Design and Performance Investigation of a Compact Catalytic Reactor Integrated with Heat Recuperator
Abstract
:1. Introduction
2. Catalytic Experiment Reactor and Integrated Catalytic Reactor Design
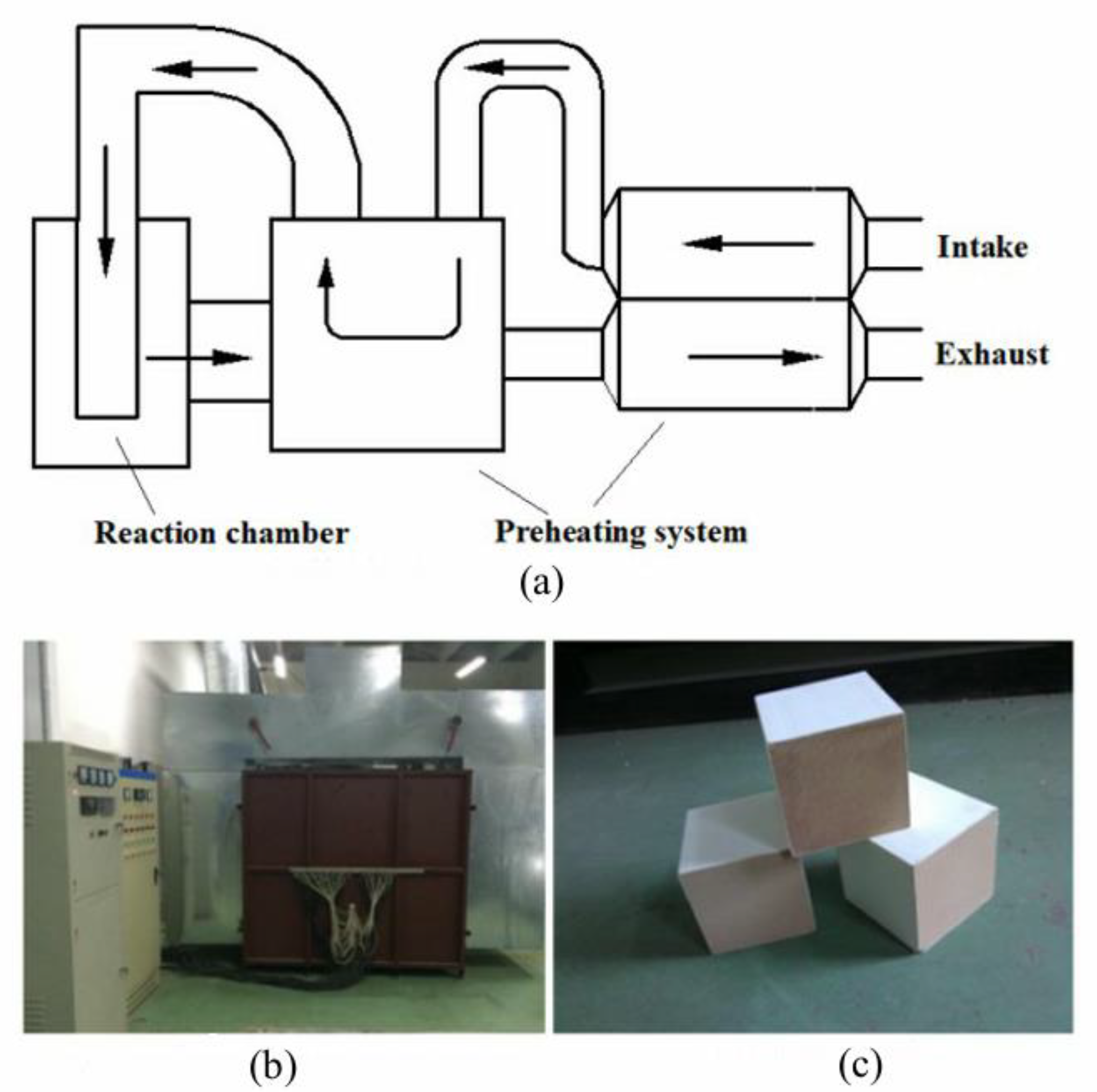
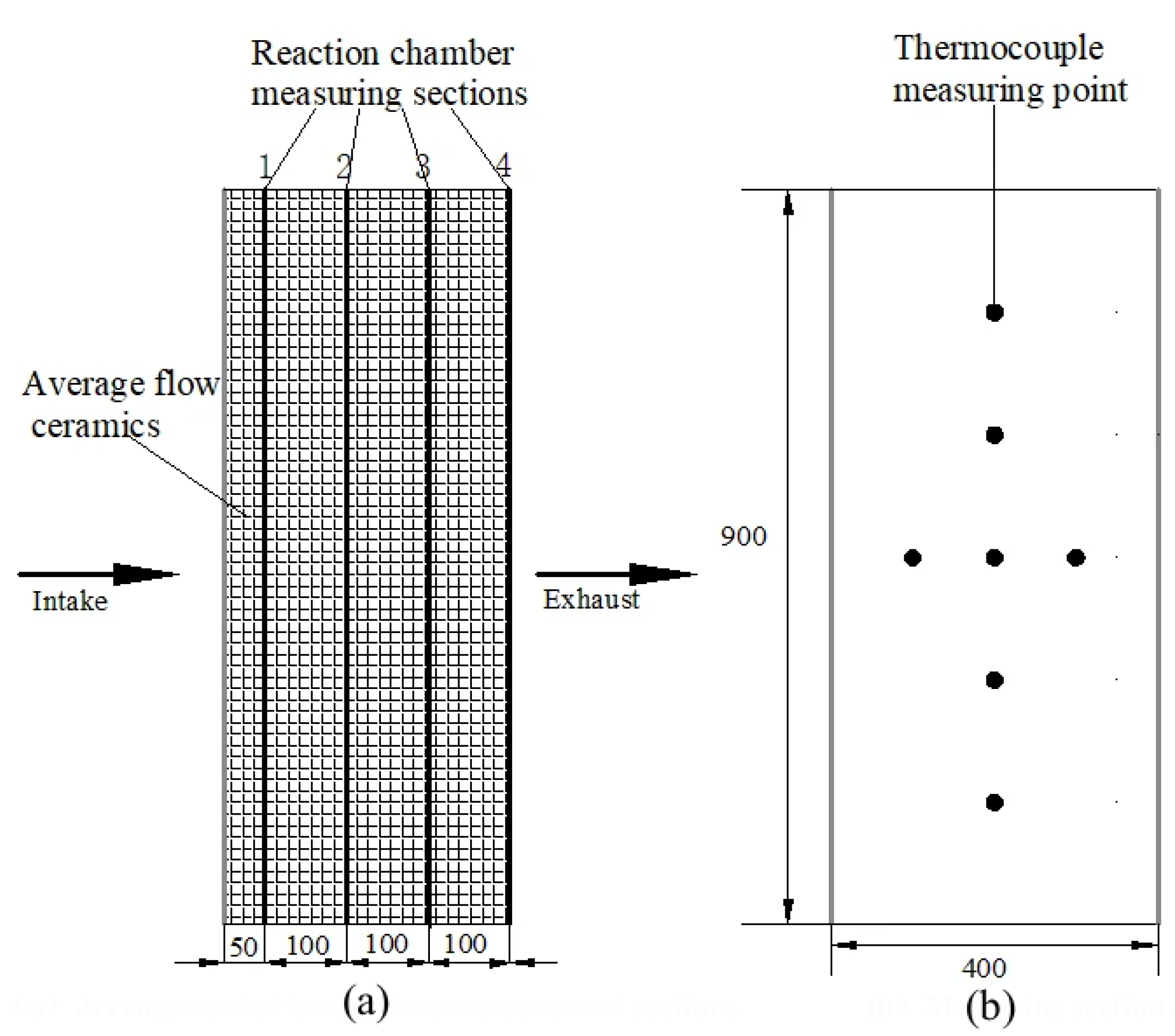
2.1. Introduction of Preheat Catalytic Reactor Test Rig
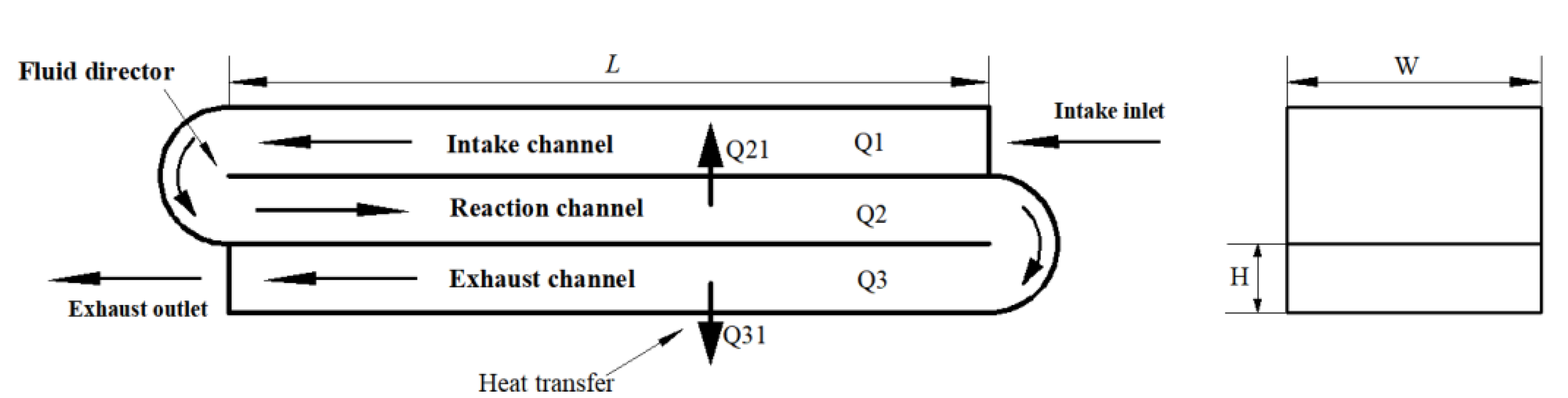
2.2. Integrated Catalytic Reactor Design
2.3. Design Process
3. Numerical Models and Simulation Approach
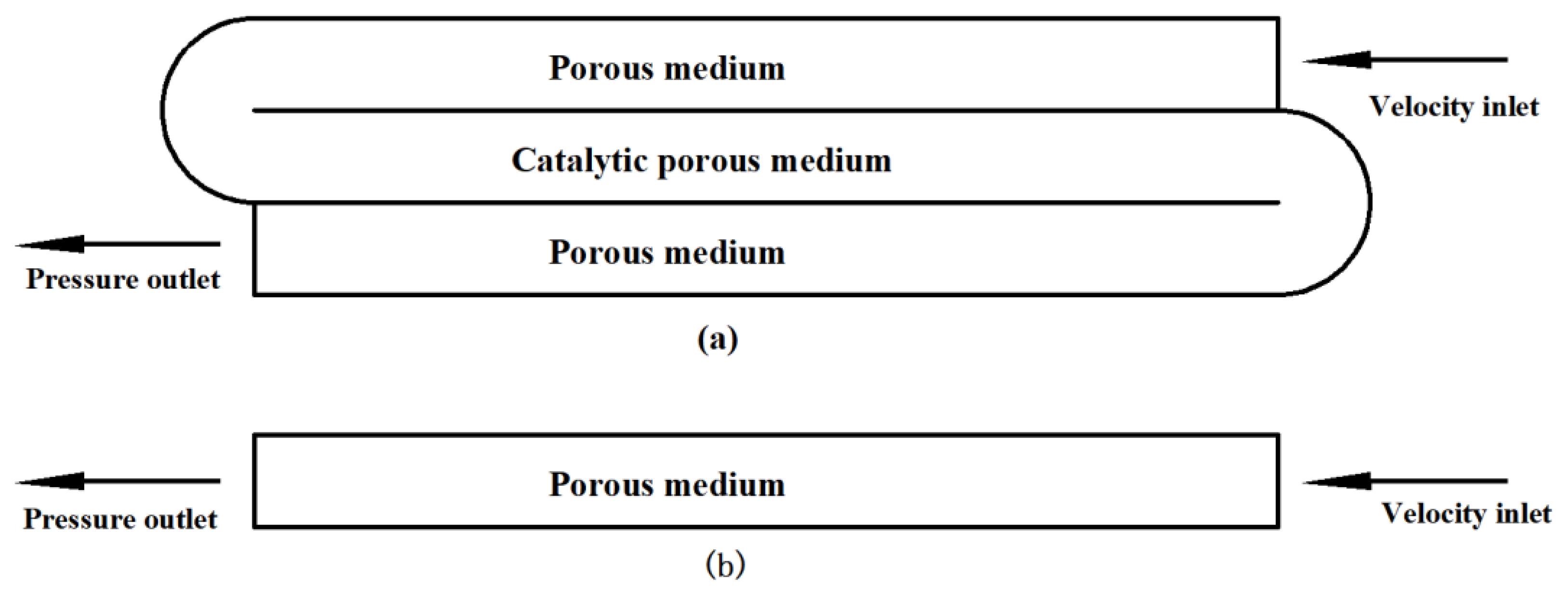
3.1. Geometric Model
3.2. Numerical Models
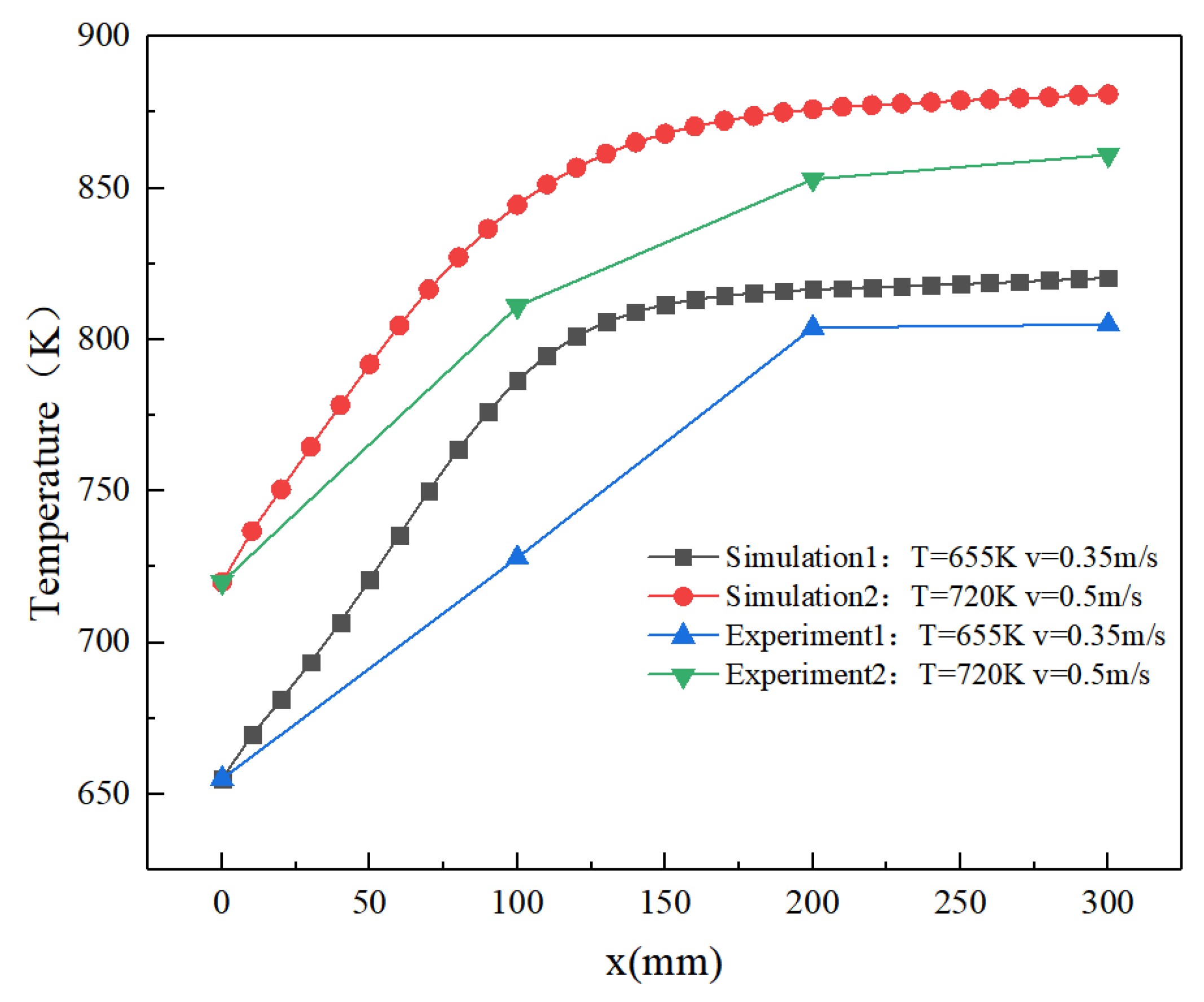
3.3. Model Validation
4. Results and Discussion
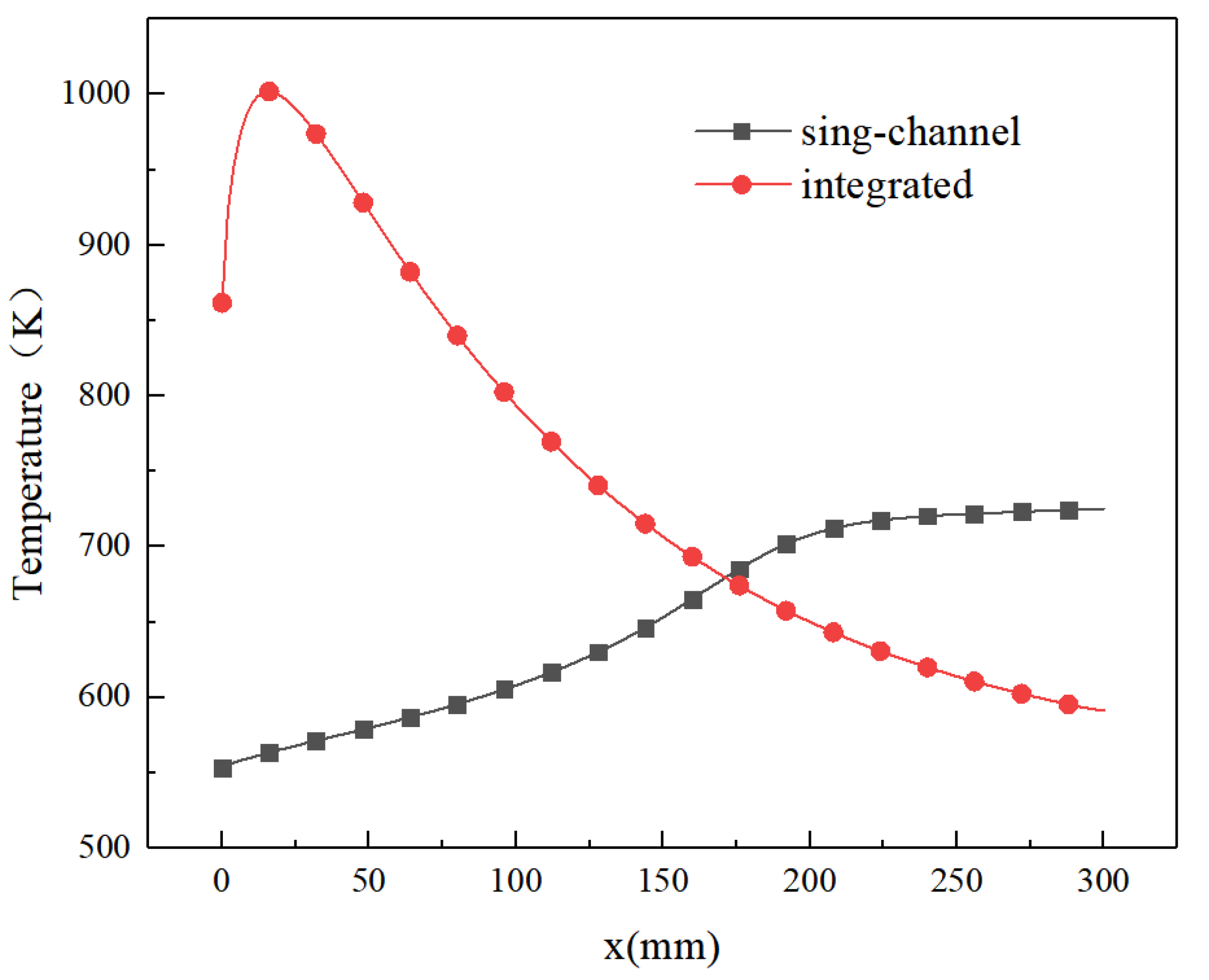
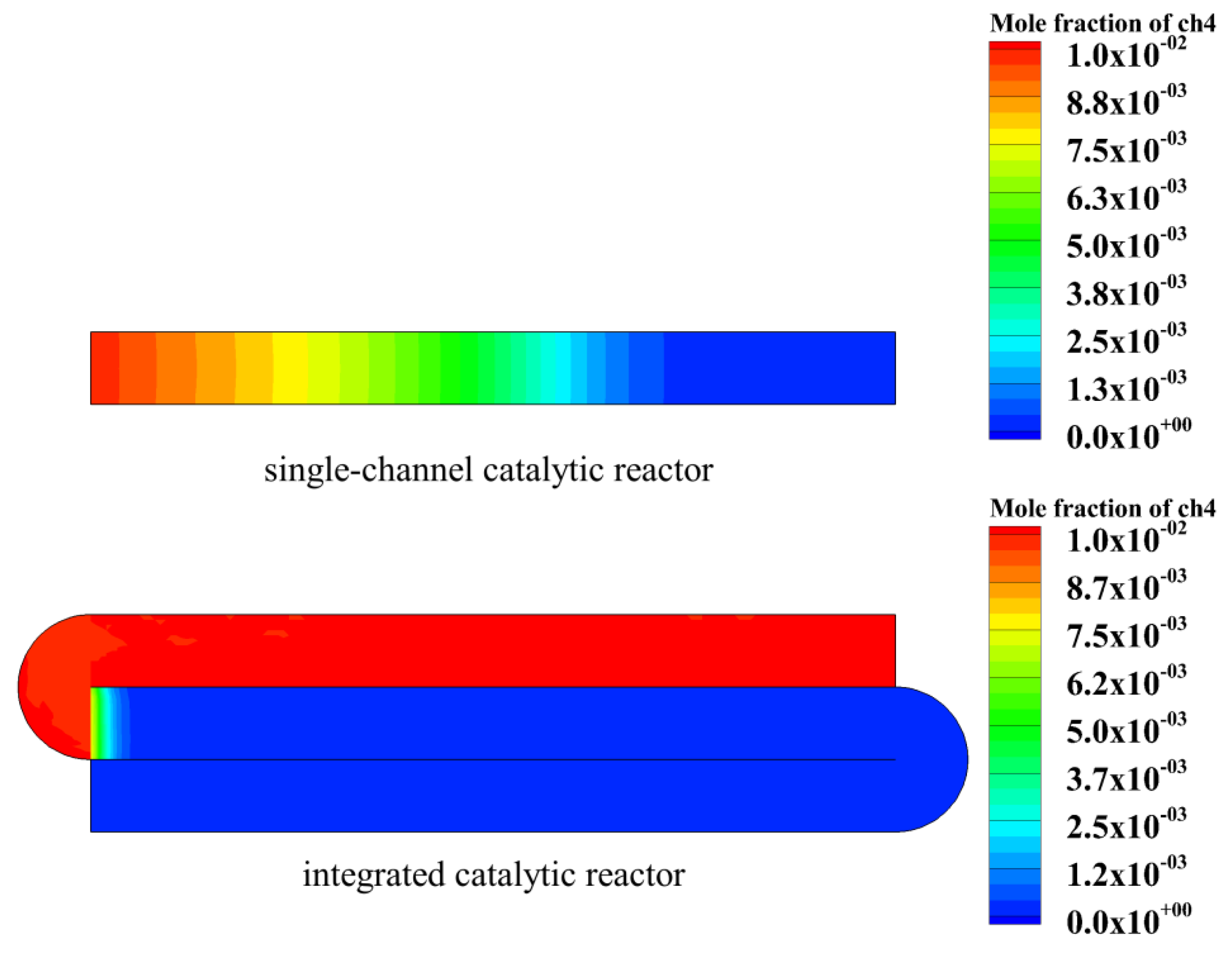
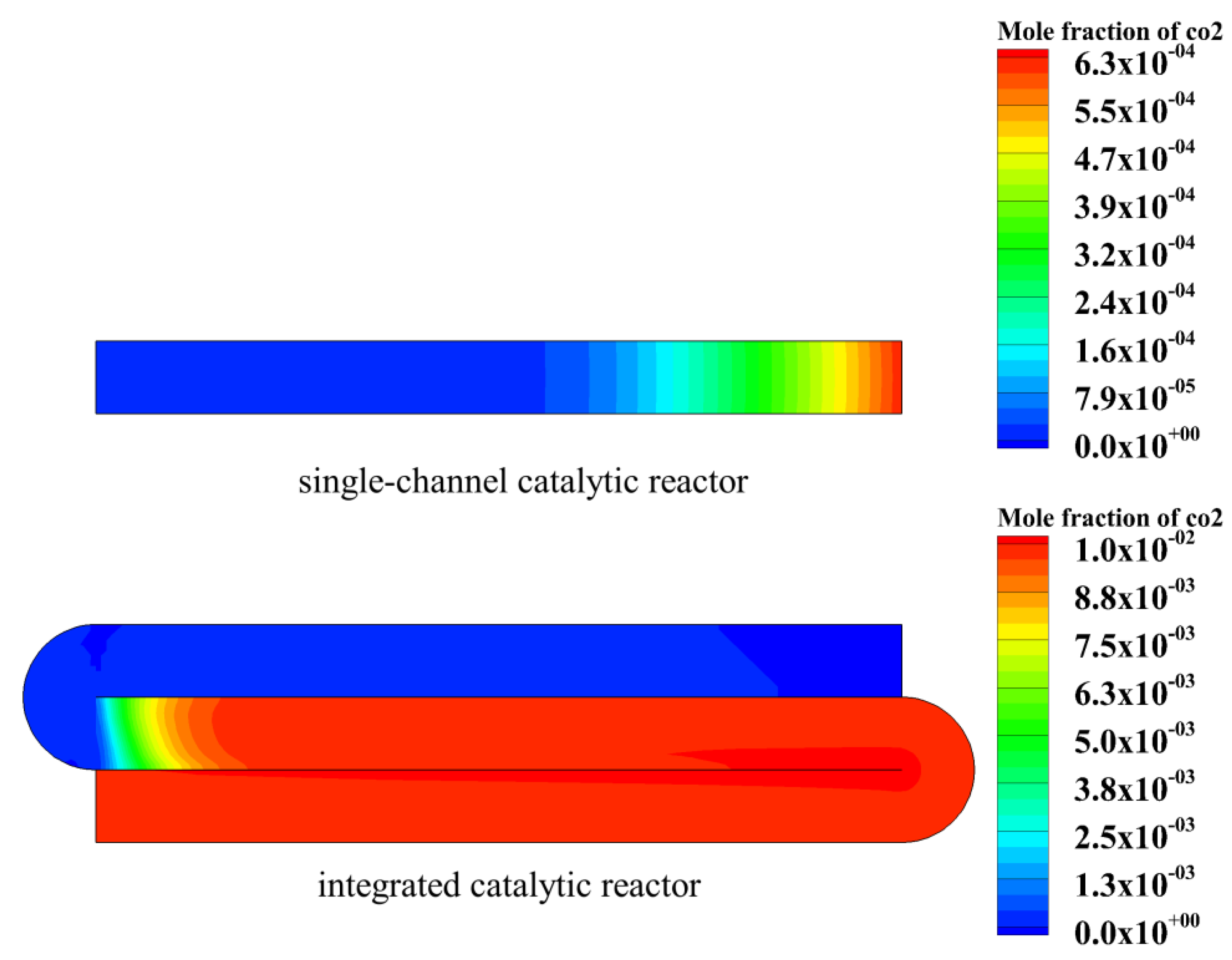
4.1. Performance Comparison between Single-Channel Catalytic Reactor and Integrated Catalytic Reactor
4.2. Effect of Inlet Parameters on Performance of the Integrated Catalytic Reactor
4.2.1. The Effect of Inlet Temperature
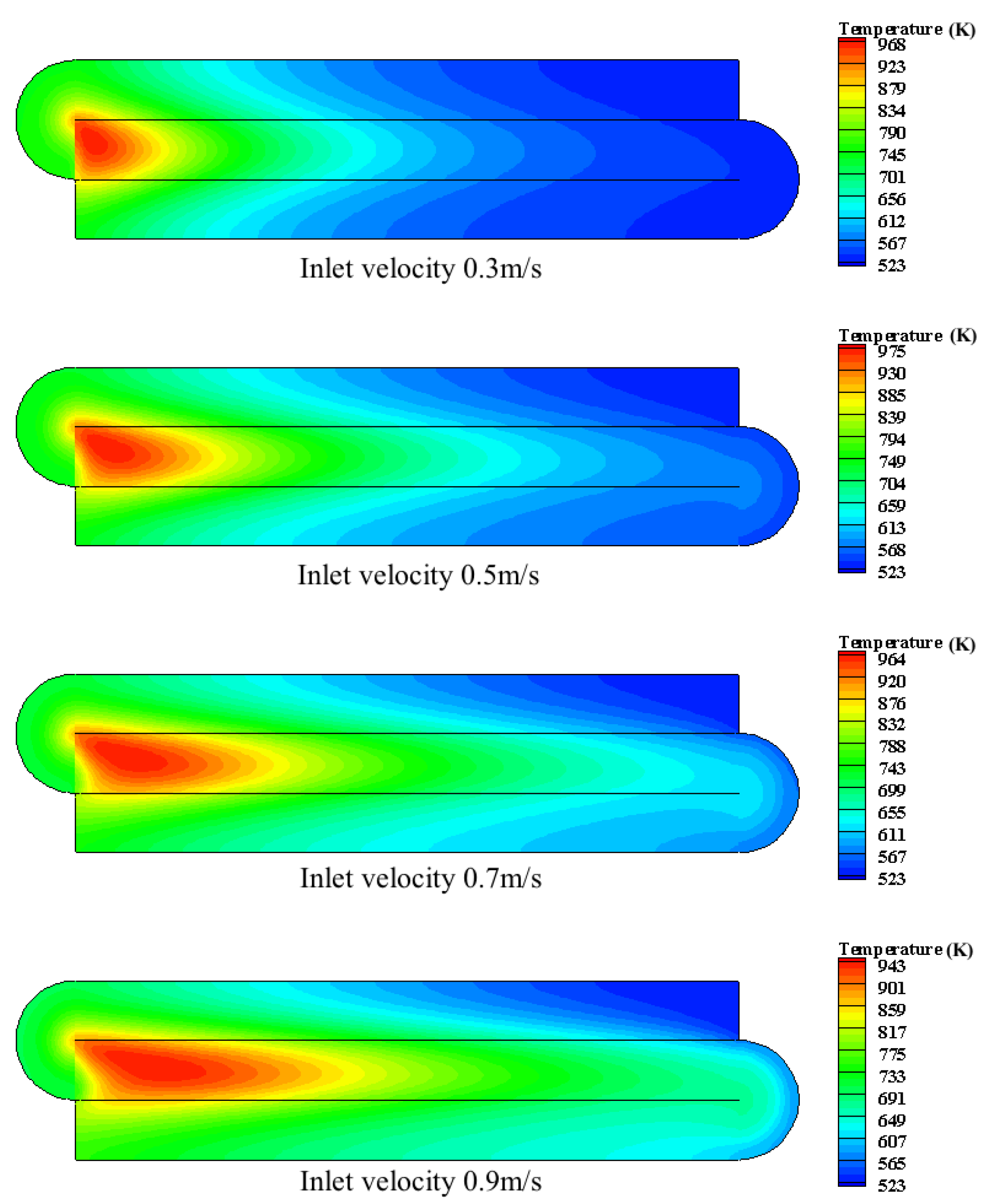
4.2.2. The Effect of Inlet Velocity
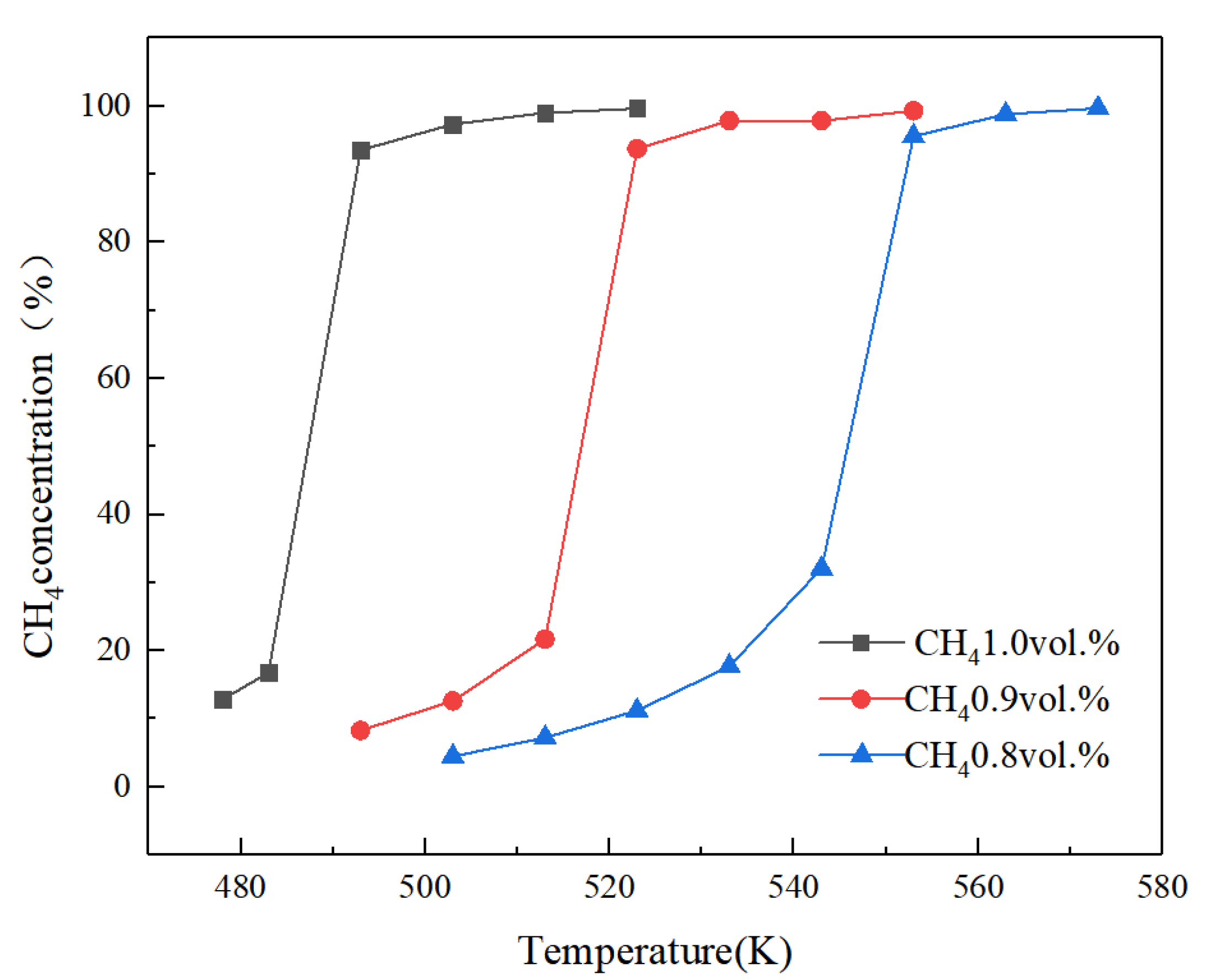
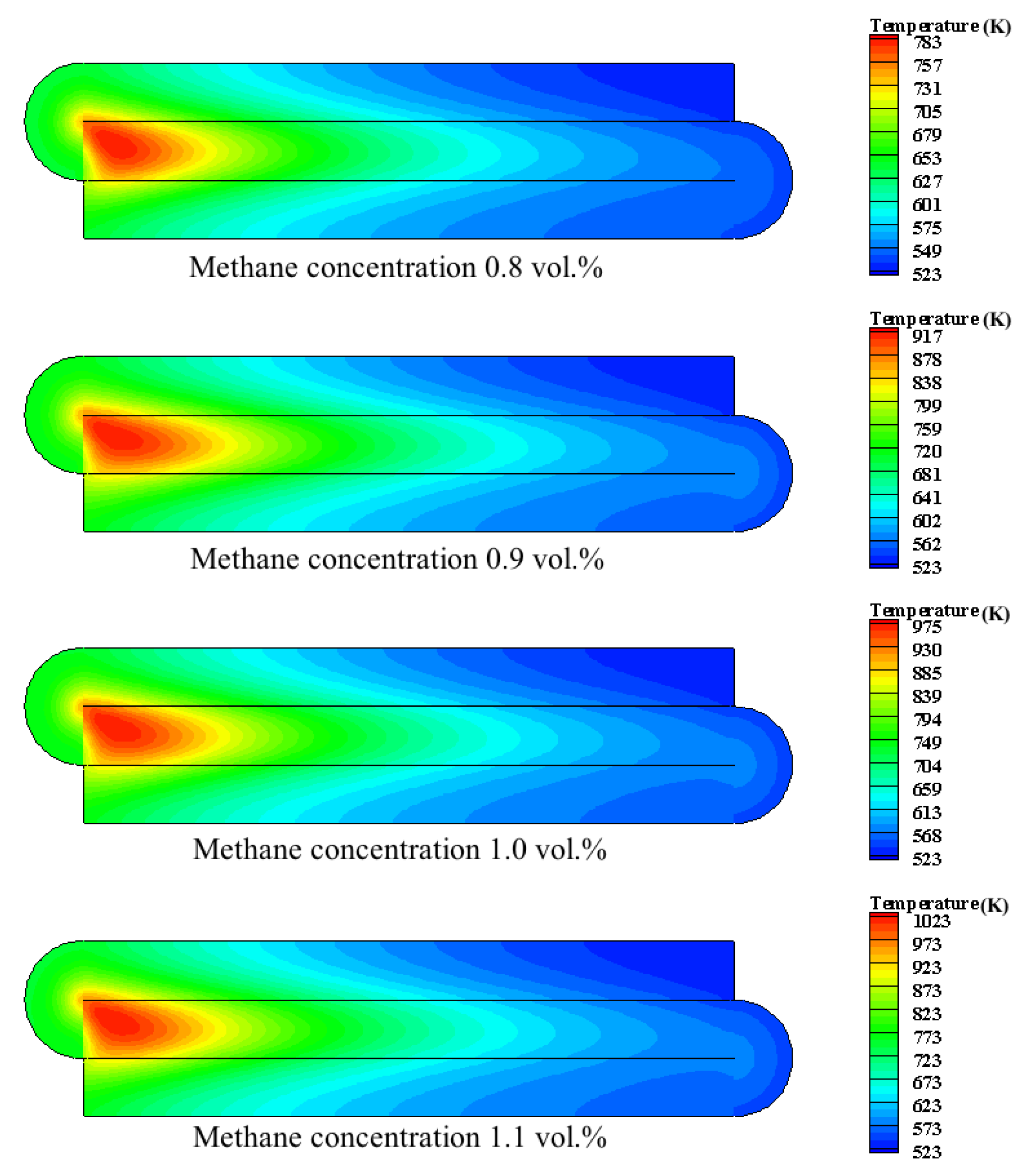
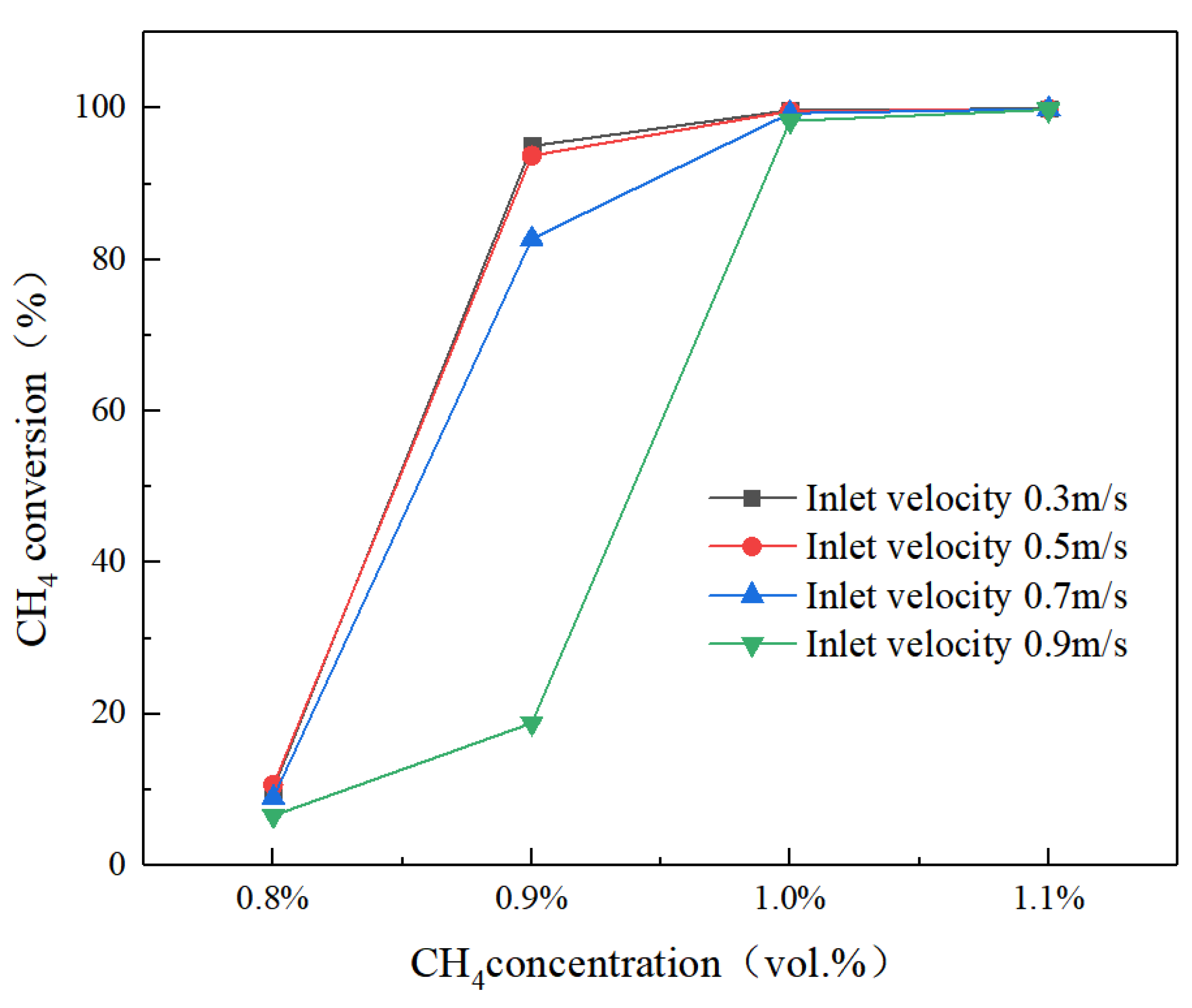
4.2.3. Effect of Feed Concentration
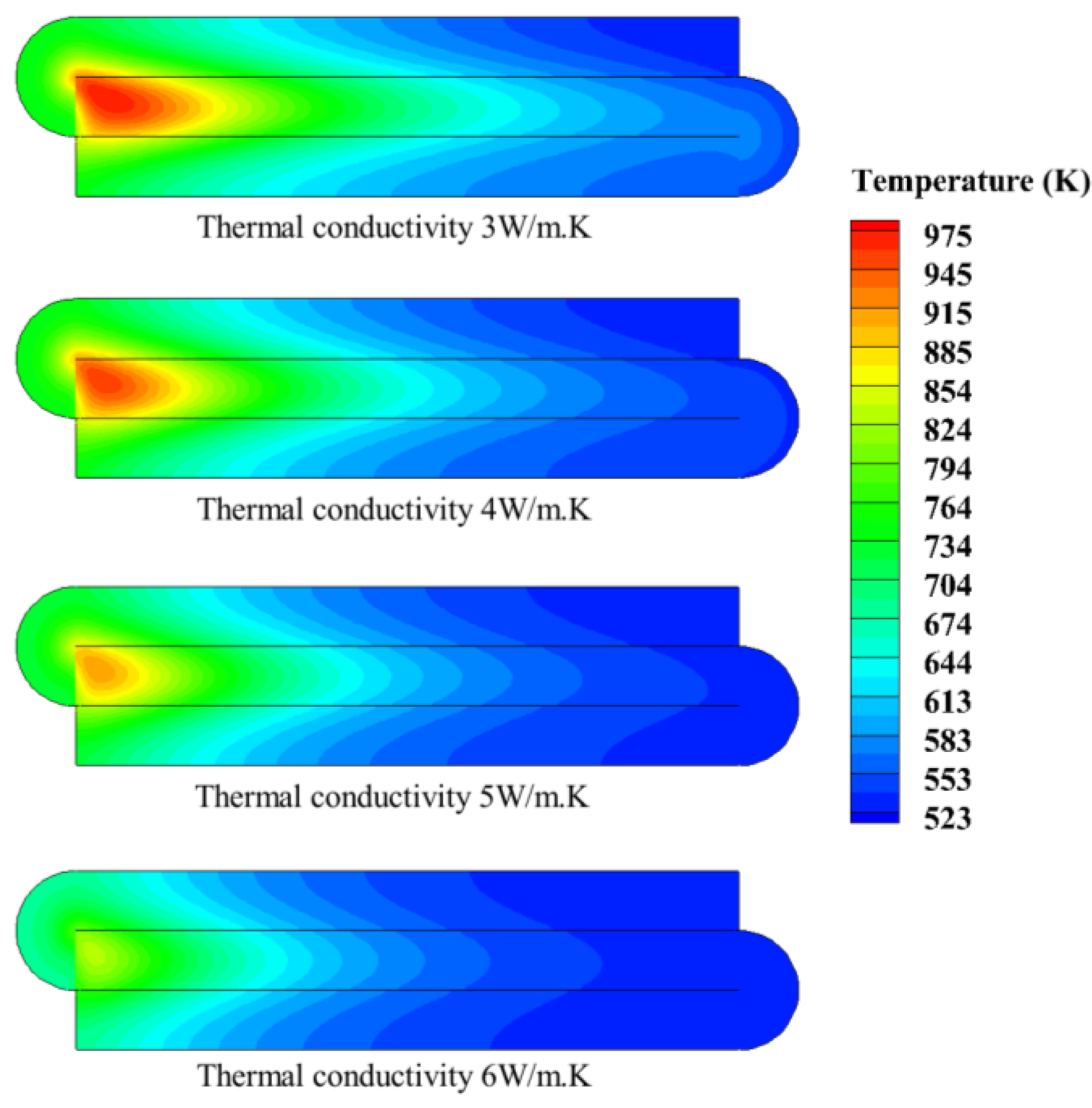
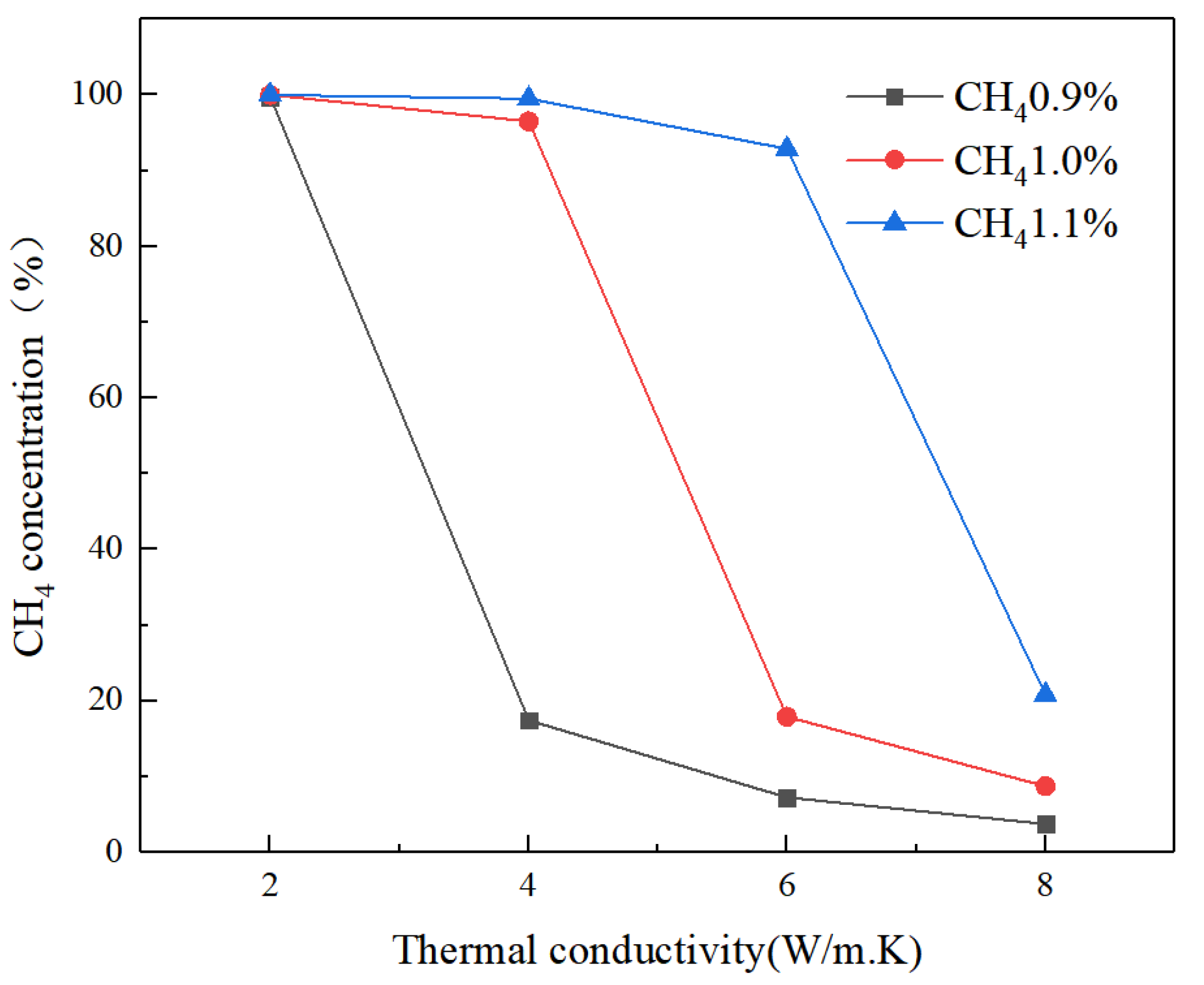
4.3. Effect of Thermal Conductivity of Porous Media
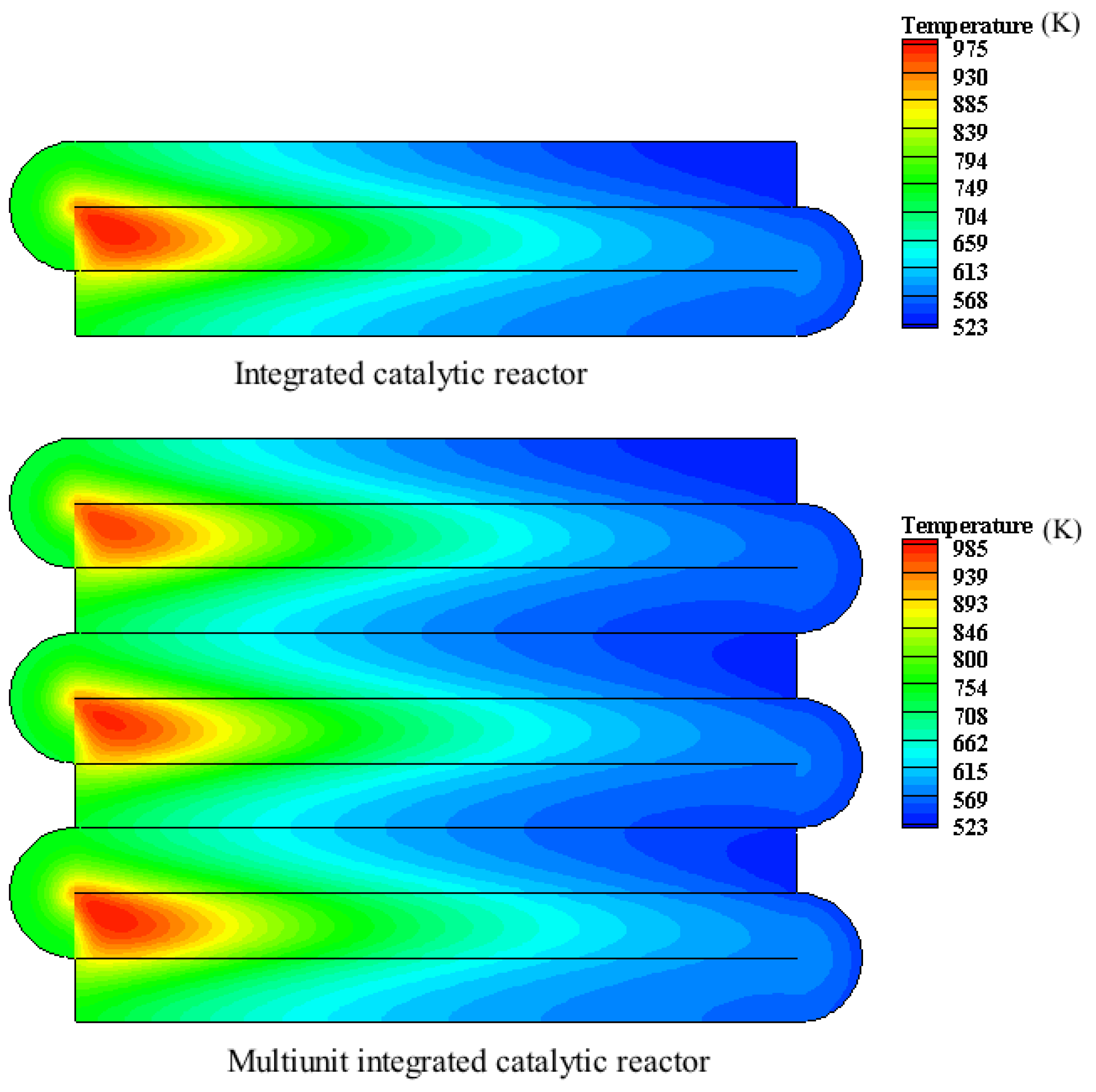
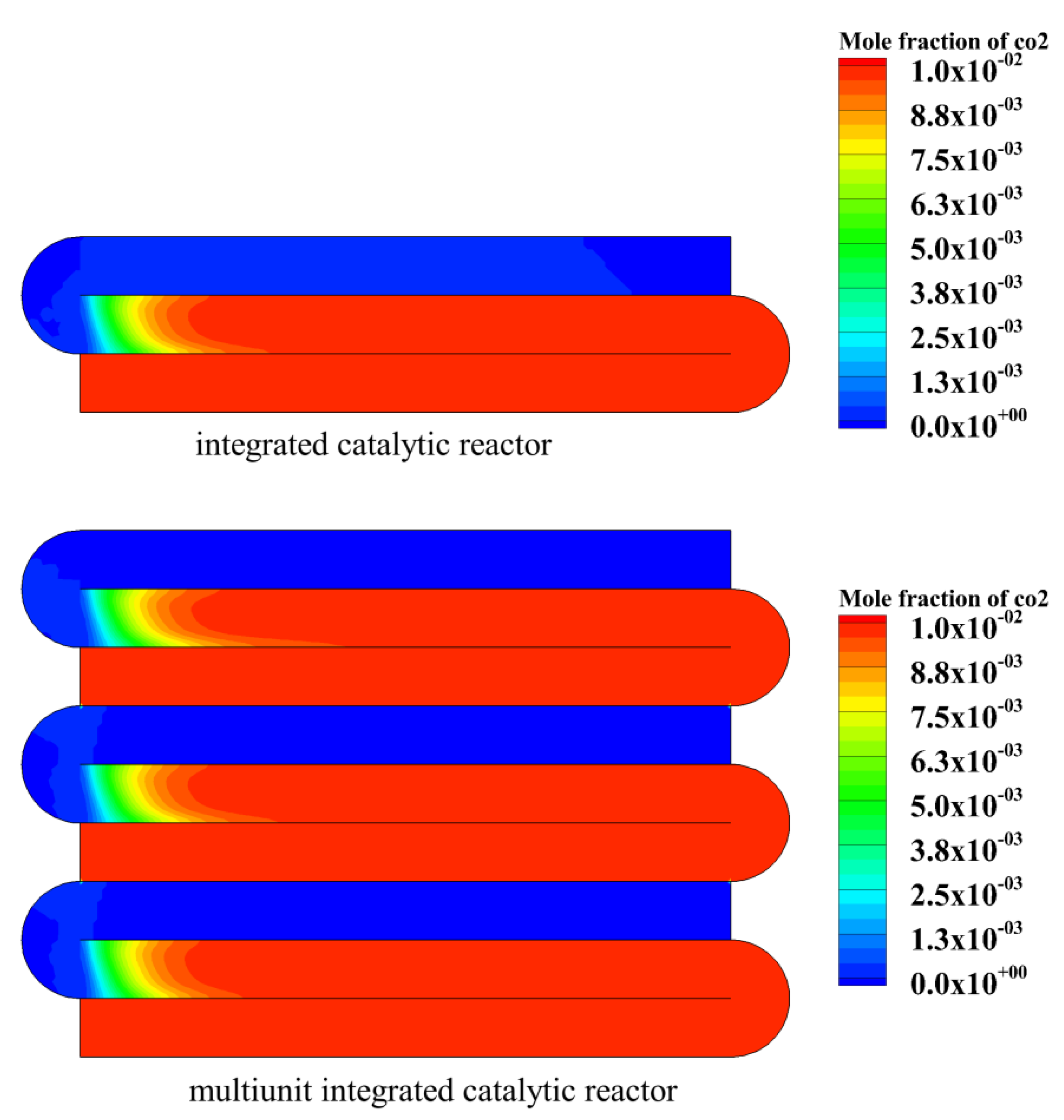
4.4. Study on the Performance of Multiunit Integrated Catalytic Reactor
5. Conclusions
- Under the same inlet conditions, the integrated catalytic reactor enables efficient conversion of methane at lower inlet temperatures compared to a single-channel catalytic reactor.
- The inlet temperature and inlet concentration of the integrated catalytic reactor have important effects on the conversion of methane. High inlet temperature and high methane concentration are more favorable for the conversion of methane; the inlet velocity affects the temperature distribution of the reactor and the preheating of the feed gas in the inlet channel.
- When the thermal conductivity of porous media is low, thermal reflux promotes the intake preheat and catalytic combustion. However, when the thermal conductivity of porous media is too high, excessive heat dissipation weakens the catalytic combustion.
- Under the same conditions, the maximum temperature of the multiunit integrated catalytic reactor is higher than that of the integrated catalytic reactor. The reason is that it further enhances the heat recovery efficiency.
Author Contributions
Funding
Conflicts of Interest
Appendix A
- (1)
- The calculation of the gas–solid heat exchange.
- (2)
- The calculation of average temperature difference.
- (3)
- The calculation of comprehensive heat transfer coefficient.
- (4)
- The calculation of size of reactor.
Appendix B
- The methane–air mixture and its products in the model are considered to be incompressible ideal gases.
- The porosity of the porous media is constant.
- The catalyst is evenly coated and the reaction on the surface of the catalyst is uniform.
- The dispersion effect of gas in the porous media is ignored.
- The heat radiation in the porous media is ignored.
- The effect of gas gravity is ignored.
References
- Gosiewski, K.; Pawlaczyk, A. Catalytic or thermal reversed flow combustion of coal mine ventilation air methane: What is better choice and when? Chem. Eng. J. 2014, 238, 78–85. [Google Scholar] [CrossRef]
- Karakurt, I.; Aydin, G.; Aydiner, K. Mine ventilation air methane as a sustainable energy source. Renew. Sustain. Energy Rev. 2010, 15, 1042–1049. [Google Scholar] [CrossRef]
- Pengfei, G. Development and present situation of utilization technology of Ventilation air methane (VAM) enrichment and utilization. IOP Conf. Ser. Earth Environ. Sci. 2019, 242, 022060. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Doroodchi, E.; Moghtaderi, B. Utilization of ventilation air methane as an oxidizing agent in chemical looping combustion. Energy Convers. Manag. 2014, 85, 839–847. [Google Scholar] [CrossRef]
- Baiquan, L.; Huaming, D.; Chaoqun, W.; Qingzhao, L.; Ke, W.; Yuanzhen, Z. Combustion characteristics of low concentration coal mine methane in divergent porous media burner. Int. J. Min. Sci. Technol. 2014, 24, 671–676. [Google Scholar]
- Hayes, R.E.; Kolaczkowski, S.T. Introduction to Catalytic Combustion (1997); Routledge: London, UK, 2021. [Google Scholar]
- Irandoust, S.; Andersson, B. Monolithic Catalysts for Nonautomobile Applications. Catal. Rev. 1988, 30, 341–392. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, J.; Li, C.; Ji, S. Conceptual design and CFD simulation of a novel metal-based monolith reactor with enhanced mass transfer. Catal. Today 2005, 105, 401–406. [Google Scholar] [CrossRef]
- Mei, H.; Li, C.; Liu, H. Simulation of heat transfer and hydrodynamics for metal structured packed bed. Catal. Today 2005, 105, 689–696. [Google Scholar] [CrossRef]
- Brooks, K.P.; Hu, J.; Zhu, H.; Kee, R.J. Methanation of carbon dioxide by hydrogen reduction using the Sabatier process in microchannel reactors. Chem. Eng. Sci. 2006, 62, 1161–1170. [Google Scholar] [CrossRef]
- Liu, Z.; Chu, B.; Zhai, X.; Jin, Y.; Cheng, Y. Total methanation of syngas to synthetic natural gas over Ni catalyst in a micro-channel reactor. Fuel 2012, 95, 599–605. [Google Scholar] [CrossRef]
- Min, D.K.; Shin, H.D. Laminar premixed flame stabilized inside a honeycomb ceramic. Int. J. Heat Mass Transf. 1991, 34, 341–356. [Google Scholar]
- Hashemi, S.M.; Hashemi, S.A. Flame stability analysis of the premixed methane-air combustion in a two-layer porous media burner by numerical simulation. Fuel 2017, 202, 56–65. [Google Scholar] [CrossRef]
- Song, F.; Wen, Z.; Dong, Z.; Wang, E.; Liu, X. Ultra-low calorific gas combustion in a gradually-varied porous burner with annular heat recirculation. Energy 2017, 119, 497–503. [Google Scholar] [CrossRef]
- Chen, J.; Yan, L.; Song, W.; Xu, D. Effect of heat recirculation on the combustion stability of methane-air mixtures in catalytic micro-combustors. Appl. Therm. Eng. 2017, 115, 702–714. [Google Scholar] [CrossRef]
- Zheng, B.; Liu, Y.; Sun, P.; Sun, J.; Meng, J. Oxidation of lean methane in a two-chamber preheat catalytic reactor. Int. J. Hydrog. Energy 2017, 42, 18643–18648. [Google Scholar] [CrossRef]
- Sun, J.; Liu, Y.; Sun, P.; Shi, Y. Oxidation characteristics of two-chamber lean methane preheat catalytic reactor. J. China Coal Soc. 2017, 42, 172–177. [Google Scholar]
- Moallemi, F.; Batley, G.; Dupont, V.; Foster, T.J.; Pourkashanian, M.; Williams, A. Chemical modelling and measurements of the catalytic combustion of CH4 /air mixtures on platinum and palladium catalysts. Catal. Today 1999, 47, 235–244. [Google Scholar] [CrossRef]
- Li, Q.; Bai, F.; Yang, B.; Wang, Y.; Xu, L.; Chang, Z.; Wang, Z.; Hefni, B.E.; Yang, Z.; Kubo, S.; et al. Dynamic simulations of a honeycomb ceramic thermal energy storage in a solar thermal power plant using air as the heat transfer fluid. Appl. Therm. Eng. 2018, 129, 3642–3649. [Google Scholar] [CrossRef]
- Patankar, S.V.; Spalding, D.B. A calculation procedure for heat, mass and momentum transfer in three-dimensional parabolic flows. Int. J. Heat Mass Transf. 1972, 15, 787–1806. [Google Scholar] [CrossRef]
- Deutschmann, O.; Behrendt, F.; Warnatz, J. Modelling and simulation of heterogeneous oxidation of methane on a platinum foil. Catal. Today 1994, 21, 461–470. [Google Scholar] [CrossRef]



| Item | Unit | Range/Value |
|---|---|---|
| Length of intake, reaction and exhaust channels | mm | 300 |
| Height of intake, reaction and exhaust channels | mm | 27 |
| Width of intake, reaction and exhaust channels | mm | 100 |
| The flow rate of the mixed gas | m3/h | 10 |
| Inlet temperature | K | 473 |
| Porosity | / | 0.6 |
| The specific surface area | m2/m3 | 2000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Mao, M.; Gao, M.; Liu, Y.; Shi, J.; Li, J. Design and Performance Investigation of a Compact Catalytic Reactor Integrated with Heat Recuperator. Energies 2022, 15, 447. https://doi.org/10.3390/en15020447
Chen Q, Mao M, Gao M, Liu Y, Shi J, Li J. Design and Performance Investigation of a Compact Catalytic Reactor Integrated with Heat Recuperator. Energies. 2022; 15(2):447. https://doi.org/10.3390/en15020447
Chicago/Turabian StyleChen, Qiang, Mingming Mao, Min Gao, Yongqi Liu, Junrui Shi, and Jia Li. 2022. "Design and Performance Investigation of a Compact Catalytic Reactor Integrated with Heat Recuperator" Energies 15, no. 2: 447. https://doi.org/10.3390/en15020447
APA StyleChen, Q., Mao, M., Gao, M., Liu, Y., Shi, J., & Li, J. (2022). Design and Performance Investigation of a Compact Catalytic Reactor Integrated with Heat Recuperator. Energies, 15(2), 447. https://doi.org/10.3390/en15020447





