Thermodynamic Analysis and Optimization Design of a Molten Salt–Supercritical CO2 Heat Exchanger
Abstract
1. Introduction
2. The Computational Model and Model Validation
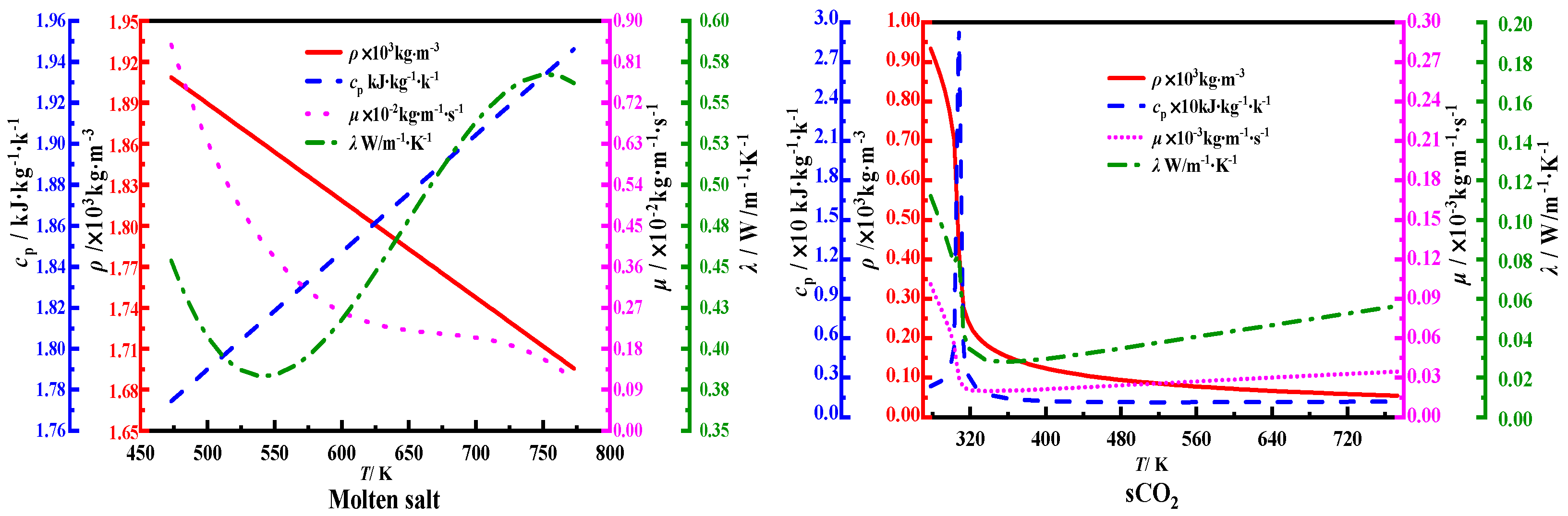
2.1. Physical Model and Heat Transfer Fluid
2.2. The Computational Model
2.3. Model Validation
3. Result and Discussion
3.1. Thermodynamic Analysis under Constant Heat Transfer Area
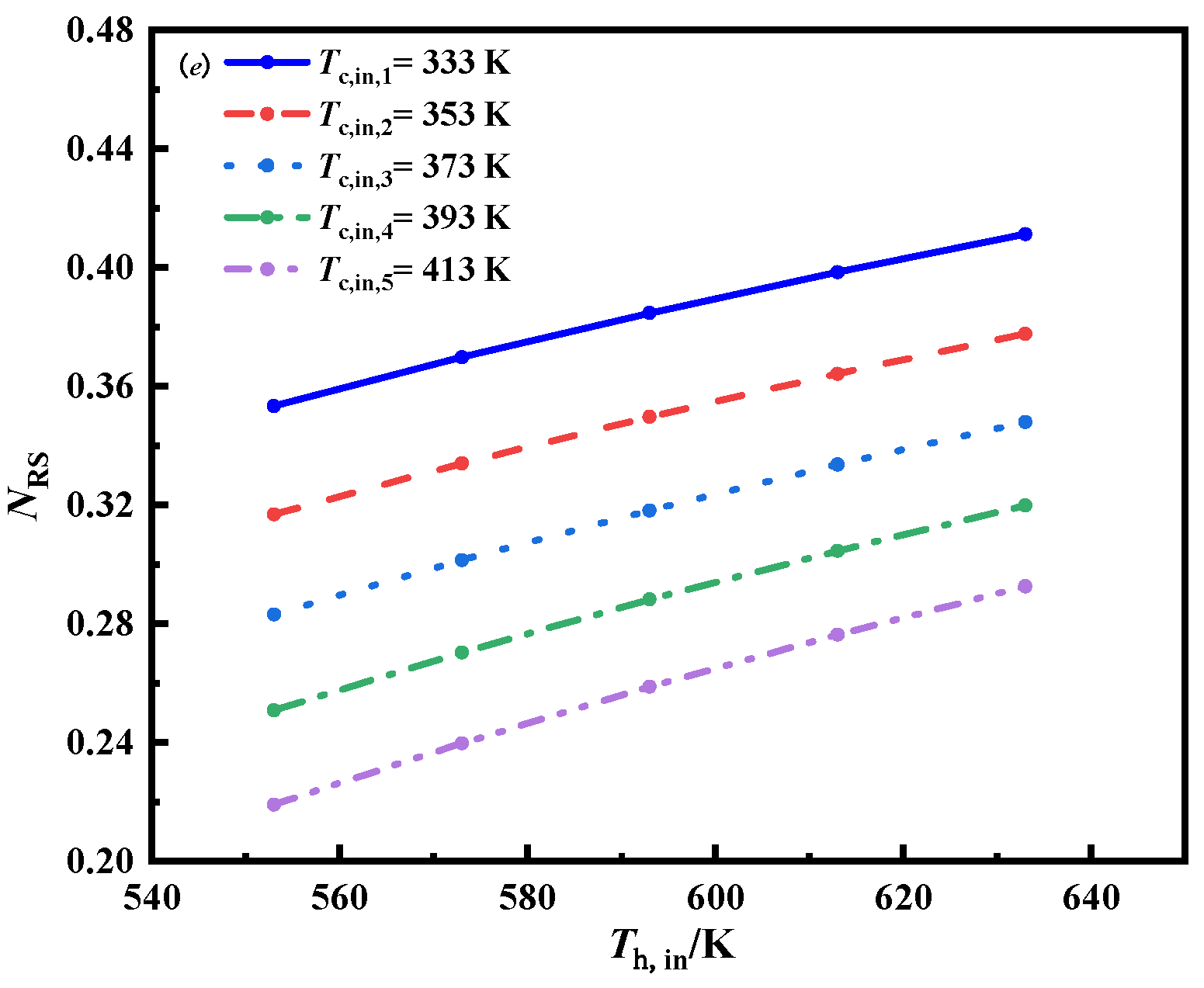
3.1.1. The Effect of Inlet Temperature and Inlet Mass Flow Rate of Molten Salt
3.1.2. The Effect of Inlet Temperature of sCO2
3.1.3. The Effect of Inlet Mass Flow Rate of sCO2
3.2. Thermodynamic Analysis under Constant Heat Flux
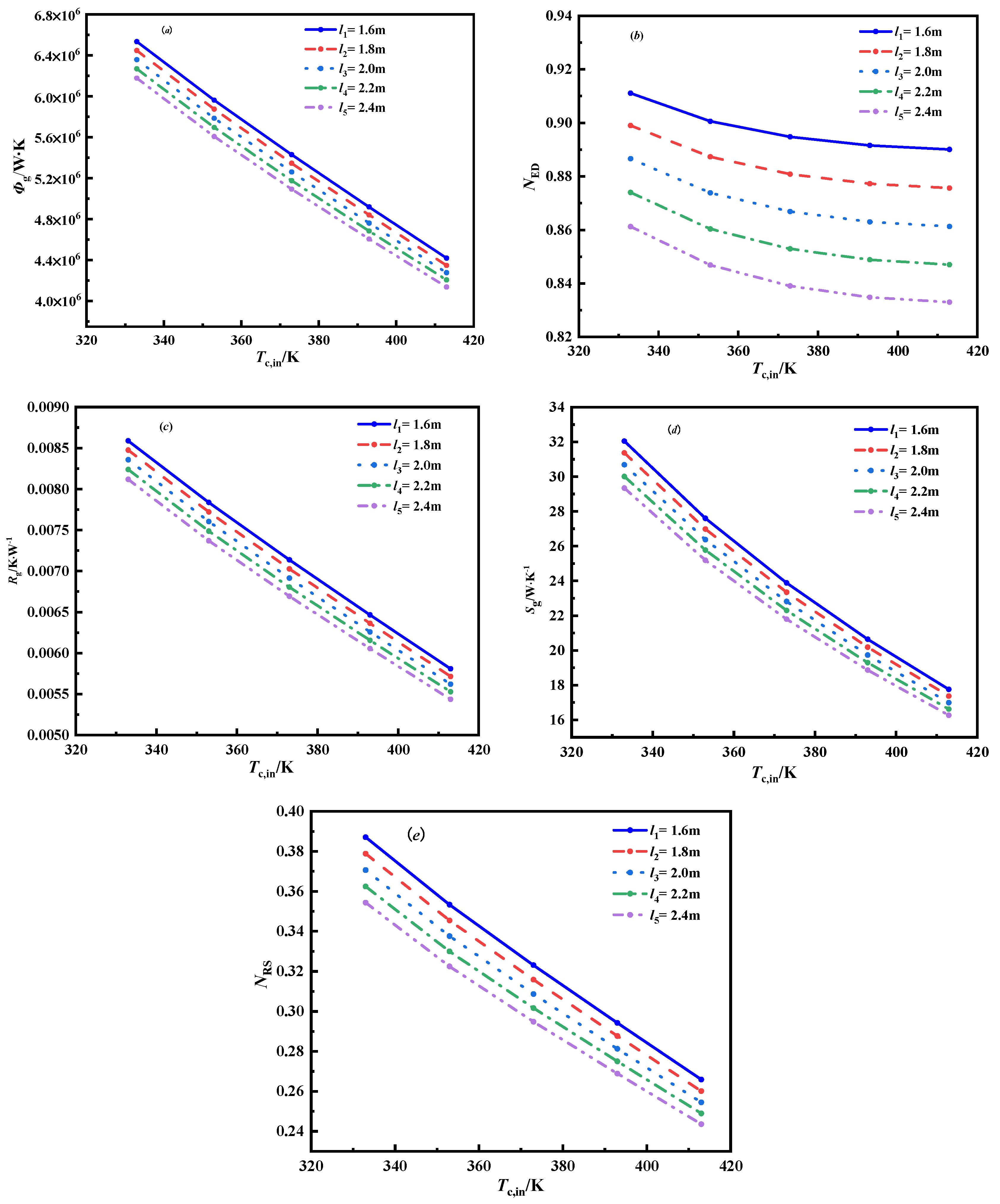
3.2.1. The Effect of the Length of the Tube
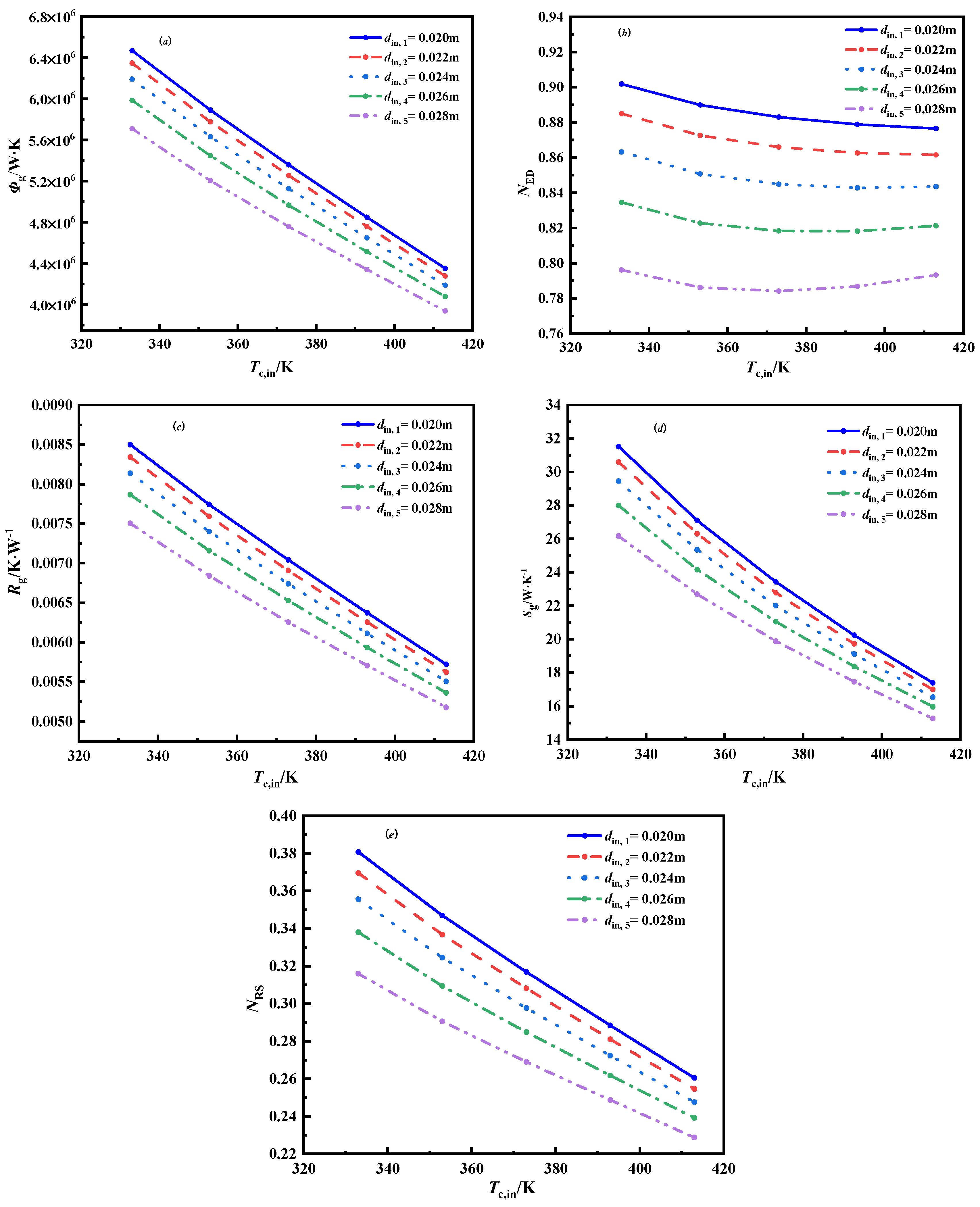
3.2.2. The Effect of the Inner Diameter
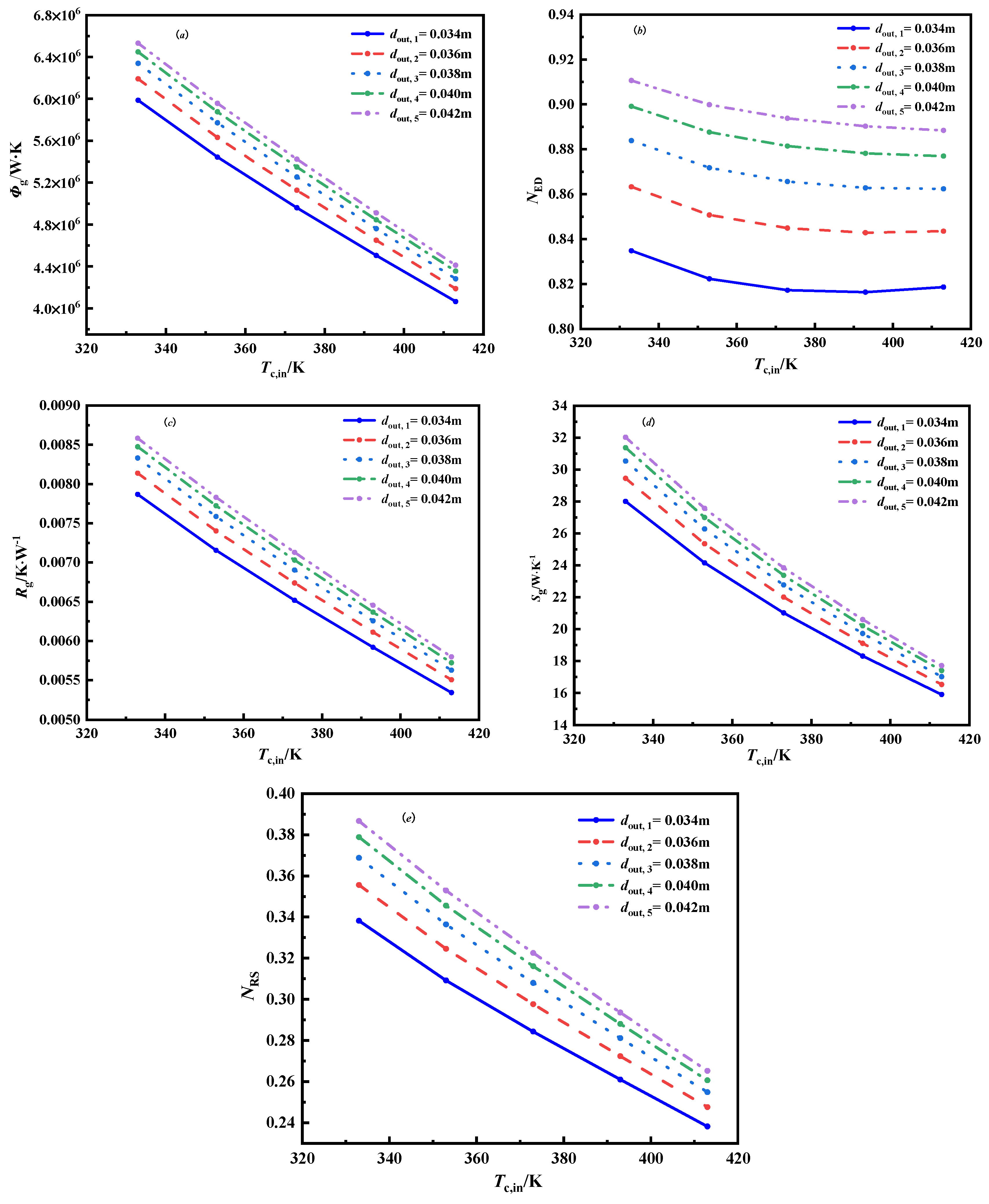
3.2.3. The Effect of Outer Diameter
3.3. Optimization Analysis of the Concentric Tube Heat Exchanger
3.3.1. Single-Objective Optimization Analysis
3.3.2. Multi-Objective Optimization Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| A | cross-sectional area, m2 | C | thermal capacity, J·K−1 |
| cp | specific heat capacity, J·kg−1·K−1 | din | inner diameter, m |
| dout | outer diameter, m | D | equivalent diameter, m |
| F | total heat exchange area, m2 | f | the resistance coefficient |
| h | specific enthalpy, J∙kg−1 | l | the length of heat exchanger, m |
| m | mass flow rate, kg·s−1 | Nu | Nusselt number |
| NED | entransy dissipation number | NRS | improve entropy production number |
| Np | pressure entransy dissipation number | P | pressure, Pa |
| ΔP | pressure drop, Pa | Pr | Prandlt number |
| Q | heat transfer rate, W | Re | Reynold number |
| Rg | entransy dissipation thermal resistance, K·W−1 | Sg | entropy production rate, W·K−1 |
| sCO2 | supercritical CO2 | T | temperature, K |
| ΔT | temperature difference, K | ΔTm | logarithmic mean temperature difference, K |
| u | velocity, m·s−1 | x | x-direction component, m |
| Greek symbols | |||
| heat transfer coefficient, W·m−2·K−1 | ε | heat transfer effectiveness | |
| λ | thermal conductivity, W·m−1·K−1 | μ | dynamic viscosity, kg·m−1·s−1 |
| ρ | density, kg·m−3 | ϕg | entransy dissipation rate, W·K |
| Subscript | |||
| ave | average | c | cold |
| h | hot | in | inside |
| max | maximum | out | outside |
| w | wall | ||
References
- Mehos, M.; Turchi, C.; Vidal, J. Concentrating Solar Power Gen 3 Demonstration Roadmap; Technical Report; NREL: Oslo, Norway, 2017. [Google Scholar]
- Han, Y.; Zhang, C.C.; Wu, Y.T.; Lu, Y.W. Investigation on thermal performance of quaternary nitrate-nitrite mixed salt and solar salt under thermal shock condition. Renw. Energyi. 2022, 175, 1041–1051. [Google Scholar] [CrossRef]
- Zhang, C.C.; Han, S.T.; Wu, Y.T.; Zhang, C.Y.; Guo, H. Investigation on convection heat transfer performance of quaternary mixed molten salt based nanofluids in smooth tube. Int. J. Therm. Sci. 2022, 177, 107534. [Google Scholar] [CrossRef]
- Bhuvaneswari, S.; Elatharasan, G. A Study of the Literature Review on Heat Transfer in A Helically Coiled Heat Exchanger. Int. J. Eng. Res. Technol. 2019, 7, 1–3. [Google Scholar]
- Mylavarapu, S.K.; Figley, J.; Sun, X.; Christensen, R.N.; Needler, N.J.; Hajek, B.K. Development and testing of printed circuit heat exchanger for generation IV reactors. Trans. Am. Nucl. Soc. 2008, 98, 626–627. [Google Scholar]
- Bejan, A. Entropy Generation Minimization: The New Thermodynamics of Finite-Size Devices and Finite-Time Processes. J. Appl. Phys. 1996, 79, 1191–1218. [Google Scholar] [CrossRef]
- Bejan, A. Second-Law Analysis in Heat Transfer and Thermal Design; Elsevier: New York, NY, USA, 1982; pp. 1–58. [Google Scholar]
- Hesselgreaves, J.E. Rationalisation of second law analysis of heat exchangers. Int. J. Heat Mass Transf. 2000, 43, 4189–4204. [Google Scholar] [CrossRef]
- Ogiso, K. Duality of heat exchanger performance in balanced counter-flow systems. J. Heat Transf.-Trans. AMSE 2003, 125, 530–532. [Google Scholar] [CrossRef]
- Shah, R.K.; Skiepko, T. Entropy generation extrema and their relationship with heat exchanger effectiveness-number of transfer unit behavior for complex flow arrangements. J. Heat Transf.-Trans. AMSE 2004, 126, 994–1002. [Google Scholar] [CrossRef]
- Guo, Z.Y.; Zhu, H.Y.; Liang, X.G. Entransy: A Physical Quantity Describing Heat Transfer Ability. Int. J. Heat Mass Transf. 2007, 50, 2545–2556. [Google Scholar] [CrossRef]
- Guo, F.; Cheng, L.; Xu, M.T. Entransy Dissipation Number and Its Application to Heat Exchanger Performance Evaluation. Chin. Sci. Bull. 2009, 54, 2998–3002. [Google Scholar] [CrossRef]
- Lerou, P.P.P.M.; Veenstra, T.T.; Burger, J.F.; ter Brake, H.J.; Rogalla, H. Optimization of counterflow heat exchanger geometry through minimization of entropy generation. Cryogenics 2005, 45, 659–669. [Google Scholar] [CrossRef]
- Chen, Q. Entransy dissipation-based thermal resistance method for heat exchanger performance design and optimization. Int. J. Heat Mass Transf. 2013, 60, 156–162. [Google Scholar] [CrossRef]
- Wang, S.M.; Jian, G.P.; Sun, J.R. Application of entransy-dissipation-based thermal resistance for performance optimization of spiral-wound heat exchanger. Int. J. Heat Mass Transf. 2018, 116, 743–750. [Google Scholar] [CrossRef]
- Guo, J.F.; Xu, M.T. The application of entransy dissipation theory in optimization design of heat exchanger. Appl. Therm. Eng. 2012, 36, 227–235. [Google Scholar] [CrossRef]
- Qian, X.D.; Li, X.Z. Analysis of entransy dissipation in heat exchangers. Int. J. Therm. Sci. 2011, 50, 608–614. [Google Scholar] [CrossRef]
- Cheng, X.T.; Zhang, Q.Z.; Liang, X.G. Analyses of entransy dissipation, entropy generation and entransy-dissipation-based thermal resistance on heat exchanger optimization. Appl. Therm. Eng. 2012, 38, 31–39. [Google Scholar] [CrossRef]
- Liu, X.B.; Meng, J.A.; Guo, Z.Y. Entropy generation extremum and entransy dissipation extremum for heat exchanger optimization. Chin. Sci. Bull. 2009, 54, 943–947. [Google Scholar] [CrossRef]
- Guo, J.F.; Xu, M.T.; Cheng, L. Principle of equipartition of entransy dissipation for heat exchanger design. Sci China Tech Sci 2010, 53, 1309–1314. [Google Scholar] [CrossRef]
- Xie, G.N.; Song, Y.D.; Asadi, M.; Lorenzini, G. Optimization of Pin-Fins for a Heat Exchanger by Entropy Generation Minimization and Constructal Law. J. Heat Transf. Trans. Asme 2015, 137, 1–9. [Google Scholar] [CrossRef]
- Guo, Z.Y.; Liu, X.B.; Tao, W.Q. Effectiveness–thermal resistance method for heat exchanger design and analysis. International J. Heat Mass Transf. 2010, 53, 2877–2884. [Google Scholar] [CrossRef]
- Li, K.; Wen, J.; Liu, Y.C.; Liu, H.; Wang, S.; Tu, J. Application of entransy theory on structure optimization of serrated fin in plate-fin heat exchanger. Appl. Therm. Eng. 2020, 173, 114809. [Google Scholar] [CrossRef]
- Han, Z.X.; Guo, J.F.; Huai, X.L. Research on optimization method of new airfoil heat exchanger based on entransy dissipation theory. Chin. Sci. Technol. Sci. 2021, 51, 1219–1230. [Google Scholar]
- Han, Y.; Zhang, C.C.; Zhu, Y.J.; Wu, X.H.; Jin, T.X.; Li, J.N. Investigation of heat transfer Exergy loss number and its application in optimization for the shell and helically coiled tube heat exchanger. Appl. Therm. Eng. 2022, 211, 118424. [Google Scholar] [CrossRef]
- Linstrom, P.J.; Mallard, W.G. National Institute of Standards and Technology: Gaithersburg, MD, USA, 2005, 2011. Available online: http://webbook.nist.gov/chemistry (accessed on 5 September 2022).
- Gnielinski, V. New Equations for Heat and Mass Transfer in Turbulent Pipe and Channel Flows. Int. J. Chem. Eng. 1976, 16, 359–368. [Google Scholar]
- Hall, W.B.; Jackson, J.D.; Watson, A. Forced convection heat transfer to fluids at supercritical pressure. In Turbulent Forced Convection in Channels and Bundles; Hemisphere: New York, NY, USA, 1979; Volume 2, pp. 563–611. [Google Scholar]
- Chen, H.; Chen, X.; Wu, Y.T.; Lu, Y.W.; Wang, X.; Ma, C.F. Experimental study on forced convection heat transfer of KNO3–Ca(NO3)2 + SiO2 molten salt nanofluid in circular tube. Sol. Energy 2020, 206, 900–906. [Google Scholar] [CrossRef]





| Number of Elements | ||
|---|---|---|
| 50 | 617.83 | 423.84 |
| 100 | 617.83 | 423.85 |
| 150 | 617.83 | 423.85 |
| 200 | 617.83 | 423.85 |
| 300 | 617.83 | 423.85 |
| 500 | 617.83 | 423.85 |
| Fluids | d/m | l/m | [29] | Deviation | |||
|---|---|---|---|---|---|---|---|
| Molten salt | 0.02 | 1 | 623.95 | 0.86 | 615.45 | 617.83 | 0.39% |
| oil | 0.034 | 1 | 425.35 | 0.465 | 425.35 | 423.84 | 0.36% |
| Parameters | Ranges | Parameters | Ranges |
|---|---|---|---|
| /m | 0.015–0.035 | /m | 0.030–0.050 |
| /m | 1.6–2.4 | /K | 543.15–583.15 |
| /K | 323.15–363.15 | /kg/s | 0.8–1.0 |
| /kg/s | 1.0–1.2 |
| Single Objective Optimization Objective Function | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ref | ||||||||||
| /m | 0.02 | 0.015 | 0.017 | 0.018 | 0.015 | 0.02 | 0.022 | 0.015 | 0.015 | 0.017 |
| /m | 0.034 | 0.05 | 0.031 | 0.032 | 0.05 | 0.034 | 0.05 | 0.03 | 0.03 | 0.031 |
| /m | 2 | 1.6 | 2.4 | 2.397 | 1.6 | 2.4 | 1.647 | 1.609 | 2.4 | 2.4 |
| /K | 563.15 | 543.15 | 583.14 | 583.12 | 543.15 | 543.15 | 574.31 | 583.13 | 583.15 | 583.15 |
| /K | 343.15 | 346.15 | 363.15 | 363.14 | 363.15 | 363.15 | 323.25 | 363.14 | 323.15 | 363.15 |
| /kg/s | 0.8 | 0.8 | 0.8 | 0.999 | 0.8 | 0.998 | 0.839 | 1 | 1 | 1 |
| kg/s | 1.2 | 1 | 1 | 1.199 | 1 | 1.001 | 1.024 | 1.2 | 1.2 | 1.001 |
| 7,491,097 | 1,798,047 | 8,245,213 | 9,817,459 | 1,798,047 | 5,624,994 | 4,919,799 | 7,083,193 | 14,124,153 | 8,985,178 | |
| 0.891 | 0.957 | 0.849 | 0.854 | 0.957 | 0.862 | 0.957 | 0.901 | 0.891 | 0.850 | |
| 0.00513 | 0.01651 | 0.00423 | 0.00359 | 0.01651 | 0.00428 | 0.01174 | 0.00554 | 0.00380 | 0.00390 | |
| 38.496 | 9.074 | 38.136 | 45.376 | 9.074 | 27.960 | 26.484 | 32.950 | 74.511 | 41.263 | |
| 0.346 | 0.316 | 0.314 | 0.315 | 0.316 | 0.280 | 0.418 | 0.335 | 0.395 | 0.312 | |
| 9.17 × 10−6 | 3.08 × 10−6 | 1.14 × 10−5 | 1.49 × 10−5 | 3.08 × 10−6 | 1.24 × 10−5 | 4.64 × 10−7 | 1.44 × 10−5 | 5.98 × 10−6 | 1.13 × 10−5 | |
| 1563.287 | 804.2732 | 1867.839 | 2079.548 | 804.2732 | 1565.425 | 749.1298 | 2391.515 | 2207.722 | 2027.949 | |
| /W | 38,213.33 | 10,437.41 | 44,146.8 | 52,291.34 | 10,437.41 | 36,248.61 | 20,467.84 | 35,746.22 | 60,983.57 | 48,021.55 |
| 0.119 | 0.046 | 0.168 | 0.166 | 0.046 | 0.167 | 0.053 | 0.112 | 0.128 | 0.183 | |
| Single-Objective Optimization Objective Function | Multi-Objective Optimization Objective Function | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ref | + | + | + | + | |||||
| /m | 0.02 | 0.017 | 0.018 | 0.02 | 0.017 | 0.0213 | 0.0285 | 0.0255 | 0.0208 |
| /m | 0.034 | 0.031 | 0.032 | 0.034 | 0.031 | 0.035 | 0.043 | 0.040 | 0.035 |
| /m | 2 | 2.4 | 2.397 | 2.4 | 2.4 | 2.398 | 2.392 | 2.397 | 2.361 |
| /K | 563.15 | 583.14 | 583.11 | 543.152 | 583.15 | 547.17 | 566.59 | 543.21 | 566.39 |
| /K | 343.15 | 363.15 | 363.14 | 363.14 | 363.15 | 344.65 | 324.149 | 363.15 | 323.60 |
| 0.8 | 0.8 | 0.999 | 0.998 | 1 | 0.993 | 1 | 0.894 | 0.820 | |
| kg/s | 1.2 | 1 | 1.199 | 1.001 | 1.001 | 1.021 | 1.171 | 1.016 | 1.182 |
| 7,491,097 | 8,245,213 | 9,817,459 | 5,624,994 | 8,985,178 | 7,188,210 | 10,923,844 | 5,113,361 | 10,479,204 | |
| 0.891 | 0.849 | 0.854 | 0.862 | 0.850 | 0.875 | 0.903 | 0.872 | 0.896 | |
| 0.00513 | 0.004231 | 0.00359 | 0.004281 | 0.003896 | 0.004366 | 0.004389 | 0.004818 | 0.004516 | |
| 38.496 | 38.136 | 45.376 | 27.960 | 41.263 | 37.443 | 59.197 | 25.524 | 57.252 | |
| 0.345692 | 0.314 | 0.315 | 0.280 | 0.312 | 0.318 | 0.385 | 0.285 | 0.385 | |
| 9.17 × 10−6 | 1.14 × 10−5 | 1.49 × 10−5 | 1.24 × 10−5 | 1.13 × 10−5 | 7.28 × 10−6 | 2.74 × 10−6 | 9.34 × 10−6 | 4.27 × 10−6 | |
| 1563.287 | 1867.839 | 2079.548 | 1565.425 | 2027.949 | 1441.761 | 1071.811 | 1091.082 | 1449.806 | |
| /W | 38,213.33 | 44,146.8 | 52,291.34 | 36,248.61 | 48,021.55 | 40,577.45 | 49,887.39 | 32,577.88 | 48,170.41 |
| 0.119 | 0.168 | 0.166 | 0.1676 | 0.1836 | 0.146 | 0.113 | 0.147 | 0.133 | |
| 5.017 | 12.580 | 37.607 | 9.212 | 28.091 | 6.689 | 1.628 | 2.414 | 5.163 | |
| 28.911 | 14.084 | 48.759 | 18.516 | 38.893 | 24.539 | 16.155 | 19.511 | 24.305 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Zhang, C.; Wu, Y.; Lu, Y.; Ma, C. Thermodynamic Analysis and Optimization Design of a Molten Salt–Supercritical CO2 Heat Exchanger. Energies 2022, 15, 7398. https://doi.org/10.3390/en15197398
Dong X, Zhang C, Wu Y, Lu Y, Ma C. Thermodynamic Analysis and Optimization Design of a Molten Salt–Supercritical CO2 Heat Exchanger. Energies. 2022; 15(19):7398. https://doi.org/10.3390/en15197398
Chicago/Turabian StyleDong, Xiaoming, Cancan Zhang, Yuting Wu, Yuanwei Lu, and Chongfang Ma. 2022. "Thermodynamic Analysis and Optimization Design of a Molten Salt–Supercritical CO2 Heat Exchanger" Energies 15, no. 19: 7398. https://doi.org/10.3390/en15197398
APA StyleDong, X., Zhang, C., Wu, Y., Lu, Y., & Ma, C. (2022). Thermodynamic Analysis and Optimization Design of a Molten Salt–Supercritical CO2 Heat Exchanger. Energies, 15(19), 7398. https://doi.org/10.3390/en15197398






