CO2 Capture from IGCC by Low-Temperature Synthesis Gas Separation
Abstract
:1. Introduction
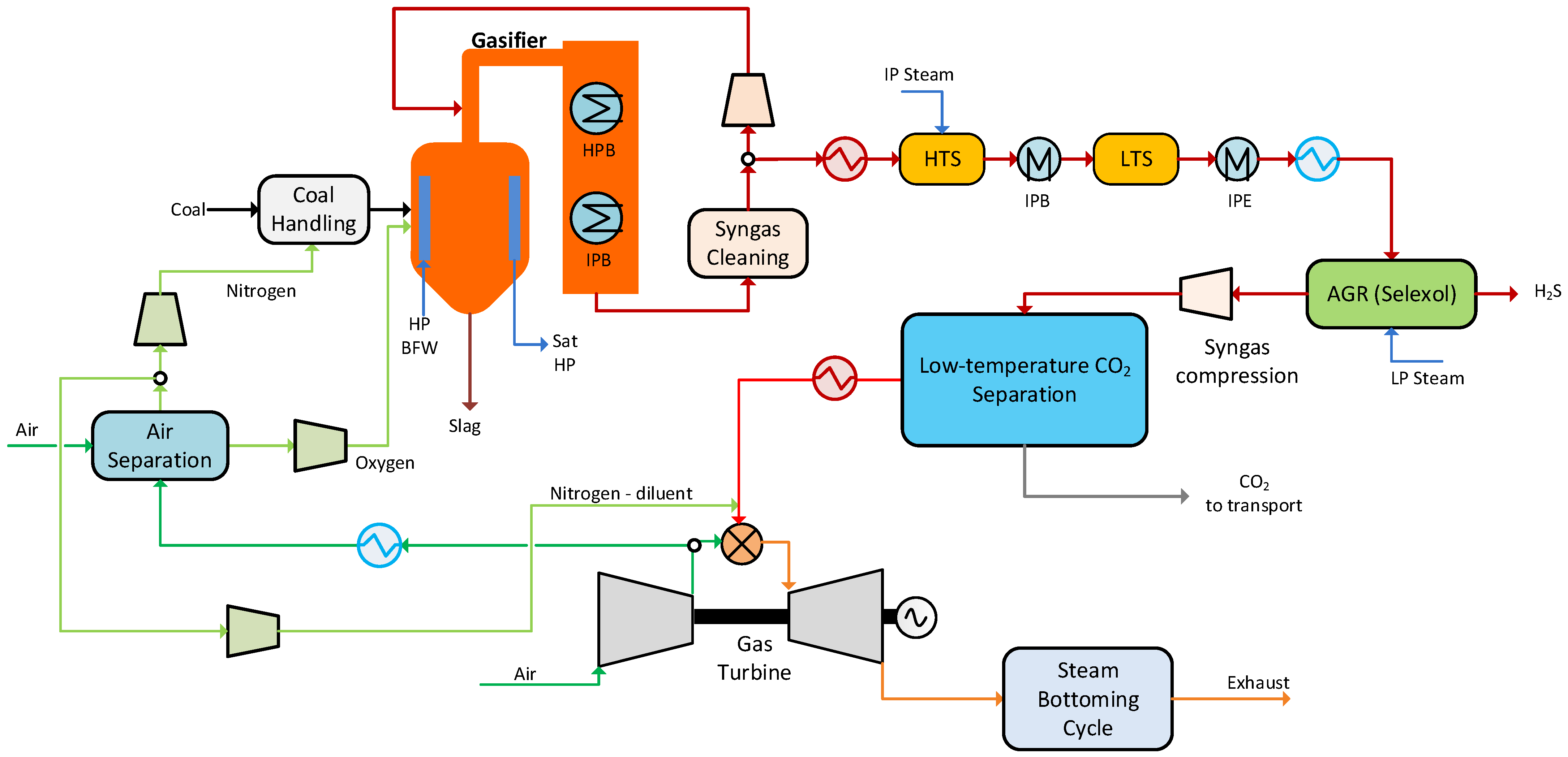
2. Capture Source: Integrated Gasification Combined Cycle
3. Thermodynamic Models, Methodology and Simulation Tools
3.1. Synthesis Gas Data
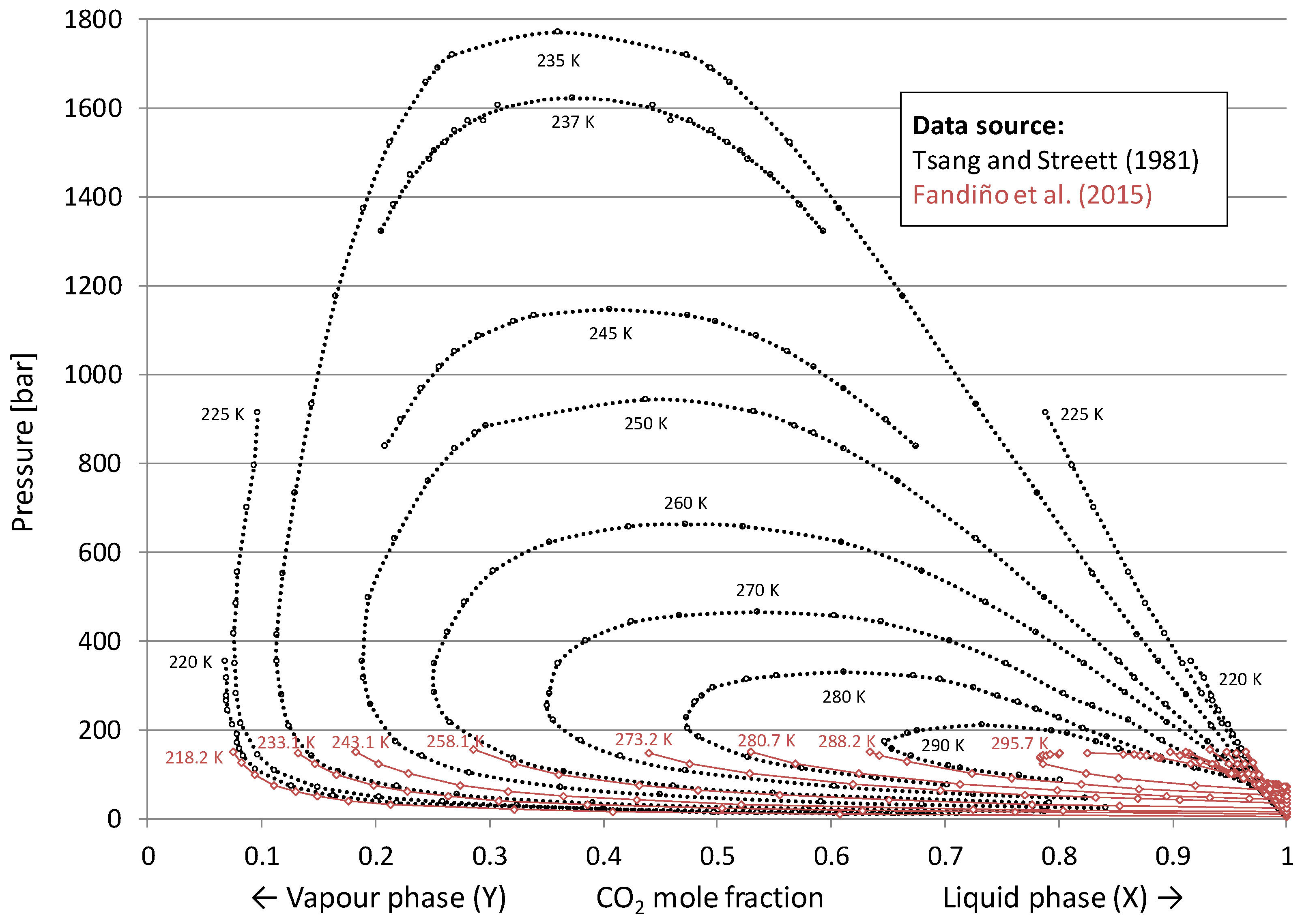
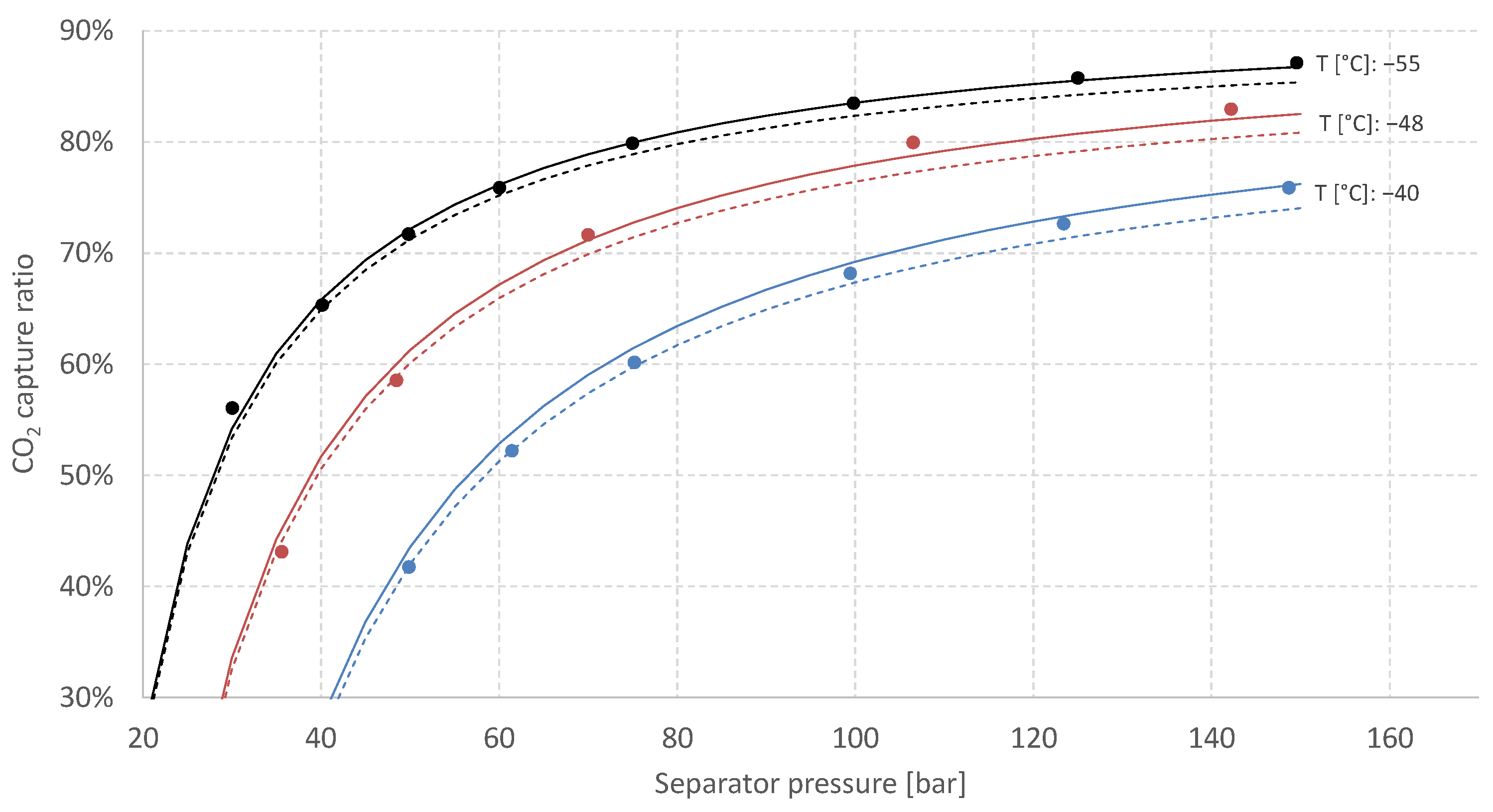
3.2. Vapour–Liquid Equilibrium for the H2/CO2 System
- Solid lines are estimates for binary mixtures (38 mol% CO2, 62 mol% H2) generated by the process simulation software Aspen HYSYS using the Peng–Robinson equation of state.
- Dashed lines are estimates for the actual five-component syngas mixture (see Table 1) generated by Aspen HYSYS using Peng–Robinson equation of state. These multi-component estimates indicate that while keeping the CO2 fraction constant, the inclusion of the additional diluents in Table 1 (CO, Ar and predominantly N2) leads to a reduction in estimated CCR.
3.3. Simulation Software
3.4. CO2 Freezing Point Estimation and Implications on Operating Temperature
4. Low-Temperature Capture Processes
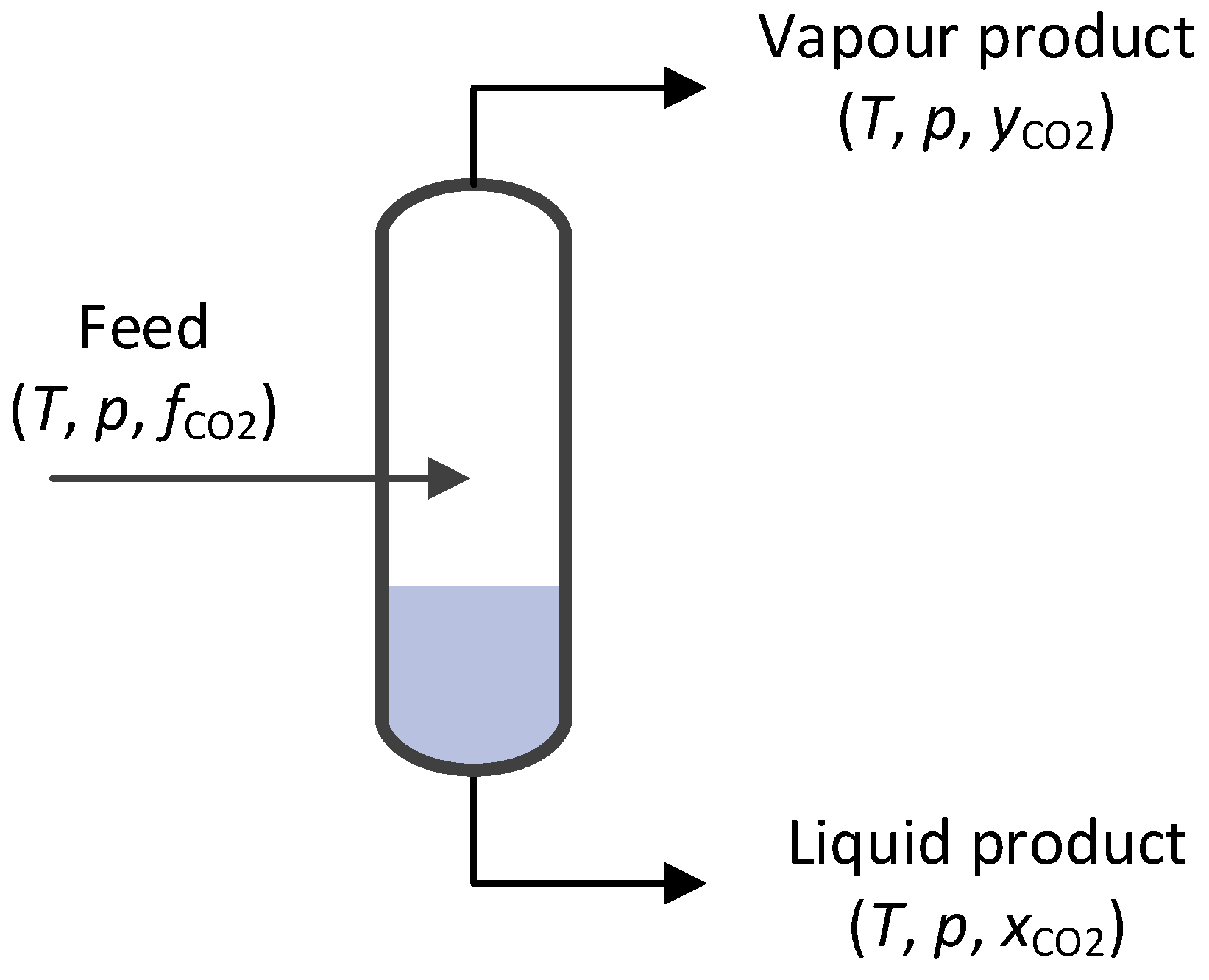
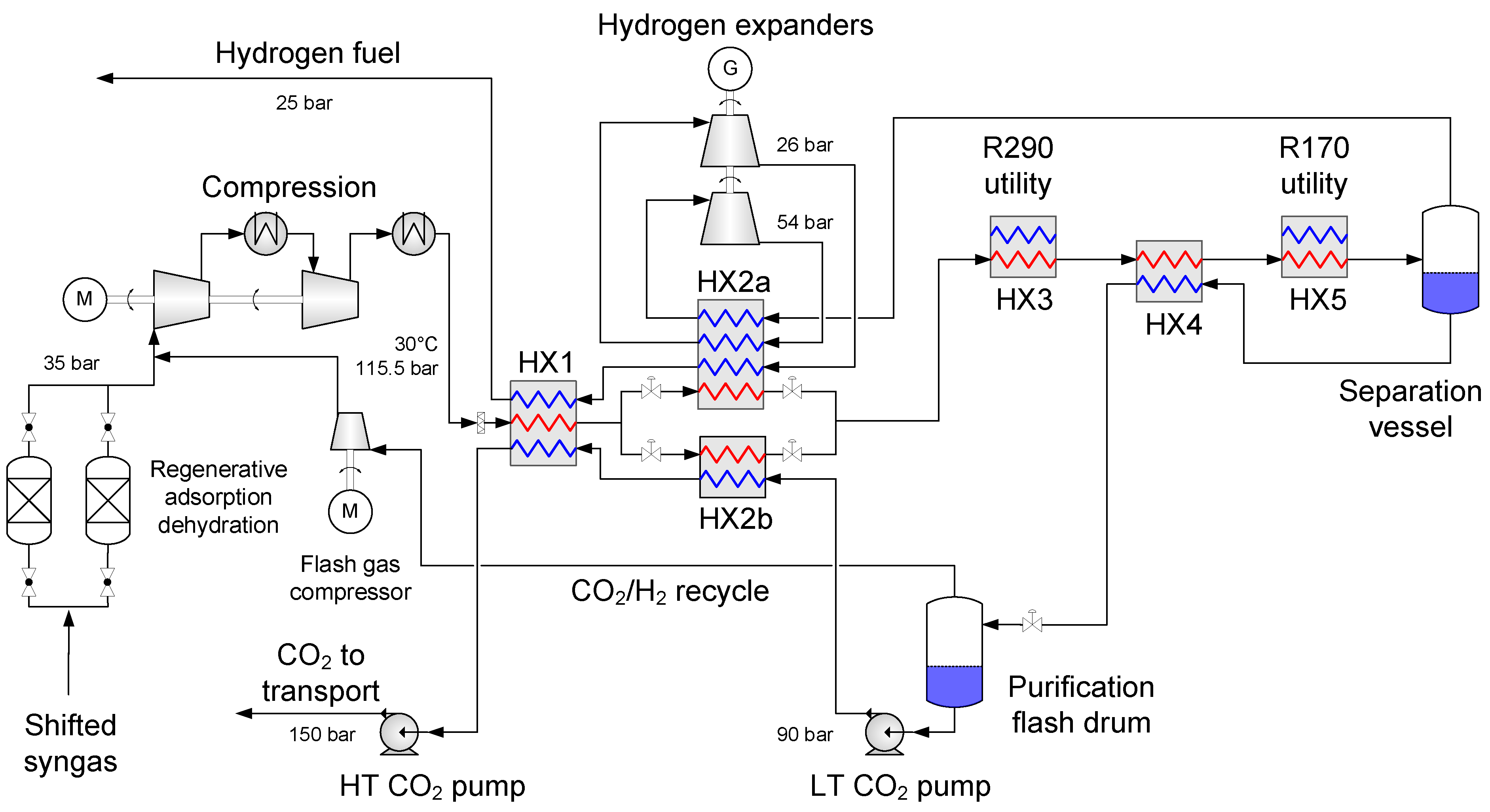
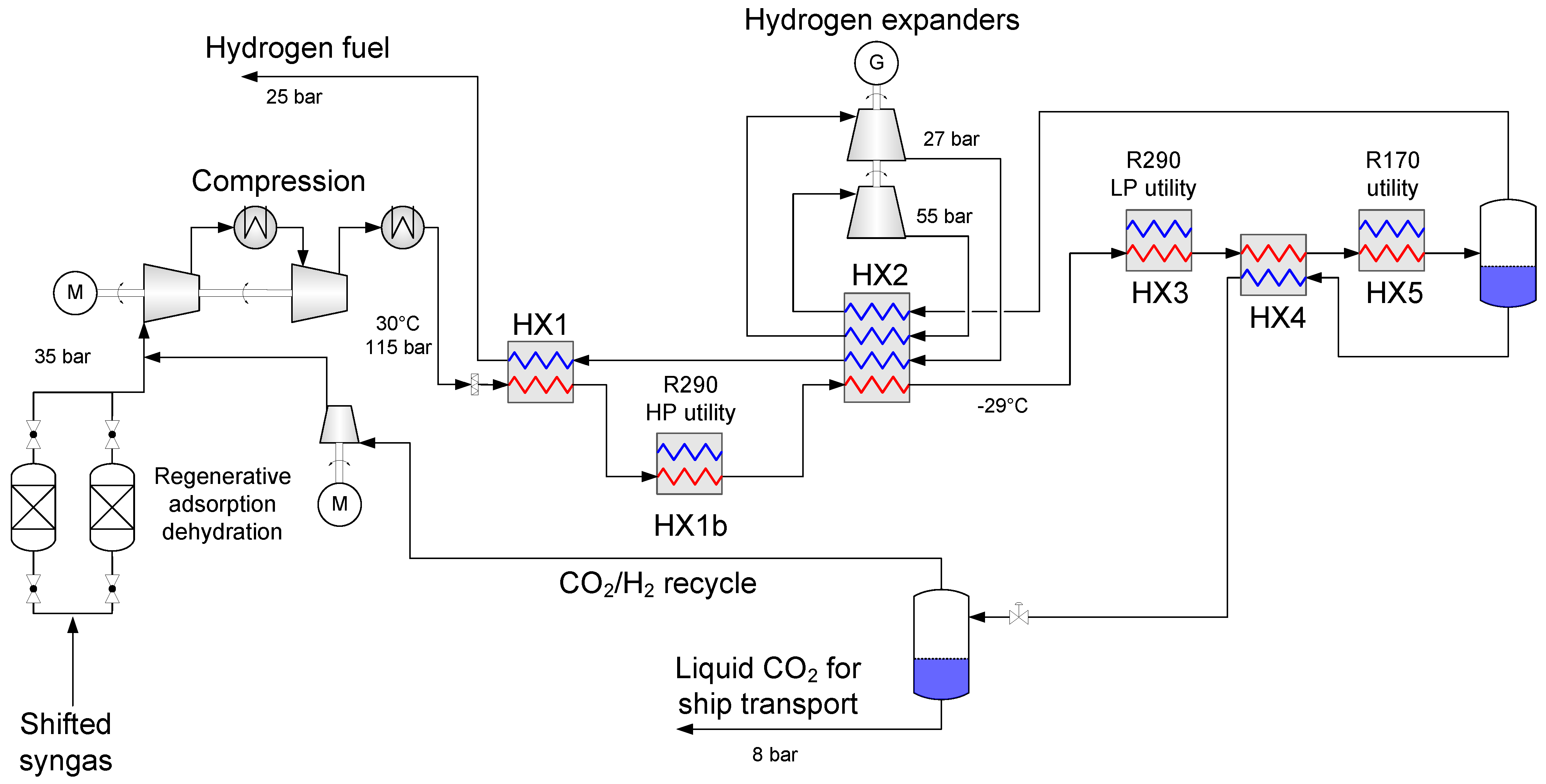
4.1. Baseline Low-Temperature CO2 Separation Process
- Enabling a temperature drop to about −53 °C so that the hydrogen-rich fuel stream can be utilized as much as possible to provide cold duty to heat exchanger HX2a.
- Generating recoverable power to be utilised in the process or increase the net power output.
4.2. Process Component Design and Performance
4.2.1. Synthesis Gas Dehydration
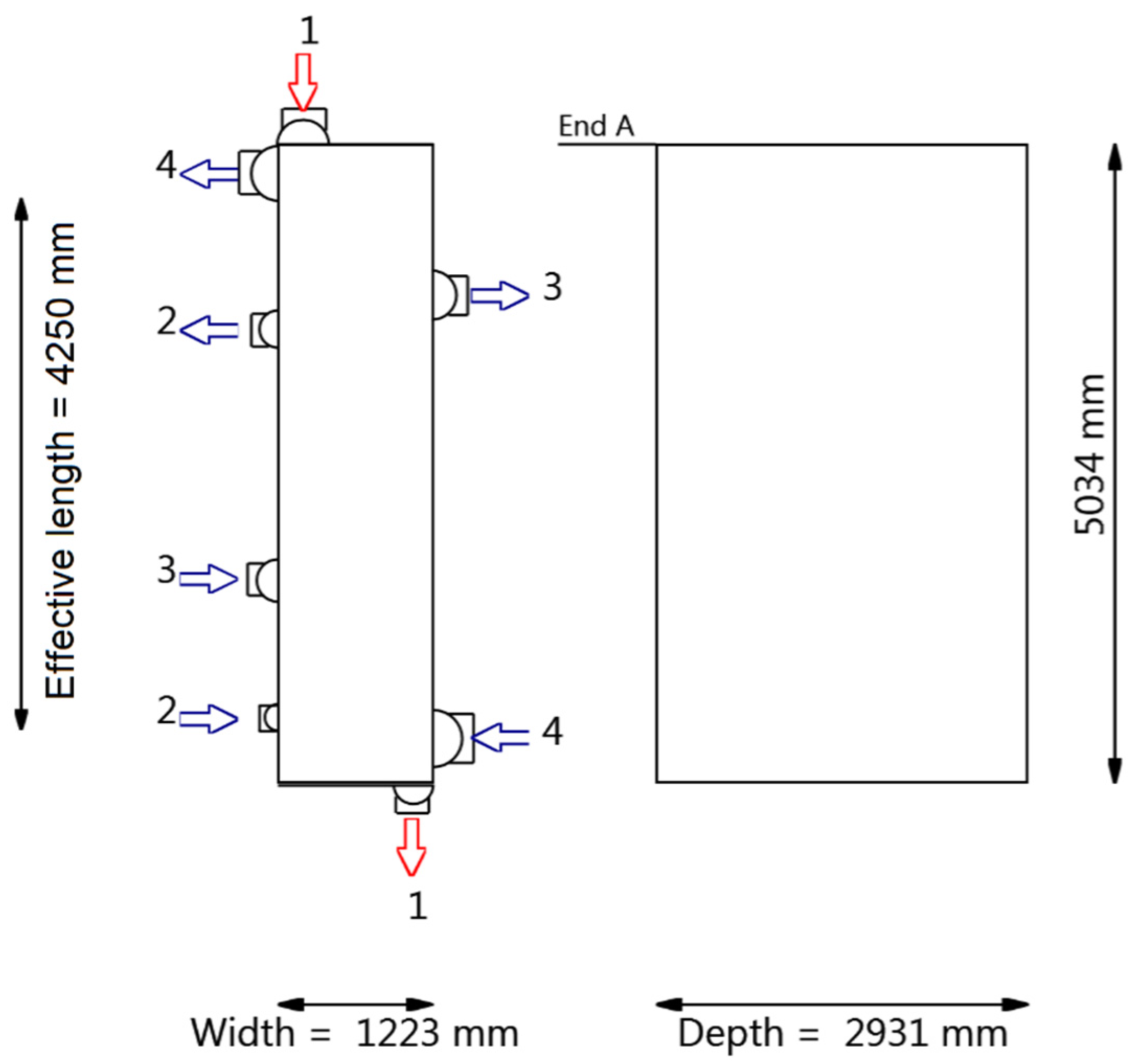
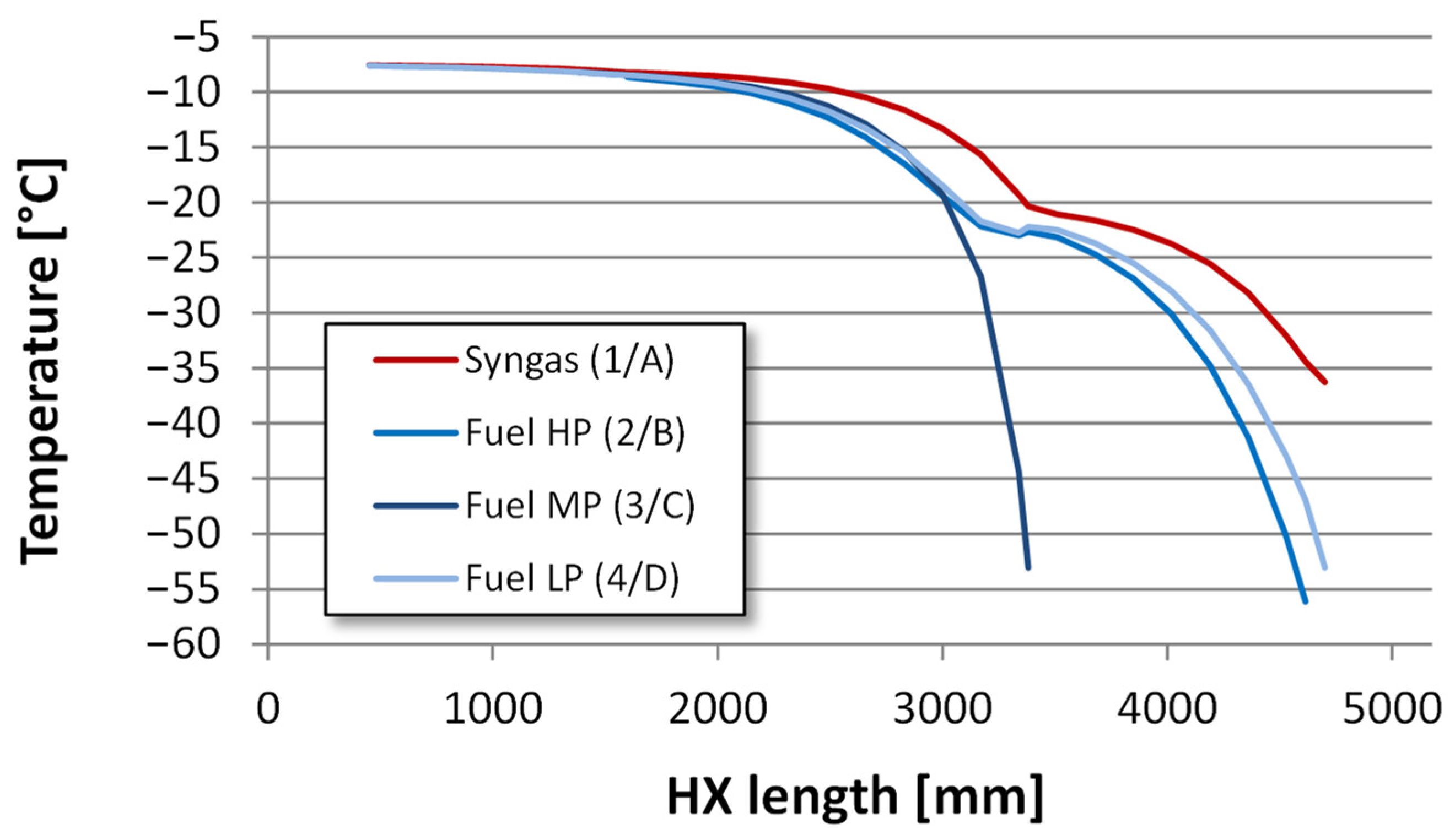
4.2.2. Heat Exchanger Design
4.2.3. Synthesis Gas Compressors
4.2.4. Fuel Expanders
4.2.5. Auxiliary Refrigeration
4.3. Alternative Process Layout: High-Temperature Expanders for Improved Operability
4.4. Alternative Process Layout: CO2 Liquefaction for Ship Transport
5. Results, Discussion and Sensitivity Analysis
5.1. Baseline Energy Results
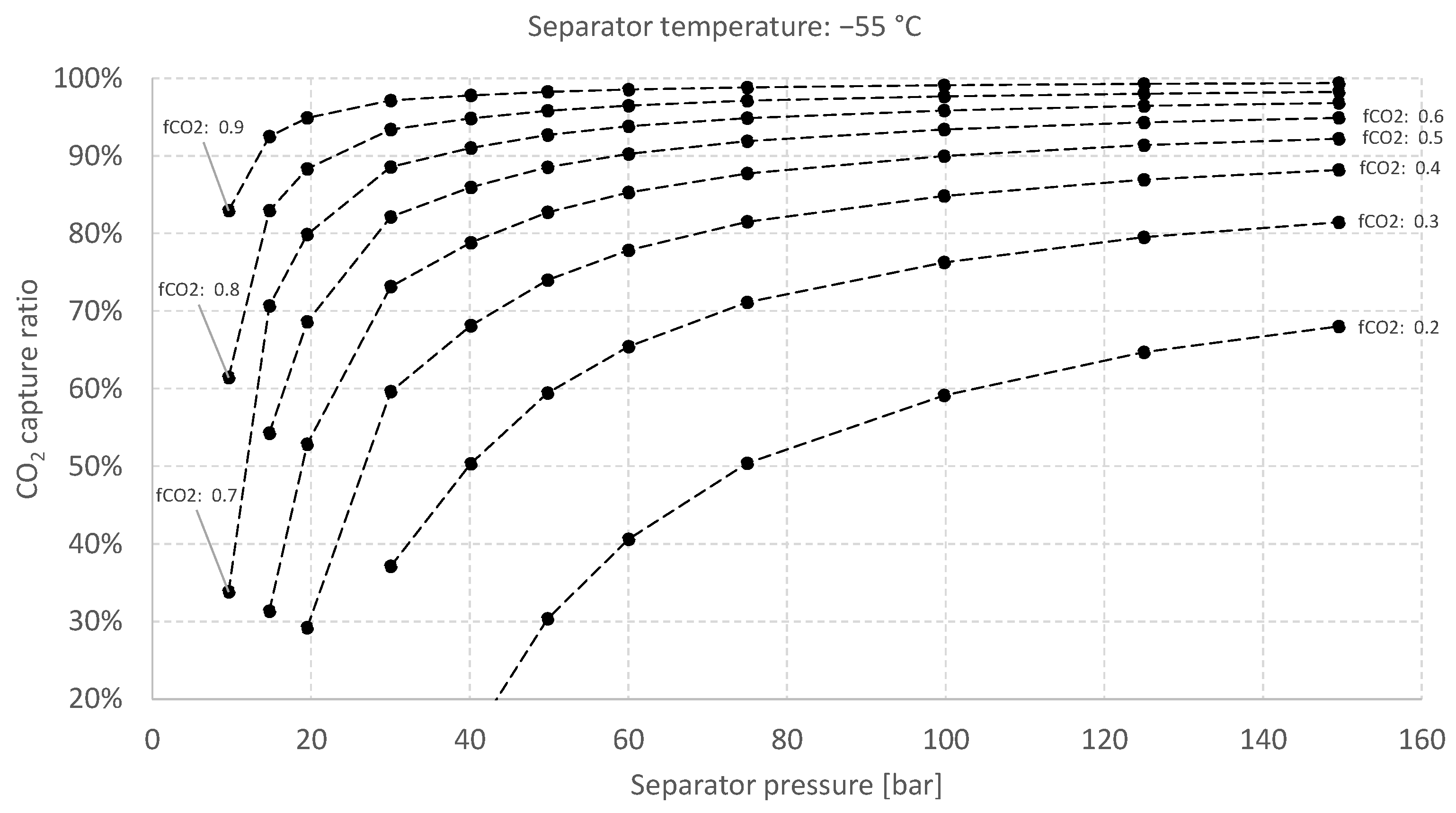
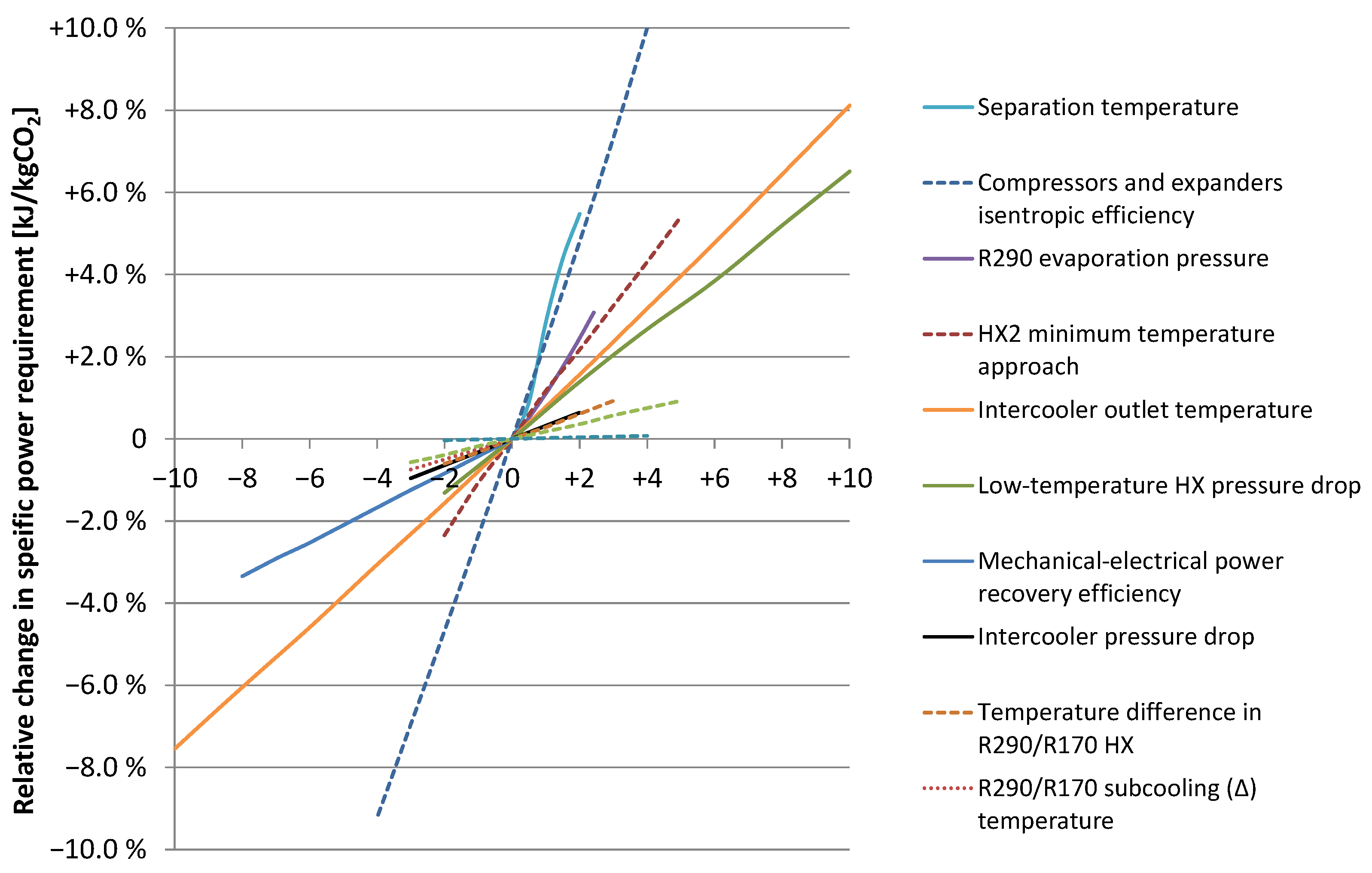
5.2. Sensitivity Analysis for the Baseline Case
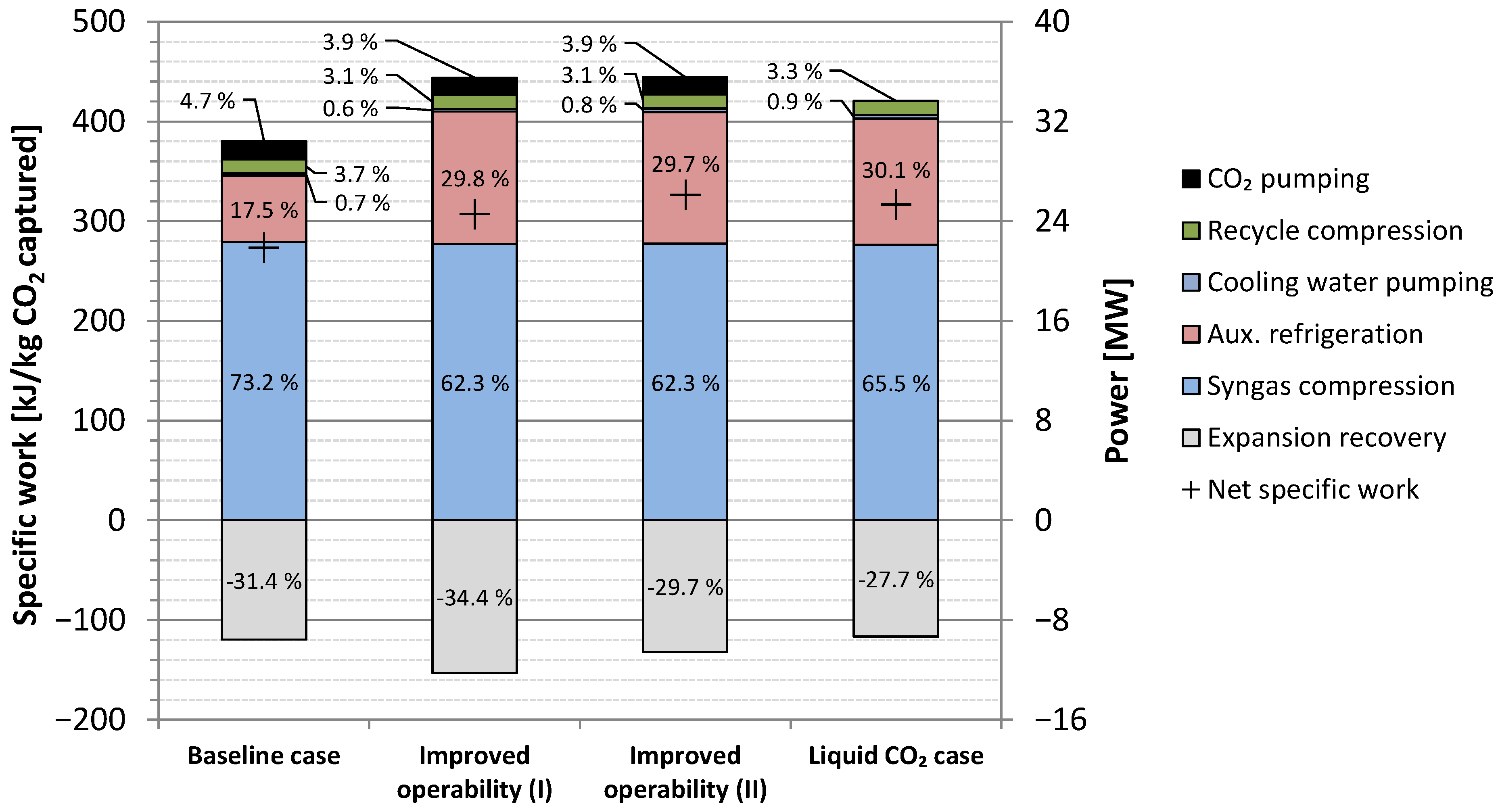
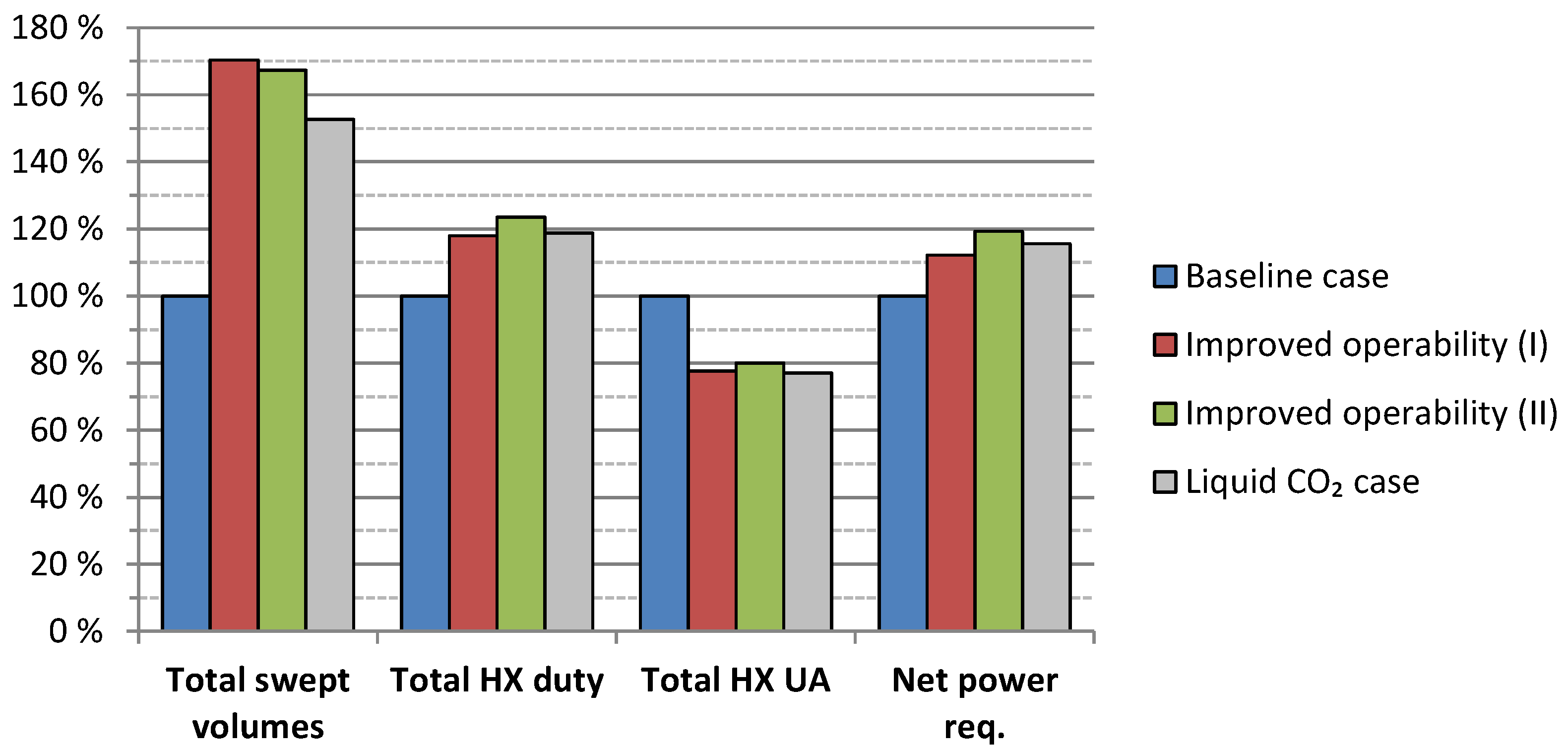
5.3. Results Summary
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Berstad, D.; Anantharaman, R.; Nekså, P. Low-temperature CO2 capture technologies—Applications and potential. Int. J. Refrig. 2013, 36, 1403–1416. [Google Scholar] [CrossRef]
- Nord, L.O.; Bolland, O. Plant flexibility of a pre-combustion CO2 capture cycle. Energy Procedia 2011, 4, 2556–2563. [Google Scholar] [CrossRef] [Green Version]
- Najmi, B.; Bolland, O. Operability of Integrated Gasification Combined Cycle power plant with SEWGS technology for pre-combustion CO2 capture. Energy Procedia 2014, 63, 1986–1995. [Google Scholar] [CrossRef] [Green Version]
- Moioli, S.; Giuffrida, A.; Romano, M.C.; Pellegrini, L.A.; Lozza, G. Assessment of MDEA absorption process for sequential H2S removal and CO2 capture in air-blown IGCC plants. Appl. Energy 2016, 183, 1452–1470. [Google Scholar] [CrossRef]
- Kunze, C.; Spliethoff, H. Assessment of oxy-fuel, pre- and post-combustion-based carbon capture for future IGCC plants. Appl. Energy 2012, 94, 109–116. [Google Scholar] [CrossRef]
- Melchior, T.; Madlener, R. Economic evaluation of IGCC plants with hot gas cleaning. Appl. Energy 2012, 97, 170–184. [Google Scholar] [CrossRef]
- Chen, S.; Xiang, W.; Wang, D.; Xue, Z. Incorporating IGCC and CaO sorption-enhanced process for power generation with CO2 capture. Appl. Energy 2012, 95, 285–294. [Google Scholar] [CrossRef]
- Franz, J.; Maas, P.; Scherer, V. Economic evaluation of pre-combustion CO2-capture in IGCC power plants by porous ceramic membranes. Appl. Energy 2014, 130, 532–542. [Google Scholar] [CrossRef]
- Ishii, H.; Hayashi, T.; Tada, H.; Yokohama, K.; Takashima, R.; Hayashi, J. Critical assessment of oxy-fuel integrated coal gasification combined cycles. Appl. Energy 2019, 233–234, 156–169. [Google Scholar] [CrossRef]
- Roussanaly, S.; Vitvarova, M.; Anantharaman, R.; Berstad, D.; Hagen, B.; Jakobsen, J.; Novotny, V.; Skaugen, G. Techno-economic comparison of three technologies for precombustion CO2 capture from a lignite-fired IGCC. Front. Chem. Sci. Eng. 2020, 14, 436–452. [Google Scholar] [CrossRef]
- Jordal, K.; Anantharaman, R.; Peters, T.A.; Berstad, D.; Morud, J.; Nekså, P.; Bredesen, R. High-Purity H2 Production with CO2 Capture Based on Coal Gasification. Energy 2015, 88, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Consonni, S.; Viganò, F.; Kreutz, T.; De Lorenzo, L. CO2 Capture in IGCC Plants via Cryogenic Separation. In Proceedings of the Sixth Annual Conference on Carbon Capture & Sequestration, Pittsburgh, PA, USA, 7–10 May 2007. [Google Scholar]
- Brouwers, J.J.H.; van Kemenade, H.P. Condensed Rotational Separation for CO2 capture in coal gasification processes. In Proceedings of the 4th International Freiberg Conference on IGCC & XtL Technologies, Dresden, Germany, 3–6 May 2010; pp. 1–18. [Google Scholar]
- Berstad, D.; Anantharaman, R.; Nekså, P. Low-temperature CCS from an IGCC power plant and comparison with physical solvents. Energy Procedia 2013, 37, 2204–2211. [Google Scholar] [CrossRef] [Green Version]
- Berstad, D.; Roussanaly, S.; Skaugen, G.; Anantharaman, R.; Nekså, P.; Jordal, K. Energy and Cost Evaluation of a Low-temperature CO2 Capture Unit for IGCC plants. Energy Procedia 2014, 63, 2031–2036. [Google Scholar] [CrossRef] [Green Version]
- Peampermpool, R.; Teh, C.J.; Tade, M.; Qader, A.; Barifcani, A. More Energy-Efficient CO2 Capture from IGCC GE Flue Gases. C 2017, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Mori, Y.; Forsyth, J. High Performance CO2 Capture by Autothermal AGR. Energy Procedia 2013, 37, 2284–2292. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Berstad, D.; Anantharaman, R.; Straus, J.; Peters, T.A.; Gundersen, T. Low Temperature Applications for CO2 Capture in Hydrogen Production. Comput. Aided Chem. Eng. 2020, 48, 445–450. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Carbon Capture and Storage; Special Report of the IPCC; Cambridge University Press: Cambridge, UK, 2005; p. 404. [Google Scholar]
- International Institute of Refrigeration. International Dictionary of Refrigeration; Peeters Publishers: Leuven, Belgium, 2007. [Google Scholar]
- Tuinier, M.; van Sint Annaland, M.; Kramer, G.J.; Kuipers, J. Cryogenic CO2 capture using dynamically operated packed beds. Chem. Eng. Sci. 2010, 65, 114–119. [Google Scholar] [CrossRef]
- Berger, A.H.; Hoeger, C.; Baxter, L.; Bhown, A. Evaluation of Cryogenic Systems for Post Combustion CO2 Capture. In Proceedings of the 14th Greenhouse Gas Control Technologies Conference (GHGT-14), Melbourne, Australia, 21–26 October 2018. [Google Scholar]
- Anantharaman, R.; Bolland, O.; Booth, N.; Dorst, E.V.; Ekstrom, C.; Franco, F.; Macchi, E.; Manzolini, G.; Nikolic, D.; Pfeffer, A.; et al. D1.4.3 European best practice guidelines for assessment of CO2 capture technologies. DECARBit 2011. Available online: https://www.sintef.no/globalassets/project/decarbit/d-1-4-3_euro_bp_guid_for_ass_co2_cap_tech_280211.pdf (accessed on 21 October 2021).
- Li, H.; Dong, B.; Yu, Z.; Yan, J.; Zhu, K. Thermo-physical properties of CO2 mixtures and their impacts on CO2 capture, transport and storage: Progress since 2011. Appl. Energy 2019, 255, 113789. [Google Scholar] [CrossRef]
- Spano, J.O.; Heck, C.K.; Barrick, P.L. Liquid-vapor equilibria of the hydrogen-carbon dioxide system. J. Chem. Eng. Data 1968, 13, 168–171. [Google Scholar] [CrossRef]
- Tsang, C.Y.; Streett, W.B. Phase equilibria in the H2/CO2 system at temperatures from 220 to 290 K and pressures to 172 MPa. Chem. Eng. Sci. 1981, 36, 993–1000. [Google Scholar] [CrossRef]
- Bezanehtak, K.; Combes, G.B.; Dehghani, F.; Foster, N.R.; Tomasko, D.L. Vapor−Liquid Equilibrium for Binary Systems of Carbon Dioxide + Methanol, Hydrogen + Methanol, and Hydrogen + Carbon Dioxide at High Pressures. J. Chem. Eng. Data 2002, 47, 161–168. [Google Scholar] [CrossRef]
- Qian, J.-W.; Jaubert, J.-N.; Privat, R. Phase equilibria in hydrogen-containing binary systems modeled with the Peng–Robinson equation of state and temperature-dependent binary interaction parameters calculated through a group-contribution method. J. Supercrit. Fluids 2013, 75, 58–71. [Google Scholar] [CrossRef]
- Fandiño, O.; Martin Trusler, J.P.; Vega-Maza, D. Phase behavior of (CO2 + H2) and (CO2 + N2) at temperatures between (218.15 and 303.15) K at pressures up to 15 MPa. Int. J. Greenh. Gas Control 2015, 36, 78–92. [Google Scholar] [CrossRef] [Green Version]
- Trædal, S.; Berstad, D.; Stang, J. Experimental Investigation of Low Temperature CO2 Liquefaction and Phase-Separation for Carbon Capture. Refrig. Sci. Technol. 2019, F147717, 147–153. [Google Scholar]
- Wu, H.; Xu, M.; Li, Y.; Wu, J.; Shen, J.; Liao, H. Experimental research on the process of compression and purification of CO2 in oxy-fuel combustion. Appl. Energy 2020, 259, 114123. [Google Scholar] [CrossRef]
- Wexler, A. Vapor Pressure Formulation for Ice. J. Res. Natl. Bur. Stand. 1977, 81A, 5–20. [Google Scholar] [CrossRef]
- ALPEMA. The Standards of the Brazed Aluminium Plate-Fin Heat Exchanger Manufacturers’ Association, 2nd ed.; ALPEMA: Houston, TX, USA, 2000. [Google Scholar]
- Bischoff, S.; Decker, L. First operating results of a dynamic gas bearing turbine in an industrial hydrogen liquefier. In Proceedings of the Advances in Cryogenic Engineering: Transactions of the Cryogenic Engineering Conference, Hong Kong, China, 17–19 March 2010. [Google Scholar]
- Roussanaly, S.; Bureau-Cauchois, G.; Husebye, J. Costs benchmark of CO2 transport technologies for a group of various size industries. Int. J. Greenh. Gas Control 2013, 12C, 341–350. [Google Scholar] [CrossRef] [Green Version]
- MACH-2—Membrane-Assisted CO2 Capture through Liquefaction for Clean H2 Production. Available online: https://www.sintef.no/en/projects/2019/mach-2-membrane-assisted-co2-capture-through-liquefaction-for-clean-h2-production/ (accessed on 19 November 2021).
- NCCS—Norwegian CCS Research Centre. Available online: https://www.sintef.no/nccs/ (accessed on 19 November 2021).
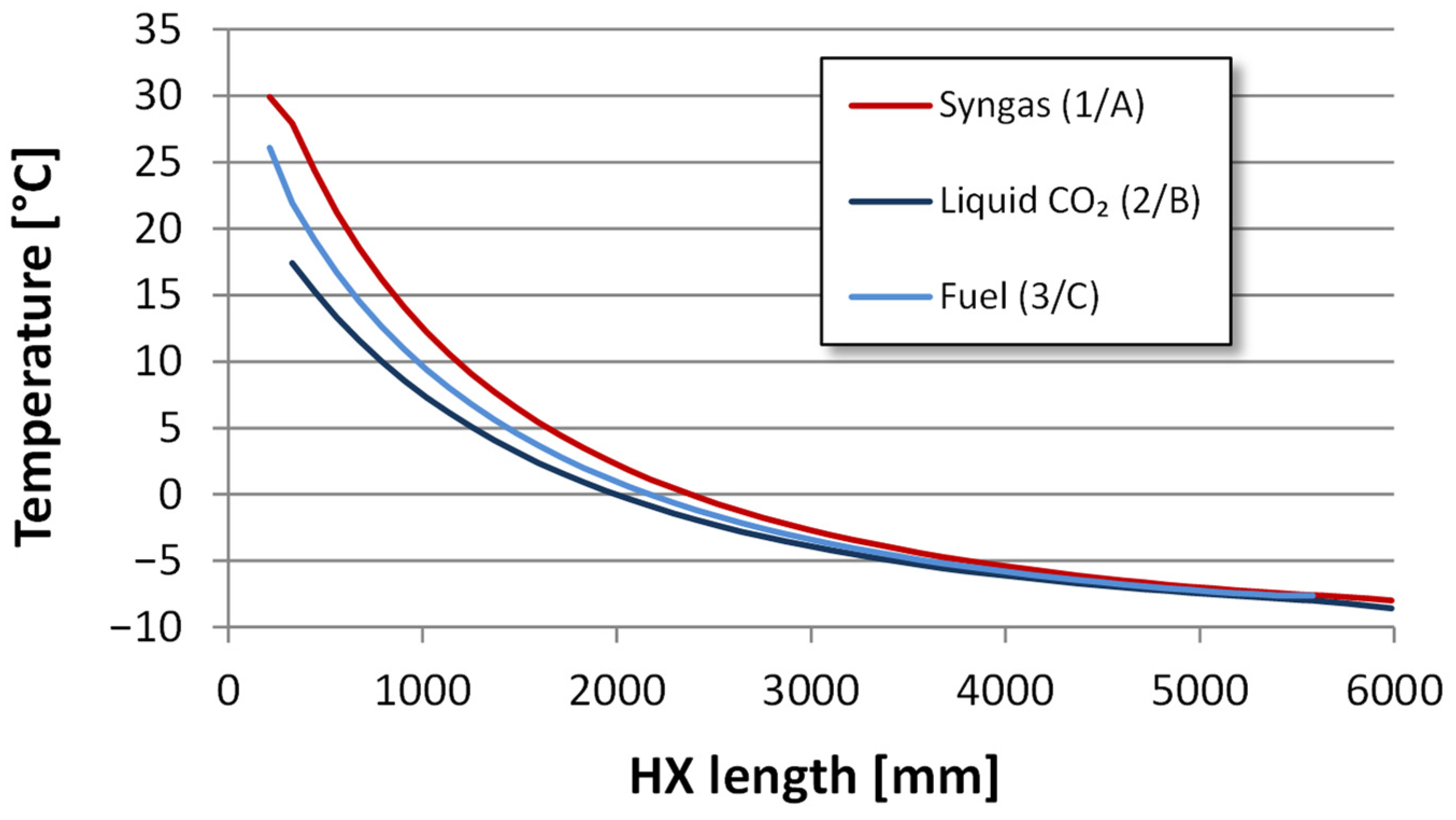
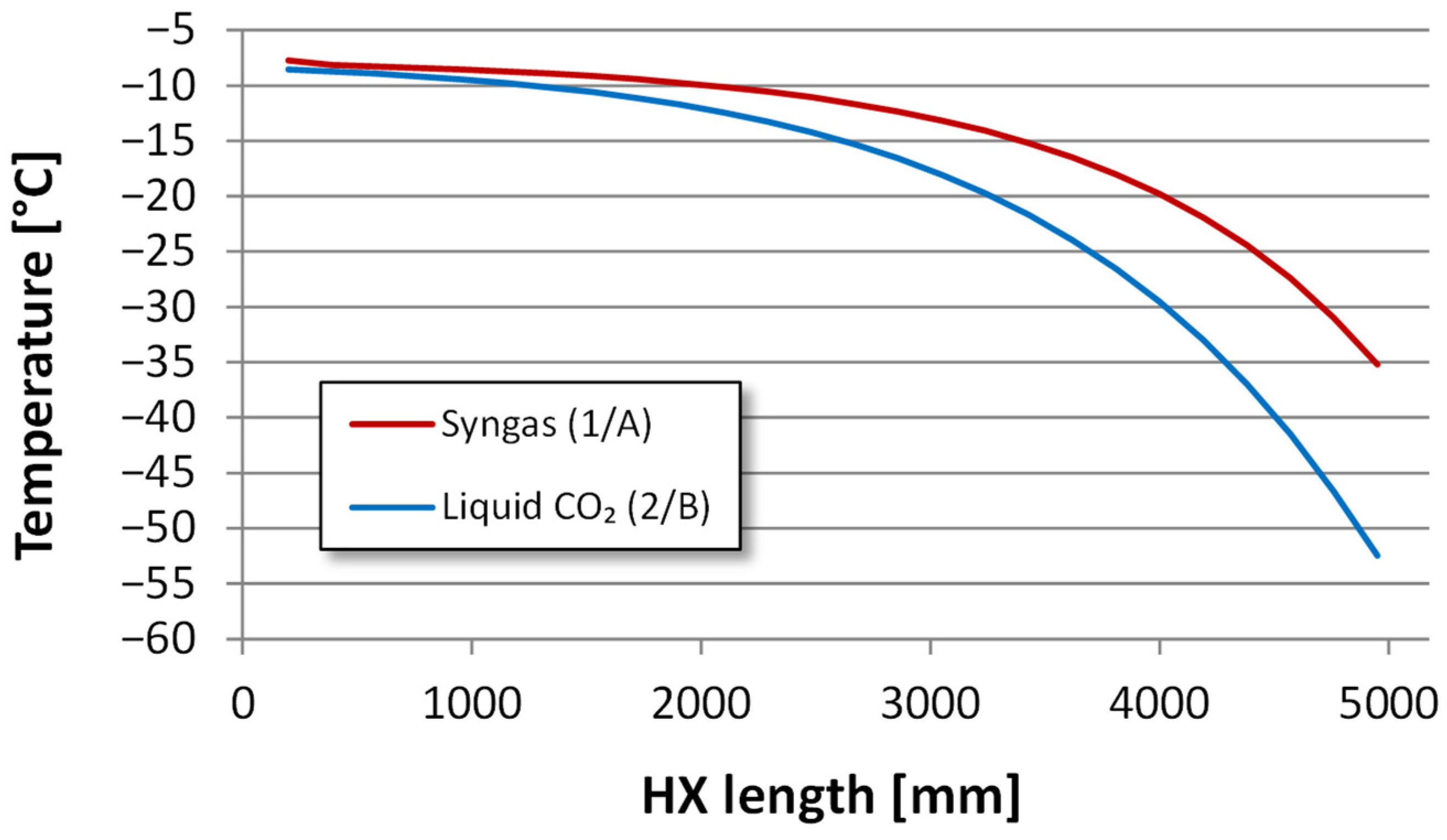
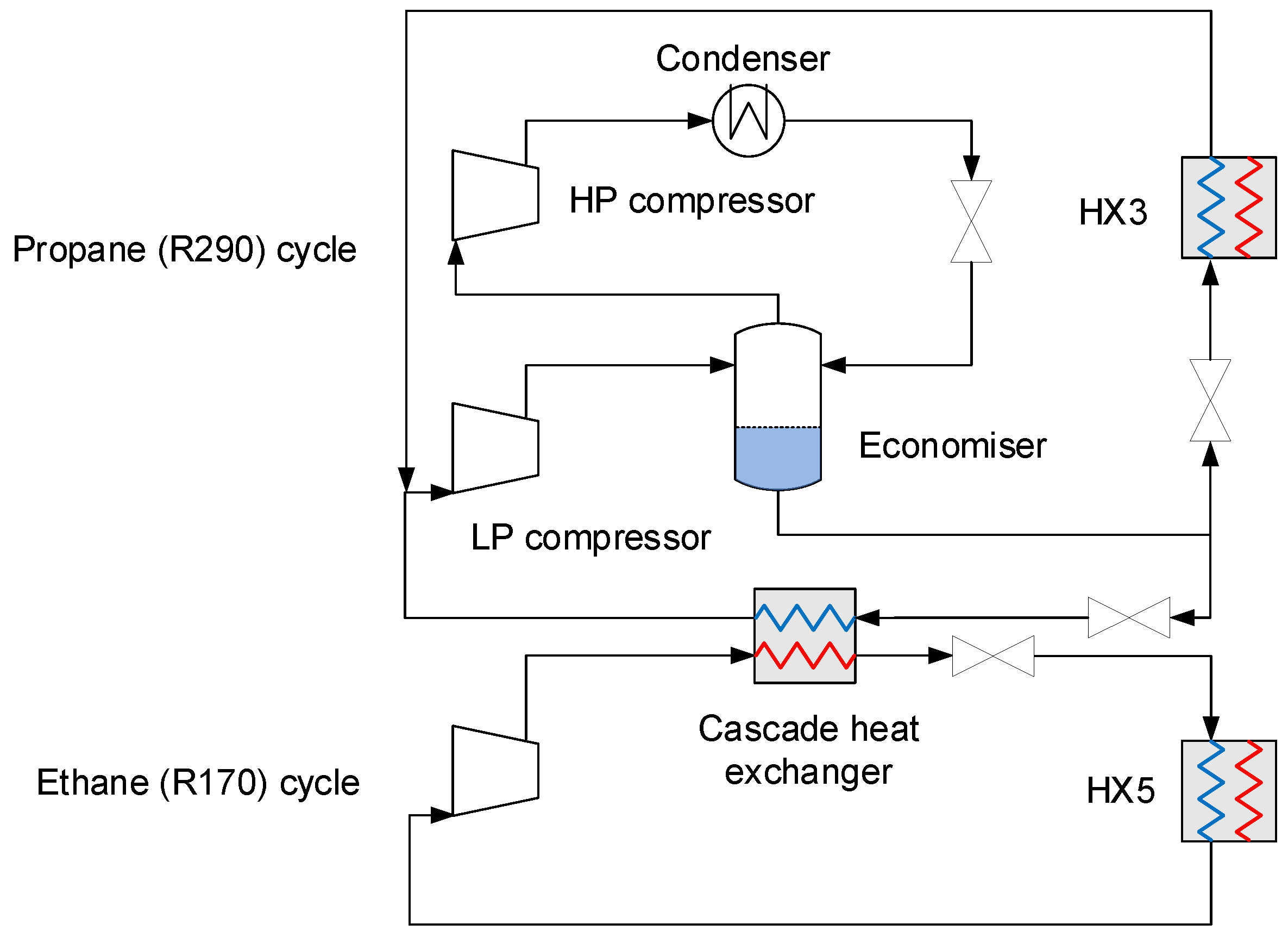
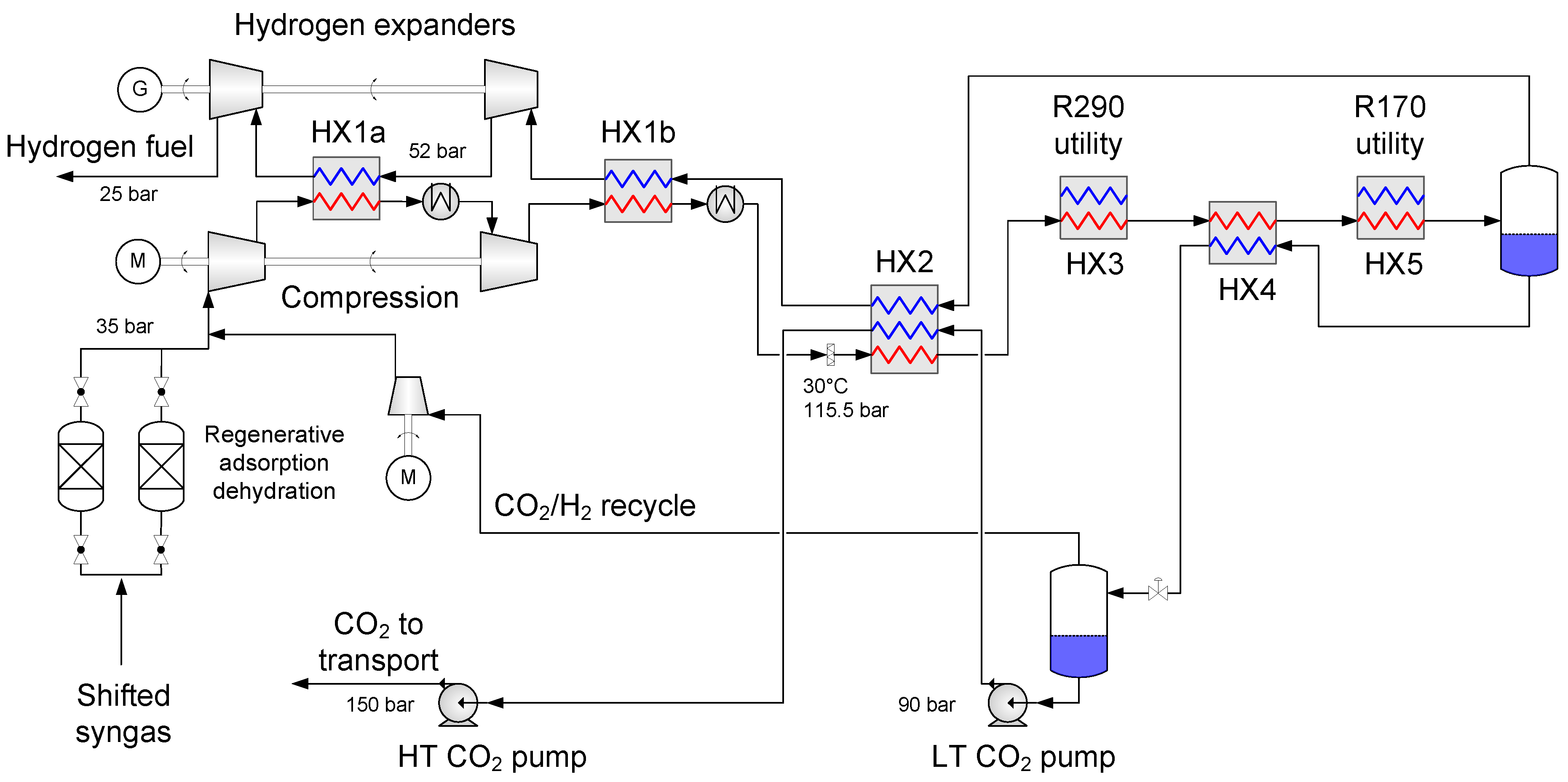
| Component | H2 | CO2 | CO | N2 | Ar |
|---|---|---|---|---|---|
| Mole fraction | 0.5375 | 0.3804 | 0.0160 | 0.0571 | 0.0090 |
| Unit | Duty MW | LMTD °C | UA kJ/(K·s) |
|---|---|---|---|
| HX1 | 9.10 | 2.55 | 3571 |
| HX2a | 16.1 | 4.37 | 3683 |
| HX2b | 7.01 | 5.02 | 1396 |
| HX3 | 1.95 | 4.44 | 438.3 |
| HX4 | 2.36 | 6.74 | 350.0 |
| HX5 | 6.53 | 7.90 | 826.8 |
| Shaft Power MW | Isentropic Efficiency % | Volumetric Flowrate m3/h | Pressure Ratio | Molar Mass g/mol | |
|---|---|---|---|---|---|
| Syngas compressor, 1st stage | 11.6 | 85 | 15,000 | 1.85 | 20.9 |
| Syngas compressor, 2nd stage | 10.8 | 82 | 7800 | 1.77 | 20.9 |
| Recycle compressor | 1.12 | 80 | 2300 | 4.55 | 34.2 |
| LP R290 compressor | 1.77 | 85 | 41,000 | 3.41 | 44.1 |
| HP R290 compressor | 2.75 | 85 | 19,000 | 3.51 | 44.1 |
| R170 compressor | 0.90 | 75 | 7600 | 2.18 | 30.1 |
| LT CO2 pump | 0.71 | 80 | 250 | 11.5 | 44.0 |
| HT CO2 pump | 0.74 | 80 | 350 | 1.71 | 44.0 |
| Fuel expander, 1st stage | 4.74 | 85 | 4600 1 | 2.09 | 8.85 |
| Fuel expander, 2nd stage | 4.86 | 88 | 9600 1 | 2.07 | 8.85 |
| Syngas compressor, 1st stage | 11.6 | 85 | 15,000 | 1.85 | 20.9 |
| Item | Unit | Value |
|---|---|---|
| Syngas compression | MWe | 22.39 |
| Auxiliary refrigeration | MWe | 5.341 |
| Cooling water pumping | MWe | 0.203 |
| Recycle compression | MWe | 1.123 |
| CO2 pumping | MWe | 1.445 |
| Hydrogen loss 1 | MWe | 0.081 |
| Fuel expanders 2 | MWe | 8.639 |
| Net power requirement | MWe | 21.94 |
| Specific power requirement | kJe/kg CO2 captured | 273.4 |
| Parameter | Unit | Baseline Value 1 | Unit on X-Axis 1 |
|---|---|---|---|
| Separation temperature | °C | −56 | 1 °C |
| Isentropic efficiency (vector) | % | See Table 3 | (−1)%-points |
| R290 evaporation pressure | bar | 1.015 | 0.1 bar |
| HX2 minimum temperature approach | °C | 3 | 1 °C |
| Intercooler outlet temperature | °C | 30 | 1 °C |
| Low-temperature HX pressure drop | bar | 0.5; 1.0 2 | 0.1 bar |
| Mech.-to-electrical conversion efficiency | % | 90 | (−1)%-points |
| Intercooler pressure drop | bar | 0.5 | 0.1 bar |
| Temperature difference in R290/R170 HX | °C | 5 | 1 °C |
| R290/R170 subcooling (∆) temperature | °C | 3 | 1 °C |
| Specific cooling water pumping power | kWel/MWth | 5 | 1 kWel/MWth |
| R290/R170 superheat (∆) temperature | °C | 3 | 1 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berstad, D.; Skaugen, G.; Roussanaly, S.; Anantharaman, R.; Nekså, P.; Jordal, K.; Trædal, S.; Gundersen, T. CO2 Capture from IGCC by Low-Temperature Synthesis Gas Separation. Energies 2022, 15, 515. https://doi.org/10.3390/en15020515
Berstad D, Skaugen G, Roussanaly S, Anantharaman R, Nekså P, Jordal K, Trædal S, Gundersen T. CO2 Capture from IGCC by Low-Temperature Synthesis Gas Separation. Energies. 2022; 15(2):515. https://doi.org/10.3390/en15020515
Chicago/Turabian StyleBerstad, David, Geir Skaugen, Simon Roussanaly, Rahul Anantharaman, Petter Nekså, Kristin Jordal, Stian Trædal, and Truls Gundersen. 2022. "CO2 Capture from IGCC by Low-Temperature Synthesis Gas Separation" Energies 15, no. 2: 515. https://doi.org/10.3390/en15020515
APA StyleBerstad, D., Skaugen, G., Roussanaly, S., Anantharaman, R., Nekså, P., Jordal, K., Trædal, S., & Gundersen, T. (2022). CO2 Capture from IGCC by Low-Temperature Synthesis Gas Separation. Energies, 15(2), 515. https://doi.org/10.3390/en15020515







