Abstract
Modern healthcare is transforming from hospital-centric to individual-centric systems. Emerging implantable and wearable medical (IWM) devices are integral parts of enabling affordable and accessible healthcare. Early disease diagnosis and preventive measures are possible by continuously monitoring clinically significant physiological parameters. However, most IWM devices are battery-operated, requiring replacement, which interrupts the proper functioning of these devices. For the continuous operation of medical devices for an extended period of time, supplying uninterrupted energy is crucial. A sustainable and health-compatible energy supply will ensure the high-performance real-time functioning of IWM devices and prolong their lifetime. Therefore, harvesting energy from the human body and ambient environment is necessary for enduring precision healthcare and maximizing user comfort. Energy harvesters convert energy from various sources into an equivalent electrical form. This paper presents a state-of-the-art comprehensive review of energy harvesting techniques focusing on medical applications. Various energy harvesting approaches, working principles, and the current state are discussed. In addition, the advantages and limitations of different methods are analyzed and existing challenges and prospects for improvement are outlined. This paper will help with understanding the energy harvesting technologies for the development of high-efficiency, reliable, robust, and battery-free portable medical devices.
1. Introduction
Precision medicine enables individual-centric healthcare with flexibility, portability, and reduced cost in clinical decision support systems [1]. The accurate measurements of physiological parameters define the current health condition of the individual and aid medical professionals in a timely clinical decision-making process. Implantable and wearable medical (IWM) devices are a crucial part of nursing, physical activity monitoring, rehabilitation, and clinical early diagnosis of diseases, as well as monitoring of disease progression [2,3,4]. Besides extensive usage in precision medicine, IWM devices are useful in physical and mental condition assessment in the military [5,6]. For example, wearable photoplethysmography (PPG) sensors presented in [7] can measure the human heartbeat extracted from the blood flow in the cardiovascular system. Smart textiles allow the integration of wearable medical devices (WMDs) in dresses to record vital signs and electrocardiogram (ECG) signals [8,9].
The demand for incorporating portable medical devices in healthcare is rapidly growing due to: (i) the prevalence of chronic diseases, (ii) the increase in the number of elderly people, (iii) enabling easy accessibility to the personal health record, (iv) real-time monitoring of health conditions, and (v) cost-effectiveness. In addition, recent advances in integrated circuits, sensor technologies, and improved interfacing of biology and electronics have enabled the widespread adoption of IWM devices [10,11,12,13]. The improvement in front-end electronics and wireless communications for bio-signal acquisition facilitates continuous monitoring, quantitative measurements, and recording of high-quality clinical data.
Uninterrupted operation of IWM devices is contingent upon continuous powering, which is critical for many applications such as fall detection of sick people, heart activity monitoring to prevent myocardial infarction, etc. [14]. However, energy remains the major constraint, and most IWM devices are still battery-operated. Although significant improvement in designing miniaturized and high-storage-capacity batteries have been made over the past few years, the limited lifetime of batteries remains a burden. Other limitations of powering IWM devices using batteries are (i) the requirement of recharge or replacement often through surgery in the case of implantable medical devices (IMDs), (ii) the increased size and weight of the IWM devices to accommodate high-capacity batteries, resulting in user discomfort, and (iii) the requirement of manual human intervention for continuous operation [15]. The next generation of IWM devices should include improved functionality, ensure user comfort, and longer uninterrupted lifetime. Harvesting sufficient energy from the human body and the environment is the most viable solution [16,17]. Integrating energy harvesting in IWM devices will reduce the device sizes and ensure uninterrupted operation with minimal manual intervention and maintenance.
Energy harvesting is the conversion of available energy from the human body and ambient environment into a usable electrical form to facilitate a maintenance-less, long-lasting, and green energy supply in IWM devices. Electrical energy can be generated from biochemical reactions and motions associated with the human body. Solar light, radio frequency (RF) signals, etc., are a few significant environmental energy sources. The recent advancement in energy harvesting techniques has paved the way for full or partial power support for IWM devices.
There are a few review papers available providing detailed studies on discrete technologies such as extracting kinetic energy from body motion [18,19], wireless power transfer [20,21,22], and scavenging energy from the environment [23,24,25]. However, due to the rapid growth of energy harvesting technologies and radical paradigm shifts in implants and wearables, a state-of-the-art review is crucial. This paper presents a comprehensive review of the latest improvements in energy harvesting research focusing on implantable and wearable medical devices to achieve sustainable, robust, reliable, and enduring precision healthcare.
The rest of the paper is organized into different sections. Section 2 provides an overview of the implantable and wearable medical devices and discusses their role in precision medicine and healthcare. Section 3 discusses various energy harvesting techniques, energy sources, wireless power transfer, and an analysis of the advantages and limitations of each approach. Section 4 presents maximum power extraction methods from the energy harvesters. Recently, there has been a boost in adopting machine learning (ML) techniques in energy-harvesting-enabled IWM devices, which are included in Section 5. Section 6 discusses a few use cases and in vivo experiments of energy harvesters. The interaction of energy harvesters with the human body and potential biological effects are explained in Section 7. Existing challenges and future research directions are provided in Section 8. An overall discussion is presented in Section 9, and conclusions are drawn in Section 10.
2. Implantable and Wearable Medical Devices in Precision Healthcare
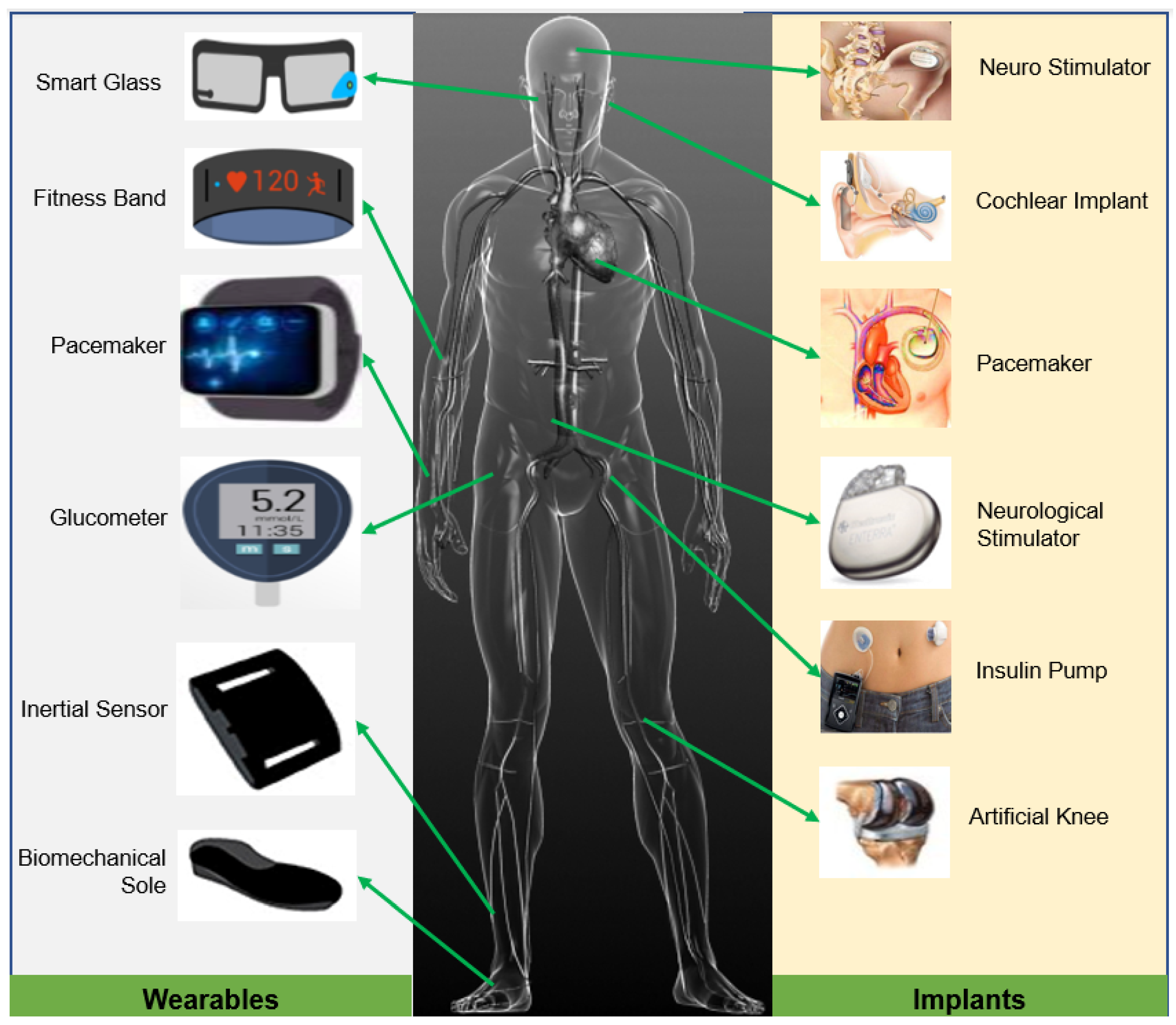
Recent progress in micro-fabrication has stimulated the miniaturization of implantable devices [26]. The pacemaker, insulin pump, neurostimulator, cochlear implant, cardiac defibrillator, and retinal implants are a few examples of clinically well-established IMDs used in precision healthcare [27]. An overview of different implantable and wearable medical devices and their usual placement in the human body are presented in Figure 1. In general, the energy system of the IMDs has three parts: energy source, storage, and power management unit (PMU) [28]. Depending on the application, the power requirement of implants varies from a few microwatts to milliwatts. The continuity and longevity of the operations of IMDs largely depend on an uninterrupted energy supply. Although the severity of the consequences of power interruption in implants varies, sometimes, it could lead to life-threatening events in the case of total artificial hearts, pacemakers, etc.
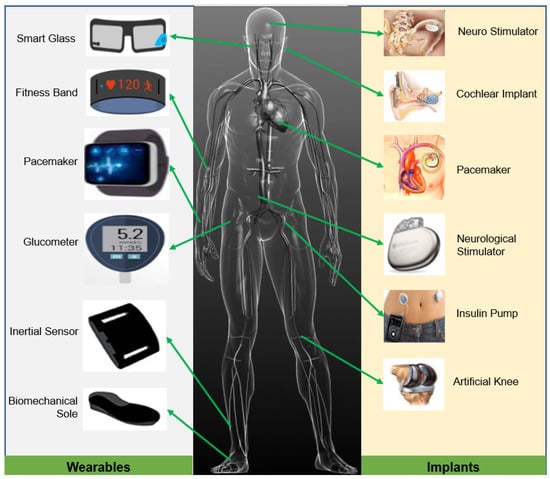
Figure 1.
Different wearable and implantable medical devices typically used in precision healthcare.
Although wearable devices are used in daily life events and fitness tracking, the adoption of wearables in healthcare is relatively slow. There are enormous opportunities to bring wearable devices to stimulate real-time, robust, reliable, and accurate monitoring of personalized health conditions. Driven by the increasing elderly population, increased economic burden, and prevalence of chronic diseases, a cost-effective, portable, and easily accessible personalized healthcare system is immensely important [2,29,30]. Therefore, the idea of care and treatment in hospitals is transferring towards more individual-centric healthcare services enabling early diagnosis and treatment initiatives [31,32]. Wearable medical devices (WMDs) are the key enabling technologies of individual-centric precision healthcare systems [33,34]. Different WMDs have been successfully implemented over the last few years such as non-invasive monitoring of the ECG, blood pressure, oxygen saturation, and respiratory rate for the prediction and prevention of diseases. Accelerometers and gyroscopes can monitor body motion and activities for ambient assistive living [4,35,36,37]. Different body fluids (blood, sweat, etc.) are also actively studied using wearable sensors to detect diabetes [38] and other metabolic disorders [39,40]. The wearables are commonly body-worn and often integrated into gloves, textiles, headsets, and patches. Appropriate management and control of many neurological and cardiovascular diseases such as Parkinson’s disease, heart failure, etc., require early detection and continuous monitoring of different clinical symptoms to maintain health in a stable condition. The wearable medical devices can mitigate the risk of catastrophic events from happening by (i) predicting disease through health monitoring, (ii) real-time diagnosis using on-site data processing, and (iii) informing concerned caregivers through telemedicine [41,42].
3. Energy Harvesting Techniques
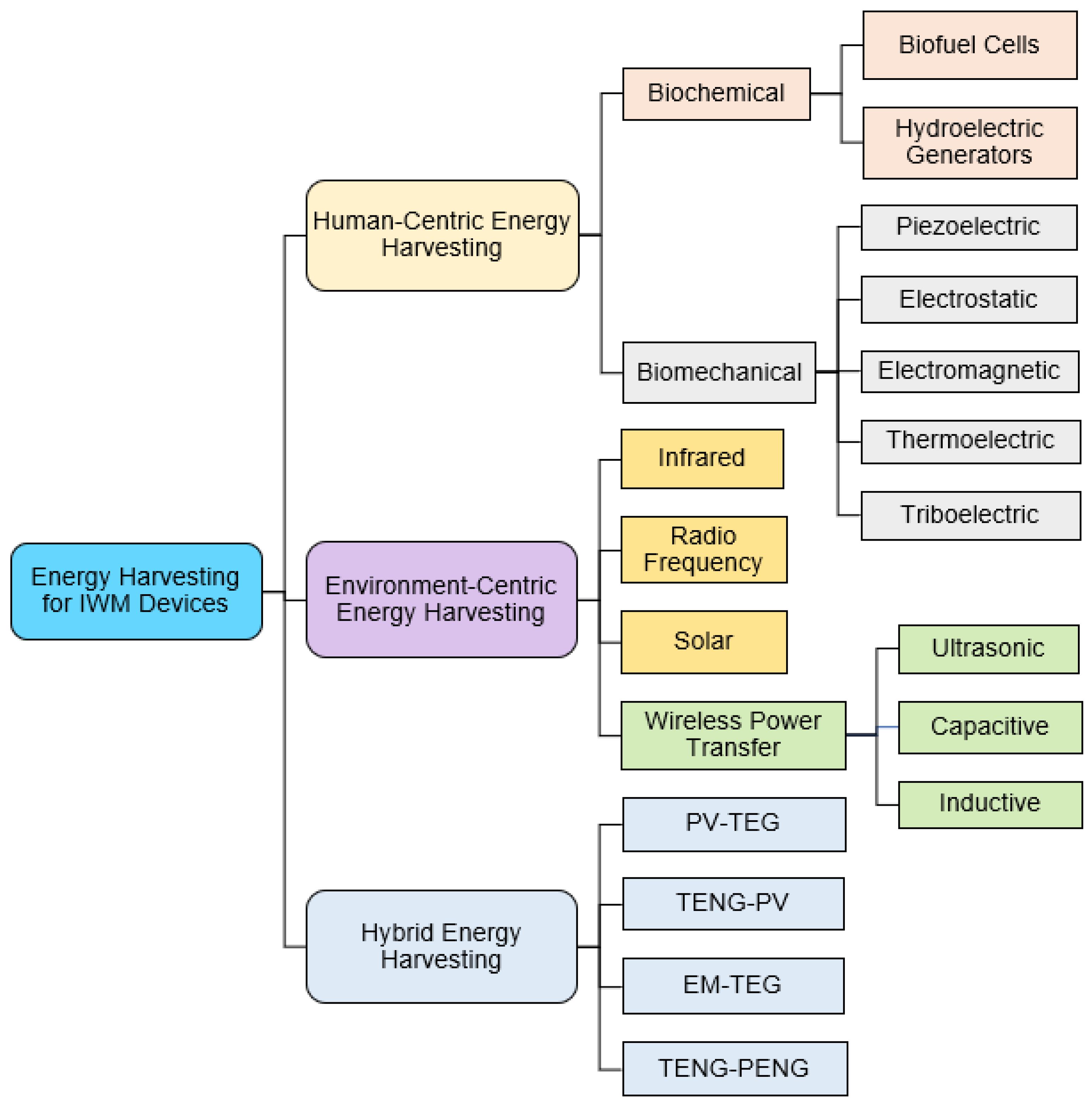
This review focuses on energy harvesting for IWM devices from two sources: (i) the human body and (ii) the environment. Human body motion and heat are a few examples of human-centric energy sources exploited for energy harvesting. A general classification of different energy harvesting techniques is presented in Figure 2. Thermoelectric energy generators (TEGs) use the temperature gradient between the human body and the surrounding environment to scavenge energy [43]. In addition, the heat dissipated from the human body has also demonstrated potential in energy harvesting using photovoltaic (PV) cells [44]. Various vibrations associated with the human body such as walking, running, and breathing are other sources of energy [45]. Researchers effectively transduced these body vibrations into electrical energy by electrostatic, triboelectric, electromagnetic (EM), and piezoelectric processes [46]. In addition, energy sources from the environment such as infrared radiation, solar energy, and radio frequency (RF) signals have shown promise as excellent sources of energy [47]. However, the reliability of environment-centric energy sources is often contingent upon their availability and requires additional storage to facilitate the continuous operation of IWM devices. Energy harvesting techniques can combine sources from the human body and the environment to obtain more robust energy solutions. Such hybrid energy harvesters are also briefly discussed in this section.
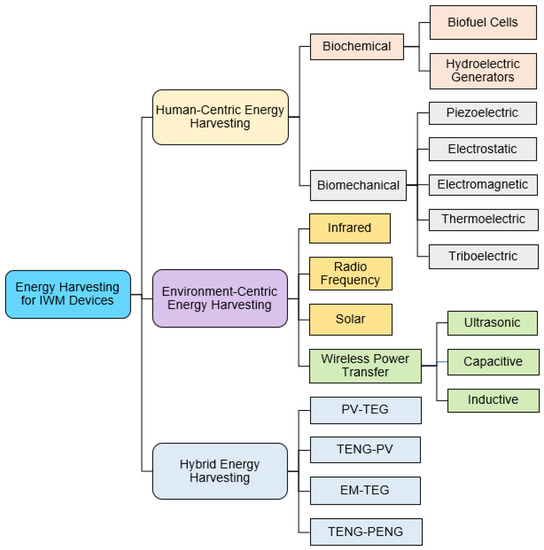
Figure 2.
Classification of human- and environment-centric energy harvesting techniques exploited in implantable and wearable medical devices.
3.1. Human-Centric Energy Harvesting
Human-centric energy harvesting has received significant attention in recent years. Researchers have shown that the human body can generate more energy than a battery cell, self-sufficient to operate IWM devices [14,48]. Harvesting this energy can minimize the burden of individuals undergoing repeated invasive procedures to replace the batteries in IWM devices and operate without any batteries. Human-centric energy harvesters often convert external body motion, temperature, and internal chemical reactions into electrical energy. While motion, vibration, temperature gradients, and enzymatic reactions can be excellent sources of energy, there is an increasing challenge because of the size constraints of the harvester in the human scenario. However, it has been shown as a proof-of-concept that even regular daily life activities are sources of different amounts of energy. For example, approximately 81 mW and 1630 mW of power can be harnessed from the human body while sleeping and walking, respectively [49]. In addition, the biofluids in the human body can act as a source of sustainable energy through their natural operation [50]. Major efforts have thus been put forward to harvest these human-centric energy, be it from the chemical reactions inside the human body or its mechanical motion. In this review, human-centric energy harvesting techniques are categorized into biochemical and biomechanical processes.
3.1.1. Biochemical Energy Harvesting
The human body contains numerous enzymes and agents to maintain the proper balance of biofluids [51]. Due to internal chemical reactions in the human body, the formation and breaking of chemical bonds generate an enormous amount of energy. This level of energy is sufficient to some extent to operate low-power implantable devices. The amount of harvested energy depends on many factors such as age, health, and food intake. In addition, the interaction of water with novel materials can generate usable energy using the hydro-voltaic effect. Biochemical energy harvesting is discussed in the following two categories: biofuel cells and hydroelectric generators (HEGs).
Biofuel Cells
Glucose in body fluid is an excellent source of energy. It releases a decent amount of energy through redox reactions in the metabolism process. Recently, the glucose biofuel (GBF) cell has garnered attention as an alternative energy source due to biocompatibility and abundance in the human body [52,53]. The GBF cell can store more energy compared to conventional lithium-ion batteries. For instance, a GBF cell implanted in the abdomen of a rat demonstrated 38.7 W of power at the output with a power density of 193.5 W/cm [54]. Experiments demonstrated that up to 100 W/cm can be generated from the GBF in the human body [55,56]. Recently, a significant improvement of the GBF cell was achieved in [57], obtaining 3.7 mW/cm of power density coating a metallic cotton fiber in glucose oxidase (GOx). However, there has been ongoing research to increase the power density of a biofuel cells (BFCs) [58]. Researchers have loaded an oxidizing enzyme and glucose dehydrogenase into a coating. The coating is made of redox polymers and polyethyleneimine, which act as a supercapacitor. Charges stored in the supercapacitor provide high-energy bursts when needed [50]. Besides, BFCs are implanted inside the body for extracting body fuel to produce electricity [59,60,61,62]. The bio-implantable fuel cell reported in [56] is powered by extracellular glucose in the cerebrospinal fluid surrounding the brain. Studies have shown the conceivability of using GOx for BFCs [63,64]. For these BFCs, the use of nanomaterial-modified electrodes with a large electrochemically active surface area (ECSA) enables a high loading and promotes power generation. Reduced graphene oxide (rGO) films, nickel microstructures, and Meldola’s blue-tetrathiafulvalene-modified carbon nanotubes (MDB-TTF-CNT) for the enzymatic anode have been reported to increase the ECSA by 3000× [65]. However, there are some limitations to the use of this enzyme due to safety concerns. Most of the concerns include the generation of hydrogen peroxide by GOx affecting the stability of the enzyme, which deteriorates the performance of the system, and most importantly, long-term use of these enzymes may be harmful to the body [66]. A few limitations of glucose as a biofuel include: (i) temperature and pH variation can disrupt glucose metabolism and (ii) potential chances of bacterial infection. In addition, biofouling often occurs in GBF cells, leading to biohazards [54]. Due to safety concerns, GBF cells are not yet usable in medical implants.
Lactates such as sweat and saliva are another energy source available in body fluids. For instance, in [50], a tattoo was designed to harvest energy from lactate during physical activity, generating 70 W/cm. Besides, pH differences can also act as a source of electrostatic energy [67]. The soft e-skin-based BFC reported in [68] extracts chemical energy from human sweat. This wearable e-skin-based BFC produces a power density of 1.2 mW/cm at 0.2 V by using sweat lactate from the human body. An extensive study on biosensor and biofuel cells is reported in [69]. This research team developed a BFC using passive sweat from the fingertips to continuously supply energy without requiring physical motion. In a recent attempt, researchers showed that 13 W of power can be achieved by a textile-based enzymatic BFC [70]. A “wearable microgrid” shirt has also been demonstrated, which incorporates sweat-powered BFCs, motion-powered triboelectric nanogenerators (TENGs), and supercapacitors to store the converted electric energy [71]. Apart from the fluids, the endocochlear potential (EP) in the inner ear can generate low power ranging between 1.1 and 6.3 nW [72].
EP found in the mammalian inner ear is a battery-like electrochemical gradient that can be used as an energy harvester. The EP occurs due to the ionic concentration difference between endolymph and perilymph. A CMOS integrated energy harvester was designed in [72] that can extract a minimum of 1.12 nW of power from the EP of a guinea pig for 5 h and also sense the change in EP.
A yarn-based biobattery utilizes the bacterial respiration system to generate green electricity [73]. The biobattery comprises polystyrene-sulfonate-based anodic and one cathodic yarn, which ensures maximum electron transfer from the microorganism to the electrode. Multiple yarns are arranged in parallel, making the biobattery easily scalable. The maximum current densities for typical 3-series, 2-parallel, and single biobatteries are 22.10 W/m, 19.14 W/m, and 2.12 W/m, respectively.
Hydroelectric Generators
The use of the hydro-voltaic effect for energy harvesting is an emerging area that falls into the biochemical category. The hydroelectric generator (HEG) uses the interaction between the water molecules and nanomaterials [74,75]. Low-dimensional materials such as carbon nanotubes and graphene can generate electricity at the solid–liquid interface while interacting with the water molecules.
Electricity generation by water evaporation and humidity does not require any additional mechanical force [76,77]. For instance, the flow of water molecules in nanomaterials’ gaps in the evaporation process induce electrical voltage and current. An electrical double-layer is formed at the channel wall surface due to the attraction between hydronium ions and negatively charged nanocarbon layers. During evaporation, the channel hydronium ion moves in the water flow direction, generating a potential difference and a resultant output current flow [75]. For example, up to 1.0 V and 150 nA of current can be generated using the water evaporation process on the annealed and plasma-treated carbon black film surface [78]. Another electricity generator using the water evaporation process was presented in [79], which uses silicon nanowire arrays. An output power density, current density, and voltage of 6 Wcm, 55 A cm, and 400 mV, respectively, were achieved while the ambient temperature was 65 °C and the relative humidity was 45%. The device used silicon nanowires as the active layer, graphite as the cathode, and silver as the anode.
The concept of humidity-induced electricity is the reversible process of hydration and dehydration. The free hydrated ion in this process migrates due to a concentration gradient triggered by moisture, creating an electrical output [77,80]. An output power density of 0.246 mW cm is achieved in a moisture-enabled HEG utilizing humidity variation from 5% to 95% [81]. A porous membrane captures the moisture, triggering the hydration and ion transport, and an electric potential is generated by the movement of the cations and anions. In an HEG using an arrangement of silver wire, graphene oxide can achieve a 0.21 Wcm output power density under a 70% humidity change [81].
A wearable perspiration analyzer was presented in [82], which uses the coupling of enzyme reactions and an HEG. The system consists of ZnO nanowire arrays modified by lactate oxidase and a flexible PDMS substrate. While attached to the skin, sweat flows through the microchannels to the ZnO array, producing an electrical current using the HEG principle. An electronic sensor using moisture-induced electricity was presented in [83]. The protein films of gelatin molecules serve as the active materials and generate considerable electrical output while the humidity level exceeds 55%. This device demonstrates potential application in wearable masks to monitor users’ breathing conditions. In addition, it finds application in evaluating the finger wound healing condition [83], detecting alcohol consumption [84], etc.
3.1.2. Biomechanical Energy Harvesting
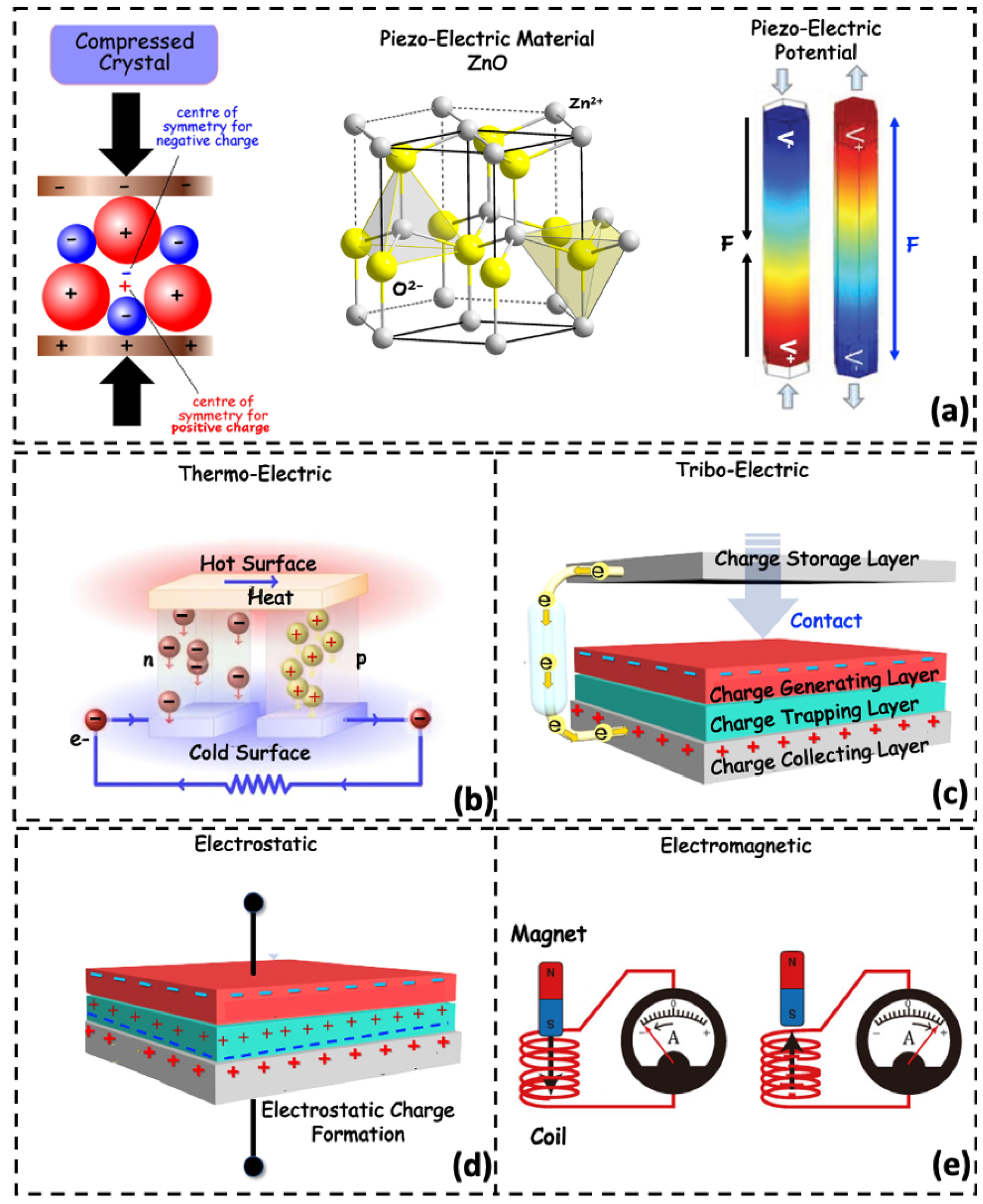
Human body motion can generate electrical energy. For instance, foot strikes, muscle movements, etc., can be converted to an electrical form, sufficient to operate wearable devices [85]. In addition, involuntary periodic activities such as cyclic breathing and rhythmic heart motion can generate electric energy for implantable devices and can be enhanced to operate on wearables. A low-power cardiac pacemaker was presented in [86], which achieves 11.1 J of electrical energy from a 90 bpm heart rhythm. The air pressure gradient due to breathing cycles develops a miniaturized wind turbine to operate wearables placed in face masks [87]. Human-centric energy harvesting from kinetic energy is discussed in this section identifying five techniques: piezoelectric, electrostatic, electromagnetic, thermoelectric, and triboelectric energy generators [88,89]. The energy conversion principles of different biomechanical energy harvesting processes are illustrated in Figure 3 and discussed in the subsequent sections.
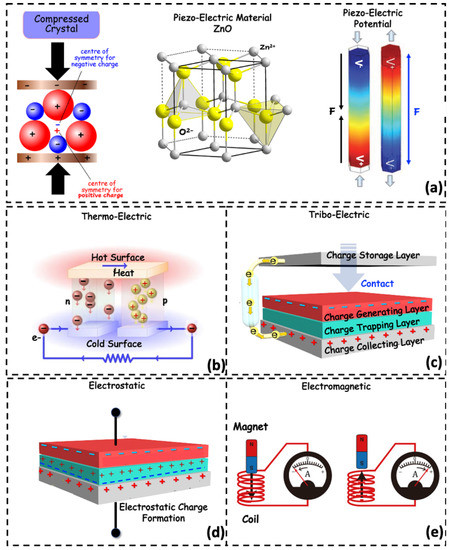
Figure 3.
Schematic illustration for working principle of various types of biomechanical energy harvesting schemes. (a) Development of electric potential while the piezoelectric crystals are compressed/strained. (b) The temperature gradient between a hot and a cold surface on a thermoelectric material develops an electric potential and a resultant current flow. (c) Charge generation at the interface of triboelectric pair materials by contact friction. (d) Charge storage on the dielectric material inside two metal contacts by electrostatic action. The charges deposited in the triboelectric and electrostatic scheme store energy by capacitive coupling. (e) A moving magnet inside a coil produces a potential difference by Faraday action.
Piezoelectric Energy Generators
Advancements in microelectronics led to the development of implantable electronics (IMEs). Besides, emerging materials helped revolutionize the energy harvesting applications for IWM devices. One such material is piezoelectric materials, which directly convert mechanical energy into electricity without requiring conversion. An electric field, thus voltage, is generated while the piezoelectric materials experience mechanical stress, which is used in energy harvesting [90]. A schematic illustration of the piezoelectric energy harvesting principle is shown in Figure 3a. For instance, while running, the piezoelectric materials integrated with the sole and heel can produce 2 mW and 8 mW of electrical power, respectively [91,92]. In a broad sense, piezoelectric materials are classified as crystals and ceramics [93]. The ceramic polycrystalline lead zirconate titanate (PZT) is one of the popular materials due to its high piezoelectric coefficient and low fabrication cost [93].
Researchers have demonstrated that a magnetic microgenerator placed on the heel can generate around 1 W of power [91]. This system is appropriate only for users capable of walking, and the placement in shoes might induce user discomfort. An energy harvester for a total knee replacement (TKR) implant was presented in [94], generating 4.8 mW of power with an excitation load of 900 N using three PZT stacks. The sensitivity of ceramic piezoelectric materials degrades over time. In recent studies, single-crystal materials such as lead magnesium niobate–lead titanate (PMN-PT) and lead–zinc niobate–lead titanate (PZN-PT) have demonstrated promising results [95,96]. The lead-integrated piezoelectric materials are toxic and may stimulate health risks. Because of that, single-crystal zinc oxide (ZnO) materials have gained attention for their non-toxic and biocompatible behavior. In [97], ZnO-based nanogenerators harvests energy from rat heartbeat. However, the amount of energy is insufficient to operate IMDs, and an array arrangement of ZnO nanowires is necessary to obtain enough energy, which increases the cost [98].
It is to be noted that IWM devices need flexible, extremely slim, and lightweight media for successful attachment and operation. Researchers have been developing high-performance piezoelectric energy harvesters using organic and inorganic materials such as polymers, ceramics, and single crystals for attaining such features. Polyvinylidene difluoride (PVDF) is a flexible piezoelectric polymer, suitable for energy harvesting. The limitations of piezoelectric polymers are the low power density and coupling coefficient [93]. A hybrid material using both ceramics and polymer piezoelectric materials resulted in improved performance showing an output of 3.8 W/mm from the movement of a hand [99]. To this date, flexible thin film piezoelectric materials remain an appropriate choice for energy harvesting due to their soft nature and adaptability to human organ shapes [93,100].
Mechanical-pressure-induced piezoelectricity due to blood pressure variations was demonstrated in [101], generating 2.3 W of power. Similar experiments were carried out in [102] using PVDF transducers to generate energy from blood pressure gradients. Piezoelectric ceramics embedded into orthopedic implants at different body locations have shown promising results of 4.8 mW [103], 1.2 mW [104], and 1.81 mW [105], respectively, depending on the size and thickness of the piezoelectric materials. In [106,107], 40 W and 60 W piezoelectric generators were presented using zirconate titanate and aluminum nitride, respectively.
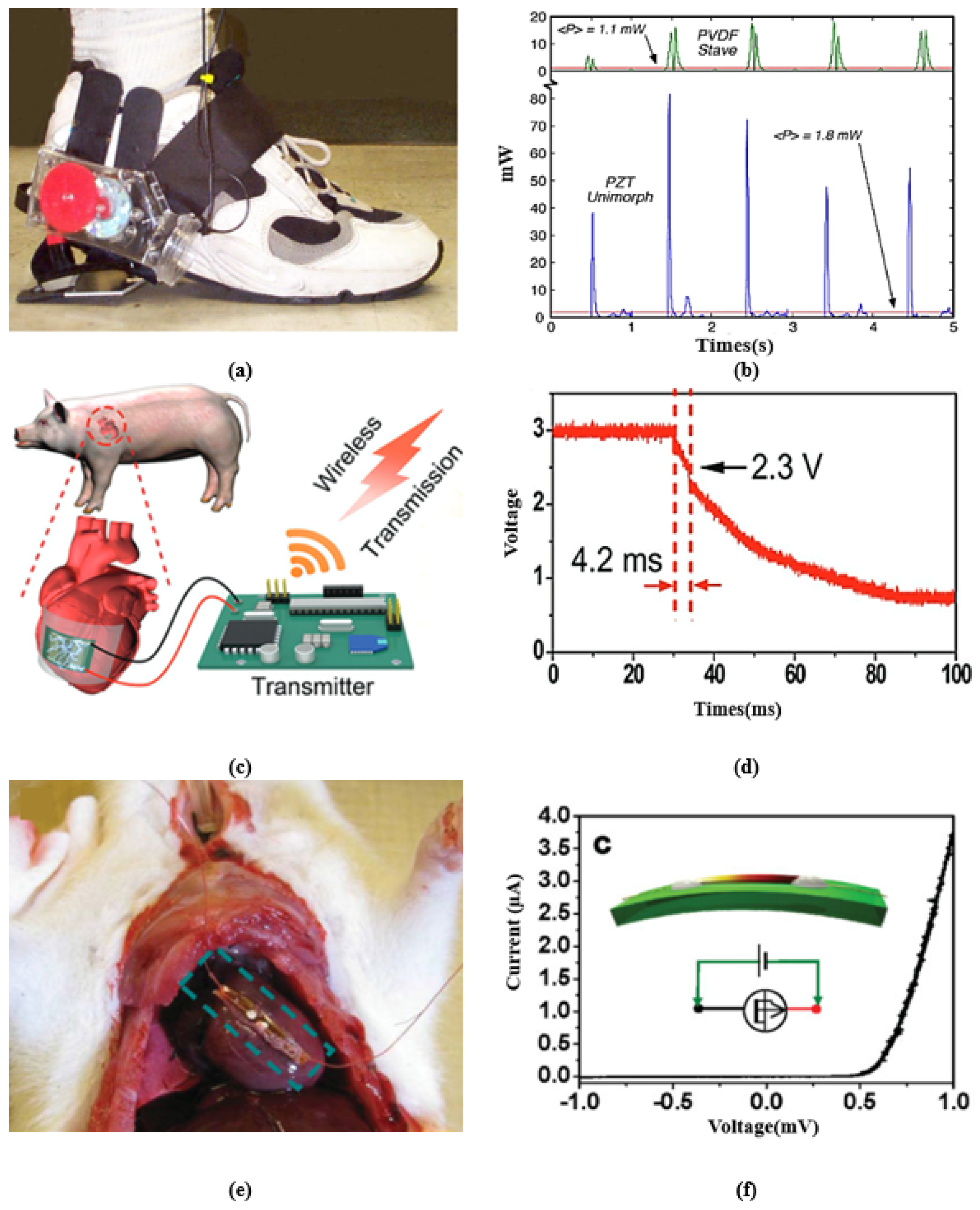
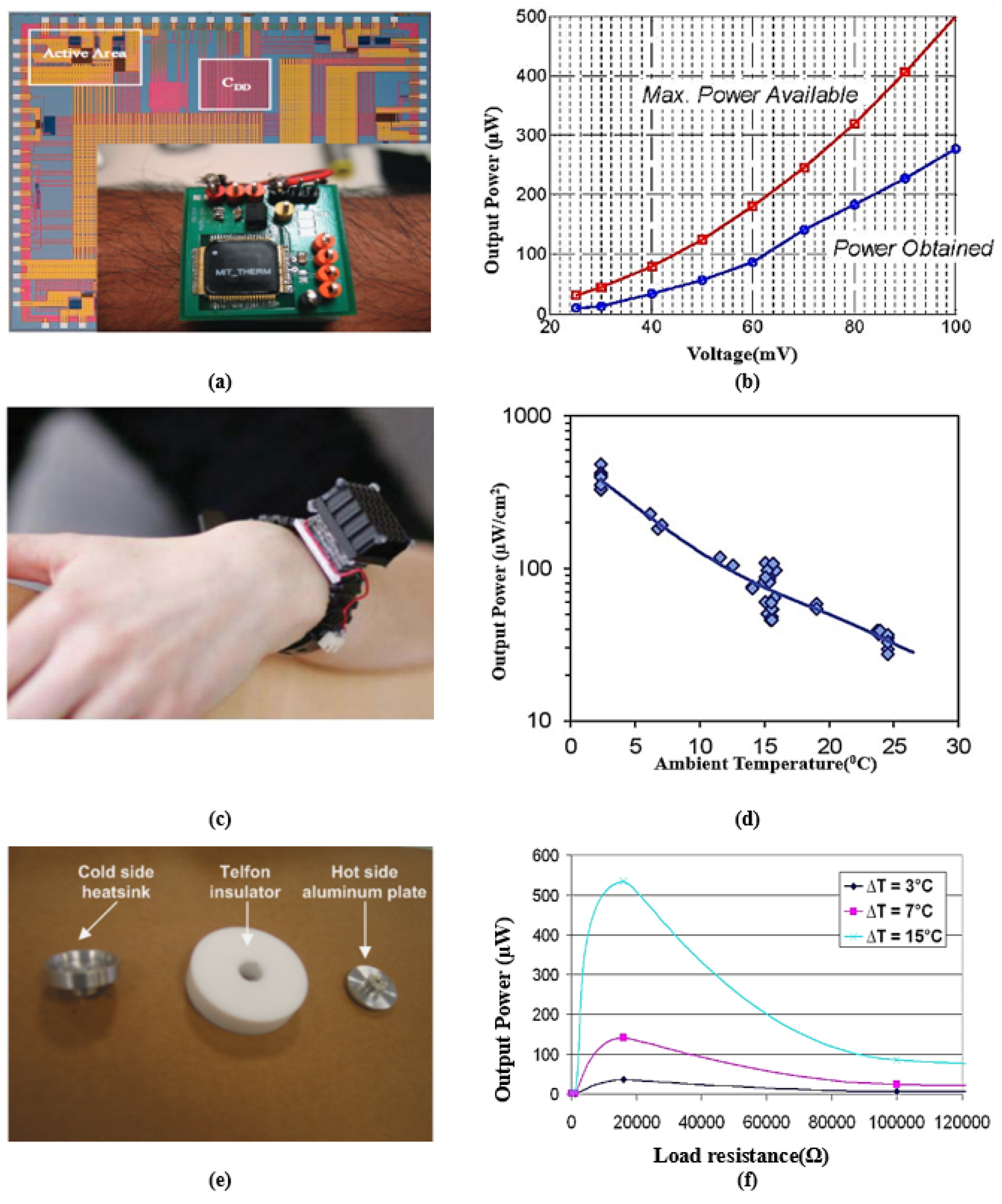
A single-wire nanogenerator driven by heart muscles’ motion was designed in [97], producing an average voltage of 3 mV and a current of 30 pA. A wearable PVDF-array-based device developed in [108] can harvest energy during running with 8.6 mW of output power. A belt-type piezoelectric energy harvester was presented in [109], which generates an output from the waist movement, producing an open-circuit voltage in the range of 1.5–1.8 V for normal and fast respiration processes. A flexible PEH-based sensor was developed in [110], which scavenges energy from respiration or shouting and the movement of muscles. Figure 4 presents some remarkable examples of piezoelectric energy harvesting in IWM devices from walking, cardiac motion, and breathing with their output characteristics. Figure 4a presents a system to harvest parasitic power in shoes. The system holds a generator in such a way as to give full pressure to the lever at the hinged heel plate. Figure 4b presents the resulting power delivered to the load by these systems. A flexible energy harvester is presented in Figure 4c, which is attached to a porcine heart, generating power from cardiac contraction and relaxation motions. The capacitor stores energy obtained from cardiac motion, finally transmitting the data to the receiver wirelessly. Figure 4d depicts the change in voltage activated by wireless data transmission where the steep slope in time from 29.8 to 34 ms implies the successful transmission of data. Figure 4e demonstrates the implantation of a single-wire generator (SWG) in a live rat to scavenge energy generated by its breath and heartbeat. This study illustrates that harvesting low-frequency dynamic muscle motion drives nanogenerators to bend periodically and generate an AC power output. Figure 4f gives the I-V characteristics of the nanogenerator.
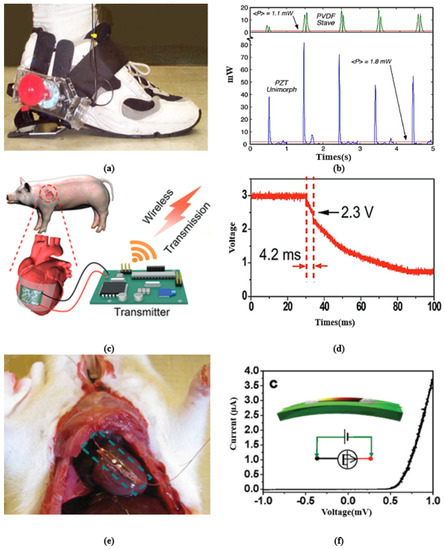
Figure 4.
(a) A shoe-mounted rotary energy generator. (b) Output power from shoe-mounted rotary energy generator. Reprinted with permission from [91]. Copyright 1998 IEEE. (c) Flexible energy harvester using cardiac motions with storage capacitor and wireless transmission. (d) The resultant output voltage from the generator reported in [95]. Adapted with permission from [95]. Copyright 2017 WILEY-VCH Verlag GmbH & Co. (e) Single-wire energy generator from the beating heart of a living rat’s diaphragm. (f) I-V characteristic of the generator. Adapted with permission from [97]. Copyright 2010 WILEY-VCH Verlag GmbH & Co.
Being one of the most popular choices in biomechanical energy harvesting, the piezoelectric nanogenerator (PENG) drew much attention in recent times. In a broad sense, the four-circuit category has been discussed and incrementally improved over the last two decades for coupling interfaces of PENG. These are standard energy harvesting (SEH), synchronized charge extraction (SCE), parallel synchronized switch harvesting on inductor (P-SSHI), and series synchronized switch harvesting on inductor (S-SSHI) circuits. Different electro-mechanical coupling conditions can alter the charging of the energy storage element. A numerical study was performed considering numerous design space factors [111]. Recently, a self-powered array interface circuit based on synchronized switching and discharging a storage capacitor through an inductive coupling technique (SSDCI) was presented [112]. The SSDCI array circuit achieves an efficiency of 82.3% and allows a gain of up to 300% in comparison to the maximum output power generated by the full-bridge rectifier. Furthermore, a self-adapting synchronized switching interface circuit using a 180 nm CMOS process node [113] has been reported, which could achieve 500% more power compared to an ideal full-bridge rectifier. In principle, the piezo-electronic energy harvester uses inductive coupling techniques for power transfer, which is discussed later in this review.
Overall, the piezoelectric-based energy harvesters produce the highest power densities among the energy harvesters that use human vibrations. The limitations of piezoelectric energy harvesters are their frequency-dependent nature and inefficiency at low-frequency human motion.
Electrostatic Transduction
Mechanical vibration can generate electrical energy by transducing electrostatic energy suitable for implantable biosensors requiring ultra-low power. Electrostatic-transduction-based energy harvesters follow the fundamental principle of Coulomb’s Law. The capacitance changes between two parallel plates, typically electrically separated by air, a vacuum, or a dielectric. The forces of attraction between these oppositely charged plates vary with gap distance or area modulation. Therefore, by changing the capacitance of these vibration-dependent conductors, the mechanical energy from the vibrations can be converted into electrical energy. This principle is illustrated in Figure 3d. Typically, the power efficiency of electrostatic devices is improved by lowering the surface region or volume of the plate. Hence, the electrostatic generators are efficient at smaller scales and well suited to microelectromechanical systems’ (MEMS) fabrication. For example, a MEMS sensor with a parallel plate capacitor can generate 8 W of power through electrostatic induction [114]. Various comb structures have been explored such as finger teeth in an interdigitated shape [115] or a symmetrical three-port structure to increase the power output density up to 270.22 Wcm [116].
The limitations of a capacitor-based system are the requirement of recharging, low energy efficiency, and being suitable only for low-power implants. Integrating a MEMS with a variable capacitor ranging from 32 nF to 200 nF demonstrated 58 W of power generation from cardiac motion [117]. Another MEMS-based electrostatic inducer for energy harvesting in biomedical IWM devices was presented in [118], generating 80 W of power. Electrostatic generators/transducers have shown promise and commercial usability in numerous biomedical applications.
Recently, electrostatic energy harvesting has gained attention for delivering energy to implantable medical devices. For instance, ventricular motion and heartbeat energy can produce 36 and 58 W of power, respectively, to operate cardiac pacemakers using a variable capacitor electrostatic generator [117]. However, the variable-capacitor-type resonant structure is ineffective in generating energy from low-frequency human motion. A non-resonant MEMS structure was studied in [118], producing 80 W of power from nonperiodic human motion. In [119], generators using microfluidics were demonstrated to obtain 230 mW of power using the electrostatic harvesting approach from chest diaphragm motion.
Apart from MEMS, inexpensive, high-performance variable capacitance mechanism devices are an option. Such devices use printed dielectric ink made of nanocomposite materials in combination with commercially available conductive elastomers. These elastomers utilize a dielectric membrane with flexible electrodes. The deformation of this uniform membrane can act like a dielectric elastomer generator system (DEGS) [120]. Low leakage current and high cycling stability have been reported for such flexible electrostatic energy harvester prototypes. One provided a power density of 160 Wcm, over 1000 cycles at a low frequency of 0.5 Hz, making the device feasible for practical wearable applications [121]. Liquid metals were recently explored to use as the electrode material in addition to a dielectric layer. The upper limit for the relative permittivity MEMS and elastomer harvesters is 1–3, respectively, whereas liquid metals can easily accommodate the high efficiency of the device by using high-dielectric-constant (high-k) materials. The harvester electrodes have been developed with mercury [122] and, subsequently, with room temperature liquid metal alloys (Galinstan) [123]. However, mercury is a toxic material having a potential risk of oxidation of liquid metals. Researchers are looking into replacing the droplet electrode with an electrically conducting elastomer. Currently, with revolutionary advancements in printed electronics, it is even possible to print complex nanocomposite formulations for electrode structures in applications of IWM devices.
Another cutting-edge research is the electrostatic rotational energy harvester. A stochastic model of a rotational energy harvester based on arm swing was presented in [124]. Similar works for harvesting energy from human motion was demonstrated in [125,126] by using metal ball bearings. The system can scavenge up to 80 W of power from the arm swing while walking at a speed of 1.45 m/s. One of the main disadvantages of using this technology is the requirement of some seed input of energy. The addition of an electret layer, which acts as a permanent charge buried within a dielectric layer made of CYTOP polymer material, has been shown to overcome this challenge [127].
The advantages of electrostatic energy harvesters include (i) compatibility with micro-scale IMDs, (ii) high efficiency in ultra-low-power IMDs, and (iii) high efficiency in low-frequency human-centric (heartbeat, ankle, hip, knee, etc.) kinetic energy harvesting [26,28]. The necessity of pre-charging, the high output impedance, and the low output current are the challenges of using electrostatic energy harvesting for IWM devices.
Electromagnetic Induction
Oscillating magnetic fluxes induce an electric field in a winding based on Faraday’s Law, which is the working principle of electromagnetic induction. The electrical output is dependent on the strength of the magnetic field, the relative motion, and the number of turns in the winding [128]. The basic working principle is shown in Figure 3e. For instance, an electromagnetic generator was presented in [129], where a permanent magnet in the abdomen oscillates relative to the conductive coil due to motion. The system can generate 1.1 mW of power utilizing 0.3 Hz human motion. An electromagnetic generator using heart motion was presented in [130], producing 6 W of power. There has been another demonstration of an electromagnetic generator utilizing heartbeat, sewn into a sheep’s heart, producing 16.7 W by the electromagnetic oscillation [86]. Blood flow and pressure were also demonstrated to be effectively converted to 3.4 mW of power in [131] using an electromagnetic generator. Using the heart movement to design an energy harvester can minimize life threats, avoiding multiple replacements of cardiac pacemakers. Energy harvester for endocardial implantation was presented in [132,133,134]. The device operates on the electromagnetic induction principle, containing a microgenerator producing 4.2 W of output power. Furthermore, the same group proposed an energy harvester, in which an eccentric oscillating mass captures external heart excitation and converts it into the rotation of an electromagnetic generator, harvesting an output power of 6W. A curved electromagnetic energy harvesting system that can capture energy through human oscillatory walking motion was presented in [135]. The experimental results showed that the harvester can produce 5.185 mW of power. An energy harvester is placed in a backpack to utilize low-frequency and high-amplitude vibrations, which leads to an output peak power of up to 32 mW while walking at a speed of 3.58 m/s [136]. The significant weight and flexibility of the electromagnetic energy harvester are the main challenges for proper energy supply to IWM devices without causing damage. For the benefit of size, research efforts have been devoted to utilizing MEMS in electromagnetic energy harvesting [137,138]. For example, a MEMS-sensor-based velocity-damped resonant generator was demonstrated to generate 10 W of power [139]. Similarly, 3.9 W of power was obtained from the ankle motion using a magnetic induction generator for miniaturized IWM devices [140]. Flexible electromagnetic devices are gaining huge popularity due to device compatibility with energy-scavenging applications. A low-cost, flexible electromagnetic energy harvester was designed in [141] utilizing a polymeric magnetostrictive ribbon and poly-vinylidene fluoride (PVDF), which gives an output power and power density of 6.4 W and 1.5 mW/cm, respectively. Nonlinear dynamic characteristics are studied in [142], where the vibration energy harvester generating an output power of 620 W was designed using the Terfenol-D/PZT composite proposed. A similar type of vibration harvester was designed with micro-machined flexible polyimide films producing a 5 W peak output power [143]. However, MEMS fabrication is a complicated process, and the energy efficiency is low, which limits its widespread adoption.
Furthermore, the kinetic energy associated with blood flow can be a source of energy harvesting. An electromagnetic (EM) generator made of metal coils and permanent magnets has been demonstrated to harvest energy from blood flow [144]. A stack of magnets oscillating under the influence of external body acceleration would generate electrical energy based on EM induction. An EM generator device implanted in the right ventricular cavity of a pig and fixed on the endocardium could achieve a power of 0.78 W. Another EM generator placed in the pig heart through a catheter utilized intra-cardiac turbine motion to harvest energy from blood flow [133]. The device weighed about 1.3 g and output power in the 10.2 ± 4.8 W range.
Over the years, EM generators have been proven to be a sustainable method for kinetic energy harvesting. Traditionally, these energy harvesters are designed and fabricated using a resonant structure [145]. However, the resonant frequency ( 100 Hz) is much higher than the frequency of human motions (<5 Hz). This difference leads to a non-resonant condition and may cause poor efficiency in energy conversion. Thus, they need a coupling interface and an energy extraction circuit for extracting magnetic flux in synchronization. This circuit enables the rectification and the amplification of the voltages produced by an EM energy harvester at optimum levels. Apart from the popular choice of a nonlinear coupling interface [146], researchers have also demonstrated energy harvesting with a linear energy extraction circuit comprising a full-wave bridge rectifier. Frequency up-conversion structures [147] and rotational structures [148] have also been reported for energy harvesting at an ultra-low frequency.
In general, more energy can be harvested in an electromagnetic generator compared to an electrostatic generator. However, the reported output energy is comparatively low than the piezoelectric energy harvesters. The limitations of electromagnetic energy harvesters include low output power and low impedance, resulting in high output current [89]. Therefore, extra circuitry is required to obtain power conditioning. In addition, the EM generators can cause electromagnetic interference (EMI) with other medical devices, which can limit the application of this type of energy harvester.
Thermoelectric Methods
The concept of a thermoelectric energy harvester is the Seebeck effect, which uses temperature differences to generate electric energy. The temperature gradient between the body and the ambient environment is converted to a voltage output. A system overview of this category of energy harvesting is shown in Figure 3b. Both metals and semiconductors can be utilized to harness energy using the Seebeck effect. However, the Seebeck effect is much more prominent in semiconductors. A basic thermoelectric generator (TEG) for IWM devices consists of a series of connected n-type and p-type semiconductor materials, resulting in electron movement from the hot terminal to the cold one. Such electron movement produces a potential difference, resulting in a current flow in the closed circuit. Bismuth telluride, calcium manganese oxide, and lead telluride are a few examples of thermoelectric materials. The advantages of these materials are low thermal and high electric conductivity, compact size, light weight, long operational lifetime, and high Seebeck coefficient [27]. The output voltage of TEGs can be boosted using multiple p-n semiconductor connections in series, forming thermopiles [149]. The Seebeck effect can potentially generate sufficient power to operate implants such as nerve and muscle stimulators, hearing aids, etc. [150,151]. However, when the temperature gradient is below 5 K, only a few hundred microwatts of power can be extracted from the human body. For instance, exploiting the 5 K thermal difference, W [152] and W [153] of power is generated using a TEG of a size of 0.19 cm and 1 cm, respectively. A recently reported TEG demonstrated an output power of around W, exploiting the 5 K temperature gradient. Thermopiles can be integrated into wearable textiles to generate electrical energy from body heat [154]. For example, a cold plate of 4 cm in diameter was glued to carbon fabric, generating up to mW of power, where the ambient environment temperature varied from 15 °C to 27 °C. In [155], an IMD with an integrated accelerometer powered by a TEG to detect falls was presented, scavenging W of power at 15 °C. Illustrations of different TEGs are presented in Figure 5. Figure 5a shows an interfacing circuit for a batteryless thermometric energy harvester fabricated in a m CMOS process. An efficient control circuit was designed for transferring the obtained energy to a capacitor, which controls the output voltage at 1.8 V. Figure 5b presents the measured output power achieved from the buck converter compared to the maximum power extracted from the TEG. Figure 5c demonstrates a wearable TEG. The non-uniformity of skin temperature is dependent on the ambient temperature. Hence, the output power extracted from the TEG depends on the location and clothes. Figure 5e presents the design of a TEG structure using Aluminum and Teflon. Aluminum is used to design the hot plate for fast heat collection, while the cold plate works as a heat diffuser. Teflon works as an insulator sandwiched between the hot and cold plates, reducing the convection and radiation of heat to prevent warming of the device. Figure 5f presents the output power curve, which varies between 40 W to 520 W.
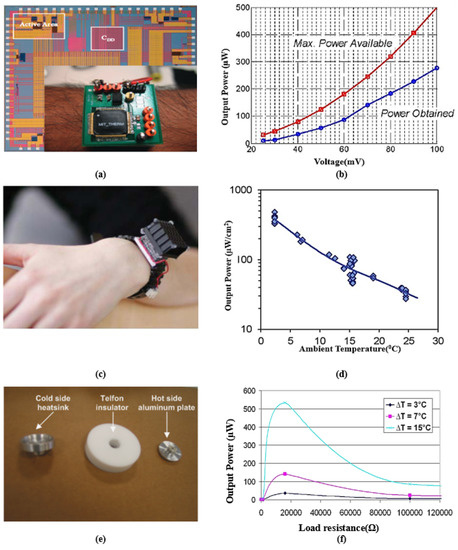
Figure 5.
(a) Interface circuit of thermal energy harvester where the inset presents the thermoelectric generator (TEG) chip worn on the arm. (b) Measured electrical power from the buck converter vs. maximum power available from the TEG. Reprinted with permission from [149]. Copyright 2010 IEEE. (c) Wearable TEGs with a cm radiator. (d) Output power variation with ambient temperature for a TEG. Reprinted with permission from [154]. Copyright 2013 IEEE. (e) A prototype of a TEG, harvesting energy from the human body. (f) Output power obtained from a TEG at different loading conditions. Reprinted with permission from [155]. Copyright 2009 IEEE.
Flexible and wearable electronic devices have gained significant attention owing to their light weight, easy accessibility, and ability to endure mechanical deformation. Polymer-based TEGs have gained widespread popularity due to their low cost, light weight, fast processing, mechanical flexibility, and low thermal conductivity. Organic thermoelectric nanomaterials were presented in [156], where single-wall carbon nanotubes (SWCNTs) were stabilized by poly. A high-performance thermoelectric poly (3-hexyl-thiophene)/CNT (P3HT/CNT) nanoparticle and flexible P3HT/CNT organic TEG were prepared in [157], using a spray-printing method, which exhibits excellent thermoelectric performance. A printable TEG composed of organic materials, graphene, PANi, and a double-wall nanotube (DWNT) was developed in [158] using a layer-by-layer assembly technique. Formulating an n-type SWCNT by doping diethylenetriamine (DETA) on the pristine SWCNT (p-type) was presented [159]. The pristine CNT presented a Seebeck coefficient of 43 V/K, and the DETA-doped CNT exhibited a negative Seebeck coefficient of V/K.
The fabrication of p- and n-type thin films for thermoelectric applications was demonstrated in [160] by doping tetrathiotetracene (TTT). A novel wet-spinning process was explained in [161] to develop an organic TEG using nanocomposite materials. The design of flexible thermoelectric threads using 3D-printable composite inks was introduced in [162]. A TEG prototype based on nanofibers was assembled in [163], which generated an output power density of Wcm from human body heat. This work forms the pathway to developing high-performance polymeric and flexible materials for TEGs.
Because of the low conversion efficiency, it is crucial to have a well-designed power conditioning module and coupling interface. Researchers have demonstrated a CMOS interface circuit in the 130 nm process, gaining up to 90% efficiency employing a single inductor-based buck–boost converter [164]. The proposed system also employed an automated zero-crossing detection feedback scheme to improve power efficiency. In addition, there has been a demonstration of an all-integrated MEMS thermoelectric power chip [165].
The limitations of TEGs are their low conversion efficiency and the high cost of realizing these devices. For instance, with a K temperature gradient in the human abdomen, one thousand thermocouples are needed to achieve W of power, requiring an area of cm [166]. These large area requirements in implantable and wearable devices are not practically feasible. In addition, due to low output voltage, TEGs require extra circuitry for boosting the converter, control, and initial start-up.
In achieving a high power density, researchers have been looking for newer materials. In simulation, different emerging materials are explored for better efficiency. It has been reported that phase-change materials can be used to enhance the thermal performance of a TEG by around 30% when the heat input is at around W [167]. Therefore, improved power conditioning methods, low-power start-up, efficient MPPT design, and novel thermoelectric material design are a few research prospects to commercialize TEGs.
Triboelectric Energy Harvesting
Triboelectric energy harvesting is an emerging technique to meet the energy needs of implantable and wearable medical devices [168]. The triboelectric nanogenerator (TENG) uses the electrification effect to generate energy. The charge is produced by the friction at an interface of two different materials, also called the triboelectric pair [169]. Electrodes are placed on each material, and the electrification effect is combined with the electrostatic induction to obtain a usable amount of power [170]. A representative working principle is shown in Figure 3c. Significant improvement has been observed in TENGs over the last few years, enabling the conversion of energy from gentle touches [171], palm tapping or walk [172], eye blinks [173], and vibration [174] into electrical energy. For example, human motion due to mild running at a speed of 5 m/s can generate 5.28 W of power [175]. A biodegradable TENG made of organic materials based on gelatin film was shown in [176]. This prototype can generate an output voltage, current, and power density of up to 500 V, mA/m, and 5 W/m, respectively.
Triboelectric materials are flexible and cost-effective. In addition, the high power density, pressure sensitivity, and conversion efficiency of TENGs have made them a promising energy harvesting technique for IWM devices [170]. For instance, a heart rate monitor was presented in [177] using a TENG to meet the energy need. The system can generate 2.28 mW of power, having an excellent 57.9% conversion efficiency using human walking motion of 10 Hz. However, the surface charge density retained in the dielectric layer of the TENG depends on the dielectric breakdown of the air, limiting the amount of harvested energy [178]. There has been ongoing research for various composite materials for TENGs. Nanowire arrays made of cobalt selenide on a Kevlar fiber and reduced graphene oxide dispersed in PDMS have been reported to output 1.1 mW cm, even at a low frequency of 5 Hz [179].
In addition, the pulsed output power, low current, and frictional damage in the contact surface are a few other limitations of TENGs. The triboelectric energy harvesting technique is still in the development phase and improvements are necessary to adopt it in powering IWM devices.
A summary of representative research in biomechanical energy harvesting is presented in Table 1. There is a lack of a universal figure of merit (FOM) for a fair comparison of energy harvesters. The performance of energy harvesters often depends on techniques, device architectures, materials, circuits, and various operational modes. The output power per unit volume, termed output power density, is a widely used metric for energy harvesters. Energy conversion efficiency is another FOM, which is the ratio of the output of the electrical form to the input, which can be biochemical, mechanical, light, heat, etc. The output voltage, current, power, power conversion efficiency, device dimension, weight, open circuit voltage, operating frequency, etc., are often used to quantify the performance of different energy harvesters. Due to wide acceptability and availability in the literature, the output power density is summarized in this review paper to provide an idea of the performance of representative energy harvesting research.

Table 1.
Summary of representative research on human-centric biomechanical energy harvesting.
Overall, biomechanical systems utilizing the kinetic energy from human body motion require a relatively larger size, producing low power. The output power of thermoelectric generators is low, which is insufficient for most IWM devices. There are challenges, as well as research opportunities to improve these energy harvesting techniques, as discussed in Section 8.
3.2. Environment-Centric Energy Harvesting
Energy harvesting from the environment scavenges electrical energy from various sources, including solar, infrared, RF, and wireless power transfer (WPT). This section presents different energy sources and state-of-the-art research to harvest energy from the environment.
3.2.1. Infrared Radiation
Earth continuously emits infrared heat into outer space in the range of Gigawatts [180]. Capturing even a fraction of that would end energy woes. Like the Seebeck effect in TEGs, whenever heat flows from a hotter to a colder body, energy can be harvested. One way to attain this is to treat waste or infrared heat as high-frequency EM waves. Appropriate antennas can collect these EM waves for further processing and use in IWM devices.
Some implantable devices have experimented with harvested energy from infrared radiation such as cardiac and brain-implanted pacemakers. A photodiode is usually implemented as part of implantable medical devices to capture infrared radiation [181]. For example, an implantable cardiac pacemaker presented in [182] achieved mW of power from infrared radiation. Biological tissue works as a source of wireless power transfer (WPT) in the near-infrared region (NIR) within the optical range of 650–1350 nm [183,184,185].
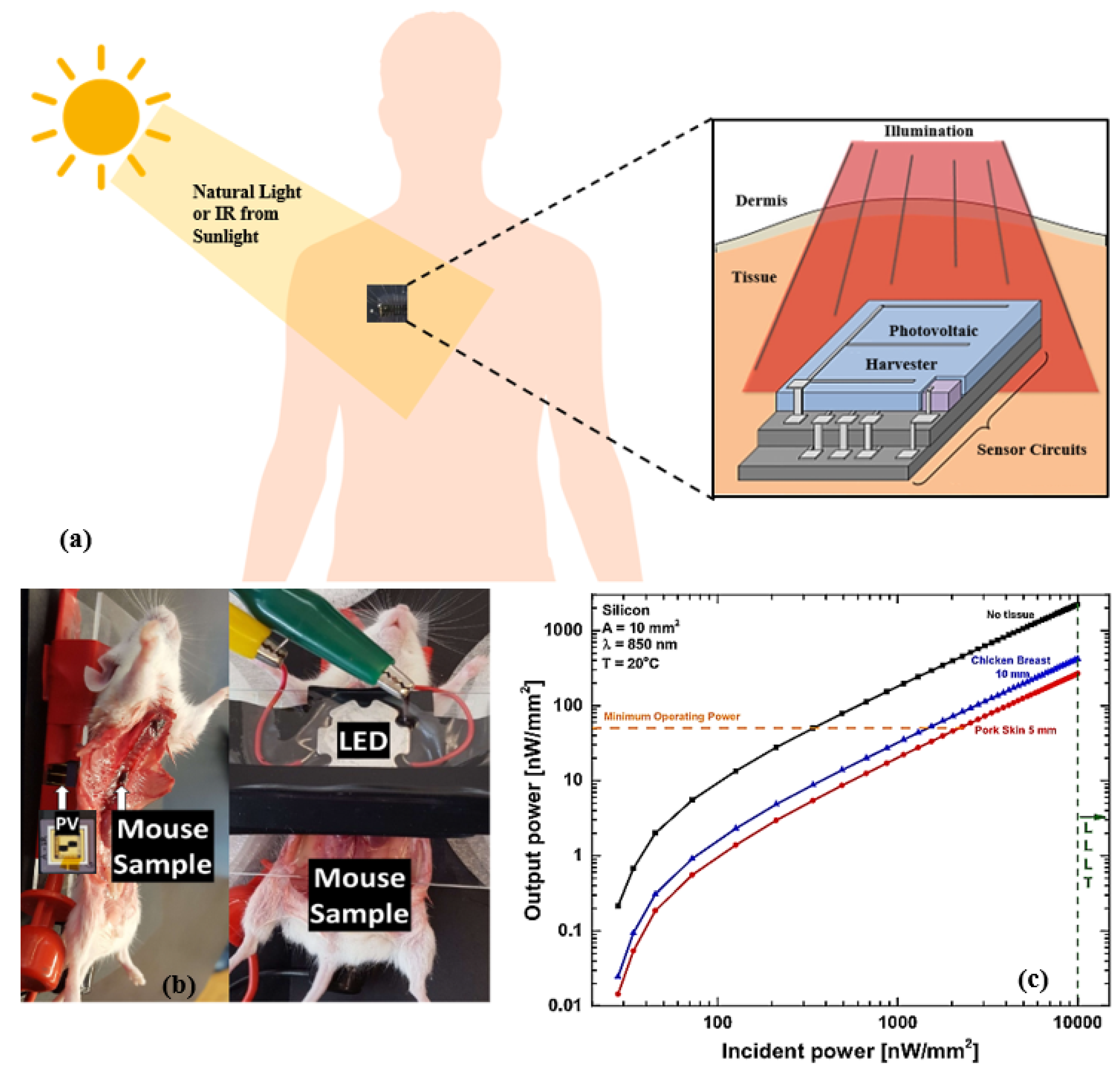
Figure 6 illustrates the concept of NIR subcutaneous energy harvesting in human, mouse, and output power variation in a photovoltaic (PV) cell implanted in a chicken breast. Silicon or GaAs PV cells convert energy in the NIR with an approximate external quantum efficiency of 100% [186,187]. As NIR can penetrate through the skin, researchers demonstrated its application in powering biomedical devices [188,189,190,191,192,193]. An artificial NIR light is attached outside the skin, which can be used as a reliable energy source. A recent work in this area presented a design of a rear reflector based on the bandgap of implanted PV cell materials, which utilize the transmission efficiency of NIR light underneath the skin [194]. The design in [192] includes a PV cell for biomedical implants, which uses infrared irradiation (850 nm), giving an output power density below 1.06 W/mm. Figure 6b illustrates the dissected sample of mouse placed between the LED and PV cell. Extracted energy is measured using LED irradiance and PV cells placed underneath the mouse. Relevant work on subcutaneous infrared energy harvesting for IMDs is reported in [190,191] with an output power of 60 W and 8.2 W, respectively. However, the limitations of using infrared radiation for IWM devices include increased skin heating, stimulating tissue damage, a low amount of harvested energy, and large device size occupying a large area, resulting in high power consumption.
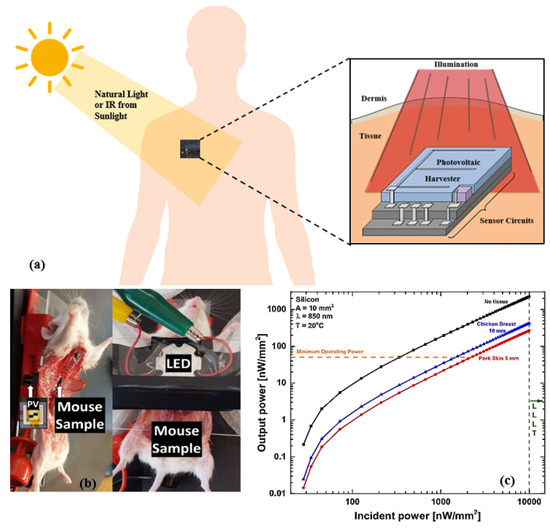
Figure 6.
(a) Schematic of photovoltaic energy harvesting through subcutaneous tissue. (b) NIR energy measurement from photovoltaic cells implanted in mouse. (c) Incident versus output power of a silicon photovoltaic cell implanted at a 5 mm depth in porcine skin and a 10 mm depth in chicken breast using low-level laser therapy. Adapted with permission from [192]. Copyright 2017 IEEE.
IR transmission possesses several advantages in terms of the design of the coupling interface. These advantages include simple and relatively low-cost circuit fabrication, low power consumption, high noise immunity, etc. Infrared or near-infrared is usually employed for harvesting energy for under-skin IMDs. For those applications, regular IR transmitter–receiver systems have shown satisfactory performance [181]. It has been reported that transmitted power is dissipated in tissue by radiation and in coupling [212], causing permanent tissue damage. The transmitted IR radiation heavily depends on the distance between the source and target. The coupling interface and power transfer in IR are active research areas.
3.2.2. Radio Frequency Signal
Due to the widespread deployment and recent advancement in wireless communications technologies, energy harvesting from RF is gaining attention [213]. The EM waves used in wireless communications are available in ample amounts, which can be used in energy harvesting. For selecting a suitable operating frequency, different compact rectifying antennas (rectennas) have been designed using techniques such as dual polarization [214] or using emerging materials such as graphene [215]. The conversion performance of the designed rectenna is 80.23% with a signal input power of dBm. The size constraint of these invasive foreign substances in the human body led researchers to look into more compact solutions. A nanocomposite single-sided printed antenna can obtain an efficiency of 62.5% at 1.8 GHz [215]. However, the implantation of the rectenna is always a concern, leading to the proposal of a textile-based rectenna array [216]. The rectenna array could achieve a conversion efficiency of 70%. It has been demonstrated as a proof-of-concept that the system could power three LEDs at a distance of cm. A complete textile-based approach with an RF energy harvester and supercapacitor was shown in [217]. The system achieved a power conversion efficiency (PCE) of up to 80%. It can charge the supercapacitor to V in four minutes at a distance of m from the source. Moreover, wireless radio power and control systems employing thin, mechanically soft, implantable, stretchable RF energy harvesting schemes were used in neural implants using light-sensitive proteins [65]. An in-vivo demonstration in rat implants showed that the system successfully modulated peripheral and spinal pain circuitry.
One of the main limitations in an RF system is that it requires much peripheral circuitry, and sometimes, the size of those additional things might be even bigger than the sensor itself [65]. For that, researchers have looked into solid-state electronics solutions. For instance, Schottky barrier diodes are popular choices in rectification due to their low built-in voltage [218]. In [219], a wearable sensor tag was designed, which can monitor physiological signals such as PPG, body temperature, blood pressure, and respiratory sounds. The wearable sensors are powered by harvesting the RF energy from an mW source using a two-stage CMOS rectifier, generating 15 μW power at a m distance. Similar technologies were studied in [220,221], harvesting RF energy at different frequencies using CMOS circuits to power up IWM devices.
RF can provide a relatively predictable amount of energy, unlike other energy sources, which are dependent on natural forces. The near-field and far-field RF signals utilize different coupling interfaces. Inductive and magnetic resonance coupling is used in the near-field RF to extract the energy. In the near-field, a power conversion efficiency (PCE) of up to 50% can be achieved while having a limited transmission distance [222]. On the other hand, far-field RF harvesting technology uses radiative coupling. Power transfer in the electric field of the propagated waves can be specifically suitable for IMDs. However, this technology needs extra assisting circuitry such as a buck–boost converter, rectenna, and supercapacitor as coupling interfaces [223].
Wide abundance of RF sources is main advantage of harvesting energy from RF signals. However, the devices have low efficiency; the output efficiency sometimes can be unpredictable and depends on the distance between the source and the harvester and many other factors. The types of diodes used in the rectifier block and the impedance matching network, the shape, and the antenna’s size are a few of the challenging design blocks in the system.
3.2.3. Solar Energy Harvesting
Solar energy harvesters are a mature technology on the macro scale inspired by the photosynthesis process in plants. In a regular solar cell, an anode and a cathode are placed in a molecular dye. This arrangement can readily convert solar energy into electrons, thereby electrical energy, upon the availability of proper sunlight. Three types of materials widely used in solar energy harvesting in photovoltaic cells are amorphous, monocrystalline, and polycrystalline silicon. Amorphous silicon is suitable for wearable devices due to its flexibility, low cost, and high sensitivity to natural light, but it lacks widespread adoption because of its low energy efficiency of around 10%. On the other hand, crystalline silicon has a high conversion efficiency of approximately 15–20% in outdoor solar lights. The amount of harvested electrical energy is linearly related to the size of the solar cell and the amount of light illumination. For example, up to 100 mW/cm of energy with an efficiency of 15–20% can be achieved during the daytime [224]. For an indoor environment, the conversion efficiency of 8% and an energy density ranging from 10–100 W/mm is achievable.
Consumer electronics such as calculators and wristwatches have efficiently been using solar energy for several decades [225]. Although solar energy is considered one of the prominent renewable energy sources, sufficient electrical energy can be harvested in miniaturized photovoltaic cells to operate low-power electronics. The typical solar cell consists of nonlinear semiconductor devices that generate electricity while exposed to light.
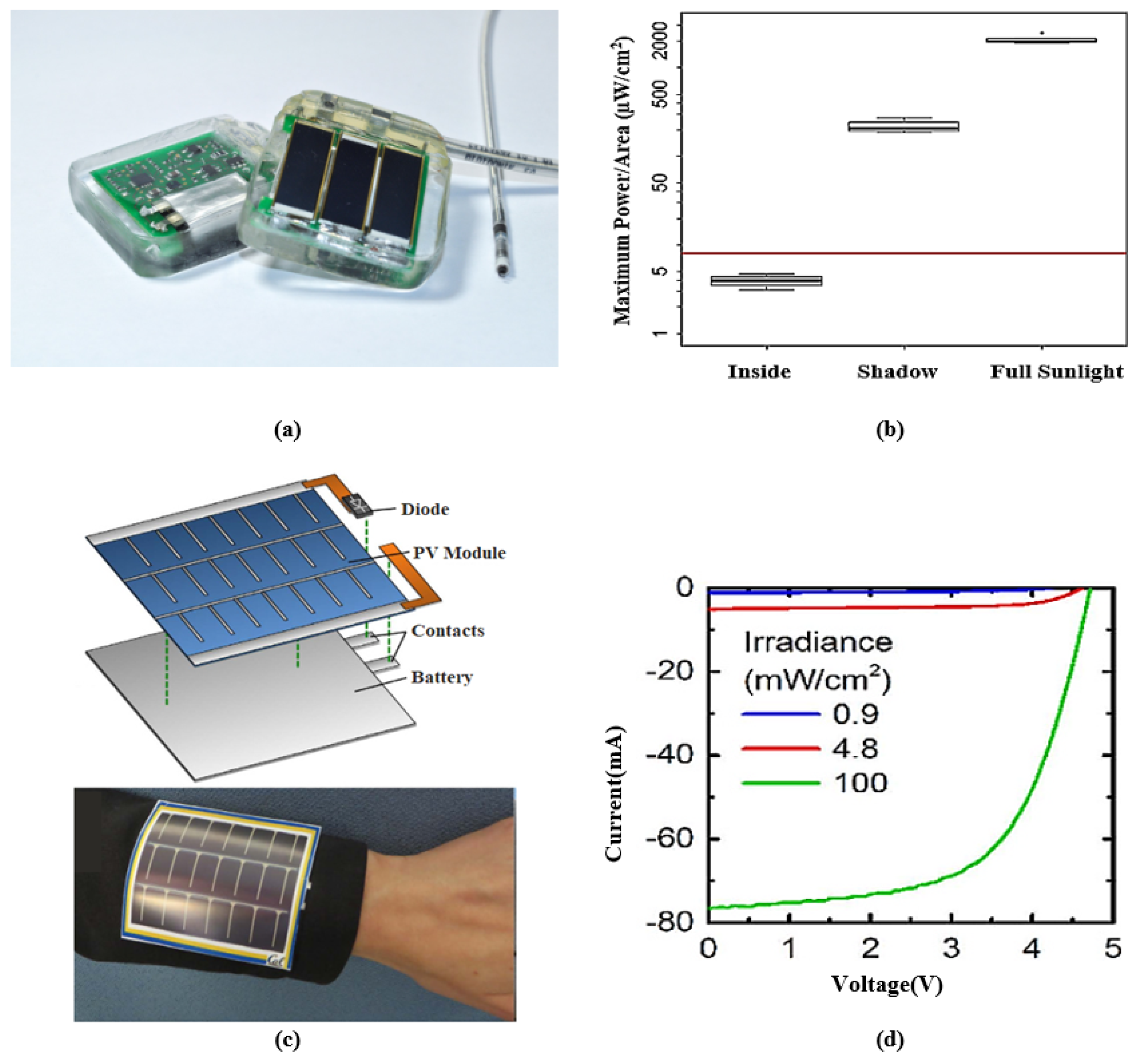
Although solar energy harvesting for IMDs remains challenging, the high conversion efficiency and availability make solar energy an attractive source for wearable devices. For instance, a wearable medical device was presented in [226], which uses monocrystalline PV cells for energy harvesting. The device can continuously monitor heart rate and blood oxygen saturation. An onboard mAh LiPo battery and a supercapacitor (which can deliver mW of power for up to 4 h) are charged when solar energy is available. A solar powered cardiac pacemaker and a flexible PV cell in a wristband are presented in Figure 7 with their output characteristics. Figure 7a presents a battery-less single-chamber pacemaker driven by solar energy harvesting. The PV cells are placed under pig skin flaps and exposed to different irradiation. The measured median output power presented in Figure 7b was found to be W/mm, W/mm, and W/mm for full sunlight outdoors, shade outdoors, and indoors, respectively. Figure 7c presents a wearable pulse oximeter with a flexible thin film amorphous silicon PV module. A flexible thin film lithium-ion battery was designed to operate this pulse oximeter. Since the PV module and battery were designed with flexible material, the system can be bent and attached to curved surfaces. Figure 7d illustrates the current–voltage (I-V) characteristics of the PV module under three lighting conditions.
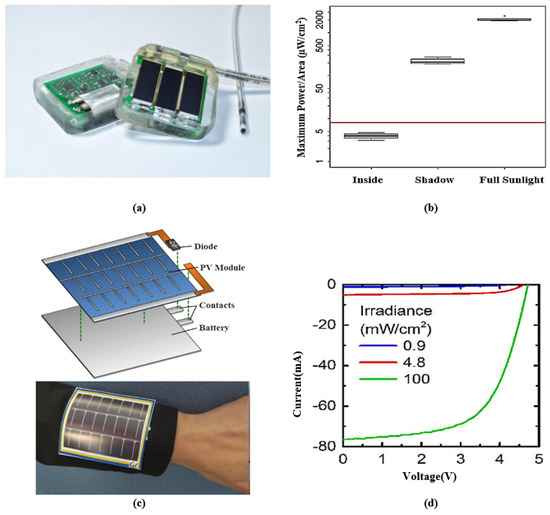
Figure 7.
(a) A batteryless solar-powered cardiac pacemaker prototype; the rear part is lithium-polymer storage (silver color). (b) Boxplot demonstrates the output power at various irradiances. Reprinted from [232]. (c) Photovoltaic energy harvesting system with the flexible battery mounted on the curved surface (sleeves). (d) I-V characteristics of photovoltaic cell charging the flexible battery. Adapted from [227].
Amorphous silicon has allowed the development of flexible photovoltaic cells suitable for wearable devices [227,228]. For instance, a flexible printed circuit board (PCB) was presented in [228] to monitor body temperature, powered by solar energy. The excess energy is stored by incorporating a power management circuit. The device can operate uninterrupted for up to 15 h in indoor environments, requiring only about of light intensity. In addition, it can wirelessly transmit recorded information to a remote node at a GHz frequency. Moreover, incorporating flexible PV cells facilitates the reduced size and weight of the wearable device. Another flexible wearable device was presented in [229], which monitors heart rate, while an integrated accelerometer is employed for fall detection. Researchers have also explored organic materials as energy sources for IMDs. They offer cost-effectiveness, superior biocompatibility, and high sensitivity to NIR light. For example, printed organic solar cells have shown 7.6% PCE and 2.9% storage efficiency of supercapacitors for retinal implant, generating mJ of energy and mW of power [230].
In the NIR, the skin and subcutaneous tissues of the human body behave transparently to light illumination [192]. Hence, solar energy can be effectively converted to electrical energy in IMDs, using the photoelectric effects. Due to recent advances in CMOS technology, on-chip photovoltaic cells are feasible [231]. A PCE of 17% and 31% is achievable in silicon and GaAs PV cells, respectively, using 1.06 W/mm NIR light at a wavelength of nm. However, the GaAs material is toxic to the human body and requires proper encapsulation. Polymers such as silicone [232,233], polydimethylsiloxane [193], and diamond are often used in encapsulation due to their high flexibility.
The characteristic features of photovoltaic energy harvesting are high output power density, low fabrication cost, and telemedicine capacity through the optical link. However, the low irradiance in indoor conditions and internal non-uniform body temperature result in low output power and poor energy conversion efficiency. In addition, the irradiance might be interrupted by the clothing and placement of the IWM devices, which becomes prominent in small-scale devices. Moreover, the PV-powered implantable devices cannot be placed deep inside the body due to area constraints and the decrease in incident solar energy with tissue thickness. Due to the nonlinear characteristics of PV cells, maximum power point tracking (MPPT) is often incorporated into solar energy harvesting to maximize conversion efficiency. However, the requirement of additional circuitry and the power consumption of MPPT limit their applications in IWM devices.
3.2.4. Wireless Power Transfer
Wireless power transfer (WPT) is a sustainable powering solution for IWM devices. WPT minimizes the finite capacity of batteries problem and avoids transdermal wiring, which can cause infection. WPT works on Faraday’s concept of transferring power from one coil to another without requiring any conducting medium. Initial works on powering transcutaneous implants were based on building a near-field inductive coupling between transmitting and receiving coils at resonance. Three categories of WPT for IWM devices are discussed in this section.
Ultrasonic Techniques
Energy harvesting using sound waves is comparatively safe for the human body with minimal chances of electrical interference with other medical devices. Pulsed ultrasonic waves were studied in [234] to provide currents in the milliampere range in piezoelectric devices. Generating high-frequency current using ultrasound waves was demonstrated in [235]. The ultrasound wave generator is placed on the skin to couple energy to a MEMS sensor implanted inside the body, providing electrical charge output. Another significant improvement in this area was presented in [236], generating 21.4 nW of power in an implanted sensor using ultrasound waves. A MEMS-based piezoelectric ultrasonic energy harvester (PUEH) was reported in [237], which increased the battery lifetime of a pacemaker.
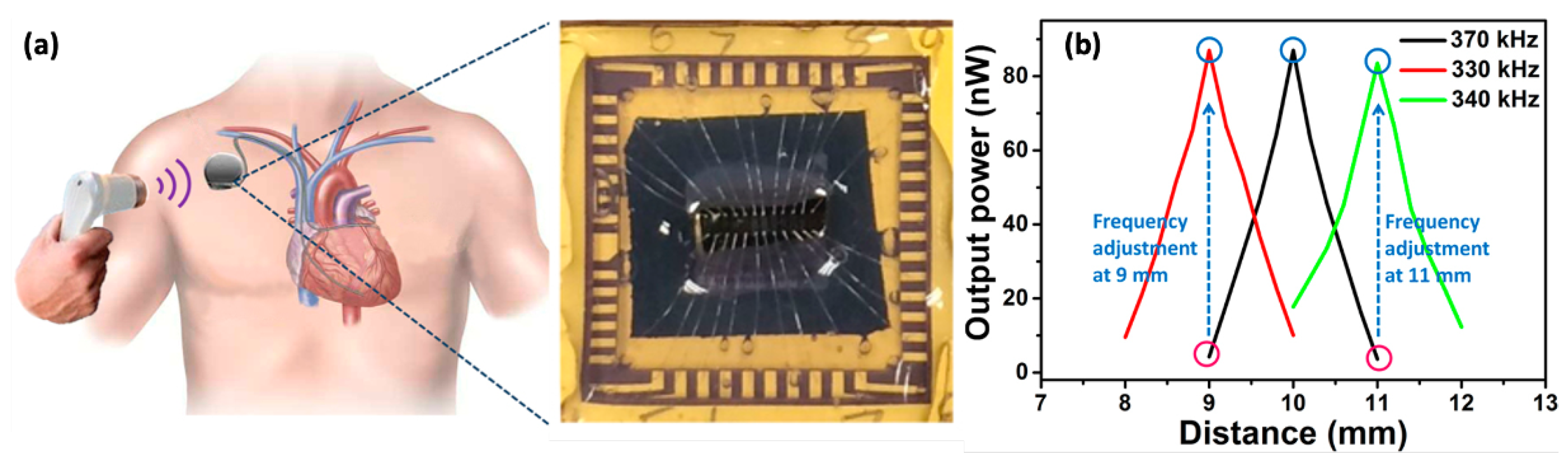
Figure 8 shows the concept of a PUEH driving a pacemaker inside the human body. The reported PUEH collects energy from an external ultrasound transducer used in hospitals for diagnostic purposes. The designed harvester provides a large bandwidth due to the two overlapping resonant modes, which increase the power density from 0.59 W/mm to W/mm at an ultrasound intensity of 1 mW/cm. A novel Samarium (Sm)-doped (Sm-PMN-PT) implantable PUEH was introduced in [238], giving an output power density of 1.1 W/cm. Electrophysiological experiments on rats confirmed its sustainability for deep brain stimulation and analgesia applications. A flexible-membrane-based PUEH was studied in [239], where the wireless charging is performed via ultrasound, producing a continuous voltage and current outputs of 2 and , respectively, on both planar and curved surfaces. A multilayered PUEH presented in [240] gives a peak output power of around 13.13 mW. However, the ultrasound energy harvesting to operate IWM devices is still in its infancy and limited by the insignificant amount of harvested energy and the requirement of large MEMS devices.
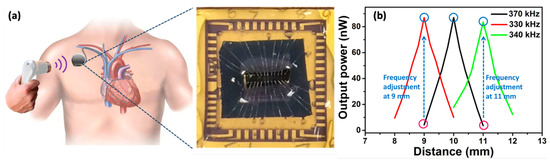
Figure 8.
(a) Ultrasonic energy harvester integrated with a pacemaker; the fabricated chip is shown at the right side. (b) Frequency adjustment for achieving high transfer power and efficiency due to breathing and muscle movement for a 1 mm fluctuation in distance. Reprinted from [237].
Capacitive Coupling
The basic principle of capacitive coupling to power IMDs is parallel plate capacitors. One of the plates is placed on the external skin, and the other is internal to the human body and attached to the implants. For instance, capacitive coupling was used in [241] to transfer power to implantable devices. The skin in between the parallel plates behaves like the dielectric, and the electric field works as the carrier to transfer power. A new approach to capacitive power transfer for implants was presented in [242]. The system transfers the power through a tissue layer, which acts as a return path for the current. This method reduces the size of the IMD while increasing power transmission efficiency. The data transmission through a capacitive coupling link between brain implants was studied in [243]. The designed capacitive link uses metal plates to attach to the bottom and side wall of the implant. The reported minimum input power required to establish the data link is 31 nW at an operating frequency of MHz. The limitations of capacitive coupling for WPT are tissue heating and user discomfort [244].
Inductive Coupling
The concept of inductive coupling is the use of magnetic fields to provide sufficient power to the IMDs [245]. Most inductive coupling techniques operate below a 20 MHz frequency to avoid tissue heating [246,247]. The overall system comprises two inductive coils: one placed outside the human body, while the other is attached to the implants. Both coils should be tuned at the same resonant frequency to achieve an efficient power transfer. The amount of power transfer is controllable by varying the number of coil turns and shapes. Most studies on inductive links reported a frequency range lower than 20 MHz [247,248] for avoiding tissue heating, which results from power absorption in tissue. The efficiency of the power transmission of a system depends on the coil shapes and sizes, the quality factor of the coil, the coupling coefficient, and alignment location. A hybrid inductive–ultrasonic wireless power transmission link was proposed in [249] for biomedical implants, which can achieve high power transfer efficiency. For instance, at 1.1 MHz, it can wirelessly power a mm ultrasonic transducer. The wireless inductive power transmission system finds potential application in the brain and spinal cord stimulants. A stimulator was designed in [250], operating at a 198 MHz resonant frequency at a distance of 14 cm from the body. The designed stimulator collects the energy from a switched capacitor and supplies it for boosting the output when the voltage reaches its threshold. Another inductive coupling work was presented in [251] to operate electromyography (EMG) implants in the gastrocnemius muscle of a rat. The system achieves a power transfer efficiency of % at 60 MHz and provides 1.3 mW of output power to the implanted device.
Table 2 summarizes the state-of-the-art environment-centric energy harvesting methods, generated output power, power density, and sizes. For subcutaneous IMDs, inductive coupling is a suitable technique at the ISM frequency band. On the other hand, several methods including capacitive coupling are still in the development phase and potentially can cause hazards to the human body.

Table 2.
Summary of representative works on environment-centric energy harvesting techniques.
3.3. Hybrid Energy Harvesting
Hybrid energy harvesting incorporates multiple energy sources to generate a larger electrical output. For example, the solar cell uses only a portion of the incident light, and photons outside the PV cell material band gap are wasted as heat dissipation. Therefore, combining photovoltaic systems with thermoelectric methods can utilize most of the incident energy, which increases the output power [262]. The PV-TEG combination requires a sufficient temperature gradient across TEGs [263]. The mathematical modeling and numerical analysis of heat transfer and temperature distribution to calculate the PV-TEG hybrid energy generation were presented in [264]. An energy efficiency of 23% is theoretically possible in PV-TEGs, higher than a single energy harvesters. A flexible PV-TEG was presented in [265], which utilizes solar energy and the human body dissipated heat to provide power support to WMDs. A lossless coupling is feasible in the PV-TEG combination. The system can achieve a power conversion efficiency of 16.3% utilizing a 15 °C temperature gradient in a TEG with a PV system [266]. As the PV-TEG combination is power-efficient and inexpensive, it can serve the energy demands of WMDs.
A hybrid energy harvester combining solar energy with human body motion was presented in [266] for self-charging textiles using a supercapacitor for energy storage. It contains a fiber-shaped TENG to use motion energy and fiber-shaped dye-sensitized solar cells to capture the solar energy. Due to the all-fiber structure, the system is flexible and can be integrated into clothes to serve the energy needs of WMDs.
A hybrid architecture combining a TENG with piezoelectric nanogenerators (PENGs) can utilize human body motion and ambient mechanical energy. For example, a TENG-PENG combination demonstrates sufficient energy generation from wind to operate digital watches [267]. Besides, TENG-PENG hybrid energy harvesters use hand vibrations to provide power support for cell phones [268]. Despite the increased output power, the challenges of hybrid energy harvesters include inflexibility, high cost, and complex mechanisms. Therefore, designing human-body-compatible hybrid energy harvesting for precision healthcare is still in the development phase.
Hybrid energy harvesting offers an excellent solution of taking the good features of multiple energy harvesting techniques. However, that makes the design of the coupling interface and power conditioning circuit much more complex. The challenges of the TENG-TEG and TENG-PV coupling effect were discussed for a metal–semiconductor interface in [269]. It has been also demonstrated that a circuit based on parallel-synchronous switch harvesting on an inductor (SSHI) can simultaneously harvest power from a PZT and a TEG, this too with a single shared inductor [270]. There have been demonstrations of highly integrated multi-source energy harvesters with the coupling technique and multiplexing schemes, which can simultaneously contribute to triboelectric, photovoltaic, and thermoelectric power generation [271].
4. Extraction of Maximum Power from Energy Harvesters
In IWM devices, capturing the maximum power from the harvester is immensely important as energy harvesters intermittently generate energy based on the availability of the source, and often, the generated power is low. To obtain the maximum power point, an impedance matching between the energy harvester and the load is crucial [272].
The two main techniques for MPPT are perturb and observe (P&O) and fractional open-circuit voltage (FOCV) [272,273]. The P&O method is also called the hill-climbing technique, mainly used in large-scale energy harvesting. The harvested voltage and current are repeatedly sensed to capture the maximum power. In addition, the duty cycle and switching frequency of the DC-DC converter are dynamically varied [273]. The P&O technique is highly accurate in finding the maximum power point. However, the P&O technique is complex and power-hungry due to digital controllers. Different techniques have been developed to minimize the power consumption of the controllers [274,275,276]. For example, maintaining a low controller duty cycle to maintain a longer sleep mode and short active period can minimize the power consumption [274,277].
The power consumption of the FOCV-based MPPT controller is relatively lower than that of the P&O technique. The FOCV controller can be implemented using analog or mixed signal circuitry having minimum complexity [278,279]. In the FOCV technique, the maximum power point is a fixed ratio of the open-circuit voltage (VOC) of the energy harvester [273]. For instance, in thermoelectric, radio frequency, and piezoelectric microgenerators, the MPP point is approximately 50% of the VOC. On the other hand, for photovoltaic cells, the MPP point is obtained as around 75–80% of the VOC [278]. To obtain the VOC, a voltage divider configuration or switched capacitor is frequently used [274]. The limitations of the FOCV-based MPPT method are the low conversion efficiency due to the frequent disconnection of the energy harvester and load to measure the VOC, which, in turn, reduces the power [279]. Table 3 presents the power consumption of different P&O- and FOCV-based MPPT systems for energy harvesting applications.

Table 3.
Summary of maximum power point tracking approaches and their power consumption.
The P&O and FOCV techniques use resistive impedance matching, which is inappropriate for electromagnetic and piezoelectric energy harvesters. The energy storage in parasitic elements limits the maximum real power transfer to load using resistive impedance matching. Different nonlinear MPPTs have been implemented in recent years. Among them, the synchronized switch harvesting on the inductor (SSHI) and synchronous electric charge extraction (SECE) methods are most commonly used to overcome the limitations of linear impedance matching [279].
5. Machine Learning for Emerging Energy Harvesters
Machine learning (ML) is a field of artificial intelligence that learns from data for automatic predictions and decision-making [286]. Recent developments in ML algorithms resulted in successful integration in precision medicine, healthcare, natural language processing, computer vision, etc. Designing robust energy harvesters with a high output power density, light weight, and miniaturized size requires manual analysis of numerous design parameters. Automatic on-demand design techniques combining the power of ML methods for structural design have shown promise in real-time adjustments [287,288,289]. Other applications of ML algorithms in energy harvesting include predicting the availability of energy sources, harvested energy management, energy intensity forecasting, and weather prediction, as discussed in the survey in [290].
Predicting the availability of energy sources is a prominent application of ML methods in energy harvesting. For instance, linear regression and artificial neural network (ANN) were used in [291] to sense and switch between intended and unintended RF energy sources to maximize energy harvesting through the appropriate wake-up and sleep schedule. AdaEM [292] uses ML to predict user activities, energy usage patterns, and energy harvesting availability for energy management in wearable devices. A prototype design demonstrated increased recharging intervals for energy harvesting from light and motion. Materials informatics integrates ML for advanced materials search. For instance, ML can discover novel materials for various thermoelectric energy harvesters [293]. A vibrational energy harvester was presented in [293], which uses ML techniques for gait signal analysis. In addition, energy harvesting signals are analyzed to find the regularities in the environment for predicting future energy availability. Different ML algorithms such as linear regression, support vector machine, random forest, and decision tree were used in [294] to predict the availability of RF energy at various nodes of a wireless sensor network. In the RF frequency band between 1805 and 1880 MHz, the linear regression model sets a threshold to turn on the energy harvester or initiate the sleep mode to ensure optimum usage [294]. A kinetic energy harvesting system was presented in [295], which uses ML for energy expenditure analysis. The output voltage of the energy harvester classifies the activity intensity. Then, activity-specific regression and random forest models estimate calorie expenditure. This energy harvester demonstrates suitability for application in human physical activity assessment [295].
Wearable and assistive devices use energy harvesting to power the devices and often integrate ML techniques for added functionalities. A walking stick was designed in [296], which harvests energy from ultra-low frequency human walking motion using triboelectric sensors. Deep learning (DL) facilitates identity, disability, and motion recognition for high accuracy activity monitoring, IoT-enabled tracing, temperature, humidity sensing, etc. Such integrated energy harvesting with DL has shown potential in building an intelligent walking stick for motion-impaired people [296]. A glove was designed in [297], which uses triboelectric energy harvesting. The ML methods enable the glove to recognize hand gestures [297]. Another smart glove was designed in [298], which uses ML and haptic feedback for object recognition and control in a game and surgical training. The constituent elements of this glove are TENGs for sensing finger bending and palm sliding [299]. A wearable smart sock having sensing capability was presented in [300]. The sock scavenges energy using low-frequency human motion and are capable of powering a Bluetooth module for wireless data transmission. This sock uses triboelectric energy harvesting techniques and can monitor various physiological signals. Besides energy harvesting, this sock can facilitate walking pattern recognition, gait sensing, and health monitoring [300] using convolutional neural networks.
There are different applications of energy harvesters rather than medical purposes such as virtual reality/augmented reality [297], voice signal, and handwritten signal recognition [301]. The prediction of harvestable energy from indoor lights using supervised ML methods was presented in [302]. The spectral information is fed into the model to classify the energy sources. The system can make energy predictions for GaAs solar cells with less than a 5% mean absolute error. Predicting the output power of hybrid energy harvesters combining solar and TEG was shown in [303]. Five different combinations of hybrid energy harvesters and eight ML models were tested. The ANN demonstrated superior performance. A flexible hydrogen sensor operating using a TENG was presented in [304], which uses ML for improved gas sensing. A distributed energy harvesting framework for wireless networks was demonstrated in [305], which uses federated learning for efficient energy management and user scheduling. Another distributed ML framework was demonstrated in [306], where the connected devices harvest energy from the environment. In addition, different ML algorithms have been studied to enhance MPPT performance. For example, ML techniques for MPPT in various solar energy harvesters [307,308,309] and TEGs [310] have shown promise. Although ML finds potential applications in various energy harvesting techniques, it has high computational needs. Such a high demand for computation increases energy demand. Wearable and implantable medical devices are required to have a miniaturized size. Therefore, realizing ML techniques in energy harvesting for IWM devices is still in its infancy.
6. Use-Cases of Energy Harvesters
6.1. Energy Harvesters in Implantable Medical Devices
Many experiments of energy-harvesting-enabled medical devices have been carried out on animals and phantoms. Piezoelectric and triboelectric energy harvesters are suitable to place on the heart surface or implanted in the pericardium to harvest energy from the heartbeat to operate commercial pacemakers. In [311], a pacemaker implanted into the pericardium of a pig harvests energy from the heartbeat using a TENG for application in arrhythmia treatment. A TENG has also performed neuro-regulation while connected to the brain nerves of rats [312]. Besides, a TENG has shown promising results to stimulate gastric vagus nerves, for weight control [313], assist urination [314,315], and for leg muscle stimulation [316,317] while placed in rat stomach, bladder, and legs, respectively. Besides small animal experiments, researchers also experimented with energy harvesters in large animals for different applications. For example, piezoelectric energy harvesters for monitoring real-time blood pressure [318] and a TENG to power commercial pacemakers [311] are a few examples of energy harvesters in the porcine body. Despite the extensive research efforts in animal experiments, significant improvements are necessary to realize these applications in the human body.
Experiments on flexible PV cells and pulse circuitry were conducted in [232] to operate a cardiac pacemaker. The BFCs placed in lobster and human serum were reported to be a feasible solution to power up the cardiac pacemakers [319,320]. A battery and leadless cardiac pacemaker were presented in [133], which use a microgenerator placed on the right ventricle to use the intracardiac blood flow for energy harvesting.
Muscle and nerve stimulators for health monitoring and rehabilitation have been experimented with using energy harvesters. Such stimulators have extensive applications in precision healthcare: deep brain stimulation in Parkinson’s disease treatment, spinal cord stimulation to reduce chronic pain, and muscle stimulation to treat function loss. For instance, piezoelectric energy harvesters can trigger the sciatic nerve of a frog [321], the motor cortex of a rat [322], etc. The TENG demonstrates application in peripheral nerve stimulation of rat’s leg muscles [323]. An implanted TENG was presented in [313], which uses stomach motion for energy harvesting to stimulate nerves and control the weight of a rat.
Muscle stimulators can rehabilitate muscle loss due to neurological imbalance and nerve injuries. For example, a stack of TENGs can stimulate the tibia muscle of a rat [316,324]. Ultrasound energy transfer was used in [325] to trigger the spinal cord muscle of an injured rat. Although significant research has been carried out over the past few years in energy harvesting for nerve and muscle stimulators, the implantation of such harvesters in target nerves and muscles remains challenging.
The TENG has successfully been used in physiological sensors implanted in different organs to monitor heartbeat, respiration, blood pressure, etc. For example, a pressure sensor in the left ventricle [326], detection of heart motion and blood pressure estimation [327], etc., are a few examples of TENG applications in medical sensors. Besides monitoring cardiovascular functionalities, other applications include TENGs in bladder fullness detection [315] and piezoelectric energy harvesting in the diaphragm for the motion detection [328] of rats.
6.2. Energy Harvesters in Wearable Medical Devices
TEGs can be used as self-powered temperature sensors because of their relation to the temperature gradients in the human body [151]. BFCs can operate as self-powered sensors to detect various body fluids such as glucose, lactate, etc. For instance, sweat metabolism in the human body can be detected using socks with integrated BFCs [329]. The bio-mechanical energy harvesters can serve multiple functionalities by using them for motion sensing and physiological variation monitoring of the human body. A shoe based on a TENG and EMG can be used for foot movement analysis [330]. Skin patches can determine the pulse and blood pressure operated using triboelectric and piezoelectric energy harvesters [331,332]. Electronic skin using TENGs [333], electronic tattoos using BFCs [334], and micro-needle sensors [335] are a few examples of wearable devices used to monitor physiological signals.
A glucose sensor powered by a wearable TEG on the wrist was presented in [336], generating 378 W of power, while the sensor requires only 64 W. The sensor can read glucose values at a 1 min interval. A wearable TEG was also successfully used to power flexible ECG sensors [337]. The system achieved a power density of 38 W/cm for the first 10 min and 13 W/cm after 22 h.
Piezoelectric energy harvesters have shown promise in powering medical sensors for health monitoring. For example, a lead-free piezoelectric sensor obtaining a 3.25 W/m power density was presented in [338] for physical activity monitoring during sleep. A PZT-based sensor can detect artery and trachea motion [332]. Pressure sensors, blood pressure measurements, and strain sensors are a few other examples of piezoelectric energy harvester applications.
Solar energy harvesting has also found applications in wearable medical devices. For example, a medical bracelet was presented in [253] using flexible solar cells. In [339], a hybrid energy harvester based on thermoelectric and flexible solar cells was presented. The wearable system can monitor vital signs in real-time and communicate via Bluetooth. RF energy harvesters find applications in wearable textiles. For example, wearable textiles achieving 70% and 80% RF-DC conversion efficiency were presented in [217,340]. Table 4 and Table 5 list the potential applications of human-centric and environment-centric energy harvesting techniques, respectively.

Table 4.
Summary of potential applications of human-centric energy harvesters.

Table 5.
Summary of potential applications of environment-centric energy harvesters.
7. Interaction of Energy Harvesters with the Human Body
The energy harvesting devices to be incorporated in IWM devices have certain requirements such as (i) safety and reliability, (ii) flexibility and durability to adapt to body/organ shapes and to withstand muscle compression, (iii) high energy density with a miniaturized size and low weight, and (iv) effective, flexible, and long-lasting encapsulation to avoid corrosion from body fluids.
The biological organs are 3D in shape, soft, and curved, a limited space for the placement of IWM devices to minimize the interface mismatch and ensure sufficient power supply [341]. The requirements of the functional characteristics of energy harvesters vary with the device types and purposes. For instance, in short- or mid-term IMDs, biodegradable and bioabsorbable materials are highly desirable to avoid multiple surgeries, reducing the potential risk of chronic inflammation [342]. For energy storage in IMDs, Li-ion batteries are commonly used, which are inflexible, bulky, and occupy a large volume. Besides the requirement of surgical replacement, the high chances of the leakage of toxic substances pose a serious health risk. An alternate way for batteries is to connect the implants to the external power source through fine wires. However, such a skin–wire interface can cause infection. Developing biodegradable energy storage devices (batteries [341] and supercapacitors [343]) having sufficient energy density and easy integration with the IMDs remains challenging.
Biocompatibility, biodegradability, and bioresorbable are the three characteristics of IWM electronic devices to ensure biosafety. The biocompatibility of an IWM device represents its ability to remain in contact with the human body without any adverse complications or biohazards. Chemical compositions, mechanical properties, and response to biomaterials affect biocompatibility. Usually, biocompatible materials are bioinert (stainless steel, titanium alloy, silicone rubber, hydroxyapatite ceramics, etc. [344]). Biodegradable materials are disintegrated in physiological aqueous solutions at a controlled rate after a specified time. For instance, the European Committee for Standardization defines biodegradable as materials that will be dissolved more than 90% within six months [345]. The recent developments in biocompatible and biodegradable materials include water-soluble metal conductors such as magnesium, zinc, iron, molybdenum, and tungsten [346]. Dielectric polymers such as polyvinyl alcohol, poly lactic-co-glycolic acid, and polylactic acid [347,348] are biodegradable. Their hydrolyzed products exist in the human body and have shown promise in implant encapsulation. Bioresorbable represents decomposable materials in which the resultant substances can be absorbed or excreted from the human body [345]. The chemical composition of the materials, dissolution rate, and concentration are a few considerations for bioresorbable materials.
In general, thermoelectric energy harvesters do not have any significant negative impact on the human body. The TEG uses solid-state devices for energy conversion without requiring any chemical reaction. Therefore, the TEG provides added benefits such as being free of noise, vibration, and leakage. Besides flexibility and biocompatibility, biodegradability is considered for TEGs in short- and mid-term IMDs [349]. A few natural and cost-effective biodegradable materials are silk, cellulose, plant leaves, fish scales, and wood [345]. Biocompatible TEGs include all-silk material TEGs [350], all-nanofiber electronic skin TEGs [351], etc.
In piezoelectric energy harvesting, mechanical deformability, high sensitivity, and compatibility with soft materials are critical in material selection to avoid damage to biological organs. Biodegradable materials demonstrated promise in piezoelectric energy harvesting for IMDs [352]. A biodegradable piezoelectric nanogenerator was presented in [353], which uses a wood sponge to utilize walking motion. Ensuring biocompatibility and biodegradability in piezoelectric energy harvesters remains to a challenge. For example, the widely used PZT materials are toxic and need encapsulation with biocompatible materials such as polyimide or SU-8 passivation epoxy [345]. On the other hand, ZnO and PVDF have good biocompatibility, but the output energy is insignificant.
For RF energy harvesters, the IEEE standard for safe exposure to the human body should be in the 3 kHz–100 GHz frequency range [354]. In addition, the permissible RF exposure should not exceed a specific absorption rate of 2 Wkg averaged over 10 g of tissue in most parts of the human body. Therefore, to maintain health safety, the radiation wireless charging using RF typically operates in a low-frequency range to provide a power supply for IMDs. A suitable antenna design compatible with the human body is critical for using RF energy harvesting for IWM devices.
Inductive-coupling-type WPT has found significant applications in IMDs. However, the human body can be negatively affected by high-density electromagnetic exposure. The overheating generated from the inductive coupling can cause irregularities in metabolic and immune systems. In recent years, biodegradable WPT systems have shown promise [355,356].
Compared to electromagnetic coupling, ultrasound has several advantages such as high safety and low attenuation to biological tissues and deeper penetration [345]. U.S. Food and Drug Administration suggests ultrasound intensity be limited to 720 mW cm to ensure human safety [345].
The possible impact of photovoltaic energy harvesting on the human body includes tissue stress, tissue heating, skin perforation, patient discomfort, and thermal compatibility issues. In addition, most photovoltaic cells are not biodegradable, limiting their applications in temporary medical implants and bioresorbable electronic devices. There are research efforts to design flexible, biocompatible, and transparent polymers for packaging to reduce the toxicity of arsenic substances in typical photovoltaic cells [357].
The health issues related to wearable medical devices include skin irritation, burns, electromagnetic field (EMF) exposure, etc. For instance, EMF can cause headaches, tingling skin sensations, ringing ears, a static feeling in the brain, excessive fatigue, and weakened immunity of the human body [358]. The wearables often use WiFi, which can disrupt the blood–brain barrier, resulting in leakage, having health risks for children and pregnant women [359]. The RF signals produce non-ionizing radiation, which can cause underdeveloped skulls in children. Besides, people having EMF hypersensitivity can experience DNA damage, potential cancer risks, and neurological impairments [360].
8. Challenges and Future Research Directions
With the recent development in sensor technology and integrated circuits, implantable and wearable medical devices are becoming widespread [361]. In the IWM devices operated by harvested energy, power management plays a significant role in regulating the power and maximizing efficient usage. Therefore, a power management unit (PMU) is often integrated into the IWM devices, which opportunistically ensures the optimum functionality while the amount of energy is the least. In addition, an effective recharging mechanism is necessary to extend the lifetime of energy storage devices. Optimization of the use of harvested energy is a challenge. While harvesting energy for IWM devices, several factors should be considered such as the amount of energy, the efficiency of storage devices, economic aspects, and the choice of wireless communication schemes. This section presents the open research questions and prospects for developing efficient energy harvesting methods for IWM devices.
8.1. Efficient Power Management
Efficient power management is critical in energy harvesting to achieve sufficient energy efficiency with minimum power consumption. Human-centric energy harvesting depends on health conditions and body motion, while energy harvesting from the environment can be affected by the time of day, weather, location, and many other factors. Therefore, while choosing the energy source, the characteristics of the source need to be carefully investigated. For instance, to maximize the energy capture from solar sources, MPPT is frequently used. However, designing low-power MPPT circuits is a challenge. The available MPPT systems are directly not applicable for IWM devices, as often, the power consumption of MPPT circuits and PMU are higher than the harvested energy. A small start-up voltage is required to operate the micro TEGs, as the temperature gradients are sometimes inadequate to run the CMOS switches. Bulky transformers are integrated to provide the start-up voltage, which is inappropriate for IWM devices [362]. Besides designing low-power circuits, the efficient integration of energy harvesting into IWM devices is a challenge. For instance, flexible PV cells are more energy efficient than rigid body cells, as the bending angle affects the electrical output [228]. In TEGs, the interface between the skin and microgenerators affects the power density [363]. Therefore, the interfaces should be sufficiently large to ensure thermal matching without impacting the user comfort. The material selection for TEGs is also critical as they are required to tolerate thermal stress. Human-centric energy harvesting produces low energy. Thus, increasing the conversion efficiency is a challenge [364]. The energy harvesting techniques from the biochemical process are still not mature. Therefore, there are opportunities for improvement as the quality of bio-substances depends on age and health conditions.
RF energy harvesting can be a safe and efficient approach to wirelessly powering IWM devices. However, the output power density depends on the choice of antenna and appropriate frequency selection [365]. In addition, the sources of RF energy may be interrupted, which negatively impacts the operations of IWM devices.
8.2. Optimizing Power Consumption
The power consumption of IWM devices is an important issue when designing energy harvesters. The power consumption of the devices should be minimum to ensure that the generated power from energy harvesting is sufficient to meet the power budget. One way of minimizing power consumption is optimizing the computational needs through algorithmic optimization. The radio transceiver used in IWM devices is another major power-consuming component. There are different techniques developed to optimize the power consumption of radio transceivers such as duty cycle optimization [265,366,367] and wake-up radios [368]. For instance, when the IWM devices are idle, sleep mode can turn on to save energy [369]. The adaptive duty cycle based on the availability of energy sources is another alternative [370]. Turning the device from sleep to wake-up mode requires energy. Hence, there are opportunities to optimize the wake-up energy [368]. The concept of the wake-up radio is to set the event-driven wake-up rules of the radio transmitter and receiver circuits. Significant attention is necessary to optimize the power consumption to meet the energy needs of the IWM devices only through energy harvesting.
8.3. Storage of Harvested Energy
The storage of harvested energy is a critical choice in ensuring the continuous operation of IWM devices. The choice of storage depends on several factors such as (i) the type of energy source, (ii) whether the device is implantable or externally wearable, and (ii) the severity of applications, whether the IWM is for critical health problems or simple fitness tracking. There are a few requirements for energy storage such as (i) the energy storage should be rechargeable as replacement is critical and (ii) the storage should be long-lasting. For instance, batteries in cardiac pacemakers require replacement every eight years, requiring surgery which is expensive and painful for the users [371].
For IWM devices, two energy storage options are batteries and supercapacitors. For the storage of a small amount of energy, flexible batteries are being developed that eliminate the need for frequent recharging [372]. In addition, current collectors using advanced materials such as carbon nanotubes [373], conductive fabrics [374], and graphene foams [375] improve the battery storage capacity and increase the flexibility. However, the limitations of current collectors are low conductivity, limiting the discharge rate [227], the possibility of leakage, which can lead to tissue damage, overcharging, and the resulting elevated temperature, which may burn the tissue. To mitigate such problems, supercapacitors have emerged as an alternative where the electrical energy is stored in the electrode–electrolyte interface. With high pulsed power capacity, supercapacitors can effectively handle short power surges. Modern supercapacitors also use advanced materials such as graphene to offer flexibility in design and increase energy density. Besides flexibility, supercapacitors have an extended lifetime of around 10–20 years, a high power density, and a low charging time.
8.4. Energy-Efficient Communication Schemes
Building an energy-efficient network and communication schemes is necessary to ensure the mobility of IWM devices. IWM devices measure physiological signals and transmit them to a central processing node for medical professionals. Thus, continuous and reliable data communication is crucial in healthcare applications. Although most IWM devices locally store recorded data for later processing and analysis, this is not applicable for real-time applications. In addition, retrieving data from IMDs is highly detrimental due to repetitive surgical procedures. A wide variety of wireless communication schemes are exploited in IWM devices. Research efforts were made in [376] to minimize the power consumption in IMDs and propose reliable techniques ensuring portability and a low bit error rate. Researchers also use conventional communication technologies such as WiFi, Bluetooth, and ZigBee for IWMs [377]. WiFi works well when the IMDs are linked to an existing network. However, it can easily connect to other devices within the existing network, which causes significant data overhead. BLE is a widely applied power-saving technology for connecting IMDs with a designated mobile phone or computer. The MedRadio scheme introduces a cooperative network infrastructure for reliable data communication [378]. In the 5G era, millimeter waves are also actively investigated for energy-efficient connection to devices using the narrow beam. High spectral efficiency is critical due to the limited power budget in IWM devices. A customized network protocol design for data communication may reduce the energy costs. In addition, designing energy-aware routing protocols considering mobility and channel fading remains challenging due to the increased bit error rate caused by interference with numerous other devices. Synchronization is necessary for communication between sensors and external processing nodes. For example, the heartbeat follows a rhythmic pattern, which can be exploited to achieve proper time synchronization in many IWM devices [379]. The cyclic rhythm of the heartbeat is a natural process. It may eliminate the need for an external timing clock, extending the lifetime of IWM devices and saving energy costs.
8.5. Energy Need for Next-Generation IWM Devices
The recent advancement in wearable medical devices has resulted in a tremendous amount of high-quality clinical data. Big data at the user nodes opens up new potential for improved diagnosis, on-site decision-making, and connected precision healthcare. In addition, multiple sensor nodes can be connected through low-power communication protocols to ensure multi-sensory learned decisions. In addition, the implantable sensors provide valuable health information. Bidirectional communication establishes monitoring and control of the implants. The next generation of implantable and wearable medical devices will be intelligent with on-device data-driven learning and decision-making capabilities [38,380]. Such intelligence is now a reality due to the immense improvement in machine learning techniques and the emergence of various compression algorithms [286]. However, deploying artificial intelligence on the device increases power consumption. Therefore, meeting the energy demand of the next-generation intelligent wearable medical devices and integrating the on-site control for implantable devices are the new challenges. In addition, the energy need increases with the increased processing power of IWM devices required to build a connected wireless body sensor network. Moreover, wireless connectivity requires energy-hungry extra circuitry and a transceiver. Thus, the future of wearables and implants will have increased energy demand, which will facilitate the exploration of novel research avenues to tackle such increased energy demand along with the miniaturization trend.
9. Discussion
Different energy harvesting techniques focusing on applications in implantable and wearable medical devices were discussed in this paper. Each technique has characteristics, application areas, limitations, design challenges, and technical constraints. The battery is considered to be the most reliable energy source when the IWM devices can accommodate the battery size and proper safety is ensured through biocompatible encapsulation. However, besides limited lifetime and functionality, there are significant health risks of batteries, requiring surgical procedures for replacement if integrated into the implants. Therefore, energy harvesting techniques to power up the IWM devices are the most promising alternative.
The selection of an appropriate energy harvesting technique depends on factors such as IWM device size, weight, placement in the body, the probability of interference, full duplex communication, the amount of harvested energy, etc. A comparison of different human-centric energy harvesting approaches is presented in Table 6, including the typical output power density, characteristic features, limitations, and potential applications. The power requirements in IWM devices typically vary between W and 30 W. Low-power energy harvesting techniques such as TEGs, GBF cells, electromagnetic, electrostatic, and photovoltaic are suitable to power up the ultra-low-power IWM devices. However, for IWM devices requiring milliwatt-level power such as neurostimulators, cochlear implants, etc., piezoelectric methods and TENGs are the potential sources.

Table 6.
Comparison of human-centric energy harvesting techniques considered for implantable and wearable medical devices.
One of the considerations in selecting the appropriate energy harvesting technique is determining the suitable energy source in proximity to the device placement site. For example, a thermoelectric energy harvester is suitable for IWM devices near a higher temperature gradient. On the other hand, electrostatic, electromagnetic, and piezoelectric are suitable for IWM devices where the source of high motion and vibration is accessible. For wearables, the photovoltaic energy source is a good choice. If there is no human-centric and environment-centric energy source available, WPT is a better option to supply energy to the device. A comparison of available environment-centric energy harvesting techniques is presented in Table 7, summarizing the typical output power density, advantages, limitations, and potential application areas.

Table 7.
Comparison of different environment-centric energy harvesting techniques having potential application in implantable and wearable medical devices.
For implantable medical devices, another issue is the penetration depth of the device inside the body. Electromagnetic energy harvesters and inductive power transfer are inappropriate in the deep body due to significant heating. Similarly, the photovoltaic energy sources are not suitable for deep implants because of the attenuation at different levels. However, with the aid of electrical connections, sometimes, these sources can power deep implants at the expense of the potential infection issues.
The output voltage and current obtained from the energy harvesters are seldom compatible with the load electronics. Thus, voltage regulation is necessary to match the source and the load. If the harvested energy is in the form of AC voltage, a rectification step is needed to obtain the DC power supply. Sometimes, an MPPT is employed at the cost of added complexity to achieve the highest possible output power. In addition, nonlinear components in the equivalent systems of electrostatic, piezoelectric, TENGs, and photovoltaic energy harvesters limit the MPPT operation.
In summary, this review will help researchers understand the current state of the research on different energy harvesting techniques for implantable and wearable medical devices, prospective areas of improvement, and a general guideline to consider factors to choose an appropriate one while needed.
10. Conclusions
This paper presented a comprehensive review of energy harvesting techniques focusing on wearable and implantable medical applications for precision healthcare. An IWM device capable of continuous, pain-free, hazardless operations can significantly improve human life. For instance, cardiac pacemakers and defibrillators save lives. New technologies such as insulin pumps for diabetes management, neurostimulators in pain therapy, brain implants for neural stimulations, and stimulants in the throat are a few examples. The requirements of such devices are minimum power consumption, miniaturized size, and sufficient energy harvesting from external sources. In optimal energy harvesters, appropriate energy sources, power budget, wireless communication technology, and energy-aware network design are a few considerations. The rapid developments in integrated circuit technologies enable ultra-low energy consumption in energy harvester circuitry towards battery-free, long-lasting, implantable and wearable medical devices.
Author Contributions
Conceptualization, methodology, M.M.H.S.; formal analysis, writing—original draft preparation, M.M.H.S., T.T. and N.A.; writing—review and editing, M.M.H.S., T.T., N.A. and S.K.I.; visualization, T.T., M.M.H.S. and N.A.; supervision, S.K.I. All authors have read and agreed to the published version of the manuscript.
Funding
This work is partially supported by the College of Engineering, University of Missouri, Columbia, MO 65211, USA.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| IWM | Implantable and wearable medical | TENG | Triboelectric nanogenerator |
| PPG | Photoplethysmography | PCB | Printed circuit board |
| WMD | Wearable medical device | MPPT | Maximum power point tracking |
| ECG | Electrocardiogram | PCE | Power conversion efficiency |
| IMD | Implantable medical device | P&O | Perturb and observe |
| RF | Radio frequency | FOCV | Fractional open-circuit voltage |
| PMU | Power management unit | VOC | Open-circuit voltage |
| TEG | Thermoelectric energy generators | SSHI | Synchronized switch harvesting on inductor |
| GBF | Glucose biofuel | SECE | Synchronous electric charge extraction |
| PZT | Polycrystalline lead zirconate titanate | BLE | Bluetooth low energy |
| TKR | Total knee replacement | BFC | Biofuel cells |
| PMN-PT | Lead magnesium niobate–lead titanate | GOx | Glucose oxidase |
| PZN-PT | Lead–zinc niobate–lead titanate | NIR | Near-infrared region |
| ZnO | Zinc oxide | WPT | Wireless power transfer |
| PVDF | Polyvinylidene difluoride | PUEH | Piezoelectric ultrasonic energy harvester |
| MEMS | Microelectromechanical systems | EMG | Electromyography |
| EP | Endocochlear potential | HEG | Hydroelectric generator |
| ECSA | Electrochemically active surface area | rGO | Reduced graphene oxide |
| IME | Implantable electronics | SWG | Single-wire generator |
| SEH | Standard energy harvesting | SCE | Synchronized charge extraction |
| EM | Electromagnetic | EMI | Electromagnetic interference |
| SWCNT | Single wall carbon nanotubes | DWNT | Double-wall nanotube |
| DETA | Diethylenetriamine | TTT | Tetrathiotetracene |
| FOM | Figure of merit | PENG | Piezoelectric nanogenerators |
| ML | Machine learning | ANN | Artificial neural network |
| EMF | Electromagnetic field | DEGS | Dielectric elastomer generator system |
References
- Jeong, I.C.; Bychkov, D.; Searson, P.C. Wearable Devices for Precision Medicine and Health State Monitoring. IEEE Trans. Biomed. Eng. 2019, 66, 1242–1258. [Google Scholar] [CrossRef]
- Aileni, R.M.; Valderrama, A.C.; Strungaru, R. Wearable Electronics for Elderly Health Monitoring and Active Living. In Ambient Assisted Living and Enhanced Living Environments; Elsevier: Amsterdam, The Netherlands, 2017; pp. 247–269. [Google Scholar] [CrossRef]
- Ghamari, M.; Janko, B.; Sherratt, R.; Harwin, W.; Piechockic, R.; Soltanpur, C. A Survey on Wireless Body Area Networks for eHealthcare Systems in Residential Environments. Sensors 2016, 16, 831. [Google Scholar] [CrossRef] [PubMed]
- Shuvo, M.M.H.; Ahmed, N.; Nouduri, K.; Palaniappan, K. A Hybrid Approach for Human Activity Recognition with Support Vector Machine and 1D Convolutional Neural Network. In Proceedings of the 2020 IEEE Applied Imagery Pattern Recognition Workshop (AIPR), Washington, DC, USA, 13–15 October 2020; pp. 1–5. [Google Scholar] [CrossRef]
- Hoyt, R.W.; Reifman, J.; Coster, T.S.; Buller, M.J. Combat medical informatics: Present and future. In Proceedings of the AMIA Symposium, San Antonio, TX, USA, 9–13 November 2002; pp. 335–339. [Google Scholar]
- Friedl, K.E. Military applications of soldier physiological monitoring. J. Sci. Med. Sport 2018, 21, 1147–1153. [Google Scholar] [CrossRef]
- Poh, M.-Z.; Kim, K.; Goessling, A.; Swenson, N.; Picard, R. Cardiovascular Monitoring Using Earphones and a Mobile Device. IEEE Pervasive Comput. 2012, 11, 18–26. [Google Scholar] [CrossRef]
- Teichmann, D.; Kuhn, A.; Leonhardt, S.; Walter, M. The MAIN Shirt: A Textile-Integrated Magnetic Induction Sensor Array. Sensors 2014, 14, 1039–1056. [Google Scholar] [CrossRef]
- di Rienzo, M.; Meriggi, P.; Rizzo, F.; Castiglioni, P.; Lombardi, C.; Ferratini, M.; Parati, G. Textile Technology for the Vital Signs Monitoring in Telemedicine and Extreme Environments. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 711–717. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, F.; Shakhsheer, Y.; Silver, J.D.; Klinefelter, A.; Nagaraju, M.; Boley, J.; Pandey, J.; Shrivastava, A.; Carlson, E.J.; et al. A Batteryless 19 μW MICS/ISM-Band Energy Harvesting Body Sensor Node SoC for ExG Applications. IEEE J. -Solid-State Circ. 2013, 48, 199–213. [Google Scholar] [CrossRef]
- Hossain, Z.; Shuvo, M.M.H.; Sarker, P. Hardware and software implementation of real time electrooculogram (EOG) acquisition system to control computer cursor with eyeball movement. In Proceedings of the 2017 4th International Conference on Advances in Electrical Engineering (ICAEE), Dhaka, Bangladesh, 28–30 September 2017; pp. 132–137. [Google Scholar] [CrossRef]
- Macdonald, A.; Hawkes, L.A.; Corrigan, D.K. Recent advances in biomedical, biosensor and clinical measurement devices for use in humans and the potential application of these technologies for the study of physiology and disease in wild animals. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20200228. [Google Scholar] [CrossRef]
- Yi, N.; Cui, H.; Zhang, L.G.; Cheng, H. Integration of biological systems with electronic-mechanical assemblies. Acta Biomater. 2019, 95, 91–111. [Google Scholar] [CrossRef]
- Shaikh, F.K.; Zeadally, S. Energy harvesting in wireless sensor networks: A comprehensive review. Renew. Sustain. Energy Rev. 2016, 55, 1041–1054. [Google Scholar] [CrossRef]
- Dionisi, A.; Marioli, D.; Sardini, E.; Serpelloni, M. Autonomous Wearable System for Vital Signs Measurement With Energy-Harvesting Module. IEEE Trans. Instrum. Meas. 2016, 65, 1423–1434. [Google Scholar] [CrossRef]
- Paradiso, J.A.; Starner, T. Energy Scavenging for Mobile and Wireless Electronics. IEEE Pervasive Comput. 2005, 4, 18–27. [Google Scholar] [CrossRef]
- Khan, A.S.; Khan, F.U. A survey of wearable energy harvesting systems. Int. J. Energy Res. 2022, 46, 2277–2329. [Google Scholar] [CrossRef]
- Khaligh, A.; Zeng, P.; Zheng, C. Kinetic Energy Harvesting Using Piezoelectric and Electromagnetic Technologies–State of the Art. IEEE Trans. Ind. Electron. 2010, 57, 850–860. [Google Scholar] [CrossRef]
- Beeby, S.P.; Tudor, M.J.; White, N.M. Energy harvesting vibration sources for microsystems applications. Meas. Sci. Technol. 2006, 17, R175–R195. [Google Scholar] [CrossRef]
- Wise, K.D.; Anderson, D.J.; Hetke, J.F.; Kipke, D.R.; Najafi, K. Wireless Implantable Microsystems: High-Density Electronic Interfaces to the Nervous System. Proc. IEEE 2004, 92, 76–97. [Google Scholar] [CrossRef]
- Haerinia, M.; Shadid, R. Wireless Power Transfer Approaches for Medical Implants: A Review. Signals 2020, 1, 12. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; Huang, M.; Chen, Z.; Zhang, X. An Overview of Regulation Topologies in Resonant Wireless Power Transfer Systems for Consumer Electronics or Bio-Implants. Energies 2018, 11, 1737. [Google Scholar] [CrossRef]
- Schmidt, C.L.; Scott, E.R. Energy harvesting and implantable medical devices—first order selection criteria. In Proceedings of the 2011 International Electron Devices Meeting, Washington, DC, USA, 5–7 December 2011; pp. 10.5.1–10.5.4. [Google Scholar] [CrossRef]
- Torres, E.O.; Rincon-Mora, G.A. A 0.7-μm BiCMOS Electrostatic Energy-Harvesting System IC. IEEE J. -Solid-State Circ. 2010, 45, 483–496. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, Q.; Rogers, J.A. Water-soluble energy harvester as a promising power solution for temporary electronic implants. APL Mater. 2020, 8, 120701. [Google Scholar] [CrossRef]
- Olivo, J.; Carrara, S.; de Micheli, G. Energy Harvesting and Remote Powering for Implantable Biosensors. IEEE Sens. J. 2011, 11, 1573–1586. [Google Scholar] [CrossRef]
- Roy, S.; Azad, A.N.M.W.; Baidya, S.; Alam, M.K.; Khan, F. Powering Solutions for Biomedical Sensors and Implants Inside the Human Body: A Comprehensive Review on Energy Harvesting Units, Energy Storage, and Wireless Power Transfer Techniques. IEEE Trans. Power Electron. 2022, 37, 12237–12263. [Google Scholar] [CrossRef]
- Roy, S.; Azad, A.N.M.W.; Baidya, S.; Khan, F. A Comprehensive Review on Rectifiers, Linear Regulators, and Switched-Mode Power Processing Techniques for Biomedical Sensors and Implants Utilizing in-Body Energy Harvesting and External Power Delivery. IEEE Trans. Power Electron. 2021, 36, 12721–12745. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, L.; Jiang, K.; Wei, Z.; Shen, G. Reviews of wearable healthcare systems: Materials, devices and system integration. Mater. Sci. Eng. R Rep. 2020, 140, 100523. [Google Scholar] [CrossRef]
- Jagadeeswari, V.; Subramaniyaswamy, V.; Logesh, R.; Vijayakumar, V. A study on medical Internet of Things and Big Data in personalized healthcare system. Health Inf. Sci. Syst. 2018, 6, 14. [Google Scholar] [CrossRef]
- Lymberis, A. Smart wearable systems for personalised health management: Current R&D and future challenges. In Proceedings of the 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (IEEE Cat. No.03CH37439), Cancun, Mexico, 17–21 September 2003; pp. 3716–3719. [Google Scholar] [CrossRef]
- Shuvo, M.M.H.; Ahmed, N.; Islam, H.; Alaboud, K.; Cheng, J.; Mosa, A.S.M.; Islam, S.K. Machine Learning Embedded Smartphone Application for Early-Stage Diabetes Risk Assessment. In Proceedings of the 2022 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Messina, Italy, 22–24 June 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Gatzoulis, L.; Iakovidis, I. Wearable and Portable eHealth Systems. IEEE Eng. Med. Biol. Mag. 2007, 26, 51–56. [Google Scholar] [CrossRef]
- Bayo-Monton, J.-L.; Martinez-Millana, A.; Han, W.; Fernandez-Llatas, C.; Sun, Y.; Traver, V. Wearable Sensors Integrated with Internet of Things for Advancing eHealth Care. Sensors 2018, 18, 1851. [Google Scholar] [CrossRef] [PubMed]
- Findlow, A.; Goulermas, J.Y.; Nester, C.; Howard, D.; Kenney, L.P.J. Predicting lower limb joint kinematics using wearable motion sensors. Gait Posture 2008, 28, 120–126. [Google Scholar] [CrossRef]
- Hester, T.; Sherrill, D.M.; Hamel, M.; Perreault, K.; Boissy, P.; Bonato, P. Identification of Tasks Performed by Stroke Patients Using a Mobility Assistive Device. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 1501–1504. [Google Scholar] [CrossRef]
- Syed, L.; Jabeen, S.; S, M.; Alsaeedi, A. Smart healthcare framework for ambient assisted living using IoMT and big data analytics techniques. Future Gener. Comput. Syst. 2019, 101, 136–151. [Google Scholar] [CrossRef]
- Shuvo, M.M.H.; Hassan, O.; Parvin, D.; Chen, M.; Islam, S.K. An Optimized Hardware Implementation of Deep Learning Inference for Diabetes Prediction. In Proceedings of the 2021 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Glasgow, UK, 17–21 May 2021; pp. 1–6. [Google Scholar] [CrossRef]
- Connolly, P.; Cotton, C.; Morin, F. Opportunities at the skin interface for continuous patient monitoring: A reverse iontophoresis model tested on lactate and glucose. IEEE Trans. Nanobiosci. 2002, 1, 37–41. [Google Scholar] [CrossRef]
- Hassan, O.; Paul, T.; Thakker, R.; Parvin, D.; Shuvo, M.M.H.; Mosa, A.S.M.; Islam, S.K. A Multi-Sensor Based Automatic Sleep Apnea Detection System for Adults Using Neural Network Inference on FPGA. In Proceedings of the 2022 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Messina, Italy, 22–24 June 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Lymberis, A.; Olsson, S. Intelligent Biomedical Clothing for Personal Health and Disease Management: State of the Art and Future Vision. Telemed. J. e-Health 2003, 9, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, J.; Xie, Y.; Gao, F.; Xu, S.; Wu, X.; Ye, Z. Wearable Health Devices in Health Care: Narrative Systematic Review. JMIR Mhealth Uhealth 2020, 8, e18907. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, M.H.; Shnawah, D.A.; Sabri, M.F.M.; Said, S.B.M.; Hassan, M.H.; Bashir, M.B.A.; Mohamad, M. A review on thermoelectric renewable energy: Principle parameters that affect their performance. Renew. Sustain. Energy Rev. 2014, 30, 337–355. [Google Scholar] [CrossRef]
- Ghomian, T.; Kizilkaya, O.; Choi, J.-W. Lead sulfide colloidal quantum dot photovoltaic cell for energy harvesting from human body thermal radiation. Appl. Energy 2018, 230, 761–768. [Google Scholar] [CrossRef]
- Khaligh, A.; Zeng, P.; Wu, X.; Xu, Y. A hybrid energy scavenging topology for human-powered mobile electronics. In Proceedings of the 2008 34th Annual Conference of IEEE Industrial Electronics, Orlando, FL, USA, 10–13 November 2008; pp. 448–453. [Google Scholar] [CrossRef]
- Akhtar, F.; Rehmani, M.H. Energy replenishment using renewable and traditional energy resources for sustainable wireless sensor networks: A review. Renew. Sustain. Energy Rev. 2015, 45, 769–784. [Google Scholar] [CrossRef]
- Colomer-Farrarons, J.; Miribel-Catala, P.; Saiz-Vela, A.; Samitier, J. A Multiharvested Self-Powered System in a Low-Voltage Low-Power Technology. IEEE Trans. Ind. Electron. 2011, 58, 4250–4263. [Google Scholar] [CrossRef]
- Merrett, G.V.; Huang, H.; White, N.M. Modeling the Effect of Orientation on Human-Powered Inertial Energy Harvesters. IEEE Sens. J. 2015, 15, 434–441. [Google Scholar] [CrossRef]
- Paulo, J.; Gaspar, P.D. Review and future trend of energy harvesting methods for portable medical devices. In Proceedings of the World Congress on Engineering, London, UK, 30 June–2 July 2010; pp. 168–196. [Google Scholar]
- Jia, W.; Valdés-Ramírez, G.; Bandodkar, A.J.; Windmiller, J.R.; Wang, J. Epidermal Biofuel Cells: Energy Harvesting from Human Perspiration. Angew. Chem. Int. Ed. 2013, 52, 7233–7236. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, F.; Rehmani, M.H. Energy Harvesting for Self-Sustainable Wireless Body Area Networks. IT Prof. 2017, 19, 32–40. [Google Scholar] [CrossRef]
- Dong, K.; Jia, B.; Yu, C.; Dong, W.; Du, F.; Liu, H. Microbial fuel cell as power supply for implantable medical devices: A novel configuration design for simulating colonic environment. Biosens. Bioelectron. 2013, 41, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Amar, A.; Kouki, A.; Cao, H. Power Approaches for Implantable Medical Devices. Sensors 2015, 15, 28889–28914. [Google Scholar] [CrossRef]
- Zebda, A.; Alcaraz, J.P.; Vadgama, P.; Shleev, S.; Minteer, S.D.; Boucher, F.; Cinquin, P.; Martin, D.K. Challenges for successful implantation of biofuel cells. Bioelectrochemistry 2018, 124, 57–72. [Google Scholar] [CrossRef]
- Kerzenmacher, S.; Ducrée, J.; Zengerle, R.; von Stetten, F. Energy harvesting by implantable abiotically catalyzed glucose fuel cells. J. Power Sources 2008, 182, 1–17. [Google Scholar] [CrossRef]
- Rapoport, B.I.; Kedzierski, J.T.; Sarpeshkar, R. A Glucose Fuel Cell for Implantable Brain–Machine Interfaces. PLoS ONE 2012, 7, e38436. [Google Scholar] [CrossRef]
- Kwon, C.H.; Ko, Y.; Shin, D.; Kwon, M.; Park, J.; Bae, W.K.; Lee, S.W.; Cho, J. High-power hybrid biofuel cells using layer-by-layer assembled glucose oxidase-coated metallic cotton fibers. Nat. Commun. 2018, 9, 4479. [Google Scholar] [CrossRef]
- Jeerapan, I.; Sempionatto, J.R.; You, J.M.; Wang, J. Enzymatic glucose/oxygen biofuel cells: Use of oxygen-rich cathodes for operation under severe oxygen-deficit conditions. Biosens. Bioelectron. 2018, 122, 284–289. [Google Scholar] [CrossRef]
- Barton, S.C.; Gallaway, J.; Atanassov, P. Enzymatic biofuel cells for implantable and microscale devices. Chem. Rev. 2004, 104, 4867–4886. [Google Scholar] [CrossRef]
- Heller, A. Miniature biofuel cells. Phys. Chem. Chem. Phys. 2004, 6, 209–216. [Google Scholar] [CrossRef]
- Hansen, B.J.; Liu, Y.; Yang, R.; Wang, Z.L. Hybrid nanogenerator for concurrently harvesting biomechanical and biochemical energy. ACS Nano 2010, 4, 3647–3652. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; MacVittie, K. Implanted biofuel cells operating in vivo—methods, applications and perspectives – feature article. Energy Environ. Sci. 2013, 6, 2791–2803. [Google Scholar] [CrossRef]
- Cinquin, P.; Gondran, C.; Giroud, F.; Mazabrard, S.; Pellissier, A.; Boucher, F.; Alcaraz, J.P.; Gorgy, K.; Lenouvel, F.; Mathé, S.; et al. A glucose biofuel cell implantedin rats. PLoS ONE 2010, 5, e10476. [Google Scholar] [CrossRef] [PubMed]
- Sales, F.C.P.F.; Iost, R.M.; Martins, M.V.A.; Almeida, M.C.; Crespilho, F.N. An intravenous implantable glucose/dioxygen biofuel cell with modified flexible carbon fiber electrodes. Lab. Chip. 2013, 13, 468–474. [Google Scholar] [CrossRef]
- Park, S.I.; Brenner, D.S.; Shin, G.; Morgan, C.D.; Copits, B.A.; Chung, H.U.; Pullen, M.Y.; Noh, K.N.; Davidson, S.; Oh, S.J.; et al. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nat. Biotechnol. 2015, 33, 1280–1286. [Google Scholar] [CrossRef]
- Milton, R.D.; Giroud, F.; Thumser, A.E.; Minteer, S.D.; Slade, R.C.T. Hydrogen peroxide produced by glucose oxidase affects the performance of laccase cathodes in glucose/oxygen fuel cells: FAD-dependent glucose dehydrogenase as a replacement. Phys. Chem. Chem. Phys. 2013, 15, 19371–19379. [Google Scholar] [CrossRef]
- He, C.; Arora, A.; Kiziroglou, M.E.; Yates, D.C.; O’Hare, D.; Yeatman, E.M. MEMS Energy Harvesting Powered Wireless Biometric Sensor. In Proceedings of the 2009 Sixth International Workshop on Wearable and Implantable Body Sensor Networks, Berkeley, CA, USA, 3–5 June 2009; pp. 207–212. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; You, J.M.; Kim, N.H.; Gu, Y.; Kumar, R.; Mohan, A.V.; Kurniawan, J.; Imani, S.; Nakagawa, T.; Parish, B.; et al. Soft, stretchable, high power density electronic skin-based biofuel cells for scavenging energy from human sweat. Energy Environ. Sci. 2017, 10, 1581–1589. [Google Scholar] [CrossRef]
- Yin, L.; Moon, J.M.; Sempionatto, J.R.; Lin, M.; Cao, M.; Trifonov, A.; Zhang, F.; Lou, Z.; Jeong, J.M.; Lee, S.J.; et al. A passive perspiration biofuel cell: High energy return on investment. Joule 2021, 5, 1888–1904. [Google Scholar] [CrossRef]
- Seok, S.; Wang, C.; Lefeuvre, E.; Park, J. Autonomous Energy Harvester Based on Textile-Based Enzymatic Biofuel Cell for On-Demand Usage. Sensors 2020, 20, 5009. [Google Scholar] [CrossRef] [PubMed]
- Energy-Harvesting Shirt Generates Electricity from Sweat and Movement. Available online: https://newatlas.com/wearables/wearable-microgrid-energy-harvesting-shirt/ (accessed on 25 September 2022).
- Mercier, P.P.; Lysaght, A.C.; Bandyopadhyay, S.; Chandrakasan, A.P.; Stankovic, K.M. Energy extraction from the biologic battery in the inner ear. Nat. Biotechnol. 2012, 30, 1240–1243. [Google Scholar] [CrossRef]
- Gao, Y.; Cho, J.H.; Ryu, J.; Choi, S. A scalable yarn-based biobattery for biochemical energy harvesting in smart textiles. Nano Energy 2020, 74, 104897. [Google Scholar] [CrossRef]
- Yin, J.; Zhou, J.; Fang, S.; Guo, W. Hydrovoltaic Energy on the Way. Joule 2020, 4, 1852–1855. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Yin, J.; Xu, Y.; Fei, W.; Xue, M.; Wang, Q.; Zhou, J.; Guo, W. Emerging hydrovoltaic technology. Nat. Nanotechnol. 2018, 13, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, S.; Xu, W.; Wang, Z. Nanogenerators with Superwetting Surfaces for Harvesting Water/Liquid Energy. Adv. Funct. Mater. 2020, 30, 1908252. [Google Scholar] [CrossRef]
- Shen, D.; Duley, W.W.; Peng, P.; Xiao, M.; Feng, J.; Liu, L.; Zou, G.; Zhou, Y.N. Moisture-Enabled Electricity Generation: From Physics and Materials to Self-Powered Applications. Adv. Mater. 2020, 32, 2003722. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Xu, Y.; Ding, T.; Li, J.; Yin, J.; Fei, W.; Cao, Y.; Yu, J.; Yuan, L.; Gong, L.; et al. Water-evaporation-induced electricity with nanostructured carbon materials. Nat. Nanotechnol. 2017, 12, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wang, Y.; Sun, X.; Li, Y.; Xu, H.; Tan, Y.; Li, Y.; Song, T.; Sun, B. Constant Electricity Generation in Nanostructured Silicon by Evaporation-Driven Water Flow. Angew. Chem. 2020, 132, 10706–10712. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, Z.; Qu, L. Power generation from graphene-water interactions. FlatChem 2019, 14, 100090. [Google Scholar] [CrossRef]
- Shao, C.; Gao, J.; Xu, T.; Ji, B.; Xiao, Y.; Gao, C.; Zhao, Y.; Qu, L. Wearable fiberform hygroelectric generator. Nano Energy 2018, 53, 698–705. [Google Scholar] [CrossRef]
- Zhang, W.; Guan, H.; Zhong, T.; Zhao, T.; Xing, L.; Xue, X. Wearable Battery-Free Perspiration Analyzing Sites Based on Sweat Flowing on ZnO Nanoarrays. Nanomicro Lett. 2020, 12, 1–11. [Google Scholar] [CrossRef]
- Mandal, S.; Roy, S.; Mandal, A.; Ghoshal, T.; Das, G.; Singh, A.; Goswami, D.K. Protein-Based Flexible Moisture-Induced Energy-Harvesting Devices As Self-Biased Electronic Sensors. ACS Appl. Electron. Mater. 2020, 2, 780–789. [Google Scholar] [CrossRef]
- Shen, D.; Xiao, Y.; Zou, G.; Liu, L.; Wu, A.; Xiao, M.; Feng, J.; Hui, Z.; Duley, W.W.; Zhou, Y.N. Exhaling-Driven Hydroelectric Nanogenerators for Stand-Alone Nonmechanical Breath Analyzing. Adv. Mater. Technol. 2020, 5, 1900819. [Google Scholar] [CrossRef]
- Xie, L.; Cai, M. Human Motion: Sustainable Power for Wearable Electronics. IEEE Pervasive Comput. 2014, 13, 42–49. [Google Scholar] [CrossRef]
- Zurbuchen, A.; Pfenniger, A.; Stahel, A.; Stoeck, C.T.; Vandenberghe, S.; Koch, V.M.; Vogel, R. Energy Harvesting from the Beating Heart by a Mass Imbalance Oscillation Generator. Ann. Biomed. Eng. 2013, 41, 131–141. [Google Scholar] [CrossRef]
- Delnavaz, A.; Voix, J. Electromagnetic micro-power generator for energy harvesting from breathing. In Proceedings of the IECON 2012—38th Annual Conference on IEEE Industrial Electronics Society, Montreal, QC, Canada, 25–28 October 2012; pp. 984–988. [Google Scholar] [CrossRef]
- Romero, E.; Warrington, R.O.; Neuman, M.R. Energy scavenging sources for biomedical sensors. Physiol. Meas. 2009, 30, R35–R62. [Google Scholar] [CrossRef] [PubMed]
- Mitcheson, P.D.; Yeatman, E.M.; Rao, G.K.; Holmes, A.S.; Green, T.C. Energy Harvesting From Human and Machine Motion for Wireless Electronic Devices. Proc. IEEE 2008, 96, 1457–1486. [Google Scholar] [CrossRef]
- Ali, F.; Raza, W.; Li, X.; Gul, H.; Kim, K.-H. Piezoelectric energy harvesters for biomedical applications. Nano Energy 2019, 57, 879–902. [Google Scholar] [CrossRef]
- Kymissis, J.; Kendall, C.; Paradiso, J.; Gershenfeld, N. Parasitic power harvesting in shoes. In Proceedings of the Digest of Papers, Second International Symposium on Wearable Computers (Cat. No.98EX215), Pittsburgh, PA, USA, 19–20 October 1998; pp. 132–139. [Google Scholar] [CrossRef]
- Shenck, N.S.; Paradiso, J.A. Energy scavenging with shoe-mounted piezoelectrics. IEEE Micro 2001, 21, 30–42. [Google Scholar] [CrossRef]
- Li, H.; Tian, C.; Deng, Z.D. Energy harvesting from low frequency applications using piezoelectric materials. Appl. Phys. Rev. 2014, 1, 041301. [Google Scholar] [CrossRef]
- Platt, S.R.; Farritor, S.; Haider, H. On Low-Frequency Electric Power Generation With PZT Ceramics. IEEE/ASME Trans. Mechatronics 2005, 10, 240–252. [Google Scholar] [CrossRef]
- Kim, D.H.; Shin, H.J.; Lee, H.; Jeong, C.K.; Park, H.; Hwang, G.T.; Lee, H.Y.; Joe, D.J.; Han, J.H.; Lee, S.H.; et al. In Vivo Self-Powered Wireless Transmission Using Biocompatible Flexible Energy Harvesters. Adv. Funct. Mater. 2017, 27, 1700341. [Google Scholar] [CrossRef]
- Hwang, G.-T.; Park, H.; Lee, J.H.; Oh, S.; Park, K.I.; Byun, M.; Park, H.; Ahn, G.; Jeong, C.K.; No, K.; et al. Self-Powered Cardiac Pacemaker Enabled by Flexible Single Crystalline PMN-PT Piezoelectric Energy Harvester. Adv. Mater. 2014, 26, 4880–4887. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, G.; Yang, R.; Wang, A.C.; Wang, Z.L. Muscle-Driven In Vivo Nanogenerator. Adv. Mater. 2010, 22, 2534–2537. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Qin, Y.; Xu, C.; Wei, Y.; Yang, R.; Wang, Z.L. Self-powered nanowire devices. Nat. Nanotechnol. 2010, 5, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.K.; Baek, C.; Kingon, A.I.; Park, K.; Kim, S. Lead-Free Perovskite Nanowire-Employed Piezopolymer for Highly Efficient Flexible Nanocomposite Energy Harvester. Small 2018, 14, 1704022. [Google Scholar] [CrossRef]
- Todaro, M.T.; Guido, F.; Algieri, L.; Mastronardi, V.M.; Desmaële, D.; Epifani, G.; De Vittorio, M. Biocompatible, Flexible, and Compliant Energy Harvesters Based on Piezoelectric Thin Films. IEEE Trans. Nanotechnol. 2018, 17, 220–230. [Google Scholar] [CrossRef]
- Ramsay, M.J.; Clark, W.W. Piezoelectric energy harvesting for bio-MEMS applications. In Proceedings of the Smart Structures and Materials 2001: Industrial and Commercial Applications of Smart Structures Technologies, Newport Beach, CA, USA, 14 June 2001; pp. 429–438. [Google Scholar] [CrossRef]
- Sohn, J.W.; Choi, S.B.; Lee, D.Y. An investigation on piezoelectric energy harvesting for MEMS power sources. Proc. Inst. Mech. Eng. Part C: J. Mech. Eng. Sci. 2005, 219, 429–436. [Google Scholar] [CrossRef]
- Platt, S.R.; Farritor, S.; Garvin, K.; Haider, H. The Use of Piezoelectric Ceramics for Electric Power Generation Within Orthopedic Implants. IEEE/ASME Trans. Mechatronics 2005, 10, 455–461. [Google Scholar] [CrossRef]
- Chen, H.; Jia, C.; Hao, W.; Zhang, C.; Wang, Z.; Liu, C. Power harvesting with PZT ceramics and circuits design. Analog. Integr. Circ. Signal Process. 2010, 62, 263–268. [Google Scholar] [CrossRef][Green Version]
- Almouahed, S.; Gouriou, M.; Hamitouche, C.; Stindel, E.; Roux, C. Self-powered instrumented knee implant for early detection of postoperative complications. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 5121–5124. [Google Scholar] [CrossRef]
- Renaud, M.; Karakaya, K.; Sterken, T.; Fiorini, P.; van Hoof, C.; Puers, R. Fabrication, modelling and characterization of MEMS piezoelectric vibration harvesters. Sens. Actuators A Phys. 2008, 145–146, 380–386. [Google Scholar] [CrossRef]
- Elfrink, R.; Kamel, T.M.; Goedbloed, M.; Matova, S.; Hohlfeld, D.; Van Andel, Y.; Van Schaijk, R. Vibration energy harvesting with aluminum nitride-based piezoelectric devices. J. Micromech. Microeng. 2009, 19, 094005. [Google Scholar] [CrossRef]
- Gao, S.; Gain, A.K.; Zhang, L. A metamaterial for wearable piezoelectric energy harvester. Smart Mater. Struct. 2020, 30, 015026. [Google Scholar] [CrossRef]
- Beyaz, M.I.; Ahmed, N. A belt-integrated piezoelectric energy harvester for wearable electronic devices. Ferroelectrics 2021, 585, 187–197. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, S.; Jin, Y.M.; Ouyang, H.; Zou, Y.; Wang, X.X.; Xie, L.X.; Li, Z.J.S.S. Flexible piezoelectric nanogenerator in wearable self-powered active sensor for respiration and healthcare monitoring. Semicond. Sci. Technol. 2017, 32, 064004. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiang, H.; Tang, L. Modeling, analysis and comparison of four charging interface circuits for piezoelectric energy harvesting. Mech. Syst. Signal Process. 2021, 152, 107476. [Google Scholar] [CrossRef]
- Long, Z.; Wang, X.; Li, P.; Wang, B.; Zhang, X.; Chung, H.S.H.; Yang, Z. Self-Powered SSDCI Array Interface for Multiple Piezoelectric Energy Harvesters. IEEE Trans. Power Electron. 2021, 36, 9093–9104. [Google Scholar] [CrossRef]
- Chamanian, S.; Muhtaroglu, A.; Kulah, H. A self-adapting synchronized-switch interface circuit for piezoelectric energy harvesters. IEEE Trans. Power Electron. 2020, 35, 901–912. [Google Scholar] [CrossRef]
- Meninger, S.; Mur-Miranda, J.O.; Amirtharajah, R.; Chandrakasan, A.; Lang, J.H. Vibration-to-electric energy conversion. IEEE Trans. Very Large Scale Integr. Syst. 2001, 9, 64–76. [Google Scholar] [CrossRef]
- Khan, F.U.; Usman, M. A power-density-enhanced MEMS electrostatic energy harvester with symmetrized high-aspect ratio comb electrodes You may also like State-of-the-art in vibration-based electrostatic energy harvesting. J. Micromech. Microeng. 2019, 29, 084002. [Google Scholar]
- Lu, Y.; Marty, F.; Galayko, D.; Laheurte, J.M.; Basset, P. A power supply module for autonomous portable electronics: Ultralow-frequency MEMS electrostatic kinetic energy harvester with a comb structure reducing air damping. Microsyst. Nanoeng. 2018, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, R.; Kabei, N.; Katayama, K.; Ishizuka, Y.; Tsuboi, F.; Tsuchiya, K. Development of an Electrostatic Generator that Harnesses the Motion of a Living Body. Use of a Resonant Phenomenon. JSME Int. J. Ser. C 2000, 43, 916–922. [Google Scholar] [CrossRef]
- Miao, P.; Mitcheson, P.D.; Holmes, A.S.; Yeatman, E.M.; Green, T.C.; Stark, B.H. Mems inertial power generators for biomedical applications. Microsyst. Technol. 2006, 12, 1079–1083. [Google Scholar] [CrossRef]
- Daneshvar, S.H.; Maymandi-Nejad, M. A new electro-static micro-generator for energy harvesting from diaphragm muscle. Int. J. Circuit Theory Appl. 2017, 45, 2307–2328. [Google Scholar] [CrossRef]
- Moretti, G.; Rosset, S.; Vertechy, R.; Anderson, I.; Fontana, M. A Review of Dielectric Elastomer Generator Systems. Adv. Intell. Syst. 2020, 2, 2000125. [Google Scholar] [CrossRef]
- Leese, H.S.; Tejkl, M.; Vilar, L.; Georgi, L.; Yau, H.C.; Rubio, N.; Reixach, E.; Buk, J.; Jiang, Q.; Bismarck, A.; et al. High-k dielectric screen-printed inks for mechanical energy harvesting devices. Mater. Adv. 2022, 3, 1780–1790. [Google Scholar] [CrossRef]
- Krupenkin, T.; Taylor, J.A. Reverse electrowetting as a new approach to high-power energy harvesting. Nat. Commun. 2011, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Panchadar, K.; West, D.; Taylor, J.A.; Krupenkin, T. Mechanical energy harvesting using a liquid metal vortex magnetohydrodynamic generator. Appl. Phys. Lett. 2019, 114, 093901. [Google Scholar] [CrossRef]
- Tanaka, Y.; Miyoshi, T.; Suzuki, Y. Stochastic Model of Human Arm Swing Toward Standard Testing for Rotational Energy Harvester. J. Phys. Conf. Ser. 2019, 1407, 012033. [Google Scholar] [CrossRef]
- Miyoshi, T.; Adachi, M.; Suzuki, K.; Liu, Y.; Suzuki, Y. Low-profile rotational electret generator using print circuit board for energy harvesting from arm swing. In Proceedings of the IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Seoul, Korea, 21–25 January 2018; pp. 230–232. [Google Scholar]
- Miyoshi, T.; Adachi, M.; Tanaka, Y.; Suzuki, Y. Low-profile rotational electret energy harvester for batteryless wearable device. In Proceedings of the IEEE/ASME International Conference on Advanced Intelligent Mechatronics, Auckland, New Zealand, 9–12 July 2018; pp. 391–394. [Google Scholar]
- Zhang, Y.; Wang, T.; Luo, A.; Hu, Y.; Li, X.; Wang, F. Micro electrostatic energy harvester with both broad bandwidth and high normalized power density. Appl. Energy 2018, 212, 362–371. [Google Scholar] [CrossRef]
- Choi, Y.-M.; Lee, M.; Jeon, Y. Wearable Biomechanical Energy Harvesting Technologies. Energies 2017, 10, 1483. [Google Scholar] [CrossRef]
- Nasiri, A.; Zabalawi, S.A.; Jeutter, D.C. A Linear Permanent Magnet Generator for Powering Implanted Electronic Devices. IEEE Trans. Power Electron. 2011, 26, 192–199. [Google Scholar] [CrossRef]
- Zurbuchen, A.; Haeberlin, A.; Pfenniger, A.; Bereuter, L.; Schaerer, J.; Jutzi, F.; Huber, C.; Fuhrer, J.; Vogel, R. Towards Batteryless Cardiac Implantable Electronic Devices–The Swiss Way. IEEE Trans. Biomed. Circ. Syst. 2017, 11, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Yu, C.-H.; Ishiyama, K. Rotary-type electromagnetic power generator using a cardiovascular system as a power source for medical implants. IEEE/ASME Trans. Mechatronics 2015, 21, 122–129. [Google Scholar] [CrossRef]
- Franzina, N.; Zurbuchen, A.; Zumbrunnen, A.; Niederhauser, T.; Reichlin, T.; Burger, J.; Haeberlin, A. A miniaturized endocardial electromagnetic energy harvester for leadless cardiac pacemakers. PLoS ONE 2020, 15, e0239667. [Google Scholar]
- Haeberlin, A.; Rösch, Y.; Tholl, M.V.; Gugler, Y.; Okle, J.; Heinisch, P.P.; Reichlin, T.; Burger, J.; Zurbuchen, A. Intracardiac Turbines Suitable for Catheter-Based Implantation-An Approach to Power Battery and Leadless Cardiac Pacemakers? IEEE Trans. Biomed. Eng. 2020, 67, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Secord, T.W.; Audi, M.C. A Tunable Resonance Cantilever for Cardiac Energy Harvesting. Cardiovasc. Eng. Technol. 2019, 10, 380–393. [Google Scholar] [CrossRef]
- Samad, F.A.; Karim, M.F.; Paulose, V.; Ong, L.C. A Curved Electromagnetic Energy Harvesting System for Wearable Electronics. IEEE Sens. J. 2016, 16, 1969–1974. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Kim, E.S. Power generation from human body motion through magnet and coil arrays with magnetic spring. J. Appl. Phys. 2014, 115, 064908. [Google Scholar] [CrossRef]
- Roberts, P.; Stanley, G.; Morgan, J.M. Abstract 2165: Harvesting the Energy of Cardiac Motion to Power a Pacemaker. Circulation 2008, 118, S_679–S_680. [Google Scholar] [CrossRef]
- Lueke, J.; Moussa, W.A. MEMS-Based Power Generation Techniques for Implantable Biosensing Applications. Sensors 2011, 11, 1433–1460. [Google Scholar] [CrossRef]
- Li, W.J.; Ho, T.C.H.; Chan, G.M.H.; Leong, P.H.W.; Wong, H.Y. Infrared signal transmission by a laser-micromachined, vibration-induced power generator. In Proceedings of the 43rd IEEE Midwest Symposium on Circuits and Systems (Cat.No.CH37144), Lansing, MI, USA, 8–11 August 2000; pp. 236–239. [Google Scholar]
- Romero, E.; Warrington, R.O.; Neuman, M.R. Body motion for powering biomedical devices. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 2–6 September 2009; pp. 2752–2755. [Google Scholar]
- Lasheras, A.; Gutiérrez, J.; Reis, S.; Sousa, D.; Silva, M.; Martins, P.; Lanceros-Mendez, S.; Barandiarán, J.M.; Shishkin, D.A.; Potapov, A.P. Energy harvesting device based on a metallic glass/PVDF magnetoelectric laminated composite. Smart Mater. Struct. 2015, 24, 065024. [Google Scholar] [CrossRef]
- Zhang, J.; Kang, Y.; Gao, Y.; Weng, G.J. Experimental Investigation of the Magnetoelectric Effect in NdFeB-Driven A-Line Shape Terfenol-D/PZT-5A Structures. Materials 2019, 12, 1055. [Google Scholar] [CrossRef]
- Toshiyoshi, H.; Ju, S.; Honma, H.; Ji, C.H.; Fujita, H. MEMS vibrational energy harvesters. Sci. Technol. Adv. Mater. 2019, 20, 124–143. [Google Scholar] [CrossRef]
- Zurbuchen, A.; Haeberlin, A.; Bereuter, L.; Pfenniger, A.; Bosshard, S.; Kernen, M.; Heinisch, P.P.; Fuhrer, J.; Vogel, R. Endocardial energy harvesting by electromagnetic induction. IEEE Trans. Biomed. Eng. 2018, 65, 424–430. [Google Scholar] [CrossRef]
- Gupta, R.K.; Shi, Q.; Dhakar, L.; Wang, T.; Heng, C.H.; Lee, C. Broadband Energy Harvester Using Non-linear Polymer Spring and Electromagnetic/Triboelectric Hybrid Mechanism. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Arroyo, E.; Badel, A.; Formosa, F. Energy harvesting from ambient vibrations: Electromagnetic device and synchronous extraction circuit. Orig. Artic. J. Intell. Mater. Syst. Struct. 2013, 24, 2023–2035. [Google Scholar] [CrossRef]
- Li, K.; He, Q.; Wang, J.; Zhou, Z.; Li, X. Wearable energy harvesters generating electricity from low-frequency human limb movement. Microsyst. Nanoeng. 2018, 4, 1–13. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, D.; Luo, J.; Xie, S.; Pu, H.; Li, Z. Power density improvement based on investigation of initial relative position in an electromagnetic energy harvester with self-powered applications. Smart Mater. Struct. 2021, 30, 065005. [Google Scholar] [CrossRef]
- Ramadass, Y.K.; Chandrakasan, A.P. A Battery-Less Thermoelectric Energy Harvesting Interface Circuit With 35 mV Startup Voltage. IEEE J. -Solid-State Circ. 2011, 46, 333–341. [Google Scholar] [CrossRef]
- Wang, W.; Deng, J.; Liu, Y.; Lu, Y.; Jia, Y.; Yu, C. Recent advances in power supply strategies for untethered neural implants. J. Micromech. Microeng. 2021, 31, 104003. [Google Scholar] [CrossRef]
- Zou, Y.; Bo, L.; Li, Z. Recent progress in human body energy harvesting for smart bioelectronic system. Fundam. Res. 2021, 1, 364–382. [Google Scholar] [CrossRef]
- Stark, I.; Stordeur, M. New micro thermoelectric devices based on bismuth telluride-type thin solid films. In Proceedings of the Eighteenth International Conference on Thermoelectrics, Proceedings, ICT’99 (Cat. No.99TH8407), Baltimore, MD, USA, 29 August–2 September 1999; pp. 465–472. [Google Scholar] [CrossRef]
- Strasser, M.; Aigner, R.; Lauterbach, C.; Sturm, T.F.; Franosch, M.; Wachutka, G. Micromachined CMOS thermoelectric generators as on-chip power supply. Sens. Actuators A Phys. 2004, 114, 362–370. [Google Scholar] [CrossRef]
- Leonov, V. Thermoelectric Energy Harvesting of Human Body Heat for Wearable Sensors. IEEE Sens. J. 2013, 13, 2284–2291. [Google Scholar] [CrossRef]
- Hoang, D.C.; Tan, Y.K.; Chng, H.B.; Panda, S.K. Thermal energy harvesting from human warmth for wireless body area network in medical healthcare system. In Proceedings of the 2009 International Conference on Power Electronics and Drive Systems (PEDS), 2–5 November 2009; pp. 1277–1282. [Google Scholar] [CrossRef]
- Moriarty, G.P.; De, S.; King, P.J.; Khan, U.; Via, M.; King, J.A.; Coleman, J.N.; Grunlan, J.C. Thermoelectric behavior of organic thin film nanocomposites. J. Polym. Sci. B Polym. Phys. 2013, 51, 119–123. [Google Scholar] [CrossRef]
- Hong, C.T.; Kang, Y.H.; Ryu, J.; Cho, S.Y.; Jang, K.S. Spray-printed CNT/P3HT organic thermoelectric films and power generators. J. Mater. Chem. A Mater. 2015, 3, 21428–21433. [Google Scholar] [CrossRef]
- Cho, C.; Wallace, K.L.; Tzeng, P.; Hsu, J.H.; Yu, C.; Grunlan, J.C. Outstanding Low Temperature Thermoelectric Power Factor from Completely Organic Thin Films Enabled by Multidimensional Conjugated Nanomaterials. Adv. Energy Mater. 2016, 6, 1502168. [Google Scholar] [CrossRef]
- Wu, G.; Gao, C.; Chen, G.; Wang, X.; Wang, H. High-performance organic thermoelectric modules based on flexible films of a novel n-type single-walled carbon nanotube. J. Mater. Chem. A Mater. 2016, 4, 14187–14193. [Google Scholar] [CrossRef]
- Pudzs, K.; Vembris, A.; Rutkis, M.; Woodward, S. Thin Film Organic Thermoelectric Generator Based on Tetrathiotetracene. Adv. Electron. Mater. 2017, 3, 1600429. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, W.; Kang, Y.H.; Cho, S.Y.; Jang, K.S. Wet-spinning and post-treatment of CNT/PEDOT:PSS composites for use in organic fiber-based thermoelectric generators. Carbon N. Y. 2018, 133, 293–299. [Google Scholar] [CrossRef]
- Peng, J.; Witting, I.; Geisendorfer, N.; Wang, M.; Chang, M.; Jakus, A.; Kenel, C.; Yan, X.; Shah, R.; Snyder, G.J.; et al. 3D extruded composite thermoelectric threads for flexible energy harvesting. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wen, N.; Fan, Z.; Yang, S.; Zhao, Y.; Cong, T.; Xu, S.; Zhang, H.; Wang, J.; Huang, H.; Li, C.; et al. Highly conductive, ultra-flexible and continuously processable PEDOT:PSS fibers with high thermoelectric properties for wearable energy harvesting. Nano Energy 2020, 78, 105361. [Google Scholar] [CrossRef]
- Kuai, Q.; Leung, H.Y.; Wan, Q.; Mok, P.K.T. A High-Efficiency Dual-Polarity Thermoelectric Energy-Harvesting Interface Circuit With Cold Startup and Fast-Searching ZCD. IEEE J. Solid-State Circ. 2022, 57, 1899–1912. [Google Scholar] [CrossRef]
- Hu, Z.; Mu, E.; Wu, Z. MEMS thermoelectric power chip for large scale thermal energy harvesting. In Proceedings of the 2020 IEEE 8th Electronics System-Integration Technology Conference, ESTC 2020, Vestfold, Norway, 15–18 September 2020. [Google Scholar] [CrossRef]
- Kumar, P.M.; Jagadeesh Babu, V.; Subramanian, A.; Bandla, A.; Thakor, N.; Ramakrishna, S.; Wei, H. The Design of a Thermoelectric Generator and Its Medical Applications. Designs 2019, 3, 22. [Google Scholar] [CrossRef]
- Selvam, C.; Manikandan, S.; Krishna, N.V.; Lamba, R.; Kaushik, S.C.; Mahian, O. Enhanced thermal performance of a thermoelectric generator with phase change materials. Int. Commun. Heat Mass Transf. 2020, 114, 104561. [Google Scholar] [CrossRef]
- Fan, F.-R.; Tian, Z.-Q.; Wang, Z.L. Flexible triboelectric generator. Nano Energy 2012, 1, 328–334. [Google Scholar] [CrossRef]
- Zhu, G.; Pan, C.; Guo, W.; Chen, C.Y.; Zhou, Y.; Yu, R.; Wang, Z.L. Triboelectric-Generator-Driven Pulse Electrodeposition for Micropatterning. Nano Lett. 2012, 12, 4960–4965. [Google Scholar] [CrossRef]
- Zhang, X.-S.; Han, M.; Kim, B.; Bao, J.-F.; Brugger, J.; Zhang, H. All-in-one self-powered flexible microsystems based on triboelectric nanogenerators. Nano Energy 2018, 47, 410–426. [Google Scholar] [CrossRef]
- Parida, K.; Bhavanasi, V.; Kumar, V.; Wang, J.; Lee, P.S. Fast charging self-powered electric double layer capacitor. J. Power Sources 2017, 342, 70–78. [Google Scholar] [CrossRef]
- Kumar, V.; Park, S.; Parida, K.; Bhavanasi, V.; Lee, P.S. Multi-responsive supercapacitors: Smart solution to store electrical energy. Mater. Today Energy 2017, 4, 41–57. [Google Scholar] [CrossRef]
- Pu, X.; Guo, H.; Chen, J.; Wang, X.; Xi, Y.; Hu, C.; Wang, Z.L. Eye motion triggered self-powered mechnosensational communication system using triboelectric nanogenerator. Sci. Adv. 2017, 3, e1700694. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Guo, H.; Chen, L.; Wang, X.; Wei, D.; Hu, C. Double-induced-mode integrated triboelectric nanogenerator based on spring steel to maximize space utilization. Nano Res. 2016, 9, 3355–3363. [Google Scholar] [CrossRef]
- Hwang, H.J.; Jung, Y.; Choi, K.; Kim, D.; Park, J.; Choi, D. Comb-structured triboelectric nanogenerators for multi-directional energy scavenging from human movements. Sci. Technol. Adv. Mater. 2019, 20, 725–732. [Google Scholar] [CrossRef]
- Pan, R.; Xuan, W.; Chen, J.; Dong, S.; Jin, H.; Wang, X.; Li, H.; Luo, J. Fully biodegradable triboelectric nanogenerators based on electrospun polylactic acid and nanostructured gelatin films. Nano Energy 2018, 45, 193–202. [Google Scholar] [CrossRef]
- Lin, Z.; Chen, J.; Li, X.; Zhou, Z.; Meng, K.; Wei, W.; Yang, J.; Wang, Z.L. Triboelectric Nanogenerator Enabled Body Sensor Network for Self-Powered Human Heart-Rate Monitoring. ACS Nano 2017, 11, 8830–8837. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Dai, K.; Yi, F.; Han, Y.; Wang, X.; You, Z. Optimization of triboelectric nanogenerator load characteristics considering the air breakdown effect. Nano Energy 2018, 53, 706–715. [Google Scholar] [CrossRef]
- Hazarika, A.; Deka, B.K.; Jeong, C.; Park, Y.b.; Park, H.W. Biomechanical Energy-Harvesting Wearable Textile-Based Personal Thermal Management Device Containing Epitaxially Grown Aligned Ag-Tipped-NiCo Nanowires/Reduced Graphene Oxide. Adv. Funct. Mater. 2019, 29, 1903144. [Google Scholar] [CrossRef]
- Sagan, C.; Mullen, G. Earth and Mars: Evolution of Atmospheres and Surface Temperatures. Science 1972, 177, 52–56. [Google Scholar] [CrossRef]
- Murakawa, K.; Kobayashi, M.; Nakamura, O.; Kawata, S. A wireless near-infrared energy system for medical implants. IEEE Eng. Med. Biol. Mag. 1999, 18, 70–72. [Google Scholar] [CrossRef]
- Goto, K.; Nakagawa, T.; Nakamura, O.; Kawata, S. An implantable power supply with an optically rechargeable lithium battery. IEEE Trans. Biomed. Eng. 2001, 48, 830–833. [Google Scholar] [CrossRef]
- Bashkatov, A.N.; Genina, E.A.; Kochubey, V.I.; Tuchin, V.v. Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm. J. Phys. D Appl. Phys. 2005, 38, 2543. [Google Scholar] [CrossRef]
- Sordillo, L.A.; Pu, Y.; Pratavieira, S.; Budansky, Y.; Alfano, R.R. Deep optical imaging of tissue using the second and third near-infrared spectral windows. J. Biomed. Opt. 2014, 19, 056004. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Mancini, M.C.; Nie, S. Second window for in vivo imaging. Nat. Nanotechnol. 2009, 4, 710. [Google Scholar] [CrossRef] [PubMed]
- Kayes, B.M.; Nie, H.; Twist, R.; Spruytte, S.G.; Reinhardt, F.; Kizilyalli, I.C.; Higashi, G.S. 27.6% Conversion efficiency, a new record for single-junction solar cells under 1 sun illumination. In Proceedings of the Conference Record of the IEEE Photovoltaic Specialists Conference, Seattle, WA, USA, 19–24 June 2011; pp. 000004–000008. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, A.; Green, M.A.; Ferrazza, F. 19.8% efficient ‘honeycomb’ textured multicrystalline and 24.4% monocrystalline silicon solar cells. Appl. Phys. Lett. 1998, 73, 1991. [Google Scholar] [CrossRef]
- Haeberlin, A.; Zurbuchen, A.; Schaerer, J.; Wagner, J.; Walpen, S.; Huber, C.; Haeberlin, H.; Fuhrer, J.; Vogel, R. Successful pacing using a batteryless sunlight-powered pacemaker. Europace 2014, 16, 1534–1539. [Google Scholar] [CrossRef] [PubMed]
- Bereuter, L.; Williner, S.; Pianezzi, F.; Bissig, B.; Buecheler, S.; Burger, J.; Vogel, R.; Zurbuchen, A.; Haeberlin, A. Energy Harvesting by Subcutaneous Solar Cells: A Long-Term Study on Achievable Energy Output. Ann. Biomed. Eng. 2017, 45, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Yang, Z.; Meacham, K.; Cvetkovic, C.; Corbin, E.A.; Vázquez-Guardado, A.; Xue, M.; Yin, L.; Boroumand, J.; Pakeltis, G.; et al. Biodegradable Monocrystalline Silicon Photovoltaic Microcells as Power Supplies for Transient Biomedical Implants. Adv. Energy Mater. 2018, 8, 1703035. [Google Scholar] [CrossRef]
- Kim, J.; Seo, J.; Jung, D.; Lee, T.; Ju, H.; Han, J.; Kim, N.; Jeong, J.; Cho, S.; Seol, J.H.; et al. Active photonic wireless power transfer into live tissues. Proc. Natl. Acad. Sci. USA 2020, 117, 16856–16863. [Google Scholar] [CrossRef]
- Moon, E.; Blaauw, D.; Phillips, J.D. Subcutaneous Photovoltaic Infrared Energy Harvesting for Bio-implantable Devices. IEEE Trans. Electron Devices 2017, 64, 2432–2437. [Google Scholar] [CrossRef]
- Song, K.; Han, J.H.; Lim, T.; Kim, N.; Shin, S.; Kim, J.; Choo, H.; Jeong, S.; Kim, Y.C.; Wang, Z.L.; et al. Subdermal Flexible Solar Cell Arrays for Powering Medical Electronic Implants. Adv. Healthc. Mater. 2016, 5, 1572–1580. [Google Scholar] [CrossRef]
- Hu, F.; Li, W.; Zou, M.; Li, Y.; Chen, F.; Lin, N.; Guo, W.; Liu, X.Y. Subcutaneous Energy/Signal Transmission Based on Silk Fibroin Up-Conversion Photonic Amplification. ACS Nano 2021, 15, 9559–9567. [Google Scholar] [CrossRef]
- Kendall, C.J. Parasitic Power Collection in Shoe Mounted Devices. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 1998. [Google Scholar]
- Moorthy, B.; Baek, C.; Wang, J.E.; Jeong, C.K.; Moon, S.; Park, K.I.; Kim, D.K. Piezoelectric energy harvesting from a PMN–PT single nanowire. RSC Adv. 2017, 7, 260–265. [Google Scholar] [CrossRef]
- Wang, W.; Cao, J.; Bowen, C.R.; Zhou, S.; Lin, J. Optimum resistance analysis and experimental verification of nonlinear piezoelectric energy harvesting from human motions. Energy 2017, 118, 221–230. [Google Scholar] [CrossRef]
- Daneshvar, S.H.; Maymandi-Nejad, M.; Sachdev, M.; Redoute, J.-M. A Charge-Depletion Study of an Electrostatic Generator With Adjustable Output Voltage. IEEE Sens. J. 2019, 19, 1028–1039. [Google Scholar] [CrossRef]
- Risquez, S.; Woytasik, M.; Wei, J.; Parrain, F.; Lefeuvre, E. Design of a 3D multilayer out-of-plane overlap electrostatic energy harvesting MEMS for medical implant applications. In Proceedings of the 2015 Symposium on Design, Test, Integration and Packaging of MEMS/MOEMS (DTIP), Montpellier, France, 27–30 April 2015; pp. 1–5. [Google Scholar] [CrossRef]
- Naruse, Y.; Matsubara, N.; Mabuchi, K.; Izumi, M.; Suzuki, S. Electrostatic micro power generation from low-frequency vibration such as human motion. J. Micromech. Microeng. 2009, 19, 094002. [Google Scholar] [CrossRef]
- Huang, W.; Tzeng, K.; Cheng, M.; Huang, R. A silicon mems micro power generator for wearable micro devices. J. Chin. Inst. Eng. 2007, 30, 133–140. [Google Scholar] [CrossRef]
- Chen, C.; Chau, L.Y.; Liao, W.-H. A knee-mounted biomechanical energy harvester with enhanced efficiency and safety. Smart Mater. Struct. 2017, 26, 065027. [Google Scholar] [CrossRef]
- Ylli, K.; Hoffmann, D.; Willmann, A.; Becker, P.; Folkmer, B.; Manoli, Y. Energy harvesting from human motion: Exploiting swing and shock excitations. Smart Mater. Struct. 2015, 24, 025029. [Google Scholar] [CrossRef]
- Hyland, M.; Hunter, H.; Liu, J.; Veety, E.; Vashaee, D. Wearable thermoelectric generators for human body heat harvesting. Appl. Energy 2016, 182, 518–524. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, M.S.; Jo, S.E.; Kim, Y.J. Flexible thermoelectric generator for human body heat energy harvesting. Electron. Lett. 2012, 48, 1015–1017. [Google Scholar] [CrossRef]
- Leonov, V.; Fiorini, P.; Sedky, S.; Torfs, T.; van Hoof, C. Thermoelectric mems generators as a power supply for a body area network. In Proceedings of the 13th International Conference on Solid-State Sensors, Actuators and Microsystems, Seoul, Korea, 5–9 June 2005; pp. 291–294. [Google Scholar] [CrossRef]
- Sahatiya, P.; Kannan, S.; Badhulika, S. Few layer MoS2 and in situ poled PVDF nanofibers on low cost paper substrate as high performance piezo-triboelectric hybrid nanogenerator: Energy harvesting from handwriting and human touch. Appl. Mater. Today 2018, 13, 91–99. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, G.; Yang, W.; Jing, Q.; Bai, P.; Yang, Y.; Hou, T.C.; Wang, Z.L. Harmonic-Resonator-Based Triboelectric Nanogenerator as a Sustainable Power Source and a Self-Powered Active Vibration Sensor. Adv. Mater. 2013, 25, 6094–6099. [Google Scholar] [CrossRef]
- Zhang, R.; Örtegren, J.; Hummelgård, M.; Olsen, M.; Andersson, H.; Olin, H. Harvesting triboelectricity from the human body using non-electrode triboelectric nanogenerators. Nano Energy 2018, 45, 298–303. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, H.; Shi, B.; Xue, X.; Liu, Z.; Jin, Y.; Ma, Y.; Zou, Y.; Wang, X.; An, Z.; et al. In Vivo Self-Powered Wireless Cardiac Monitoring via Implantable Triboelectric Nanogenerator. ACS Nano 2016, 10, 6510–6518. [Google Scholar] [CrossRef]
- Wu, F.; Li, C.; Yin, Y.; Cao, R.; Li, H.; Zhang, X.; Zhao, S.; Wang, J.; Wang, B.; Xing, Y.; et al. A Flexible, Lightweight, and Wearable Triboelectric Nanogenerator for Energy Harvesting and Self-Powered Sensing. Adv. Mater. Technol. 2019, 4, 1800216. [Google Scholar] [CrossRef]
- Ho, J.S.; Yeh, A.J.; Neofytou, E.; Kim, S.; Tanabe, Y.; Patlolla, B.; Beygui, R.E.; Poon, A.S. Wireless power transfer to deep-tissue microimplants. Proc. Natl. Acad. Sci. USA 2014, 111, 7974–7979. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.; Islam, S.K.; To, G.; Mahfouz, M. Powering wearable sensors with a low-power CMOS piezoelectric energy harvesting circuit. In Proceedings of the 2017 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rochester, MN, USA, 7–10 May 2017; pp. 308–313. [Google Scholar] [CrossRef]
- Singh, N.; Kanaujia, B.K.; Beg, M.T.; Khan, T.; Kumar, S. A dual polarized multiband rectenna for RF energy harvesting. AEU—Int. J. Electron. Commun. 2018, 93, 123–131. [Google Scholar] [CrossRef]
- Singh, N.; Kumar, S.; Kanaujia, B.K.; Beg, M.T.; Kumar, S. A compact and efficient graphene FET based RF energy harvester for green communication. AEU—Int. J. Electron. Commun. 2020, 115, 153059. [Google Scholar] [CrossRef]
- Agrawal, S.; Parihar, M.S.; Kondekar, P.N. Broadband Rectenna for Radio Frequency Energy Harvesting Application. IETE J. Res. 2018, 64, 347–353. [Google Scholar] [CrossRef]
- Vital, D.; Bhardwaj, S.; Volakis, J.L. Textile-Based Large Area RF-Power Harvesting System for Wearable Applications. IEEE Trans. Antennas Propag. 2020, 68, 2323–2331. [Google Scholar] [CrossRef]
- Krikidis, I.; Timotheou, S.; Nikolaou, S.; Zheng, G.; Ng, D.W.K.; Schober, R. Simultaneous wireless information and power transfer in modern communication systems. IEEE Commun. Mag. 2014, 52, 104–110. [Google Scholar] [CrossRef]
- Akkermans, J.A.G.; van Beurden, M.C.; Doodeman, G.J.N.; Visser, H.J. Analytical models for low-power rectenna design. IEEE Antennas Wirel. Propag. Lett. 2005, 4, 187–190. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, H.; Zhang, L.; Zhang, C.; Wang, Z.; Chen, X. An Energy-Efficient ASIC for Wireless Body Sensor Networks in Medical Applications. IEEE Trans. Biomed. Circ. Syst. 2010, 4, 11–18. [Google Scholar] [CrossRef]
- Xia, L.; Cheng, J.; Glover, N.E.; Chiang, P. 0.56 V, –20 dBm RF-Powered, Multi-Node Wireless Body Area Network System-on-a-Chip With Harvesting-Efficiency Tracking Loop. IEEE J. -Solid-State Circ. 2014, 49, 1345–1355. [Google Scholar] [CrossRef]
- Chung, W.Y.; Le, G.T.; Tran, T.V.; Nguyen, N.H. Novel proximal fish freshness monitoring using batteryless smart sensor tag. Sens. Actuators B Chem. C 2017, 248, 910–916. [Google Scholar] [CrossRef]
- Lam, M.B.; Dang, N.T.; Nguyen, T.H.; Chung, W.Y. A Neural Network-Based Model of Radio Frequency Energy Harvesting Characteristics in a Self-Powered Food Monitoring System. Undefined 2019, 19, 8813–8823. [Google Scholar] [CrossRef]
- Roundy, S.; Steingart, D.; Frechette, L.; Wright, P.; Rabaey, J. Power Sources for Wireless Sensor Networks; Springer: Berlin/Heidelberg, Germany, 2004; pp. 1–17. [Google Scholar]
- Grätzel, M. Photoelectrochemical cells. In Materials for Sustainable Energy; Co-Published with Macmillan Publishers Ltd.: London, UK, 2010; pp. 26–32. [Google Scholar]
- Dieffenderfer, J.P.; Beppler, E.; Novak, T.; Whitmire, E.; Jayakumar, R.; Randall, C.; Qu, W.; Rajagopalan, R.; Bozkurt, A. Solar powered wrist worn acquisition system for continuous photoplethysmogram monitoring. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 3142–3145. [Google Scholar] [CrossRef]
- Ostfeld, A.E.; Gaikwad, A.M.; Khan, Y.; Arias, A.C. High-performance flexible energy storage and harvesting system for wearable electronics. Sci. Rep. 2016, 6, 26122. [Google Scholar] [CrossRef]
- Toh, W.Y.; Tan, Y.K.; Koh, W.S.; Siek, L. Autonomous Wearable Sensor Nodes With Flexible Energy Harvesting. IEEE Sens. J. 2014, 14, 2299–2306. [Google Scholar] [CrossRef]
- Wu, T.; Wu, F.; Redoute, J.-M.; Yuce, M.R. An Autonomous Wireless Body Area Network Implementation Towards IoT Connected Healthcare Applications. IEEE Access 2017, 5, 11413–11422. [Google Scholar] [CrossRef]
- Lechêne, B.P.; Cowell, M.; Pierre, A.; Evans, J.W.; Wright, P.K.; Arias, A.C. Organic solar cells and fully printed super-capacitors optimized for indoor light energy harvesting. Nano Energy 2016, 26, 631–640. [Google Scholar] [CrossRef]
- Ayazian, S.; Akhavan, V.A.; Soenen, E.; Hassibi, A. A Photovoltaic-Driven and Energy-Autonomous CMOS Implantable Sensor. IEEE Trans. Biomed. Circ. Syst. 2012, 6, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Haeberlin, A.; Zurbuchen, A.; Walpen, S.; Schaerer, J.; Niederhauser, T.; Huber, C.; Tanner, H.; Servatius, H.; Seiler, J.; Haeberlin, H.; et al. The first batteryless, solar-powered cardiac pacemaker. Heart Rhythm. 2015, 12, 1317–1323. [Google Scholar] [CrossRef]
- Wu, T.; Redoute, J.-M.; Yuce, M.R. A Wireless Implantable Sensor Design With Subcutaneous Energy Harvesting for Long-Term IoT Healthcare Applications. IEEE Access 2018, 6, 35801–35808. [Google Scholar] [CrossRef]
- Phillips, W.B.; Towe, B.C.; Larson, P.J. An ultrasonically-driven piezoelectric neural stimulator. In Proceedings of the 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (IEEE Cat. No.03CH37439), Cancun, Mexico, 17–21 September 2003; pp. 1983–1986. [Google Scholar] [CrossRef]
- Towe, B.C.; Larson, P.J.; Gulick, D.W. Wireless ultrasound-powered biotelemetry for implants. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 2–6 September 2009; pp. 5421–5424. [Google Scholar] [CrossRef]
- Zhu, Y.; Moheimani, S.O.R.; Yuce, M.R. Ultrasonic Energy Transmission and Conversion Using a 2-D MEMS Resonator. IEEE Electron Device Lett. 2010, 31, 374–376. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, T.; Lee, C. MEMS Based Broadband Piezoelectric Ultrasonic Energy Harvester (PUEH) for Enabling Self-Powered Implantable Biomedical Devices. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Zhang, T.; Liang, H.; Wang, Z.; Qiu, C.; Peng, Y.B.; Zhu, X.; Li, J.; Ge, X.; Xu, J.; Huang, X.; et al. Piezoelectric ultrasound energy–harvesting device for deep brain stimulation and analgesia applications. Sci. Adv. 2022, 8, 159. [Google Scholar] [CrossRef]
- Jian, L.G.; Yang, Y.; Chen, R.; Lu, G.; Li, R.; Li, D.; Humayun, M.S.; Shung, K.K.; Zhu, J.; Chen, Y.; et al. Flexible piezoelectric ultrasonic energy harvester array for bio-implantable wireless generator. Nano Energy 2019, 56, 216–224. [Google Scholar] [CrossRef]
- Wan, X.; Chen, P.; Xu, Z.; Mo, X.; Jin, H.; Yang, W.; Wang, S.; Duan, J.; Hu, B.; Luo, Z.; et al. Hybrid-Piezoelectret Based Highly Efficient Ultrasonic Energy Harvester for Implantable Electronics. Adv. Funct. Mater. 2022, 32, 2200589. [Google Scholar] [CrossRef]
- Culurciello, E.; Andreou, A.G. Capacitive Inter-Chip Data and Power Transfer for 3-D VLSI. IEEE Trans. Circ. Syst. II Express Briefs 2006, 53, 1348–1352. [Google Scholar] [CrossRef]
- Sambas, A.; Mamat, M.; Arafa, A.A.; Mahmoud, G.M.; Mohamed, M.A.; Sanjaya, W.S.M. A New Design of Capacitive Power Transfer Based on Hybrid Approach for Biomedical Implantable Device. Int. J. Electr. Comput. Eng. 2019, 9, 2365–2376. [Google Scholar] [CrossRef]
- Sha, X.; Zheng, P.; Karimi, Y.; Stanacevic, M. Capacitive link for data communication between free floating mm-sized brain implants. In Proceedings of the 2021 IEEE International Symposium on Medical Measurements and Applications, MeMeA 2021—Conference Proceedings, Lausanne, Switzerland, 23–25 June 2021. [Google Scholar] [CrossRef]
- Riistama, J.; Väisänen, J.; Heinisuo, S.; Harjunpää, H.; Arra, S.; Kokko, K.; Mäntylä, M.; Kaihilahti, J.; Heino, P.; Kellomäki, M.; et al. Wireless and inductively powered implant for measuring electrocardiogram. Med. Biol. Eng. Comput. 2007, 45, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Hsia, M.-L.; Tsai, Y.-S.; Chen, O.T.-C. An UHF Passive RFID Transponder Using A Low-Power Clock Generator without Passive Components. In Proceedings of the 2006 49th IEEE International Midwest Symposium on Circuits and Systems, San Juan, Puerto Rico, 6–9 August 2006; pp. 11–15. [Google Scholar] [CrossRef]
- Parramon, J.; Doguet, P.; Marin, D.; Verleyssen, M.; Munoz, R.; Leija, L.; Valderrama, E. ASIC-based batteryless implantable telemetry microsystem for recording purposes. In Proceedings of the 19th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Magnificent Milestones and Emerging Opportunities in Medical Engineering (Cat. No.97CH36136), Chicago, IL, USA, 30 October–2 November 1997; pp. 2225–2228. [Google Scholar] [CrossRef]
- Sauer, C.; Stanacevic, M.; Cauwenberghs, G.; Thakor, N. Power harvesting and telemetry in CMOS for implanted devices. IEEE Trans. Circ. Syst. I Regul. Pap. 2005, 52, 2605–2613. [Google Scholar] [CrossRef]
- Hannan, M.A.; Mutashar, S.; Samad, S.A.; Hussain, A. Energy harvesting for the implantable biomedical devices: Issues and challenges. Biomed. Eng. Online 2014, 13, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Kim, J.A.; Gurgu, A.; Khawaja, M.; Cozma, D.; Chelu, M.G. Innovations in Cardiac Implantable Electronic Devices. Cardiovasc. Drugs Ther. 2021, 36, 1–13. [Google Scholar] [CrossRef]
- Lyu, H.; Wang, J.; La, J.H.; Chung, J.M.; Babakhani, A. An Energy-Efficient Wirelessly Powered Millimeter-Scale Neurostimulator Implant Based on Systematic Codesign of an Inductive Loop Antenna and a Custom Rectifier. IEEE Trans. Biomed. Circ. Syst 2018, 12, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Mirbozorgi, S.A.; Yeon, P.; Ghovanloo, M. Robust Wireless Power Transmission to mm-Sized Free-Floating Distributed Implants. IEEE Trans. Biomed. Circ. Syst. 2017, 11, 692–702. [Google Scholar] [CrossRef]
- Ghomian, T.; Mehraeen, S. Survey of energy scavenging for wearable and implantable devices. Energy 2019, 178, 33–49. [Google Scholar] [CrossRef]
- Jokic, P.; Magno, M. Powering smart wearable systems with flexible solar energy harvesting. In Proceedings of the 2017 IEEE International Symposium on Circuits and Systems (ISCAS), Baltimore, MD, USA, 28–31 May 2017; pp. 1–4. [Google Scholar] [CrossRef]
- Ahnood, A.; Fox, K.E.; Apollo, N.V.; Lohrmann, A.; Garrett, D.J.; Nayagam, D.A.; Karle, T.; Stacey, A.; Abberton, K.M.; Morrison, W.A.; et al. Diamond encapsulated photovoltaics for transdermal power delivery. Biosens. Bioelectron. 2016, 77, 589–597. [Google Scholar] [CrossRef]
- Laube, T.; Brockmann, C.; Buß, R.; Lau, C.; Höck, K.; Stawski, N.; Stieglitz, T.; Richter, H.A.; Schilling, H. Optical energy transfer for intraocular microsystems studied in rabbits. Graefe’s Arch. Clin. Exp. Ophthalmol. 2004, 242, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Y.Z.; Chen, H.C.; Liu, C.W.; Chen, Y.S.; Lo, Y.C.; Tsao, C.S.; Huang, Y.C.; Liu, S.W.; Wong, K.T.; et al. Unveiling the underlying mechanism of record-high efficiency organic near-infrared photodetector harnessing a single-component photoactive layer. Mater. Horizons 2020, 7, 1171–1179. [Google Scholar] [CrossRef]
- Lo, Y.K.; Chen, K.; Gad, P.; Liu, W. A Fully-Integrated High-Compliance Voltage SoC for Epi-Retinal and Neural Prostheses. IEEE Trans. Biomed. Circ. Syst. 2013, 7, 761–772. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hsieh, C.H.; Yang, C.M. Wireless Front-End With Power Management for an Implantable Cardiac Microstimulator. IEEE Trans. Biomed. Circ. Syst. 2012, 6, 28–38. [Google Scholar] [CrossRef]
- Kelly, S.K.; Shire, D.B.; Chen, J.; Doyle, P.; Gingerich, M.D.; Cogan, S.F.; Drohan, W.A.; Behan, S.; Theogarajan, L.; Wyatt, J.L.; et al. A Hermetic Wireless Subretinal Neurostimulator for Vision Prostheses. IEEE Trans. Biomed. Eng. 2011, 58, 3197–3205. [Google Scholar] [CrossRef] [PubMed]
- Knecht, O.; Kolar, J.W. Performance Evaluation of Series-Compensated IPT Systems for Transcutaneous Energy Transfer. IEEE Trans. Power Electrons 2019, 34, 438–451. [Google Scholar] [CrossRef]
- Jegadeesan, R.; Nag, S.; Agarwal, K.; Thakor, N.v.; Guo, Y.-X. Enabling Wireless Powering and Telemetry for Peripheral Nerve Implants. IEEE J. Biomed. Health Inform. 2015, 19, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Deng, Y.; Wang, Y.; Shen, S.; Gulfam, R. High-performance photovoltaic-thermoelectric hybrid power generation system with optimized thermal management. Energy 2016, 100, 91–101. [Google Scholar] [CrossRef]
- Li, G.; Shittu, S.; Diallo, T.M.O.; Yu, M.; Zhao, X.; Ji, J. A review of solar photovoltaic-thermoelectric hybrid system for electricity generation. Energy 2018, 158, 41–58. [Google Scholar] [CrossRef]
- Kraemer, D.; Hu, L.; Muto, A.; Chen, X.; Chen, G.; Chiesa, M. Photovoltaic-thermoelectric hybrid systems: A general optimization methodology. Appl. Phys. Lett. 2008, 92, 243503. [Google Scholar] [CrossRef]
- Magno, M.; Brunelli, D.; Sigrist, L.; Andri, R.; Cavigelli, L.; Gomez, A.; Benini, L. InfiniTime: Multi-sensor wearable bracelet with human body harvesting. Sustain. Comput. Inform. Syst. 2016, 11, 38–49. [Google Scholar] [CrossRef]
- Park, K.-T.; Shin, S.M.; Tazebay, A.S.; Um, H.D.; Jung, J.Y.; Jee, S.W.; Oh, M.W.; Park, S.D.; Yoo, B.; Yu, C.; et al. Lossless hybridization between photovoltaic and thermoelectric devices. Sci. Rep. 2013, 3, 2123. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zi, Y.; He, X.; Guo, H.; Lai, Y.C.; Wang, J.; Zhang, S.L.; Wu, C.; Cheng, G.; Wang, Z.L. Concurrent Harvesting of Ambient Energy by Hybrid Nanogenerators for Wearable Self-Powered Systems and Active Remote Sensing. ACS Appl. Mater. Interfaces 2018, 10, 14708–14715. [Google Scholar] [CrossRef]
- Chung, J.; Yong, H.; Moon, H.; Duong, Q.V.; Choi, S.T.; Kim, D.; Lee, S. Hand-Driven Gyroscopic Hybrid Nanogenerator for Recharging Portable Devices. Adv. Sci. 2018, 5, 1801054. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Liu, X.; Wang, X.; Li, Q. An energy extraction enhanced interface circuit for piezoelectric and thermoelectric energy harvesting. IEICE Electron. Express 2019, 16. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Z.; Zhou, H.; Wang, Z.; Lin, Y.; Chen, Y.; Lv, Y.; Chen, Y.; Zhang, C. Multisource Energy Harvester with Coupling Structure and Multiplexing Mechanism. Adv. Mater. Interfaces 2022, 9, 2200468. [Google Scholar] [CrossRef]
- Zhang, Z.; He, T.; Zhao, J.; Liu, G.; Wang, Z.L.; Zhang, C. Tribo-thermoelectric and tribovoltaic coupling effect at metal-semiconductor interface. Mater. Today Phys. 2021, 16, 100295. [Google Scholar] [CrossRef]
- Mitcheson, P.D.; Toh, T.T. Power Management Electronics. In Energy Harvesting for Autonomous Systems; Artech House: Norwood, MA, USA, 2010. [Google Scholar]
- Newell, D.; Duffy, M. Review of Power Conversion and Energy Management for Low-Power, Low-Voltage Energy Harvesting Powered Wireless Sensors. IEEE Trans. Power Electron. 2019, 34, 9794–9805. [Google Scholar] [CrossRef]
- Kong, N.; Ha, D.S. Low-Power Design of a Self-powered Piezoelectric Energy Harvesting System With Maximum Power Point Tracking. IEEE Trans. Power Electron. 2012, 27, 2298–2308. [Google Scholar] [CrossRef]
- Szarka, G.D.; Burrow, S.G.; Proynov, P.P.; Stark, B.H. Maximum Power Transfer Tracking for Ultralow-Power Electromagnetic Energy Harvesters. IEEE Trans. Power Electron. 2014, 29, 201–212. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.; Kwon, C.-K.; Min, Y.-J.; Kim, C.; Kim, S.-W. An Energy-Efficient Fast Maximum Power Point Tracking Circuit in an 800-µW Photovoltaic Energy Harvester. IEEE Trans. Power Electron. 2013, 28, 2927–2935. [Google Scholar] [CrossRef]
- Sankman, J.; Ma, D. A 12 µW to 1.1 mW AIM Piezoelectric Energy Harvester for Time-Varying Vibrations With 450 nA IQ. IEEE Trans. Power Electron. 2015, 30, 632–643. [Google Scholar] [CrossRef]
- Chew, Z.J.; Zhu, M. Adaptive Maximum Power Point Finding Using Direct V OC /2 Tracking Method With Microwatt Power Consumption for Energy Harvesting. IEEE Trans. Power Electron. 2018, 33, 8164–8173. [Google Scholar] [CrossRef]
- Fang, S.; Xia, H.; Xia, Y.; Ye, Y.; Shi, G.; Wang, X.; Chen, Z. An Efficient Piezoelectric Energy Harvesting Circuit With Series-SSHI Rectifier and FNOV-MPPT Control Technique. IEEE Trans. Ind. Electron. 2021, 68, 7146–7155. [Google Scholar] [CrossRef]
- Shim, M.; Kim, J.; Jeong, J.; Park, S.; Kim, C. Self-Powered 30 µW to 10 mW Piezoelectric Energy Harvesting System With 9.09 ms/V Maximum Power Point Tracking Time. IEEE J. -Solid-State Circ. 2015, 50, 2367–2379. [Google Scholar] [CrossRef]
- Fan, S.; Wei, R.; Zhao, L.; Yang, X.; Geng, L.; Feng, P.X.-L. An Ultralow Quiescent Current Power Management System With Maximum Power Point Tracking (MPPT) for Batteryless Wireless Sensor Applications. IEEE Trans. Power Electron. 2018, 33, 7326–7337. [Google Scholar] [CrossRef]
- Shrivastava, A.; Roberts, N.E.; Khan, O.U.; Wentzloff, D.D.; Calhoun, B.H. A 10 mV-Input Boost Converter With Inductor Peak Current Control and Zero Detection for Thermoelectric and Solar Energy Harvesting With 220 mV Cold-Start and -14.5 dBm, 915 MHz RF Kick-Start. IEEE J. -Solid-State Circ. 2015, 50, 1820–1832. [Google Scholar] [CrossRef]
- Carreon-Bautista, S.; Erbay, C.; Han, A.; Sanchez-Sinencio, E. Power Management System With Integrated Maximum Power Extraction Algorithm for Microbial Fuel Cells. IEEE Trans. Energy Convers. 2015, 30, 262–272. [Google Scholar] [CrossRef]
- Doms, I.; Merken, P.; van Hoof, C.; Mertens, R.P. Capacitive Power Management Circuit for Micropower Thermoelectric Generators With a 1.4 μA Controller. IEEE J. -Solid-State Circ. 2009, 44, 2824–2833. [Google Scholar] [CrossRef]
- Liu, X.; Huang, L.; Ravichandran, K.; Sanchez-Sinencio, E. A Highly Efficient Reconfigurable Charge Pump Energy Harvester With Wide Harvesting Range and Two-Dimensional MPPT for Internet of Things. IEEE J. -Solid-State Circ. 2016, 51, 1302–1312. [Google Scholar] [CrossRef]
- Shuvo, M.M.H. Edge AI: Leveraging the Full Potential of Deep Learning. In Recent Innovations in Artificial Intelligence and Smart Applications, Studies in Computational Intelligence; Springer: Berlin, Germany, 2022; Volume 1061. [Google Scholar]
- Li, X.; Ning, S.; Liu, Z.; Yan, Z.; Luo, C.; Zhuang, Z. Designing phononic crystal with anticipated band gap through a deep learning based data-driven method. Comput. Methods Appl. Mech. Eng. 2020, 361, 112737. [Google Scholar] [CrossRef]
- Luo, C.; Ning, S.; Liu, Z.; Zhuang, Z. Interactive inverse design of layered phononic crystals based on reinforcement learning. Extrem. Mech. Lett. 2020, 36, 100651. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, W.; Wen, Z.; Torrent, D.; Djafari-Rouhani, B. Topological states in twisted pillared phononic plates. Extrem. Mech. Lett. 2020, 39, 100777. [Google Scholar] [CrossRef]
- Wahba, M.A.; Ashour, A.S.; Ghannam, R. Prediction of Harvestable Energy for Self-Powered Wearable Healthcare Devices: Filling a Gap. IEEE Access 2020, 8, 170336–170354. [Google Scholar] [CrossRef]
- Kwan, J.C.; Chaulk, J.M.; Fapojuwo, A.O. A Coordinated Ambient/Dedicated Radio Frequency Energy Harvesting Scheme Using Machine Learning. IEEE Sens. J. 2020, 20, 13808–13823. [Google Scholar] [CrossRef]
- Hussein, D.; Bhat, G.; Doppa, J.R. Adaptive Energy Management for Self-Sustainable Wearables in Mobile Health. Proc. AAAI 2022. [Google Scholar]
- Akinaga, H. Recent advances and future prospects in energy harvesting technologies. Jpn. J. Appl. Phys. 2020, 59, 110201. [Google Scholar] [CrossRef]
- Ye, Y.; Azmat, F.; Adenopo, I.; Chen, Y.; Shi, R. RF energy modelling using machine learning for energy harvesting communications systems. Int. J. Commun. Syst. 2021, 34, e4688. [Google Scholar] [CrossRef]
- Xiao, L.; Wu, K.; Tian, X.; Luo, J. Activity-specific caloric expenditure estimation from kinetic energy harvesting in wearable devices. Pervasive Mob. Comput. 2020, 67, 101185. [Google Scholar] [CrossRef]
- Guo, X.; He, T.; Zhang, Z.; Luo, A.; Wang, F.; Ng, E.J.; Zhu, Y.; Liu, H.; Lee, C. Artificial Intelligence-Enabled Caregiving Walking Stick Powered by Ultra-Low-Frequency Human Motion. ACS Nano 2021, 15, 19054–19069. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Sun, Z.; He, T.; Shi, Q.; Zhu, M.; Zhang, Z.; Li, L.; Zhang, T.; Lee, C. Machine Learning Glove Using Self-Powered Conductive Superhydrophobic Triboelectric Textile for Gesture Recognition in VR/AR Applications. Adv. Sci. 2020, 7, 2000261. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Sun, Z.; Zhang, Z.; Shi, Q.; He, T.; Liu, H.; Chen, T.; Lee, C. Haptic-feedback smart glove as a creative human-machine interface (HMI) for virtual/augmented reality applications. Sci. Adv. 2020, 6, eaaz8693. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, M.; Shi, Q.; Wen, F.; Liu, L.; Dong, B.; Haroun, A.; Yang, Y.; Vachon, P.; Guo, X.; et al. Progress in TENG technology—A journey from energy harvesting to nanoenergy and nanosystem. EcoMat 2020, 2, e12058. [Google Scholar] [CrossRef]
- Zhang, Z.; He, T.; Zhu, M.; Shi, Q.; Lee, C. Smart Triboelectric Socks for Enabling Artificial Intelligence of Things (AIoT) Based Smart Home and Healthcare. In Proceedings of the 2020 IEEE 33rd International Conference on Micro Electro Mechanical Systems (MEMS), Vancouver, BC, Canada, 18–22 January 2020; pp. 80–83. [Google Scholar] [CrossRef]
- Ji, X.; Zhao, T.; Zhao, X.; Lu, X.; Li, T. Triboelectric Nanogenerator Based Smart Electronics via Machine Learning. Adv. Mater Technol. 2020, 5, 1900921. [Google Scholar] [CrossRef]
- Politi, B.; Foucaran, A.; Camara, N. Low-Cost Sensors for Indoor PV Energy Harvesting Estimation Based on Machine Learning. Energies 2022, 15, 1144. [Google Scholar] [CrossRef]
- Park, Y.; Cho, K.; Kim, S. Performance Prediction of Hybrid Energy Harvesting Devices Using Machine Learning. ACS Appl. Mater. Interfaces 2022, 14, 11248–11254. [Google Scholar] [CrossRef]
- Zhu, J.; Cho, M.; Li, Y.; He, T.; Ahn, J.; Park, J.; Ren, T.L.; Lee, C.; Park, I. Machine learning-enabled textile-based graphene gas sensing with energy harvesting-assisted IoT application. Nano Energy 2021, 86, 106035. [Google Scholar] [CrossRef]
- Hamdi, R.; Chen, M.; Said, A.b.; Qaraqe, M.; Poor, H.V. Federated Learning over Energy Harvesting Wireless Networks. IEEE Internet Things J. 2022, 9, 92–103. [Google Scholar] [CrossRef]
- Guler, B.; Yener, A. Energy-Harvesting Distributed Machine Learning. In Proceedings of the 2021 IEEE International Symposium on Information Theory, Virtual, 11–16 July 2021; pp. 320–325. [Google Scholar] [CrossRef]
- Pervez, I.; Antoniadis, C.; Massoud, Y. A Reduced Search Space Exploration Metaheuristic Algorithm for MPPT. IEEE Access 2022, 10, 26090–26100. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Liu, Y.; Gao, K.; Yin, T.; Yu, H. Few-shot learning based multi-weather-condition impedance identification for MPPT-controlled PV converters. IET Renew. Power Gener. 2022, 16, 1345–1353. [Google Scholar] [CrossRef]
- Singh, Y.; Pal, N. Reinforcement learning with fuzzified reward approach for MPPT control of PV systems. Sustain. Energy Technol. Assessments 2021, 48, 101665. [Google Scholar] [CrossRef]
- Zafar, M.H.; Khan, N.M.; Mansoor, M.; Khan, U.A. Towards green energy for sustainable development: Machine learning based MPPT approach for thermoelectric generator. J. Clean. Prod. 2022, 351, 131591. [Google Scholar] [CrossRef]
- Ouyang, H.; Liu, Z.; Li, N.; Shi, B.; Zou, Y.; Xie, F.; Ma, Y.; Li, Z.; Li, H.; Zheng, Q.; et al. Symbiotic cardiac pacemaker. Nat. Commun. 2019, 10, 1821. [Google Scholar] [CrossRef]
- Guan, H.; Lv, D.; Zhong, T.; Dai, Y.; Xing, L.; Xue, X.; Zhang, Y.; Zhan, Y. Self-powered, wireless-control, neural-stimulating electronic skin for in vivo characterization of synaptic plasticity. Nano Energy 2020, 67, 104182. [Google Scholar] [CrossRef]
- Yao, G.; Kang, L.; Li, J.; Long, Y.; Wei, H.; Ferreira, C.A.; Jeffery, J.J.; Lin, Y.; Cai, W.; Wang, X. Effective weight control via an implanted self-powered vagus nerve stimulation device. Nat. Commun. 2018, 9, 5349. [Google Scholar] [CrossRef]
- Lee, S.; Wang, H.; Peh, W.Y.X.; He, T.; Yen, S.C.; Thakor, N.V.; Lee, C. Mechano-neuromodulation of autonomic pelvic nerve for underactive bladder: A triboelectric neurostimulator integrated with flexible neural clip interface. Nano Energy 2019, 60, 449–456. [Google Scholar] [CrossRef]
- Hassani, F.A.; Mogan, R.P.; Gammad, G.G.; Wang, H.; Yen, S.C.; Thakor, N.V.; Lee, C. Toward Self-Control Systems for Neurogenic Underactive Bladder: A Triboelectric Nanogenerator Sensor Integrated with a Bistable Micro-Actuator. ACS Nano 2018, 12, 3487–3501. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.; He, T.; He, B.; Thakor, N.v.; Lee, C. Investigation of Low-Current Direct Stimulation for Rehabilitation Treatment Related to Muscle Function Loss Using Self-Powered TENG System. Adv. Sci. 2019, 6, 1900149. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Thakor, N.v.; Lee, C. Self-Powered Direct Muscle Stimulation Using a Triboelectric Nanogenerator (TENG) Integrated with a Flexible Multiple-Channel Intramuscular Electrode. ACS Nano 2019, 13, 3589–3599. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Xue, X.; Ma, Y.; Han, M.; Zhang, W.; Xu, Z.; Zhang, H.; Zhang, H. Implantable and self-powered blood pressure monitoring based on a piezoelectric thinfilm: Simulated, in vitro and in vivo studies. Nano Energy 2016, 22, 453–460. [Google Scholar] [CrossRef]
- Southcott, M.; MacVittie, K.; Halámek, J.; Halámková, L.; Jemison, W.D.; Lobel, R.; Katz, E. A pacemaker powered by an implantable biofuel cell operating under conditions mimicking the human blood circulatory system—Battery not included. Phys. Chem. Chem. Phys. 2013, 15, 6278. [Google Scholar] [CrossRef] [PubMed]
- MacVittie, K.; Halámek, J.; Halámková, L.; Southcott, M.; Jemison, W.D.; Lobel, R.; Katz, E. From ‘cyborg’ lobsters to a pacemaker powered by implantable biofuel cells. Energy Environ. Sci. 2013, 6, 81–86. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, A.C.; Liu, Y.; Zhou, Y.; Wang, Z.L. Functional Electrical Stimulation by Nanogenerator with 58 V Output Voltage. Nano Lett. 2012, 12, 3086–3090. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.-T.; Kim, Y.; Lee, J.H.; Oh, S.; Jeong, C.K.; Park, D.Y.; Ryu, J.; Kwon, H.; Lee, S.G.; Joung, B.; et al. Self-powered deep brain stimulation via a flexible PIMNT energy harvester. Energy Environ. Sci. 2015, 8, 2677–2684. [Google Scholar] [CrossRef]
- Lee, S.; Wang, H.; Wang, J.; Shi, Q.; Yen, S.C.; Thakor, N.V.; Lee, C. Battery-free neuromodulator for peripheral nerve direct stimulation. Nano Energy 2018, 50, 148–158. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; He, T.; Li, Z.; Lee, C. Direct muscle stimulation using diode-amplified triboelectric nanogenerators (TENGs). Nano Energy 2019, 63, 103844. [Google Scholar] [CrossRef]
- Alam, M.; Li, S.; Ahmed, R.U.; Yam, Y.M.; Thakur, S.; Wang, X.Y.; Tang, D.; Ng, S.; Zheng, Y.P. Development of a battery-free ultrasonically powered functional electrical stimulator for movement restoration after paralyzing spinal cord injury. J. Neuroeng. Rehabil. 2019, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, Y.; Ouyang, H.; Shi, B.; Li, N.; Jiang, D.; Xie, F.; Qu, D.; Zou, Y.; Huang, Y.; et al. Transcatheter Self-Powered Ultrasensitive Endocardial Pressure Sensor. Adv. Funct. Mater. 2019, 29, 1807560. [Google Scholar] [CrossRef]
- Ma, Y.; Zheng, Q.; Liu, Y.; Shi, B.; Xue, X.; Ji, W.; Liu, Z.; Jin, Y.; Zou, Y.; An, Z.; et al. Self-Powered, One-Stop, and Multifunctional Implantable Triboelectric Active Sensor for Real-Time Biomedical Monitoring. Nano Lett. 2016, 16, 6042–6051. [Google Scholar] [CrossRef]
- Curry, E.J.; Ke, K.; Chorsi, M.T.; Wrobel, K.S.; Miller III, A.N.; Patel, A.; Kim, I.; Feng, J.; Yue, L.; Wu, Q.; et al. Biodegradable Piezoelectric Force Sensor. Proc. Natl. Acad. Sci. USA 2018, 115, 909–914. [Google Scholar] [CrossRef]
- Jeerapan, I.; Sempionatto, J.R.; Pavinatto, A.; You, J.-M.; Wang, J. Stretchable biofuel cells as wearable textile-based self-powered sensors. J. Mater. Chem. A Mater. 2016, 4, 18342–18353. [Google Scholar] [CrossRef]
- Jiang, D.; Ouyang, H.; Shi, B.; Zou, Y.; Tan, P.; Qu, X.; Chao, S.; Xi, Y.; Zhao, C.; Fan, Y.; et al. A wearable noncontact free-rotating hybrid nanogenerator for self-powered electronics. InfoMat 2020, 2, 1191–1200. [Google Scholar] [CrossRef]
- Ouyang, H.; Tian, J.; Sun, G.; Zou, Y.; Liu, Z.; Li, H.; Zhao, L.; Shi, B.; Fan, Y.; Fan, Y.; et al. Self-Powered Pulse Sensor for Antidiastole of Cardiovascular Disease. Adv. Mater. 2017, 29, 1703456. [Google Scholar] [CrossRef]
- Park, D.Y.; Joe, D.J.; Kim, D.H.; Park, H.; Han, J.H.; Jeong, C.K.; Park, H.; Park, J.G.; Joung, B.; Lee, K.J. Self-Powered Real-Time Arterial Pulse Monitoring Using Ultrathin Epidermal Piezoelectric Sensors. Adv. Mater. 2017, 29, 1702308. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-C.; Deng, J.; Niu, S.; Peng, W.; Wu, C.; Liu, R.; Wen, Z.; Wang, Z.L. Electric Eel-Skin-Inspired Mechanically Durable and Super-Stretchable Nanogenerator for Deformable Power Source and Fully Autonomous Conformable Electronic-Skin Applications. Adv. Mater. 2016, 28, 10024–10032. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Bandodkar, A.J.; Valdés-Ramírez, G.; Windmiller, J.R.; Yang, Z.; Ramírez, J.; Chan, G.; Wang, J. Electrochemical Tattoo Biosensors for Real-Time Noninvasive Lactate Monitoring in Human Perspiration. Anal. Chem. 2013, 85, 6553–6560. [Google Scholar] [CrossRef]
- Teymourian, H.; Moonla, C.; Tehrani, F.; Vargas, E.; Aghavali, R.; Barfidokht, A.; Tangkuaram, T.; Mercier, P.P.; Dassau, E.; Wang, J. Microneedle-Based Detection of Ketone Bodies along with Glucose and Lactate: Toward Real-Time Continuous Interstitial Fluid Monitoring of Diabetic Ketosis and Ketoacidosis. Anal. Chem. 2020, 92, 2291–2300. [Google Scholar] [CrossRef]
- Kim, J.; Khan, S.; Wu, P.; Park, S.; Park, H.; Yu, C.; Kim, W. Self-charging wearables for continuous health monitoring. Nano Energy 2021, 79, 105419. [Google Scholar] [CrossRef]
- Kim, C.S.; Yang, H.M.; Lee, J.; Lee, G.S.; Choi, H.; Kim, Y.J.; Lim, S.H.; Cho, S.H.; Cho, B.J. Self-Powered Wearable Electrocardiography Using a Wearable Thermoelectric Power Generator. ACS Energy Lett. 2018, 3, 501–507. [Google Scholar] [CrossRef]
- Vivekananthan, V.; Chandrasekhar, A.; Alluri, N.R.; Purusothaman, Y.; Kim, W.J.; Kang, C.N.; Kim, S.J. A flexible piezoelectric composite nanogenerator based on doping enhanced lead-free nanoparticles. Mater. Lett. 2019, 249, 73–76. [Google Scholar] [CrossRef]
- Mohsen, S.; Zekry, A.; Youssef, K.; Abouelatta, M. A Self-powered Wearable Wireless Sensor System Powered by a Hybrid Energy Harvester for Healthcare Applications. Wirel Pers Commun. 2021, 116, 3143–3164. [Google Scholar] [CrossRef]
- Wagih, M.; Hillier, N.; Yong, S.; Weddell, A.S.; Beeby, S. RF-Powered Wearable Energy Harvesting and Storage Module Based on E-Textile Coplanar Waveguide Rectenna and Supercapacitor. IEEE Open J. Antennas Propag. 2021, 2, 302–314. [Google Scholar] [CrossRef]
- Huang, X.; Wang, L.; Wang, H.; Zhang, B.; Wang, X.; Stening, R.Y.; Sheng, X.; Yin, L. Materials Strategies and Device Architectures of Emerging Power Supply Devices for Implantable Bioelectronics. Small 2020, 16, 1902827. [Google Scholar] [CrossRef]
- Dinis, H.; Mendes, P.M. A comprehensive review of powering methods used in state-of-the-art miniaturized implantable electronic devices. Biosens. Bioelectron. 2021, 172, 112781. [Google Scholar] [CrossRef]
- Sheng, H.; Zhou, J.; Li, B.; He, Y.; Zhang, X.; Liang, J.; Zhou, J.; Su, Q.; Xie, E.; Lan, W.; et al. A thin, deformable, high-performance supercapacitor implant that can be biodegraded and bioabsorbed within an animal body. Sci. Adv. 2021, 7, eabe3097. [Google Scholar] [CrossRef]
- Guan, Y.-S.; Thukral, A.; Zhang, S.; Sim, K.; Wang, X.; Zhang, Y.; Ershad, F.; Rao, Z.; Pan, F.; Wang, P.; et al. Air/water interfacial assembled rubbery semiconducting nanofilm for fully rubbery integrated electronics. Sci. Adv. 2020, 6, eabb3656. [Google Scholar] [CrossRef]
- Sheng, H.; Zhang, X.; Liang, J.; Shao, M.; Xie, E.; Yu, C.; Lan, W. Recent Advances of Energy Solutions for Implantable Bioelectronics. Adv. Healthc. Mater. 2021, 10, 2100199. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Koo, J.; Rogers, J.A. Inorganic materials for transient electronics in biomedical applications. MRS Bull. 2020, 45, 103–112. [Google Scholar] [CrossRef]
- Wang, P.; Hu, M.; Wang, H.; Chen, Z.; Feng, Y.; Wang, J.; Ling, W.; Huang, Y. The Evolution of Flexible Electronics: From Nature, Beyond Nature, and To Nature. Adv. Sci. 2020, 7, 2001116. [Google Scholar] [CrossRef]
- Ershad, F.; Thukral, A.; Yue, J.; Comeaux, P.; Lu, Y.; Shim, H.; Sim, K.; Kim, N.I.; Rao, Z.; Guevara, R.; et al. Ultra-conformal drawn-on-skin electronics for multifunctional motion artifact-free sensing and point-of-care treatment. Nat. Commun. 2020, 11, 3823. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.; Ouyang, H.; Jiang, D.; Fan, Y.; Li, Z. Triboelectric nanogenerator based on degradable materials. EcoMat 2021, 3, e12072. [Google Scholar] [CrossRef]
- Niu, Q.; Huang, L.; Lv, S.; Shao, H.; Fan, S.; Zhang, Y. Pulse-driven bio-triboelectric nanogenerator based on silk nanoribbons. Nano Energy 2020, 74, 104837. [Google Scholar] [CrossRef]
- Peng, X.; Dong, K.; Ye, C.; Jiang, Y.; Zhai, S.; Cheng, R.; Liu, D.; Gao, X.; Wang, J.; Wang, Z.L. A breathable, biodegradable, antibacterial, and self-powered electronic skin based on all-nanofiber triboelectric nanogenerators. Sci. Adv. 2020, 6, eaba9624. [Google Scholar] [CrossRef] [PubMed]
- Dagdeviren, C.; Hwang, S.W.; Su, Y.; Kim, S.; Cheng, H.; Gur, O.; Haney, R.; Omenetto, F.G.; Huang, Y.; Rogers, J.A. Transient, Biocompatible Electronics and Energy Harvesters Based on ZnO. Small 2013, 9, 3398–3404. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Guo, H.; Ribera, J.; Wu, C.; Tu, K.; Binelli, M.; Panzarasa, G.; Schwarze, F.W.; Wang, Z.L.; Burgert, I. Sustainable and Biodegradable Wood Sponge Piezoelectric Nanogenerator for Sensing and Energy Harvesting Applications. ACS Nano 2020, 14, 14665–14674. [Google Scholar] [CrossRef]
- Lin, J.C. A new IEEE standard for safety levels with respect to human exposure to radio-frequency radiation. IEEE Antennas Propag. Mag. 2006, 48, 157–159. [Google Scholar] [CrossRef]
- Lu, D.; Yan, Y.; Avila, R.; Kandela, I.; Stepien, I.; Seo, M.H.; Bai, W.; Yang, Q.; Li, C.; Haney, C.R.; et al. Bioresorbable, Wireless, Passive Sensors as Temporary Implants for Monitoring Regional Body Temperature. Adv. Healthc. Mater. 2020, 9, 2000942. [Google Scholar] [CrossRef]
- Guo, Q.; Koo, J.; Xie, Z.; Avila, R.; Yu, X.; Ning, X.; Zhang, H.; Liang, X.; Kim, S.B.; Yan, Y.; et al. A Bioresorbable Magnetically Coupled System for Low-Frequency Wireless Power Transfer. Adv. Funct. Mater. 2019, 29, 1905451. [Google Scholar] [CrossRef]
- Zhao, J.; Ghannam, R.; Htet, K.O.; Liu, Y.; Law, M.K.; Roy, V.A.; Michel, B.; Imran, M.A.; Heidari, H. Self-Powered Implantable Medical Devices: Photovoltaic Energy Harvesting Review. Adv. Healthc. Mater. 2020, 9, 2000779. [Google Scholar] [CrossRef]
- Kim, T.B.; Ho, C.-T.B. Validating the moderating role of age in multi-perspective acceptance model of wearable healthcare technology. Telemat. Inform. 2021, 61, 101603. [Google Scholar] [CrossRef]
- Huarng, K.-H.; Yu, T.H.-K.; Lee, C.F. Adoption model of healthcare wearable devices. Technol. Forecast. Soc. Chang. 2022, 174, 121286. [Google Scholar] [CrossRef]
- Yin, Z.; Yan, J.; Fang, S.; Wang, D.; Han, D. User acceptance of wearable intelligent medical devices through a modified unified theory of acceptance and use of technology. Ann. Transl. Med. 2022, 10, 629. [Google Scholar] [CrossRef]
- Zhao, S.; Fang, C.; Yang, J.; Sawan, M. Emerging Energy-Efficient Biosignal-Dedicated Circuit Techniques: A Tutorial Brief. IEEE Trans. Circ. Syst. II Express Briefs 2022, 69, 2592–2597. [Google Scholar] [CrossRef]
- Damaschke, J.M. Design of a low-input-voltage converter for thermoelectric generator. IEEE Trans. Ind. Appl. 1997, 33, 1203–1207. [Google Scholar] [CrossRef]
- Thielen, M.; Sigrist, L.; Magno, M.; Hierold, C.; Benini, L. Human body heat for powering wearable devices: From thermal energy to application. Energy Convers. Manag. 2017, 131, 44–54. [Google Scholar] [CrossRef]
- Mitcheson, P.D. Energy harvesting for human wearable and implantable bio-sensors. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 3432–3436. [Google Scholar]
- Borges, L.M.; Chávez-Santiago, R.; Barroca, N.; Velez, F.J.; Balasingham, I. Radio-frequency energy harvesting for wearable sensors. Healthc. Technol. Lett. 2015, 2, 22–27. [Google Scholar] [CrossRef]
- Shigeta, R.; Sasaki, T.; Quan, D.M.; Kawahara, Y.; Vyas, R.J.; Tentzeris, M.M.; Asami, T. Ambient RF Energy Harvesting Sensor Device With Capacitor-Leakage-Aware Duty Cycle Control. IEEE Sens. J. 2013, 13, 2973–2983. [Google Scholar] [CrossRef]
- Karlen, W.; Floreano, D. Adaptive Sleep–Wake Discrimination for Wearable Devices. IEEE Trans. Biomed. Eng. 2011, 58, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Jelicic, V.; Magno, M.; Brunelli, D.; Bilas, V.; Benini, L. Benefits of Wake-Up Radio in Energy-Efficient Multimodal Surveillance Wireless Sensor Network. IEEE Sens. J. 2014, 14, 3210–3220. [Google Scholar] [CrossRef]
- Anastasi, G.; Conti, M.; di Francesco, M.; Passarella, A. Energy conservation in wireless sensor networks: A survey. Ad. Hoc. Netw. 2009, 7, 537–568. [Google Scholar] [CrossRef]
- Hsu, J.; Zahedi, S.; Kansal, A.; Srivastava, M.; Raghunathan, V. Adaptive duty cycling for energy harvesting systems. In Proceedings of the 2006 International Symposium on Low Power Electronics and Design—ISLPED ’06, Bavaria, Germany, 4–6 October 2006; p. 180. [Google Scholar]
- Mallela, V.S.; Ilankumaran, V.; Rao, N.S. Trends in cardiac pacemaker batteries. Indian Pacing Electrophysiol. J. 2004, 4, 201–212. [Google Scholar] [PubMed]
- Pu, X.; Li, L.; Song, H.; Du, C.; Zhao, Z.; Jiang, C.; Cao, G.; Hu, W.; Wang, Z.L. A Self-Charging Power Unit by Integration of a Textile Triboelectric Nanogenerator and a Flexible Lithium-Ion Battery for Wearable Electronics. Adv. Mater. 2015, 27, 2472–2478. [Google Scholar] [CrossRef]
- Gaikwad, A.M.; Khau, B.v.; Davies, G.; Hertzberg, B.; Steingart, D.A.; Arias, A.C. A High Areal Capacity Flexible Lithium-Ion Battery with a Strain-Compliant Design. Adv. Energy Mater. 2015, 5, 1401389. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Kim, J.S.; Noh, J.; Lee, I.; Kim, H.J.; Choi, S.; Seo, J.; Jeon, S.; Kim, T.S.; Lee, J.Y.; et al. Wearable Textile Battery Rechargeable by Solar Energy. Nano Lett. 2013, 13, 5753–5761. [Google Scholar] [CrossRef]
- Li, N.; Chen, Z.; Ren, W.; Li, F.; Cheng, H.-M. Flexible graphene-based lithium ion batteries with ultrafast charge and discharge rates. Proc. Natl. Acad. Sci. USA 2012, 109, 17360–17365. [Google Scholar] [CrossRef]
- IEEE Std 802.15.1-2005 (Revision of IEEE Std 802.15.1-2002); IEEE Standard for Information technology—Local and metropolitan area networks—Specific requirements– Part 15.1a: Wireless Medium Access Control (MAC) and Physical Layer (PHY) specifications for Wireless Personal Area Networks (WPAN). IEEE: New York, NY, USA, 14 June 2005; pp. 1–700.
- Baker, N. ZigBee and Bluetooth strengths and weaknesses for industrial applications. IEEE Comput. Control Eng. 2005, 16, 20–25. [Google Scholar] [CrossRef]
- Hsu, J.M.; Chiang, T.C.; Yu, Y.C.; Teng, W.G.; Hou, T.W. A New Energy Efficient and Reliable MedRadio Scheme Based on Cooperative Communication for Implanted Medical Devices. Int. J. Distrib. Sens. Netw. 2015, 11, 239597. [Google Scholar] [CrossRef]
- Li, H.; Tan, J. Heartbeat-Driven Medium-Access Control for Body Sensor Networks. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 44–51. [Google Scholar] [PubMed]
- Hassan, O.; Paul, T.; Shuvo, M.M.H.; Parvin, D.; Thakker, R.; Chen, M.; Mosa, A.S.M.; Islam, S.K. Energy Efficient Deep Learning Inference Embedded on FPGA for Sleep Apnea Detection. J. Sign. Process. Syst. 2022, 94, 609–619. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).