Porous Activated Carbons Derived from Coffee Waste for Use as Functional Separators in Lithium-Sulfur Batteries
Abstract
1. Introduction
2. Experimental
2.1. Synthesis of Activated Carbons (ACs) Derived from Coffee Waste
2.2. MWCNT/S as a Cathode
2.3. Materials Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tao, T.; Lu, S.; Fan, Y.; Lei, W.; Huang, S.; Chen, Y. Anode Improvement in Rechargeable Lithium–Sulfur Batteries. Adv. Mater. 2017, 29, 1700542. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Ma, W.; Zhang, S.; Tang, B. Recent advances in shuttle effect inhibition for lithium sulfur batteries. Energy Storage Mater. 2019, 23, 707–732. [Google Scholar] [CrossRef]
- Li, J.; Wei, W.; Meng, L. Liquid-phase exfoliated-graphene-supporting nanostructural sulfur as high-performance lithium–sulfur batteries cathode. Compos. Commun. 2019, 15, 149–154. [Google Scholar] [CrossRef]
- Sun, J.; Ma, J.; Fan, J.; Pyun, J.; Geng, J. Rational design of sulfur-containing composites for high-performance lithium-sulfur batteries. APL Mater. 2019, 7, 020904. [Google Scholar] [CrossRef]
- Pan, Z.; Brett, D.J.L.; He, G.; Parkin, I.P. Progress and Perspectives of Organosulfur for Lithium–Sulfur Batteries. Adv. Energy Mater. 2022, 12, 2103483. [Google Scholar] [CrossRef]
- Zhu, J.; Zou, J.; Cheng, H.; Gu, Y.; Lu, Z. High energy batteries based on sulfur cathode. Green Energy Environ. 2019, 4, 345–359. [Google Scholar] [CrossRef]
- Sadd, M.; De Angelis, S.; Colding-Jørgensen, S.; Blanchard, D.; Johnsen, R.E.; Sanna, S.; Borisova, E.; Matic, A.; Bowen, J.R. Visualization of Dissolution-Precipitation Processes in Lithium–Sulfur Batteries. Adv. Energy Mater. 2022, 12, 2103126. [Google Scholar] [CrossRef]
- Yu, L.; Ong, S.J.H.; Liu, X.; Mandler, D.; Xu, Z.J. The importance of the dissolution of polysulfides in lithium-sulfur batteries and a perspective on high-energy electrolyte/cathode design. Electrochim. Acta 2021, 392, 139013. [Google Scholar] [CrossRef]
- Zhao, M.; Li, B.Q.; Zhang, X.Q.; Huang, J.Q.; Zhang, Q. A Perspective toward Practical Lithium-Sulfur Batteries. ACS Cent. Sci. 2020, 6, 1095–1104. [Google Scholar] [CrossRef]
- Zhao, Z.; Pathak, R.; Wang, X.; Yang, Z.; Li, H.; Qiao, Q. Sulfiphilic FeP/rGO as a highly efficient sulfur host for propelling redox kinetics toward stable lithium-sulfur battery. Electrochim. Acta 2020, 364, 137117. [Google Scholar] [CrossRef]
- Li, S.; Zhang, W.; Zheng, J.; Lv, M.; Song, H.; Du, L. Inhibition of Polysulfide Shuttles in Li–S Batteries: Modified Separators and Solid-State Electrolytes. Adv. Energy Mater. 2021, 11, 2000779. [Google Scholar] [CrossRef]
- Shen, C.; Xie, J.; Zhang, M.; Andrei, P.; Zheng, J.P.; Hendrickson, M.; Plichta, E.J. A Li-Li2S4 battery with improved discharge capacity and cycle life at low electrolyte/sulfur ratios. J. Power Sources 2019, 414, 412–419. [Google Scholar] [CrossRef]
- Li, S.; Leng, D.; Li, W.; Qie, L.; Dong, Z.; Cheng, Z.; Fan, Z. Recent progress in developing Li2S cathodes for Li–S batteries. Energy Storage Mater. 2020, 27, 279–296. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Younus, H.A.; Wang, B.; Weng, Q.; Zhang, Y.; Zhang, S. An overview of the characteristics of advanced binders for high-performance Li–S batteries. Nano Mater. Sci. J. 2021, 3, 124–139. [Google Scholar] [CrossRef]
- Deng, S.; Yan, Y.; Wei, L.; Li, T.; Su, X.; Yang, X.; Li, Z.; Wu, M. Amorphous Al2O3 with N-Doped Porous Carbon as Efficient Polysulfide Barrier in Li−S Batteries. ACS Appl. Energy Mater. 2019, 2, 1266–1273. [Google Scholar] [CrossRef]
- Kim, S.; Lim, W.G.; Cho, A.; Jeong, J.; Jo, C.; Kang, D.; Han, S.M.; Han, J.W.; Lee, J. Simultaneous Suppression of Shuttle Effect and Lithium Dendrite Growth by Lightweight Bifunctional Separator for Li-S Batteries. ACS Appl. Energy Mater. 2020, 3, 2643–2652. [Google Scholar] [CrossRef]
- Fan, L.; Li, M.; Li, X.; Xiao, W.; Chen, Z.; Lu, J. Interlayer Material Selection for Lithium-Sulfur Batteries. Joule 2019, 3, 361–386. [Google Scholar] [CrossRef]
- Chen, L.; Yu, H.; Li, W.; Dirican, M.; Liu, Y.; Zhang, X. Interlayer design based on carbon materials for lithium-sulfur batteries: A review. J. Mater. Chem. A 2020, 8, 10709–10735. [Google Scholar] [CrossRef]
- Wei, H.; Liu, Y.; Zhai, X.; Wang, F.; Ren, X.; Tao, F.; Li, T.; Wang, G.; Ren, F. Application of Carbon Nanotube-Based Materials as Interlayers in High-Performance Lithium-Sulfur Batteries: A Review. Front. Energy Res. 2020, 8, 585795. [Google Scholar] [CrossRef]
- Li, M.; Fu, K.; Wang, Z.; Cao, C.; Yang, J.; Zhai, Q.; Zhou, Z.; Ji, J.; Xue, Y.; Tang, C. Enhanced Adsorption of Polysulfides on Carbon Nanotubes/Boron Nitride Fibers for High-Performance Lithium-Sulfur Batteries. Chem. A Eur. J. 2020, 26, 17567–17573. [Google Scholar] [CrossRef]
- Xu, T.; Song, J.; Gordin, M.L.; Sohn, H.; Yu, Z.; Chen, S.; Wang, D. Mesoporous Carbon–Carbon Nanotube–Sulfur Composite Microspheres for High-Areal-Capacity Lithium–Sulfur Battery Cathodes. ACS Appl. Mater. Interfaces 2013, 5, 11355–11362. [Google Scholar] [CrossRef] [PubMed]
- Petnikota, S.; Rotte, N.K.; Srikanth, V.V.S.S.; Kota, B.S.R.; Reddy, M.V.; Loh, K.P.; Chowdari, B.V.R. Electrochemical studies of few-layered graphene as an anode material for Li ion batteries. J. Solid State Electrochem. 2014, 18, 941–949. [Google Scholar] [CrossRef]
- Suleman, M.; Othman, M.A.R.; Hashmi, S.A.; Kumar, Y.; Deraman, M.; Omar, R.; Jasni, M.R.M. Activated graphene oxide/reduced graphene oxide electrodes and low viscous sulfonium cation based ionic liquid incorporated flexible gel polymer electrolyte for high rate supercapacitors. J. Alloys Compd. 2017, 695, 3376–3392. [Google Scholar] [CrossRef]
- Tsai, W.Y.; Lin, R.; Murali, S.; Li Zhang, L.; McDonough, J.K.; Ruoff, R.S.; Taberna, P.L.; Gogotsi, Y.; Simon, P. Outstanding performance of activated graphene based supercapacitors in ionic liquid electrolyte from −50 to 80 °C. Nano Energy 2013, 2, 403–411. [Google Scholar] [CrossRef]
- Nishihara, H.; Kyotani, T. Zeolite-templated carbons—Three-dimensional microporous graphene frameworks. Chem. Commun. 2018, 54, 5648. [Google Scholar] [CrossRef]
- Chung, D.Y.; Son, Y.J.; Yoo, J.M.; Kang, J.S.; Ahn, C.Y.; Park, S.; Sung, Y.E. Coffee Waste-Derived Hierarchical Porous Carbon as a Highly Active and Durable Electrocatalyst for Electrochemical Energy Applications. ACS Appl. Mater. Interfaces 2017, 9, 41303–41313. [Google Scholar] [CrossRef]
- Zhao, P.; Shiraz, M.H.A.; Zhu, H.; Liu, Y.; Tao, L.; Liu, J. Hierarchically porous carbon from waste coffee grounds for high-performance Li–Se batteries. Electrochim. Acta 2019, 325, 134931. [Google Scholar] [CrossRef]
- Sangprasert, T.; Sattayarut, V.; Rajrujithong, C.; Khanchaitit, P.; Khemthong, P.; Chanthad, C.; Grisdanurak, N. Making use of the inherent nitrogen content of spent coffee grounds to create nanostructured activated carbon for supercapacitor and lithium-ion battery applications. Diam. Relat. Mater. 2022, 127, 109164. [Google Scholar] [CrossRef]
- Colon, M.; Nerin, C. Role of catechins in the antioxidant capacity of an active film containing green tea, green coffee, and grapefruit extracts. J. Agric. Food Chem. 2012, 60, 9842–9849. [Google Scholar] [CrossRef]
- Zhu, L.; Jiang, H.; Yang, Q.; Yao, S.; Shen, X.; Tu, F. An Effective Porous Activated Carbon Derived from Puffed Corn Employed as the Separator Coating in a Lithium–Sulfur Battery. Energy Technol. 2019, 7, 1900752. [Google Scholar] [CrossRef]
- Manoj, M.; Muhamed Ashraf, C.; Jasna, M.; Anilkumar, K.M.; Jinisha, B.; Pradeep, V.S.; Jayalekshmi, S. Biomass-derived, activated carbon-sulfur composite cathode with a bifunctional interlayer of functionalized carbon nanotubes for lithium-sulfur cells. J. Colloid Interface Sci. 2019, 535, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; De, M. Alumina based doped templated carbons: A comparative study with zeolite and silica gel templates. Microporous Mesoporous Mater. 2018, 257, 241–252. [Google Scholar] [CrossRef]
- El-Hendawy, A.N.A. An insight into the KOH activation mechanism through the production of microporous activated carbon for the removal of Pb2+ cations. Appl. Surf. Sci. 2009, 255, 3723–3730. [Google Scholar] [CrossRef]
- Xia, C.; Shi, S.Q. Self-activation for activated carbon from biomass: Theory and parameters. Green Chem. 2016, 18, 2063–2071. [Google Scholar] [CrossRef]
- Shiryaev, A.A.; Voloshchuk, A.M.; Volkov, V.V.; Averin, A.A.; Artamonova, S.D. Nanoporous active carbons at ambient conditions: A comparative study using X-ray scattering and diffraction, Raman spectroscopy and N2 adsorption. J. Phys. Conf. Ser. 2017, 848, 012009. [Google Scholar] [CrossRef]
- Bai, L.; Ge, Y.; Bai, L. Boron and nitrogen co-doped porous carbons synthesized from polybenzoxazines for high-performance supercapacitors. Coatings 2019, 9, 657. [Google Scholar] [CrossRef]
- Ma, F.; Ding, S.; Ren, H.; Liu, Y. Sakura-based activated carbon preparation and its performance in supercapacitor applications. RSC Adv. 2019, 9, 2474–2483. [Google Scholar] [CrossRef]
- de Paula, F.G.F.; Campello-Gómez, I.; Ortega, P.F.R.; Rodríguez-Reinoso, F.; Martínez-Escandell, M.; Silvestre-Albero, J. Structural flexibility in activated carbon materials prepared under harsh activation conditions. Materials 2019, 12, 1988. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-Based Supercapacitors Produced by Activation of Graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef]
- Men’Shchikov, I.; Shkolin, A.; Khozina, E.; Fomkin, A. Thermodynamics of adsorbed methane storage systems based on peat-derived activated carbons. Nanomaterials 2020, 10, 1379. [Google Scholar] [CrossRef]
- Shrestha, R.L.; Chaudhary, R.; Shrestha, T.; Tamrakar, B.M.; Shrestha, R.G.; Maji, S.; Hill, J.P.; Ariga, K.; Shrestha, L.K. Nanoarchitectonics of Lotus Seed Derived Nanoporous Carbon Materials for Supercapacitor Applications. Materials 2020, 13, 5434. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, Y.; Manthiram, A. MOF-derived Cobalt Sulfide Grown on 3D Graphene Foam as an Efficient Sulfur Host for Long-Life Lithium-Sulfur Batteries. iScience 2018, 4, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Qian, J.; Zhao, T.; Ye, Y.; Xing, Y.; Huang, Y.; Wei, L.; Zhang, N.; Chen, N.; Li, L.; et al. Boosting High-Rate Li–S Batteries by an MOF-Derived Catalytic Electrode with a Layer-by-Layer Structure. Adv. Sci. 2019, 6, 1802362. [Google Scholar] [CrossRef] [PubMed]
- Mahankali, K.; Nagarajan, S.; Thangavel, N.K.; Rajendran, S.; Yeddala, M.; Arava, L.M.R. Metal-Based Electrocatalysts for High-Performance Lithium-Sulfur Batteries: A Review. Catalysts 2020, 10, 1137. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Z.; Knibbe, R.; Luo, B.; Abdul Ahad, S.; Sun, D.; Wang, L. Cyclic Voltammetry in Lithium–Sulfur Batteries—Challenges and Opportunities. Energy Technol. 2019, 7, 1801001. [Google Scholar] [CrossRef]
- Wang, R.; Wu, R.; Ding, C.; Chen, Z.; Xu, H.; Liu, Y.; Zhang, J.; Ha, Y.; Fei, B.; Pan, H. Porous Carbon Architecture Assembled by Cross-Linked Carbon Leaves with Implanted Atomic Cobalt for High-Performance Li–S Batteries. Nano-Micro Lett. 2021, 13, 151. [Google Scholar] [CrossRef]
- Li, R.; Sun, X.; Zou, J.; He, Q. Coordination effect of biocatalyst dithiothreitol and aramid fiber interlayer for lithium-sulfur batteries. J. Mater. Sci. Mater. Electron. 2020, 31, 14233–14240. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Wan, C.; Du, K.; Xie, J.; Xu, N. Sulfur Composite Cathode Materials for Rechargeable Lithium Batteries. Adv. Funct. Mater. 2003, 13, 487–492. [Google Scholar] [CrossRef]
- Guo, J.; Liu, J. A binder-free electrode architecture design for lithium-sulfur batteries: A review. Nanoscale Adv. 2019, 1, 2104–2122. [Google Scholar] [CrossRef]
- Yan, J.; Liu, X.; Li, B. Capacity Fade Analysis of Sulfur Cathodes in Lithium–Sulfur Batteries. Adv. Sci. 2016, 3, 1600101. [Google Scholar] [CrossRef]
- Li, B.; Xie, M.; Yi, G.; Zhang, C. Biomass-derived activated carbon/sulfur composites as cathode electrodes for Li–S batteries by reducing the oxygen content. RSC Adv. 2020, 10, 2823–2829. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yang, W.; Dong, L.; Gao, X.; Wang, G.; Shao, G. Enabling immobilization and conversion of polysulfides through a nitrogen-doped carbon nanotubes/ultrathin MoS2 nanosheet core-shell architecture for lithium-sulfur batteries. J. Mater. Chem. A 2019, 7, 13103–13112. [Google Scholar] [CrossRef]
- Wu, H.; Xia, L.; Ren, J.; Zheng, Q.; Xu, C.; Lin, D. A high-efficiency N/P co-doped graphene/CNT@porous carbon hybrid matrix as a cathode host for high performance lithium-sulfur batteries. J. Mater. Chem. A 2017, 5, 20458–20472. [Google Scholar] [CrossRef]
- Qi, Y.; Li, Q.J.; Wu, Y.; Bao, S.J.; Li, C.; Chen, Y.; Wang, G.; Xu, M. A Fe3N/carbon composite electrocatalyst for effective polysulfides regulation in room-temperature Na-S batteries. Nat. Commun. 2021, 12, 3791. [Google Scholar] [CrossRef] [PubMed]
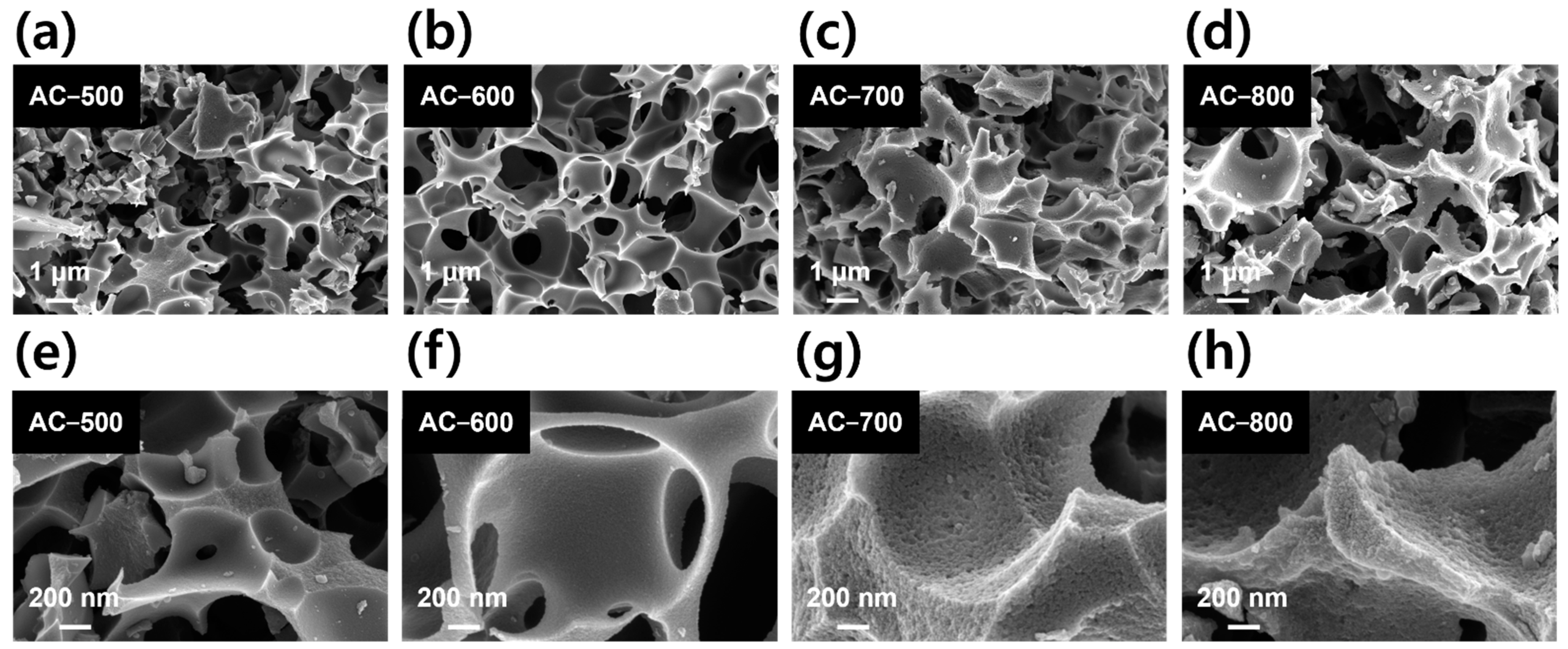
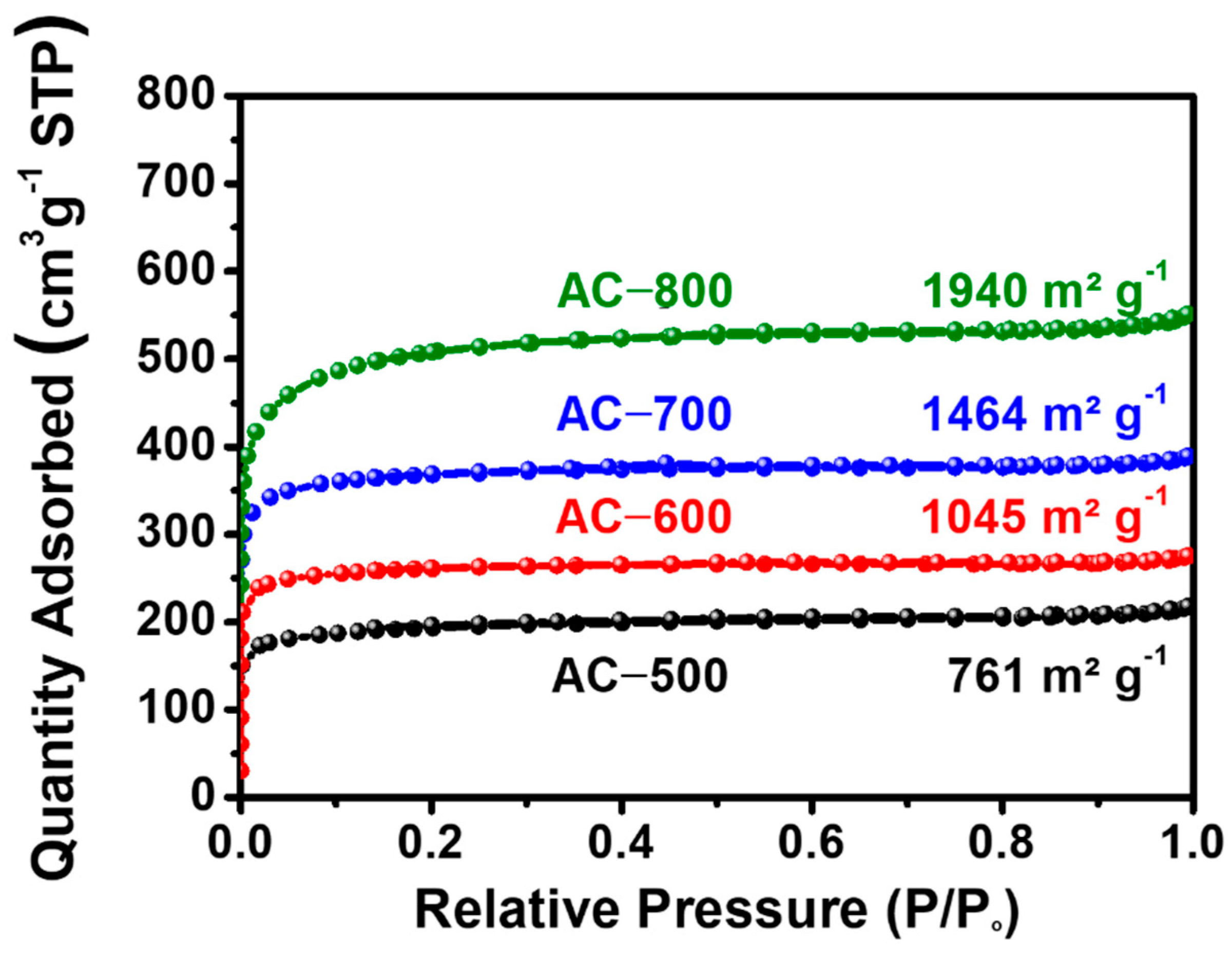
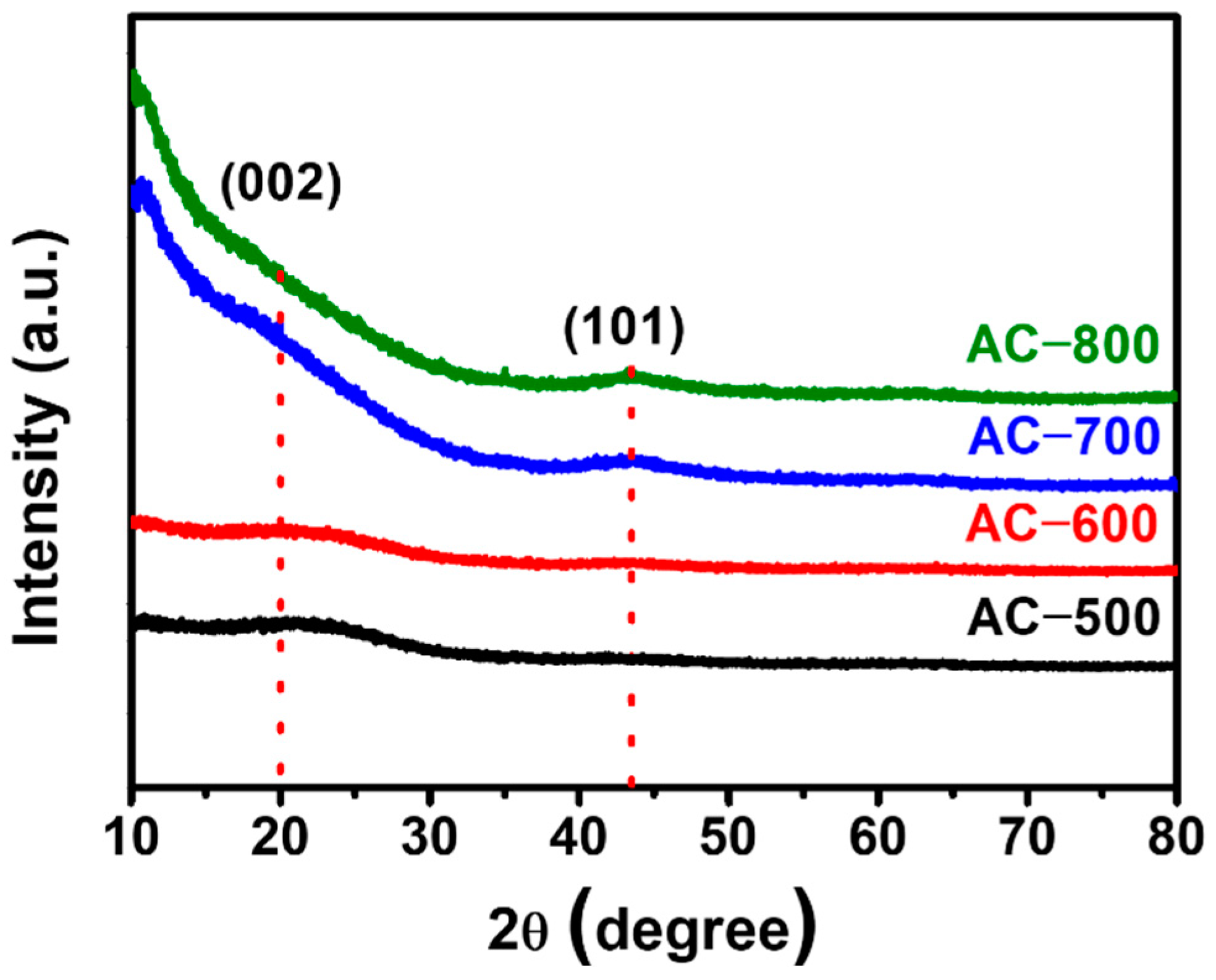
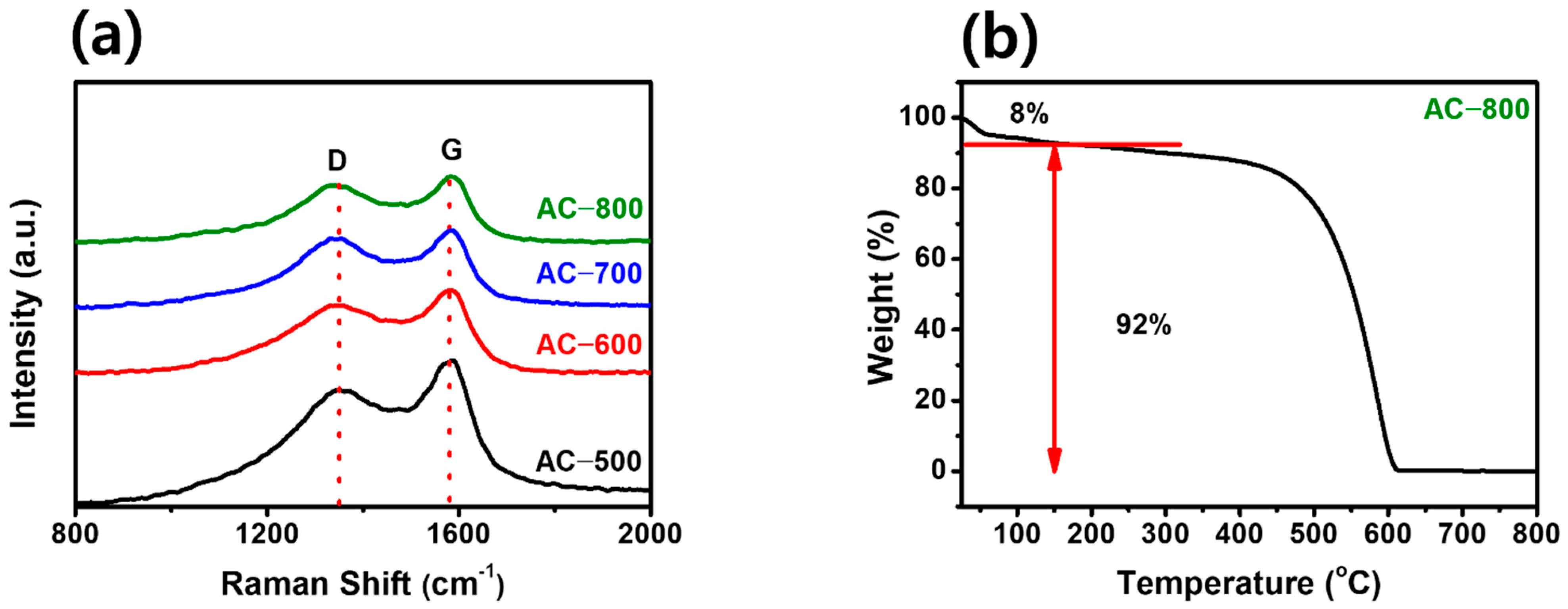

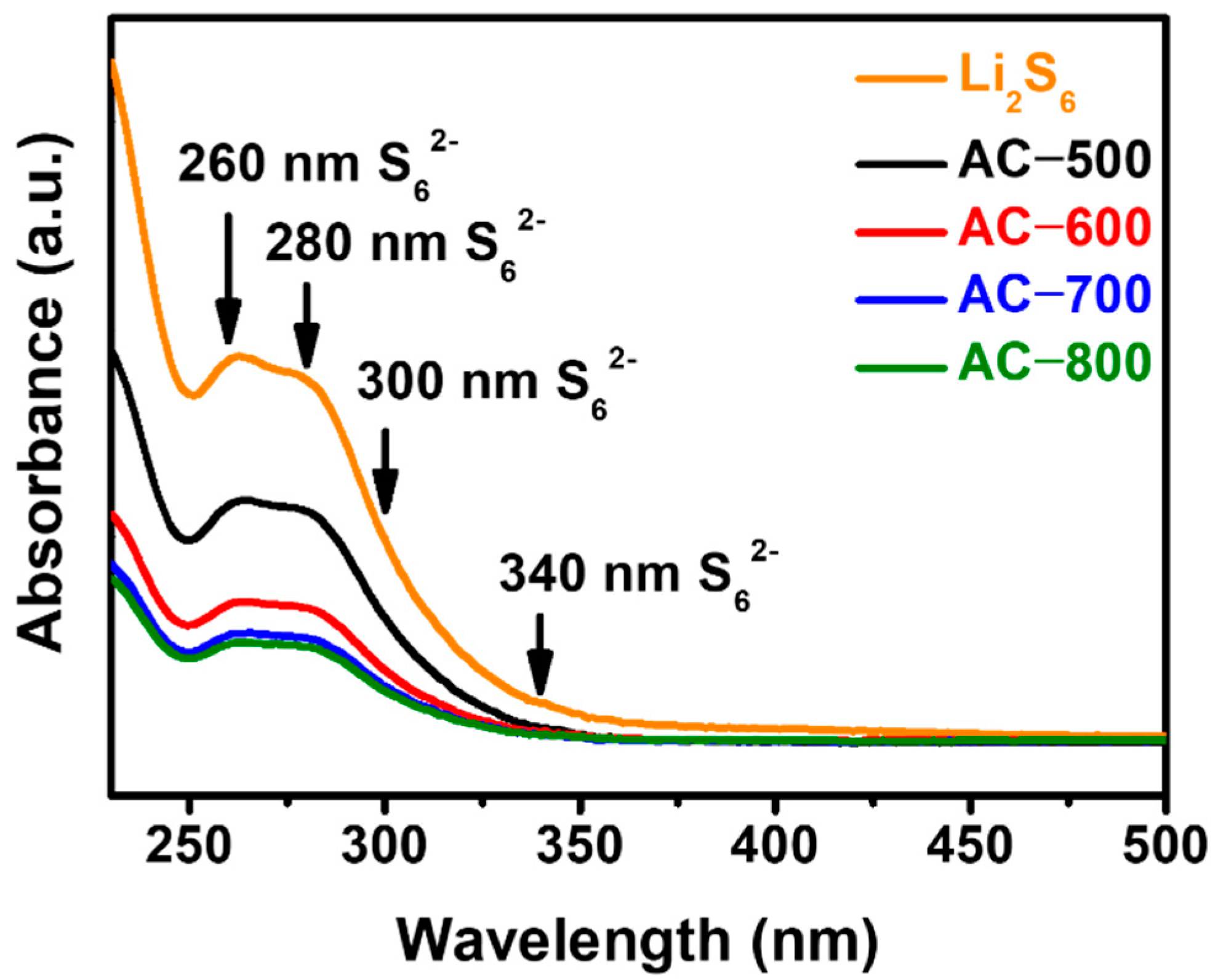

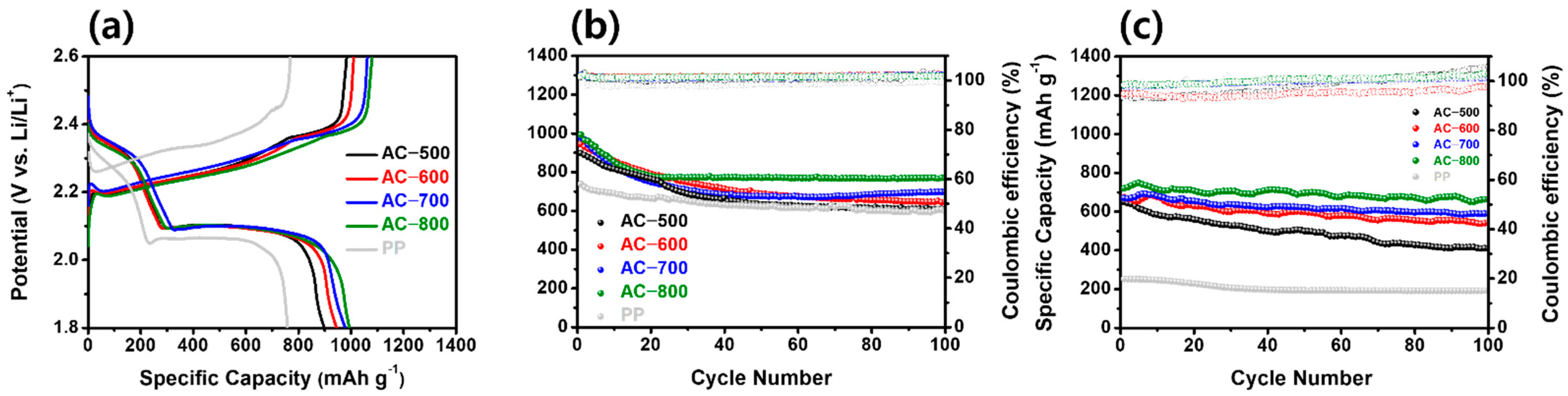
| Sample | BET Surface Area (m2 g−1) | Micro Surface Area (m2 g−1) | Micropore Volume (cm3 g−1) | Median Pore Width (nm) | Ref. |
|---|---|---|---|---|---|
| Alumina- Sodium dodecyl sulfate | 288 | 16 | 0.68 | 6.5 | [32] |
| Zeolite | 1664 | 597 | 1.0 | 1.3 | [32] |
| Silica Gel | 740 | 0 | 0.65 | 5.7 | [32] |
| Activated carbon | 1864 | 690 | 0.87 | 1.8 | [31] |
| Sample | BET Surface Area (m2 g−1) | Micro Surface Area (m2 g−1) | Micropore Volume (cm3 g−1) | Median Pore Width (nm) |
|---|---|---|---|---|
| AC-500 | 761 ± 11.89 | 629 ± 9.79 | 0.24 ± 0.005 | 0.54 ± 0.003 |
| AC-600 | 1054 ± 17.42 | 938 ± 6.70 | 0.36 ± 0.006 | 0.51 ± 0.001 |
| AC-700 | 1464 ± 25.26 | 1295 ± 12.54 | 0.49 ± 0.004 | 0.54 ± 0.001 |
| AC-800 | 1940 ± 32.77 | 1470 ± 35.38 | 0.58 ± 0.02 | 0.61 ± 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.-H.; Park, Y.-Y.; Moon, S.-H.; Kim, J.-H.; Jang, J.-S.; Kim, S.-B.; Lee, S.-N.; Park, K.-W. Porous Activated Carbons Derived from Coffee Waste for Use as Functional Separators in Lithium-Sulfur Batteries. Energies 2022, 15, 7961. https://doi.org/10.3390/en15217961
Shin J-H, Park Y-Y, Moon S-H, Kim J-H, Jang J-S, Kim S-B, Lee S-N, Park K-W. Porous Activated Carbons Derived from Coffee Waste for Use as Functional Separators in Lithium-Sulfur Batteries. Energies. 2022; 15(21):7961. https://doi.org/10.3390/en15217961
Chicago/Turabian StyleShin, Jae-Hoon, Yu-Yeon Park, Sang-Hyun Moon, Ji-Hwan Kim, Jae-Sung Jang, Sung-Beom Kim, Seong-Nam Lee, and Kyung-Won Park. 2022. "Porous Activated Carbons Derived from Coffee Waste for Use as Functional Separators in Lithium-Sulfur Batteries" Energies 15, no. 21: 7961. https://doi.org/10.3390/en15217961
APA StyleShin, J.-H., Park, Y.-Y., Moon, S.-H., Kim, J.-H., Jang, J.-S., Kim, S.-B., Lee, S.-N., & Park, K.-W. (2022). Porous Activated Carbons Derived from Coffee Waste for Use as Functional Separators in Lithium-Sulfur Batteries. Energies, 15(21), 7961. https://doi.org/10.3390/en15217961






