Mathematical Model of Steam Reforming in the Anode Channel of a Molten Carbonate Fuel Cell
Abstract
:1. Introduction
2. Theory
2.1. Reforming Model
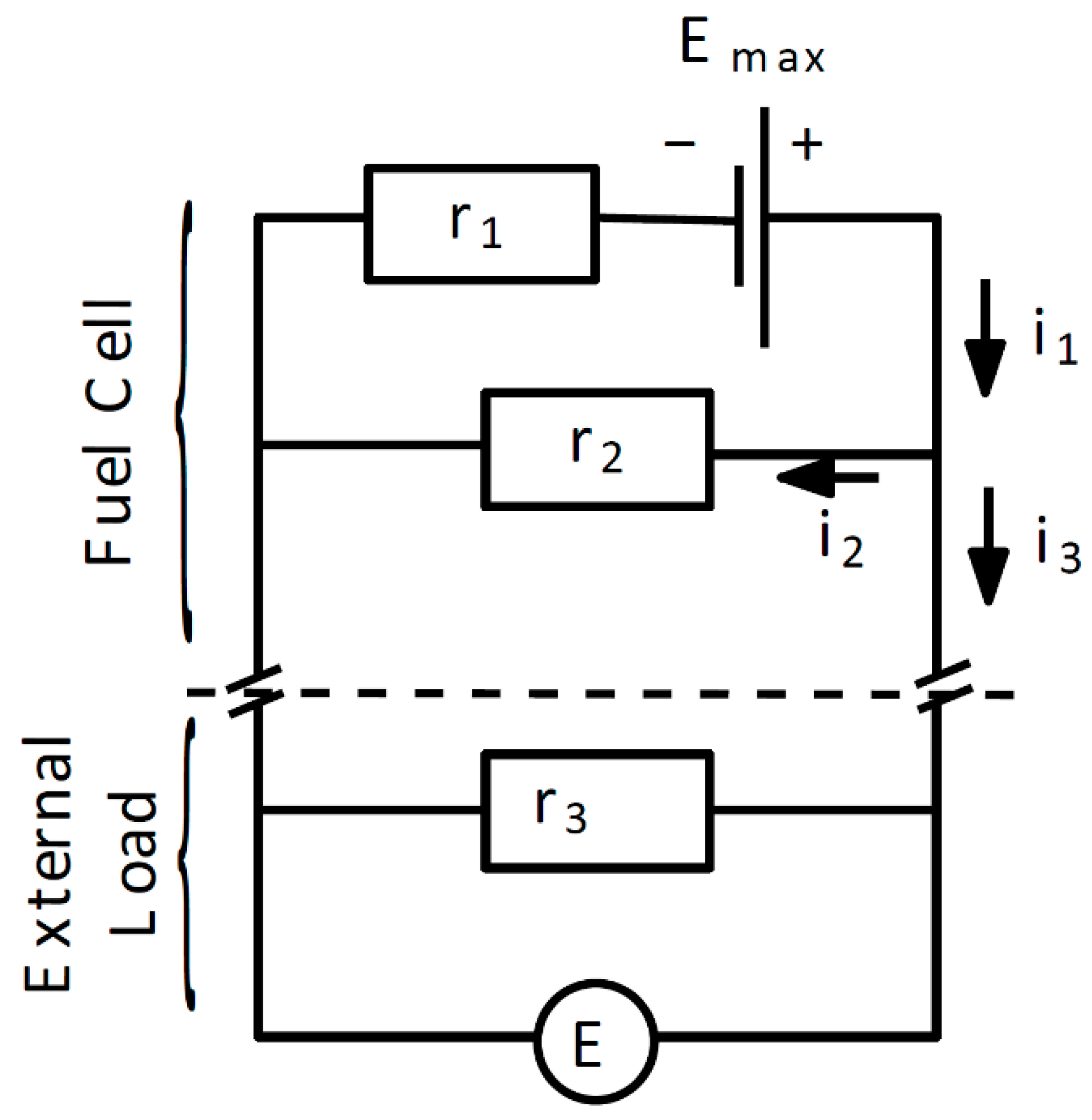
2.2. Fuel Cell Model
2.3. Maximum Voltage Emax
2.4. Maximum Current Density imax
2.5. Ionic Resistance r1
2.6. Ionic Resistance r2
3. Experiment
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szablowski, L.; Kupecki, J.; Milewski, J.; Motylinski, K. Kinetic model of a plate fin heat exchanger with catalytic coating as a steam reformer of methane, biogas, and dimethyl ether. Int. J. Energy Res. 2019, 43, 2930–2939. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Z.; Wang, F.; Li, Y.; Jing, Y. Two-Dimensional Metal Hexahydroxybenzene Frameworks as Promising Electrocatalysts for an Oxygen Reduction Reaction. ACS Sustain. Chem. Eng. 2020, 8, 7472–7479. [Google Scholar] [CrossRef]
- Li, T.; Li, M.; Zhu, X.; Zhang, J.; Jing, Y. Conductive two-dimensional M3 (C6S3O3)2 monolayers as effective electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2021, 9, 24887–24894. [Google Scholar] [CrossRef]
- McPhail, S.J.; Aarva, A.; Devianto, H.; Bove, R.; Moreno, A. SOFC and MCFC: Commonalities and opportunities for integrated research. Int. J. Hydrog. Energy 2011, 36, 10337–10345. [Google Scholar] [CrossRef]
- Milewski, J.; Cwieka, K.; Szczęśniak, A.; Szabłowski, Ł.; Wejrzanowski, T.; Skibinski, J.; Dybiński, O.; Lysik, A.; Sienko, A.; Stanger, P. Recycling electronic scrap to make molten carbonate fuel cell cathodes. Int. J. Hydrog. Energy 2021. [Google Scholar] [CrossRef]
- Clarke, S.H.; Dicks, A.L.; Pointon, K.; Smith, T.A.; Swann, A. Catalytic aspects of the steam reforming of hydrocarbons in internal reforming fuel cells. Catal. Today 1997, 38, 411–423. [Google Scholar] [CrossRef]
- Dicks, A.L. Advances in catalysts for internal reforming in high temperature fuel cells. J. Power Sources 1998, 71, 111–122. [Google Scholar] [CrossRef]
- Freni, S.; Maggio, G. Energy balance of different internal reforming MCFC configurations. Int. J. Energy Res. 1997, 21, 164–170. [Google Scholar] [CrossRef]
- Heidebrecht, P.; Sundmacher, K. Molten carbonate fuel cell (MCFC) with internal reforming: Model-based analysis of cell dynamics. Chem. Eng. Sci. 2003, 58, 1029–1036. [Google Scholar] [CrossRef]
- Campanari, S.; Manzolini, G.; Chiesa, P. Using MCFC for high efficiency CO2 capture from natural gas combined cycles: Comparison of internal and external reforming. Appl. Energy 2013, 112, 772–783. [Google Scholar] [CrossRef]
- Musa, A.; Steeman, H.J.; De Paepe, M. Performance of internal and external reforming molten carbonate fuel cell systems. J. Fuel Cell Sci. Technol. 2007, 4, 65–71. [Google Scholar] [CrossRef]
- Bove, R.; Lunghi, P. Experimental comparison of MCFC performance using three different biogas types and methane. J. Power Sources 2005, 145, 588–593. [Google Scholar] [CrossRef]
- Guerra, C.; Lanzini, A.; Leone, P.; Santarelli, M.; Brandon, N.P. Optimization of dry reforming of methane over Ni/YSZ anodes for solid oxide fuel cells. J. Power Sources 2014, 245, 154–163. [Google Scholar] [CrossRef]
- Klein, J.M.; Hénault, M.; Roux, C.; Bultel, Y.; Georges, S. Direct methane solid oxide fuel cell working by gradual internal steam reforming: Analysis of operation. J. Power Sources 2009, 193, 331–337. [Google Scholar] [CrossRef]
- Lanzini, A.; Leone, P.; Guerra, C.; Smeacetto, F.; Brandon, N.P.; Santarelli, M. Durability of anode supported Solid Oxides Fuel Cells (SOFC) under direct dry-reforming of methane. Chem. Eng. J. 2013, 220, 254–263. [Google Scholar] [CrossRef]
- Lin, Y.; Zhan, Z.; Liu, J.; Barnett, S.A. Direct operation of solid oxide fuel cells with methane fuel. Solid State Ion. 2005, 176, 1827–1835. [Google Scholar] [CrossRef]
- Lin, Y.; Zhan, Z.; Barnett, S.A. Improving the stability of direct-methane solid oxide fuel cells using anode barrier layers. J. Power Sources 2006, 158, 1313–1316. [Google Scholar] [CrossRef]
- Liu, J.; Barnett, S.A. Operation of anode-supported solid oxide fuel cells on methane and natural gas. Solid State Ion. 2003, 158, 11–16. [Google Scholar] [CrossRef]
- Ni, M. Electrolytic effect in solid oxide fuel cells running on steam/methane mixture. J. Power Sources 2011, 196, 2027–2036. [Google Scholar] [CrossRef] [Green Version]
- Ni, M. Modeling and parametric simulations of solid oxide fuel cells with methane carbon dioxide reforming. Energy Convers. Manag. 2013, 70, 116–129. [Google Scholar] [CrossRef] [Green Version]
- Pillai, M.; Lin, Y.; Zhu, H.; Kee, R.J.; Barnett, S.A. Stability and coking of direct-methane solid oxide fuel cells: Effect of CO2 and air additions. J. Power Sources 2010, 195, 271–279. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, W.; Ran, R.; Cai, R.; Shao, Z. Methane-fueled SOFC with traditional nickel-based anode by applying Ni/Al2O3 as a dual-functional layer. Electrochem. Commun. 2009, 11, 194–197. [Google Scholar] [CrossRef]
- Sá, S.; Silva, H.; Brandão, L.; Sousa, J.M.; Mendes, A. Catalysts for methanol steam reforming-A review. Appl. Catal. B Environ. 2010, 99, 43–57. [Google Scholar] [CrossRef]
- Yaakob, Z.; Kamarudin, S.K.; Daud, W.R.W.; Yosfiah, M.R.; Lim, K.L.; Kazemian, H. Hydrogen production by methanol-steam reforming using Ni-Mo-Cu/γ- alumina trimetallic catalysts. Asia-Pac. J. Chem. Eng. 2010, 5, 862–868. [Google Scholar] [CrossRef]
- Douvartzides, S.L.; Coutelieris, F.A.; Demin, A.K.; Tsiakaras, P.E. Electricity from ethanol fed SOFCs: The expectations for sustainable development and technological benefits. Int. J. Hydrog. Energy 2004, 29, 375–379. [Google Scholar] [CrossRef]
- Alberton, A.L.; Souza, M.M.V.M.; Schmal, M. Carbon formation and its influence on ethanol steam reforming over Ni/Al2O3 catalysts. Catal. Today 2007, 123, 257–264. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; Carrero, A.; Calles, J.A. Hydrogen production by ethanol steam reforming over Cu-Ni supported catalysts. Int. J. Hydrog. Energy 2007, 32, 1450–1461. [Google Scholar] [CrossRef]
- Comas, J.; Marino, F.; Laborde, M.; Amadeo, N. Bio-ethanol steam reforming on Ni/Al2O3 catalyst. Chem. Eng. J. 2004, 98, 61–68. [Google Scholar] [CrossRef]
- Resini, C.; Arrighi, L.; Delgado, M.C.H.; Vargas, M.A.L.; Alemany, L.J.; Riani, P.; Berardinelli, S.; Marazza, R.; Busca, G. Production of hydrogen by steam reforming of C3 organics over Pd-Cu/γ-Al2O3 catalyst. Int. J. Hydrog. Energy 2006, 31, 13–19. [Google Scholar] [CrossRef]
- Myint, M.; Yan, Y.; Chen, J.G. Reaction pathways of propanal and 1-propanol on fe/ni(111) and cu/ni(111) bimetallic surfaces. J. Phys. Chem. C 2014, 118, 11340–11349. [Google Scholar] [CrossRef]
- Mizuno, T.; Matsumura, Y.; Nakajima, T.; Mishima, S. Effect of support on catalytic properties of Rh catalysts for steam reforming of 2-propanol. Int. J. Hydrog. Energy 2003, 28, 1393–1399. [Google Scholar] [CrossRef]
- Wang, M.; Au, C.T.; Lai, S.Y. H2 production from catalytic steam reforming of n-propanol over ruthenium and ruthenium-nickel bimetallic catalysts supported on ceria-alumina oxides with different ceria loadings. Int. J. Hydrog. Energy 2015, 40, 13926–13935. [Google Scholar] [CrossRef]
- Yadav, A.K.; Vaidya, P.D. Reaction Kinetics of Steam Reforming of n-Butanol over a Ni/Hydrotalcite Catalyst. Chem. Eng. Technol. 2018, 41, 890–896. [Google Scholar] [CrossRef]
- Patel, R.; Patel, S. Renewable hydrogen production from butanol: A review. Clean Energy 2017, 1, 90–101. [Google Scholar] [CrossRef]
- Nahar, G.A.; Madhani, S.S. Thermodynamics of hydrogen production by the steam reforming of butanol: Analysis of inorganic gases and light hydrocarbons. Int. J. Hydrog. Energy 2010, 35, 98–109. [Google Scholar] [CrossRef]
- Schwengber, C.A.; Alves, H.J.; Schaffner, R.A.; Da Silva, F.A.; Sequinel, R.; Bach, V.R.; Ferracin, R.J. Overview of glycerol reforming for hydrogen production. Renew. Sustain. Energy Rev. 2016, 58, 259–266. [Google Scholar] [CrossRef]
- Dou, B.; Dupont, V.; Rickett, G.; Blakeman, N.; Williams, P.T.; Chen, H.; Ding, Y.; Ghadiri, M. Hydrogen production by sorption-enhanced steam reforming of glycerol. Bioresour. Technol. 2009, 100, 3540–3547. [Google Scholar] [CrossRef] [Green Version]
- Dou, B.; Wang, C.; Song, Y.; Chen, H.; Xu, Y. Activity of Ni-Cu-Al based catalyst for renewable hydrogen production from steam reforming of glycerol. Energy Convers. Manag. 2014, 78, 253–259. [Google Scholar] [CrossRef]
- Sánchez, E.A.; D’Angelo, M.A.; Comelli, R.A. Hydrogen production from glycerol on Ni/Al2O3 catalyst. Int. J. Hydrog. Energy 2010, 35, 5902–5907. [Google Scholar] [CrossRef]
- Adhikari, S.; Fernando, S.D.; Haryanto, A. Hydrogen production from glycerol: An update. Energy Convers. Manag. 2009, 50, 2600–2604. [Google Scholar] [CrossRef]
- Cheng, C.K.; Foo, S.Y.; Adesina, A.A. Glycerol steam reforming over bimetallic Co-Ni/Al2O3. Ind. Eng. Chem. Res. 2010, 49, 10804–10817. [Google Scholar] [CrossRef]
- Lytkina, A.A.; Zhilyaeva, N.A.; Ermilova, M.M.; Orekhova, N.V.; Yaroslavtsev, A.B. Influence of the support structure and composition of Ni-Cu-based catalysts on hydrogen production by methanol steam reforming. Int. J. Hydrog. Energy 2015, 40, 9677–9684. [Google Scholar] [CrossRef]
- Pomfret, M.B.; Steinhurst, D.A.; Owrutsky, J.C. Methanol and ethanol fuels in solid oxide fuel cells: A thermal imaging study of carbon deposition. Energy Fuels 2011, 25, 2633–2642. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Assabumrungrat, S. Catalytic steam reforming of methane, methanol, and ethanol over Ni/YSZ: The possible use of these fuels in internal reforming SOFC. J. Power Sources 2007, 163, 943–951. [Google Scholar] [CrossRef]
- Lwin, Y.; Daud, W.R.W.; Mohamad, A.B.; Yaakob, Z. Hydrogen production from steam-methanol reforming: Thermodynamic analysis. Int. J. Hydrog. Energy 2000, 25, 47–53. [Google Scholar] [CrossRef]
- Young, D.C. Computational Chemistry: A Practical Guide for Applying Techniques to Real World Problems; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Milewski, J.; Wołowicz, M.; Miller, A.; Bernat, R. A reduced order model of molten carbonate fuel cell: A proposal. Int. J. Hydrog. Energy 2013, 38, 11565–11575. [Google Scholar] [CrossRef]
- Sundmacher, K.; Kienle, A.; Pesch, H.J.; Berndt, J.F.; Huppmann, G. Molten Carbonate Fuel Cells: Modeling, Analysis, Simulation, and Control; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007. [Google Scholar]
- Morita, H.; Komoda, M.; Mugikura, Y.; Izaki, Y.; Watanabe, T.; Masuda, Y.; Matsuyama, T. Performance analysis of molten carbonate fuel cell using a Li/Na electrolyte. J. Power Sources 2002, 112, 509–518. [Google Scholar] [CrossRef]
- Baron, R.; Wejrzanowski, T.; Milewski, J.; Szabłowski, Ł.; Szczęśniak, A.; Fung, K.Z. Manufacturing of Γ-LiAlO2 matrix for molten carbonate fuel cell by high-energy milling. Int. J. Hydrog. Energy 2018, 43, 6696–6700. [Google Scholar] [CrossRef]







| Chemical Reaction | A | Ea, kJ/kmol |
|---|---|---|
| 1 × 105 | 107,300 | |
| 1 × 1010 | 114,800 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szablowski, L.; Dybinski, O.; Szczesniak, A.; Milewski, J. Mathematical Model of Steam Reforming in the Anode Channel of a Molten Carbonate Fuel Cell. Energies 2022, 15, 608. https://doi.org/10.3390/en15020608
Szablowski L, Dybinski O, Szczesniak A, Milewski J. Mathematical Model of Steam Reforming in the Anode Channel of a Molten Carbonate Fuel Cell. Energies. 2022; 15(2):608. https://doi.org/10.3390/en15020608
Chicago/Turabian StyleSzablowski, Lukasz, Olaf Dybinski, Arkadiusz Szczesniak, and Jaroslaw Milewski. 2022. "Mathematical Model of Steam Reforming in the Anode Channel of a Molten Carbonate Fuel Cell" Energies 15, no. 2: 608. https://doi.org/10.3390/en15020608






