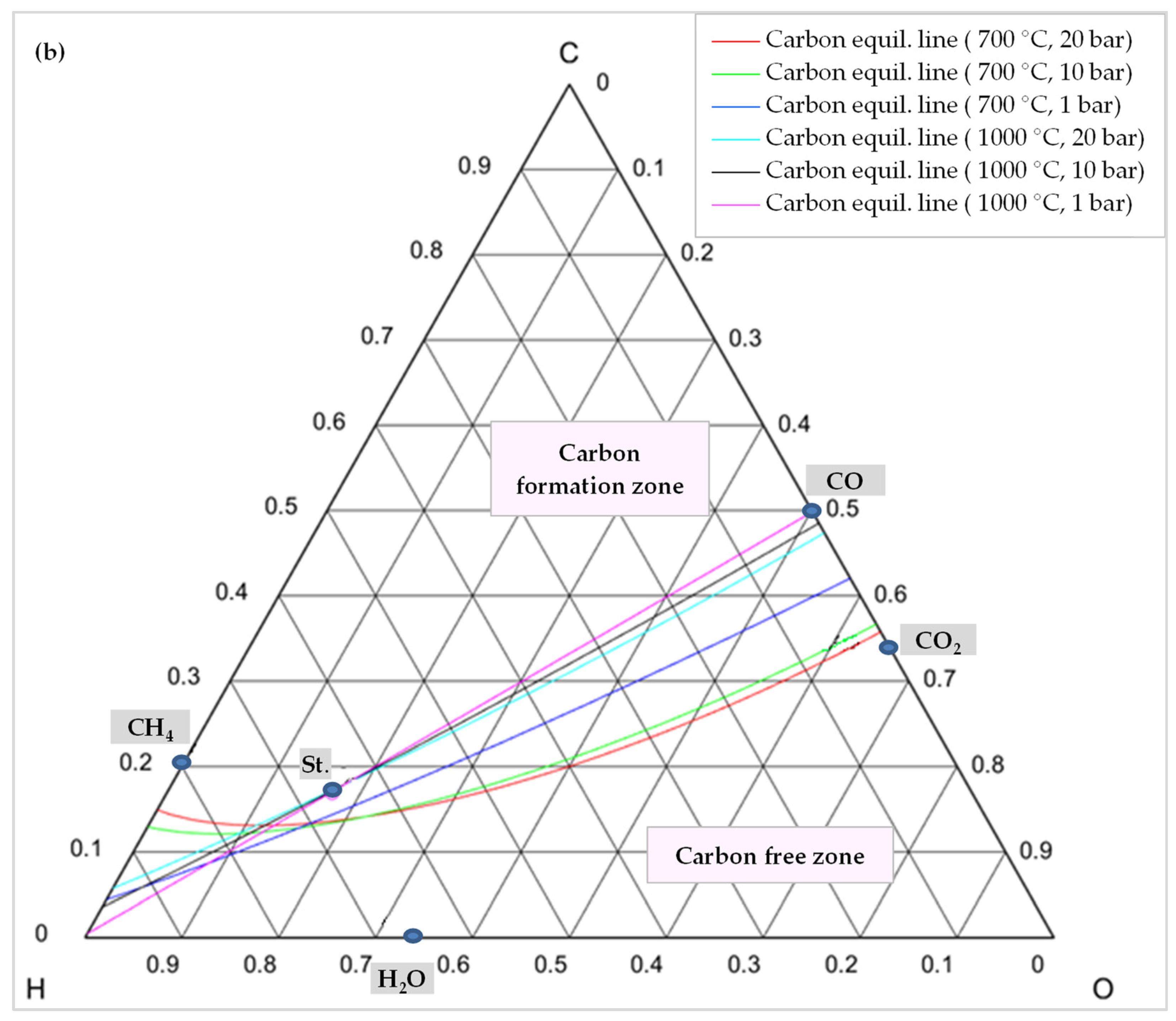
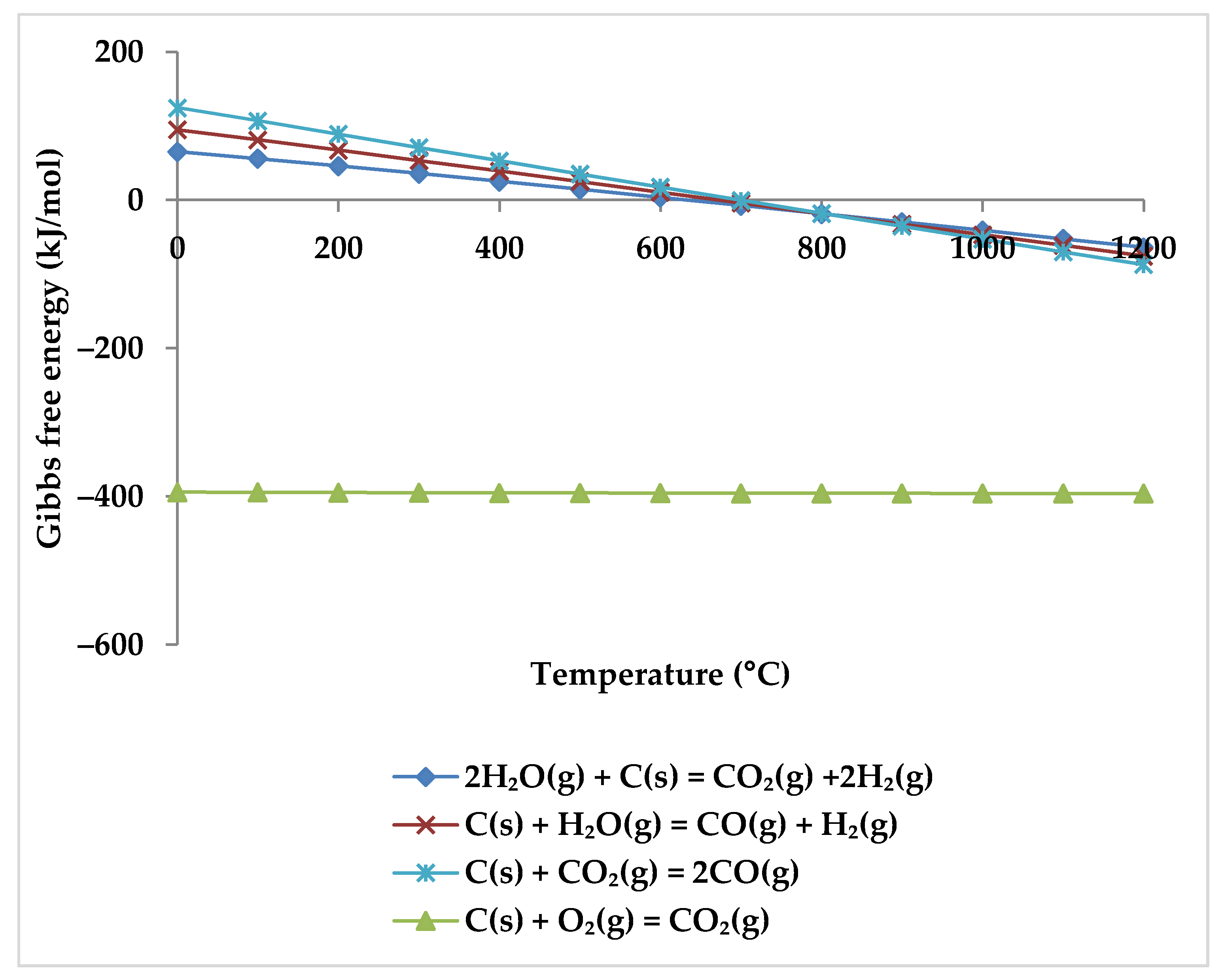


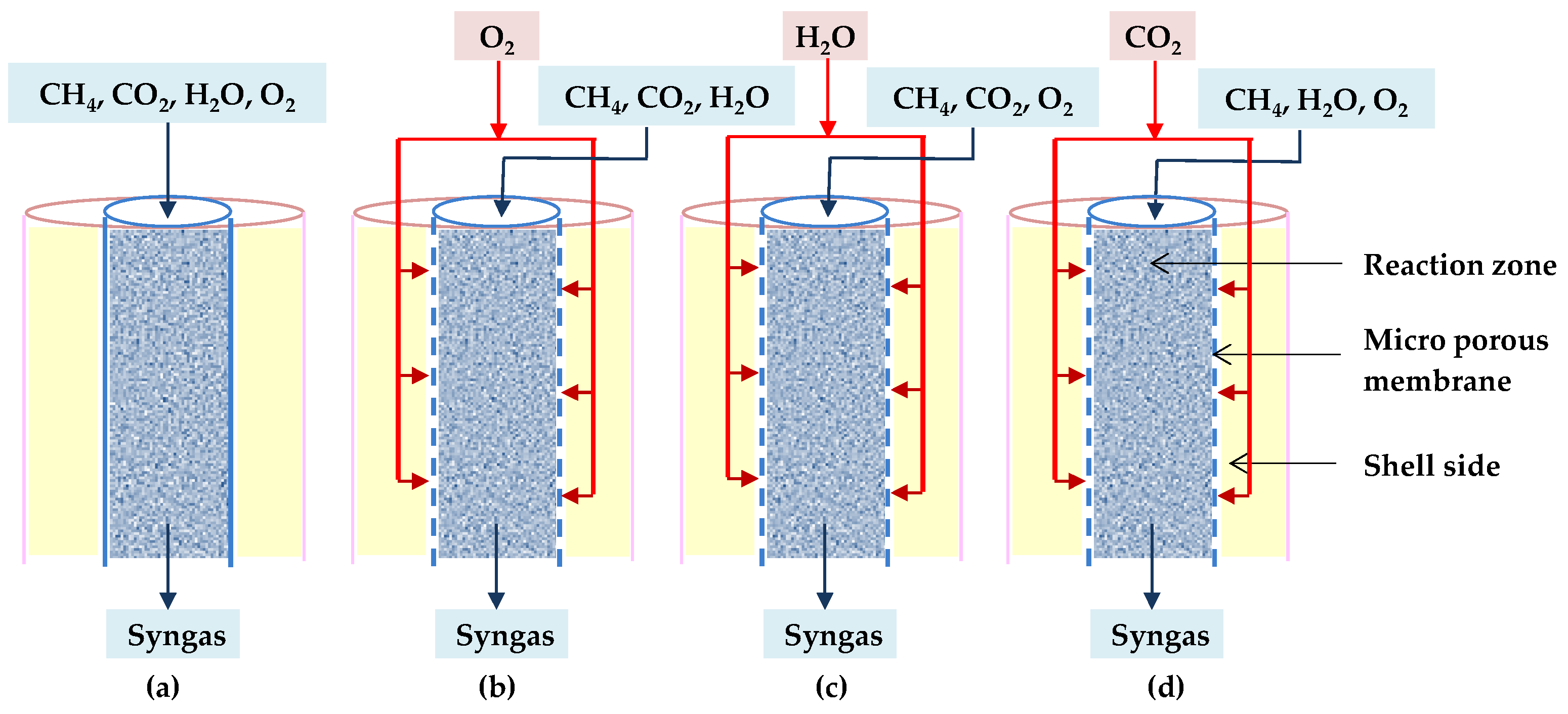
| Process Conditions | Feed Gas Ratio CH4:CO2:H2O:O2 | Minimization Method | Property Methods | ASPEN Technologies | |
|---|---|---|---|---|---|
| Temperature | Pressure | ||||
| 400–1000 °C | 1 bar | 1:0.3:0.3:0.2 | Gibbs free energy reactor | UNIFAC + STEAMNBS | ASPEN plus, V.12.0 (38.0.0.380), ASPEN Technology, Bedford, MA, USA |
| Process Conditions | Feed Gas Ratio CH4:CO2:H2O:O2 | Minimization Method | Property Methods | ASPEN Technologies | |
|---|---|---|---|---|---|
| Temperature | Pressure | ||||
| 0–850 °C | 1, 3, 5, 7, 10 bar | 1:0.3:0.3:0.2 | Gibbs free energy reactor | UNIFAC + STEAMNBS | ASPEN plus, V.12.0 (38.0.0.380), ASPEN Technology, Bedford, MA, USA |
| CO2 | CH4 | H2O | H2/CO | C Formation | Optimum Ratio | Comments | |
|---|---|---|---|---|---|---|---|
| Temperature | D 1 | D | D | I2 | I | 800 °C | |
| Pressure | I | I | I | D | I or D (temperature-dependent) | 1 bar | At low temperatures, carbon deposition reduces as pressure rises, but at temperatures above 600 °C, it increases considerably with pressure. |
| CH4/flue gas | I or D (temperature-dependent) | Between 0.4 and 1.0 | When the temperature is lower than 550 °C, CO2 molar flow increases by increasing the CH4/flue gas ratio but after that it decreases. | ||||
| O2/CH4 | I | D | I | I or D (depending on O2/CH4 ratio) | I | Between 0.45 and 0.50 | When this ratio is between 0.1 and 0.4, the H2 yield is increased by the O2/CH4 ratio, and when the O2/CH4 ratio is greater than 0.4, the H2 yield gradually decreases. |
| H2O/CH4 | I | I or D (temperature-dependent) | I or D (temperature-dependent) | I or D (temperature-dependent) | I | 1 | When the temperature exceeds 650 °C, the H2O conversion and H2/CO ratio decrease but when the temperature drops below 650 °C, the opposite is true. |
| CO2/CH4 | I | D | I | I | 1.43 | ||
| O2/CO2 | I | D | I | 0.17 for a high CO2 conversion | |||
| H2O/CO2 | I | D | I |
| Process Conditions | Optimum Feed Composition CH4:CO2:H2O:O2 | Conversions | H2/CO | Ref. | |
|---|---|---|---|---|---|
| Temperature | Pressure | ||||
| 850 °C | 1 atm | 1:0.291:0.576:0.088 | CO2 > 90% | 2.0 | [19] |
| 850 °C | 1 atm | 1:0.282:0.574:0.1 | CO2 > 90% CH4 > 90% | 2.0 | [36] |
| 1000 °C | 30 bar | 1:0.2:0.35:0.48 | CO2 = 50% | 2.32 | [75] |
| 750 °C | 1 bar | 1:1:0.4:0.3 | CO2 = 47.8% | - | [51] |
| Type of Membrane Reactor | Distributed Gas through the Membrane | Reason to Be Longer for Desired H2/CO Ratio |
|---|---|---|
| O-TRM | O2 | To provide heat for reforming processes |
| C-TRM | CO2 | To accommodate the consumption of CO2 diffused through the catalytic bed |
| H-TRM | H2O | To achieve a favorable H2/CO ratio |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soleimani, S.; Lehner, M. Tri-Reforming of Methane: Thermodynamics, Operating Conditions, Reactor Technology and Efficiency Evaluation—A Review. Energies 2022, 15, 7159. https://doi.org/10.3390/en15197159
Soleimani S, Lehner M. Tri-Reforming of Methane: Thermodynamics, Operating Conditions, Reactor Technology and Efficiency Evaluation—A Review. Energies. 2022; 15(19):7159. https://doi.org/10.3390/en15197159
Chicago/Turabian StyleSoleimani, Samira, and Markus Lehner. 2022. "Tri-Reforming of Methane: Thermodynamics, Operating Conditions, Reactor Technology and Efficiency Evaluation—A Review" Energies 15, no. 19: 7159. https://doi.org/10.3390/en15197159
APA StyleSoleimani, S., & Lehner, M. (2022). Tri-Reforming of Methane: Thermodynamics, Operating Conditions, Reactor Technology and Efficiency Evaluation—A Review. Energies, 15(19), 7159. https://doi.org/10.3390/en15197159







