Abstract
A large portion of food loss and waste (FSL) is comprised of seeds and stones. Exotic fruits such as mangoes, lychees and avocados, in which the seeds account for a significant part of the weight and volume of the entire product, are most affected by this problem. The seeds contain a large quantity of polyphenols and essential nutrients, which makes them a good material for extraction. However, conventional extraction techniques are considered time-consuming, and therefore significantly limit their use on an industrial scale. An alternative method of managing the seeds may be their energy utilization. In this study, torrefaction was proposed as a method for the valorization of exotic fruit seeds (mango, lychee, avocado). Thus, the influence of torrefaction temperature (200–300 °C) on the physical-chemical properties of substrates was investigated. The obtained results revealed that, in relation to the unprocessed raw materials, the torreficates are characterized by improved hydrophobic properties (all materials are classified as extremely hydrophobic), higher heating value (at 300 °C the values increased from 17,789 to 24,842 kJ∙kg−1 for mango, from 18,582 to 26,513 kJ∙kg−1 for avocado, and from 18,584 to 25,241 kJ∙kg−1 for lychee), higher fixed carbon content (which changed from 7.87–15.38% to 20.74–32.47%), and significant mass loss, by 50–60%. However, as a side effect of thermal treatment, an increase in ash content (approx. 2–3 times but still less than in coal) was observed. Therefore, the torreficates may be competitive with coal. The possibility of using residues from the food processing sector as a substrate for energy purposes is important from the point of view of environment protection and is a part of the functioning of the circular economy.
1. Introduction
Food loss and waste (FSL) is considered to be one of the most important threats related to food safety, the economy and environmental protection issues [1,2]. According to FAO estimates, about 30% of the food produced for human consumption is lost or wasted somewhere along the supply chain [3]. This phenomenon, in principle, occurs at all stages of the logistics chain—agriculture (harvest operation and subsequent sorting and grading), post-harvest (handling, transportation and storage immediately after harvest and before processing), processing, distribution and consumption. The annual value of this large amount of food lost and wasted during these operations may exceed USD 1 trillion [3,4].
However, depending on the commodity and morphological components, the amount and type of FSL can vary significantly. The unused and unconsumed part of a fruit, a vegetable or other food stuff may consist of various components, such as peels, leaves, roots, tubers, skin, pulps and pomace [5,6]. A large portion of FSL is also comprised of seeds and stones, which, in many vegetables and fruits, are not consumed and constitute unnecessary material from the consumer’s point of view. This problem, in particular, concerns tropical/exotic fruits, in which seeds and stones account for a significant part of the mass and volume; these fruits include mango, lychee, papaya, avocado and rambutan [5,6,7,8,9,10,11]. Globally, the total production of the main tropical fruits in 2017 was estimated at 93.7 million tonnes, of which 58% was mango and avocado [12]. This high level of production, combined with a high share of seeds in the final product, means that interesting methods are being sought that allow to fully use the potential of waste biomass, while eliminating the problems associated with seed management.
Due to the fact that numerous studies have shown that exotic/tropical fruit seeds contain numerous quantities of phytochemicals and essential nutrients, extraction is currently the primary method of their disposal and processing [13]. For example, chemical characteristics of avocado seeds reflect a large number of polyphenols (i.e., catechins, procyanidins and other tannins), flavonoids, triterpenes and unsaturated fatty acids [14,15,16]. However, current conventional extraction techniques (i.e., maceration), are time-consuming, which significantly limits the use of this process on an industrial scale [14]. Thus, the use of exotic fruit seeds as raw materials to obtain extracts with desired and valuable bioactive ingredients is problematic. Fidelis et al. [14] indicated that, in order to improve this process and thus contribute to the commercialization of extracts that can be used in the pharmaceutical and food sectors, a number of additional studies are required to optimize the conditions and techniques of extraction.
An alternative to the management of waste biomass, compared to the extraction process, may be the energetic use or thermal valorization of exotic/tropical fruit seeds. From a practical point of view, the energy use of wasted and lost food has many advantages. First, increasing the degree of electricity/heat production from such substrates may significantly mitigate competition for soil resources. These operations would reduce the increasing pressure on natural resources and ecosystems [17,18,19]. Furthermore, recent studies show that, despite the introduction of restrictive rules of forest management, in addition to stringent timber-harvesting requirements, the production of biofuels can significantly cause deforestation of large areas of land [20]. This problem particularly affects developing countries. Hence, the use of waste substrates in the process of generating energy, such as tropical/exotic fruit seeds, would not adversely affect soil competition where human and animal edible food is grown [21]. This process would use the synergy resulting from the potential use of valuable organic matter contained in the seeds of exotic fruit, while at the same time solving the problems related to the management of this type of organic waste. This would also be in line with the idea of the operation of the circular economy.
However, fresh and unprocessed biomass is usually distinguished by a high moisture content, which lowers its higher heating value (the average calorific value of raw biomass is 50% of that of bituminous coal). Additional barriers are the low bulk density and low energy density, which are about 2–7% of the value of coal [22]. It should also be emphasized that biomass tends to excessively absorb moisture, which makes it vulnerable to microbiological degradation [22].
The above barriers mean that, in order to adapt the material for energy use and stabilize the physical-chemical properties of fresh, unprocessed biomass (including exotic fruit seeds), alternative methods of waste biomass valorization should be applied. One of the potential options for improving the properties of exotic/tropical fruit seeds is torrefaction, which is a thermochemical process carried out at temperatures ranging from 200 to 350 °C in an inert atmosphere. The heating rate during the torrefaction process should not exceed 50 °C∙min−1 [23], and the necessary processing time in typical reactors is 0.5–2 h. After completion of the torrefaction process, the obtained products are characterized by an increase in fixed carbon content, higher heating value, better grindability, and reduced moisture content, hygroscopicity and heterogeneity [24,25,26,27].
Many studies are available in the literature that take into account the impact of torrefaction on the physical-chemical properties of waste biomass from the agri-food industry, but still little information is dedicated to the seeds of exotic fruit. These data are important with regard to further application possibilities of the concept’s implementation, consisting of increasing electricity production from exotic fruit seeds, in addition to modeling and optimization of the torrefaction process.
However, the current state of knowledge regarding the torrefaction of exotic fruit seeds is not sufficient. In fact, there are positions in the literature that propose torrefaction as a method of valorization of this type of biomass, but they do not cover a wide range of physical-chemical characteristics, focusing mainly on the possibility of their energy utilization. Domínguez et al. [28] performed pyrolysis of avocado seeds in the range of 150–900 °C. In the case of torrefaction at 300 °C, these authors obtained material with properties similar to those of carbon (0.66 fixed carbon/volatile matter, HHV of 23.3 MJ∙kg−1). Similar results for this type of fruit were also obtained by Sanchez et al. [29] by conducting the torrefaction process at 304 °C (36.7% FC, HHV of 24.3 MJ∙kg−1). In the case of mango seeds, Ganeshan et al. [30] found that their conventional pyrolysis performed in the temperature range of 300–500 °C allowed the acquisition of products with added value. Santanna et al. [31] torrefied urban waste from Brazil, which consisted of a mixture of six different wood species (including mango and avocado), resulting in a valuable high-calorific product, but did not focus fully on single substrates. In turn, Lin and Zheng [32] found that the addition of mango seeds to optoelectronic sludge in various proportions increases the quality of biofuel obtained by torrefaction. In another work [33], the same authors performed torrefaction of mango seed and passion fruit shell, The obtained biochars were characterized by higher heating values of 19.2–28.6 MJ∙kg−1. Moreover, the biochar samples showed an increased fuel ratio index (FR = 0.02–0.17) and a high energy return on investment (EROI) index (13.3–35.9). However, in order to be able to use torrefaction in the future as a method of valorization of waste seeds of exotic fruit, it is necessary to perform additional studies, including the analysis other physical properties, such as bulk density, specific density porosity and hydrophobicity propensities, which are also very important in terms of storage, transport and energy utilization of biomass. In particular, the hydrophobic properties of exotic fruit seeds are crucial for the whole supply chain, because they have a significant impact on the long-term quality of torreficates. This data is still missing, creating a space for novel and more detailed study of these materials.
Taking into account the above arguments, this study aimed to investigate the impact of the torrefaction temperature on: (i) the change in proximate analysis of raw and torrefied exotic fruit seeds; (ii) the change in physical properties of raw and torrefied exotic fruit seeds; and (iii) the change in hydrophobic properties of raw and torrefied exotic fruits seeds.
2. Materials and Methods
2.1. Materials
In the experiment, the waste biomass from exotic fruits were used. Three types of organic residues were evaluated: mango seeds, avocado seeds and lychee seeds.
2.2. Samples Preparation and Torrefaction Procedure
The collected waste biomass was dried for 24 h in a KBC–65 W drying chamber (WAMED, Warszawa, Poland) at the temperature of 105 °C, in accordance with the PN-EN ISO 18134-2:2017-03 standard. The dried material was ground with a LMN 400 knife mill (TESTCHEM, Pszów, Poland) with a sieve diameter of 1 mm. Then, 50 g samples of the material were prepared using an RADWAG AS 220.R2 (RADWAG, Radom, Poland) scale.
The torrefaction process was carried out in an SNOL 8.2/1100 (SNOL, Utena, Lithuania) muffle furnace. The torrefaction time was 60 min. The torrefaction was carried out at the temperatures of 200, 220, 240, 260, 280, and 300 °C in 5 replications. Carbon dioxide from a gas cylinder was introduced into the furnace to ensure an inert atmosphere during the process (90 mL∙min−1).
2.3. Proximate Analysis
In order to determine the properties of torrefied exotic fruit seeds, proximate analysis was carried out. All experiments were performed according to ISO standards (Table 1). The following parameters were evaluated: ash content (AC), volatile matter content (VMC), higher heating value (HHV) and fixed carbon content (FCC). The measurements were repeated three times.

Table 1.
Standards for performed tests of proximate analysis.
2.4. Physical Analysis
Additionally, the following physical features of exotic fruit biomass were evaluated before and after the torrefaction process: the mass loss, bulk density, specific density and porosity. Each test was repeated three times.
The mass loss was calculated on the basis of the difference in mass of the sample before and after torrefaction of a given type of biomass, according to the formula:
where: ML—mass loss during torrefaction process (%); m1—mass of the sample before torrefaction process (g); m2—mass of the sample after torrefaction process (g).
The bulk density was determined using a 25 cm3 vessel. After filling with a given type of waste biomass, the bulk density was calculated according to the formula:
where: n—bulk density (kg∙m−3); mb—mass of the biomass in vessel (g); V—volume of the vessel (cm3).
The specific density was determined using a gas pycnometer, supplied with argon from a cylinder. The dried material was placed in a vessel of known volume and weighed on a laboratory balance. The porosity was calculated according to PN-EN 1936:2010 using the following formula:
where: ε—porosity of the material in the dry analytical state (%); ρb—bulk density of the material in the dry analytical state (kg·m−3); and ρs—specific density of material in the dry analytical state (kg·m−3).
2.5. Hydrophobicity Analysis
Hydrophobic properties of torreficates and primary materials were determined by measuring water drop penetration time (WDPT) [34]. The material was spread evenly on glass to create a layer. Next, a few drops of demineralized water were placed (using a pipette) on the material. Using a stopwatch, the time taken for a drop of water to soak into the material was measured. The hydrophobic properties were determined on the basis of the water droplet penetration time according to the classification shown in Table 2.

Table 2.
Classification criterion of hydrophobic properties [35,36].
2.6. Fourier Transform InfraRed (FTIR) Analysis
The FTIR analyses were performed using the Thermo Scientific Nicolet iZ10 FT-IR spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Each spectrum of the torreficates was the average of 32 scans in the 400–4000 cm−1 wavenumber range at the 4 cm−1 spectral resolution. Spectrums are presented using OMNIC ™ Specta Software (Thermo Fisher Scientific, Waltham, MA, USA).
2.7. Statistical Analysis
A statistical analysis (at p-value < 0.05) involving a one-way analysis of variance (ANOVA) was carried out. The test was focused on the elaboration of statistical significance of the influence of the torrefaction temperature on the physical-chemical properties of the exotic fruit seeds under analysis. The differences between the levels of factor were determined by a post hoc test, using a Fisher LSD test. The statistical analysis was developed using the software STATISTICA (StatSoft—DELL Software, Round Rock, TX, USA).
3. Results and Discussion
3.1. Results of the Proximate Analysis
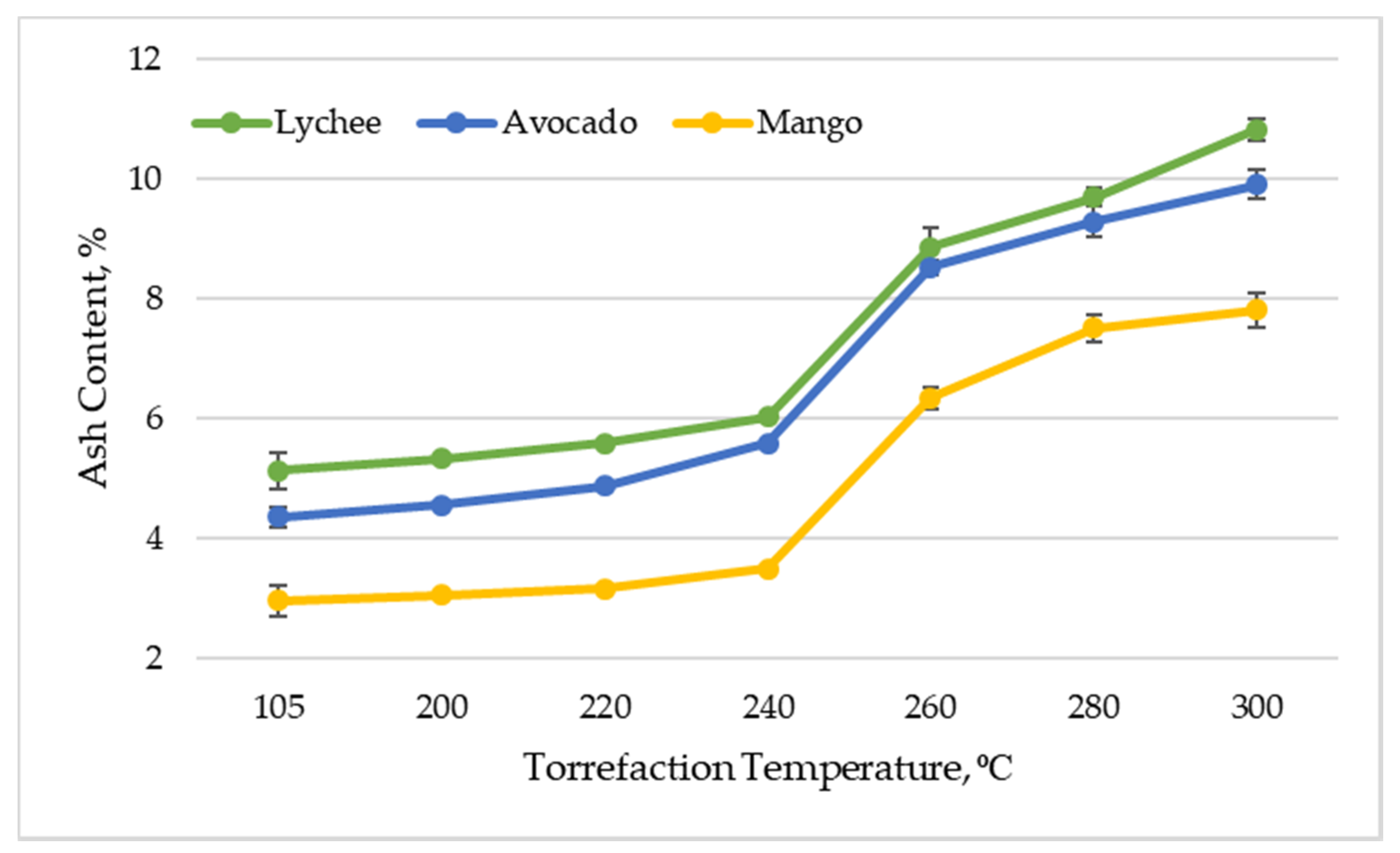
In the dry state (drying at 105 °C for 24 h), the ash content of the substrates was as follows: for mango seeds—2.96% (±0.26%), for avocado seeds—4.36% (±0.17%) and for lychee seeds—5.12% (±0.30%). After the torrefaction process, these values increased significantly. Finally, after thermal treatment at 300 °C, the highest ash content was recorded for lychee seeds—10.81% (±0.18%), and the lowest for mango seeds—7.80% (±0.29%). The ash content for avocado seeds was 9.90% (±0.24%). It should also be noted that the ash content increased with increasing temperature of the torrefaction process (Figure 1). Lin and Zheng [33] also obtained a significant increase of ash content in mango seeds after torrefaction.
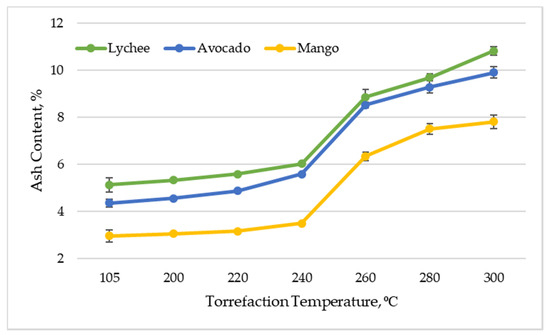
Figure 1.
Ash content vs. torrefaction temperature of exotic fruit seeds.
Ultimately, as a result of torrefaction, the ash content in all types of exotic fruit seeds more than doubled. This behavior is related to the release of volatile substances during thermal decomposition. Due to the effect of significant mass loss, the percentage of non-combustible parts (ash) in the total material increases. This phenomenon is commonly observed during research on the process of biomass torrefaction [37,38,39]. From a technical point of view, it is desirable that the ash content is as low as possible, as the high proportion of this element in the raw materials subjected to thermochemical transformations is associated with several significant technical barriers. These thermochemical transformations are associated with the formation of slag, deterioration of the quality of products, corrosion, and additional financial expenses related to the need to process the raw material [40]. Nonetheless, a small part of the ash contained in the biomass can have a positive effect on the pyrolysis process. This is due to the fact that this element can provide catalytic activity for the ameliorated transformation of biomass into valuable products [41]. The values of ash content in torreficates obtained in the experiment (made of exotic fruit seeds) are similar to the ash content in thermochemically untreated wood/agricultural biomass fuels in the form of pellets (ash content in the range of a few tenths of a percent up to a few percent) [42]. This proves the possibility of using torrefied exotic fruit seeds as energy fuel. It is good to note that the ash content in coal may reach even 35% [43], which additionally emphasizes the competitiveness of the proposed solution. The value of ash content in torreficates is, however, slightly higher than that for industrial charcoal, for which the value of the parameter usually ranges from 0.4 to 4% [44]. The added value is that biomass ash can be used for fertilization purposes, which eliminates the problem of furnace waste management, as is the case with coal combustion. The appropriate dose of biomass ash may have a favorable result on the chemical properties of the soil, thus contributing to increasing plant yields [45]. For this reason, ash additives to biochar can serve as an interesting alternative to traditional mineral fertilizers [45,46].
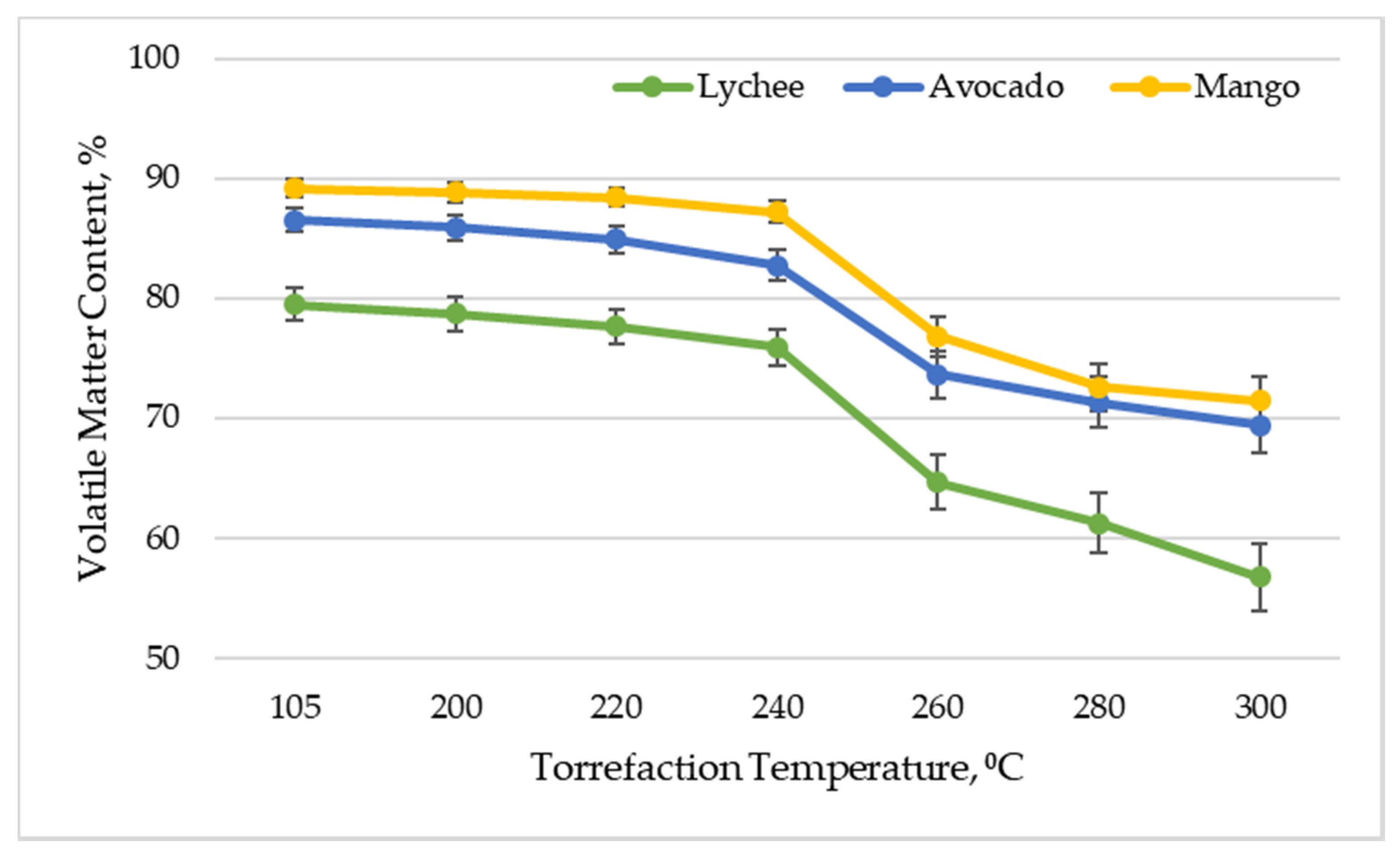
Figure 2 shows the content of volatile matter (combustible part of the fuel) of exotic fruit seeds depending on the torrefaction temperature. This parameter is an important issue in the context of the energetic use of the material. It is responsible for the ignition and combustion stability in the furnace of the boiler.
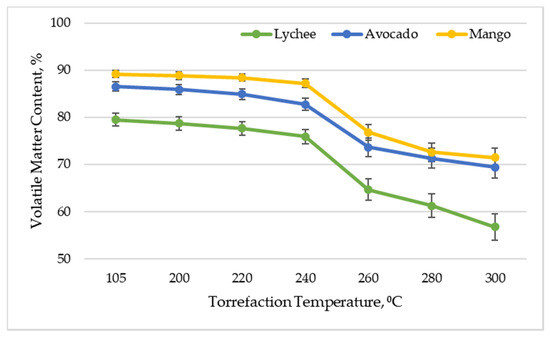
Figure 2.
Volatile matter content vs. torrefaction temperature of exotic fruit seeds.
The conducted analysis showed that, as a result of increasing the temperature of the torrefaction process, the content of volatile matter decreases. In the case of mango seeds, the content of volatile matter decreased from 89.17% ± 0.76% for dried material to 71.45% ± 2.01% for torrefied material at 300 °C. Similar relationships were obtained for other types of biomass. For avocado seeds, the volatile matter content decreased from 86.50% ± 1.00% to 69.36% ± 2.27%, and for lychee seeds, the volatile matter content decreased from 79.50% ± 1.32% to 56.71% ± 2.79%. After torrefaction (300 °C), Lin and Zheng [33] also reported a decrease in VMC by almost 20 percentage points.
The kinetics of the process of lowering the content of volatile matter in the biomass, depending on the torrefaction temperature, is consistent with the data observed in the literature. By examining the effect of torrefaction on the physical and chemical parameters of biomass (wood chips and oil palm fronds), Homdoung et al. [47] obtained the content of volatile matter at the level of 70.31–73.09% in the raw material and 41.9–43.5% after torrefaction in a similar temperature range. Similar values were also obtained by Akhtar et al. [48] when investigating the impact of torrefaction on the properties of waste biomass from the agricultural industry (cotton stalks, corn cobs, and sunflower). It is worth noting that the torrefied seeds of exotic fruit are still characterized by twice as much volatile matter as that in bituminous coal and five times as much as that in charcoal [49].
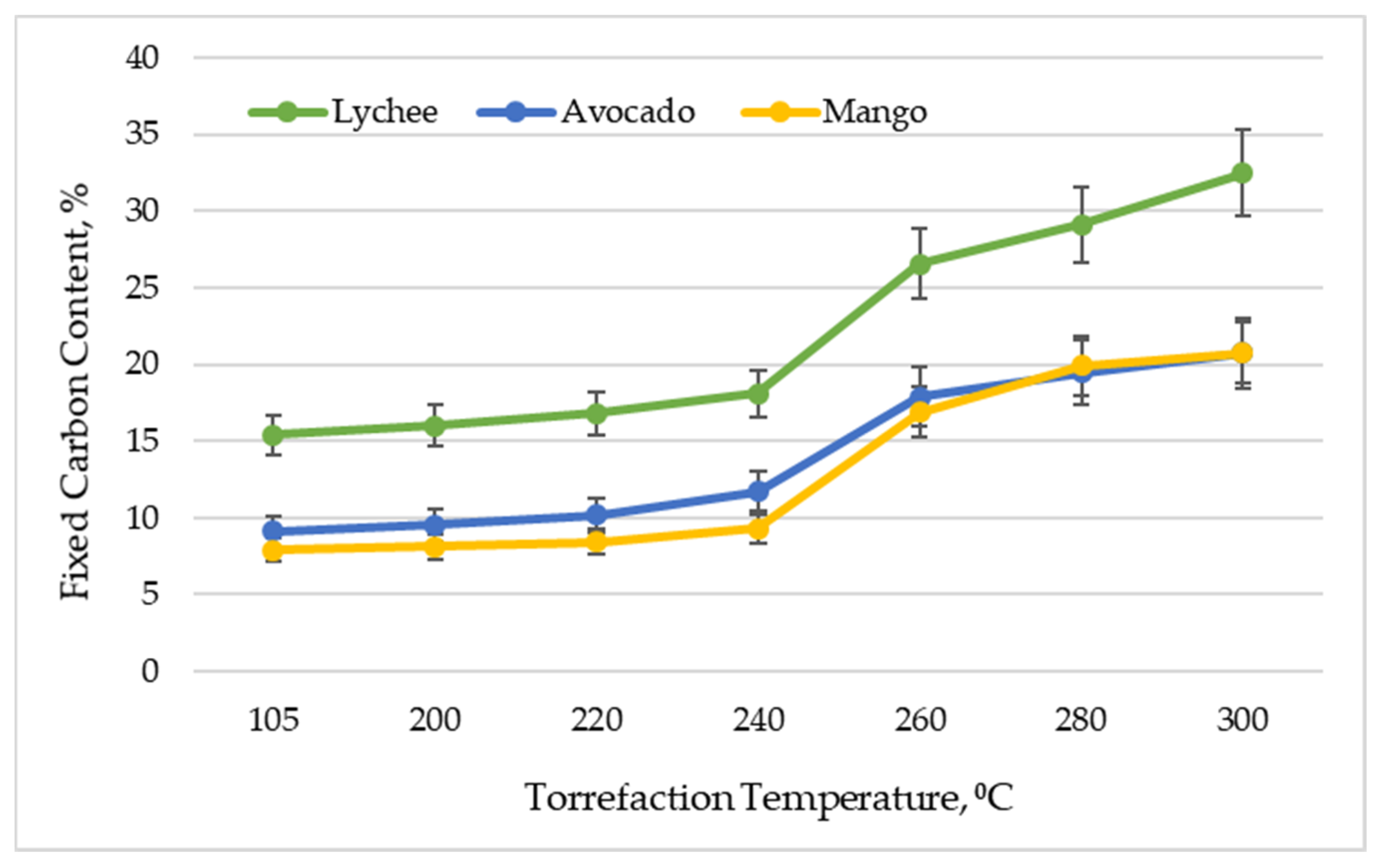
Figure 3 shows the fixed carbon content in exotic fruit seeds depending on the torrefaction temperature. This parameter reflects the solid combustible parts in the material. As expected, along with the increase of the torrefaction temperature, an increase in the content of fixed carbon [50] was observed. The highest content of fixed carbon in the raw material was found in lychee seeds—15.38% ± 1.32%, and the lowest for mango seeds—7.87% ± 0.76%. It should be noted, however, that this material is characterized by high differentiation, because in the work [33] the authors obtained FCC at the level of 2.2% (dried raw material), and after torrefaction (300 °C)—4.7%. Avocado seeds had a fixed carbon content of 9.14% ± 1.00%. As a result of torrefaction (300 °C), the values of this parameter improved to 32.47% ± 2.79% for lychee and 20.74% ± 2.01% for mango and 20.74% ± 2.27% for avocado. Generally, a higher fixed carbon content in the biomass is desirable due to the potentially greater heat yields that can be generated by utilizing the material [51]. The values of bound carbon obtained in the experiment coincide with other types of biomass (including straw, carthamus chips, cardoon chips, olive and wine pruning, residues from the maintenance/cleaning of rivers), analyzed by various authors [52]. Nonetheless, the value of this parameter is much lower (even after torrefaction) than that of coal, in which it oscillates around 60% [53], and industrial charcoal, in which it oscillates around 65–85% [44].
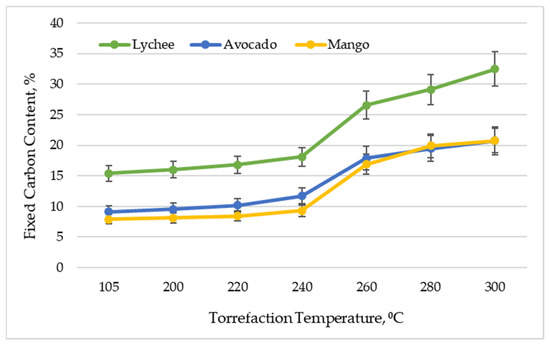
Figure 3.
Fixed carbon content vs. torrefaction temperature of exotic fruit seeds.
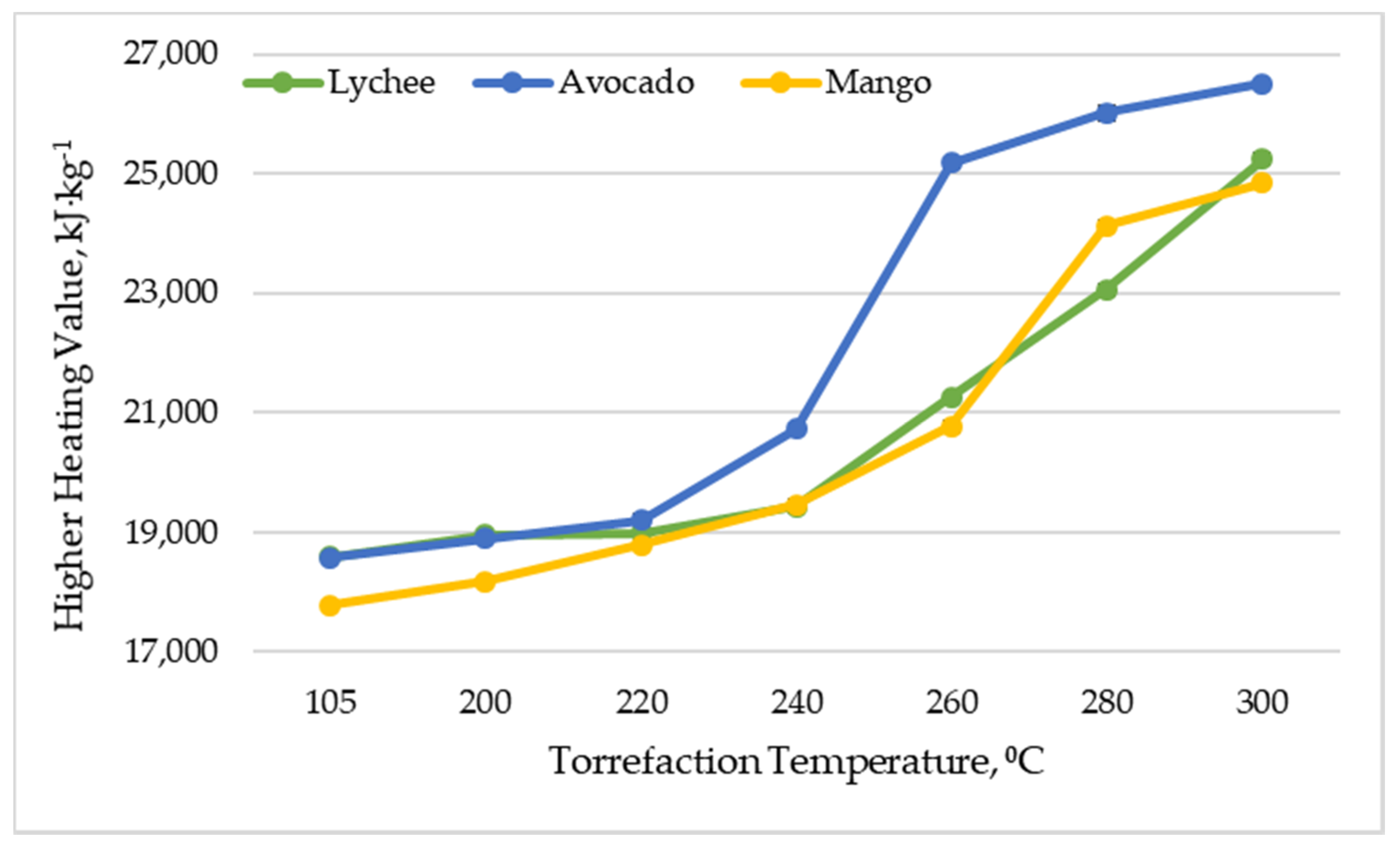
The higher heating value (HHV) is a parameter relating to the heat released from fuel combustion with primary water and produced in a condensed state [54,55,56]. It is considered to be one of the key properties of fuels, and explains the fuel’s energy content and determines its efficient use (renewable or non-renewable) [55]. The higher heating value of dried lychee, mango and avocado seeds ranged from 17,789 to 18,584 kJ∙kg−1 (Figure 4), which is a result similar to that of other types of biomass, such as corncob, corn stover, and sunflower shell (17.0–18.0 MJ∙kg−1), but slightly lower than that of others, such as beech wood, hazelnut shell, wood bark, olive husk, and walnut shell (19.2–21.6 MJ∙kg−1) [55]. Along with increasing the torrefaction temperature, an increase in the higher calorific value of the substrates was noted. As a result of torrefaction in 300 °C, avocado seeds (26,513 kJ∙kg−1 ± 53.75 kJ∙kg−1) had the highest HHV, and mango seeds had the lowest (24,842 kJ∙kg−1 ± 55.86 kJ∙kg−1). The obtained values are slightly lower than those presented in the work [33], where the authors obtained HHV at 20–29 MJ∙kg−1 in the temperature range of 210–300 °C by torrefaction of mango seeds. Lychee seeds had a higher heating value of 25,241 kJ∙kg−1 ± 96.87 kJ∙kg−1. The effect of torrefaction temperature on higher calorific value was also confirmed in other research studies, where it was found that torrefaction influences the release of volatile substances, which results in high energy release; hence, a higher torrefaction reaction will produce greater biomass a higher heating value [57,58,59].
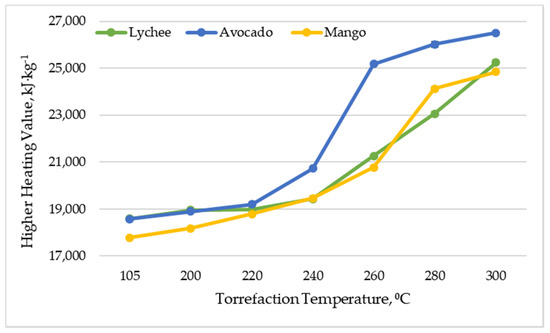
Figure 4.
Higher heating value vs. torrefaction temperature of exotic fruit seeds.
3.2. Results of the Physical Analysis
Due to the fact that the torrefaction process significantly affects the physical parameters of biomass [60], the mass loss, bulk density, specific density and porosity were evaluated in relation to the process temperature.
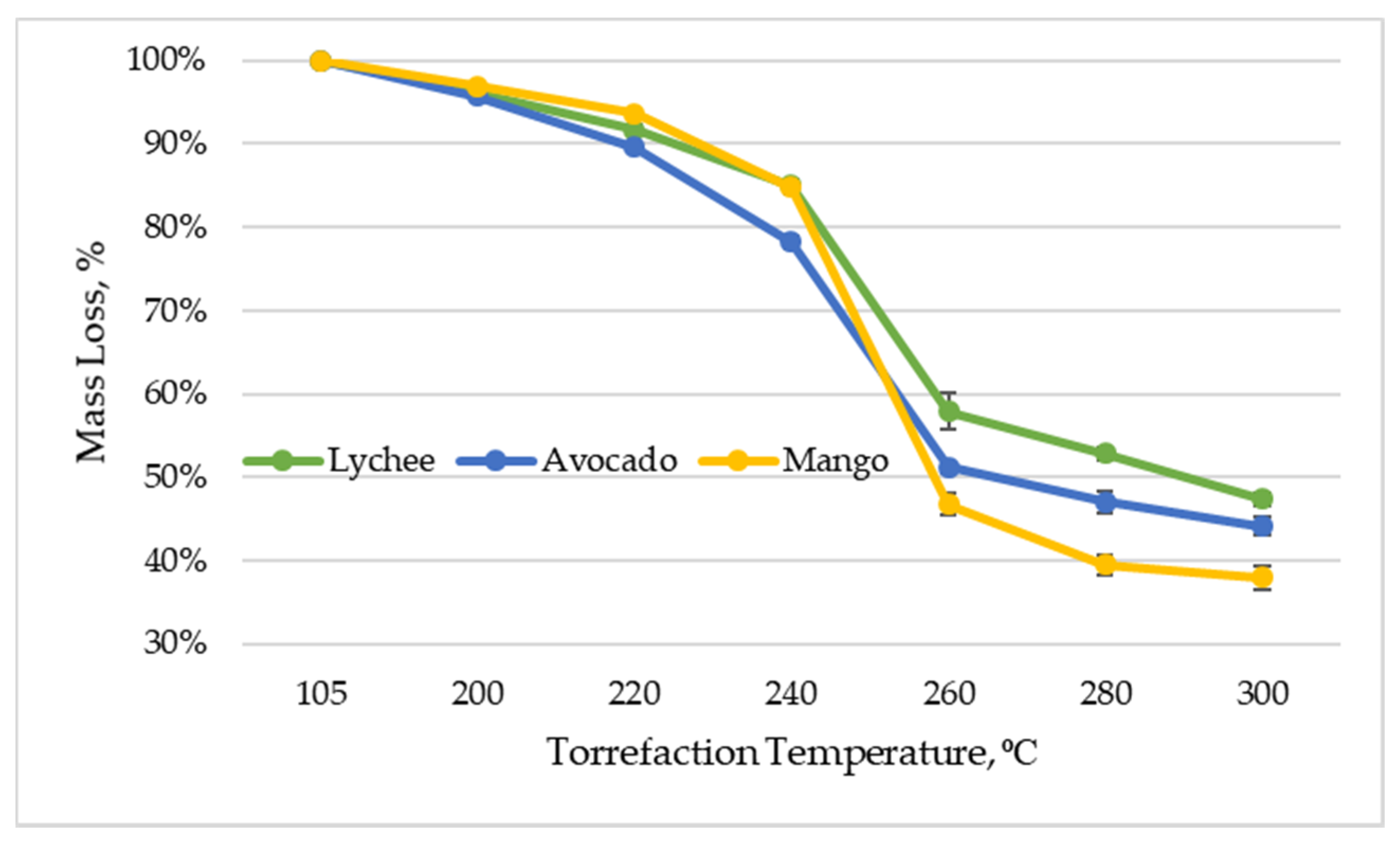
As mentioned earlier, as a result of torrefaction, volatile substances are released during the thermal decomposition of biomass, which results in a mass loss in relation to the original material. Figure 5 shows the percentage of mass loss in relation to the mass of dried material, depending on the temperature of the torrefaction process. It should be mentioned that the mass yield in all torrefied exotic fruit seeds decreased by more than 50% after the process at a temperature of 300 °C. The mass yield of lychee seeds was 47.36% (±0.80%), mango seeds—37.94% (±1.34%), and avocado seeds—44.06% (±1.08%). These results are consistent with the research of other authors, where the loss in the mass of waste fruit as a result of the torrefaction process was also evaluated, and the mass yield after the torrefaction process was 56% at 300 °C [61]. It is worth noting, however, that the weight loss may differ significantly depending on the type of biomass; an example is waste biomass from the forestry industry, where, after torrefaction at 320 °C, the mass loss of oak acorns, horse chestnuts, and spruce cones was 70.6–84.4% [51].
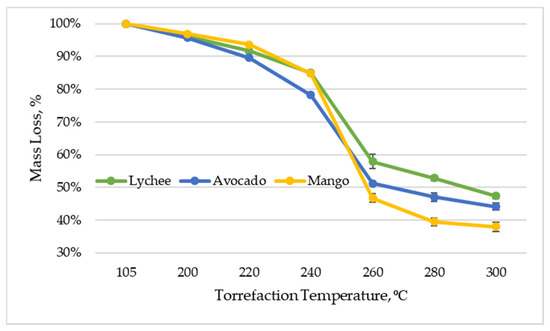
Figure 5.
Mass loss vs. torrefaction temperature of exotic fruit seeds.
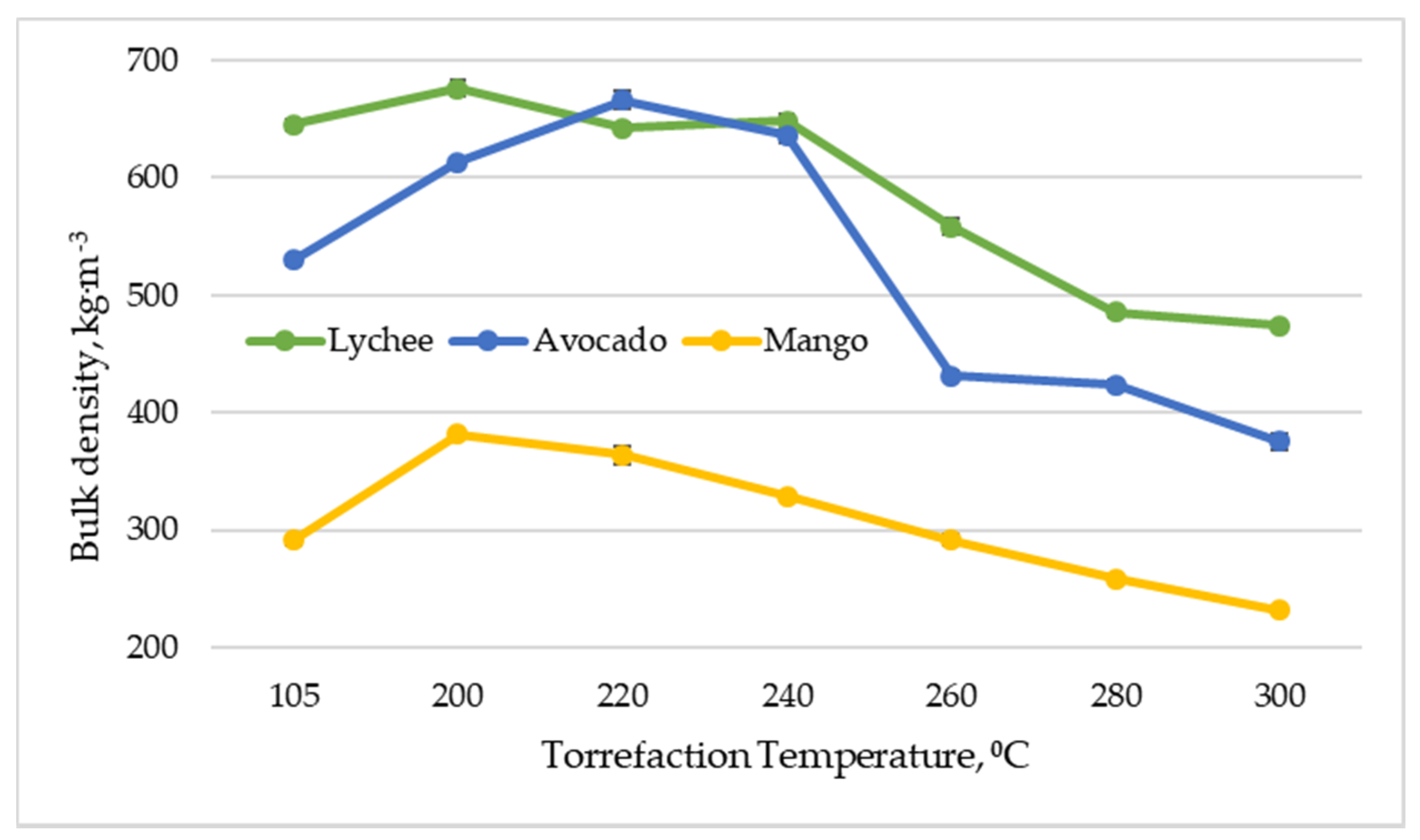
Bulk density is defined as the key biomass quality parameter due to the fact that it is used to estimate the spatial requirements for handling, storage and transport, in addition to transport and storage costs [62]. The bulk density of dried exotic fruit seeds varied and ranged from 292 to 645 kg∙m−3, with mango seeds having the lowest value and lychee seeds the highest (Figure 6). In general, the bulk density of the torreficates was much higher than that observed for industrial charcoal (180–350 kg∙m−3) [44]. In most cases (except for the minimum decrease at 240 °C for lychee seeds), the torrefaction process at 240 °C had a positive effect on the value of the bulk density. The highest values of the parameter for lychee and mango seeds were obtained at 200 °C (676 and 382 kg∙m−3, respectively), and for avocado seeds at 220 °C (666 kg∙m−3). Then, a decrease in bulk density was observed for all types of biomass.
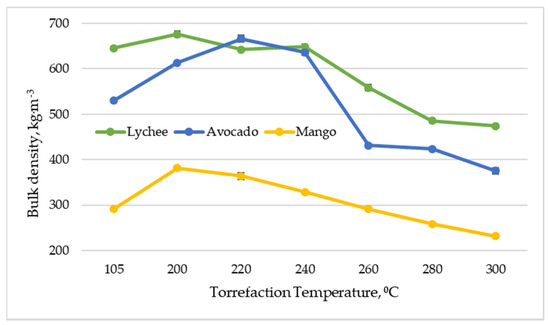
Figure 6.
Bulk density depending vs. torrefaction temperature of exotic fruit seeds.
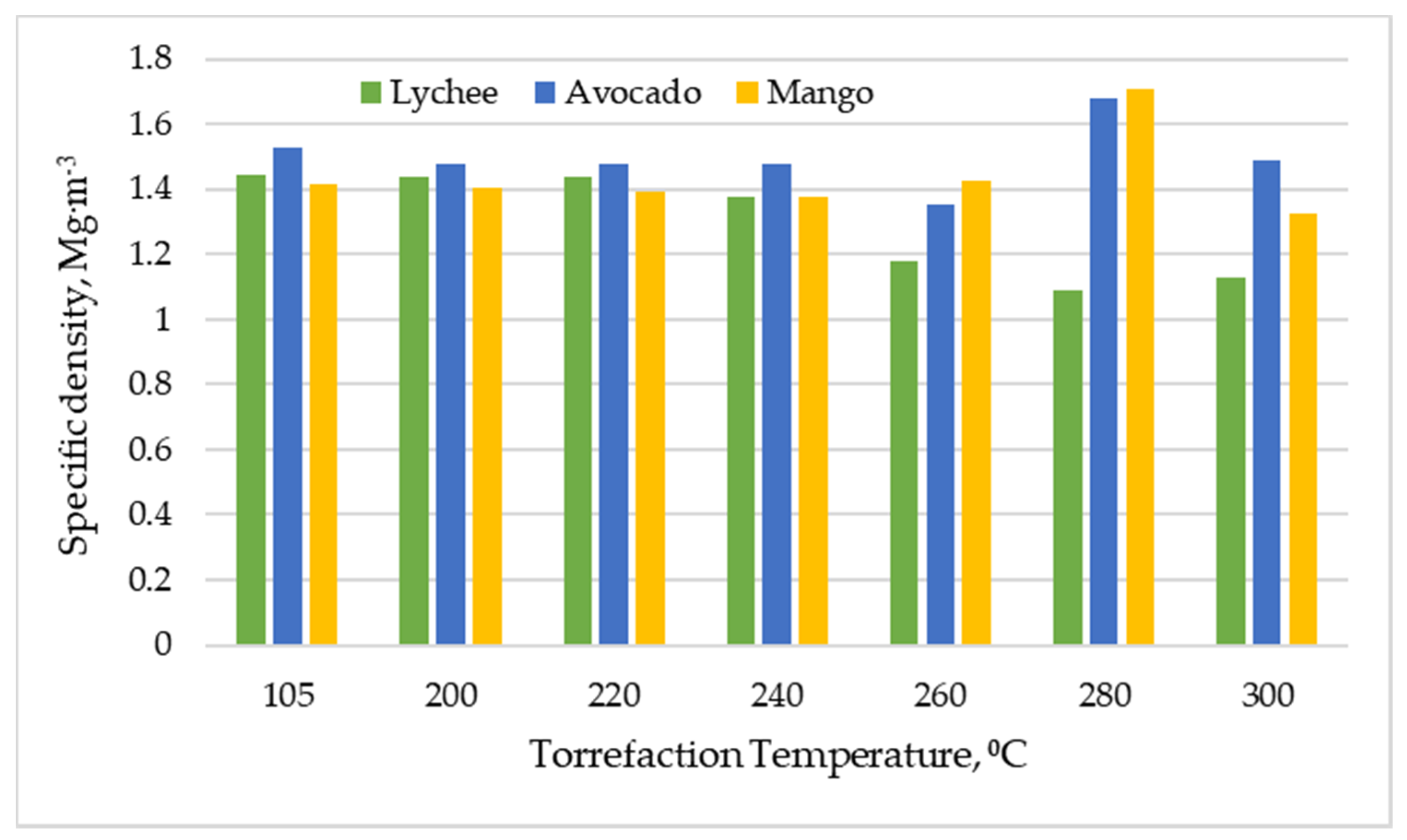
Torrefaction also did not notably affect the specific density of the biomass when the process was performed at temperatures of 200 to 240 °C. As can be seen in Figure 7, for lychee seeds, the specific density dropped to the level of 1.08 to 1.18 Mg∙m−3, whereas for mango and avocado seeds it increased to a maximum of 1.71 and 1.68 Mg∙m−3, respectively (at 280 °C). This is probably related to the complex processes of decomposition of organic compounds in the tested materials and the significant expansion of the active surface of the particles.
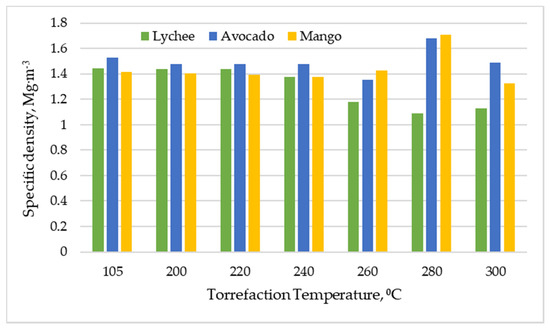
Figure 7.
Specific density vs. torrefaction temperature of exotic fruit seeds.
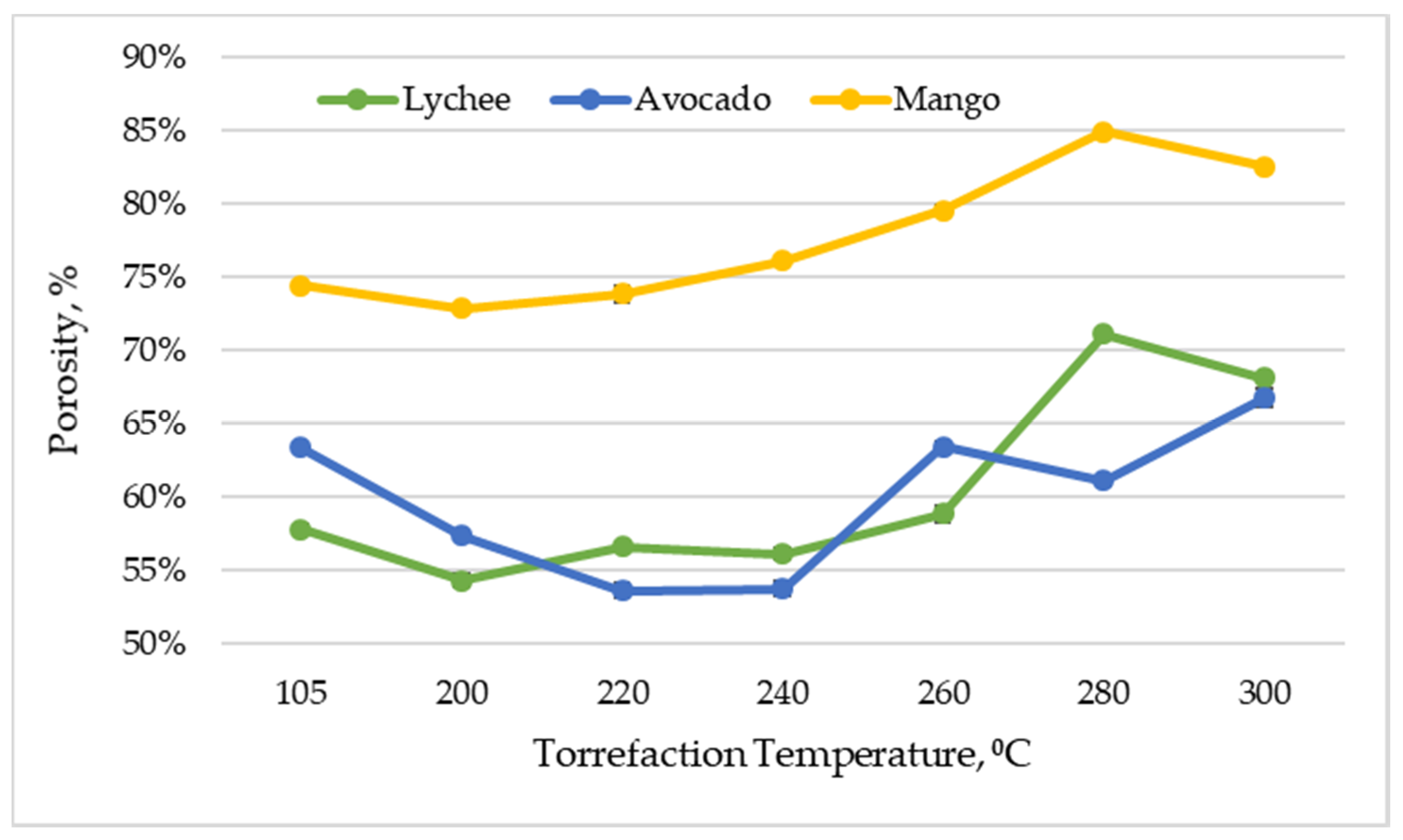
Figure 8 shows the effect of the torrefaction temperature on the porosity of the analyzed materials. The porosity of the materials used in the experiment ranged from 72.79% (±0.23%) to 84.86% (±0.18%) for mangoes, 53.59% (±0.52%) to 66.70% (±0.61%) for avocados, and 54.29% (±0.48%) to 71.05% (±0.20%) for lychees. In general, the level of porosity in the materials was different at different torrefaction temperatures, but a growing trend is noticeable, which results from the fact that the torrefaction process significantly increases the porosity of the biomass due to the degradation of its components [39].
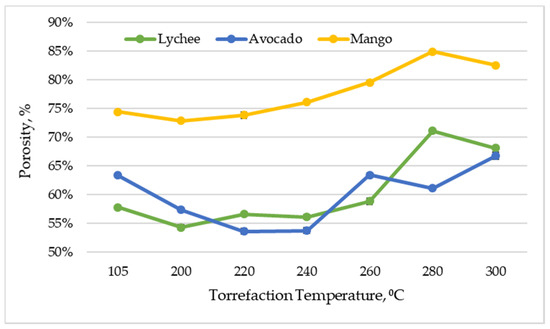
Figure 8.
Porosity vs. torrefaction temperature of exotic fruit seeds.
3.3. Results of the Hydrophobicity Analysis
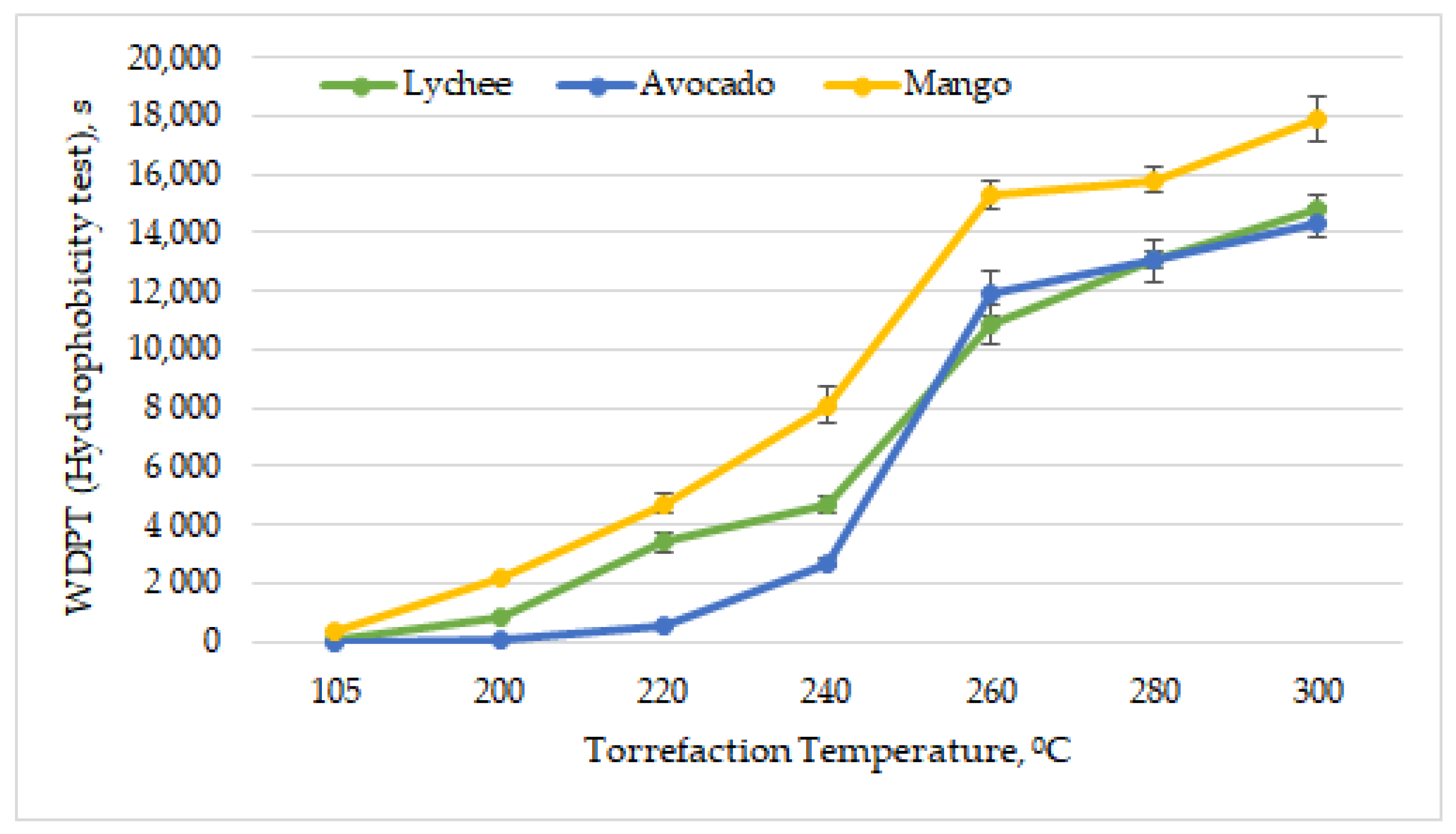
The ability of biomass to absorb moisture is an important parameter from the point of view of harvesting, handling, transport and storage of biomass [63]. Dried exotic fruit seeds were characterized by various hydrophobic properties. The most resistant to the absorption of water drops was found to be the dried mango seeds, the character of which was determined to be highly hydrophobic (WDPT = 330 s ± 19 s). Dried lychee seeds were found to be slightly hydrophobic (WDPT = 43 s ± 3 s) and avocado seeds to be hydrophilic (WDPT = 4.4 s ± 0.5 s). As can be observed in Figure 9, with increasing torrefaction temperature, the penetration time of water droplets was greater. Exotic fruit pits obtained an extremely hydrophobic status (WDPT > 3600 s, according to Table 2) at relatively low temperatures. Mango seeds achieved extremely hydrophobic status after torrefaction at 220 °C, lychee seeds at 240 °C, and avocado seeds ay 260 °C.
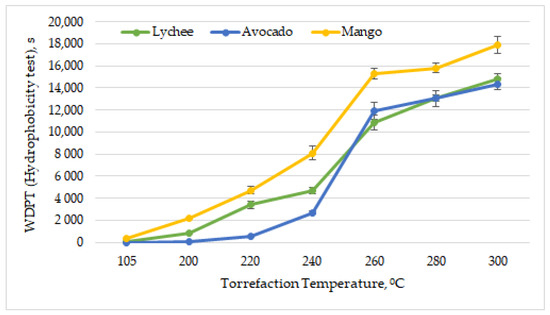
Figure 9.
Hydrophobicity vs. torrefaction temperature of exotic fruit seeds.
Previous research works also confirmed that, due to torrefaction, the biomass gains hydrophobic properties [64]. In most cases, along with increasing the torrefaction temperature, the hydrophobic properties of biomass improve [65,66]; however, there are also research works in which this rule does not apply [67]. This is mainly because, during the torrefaction process, a common deodorization reaction takes place, and the carbon dioxide is formed on the decarboxylation of unstable carboxyl groups in the hemicellulose structure [68]. Hence, a relationship was found between the presence of hydroxyl groups and the thermal treatment of the substrate [69]. By increasing the torrefaction temperatures, more O-H bonds are dissolved and dehydrated, making the material more hydrophobic [70,71].
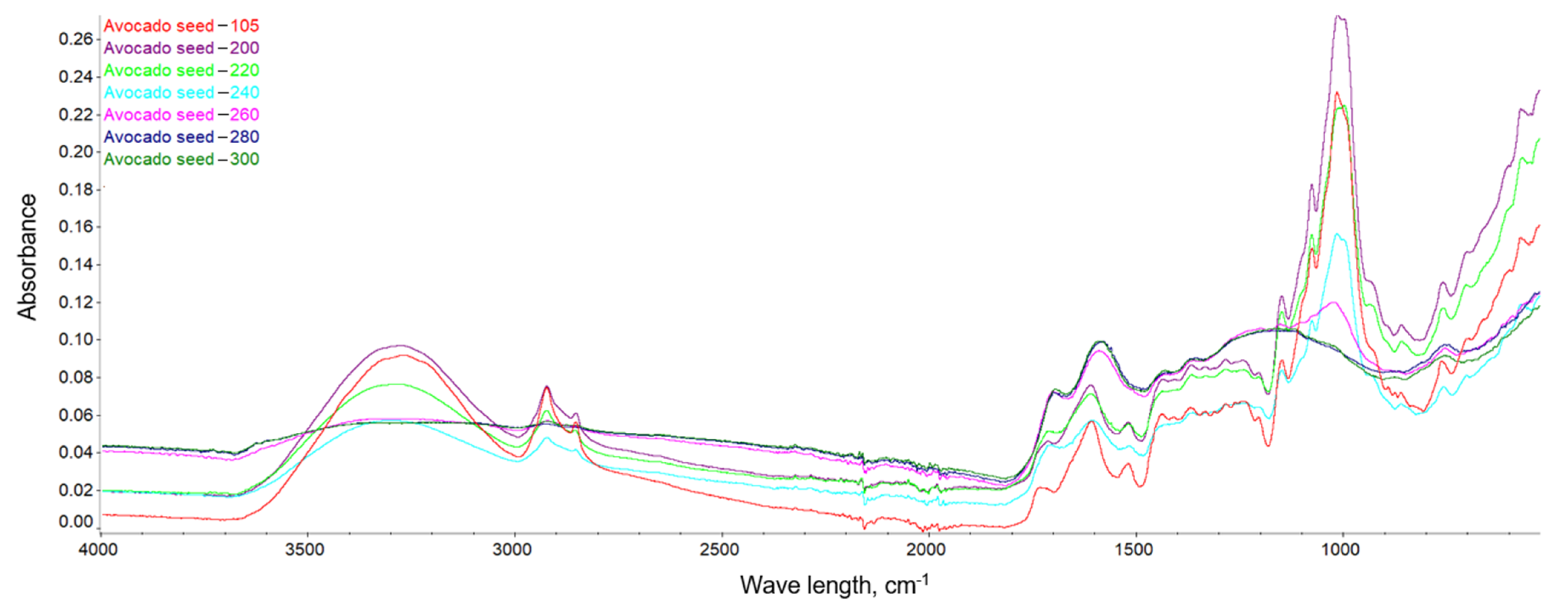
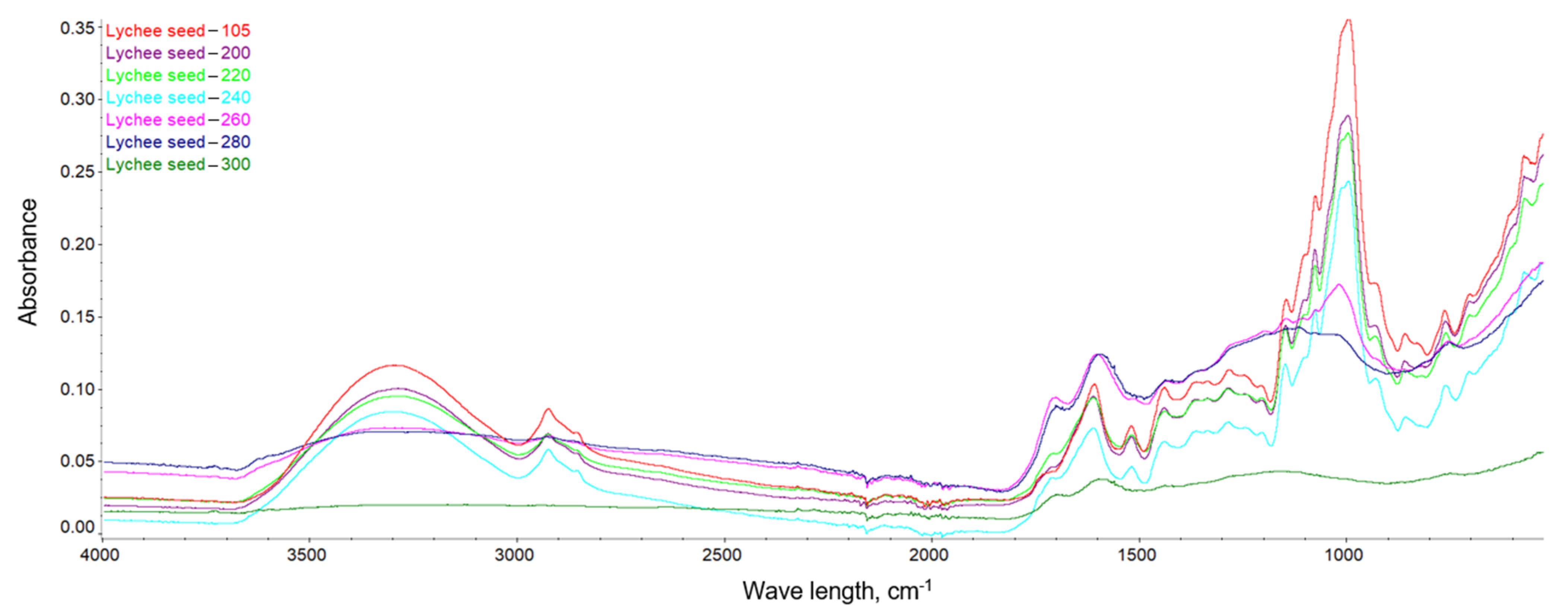
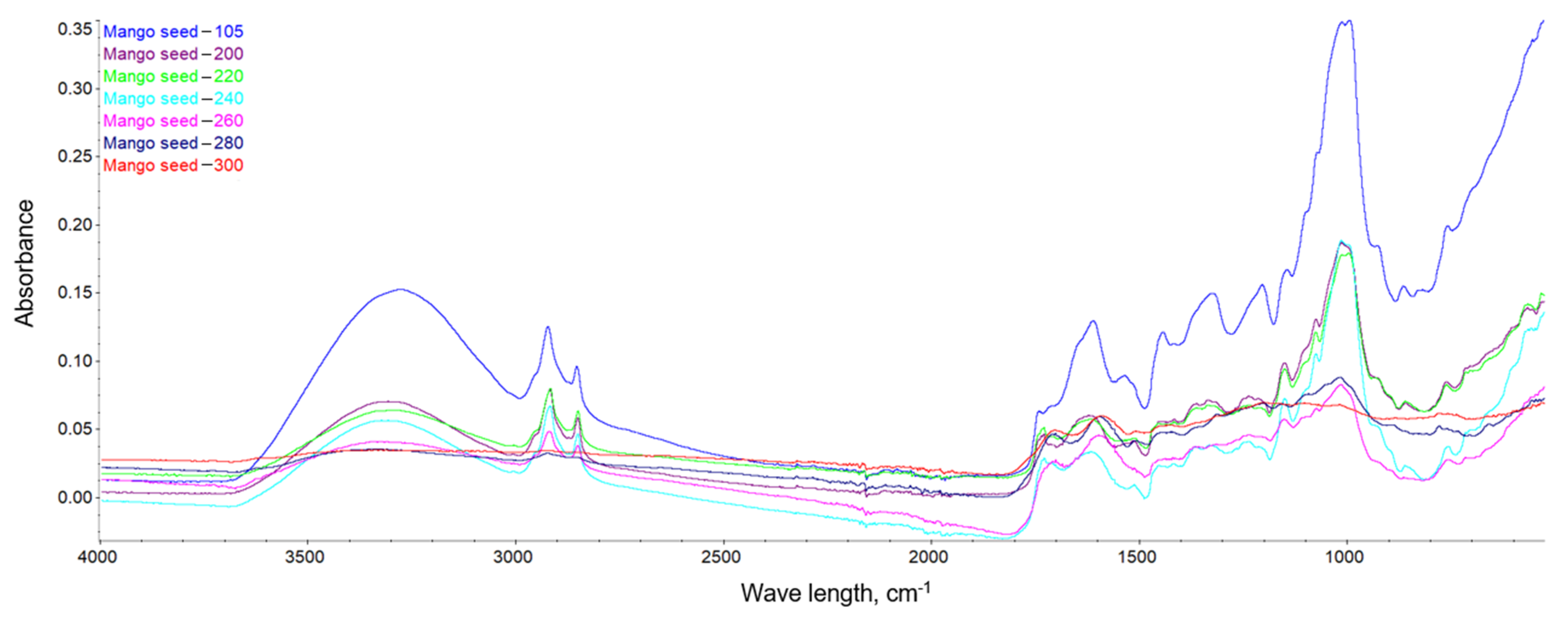
This dependence can be observed in Figure 10, Figure 11 and Figure 12, where the intensification of the particular absorption peaks of the -OH groups was 3400–3200 cm−1. In general, it can be seen that the torrefaction process caused a reduction of hydroxyl groups in the material resulting from the reduction of cellulose and hemicellulose contained in the substrates. As the temperature increases, the absorbance of the sample decreases, indicating the breakdown of the structures of hydroxyl groups, which play a key role in the process of binding water in the material [65]. The greatest dependencies are observed between extreme temperatures of 200 and 300 °C. However, between individual temperatures there is also a noticeable difference in the level of absorbance; thus, it can be concluded that a higher temperature of the torrefaction process improves the hydrophobic properties.
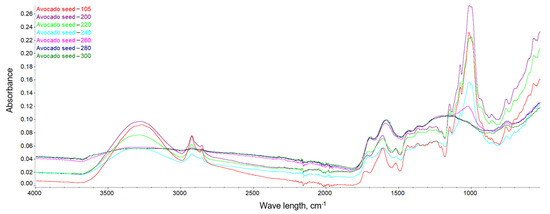
Figure 10.
FTIR spectra of the avocado seeds.
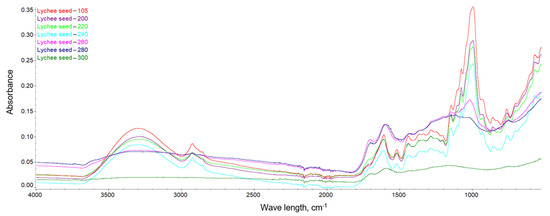
Figure 11.
FTIR spectra of the lychee seeds.
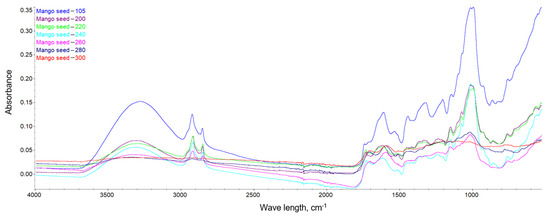
Figure 12.
FTIR spectra of the mango seeds.
Detailed results of all analysis are shown in the Supplementary Materials (Tables S1–S9).
3.4. Main Results of Statistical Analysis
Table 3 shows the results of the one-way ANOVA for the dependent variable, torrefaction temperature (TT). The performed analysis showed that the torrefaction temperature significantly influenced each of the eight analyzed traits in all considered exotic fruit seeds. For each case, the p-value was 0.00. Detailed results of the analysis of variance and Fisher’s LSD post hoc test, which show the statistical differences between the torrefaction temperatures in mango, lychee and avocado seeds, are included in Supplementary Materials (Tables S10–S34).

Table 3.
Results of analysis of variance (one-way ANOVA) for the dependent variable (TT).
4. Conclusions
The energy utilization of wasted and lost food is an interesting alternative to the management of waste biomass. This is because the raw materials used do not constitute direct competition for soil resources and can help fight climate change by improving the state of the environment through ecological balance and developing a circular bioeconomy. However, due to the unfavorable physical and chemical properties of waste biomass from the food sector, methods of biomass valorization must be used in order to improve and enable their utilization in conventional coal-fed power plants.
In this research, torrefaction, as a method of valorizing biomass residues (avocado, mango and lychee seeds), was applied. The results showed that torrefaction significantly improves the physical-chemical properties of materials. Exotic fruit seed torreficates are characterized by increased resistance to water absorption, higher heating value and higher carbon content, which allows them to be classified as an attractive alternative fuel to wooden biomass or coal. It is also worth mentioning that, compared to coal, the potential use of exotic fruit seeds as an alternative fuel can have significant economic benefits. Although pre-heat treatment is required for their valorization, the added value lies in the efficient use of bio-waste, lack of extraction costs (unlike in the case of fossil fuels) and waste management, and a lower environmental burden.
However, the selection of the torrefaction temperature should be strictly determined by an analysis of the fuel properties, to properly optimize the biomass valorization process, which will also translate into financial results related to the reduction in energy inputs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en15020612/s1, Table S1. Ash content depending on the torrefaction temperature of exotic fruit seeds; Table S2. Volatile matter content depending on the torrefaction temperature of exotic fruit seeds; Table S3. Fixed carbon content depending on the torrefaction temperature of exotic fruit seeds; Table S4. Higher heating value depending on the torrefaction temperature of exotic fruit seeds; Table S5. Mass loss depending on the torrefaction temperature of exotic fruit seeds; Table S6. Bulk density depending on the torrefaction temperature of exotic fruit seeds; Table S7. Specific density depending on the torrefaction temperature of exotic fruit seeds; Table S8. Porosity depending on the torrefaction temperature of exotic fruit seeds; Table S9. WDPT (Hydrophobicity test) depending on the torrefaction temperature of exotic fruit seeds. Table S10. Result of the Fisher LSD test for the content of lychee ash at a variable torrefaction temperature; Table S11. Result of the Fisher LSD test for the content of mango ash at a variable torrefaction temperature; Table S12. Result of the Fisher LSD test for the content of avocado ash at a variable torrefaction temperature; Table S13. Result of the Fisher LSD test for the content of lychee volatile matter at a variable torrefaction temperature; Table S14. Result of the Fisher LSD test for the content of mango volatile matter at a variable torrefaction temperature; Table S15. Result of the Fisher LSD test for the content of avocado volatile matter at a variable torrefaction temperature; Table S16. Result of the Fisher LSD test for the content of mango fixed carbon at a variable torrefaction temperature; Table S17. Result of the Fisher LSD test for the content of avocado fixed carbon at a variable torrefaction temperature; Table S18. Result of the Fisher LSD test for the content of lychee fixed carbon at a variable torrefaction temperature; Table S19. Result of the Fisher LSD test for the content of lychee HHV at a variable torrefaction temperature; Table S20. Result of the Fisher LSD test for the content of mango HHV at a variable torrefaction temperature; Table S21. Result of the Fisher LSD test for the content of avocado HHV at a variable torrefaction temperature; Table S22. Result of the Fisher LSD test for the mass loss of lychee at a variable torrefaction temperature; Table S23. Result of the Fisher LSD test for the mass loss of mango at a variable torrefaction temperature; Table S24. Result of the Fisher LSD test for the mass loss of avocado at a variable torrefaction temperature; Table S25. Result of the Fisher LSD test for the bulk density of lychee at a variable torrefaction temperature; Table S26. Result of the Fisher LSD test for the bulk density of mango at a variable torrefaction temperature; Table S27. Result of the Fisher LSD test for the bulk density of avocado at a variable torrefaction temperature; Table S28. Result of the Fisher LSD test for the porosity of lychee at a variable torrefaction temperature; Table S29. Result of the Fisher LSD test for the porosity of mango at a variable torrefaction temperature; Table S30. Result of the Fisher LSD test for the porosity of avocado at a variable torrefaction temperature; Table S31. Result of the Fisher LSD test for the hydrophobicity of lychee at a variable torrefaction temperature; Table S32. Result of the Fisher LSD test for the hydrophobicity of mango at a variable torrefaction temperature; Table S33. Result of the Fisher LSD test for the hydrophobicity of avocado at a variable torrefaction temperature; Table S34. Results of analysis of variance (one-way ANOVA) for the dependent variable (TT).
Author Contributions
Conceptualization, A.D.; Data curation, A.D. and Ł.S.; Formal analysis, Ł.S., A.D., T.N. and J.M.; Funding acquisition, A.D.; Investigation, Ł.S., T.N. and J.M.; Methodology, A.D., T.N. and Ł.S.; Resources, J.M.; Software, Ł.S.; Supervision, A.D.; Validation, A.D. and Ł.S.; Visualization, Ł.S. and A.D.; Writing—original draft, Ł.S. and A.D.; Writing—review and editing, A.D. All authors have read and agreed to the published version of the manuscript.
Funding
Publication financed by the project “Access to scientific resources for 2020” Order No. 78/2020 (3 March 2020) introduced by the Rector of Wrocław University of Environmental and Life Sciences.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ishangulyyev, R.; Kim, S.; Lee, S.H. Understanding Food Loss and Waste—Why Are We Losing and Wasting Food? Foods 2019, 8, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abiad, M.G.; Meho, L. Food loss and food waste research in the Arab world: A systematic review. Food Secur. 2018, 10, 311–322. [Google Scholar] [CrossRef]
- FAO. Global Initiative on Food Loss and Waste Reduction; FAO: Rome, Italy, 2015. [Google Scholar]
- Rezaei, M.; Liu, B. Food loss and waste in the food supply chain. Nutfruit 2017, 71, 26–27. [Google Scholar]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panouillé, M.; Ralet, M.-C.; Bonnin, E.; Thibault, J.-F. Recovery and reuse of trimmings and pulps from fruit and vegetable processing. In Handbook of Waste Management and Co-Product Recovery in Food Processing; Woodhead Publishing: Abington Hall, UK, 2007; pp. 417–447. [Google Scholar]
- Mitra, S.; Pathak, P.; Lembisana Devi, H.; Chakraborty, I. Utilization of seed and peel of mango. In Proceedings of the IX International Mango Symposium 992, Sanya, China, 8–12 April 2010; pp. 593–596. [Google Scholar]
- Lee, W.-J.; Lee, M.-H.; Su, N.-W. Characteristics of papaya seed oils obtained by extrusion-expelling processes. J. Sci. Food Agric. 2011, 91, 2348–2354. [Google Scholar] [CrossRef] [PubMed]
- Parni, B.; Verma, Y. Biochemical properties in peel, pulp and seeds of Carica papaya. Plant Arch. 2014, 14, 565–568. [Google Scholar]
- Sirisompong, W.; Jirapakkul, W.; Klinkesorn, U. Response surface optimization and characteristics of rambutan (Nephelium lappaceum L.) kernel fat by hexane extraction. LWT-Food Sci. Technol. 2011, 44, 1946–1951. [Google Scholar] [CrossRef]
- Issara, U.; Zzaman, W.; Yang, T. Rambutan seed fat as a potential source of cocoa butter substitute in confectionary product. Int. Food Res. J. 2014, 21, 25–31. [Google Scholar]
- Altendorf, S. Major Tropical Fruits Market Review 2017; FAO: Rome, Italy, 2019; p. 10. [Google Scholar]
- Sayago-Ayerdi, S.; García-Martínez, D.L.; Ramírez-Castillo, A.C.; Ramírez-Concepción, H.R.; Viuda-Martos, M. Tropical Fruits and Their Co-Products as Bioactive Compounds and Their Health Effects: A Review. Foods 2021, 10, 1952. [Google Scholar] [CrossRef] [PubMed]
- Fidelis, M.; De Moura, C.; Junior, T.K.; Pap, N.; Mattila, P.H.; Mäkinen, S.; Putnik, P.; Kovačević, D.B.; Tian, Y.; Yang, B.; et al. Fruit Seeds as Sources of Bioactive Compounds: Sustainable Production of High Value-Added Ingredients from By-Products within Circular Economy. Molecules 2019, 24, 3854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabas, D.; Shegog, R.; Ziegler, G.; Lambert, J. Avocado (Persea americana) Seed as a Source of Bioactive Phytochemicals. Curr. Pharm. Des. 2013, 19, 6133–6140. [Google Scholar] [CrossRef] [PubMed]
- Kosińska, A.; Karamać, M.; Estrella, I.; Hernández, T.; Bartolomé, B.; Dykes, G. Phenolic Compound Profiles and Antioxidant Capacity of Persea americana Mill. Peels and Seeds of Two Varieties. J. Agric. Food Chem. 2012, 60, 4613–4619. [Google Scholar] [CrossRef] [PubMed]
- Garnett, T.; Roos, E.; Little, D.C. Lean, Green, Mean, Obscene...? What Is Efficiency? And Is It Sustainable? Animal Production and Consumption Reconsidered; Food Climate Research Network, University of Oxford: Oxford, UK, 2015. [Google Scholar]
- Haberl, H.; Erb, K.-H.; Krausmann, F. Human Appropriation of Net Primary Production: Patterns, Trends, and Planetary Boundaries. Annu. Rev. Environ. Resour. 2014, 39, 363–391. [Google Scholar] [CrossRef]
- Karlberg, L.; Hoff, H.; Flores-López, F.; Goetz, A.; Matuschke, I. Tackling biomass scarcity—From vicious to virtuous cycles in sub-Saharan Africa. Curr. Opin. Environ. Sustain. 2015, 15, 1–8. [Google Scholar] [CrossRef]
- Cowie, A.; Berndes, G.; Jungigner, M.; Ximenes, F. Response to Chatham House Report “Woody Biomass for Power and Heat: Impacts on the Global Climate”; IEA Bioenergy: Lismore, Australia, 2017. [Google Scholar]
- Muscat, A.; de Olde, E.M.; de Boer, I.; Ripoll-Bosch, R. The battle for biomass: A systematic review of food-feed-fuel competition. Glob. Food Secur. 2019, 25, 100330. [Google Scholar] [CrossRef]
- Cocker-Maciejewska, A. Obróbka wstępna biomasy na potrzeby systemów energetycznych (Biomass pre-treatment for energy purposes). Ochr. Śródowiska Zasobów Nat. 2007, 30, 133–141. (In Polish) [Google Scholar]
- Bergman, P.C.; Kiel, J.H. Torrefaction for Biomass Upgrading. In Proceedings of the 14th European Biomass Conference and Exhibition, Paris, France, 17–21 October 2005. [Google Scholar]
- van der Stelt, M.J.C.; Gerhauser, H.; Kiel, J.H.A.; Ptasinski, K.J. Biomass upgrading by torrefaction for the production of biofuels: A review. Biomass Bioenergy 2011, 35, 3748–3762. [Google Scholar] [CrossRef]
- Esteves, B.M.; Pereira, H.M. Wood modification by heat treatment: A review. BioResources 2009, 4, 370–404. [Google Scholar] [CrossRef]
- Medic, D.; Darr, M.; Shah, A.; Potter, B.; Zimmerman, J. Effects of torrefaction process parameters on biomass feedstock upgrading. Fuel 2012, 91, 147–154. [Google Scholar] [CrossRef]
- Du, S.-W.; Chen, W.-H.; Lucas, J. Pretreatment of biomass by torrefaction and carbonization for coal blend used in pulverized coal injection. Bioresour. Technol. 2014, 161, 333–339. [Google Scholar] [CrossRef]
- Domínguez, M.P.; Araus, K.; Bonert, P.; Sánchez, F.; Miguel, G.S.; Toledo, M. The Avocado and Its Waste: An Approach of Fuel Potential/Application. In Environment, Energy and Climate Change II, 1st ed.; Lefebvre, G., Jiménez, E., Cabañas, B., Eds.; Springer: New York, NY, USA, 2016; Volume 34, pp. 199–223. [Google Scholar]
- Sánchez, F.; Araus, K.; Domínguez, M.P.; Miguel, G.S. Thermochemical Transformation of Residual Avocado Seeds: Torrefaction and Carbonization. Waste Biomass Valorization 2016, 8, 2495–2510. [Google Scholar] [CrossRef]
- Ganeshan, G.; Shadangi, K.P.; Mohanty, K. Thermo-chemical conversion of mango seed kernel and shell to value added products. J. Anal. Appl. Pyrolysis 2016, 121, 403–408. [Google Scholar] [CrossRef]
- Santanna, M.S.; Silveira, E.A.; Caldeira-Pires, A. Termochemical pathways for municipal lignocellulosic waste as biofuel. In Proceedings of the 29th European Biomass Conference and Exhibition, Online, 26–29 April 2021. [Google Scholar]
- Lin, Y.-L.; Zheng, N.-Y. Biowaste-to-biochar through microwave-assisted wet co-torrefaction of blending mango seed and passion shell with optoelectronic sludge. Energy 2021, 225, 120213. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Zheng, N.-Y. Orrefaction of fruit waste seed and shells for biofuel production with reduced CO2 emission. Energy 2021, 225, 120226. [Google Scholar] [CrossRef]
- Doerr, S.H. On Standardizing the “Water Drop Penetration Time” and the “Molarity of An Ethanol Droplet” Techniques to Classify Soil Hydrophobicity: A Case Study Using Medium Textured Soils. Earth Surf. Process. Landf. 1998, 23, 663–668. [Google Scholar] [CrossRef]
- Guatam, R.; Ashwath, N. Hydrophobicity of 43 Potting Media: Its Implications for Raising Seedlings in Revegetation Programs. J. Hydrol. 2012, 430–431, 111–117. [Google Scholar] [CrossRef]
- Bisdom, E.; Dekker, L.; Schoute, J. Water repellency of sieve fractions from sandy soils and relationships with organic material and soil structure. Geoderma 1993, 56, 105–118. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, B.-J.; Colin, B.; Chang, J.-S.; Pétrissans, A.; Bi, X.; Pétrissans, M. Hygroscopic transformation of woody biomass torrefaction for carbon storage. Appl. Energy 2018, 231, 768–776. [Google Scholar] [CrossRef]
- Mafu, L.D.; Neomagus, H.W.; Everson, R.C.; Carrier, M.; Strydom, C.A.; Bunt, J.R. Structural and chemical modifications of typical South African biomasses during torrefaction. Bioresour. Technol. 2016, 202, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Cahyanti, M.; Doddapaneni, T.; Madissoo, M.; Pärn, L.; Virro, I.; Kikas, T. Torrefaction of Agricultural and Wood Waste: Comparative Analysis of Selected Fuel Characteristics. Energies 2021, 14, 2774. [Google Scholar] [CrossRef]
- Liu, H.; Feng, Y.; Wu, S.; Liu, D. The role of ash particles in the bed agglomeration during the fluidized bed combustion of rice straw. Bioresour. Technol. 2009, 100, 6505–6513. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Bakar, M.; Razzaq, A.; Hidayat, S.; Jamil, F.; Amin, M.; Sukri, R.; Shah, N.; Park, Y.-K. Characterization and Thermal Behavior Study of Biomass from Invasive Acacia mangium Species in Brunei Preceding Thermochemical Conversion. Sustainability 2021, 13, 5249. [Google Scholar] [CrossRef]
- Dyjakon, A.; Noszczyk, T. The Influence of Freezing Temperature Storage on the Mechanical Durability of Commercial Pellets from Biomass. Energies 2019, 12, 2627. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Zhu, L.; Liu, C.; Bai, Q. Optimization of the oil agglomeration for high-ash content coal slime based on design and analysis of response surface methodology (RSM). Fuel 2019, 254, 115560. [Google Scholar] [CrossRef]
- Surup, G.R.; Trubetskaya, A.; Tangstad, M. Charcoal as an Alternative Reductant in Ferroalloy Production: A Review. Processes 2020, 8, 1432. [Google Scholar] [CrossRef]
- Wierzbowska, J.; Sienkiewicz, S.; Żarczyński, P.; Krzebietke, S. Environmental Application of Ash from Incinerated Biomass. Agronomy 2020, 10, 482. [Google Scholar] [CrossRef] [Green Version]
- Saletnik, B.; Zagula, G.; Bajcar, M.; Czernicka, M.; Puchalski, C. Biochar and Biomass Ash as a Soil Ameliorant: The Effect on Selected Soil Properties and Yield of Giant Miscanthus (Miscanthus × giganteus). Energies 2018, 11, 2535. [Google Scholar] [CrossRef] [Green Version]
- Homdoung, N.; Sasujit, K.; Uttaruean, J.; Wongsiriamnuay, T.; Tippayawong, N. Influence of torrefaction temperature and time on the yields and properties of torrefied biomass. Eng. Appl. Sci. Res. 2019, 46, 170–175. [Google Scholar]
- Akhtar, J.; Imran, M.; Ali, A.; Nawaz, Z.; Muhammad, A.; Butt, R.; Jillani, M.; Naeem, H. Torrefaction and Thermochemical Properties of Agriculture Residues. Energies 2021, 14, 4218. [Google Scholar] [CrossRef]
- Batidzirai, B.; Mignot, A.; Schakel, W.; Junginger, M.; Faaij, A. Biomass torrefaction technology: Techno-economic status and future prospects. Energy 2013, 62, 196–214. [Google Scholar] [CrossRef]
- Dong, L. Impact of Torrefaction on Grindability, Hydrophobicity and Fuel Characteristics of Biomass Relevant to Hawai‘i. Master’s Thesis, University of Hawai‘i at Mānoa, Mānoa, HI, USA, 2015. [Google Scholar]
- Dyjakon, A.; Noszczyk, T. Alternative Fuels from Forestry Biomass Residue: Torrefaction Process of Horse Chestnuts, Oak Acorns, and Spruce Cones. Energies 2020, 13, 2468. [Google Scholar] [CrossRef]
- Correia, R.; Gonçalves, M.; Nobre, C.; Mendes, B. Impact of torrefaction and low-temperature carbonization on the properties of biomass wastes from Arundo donax L. and Phoenix canariensis. Bioresour. Technol. 2017, 223, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Riaza, J.; Gibbins, J.; Chalmers, H. Ignition and combustion of single particles of coal and biomass. Fuel 2017, 202, 650–655. [Google Scholar] [CrossRef]
- Sheng, C.; Azevedo, J. Estimating the higher heating value of biomass fuels from basic analysis data. Biomass Bioenergy 2005, 28, 499–507. [Google Scholar] [CrossRef]
- Acar, S.; Ayanoglu, A. Determination of higher heating values (HHVs) of biomass fuels. Energy Educ. Sci. Technol. Part A Energy Sci. Res. 2012, 28, 749–758. [Google Scholar]
- Enes, T.; Aranha, J.; Fonseca, T.; Lopes, D.; Alves, A.; Lousada, J. Thermal Properties of Residual Agroforestry Biomass of Northern Portugal. Energies 2019, 12, 1418. [Google Scholar] [CrossRef] [Green Version]
- Anukam, A.; Mamphweli, S.; Okoh, O.; Reddy, P. Influence of Torrefaction on the Conversion Efficiency of the Gasification Process of Sugarcane Bagasse. Bioengineering 2017, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Uemura, Y.; Sellappah, V.; Trinh, T.H.; Komiyama, M.; Hassan, S.; Tanoue, K. Improvement of energy density and energy yield of oil palm biomass by torrefaction in combustion gas. IOP Conf. Ser. Mater. Sci. Eng. 2018, 458, 012061. [Google Scholar] [CrossRef]
- Sellappah, V.; Uemura, Y.; Hassan, S.; Sulaiman, M.H.; Lam, M.K. Torrefaction of Empty Fruit Bunch in the Presence of Combustion Gas. Procedia Eng. 2016, 148, 750–757. [Google Scholar] [CrossRef] [Green Version]
- Phusunti, N.; Phetwarotai, W.; Tekasakul, S. Effects of torrefaction on physical properties, chemical composition and reactivity of microalgae. Korean J. Chem. Eng. 2017, 35, 503–510. [Google Scholar] [CrossRef]
- Uemura, Y.; Omar, W.N.; Tsutsui, T.; Yusup, S.B. Torrefaction of oil palm wastes. Fuel 2011, 90, 2585–2591. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.S.; Bajwa, S.G. Properties of densified solid biofuels in relation to chemical composition, moisture content, and bulk density of the biomass. BioResources 2019, 14, 4996–5015. [Google Scholar]
- Cai, J.; He, Y.; Yu, X.; Banks, S.; Yang, Y.; Zhang, X.; Yu, Y.; Liu, R.; Bridgwater, T. Review of physicochemical properties and analytical characterization of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2017, 76, 309–322. [Google Scholar] [CrossRef] [Green Version]
- Pouzet, M.; Dubois, M.; Charlet, K.; Petit, E.; Béakou, A.; Dupont, C. Fluorination/Torrefaction Combination to Further Improve the Hydrophobicity of Wood. Macromol. Chem. Phys. 2019, 220, 1900041. [Google Scholar] [CrossRef]
- Dyjakon, A.; Noszczyk, T.; Smędzik, M. The Influence of Torrefaction Temperature on Hydrophobic Properties of Waste Biomass from Food Processing. Energies 2019, 12, 4609. [Google Scholar] [CrossRef] [Green Version]
- Acharjee, T.C.; Coronella, C.J.; Vasquez, V.R. Effect of thermal pretreatment on equilibrium moisture content of lignocellulosic biomass. Bioresour. Technol. 2011, 102, 4849–4854. [Google Scholar] [CrossRef] [PubMed]
- Dyjakon, A.; Noszczyk, T.; Sobol, Ł.; Misiakiewicz, D. Influence of Torrefaction Temperature and Climatic Chamber Operation Time on Hydrophobic Properties of Agri-Food Biomass Investigated Using the EMC Method. Energies 2021, 14, 5299. [Google Scholar] [CrossRef]
- Chang, S.; Zhao, Z.; Zheng, A.; He, F.; Huang, Z.; Li, H. Characterization of Products from Torrefaction of Sprucewood and Bagasse in an Auger Reactor. Energy Fuels 2012, 26, 7009–7017. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, B.; Yang, H.; Yang, Q.; Chen, H. Evolution of functional groups and pore structure during cotton and corn stalks torrefaction and its correlation with hydrophobicity. Fuel 2014, 137, 41–49. [Google Scholar] [CrossRef]
- Chen, D.; Gao, A.; Cen, K.; Zhang, J.; Cao, X.; Ma, Z. Investigation of biomass torrefaction based on three major components: Hemicellulose, cellulose, and lignin. Energy Convers. Manag. 2018, 169, 228–237. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Ru, B.; Zhao, Y.; Wang, X.; Xiao, G.; Luo, Z. Influence of Torrefaction on the Characteristics and Pyrolysis Behaviour of Cellulose. Energy 2017, 120, 864–871. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).