Prediction of Oxidation Stability of Biodiesel Derived from Waste and Refined Vegetable Oils by Statistical Approaches
Abstract
:1. Introduction
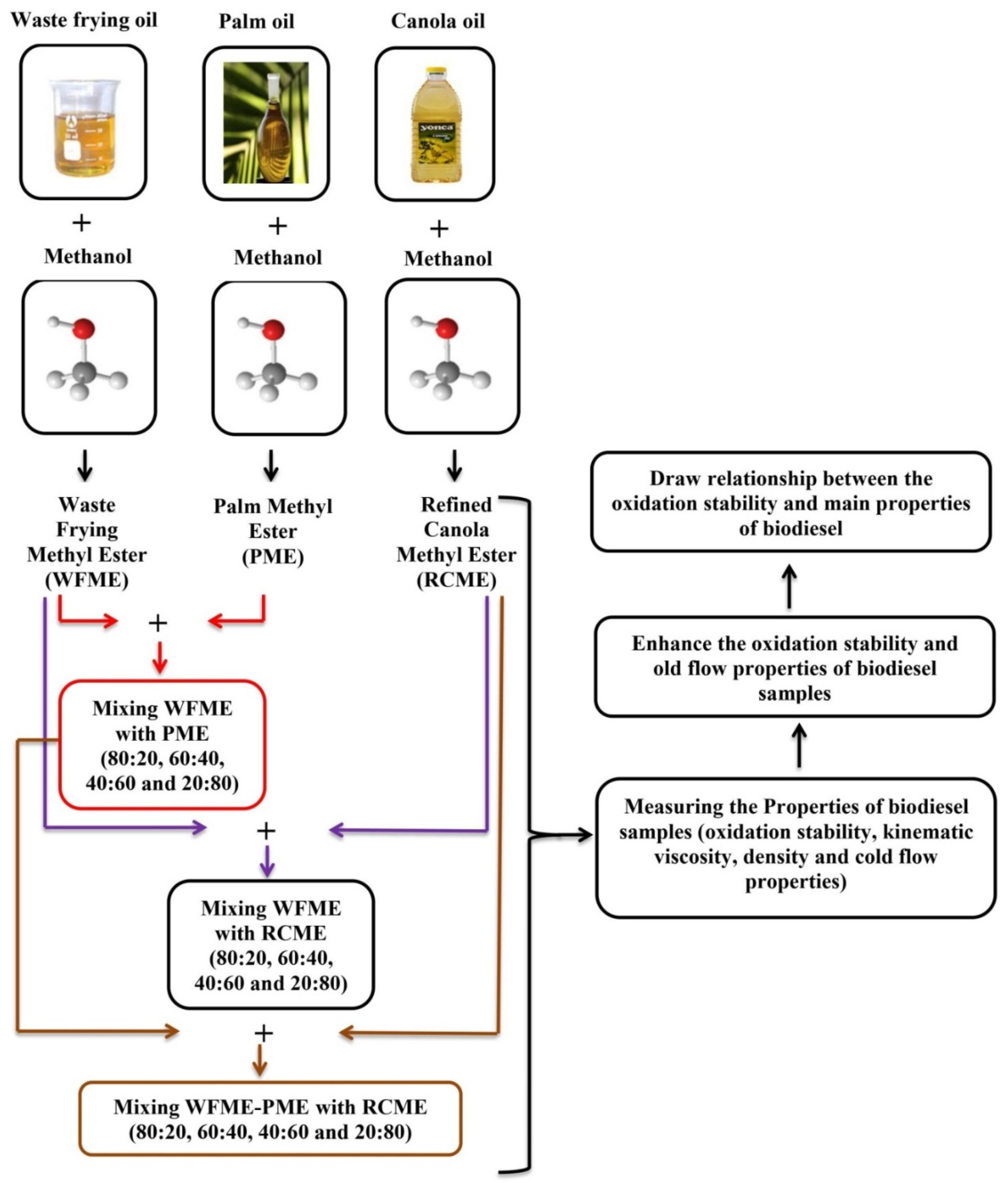
2. Materials and Methods
2.1. Raw Materials
2.2. Instruments
2.3. Fuel Characteristics
2.4. Blend Preparation
2.5. Empirical Models
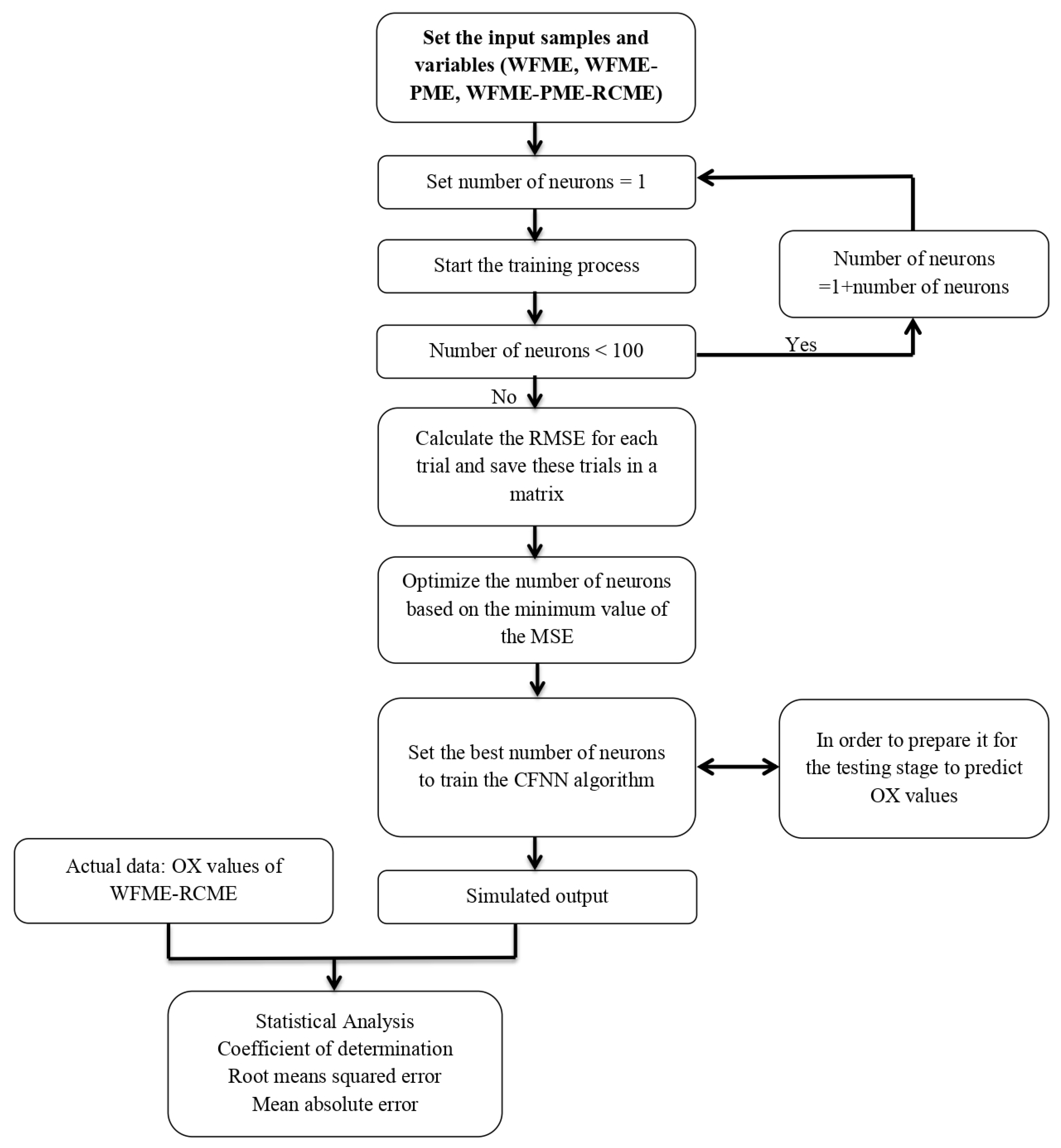
2.5.1. Artificial Neural Networks (ANN)
- (a)
- Multilayer feedforward neural network (MFFNN)
- (b)
- Cascade feedforward neural network (CFNN)
- (c)
- Radial basis neural networks (RBFNN)
- (d)
- Elmanneural network (ENN)
2.5.2. Poisson Regression Model (PRM)
2.5.3. Model Performance Criteria
3. Results
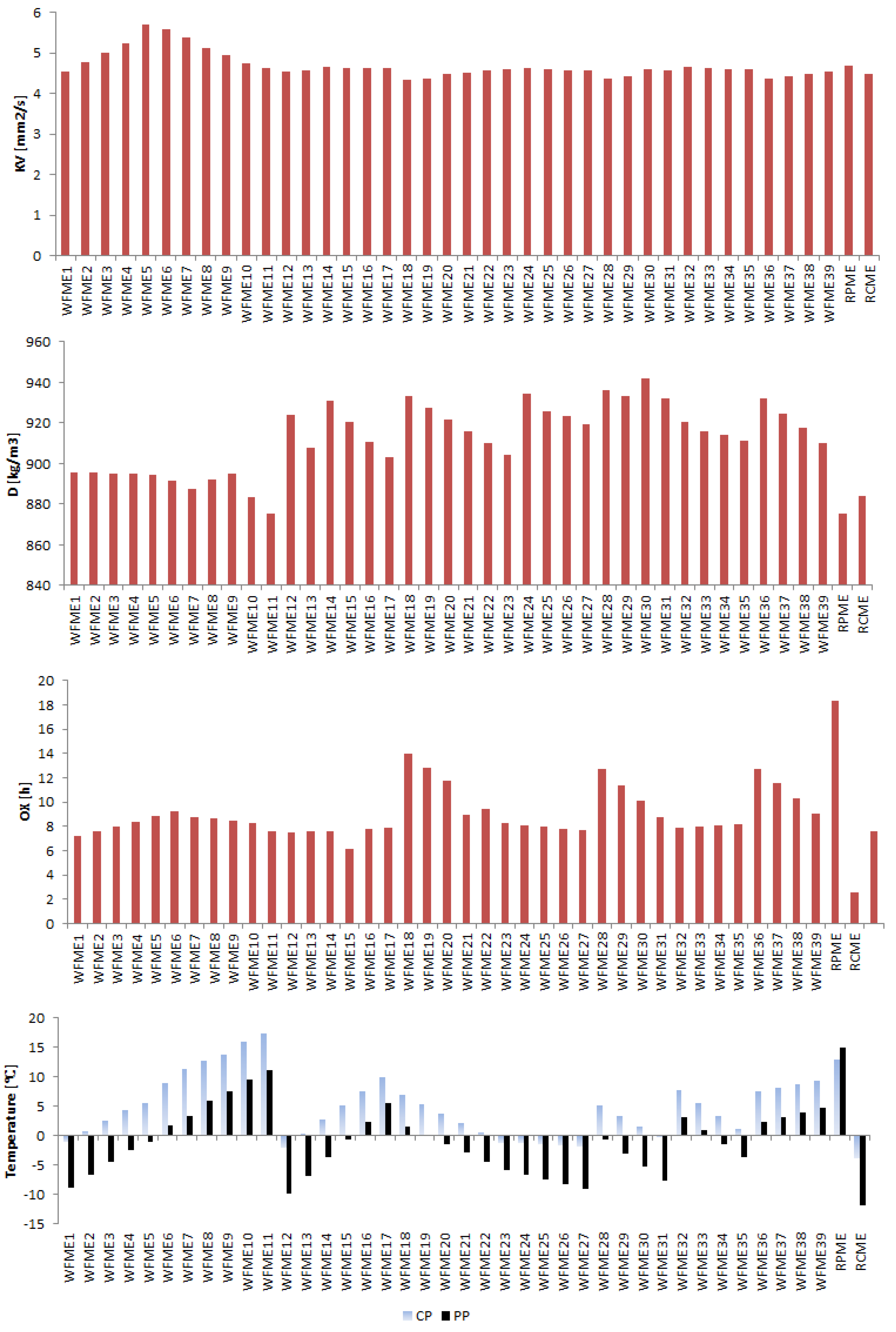
3.1. Fuel Properties
3.2. Empirical Models
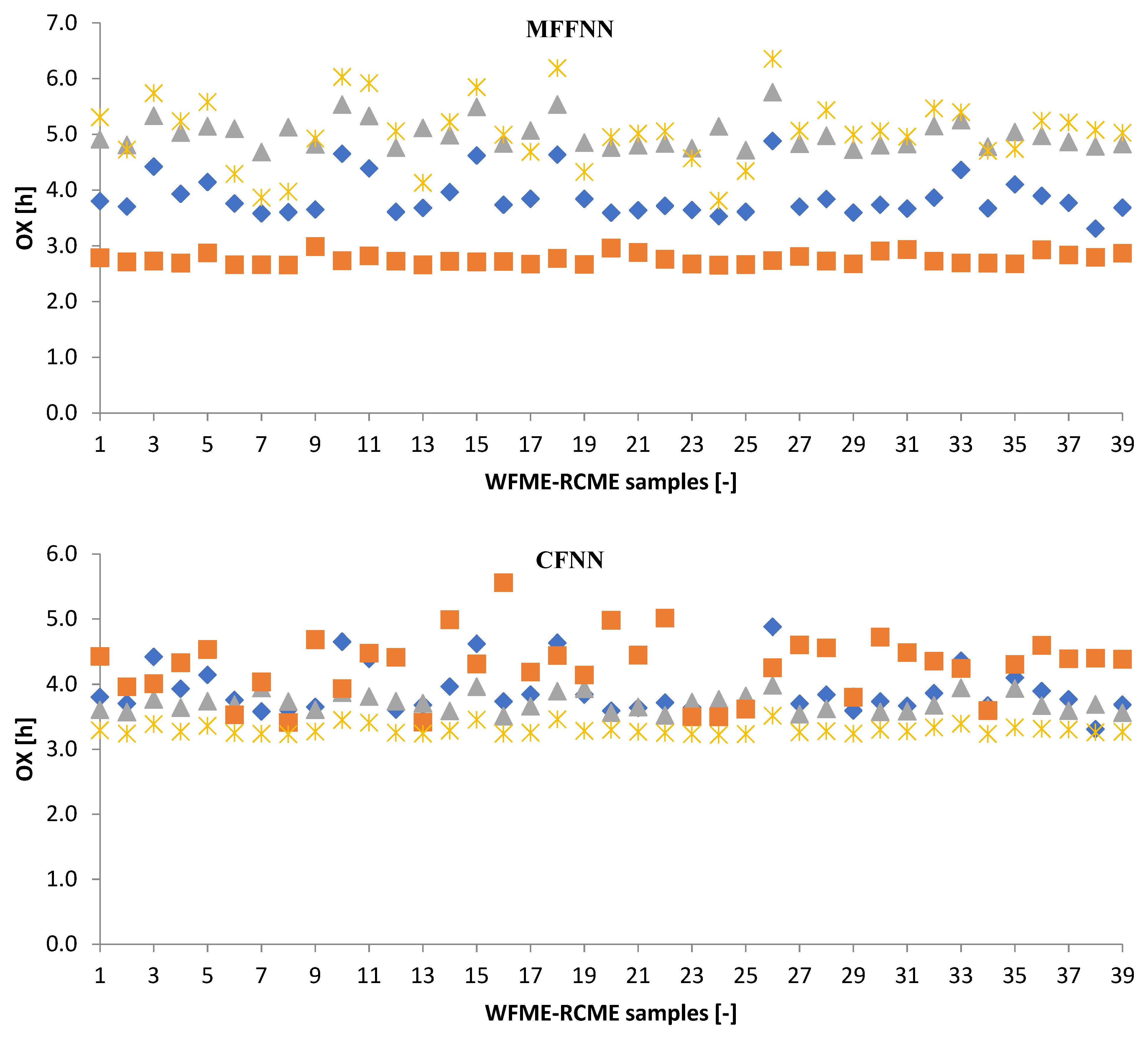
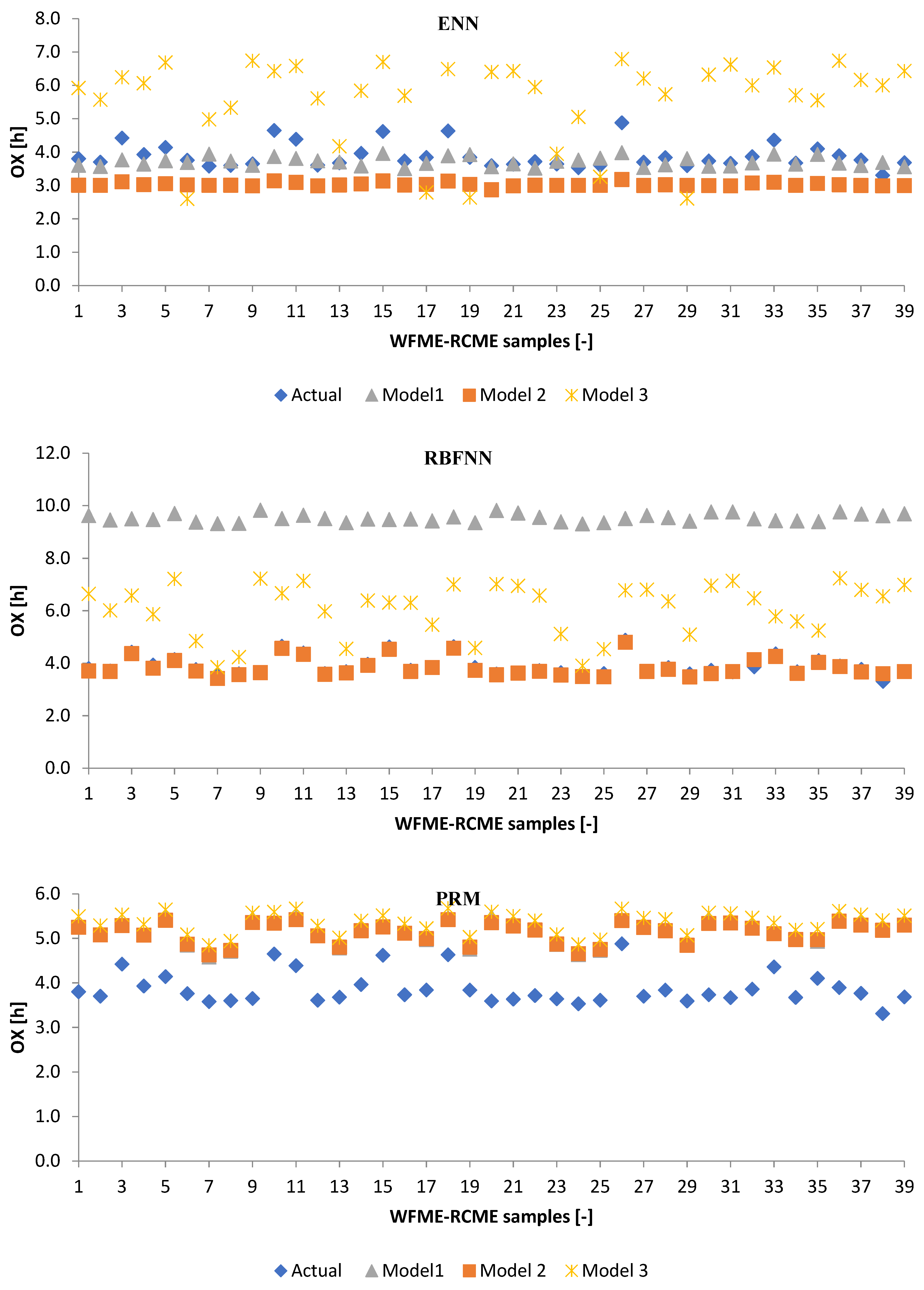
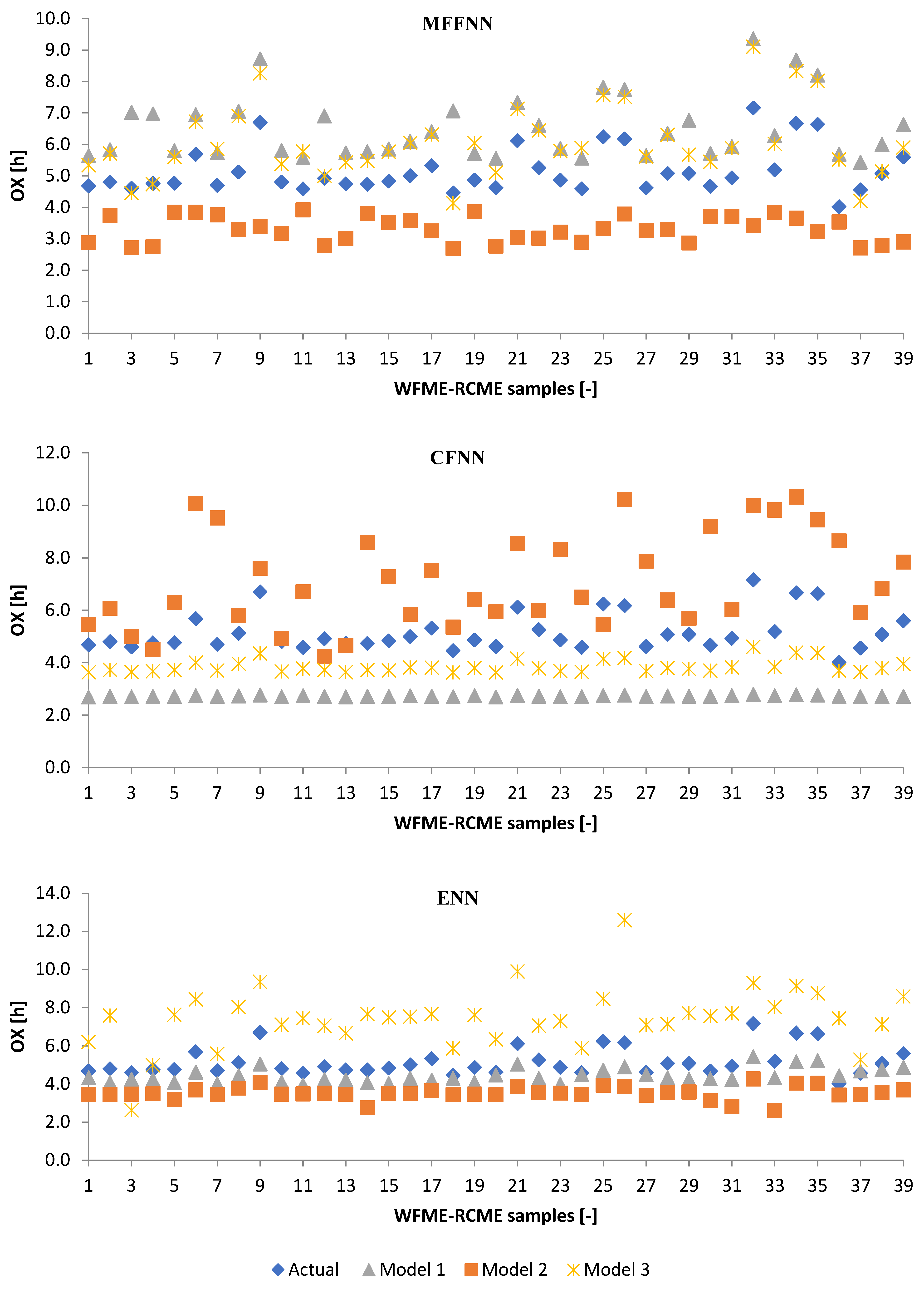
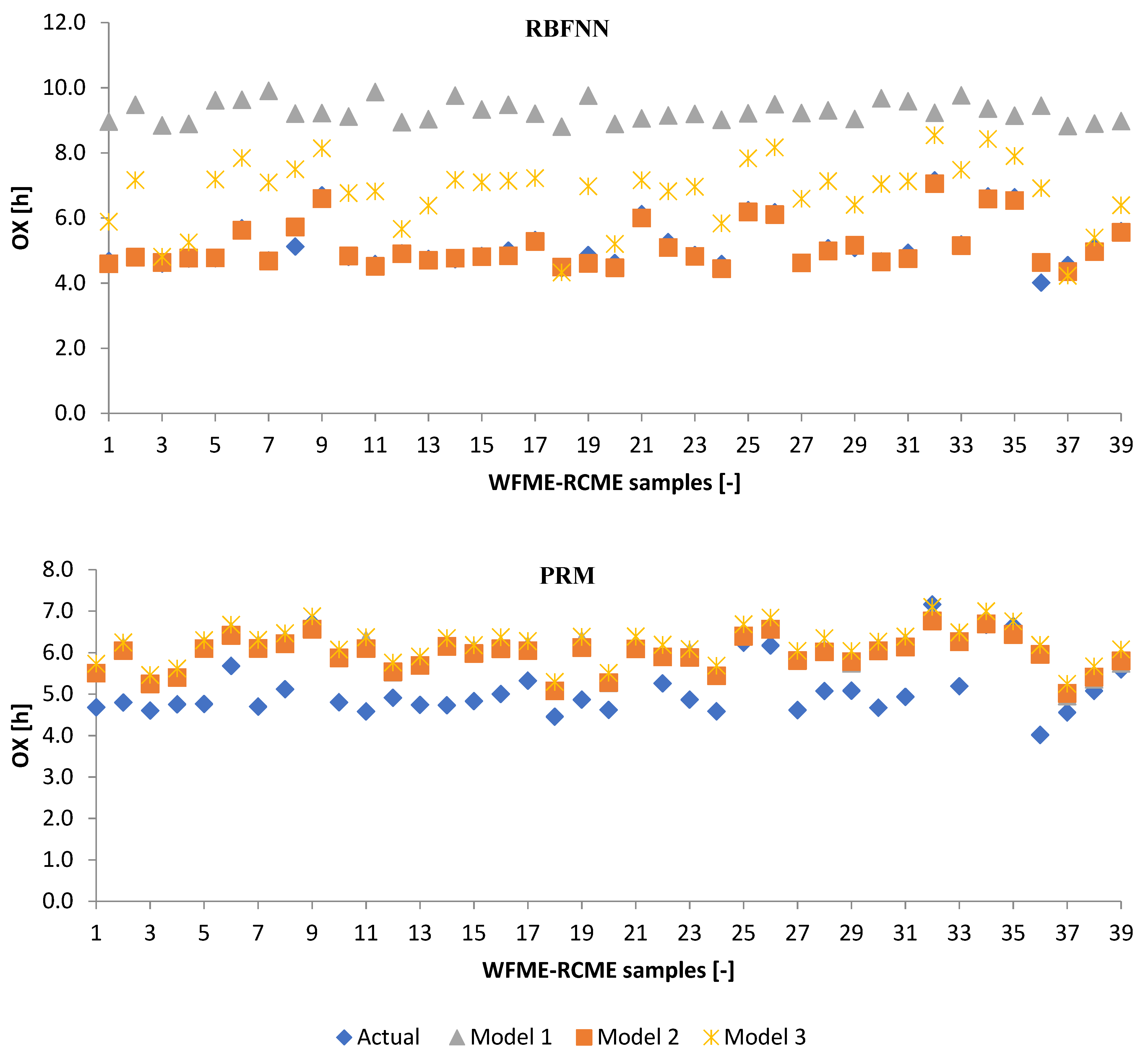
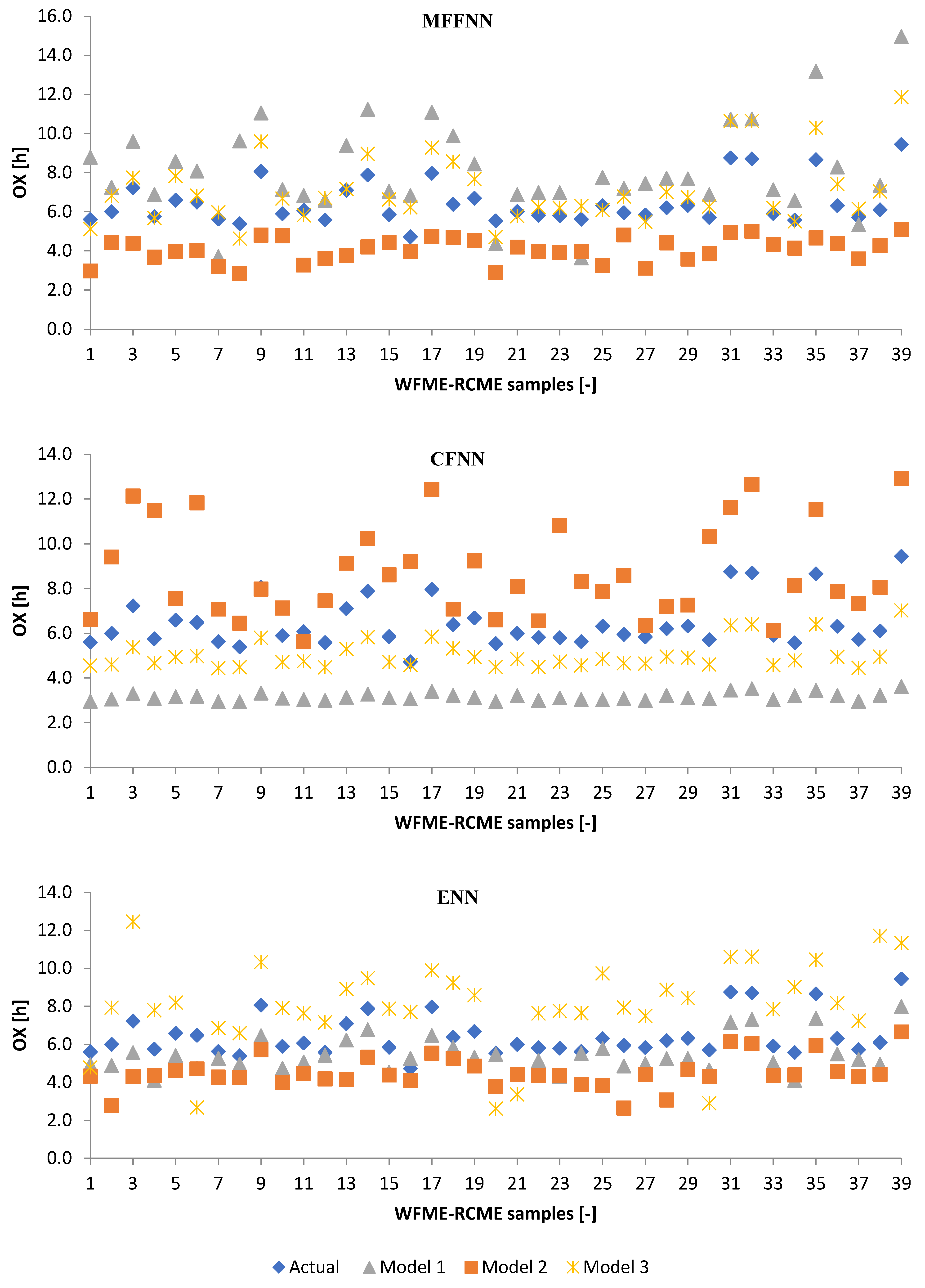
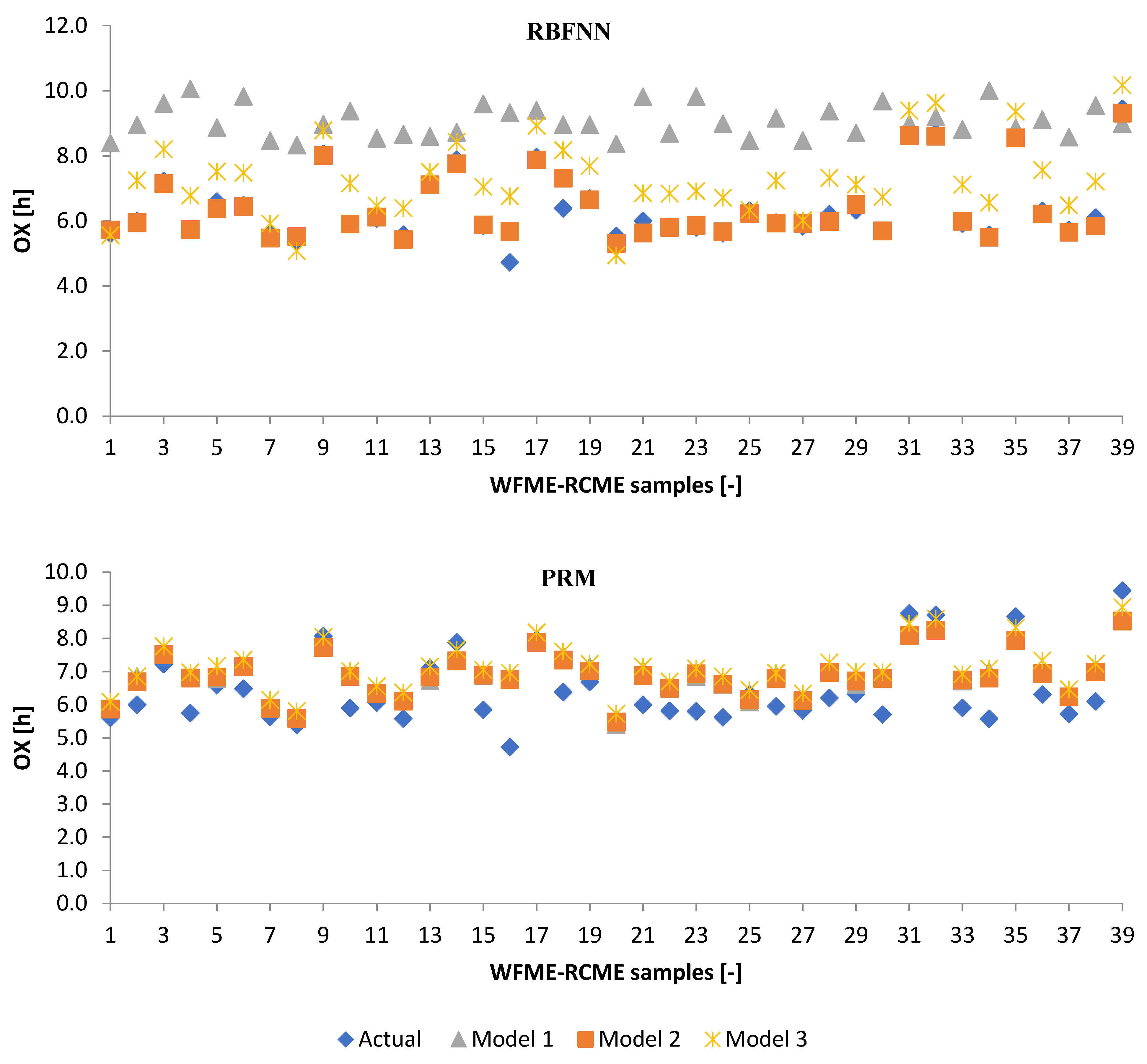
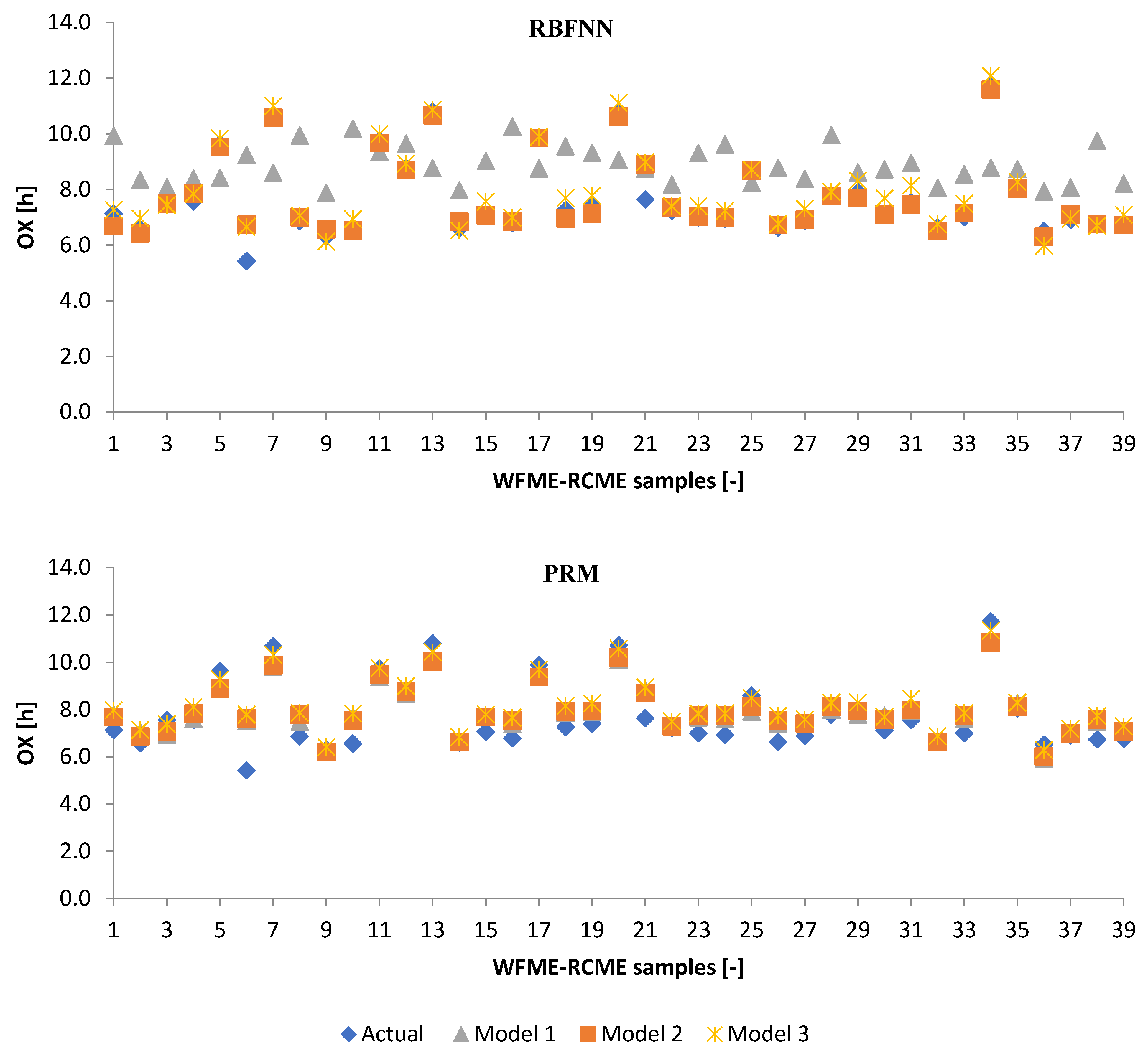
3.2.1. Machine Learning Models
3.2.2. Poisson Regression Model (PRM)
3.3. Performance Evaluation of Artificial Models and Mathematical Models for Testing Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, A.; Murugan, S. Effect of blending waste tyre derived fuel on oxidation stability of biodiesel and performance and emission studies of a diesel engine. Appl. Therm. Eng. 2017, 118, 365–374. [Google Scholar] [CrossRef]
- Ong, H.C.; Tiong, Y.W.; Goh, B.H.H.; Gan, Y.Y.; Mofijur, M.; Fattah, I.R.; Alam, M.A.; Lee, H.V.; Silitonga, A.S.; Mahlia, T.M.I.; et al. Recent advances in biodiesel production from agricultural products and microalgae using ionic liquids: Opportunities and challenges. Energy Convers. Manag. 2021, 228, 113647. [Google Scholar] [CrossRef]
- Lahane, S.; Subramanian, K.A. Impact of nozzle holes configuration on fuel spray, wall impingement and NOx emission of a diesel engine for biodiesel–diesel blend (B20). Appl. Therm. Eng. 2014, 64, 307–314. [Google Scholar] [CrossRef]
- Babu, D.; Karvembu, R.; Anand, R. Impact of split injection strategy on combustion, performance and emissions characteristics of biodiesel fuelled common rail direct injection assisted diesel engine. Energy 2018, 165, 577–592. [Google Scholar] [CrossRef]
- Babu, D.; Thangarasu, V.; Ramanathan, A. Artificial neural network approach on forecasting diesel engine characteristics fuelled with waste frying oil biodiesel. Appl. Energy 2020, 263, 114612. [Google Scholar] [CrossRef]
- Fawaz, E.G.; Salam, D.A. Preliminary economic assessment of the use of waste frying oils for biodiesel production in Beirut, Lebanon. Sci. Total Environ. 2018, 637, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Nezhad, M.K.; Aghaei, H. Tosylated cloisite as a new heterofunctional carrier for covalent immobilization of lipase and its utilization for biodiesel production from waste frying oil. Renew. Energy 2021, 164, 876–888. [Google Scholar] [CrossRef]
- Wang, Y.; Heydari, H. Developing an Extreme Learning Machine-Based Model for Estimating the Isothermal Compressibility of Biodiesel. Int. J. Chem. Eng. 2021, 2021, 6099019. [Google Scholar] [CrossRef]
- Gupta, K.K.; Kalita, K.; Ghadai, R.K.; Ramachandran, M.; Gao, X.Z. Machine Learning-Based Predictive Modelling of Biodiesel Production—A Comparative Perspective. Energies 2021, 14, 1122. [Google Scholar] [CrossRef]
- Sultana, N.; Hossain, S.Z.; Abusaad, M.; Alanbar, N.; Senan, Y.; Razzak, S.A. Prediction of biodiesel production from microalgal oil using Bayesian optimization algorithm-based machine learning approaches. Fuel 2022, 309, 122184. [Google Scholar] [CrossRef]
- Kumar, V.; Kalita, K.; Madhu, S.; Ragavendran, U.; Gao, X.Z. A Hybrid Genetic Programming–Gray Wolf Optimizer Approach for Process Optimization of Biodiesel Production. Processes 2021, 9, 442. [Google Scholar] [CrossRef]
- Yaşar, F. Comparision of fuel properties of biodiesel fuels produced from different oils to determine the most suitable feedstock type. Fuel 2020, 264, 116817. [Google Scholar] [CrossRef]
- Giakoumis, E.G.; Sarakatsanis, C.K. Estimation of biodiesel cetane number, density, kinematic viscosity and heating values from its fatty acid weight composition. Fuel 2018, 222, 574–585. [Google Scholar] [CrossRef]
- Alviso, D.; Artana, G.; Duriez, T. Prediction of biodiesel physico-chemical properties from its fatty acid composition using genetic programming. Fuel 2020, 264, 116844. [Google Scholar] [CrossRef]
- Razavi, R.; Bemani, A.; Baghban, A.; Mohammadi, A.H.; Habibzadeh, S. An insight into the estimation of fatty acid methyl ester based biodiesel properties using a LSSVM model. Fuel 2019, 243, 133–141. [Google Scholar] [CrossRef]
- Kumar, N. Oxidative stability of biodiesel: Causes, effects and prevention. Fuel 2017, 190, 328–350. [Google Scholar] [CrossRef]
- Pullen, J.; Saeed, K. Experimental study of the factors affecting the oxidation stability of biodiesel FAME fuels. Fuel Process. Technol. 2014, 125, 223–235. [Google Scholar] [CrossRef]
- Knothe, G.; Dunn, R.O. Dependence of oil stability index of fatty compounds on their structure and concentration and presence of metals. J. Am. Oil Chem. Soc. 2003, 80, 1021–1026. [Google Scholar] [CrossRef]
- McCormick, R.L.; Ratcliff, M.; Moens, L.; Lawrence, R. Several factors affecting the stability of biodiesel in standard accelerated tests. Fuel Process. Technol. 2007, 88, 651–657. [Google Scholar] [CrossRef]
- Dunn, R.O. Antioxidants for improving storage stability of biodiesel. Biofuels Bioprod. Biorefin. Innovation Sustain. Economy 2008, 2, 304–318. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, D.K.; Lee, J.P.; Park, S.C.; Kim, Y.J.; Lee, J.S. Blending effects of biodiesels on oxidation stability and low temperature flow properties. Bioresour. Technol. 2008, 99, 1196–1203. [Google Scholar] [CrossRef]
- Knothe, G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process. Technol. 2005, 86, 1059–1070. [Google Scholar] [CrossRef]
- Pereira, G.G.; Garcia, R.K.; Ferreira, L.L.; Barrera-Arellano, D. Soybean and soybean/beef-tallow biodiesel: A comparative study on oxidative degradation during long-term storage. J. Am. Oil Chem. Soc. 2017, 94, 587–593. [Google Scholar] [CrossRef]
- Zhou, J.; Xiong, Y.; Liu, X. Evaluation of the oxidation stability of biodiesel stabilized with antioxidants using the Rancimat and PDSC methods. Fuel 2017, 188, 61–68. [Google Scholar] [CrossRef]
- Neuana, N.F.; de Sousa Barboza, J.C.; dos Santos, E.P.; da Silva, M.L.C.P. A novel application of Mangifera indica L and Eugenia uniflora L extracts as antioxidants to control biodiesel oxidation stability. Environ. Prog. Sustain. Energy 2021, 40, e13540. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar, J.M.; González, J.F. Safflower Biodiesel: Improvement of its Oxidative Stability by using BHA and TBHQ. Energies 2019, 12, 1940. [Google Scholar] [CrossRef] [Green Version]
- Çamur, H.; Alassi, E. Physicochemical Properties Enhancement of Biodiesel Synthesis from Various Feedstocks of Waste/Residential Vegetable Oils and Palm Oil. Energies 2021, 14, 4928. [Google Scholar] [CrossRef]
- Kassem, Y.; Çamur, H.; Alassi, E. Biodiesel production from four residential waste frying oils: Proposing blends for improving the physicochemical properties of methyl biodiesel. Energies 2020, 13, 4111. [Google Scholar] [CrossRef]
- Kassem, Y.; Çamur, H. Effects of storage under different conditions on the fuel properties of biodiesel admixtures derived from waste frying and canola oils. Biomass Convers. Biorefinery 2018, 8, 825–845. [Google Scholar] [CrossRef]
- Saeed RH, S.; Kassem, Y.; Çamur, H. Effect of Biodiesel Mixture Derived from Waste Frying-Corn, Frying-Canola-Corn and Canola-Corn Cooking Oils with Various Ages on Physicochemical Properties. Energies 2019, 12, 3729. [Google Scholar] [CrossRef] [Green Version]
- ASTM D445–09; Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity). ASTM: West Conshohocken, PA, USA, 2009.
- ASTM D854-14; Standard Test Methods for Specific Gravity of Soil Solids by Water Pycnometer. ASTM: West Conshohocken, PA, USA, 2014.
- ASTM D2500-17a; Standard Test Method for Cloud Point of Petroleum Products. ASTM: West Conshohocken, PA, USA, 2017.
- ASTM D97-17b; Standard Test Method for Pour Point of Petroleum Products. ASTM: West Conshohocken, PA, USA, 2017.
- EN 14112; Fat and Oil Derivatives. Fatty Acid Methyl Esters (FAME). Determination of Oxidation Stability (Accelerated Oxidation Test). National Standards Authority of Ireland: Dublin, Ireland, 2003.
- Magalhães, A.M.; Pereira, E.; Meirelles, A.J.; Sampaio, K.A.; Maximo, G.J. Proposing blends for improving the cold flow properties of ethylic biodiesel. Fuel 2019, 253, 50–59. [Google Scholar] [CrossRef]
- Verma, P.; Sharma, M.P.; Dwivedi, G. Evaluation and enhancement of cold flow properties of palm oil and its biodiesel. Energy Rep. 2016, 2, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Ali, O.M.; Mamat, R.; Abdullah, N.R.; Abdullah, A.A. Analysis of blended fuel properties and engine performance with palm biodiesel–diesel blended fuel. Renew. Energy 2016, 86, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Moser, B.R. Influence of extended storage on fuel properties of methyl esters prepared from canola, palm, soybean and sunflower oils. Renew. Energy 2011, 36, 1221–1226. [Google Scholar] [CrossRef]
- Öztürk, E. Performance, emissions, combustion and injection characteristics of a diesel engine fuelled with canola oil–hazelnut soapstock biodiesel mixture. Fuel Process. Technol. 2015, 129, 183–191. [Google Scholar] [CrossRef]
- Uyaroğlu, A.; Uyumaz, A.; Çelikten, İ. Comparison of the combustion, performance, and emission characteristics of inedible Crambe abyssinica biodiesel and edible Hazelnut, Corn, soybean, sunflower, and Canola biodiesels. Environ. Prog. Sustain. Energy 2018, 37, 1438–1447. [Google Scholar] [CrossRef]
- Kassem, Y.; Gokcekus, H. Do quadratic and Poisson regression models help to predict monthly rainfall? Desalination Water Treat. 2021, 215, 288–318. [Google Scholar] [CrossRef]
- Gündoğdu, S.; Elbir, T. Application of feedforward and cascade forward neural network models for prediction of hourly ambient air temperature based on MERRA-2 reanalysis data in a coastal area of Turkey. Meteorol. Atmos. Phys. 2021, 133, 1481–1493. [Google Scholar] [CrossRef]
- Alkhasawneh, M.S.; Tay, L.T. A hybrid intelligent system integrating the cascade forward neural network with Elman neural network. Arab. J. Sci. Eng. 2018, 43, 6737–6749. [Google Scholar] [CrossRef]
- Jianyu, L.; Siwei, L.; Yingjian, Q.; Yaping, H. Numerical solution of elliptic partial differential equation using radial basis function neural networks. Neural Netw. 2003, 16, 729–734. [Google Scholar] [CrossRef]
- Guo, C.; Lu, J.; Tian, Z.; Guo, W.; Darvishan, A. Optimization of critical parameters of PEM fuel cell using TLBO-DE based on Elman neural network. Energy Convers. Manag. 2019, 183, 149–158. [Google Scholar] [CrossRef]
- Ren, G.; Cao, Y.; Wen, S.; Huang, T.; Zeng, Z. A modified Elman neural network with a new learning rate scheme. Neurocomputing 2018, 286, 11–18. [Google Scholar] [CrossRef]
- Sadler, J.M.; Goodall, J.L.; Morsy, M.M.; Spencer, K. Modeling urban coastal flood severity from crowd-sourced flood reports using Poisson regression and Random Forest. J. Hydrol. 2018, 559, 43–55. [Google Scholar] [CrossRef]
- Coxe, S.; West, S.G.; Aiken, L.S. The analysis of count data: A gentle introduction to Poisson regression and its alternatives. J. Personal. Assess. 2009, 91, 121–136. [Google Scholar] [CrossRef]
- Evcil, A.; Al-Shanableh, F.; Savas, M.A. Variation of solid fraction with cold flow properties of biodiesel produced from waste frying oil. Fuel 2018, 215, 522–527. [Google Scholar] [CrossRef]
- El-Araby, R.; Amin, A.; El Morsi, A.K.; El-Ibiari, N.N.; El-Diwani, G.I. Study on the characteristics of palm oil–biodiesel–diesel fuel blend. Egypt. J. Pet. 2017, 27, 187–194. [Google Scholar] [CrossRef]
- Dunn, R.O. Fuel properties of biodiesel/ultra-low sulfur diesel (ULSD) blends. J. Am. Oil Chem. Soc. 2011, 88, 1977–1987. [Google Scholar] [CrossRef]
- Doğan, T.H. The testing of the effects of cooking conditions on the quality of biodiesel produced from waste cooking oils. Renew. Energy 2016, 94, 466–473. [Google Scholar] [CrossRef]
- Fu, J.; Turn, S.Q.; Takushi, B.M.; Kawamata, C.L. Storage and oxidation stabilities of biodiesel derived from waste cooking oil. Fuel 2016, 167, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Mejía, J.D.; Salgado, N.; Orrego, C.E. Effect of blends of Diesel and Palm-Castor biodiesels on viscosity, cloud point and flashpoint. Ind. Crops Prod. 2013, 43, 791–797. [Google Scholar] [CrossRef]
- Atabani, A.E.; Mahlia TM, I.; Masjuki, H.H.; Badruddin, I.A.; Yussof, H.W.; Chong, W.T.; Lee, K.T. A comparative evaluation of physical and chemical properties of biodiesel synthesized from edible and non-edible oils and study on the effect of biodiesel blending. Energy 2013, 58, 296–304. [Google Scholar] [CrossRef]
- Campus, P. Comparative analysis of fuel characteristics of bio-diesel produced from selected oil-bearing seeds in Nigeria. Eur. J. Sci. Res. 2011, 58, 238–246. [Google Scholar]
- Balat, M. Potential alternatives to edible oils for biodiesel production–A review of current work. Energy Convers. Manag. 2011, 52, 1479–1492. [Google Scholar] [CrossRef]
- Karmakar, A.; Karmakar, S.; Mukherjee, S. Properties of various plants and animals feedstocks for biodiesel production. Bioresour. Technol. 2010, 101, 7201–7210. [Google Scholar] [CrossRef]
- Gui, M.M.; Lee, K.T.; Bhatia, S. Feasibility of edible oil vs. non-edible oil vs. waste edible oil as biodiesel feedstock. Energy 2008, 33, 1646–1653. [Google Scholar]
- Zuleta, E.C.; Rios, L.A.; Benjumea, P.N. Oxidative stability and cold flow behavior of palm, sacha-inchi, jatropha and castor oil biodiesel blends. Fuel Process. Technol. 2012, 102, 96–101. [Google Scholar] [CrossRef]
- Dey, S.; Reang, N.M.; Das, P.K.; Deb, M. A comprehensive study on prospects of economy, environment, and efficiency of palm oil biodiesel as a renewable fuel. J. Clean. Prod. 2021, 286, 124981. [Google Scholar] [CrossRef]
- Mosarof, M.H.; Kalam, M.A.; Masjuki, H.H.; Ashraful, A.M.; Rashed, M.M.; Imdadul, H.K.; Monirul, I.M. Implementation of palm biodiesel based on economic aspects, performance, emission and wear characteristics. Energy Convers. Manag. 2015, 105, 617–629. [Google Scholar] [CrossRef]
- Abd Wafti, N.S.; Lau HL, N.; Choo, Y.M. Production technology of biodiesel from palm fatty acid distillate using mild acid catalyst. J. Oil Palm Res. 2015, 27, 352–359. [Google Scholar]
- May, C.Y.; Liang, Y.C.; Foon, C.S.; Ngan, M.A.; Hook, C.C.; Basiron, Y. Key fuel properties of palm oil alkyl esters. Fuel 2005, 84, 1717–1720. [Google Scholar] [CrossRef]
- Kalam, M.A.; Masjuki, H.H. Biodiesel from palmoil—An analysis of its properties and potential. Biomass Bioenergy 2002, 23, 471–479. [Google Scholar] [CrossRef]
- Monirul, I.M.; Masjuki, H.H.; Kalam, M.A.; Zulkifli, N.W.M.; Rashedul, H.K.; Rashed, M.M.; Mosarof, M.H. A comprehensive review on biodiesel cold flow properties and oxidation stability along with their improvement processes. RSC Adv. 2015, 5, 86631–86655. [Google Scholar] [CrossRef]
- Can, Ö.; Öztürk, E.; Yücesu, H.S. Combustion and exhaust emissions of canola biodiesel blends in a single-cylinder DI diesel engine. Renew. Energy 2017, 109, 73–82. [Google Scholar] [CrossRef]
- Uzun, B.B.; Kılıç, M.; Özbay, N.; Pütün, A.E.; Pütün, E. Biodiesel production from waste frying oils: Optimization of reaction parameters and determination of fuel properties. Energy 2012, 44, 347–351. [Google Scholar] [CrossRef]
- Bharti, R.; Singh, B. Green tea (Camellia assamica) extract as an antioxidant additive to enhance the oxidation stability of biodiesel synthesized from waste cooking oil. Fuel 2020, 262, 116658. [Google Scholar] [CrossRef]
- Betiku, E.; Odude, V.O.; Ishola, N.B.; Bamimore, A.; Osunleke, A.S.; Okeleye, A.A. Predictive capability evaluation of RSM, ANFIS and ANN: A case of reduction of high free fatty acid of palm kernel oil via esterification process. Energy Convers. Manag. 2016, 124, 219–230. [Google Scholar] [CrossRef]
- Hariram, V.; Bose, A.; Seralathan, S. Dataset on optimized biodiesel production from seeds of Vitis vinifera using ANN, RSM and ANFIS. Data Brief 2019, 25, 104298. [Google Scholar] [CrossRef] [PubMed]




| Samples | |||||||
|---|---|---|---|---|---|---|---|
| WFME1 | 7.74 | 64.01 | 28.33 | 1.50 | 120.67 | 1.81 | 11.93 |
| WFME2 | 9.50 | 62.83 | 27.81 | 1.43 | 118.46 | 2.01 | 9.54 |
| WFME3 | 11.27 | 61.65 | 27.30 | 1.35 | 116.25 | 2.20 | 7.90 |
| WFME4 | 13.03 | 60.47 | 26.78 | 1.28 | 114.04 | 2.40 | 6.70 |
| WFME5 | 16.55 | 58.11 | 25.75 | 1.13 | 109.62 | 2.79 | 5.07 |
| WFME6 | 20.08 | 55.75 | 24.72 | 0.98 | 105.20 | 3.19 | 4.01 |
| WFME7 | 25.38 | 52.22 | 23.18 | 0.75 | 98.58 | 3.78 | 2.97 |
| WFME8 | 30.65 | 48.68 | 21.63 | 0.53 | 91.93 | 4.37 | 2.29 |
| WFME9 | 34.20 | 46.32 | 20.60 | 0.38 | 87.52 | 4.77 | 1.96 |
| WFME10 | 39.47 | 42.78 | 19.05 | 0.15 | 80.88 | 5.35 | 1.57 |
| WFME11 | 42.99 | 40.42 | 18.02 | 0.00 | 76.46 | 5.75 | 1.36 |
| WFME12 | 7.74 | 65.43 | 27.33 | 1.92 | 120.09 | 1.81 | 11.98 |
| WFME13 | 14.79 | 60.43 | 25.27 | 1.54 | 110.96 | 2.60 | 5.79 |
| WFME14 | 21.84 | 55.43 | 23.21 | 1.15 | 101.84 | 3.38 | 3.60 |
| WFME15 | 28.89 | 50.42 | 21.14 | 0.77 | 92.71 | 4.17 | 2.48 |
| WFME16 | 35.94 | 45.42 | 19.08 | 0.38 | 83.59 | 4.96 | 1.79 |
| WFME17 | 42.99 | 40.42 | 17.02 | 0.00 | 74.46 | 5.75 | 1.34 |
| WFME18 | 22.13 | 52.07 | 25.48 | 1.31 | 103.03 | 4.13 | 3.50 |
| WFME19 | 22.70 | 51.00 | 26.22 | 1.29 | 103.43 | 4.20 | 3.40 |
| WFME20 | 23.28 | 49.93 | 26.95 | 1.27 | 103.83 | 4.28 | 3.30 |
| WFME21 | 23.85 | 48.85 | 27.69 | 1.26 | 104.23 | 4.35 | 3.21 |
| WFME22 | 24.43 | 47.78 | 28.42 | 1.24 | 104.63 | 4.43 | 3.12 |
| WFME23 | 25.00 | 46.71 | 29.16 | 1.22 | 105.03 | 4.50 | 3.03 |
| WFME24 | 21.55 | 50.45 | 28.79 | 1.36 | 108.04 | 3.96 | 3.68 |
| WFME25 | 18.10 | 54.20 | 28.43 | 1.50 | 111.05 | 3.42 | 4.57 |
| WFME26 | 14.64 | 57.94 | 28.06 | 1.64 | 114.07 | 2.89 | 5.87 |
| WFME27 | 11.19 | 61.69 | 27.70 | 1.78 | 117.08 | 2.35 | 7.99 |
| WFME28 | 19.25 | 54.74 | 25.85 | 1.43 | 106.44 | 3.66 | 4.19 |
| WFME29 | 16.37 | 57.41 | 26.22 | 1.55 | 109.85 | 3.20 | 5.11 |
| WFME30 | 13.50 | 60.09 | 26.59 | 1.68 | 113.27 | 2.74 | 6.42 |
| WFME31 | 10.62 | 62.76 | 26.96 | 1.80 | 116.68 | 2.27 | 8.45 |
| WFME32 | 39.39 | 41.68 | 19.45 | 0.24 | 80.57 | 5.50 | 1.55 |
| WFME33 | 35.79 | 42.94 | 21.88 | 0.49 | 86.69 | 5.25 | 1.81 |
| WFME34 | 32.20 | 44.19 | 24.30 | 0.73 | 92.80 | 5.00 | 2.13 |
| WFME35 | 28.60 | 45.45 | 26.73 | 0.98 | 98.92 | 4.75 | 2.52 |
| WFME36 | 26.30 | 49.74 | 23.79 | 1.05 | 97.32 | 4.45 | 2.80 |
| WFME37 | 30.47 | 47.41 | 22.10 | 0.79 | 91.60 | 4.78 | 2.28 |
| WFME38 | 34.65 | 45.08 | 20.40 | 0.52 | 85.89 | 5.10 | 1.89 |
| WFME39 | 38.82 | 42.75 | 18.71 | 0.26 | 80.17 | 5.42 | 1.58 |
| RCME | 6.73 | 60.25 | 29.91 | 1.98 | 120.07 | 2.00 | 13.40 |
| PME | 44.90 | 43.50 | 11.20 | 0.50 | 65.90 | 6.36 | 1.22 |
| WFME: | Waste frying methyl ester | ||||||
| PME: | Palm methyl ester | ||||||
| RCME: | Refined canola methyl ester | ||||||
| Data | Variable | Explanation | SD | CV | Max. | Min. | Unit |
|---|---|---|---|---|---|---|---|
| Training | X1 | Concentration amount of WFME in the mixture | 26.47 | 47.43 | 20 | 100 | % |
| X2 | Concentration amount of PME in the mixture | 26.47 | 59.89 | 0 | 80 | % | |
| X3 | Concentration amount of RCME in the mixture | 28.98 | 131.16 | 0 | 80 | % | |
| Total saturated | 8.013 | 26.02 | 6.73 | 44.9 | wt% | ||
| Total monounsaturated | 4.751 | 9.63 | 40.42 | 65.43 | wt% | ||
| Total polyunsaturated | 3.886 | 19.75 | 11.2 | 29.91 | wt% | ||
| Very-long-chain fatty acid | 0.3461 | 38.03 | 0 | 1.98 | wt% | ||
| Degree of unsaturation | 11.64 | 13.13 | 65.9 | 120.67 | wt% | ||
| Long-chain saturated factor | 0.9306 | 19.77 | 1.808 | 6.36 | wt% | ||
| Ratio | 1.5297 | 59.16 | 1.2183 | 13.3967 | - | ||
| KV (at 40 °C) | Kinematic viscosity | 0.1859 | 3.95 | 4.3285 | 5.705 | Mm2/s | |
| D (at 15 °C) | Density | 13.84 | 1.54 | 875 | 941.79 | Kg/m3 | |
| CP | Cloud Point | 3.678 | 50.36 | −4 | 17.4 | °C | |
| PP | Pour Point | 5.203 | 120.92 | −12 | 15 | °C | |
| XO | Oxidation stability | 2.68 | 21.89 | 2.6 | 18.35 | h | |
| Testing | X1 | Concentration amount of WFME in the mixture | 22.43 | 44.87 | 20 | 80 | % |
| X2 | Concentration amount of PME in the mixture | 0 | * | 0 | 0 | % | |
| X3 | Concentration amount of RCME in the mixture | 22.43 | 44.87 | 20 | 80 | % | |
| Total saturated | 6.765 | 44.03 | 6.932 | 35.738 | wt% | ||
| Total monounsaturated | 4.399 | 7.84 | 44.386 | 64.394 | wt% | ||
| Total polyunsaturated | 2.254 | 8.28 | 19.598 | 29.76 | wt% | ||
| Very-long-chain fatty acid | 0.3567 | 23.61 | 0.396 | 1.968 | wt% | ||
| Degree of unsaturation | 8.34 | 7.54 | 83.58 | 120.55 | wt% | ||
| Long-chain saturated factor | 0.7674 | 26.06 | 1.8462 | 4.999 | wt% | ||
| Ratio | 2.974 | 45.01 | 1.79 | 13.081 | - | ||
| KV (at 40 °C) | Kinematic viscosity | 0.1895 | 4.09 | 4.4343 | 5.5595 | Mm2/s | |
| D (at 15 °C) | Density | 11.9 | 1.32 | 880.83 | 934.41 | Kg/m3 | |
| CP | Cloud Point | 3.413 | 811.73 | −3.6 | 13.12 | °C | |
| PP | Pour Point | 3.817 | −57.83 | −11.6 | 6.48 | °C | |
| XO | Oxidation stability | 1.744 | 30.06 | 3.308 | 11.72 | h |
| Model Name | Combination of Input |
|---|---|
| Model 1 | . VLCFA, DU, LCSF, and |
| Model 2 | . VLCFA, DU, LCSF, KV (at 40 °C) and D (at 15 °C) |
| Model 3 | . VLCFA, DU, LCSF, KV (at 40 °C), D (at 15 °C), CP and PP |
| Machine Learning Model | Model Name | TF | HL | NN | MSE (Training) | Epoch | R2 (Training) |
|---|---|---|---|---|---|---|---|
| MFFNN | Model 1 | 1 | 5 | TANSIG | 9.29× 10−5 | 759 | 0.9984 |
| Model 2 | 1 | 5 | TANSIG | 1.66 × 10−4 | 18 | 0.9936 | |
| Model 3 | 1 | 5 | TANSIG | 9.06 × 10−5 | 325 | 0.9962 | |
| CFNN | Model 1 | 1 | 5 | LOGSIG | 2.06 × 10−4 | 19 | 0.9911 |
| Model 2 | 1 | 5 | LOGSIG | 7.26 × 10−5 | 345 | 0.9978 | |
| Model 3 | 1 | 5 | LOGSIG | 2.02 × 10−4 | 8 | 0.9916 | |
| ENN | Model 1 | 1 | 8 | TANSIG | 3.74 × 10−5 | 999 | 0.9988 |
| Model 2 | 1 | 10 | TANSIG | 1.42 × 10−4 | 294 | 0.9897 | |
| Model 3 | 1 | 10 | TANSIG | 1.06 × 10−4 | 912 | 0.9992 |
| Model Name | Mathematical Equation |
|---|---|
| Model 1 | |
| Model 2 | |
| Model 3 |
| Volume Fraction of RCME | Statistical Indicator | FFNN | CFNN | ENN | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | ||
| 80% | R2 | 0.7991 | 0.0051 | 0.5890 | 0.3389 | 0.0060 | 0.8949 | 0.3389 | 0.8283 | 0.1349 |
| RMSE | 1.1328 | 1.1838 | 1.2243 | 0.3454 | 0.6959 | 0.6502 | 0.3454 | 0.9031 | 2.0591 | |
| MAE | 1.1195 | 1.1216 | 1.1580 | 0.2637 | 0.5601 | 0.5813 | 0.2637 | 0.8479 | 1.9408 | |
| SEP [%] | 40.5801 | 28.9035 | 23.1596 | 6.1730 | 11.1167 | 15.6565 | 8.6950 | 25.7013 | 52.2759 | |
| ADD [%] | 29.3626 | 28.3311 | 29.8648 | 6.4473 | 14.7524 | 14.4612 | 6.4473 | 21.3468 | 50.3830 | |
| 60% | R2 | 0.7916 | 0.0218 | 0.8205 | 0.7309 | 0.2397 | 0.9354 | 0.6757 | 0.5071 | 0.4765 |
| RMSE | 1.4319 | 2.0104 | 1.0372 | 2.5319 | 2.4843 | 1.4000 | 0.8627 | 1.7190 | 2.5829 | |
| MAE | 1.3492 | 1.8567 | 0.9232 | 2.4335 | 2.0343 | 1.3124 | 0.7540 | 1.6330 | 2.3980 | |
| SEP [%] | 40.8346 | 35.9766 | 14.1804 | 34.2581 | 33.6743 | 48.1172 | 21.3737 | 42.4309 | 54.0799 | |
| ADD [%] | 26.2692 | 35.0161 | 17.6570 | 46.2930 | 39.7644 | 24.6975 | 13.9619 | 31.0069 | 46.5722 | |
| 40% | R2 | 0.7252 | 0.3923 | 0.8731 | 0.8005 | 0.4275 | 0.9440 | 0.7737 | 0.6157 | 0.3441 |
| RMSE | 2.2280 | 2.5151 | 1.0236 | 3.4191 | 2.7897 | 1.4965 | 1.1222 | 2.0331 | 2.4568 | |
| MAE | 1.9370 | 2.3690 | 0.8338 | 3.2888 | 2.3318 | 1.4146 | 1.0241 | 1.9195 | 2.2607 | |
| SEP [%] | 47.5647 | 36.7778 | 10.2969 | 37.6053 | 32.8045 | 44.8733 | 22.6488 | 38.1872 | 41.5207 | |
| ADD [%] | 29.4424 | 36.0984 | 12.5047 | 50.1892 | 36.6401 | 21.4080 | 15.7027 | 29.4800 | 35.9919 | |
| 20% | R2 | 0.7640 | 0.7305 | 0.8968 | 0.9158 | 0.7493 | 0.9552 | 0.8699 | 0.4941 | 0.8288 |
| RMSE | 2.7585 | 2.4453 | 0.7200 | 2.8433 | 1.8142 | 0.7945 | 0.9900 | 1.7729 | 1.1751 | |
| MAE | 2.3567 | 2.3227 | 0.5572 | 2.7589 | 1.5783 | 0.7510 | 0.8637 | 1.3976 | 1.0920 | |
| SEP [%] | 43.6498 | 30.0795 | 5.5680 | 29.6656 | 19.7853 | 15.1332 | 13.6207 | 19.9475 | 15.8655 | |
| ADD [%] | 29.8220 | 29.8999 | 7.2084 | 35.4254 | 20.9252 | 9.6503 | 11.2800 | 17.6835 | 14.8233 | |
| 80% | R2 | 0.0002 | 0.9427 | 0.1020 | 0.1594 | 0.2171 | 0.2508 | |||
| RMSE | 5.6655 | 0.0942 | 2.3867 | 1.3278 | 1.2901 | 1.5098 | ||||
| MAE | 5.6520 | 0.0689 | 2.1923 | 1.2798 | 1.2486 | 1.4761 | ||||
| SEP [%] | 147.0920 | 1.2357 | 22.9985 | 24.9530 | 20.5602 | 28.6048 | ||||
| ADD [%] | 147.5842 | 1.8045 | 57.0214 | 33.7886 | 32.9778 | 38.8593 | ||||
| 60% | R2 | 0.0002 | 0.9471 | 0.4375 | 0.4307 | 0.4938 | 0.5447 | |||
| RMSE | 4.1872 | 0.1676 | 1.7904 | 1.0019 | 0.9461 | 1.1475 | ||||
| MAE | 4.1123 | 0.1023 | 1.6346 | 0.8699 | 0.8297 | 1.0388 | ||||
| SEP [%] | 80.0758 | 1.3712 | 17.6268 | 14.6157 | 12.2521 | 17.3059 | ||||
| ADD [%] | 82.7778 | 2.0723 | 32.3416 | 17.9959 | 17.1649 | 21.3994 | ||||
| 40% | R2 | 0.0002 | 0.9481 | 0.8118 | 0.6853 | 0.7123 | 0.7552 | |||
| RMSE | 2.8745 | 0.2464 | 0.9742 | 0.7856 | 0.7597 | 0.8964 | ||||
| MAE | 2.63924 | 0.14269 | 0.88132 | 0.67287 | 0.64838 | 0.76202 | ||||
| SEP [%] | 40.9905 | 1.77558 | 9.73032 | 9.78586 | 8.91425 | 11.0382 | ||||
| ADD [%] | 44.3097 | 2.38234 | 14.2071 | 11.0757 | 10.6262 | 12.8206 | ||||
| 20% | R2 | 0.00032 | 0.94878 | 0.95132 | 0.83521 | 0.83661 | 0.86433 | |||
| RMSE | 1.97698 | 0.32938 | 0.42331 | 0.66956 | 0.67909 | 0.75028 | ||||
| MAE | 1.76736 | 0.1827 | 0.32411 | 0.54833 | 0.54779 | 0.60237 | ||||
| SEP [%] | 22.7605 | 2.10091 | 3.64829 | 6.88776 | 6.8504 | 7.60394 | ||||
| ADD [%] | 24.2113 | 2.61405 | 4.47543 | 7.41835 | 7.46685 | 8.56032 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çamur, H.; Al-Ani, A.M.R. Prediction of Oxidation Stability of Biodiesel Derived from Waste and Refined Vegetable Oils by Statistical Approaches. Energies 2022, 15, 407. https://doi.org/10.3390/en15020407
Çamur H, Al-Ani AMR. Prediction of Oxidation Stability of Biodiesel Derived from Waste and Refined Vegetable Oils by Statistical Approaches. Energies. 2022; 15(2):407. https://doi.org/10.3390/en15020407
Chicago/Turabian StyleÇamur, Hüseyin, and Ahmed Muayad Rashid Al-Ani. 2022. "Prediction of Oxidation Stability of Biodiesel Derived from Waste and Refined Vegetable Oils by Statistical Approaches" Energies 15, no. 2: 407. https://doi.org/10.3390/en15020407






